9-Hydroxyaristoquinolone: A New Indole Alkaloid Isolated from Aristotelia chilensis with Inhibitory Activity of NF-κB in HMC-3 Microglia Cells
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of 9-Hydroxyaristoquinolone (3)
2.2. Analytical Data for 9-Hydroxyaristoquinolone
2.3. Evaluation of the Pharmacokinetic and Toxicity Profiles of 9-Hydroxyaristoquinolone by In Silico
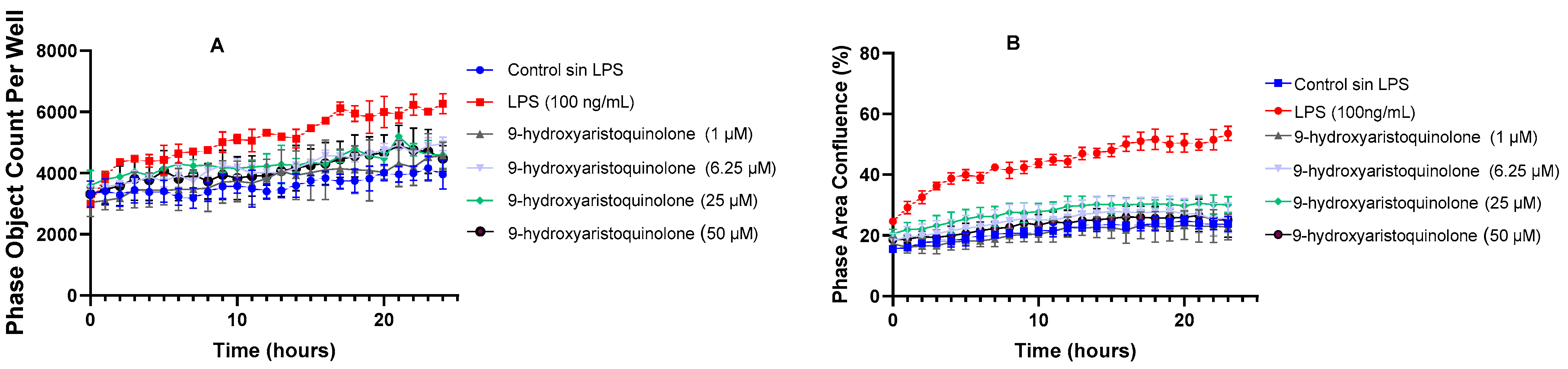
2.4. Kinetic Assays of Morphological Changes by Live Cell AnalysisIncucyte®: 9-Hydroxyaristoquinolone Inhibits LPS-Induced Microglial Reactivation
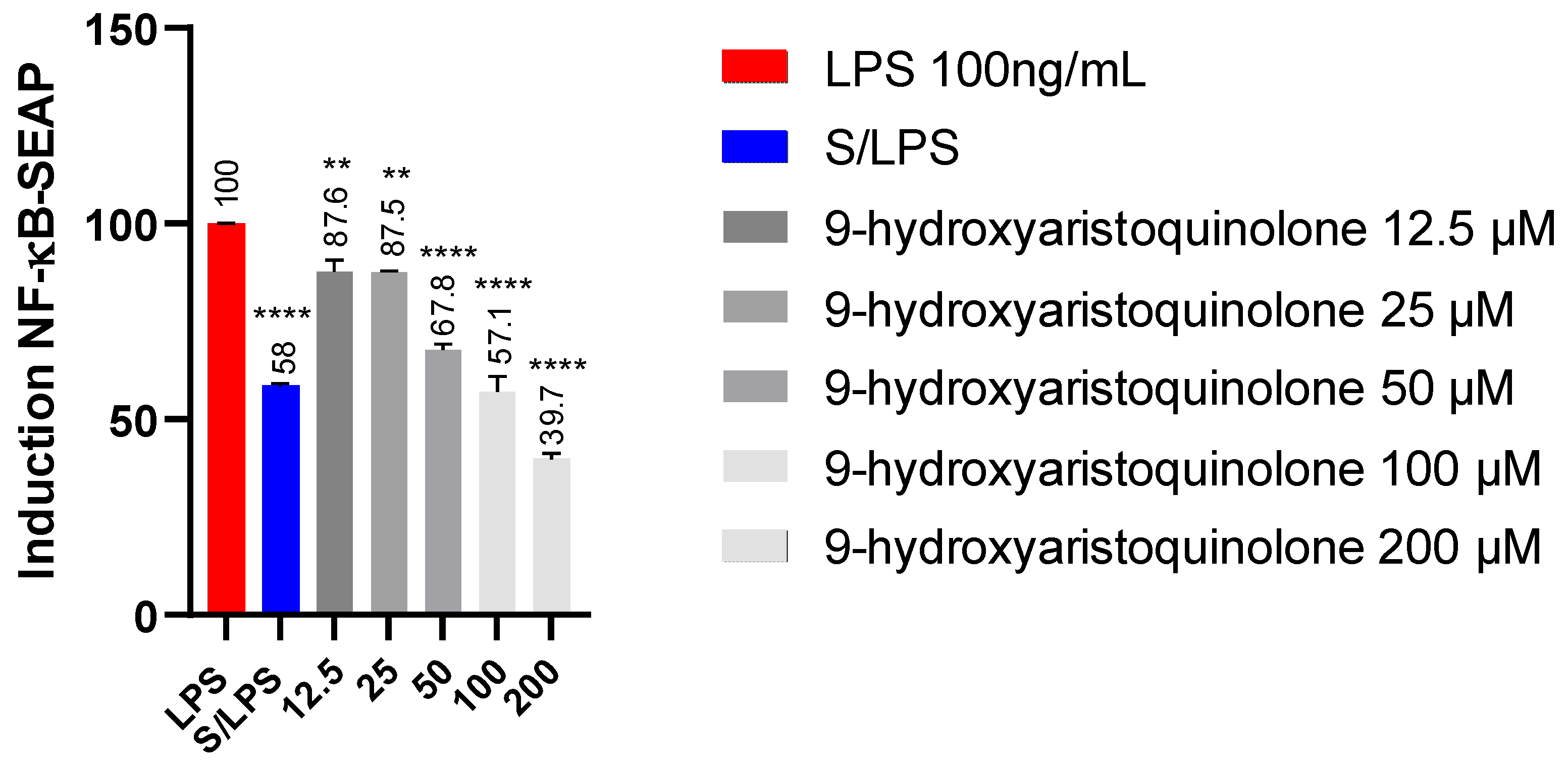
2.5. Detection of NF-kB-SEAP Levels:9-Hydroxyaristoquinolone Reduces NF-KB Pathway Activity in THP-1 Reporter Cell
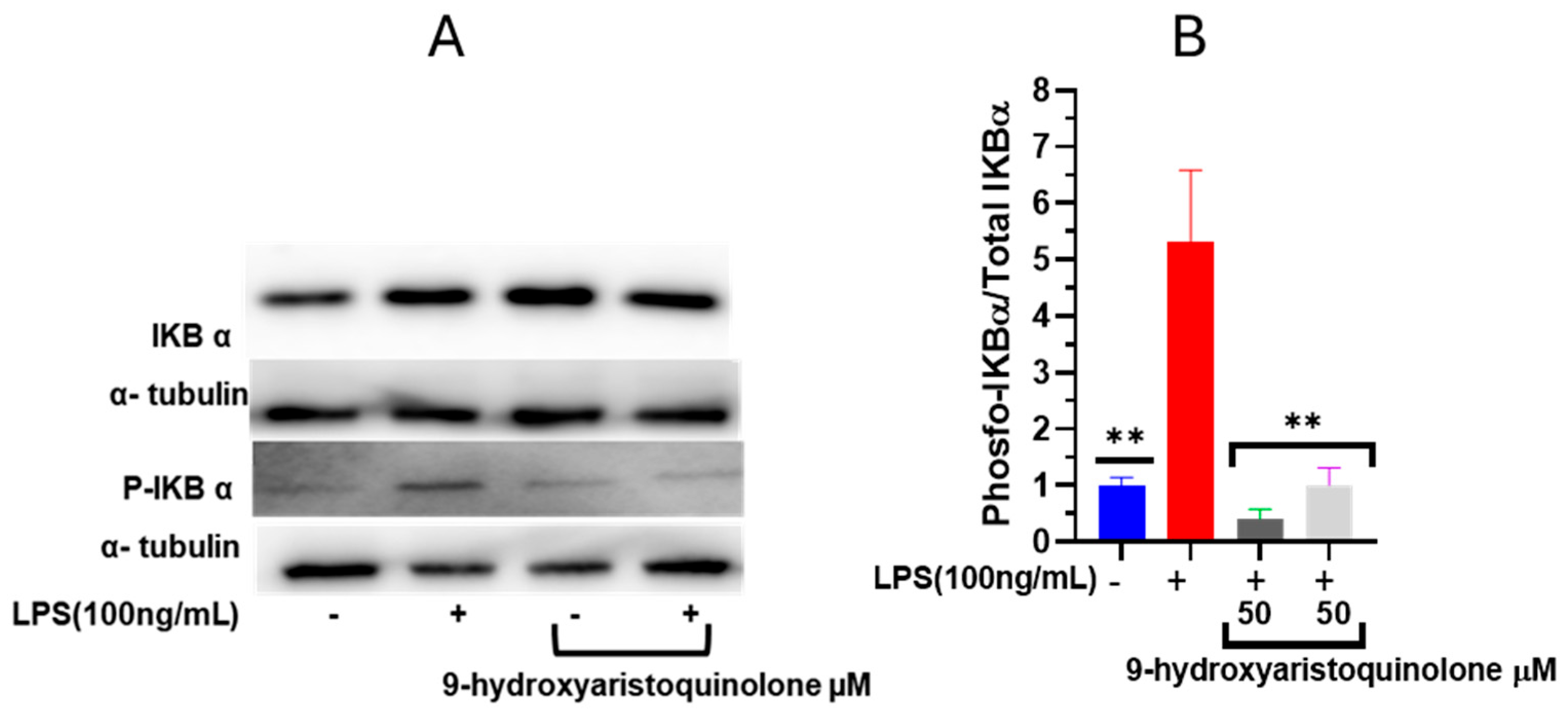
2.6. Nuclear Factor NF-κB Modulation Assay: 9-Hydroxyaristoquinolone Inhibits LPS-Induced Phosphorylation of IκB-α
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Purification of 9-Hydroxyaristoquinolone from Leaves of Aristotelia chilensis
4.3. Chemical Characterization of 9-Hydroxyaristoquinolone
4.4. Pharmacokinetic and Toxicity Profiles of 9-Hydroxyaristoquinolone
4.5. Cell Culture
4.6. Kinetic Assays of Morphological Changes by Live Cell Analysis -Incucyte®
4.7. Detection of NF-κB-SEAP Levels by THP-1-Dual Cells
4.8. Western Blot Assay on the Modulation of Nuclear Factor NF-κB
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cao, X.; Hou, Y.; Zhang, X.; Xu, C.; Jia, P.; Sun, X.; Sun, L.; Gao, Y.; Yang, H.; Cui, Z.; et al. A comparative, correlate analysis and projection of global and regional life expectancy, healthy life expectancy, and their GAP: 1995–2025. J. Glob. Health 2020, 10, 020407. [Google Scholar] [CrossRef]
- Naciones Unidas. Desafios Globales adlphwuoeg-ipcd. Available online: https://www.un.org/development/desa/pd/es/content/World-Population-Prospects-2022 (accessed on 14 October 2024).
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Zhang, X.X.; Tian, Y.; Wang, Z.T.; Ma, Y.H.; Tan, L.; Yu, J.T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Miranda, A.; Montiel, E.; Ulrich, H.; Paz, C. Selective Secretase Targeting for Alzheimer’s Disease Therapy. J. Alzheimer’s Dis. 2021, 81, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Doens, D.; Fernández, P.L. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef]
- García-Revilla, J.; Alonso-Bellido, I.M.; Burguillos, M.A.; Herrera, A.J.; Espinosa-Oliva, A.M.; Ruiz, R.; Cruz-Hernández, L.; García-Domínguez, I.; Roca-Ceballos, M.A.; Santiago, M.; et al. Reformulating Pro-Oxidant Microglia in Neurodegeneration. J. Clin. Med. 2019, 8, 1719. [Google Scholar] [CrossRef]
- Pampuscenko, K.; Morkuniene, R.; Krasauskas, L.; Smirnovas, V.; Brown, G.C.; Borutaite, V. Extracellular tau stimulates phagocytosis of living neurons by activated microglia via Toll-like 4 receptor–NLRP3 inflammasome–caspase-1 signalling axis. Sci. Rep. 2023, 13, 10813. [Google Scholar] [CrossRef]
- Hopp, S.C.; Lin, Y.; Oakley, D.; Roe, A.D.; DeVos, S.L.; Hanlon, D.; Hyman, B.T. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 269. [Google Scholar] [CrossRef]
- Patel, J.R.; Brewer, G.J. Age-related changes to tumor necrosis factor receptors affect neuron survival in the presence of beta-amyloid. J. Neurosci. Res. 2008, 86, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Braak, H.; Del Tredici, K.; Leyh, J.; Lier, J.; Khoshbouei, H.; Eisenlöffel, C.; Müller, W.; Bechmann, I. Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 2018, 66, 2550–2562. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139 (Suppl. 2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Jiang, X.; Leak, R.K.; Shi, Y.; Hu, X.; Chen, J. Microglial Responses to Brain Injury and Disease: Functional Diversity and New Opportunities. Transl. Stroke Res. 2021, 12, 474–495. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, J.A.; Kavanagh, E.; Engskog-Vlachos, P.; Engskog, M.K.; Herrera, A.J.; Espinosa-Oliva, A.M.; Joseph, B.; Hajji, N.; Venero, J.L.; Burguillos, M.A. Microglia: Agents of the CNS Pro-Inflammatory Response. Cells 2020, 9, 1717. [Google Scholar] [CrossRef]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an Inducible Regulator of Inflammation in the Central Nervous System. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB Pathway and Its Inhibitors: A Promising Frontier in the Management of Alzheimer’s Disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef]
- Muñoz, O.; Christen, P.; Cretton, S.; Backhouse, N.; Torres, V.; Correa, O.; Costa, E.; Miranda, H.; Delporte, C. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J. Pharm. Pharmacol. 2011, 63, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of antioxidant compounds and α-Glucosidase/α-Amylase inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef]
- Velázquez, L.; Quiñones, J.; Inostroza, K.; Sepúlveda, G.; Díaz, R.; Scheuermann, E.; Domínguez, R.; Lorenzo, J.M.; Velásquez, C.; Sepúlveda, N. Maqui (Aristotelia chilensis (Mol.) Stuntz): A Natural Antioxidant to Improve Quality of Meat Patties. Antioxidants 2022, 11, 1405. [Google Scholar] [CrossRef]
- Cespedes, C.; Jakupovic, J.; Silva, M.; Watson, W. Indole alkaloids from Aristotelia chilensis. Phytochemistry 1990, 29, 1354–1356. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Paredes, A.; Nwokocha, C.R.; Paz, C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules 2018, 23, 3050. [Google Scholar] [CrossRef]
- Pérez, R.; Figueredo, C.; Burgos, V.; Cabrera-Pardo, J.R.; Schmidt, B.; Heydenreich, M.; Koch, A.; Deuis, J.R.; Deuis, J.R.; Paz, C. Natural Compounds Purified from the Leaves of Aristotelia chilensis: Makomakinol, a New Alkaloid and the Effect of Aristoteline and Hobartine on NaV Channels. Int. J. Mol. Sci. 2023, 24, 15504. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Palacios, J.; Jofré, I.; Paz, C.; Nwokocha, C.R.; Paredes, A.; Cifuentes, F. Aristoteline, an Indole-Alkaloid, Induces Relaxation by Activating Potassium Channels and Blocking Calcium Channels in Isolated Rat Aorta. Molecules 2019, 24, 2748. [Google Scholar] [CrossRef] [PubMed]
- Argade, M.D.; Riley, A.P. Syntheses of Aristotelia Alkaloids: Reflections in the Chiral Pool. Synlett Acc. Rapid Commun. Synth. Org. Chem. 2022, 33, 1209–1214. [Google Scholar]
- Argade, M.D.; Straub, C.J.; Rusali, L.E.; Santarsiero, B.D.; Riley, A.P. Synthesis of Aristoquinoline Enantiomers and Their Evaluation at the α3β4 Nicotinic Acetylcholine Receptor. Org. Lett. 2021, 23, 7693–7697. [Google Scholar] [CrossRef]
- Arias, H.R.; Ortells, M.O.; Feuerbach, D.; Burgos, V.; Paz, C. Alkaloids Purified from Aristotelia chilensis Inhibit the Human α3β4 Nicotinic Acetylcholine Receptor with Higher Potencies Compared with the Human α4β2 and α7 Subtypes. J. Nat. Prod. 2019, 82, 1953–1960. [Google Scholar] [CrossRef]
- Dobler, M.; Borschberg, H.-J.; Azerad, R. Microbial hydroxylation of some synthetic Aristotelia alkaloids. Tetrahedron Asymmetry 1995, 6, 213–220. [Google Scholar] [CrossRef]
- Schön, C.; Wacker, R.; Micka, A.; Steudle, J.; Lang, S.; Bonnländer, B. Bioavailability Study of Maqui Berry Extract in Healthy Subjects. Nutrients 2018, 10, 1720. [Google Scholar] [CrossRef]
- Zúñiga, G.E.; Tapia, A.; Arenas, A.; Contreras, R.A.; Zúñiga-Libano, G. Phytochemistry and biological properties of Aristotelia chilensis a Chilean blackberry: A review. Phytochem. Rev. 2017, 16, 1081–1094. [Google Scholar] [CrossRef]
- Bribiesca-Cruz, I.; Moreno, D.A.; García-Viguera, C.; Gallardo, J.M.; Segura-Uribe, J.J.; Pinto-Almazán, R.; Guerra-Araiza, C. Maqui berry (Aristotelia chilensis) extract improves memory and decreases oxidative stress in male rat brain exposed to ozone. Nutr. Neurosci. 2021, 24, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Balbontin, C.; Avila, J.G.; Dominguez, M.; Alarcon, J.; Paz, C.; Burgos, V.; Ortiz, L.; Peñaloza-Castro, I.; Seigler, D.S.; et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 109 Pt 2, 984–995. [Google Scholar] [CrossRef]
- Hitchcock, S.A. Blood–brain barrier permeability considerations for CNS-targeted compound library design. Curr. Opin. Chem. Biol. 2008, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R. Determination of lipophilicity and its use as a predictor of blood–brain barrier penetration of molecular imaging agents. Mol. Imaging Biol. 2003, 5, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Könczöl, Á.; Müller, J.; Földes, E.; Béni, Z.; Végh, K.; Kéry, Á.; Balogh, G.T. Applicability of a blood–brain barrier specific artificial membrane permeability assay at the early stage of natural product-based CNS drug discovery. J. Nat. Prod. 2013, 76, 655–663. [Google Scholar] [CrossRef]
- Ogu, C.C.; Maxa, J.L. Drug interactions due to cytochrome P450. Bayl. Univ. Med. Cent. Proc. 2000, 13, 421–423. [Google Scholar] [CrossRef]
- Vukotić, N.T.; Đorđević, J.; Pejić, S.; Đorđević, N.; Pajović, S.B. Antidepressants- and antipsychotics-induced hepatotoxicity. Arch. Toxicol. 2021, 95, 767–789. [Google Scholar] [CrossRef]
- Ivanović, V.; Rančić, M.; Arsić, B.; Pavlović, A. Lipinski’s rule of five, famous extensions and famous exceptions. Pop. Sci. Artic. 2020, 3, 171–181. [Google Scholar] [CrossRef]
- Kim, M.; Shin, M.S.; Lee, J.M.; Cho, H.S.; Kim, C.J.; Kim, Y.J.; Choi, H.R.; Jeon, J.W. Inhibitory Effects of Isoquinoline Alkaloid Berberine on Ischemia-Induced Apoptosis via Activation of Phosphoinositide 3-Kinase/Protein Kinase B Signaling Pathway. Int. Neurourol. J. 2014, 18, 115–125. [Google Scholar] [CrossRef]
- Lu, D.; Tang, C.; Chen, Y.; Wei, I. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 2010, 110, 697–705. [Google Scholar] [CrossRef]
- Wang, H.; Liu, C.; Mei, X.; Cao, Y.; Guo, Z.; Yuan, Y.; Zhao, Z.; Song, C.; Guo, Y.; Shen, Z. Berberine attenuated pro-inflammatory factors and protect against neuronal damage via triggering oligodendrocyte autophagy in spinal cord injury. Oncotarget 2017, 8, 98312–98321. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, M.; Ma, R.; Wu, M.; Zhang, Y. Tetrandrine alleviates cerebral ischemia/reperfusion injury by suppressing NLRP3 inflammasome activation via Sirt-1. PeerJ 2020, 8, e9042. [Google Scholar] [CrossRef]
- Azam, S.; Haque, E.; Kim, I.-S.; Choi, D.-K. Microglial Turnover in Ageing-Related Neurodegeneration: Therapeutic Avenue to Intervene in Disease Progression. Cells 2021, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Aman, Y.; Ahmed, I.; Chetelat, G.; Landeau, B.; Chaudhuri, K.R.; Brooks, D.J.; Edison, P. Influence of microglial activation on neuronal function in Alzheimer’s and Parkinson’s disease dementia. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2015, 11, 608–621.e7. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Takada, Y.; Shishodia, S.; Gutierrez, A.M.; Oommen, O.V.; Ichikawa, H.; Baba, Y.; Kumar, A. Nuclear transcription factor NF-kappa B: Role in biology and medicine. Indian J. Exp. Biol. 2004, 42, 341–353. [Google Scholar]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Saegusa, M.; Hashimura, M.; Kuwata, T. Pin1 acts as a modulator of cell proliferation through alteration in NF-κB but not β-catenin/TCF4 signalling in a subset of endometrial carcinoma cells. J. Pathol. 2010, 222, 410–420. [Google Scholar] [CrossRef]
- Validation Data for THP1-Blue™ NF-κB Cells. Available online: https://www.invivogen.com/thp1-blue-nfkb (accessed on 20 September 2024).
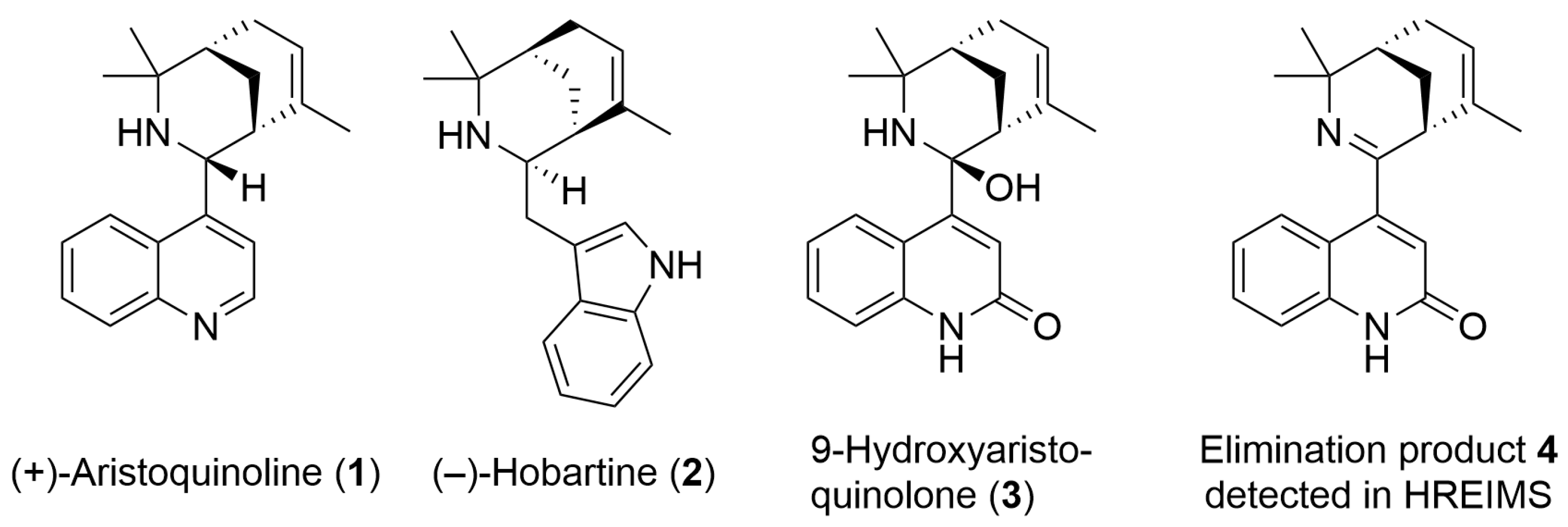
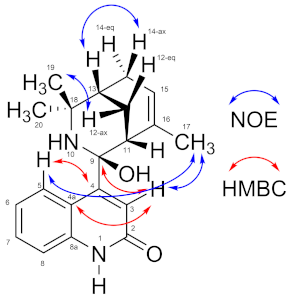
| Position | δH m (J in Hz) | δC, Type | HMBC | NOE |
|---|---|---|---|---|
| 1 | n.d. | -- | ||
| 2 | -- | 174.0, C | ||
| 3 | 5.90 s | 117.9, CH | C-2, C-4, C-4a, C-9 | H-17 |
| 4 | -- | 162.0, C | ||
| 4a | -- | 120.9, C | ||
| 5 | 7.44 d (7.6) | 123.9, CH | C-4, C-7, C-8a | H-17 |
| 6 | 6.91 ddd (7.6, 7.6, 1.0) | 121.3, CH | C-4a, C-8 | |
| 7 | 7.24 ddd (8.0, 7.6, 1.2) | 131.5, CH | C-5, C-8a | |
| 8 | 6.85 d (8.0) | 112.9, CH | C-4a, C-6 | |
| 8a | -- | 154.4, C | ||
| 9 | -- | 91.3, C | ||
| 10 | n.d. | -- | ||
| 11 | 2.12 dd (2.8, 2.8) | 45.7, CH | ||
| 12 | ax: 2.45 ddd (13.7, 3.0, 3.0)eq: 1.87 ddd (13.7, 2.9, 2.9) | 25.0, CH2 | C-16C-9, C-18 | |
| 13 | 1.67 m | 37.8, CH | C-15 | H-12ax, H-12eq, H-14ax, H-19, H-20 |
| 14 | eq: 2.64 d (19.3)ax: 2.15 dm (19.3) | 27.5, CH2 | C-15 | H-20H-13 |
| 15 | 5.49 m | 125.2, CH | H-17, H-14ax, H-14eq | |
| 16 | -- | 131.6, C | ||
| 17 | 1.51 m | 24.4, CH3 | C-16, C-15, C-11 | H-3, H-5, H-11, H-15 |
| 18 | -- | 58.9, C | ||
| 19 | 1.45 s | 29.6, CH3 | C-18, C-13, C-20 | |
| 20 | 1.89 s | 26.9, CH3 | C-18, C-13, C-19 |
| Pharmacokinetics/Druglikeness | |||
|---|---|---|---|
| Entry | 9-Hydroxyaristoquinolone | Toxicity | 9-Hydroxyaristoquinolone |
| MW | 324.424 | AMES toxicity | No |
| #Rotatable bonds | 1 | Hepatotoxicity | No |
| #H-bond acceptors | 3 | hERG I/II inhibitors | No/Yes |
| #H-bond donors | 3 | Skin sensitization | No |
| TPSA | 65.12 | ||
| Consensus Log Po/w | 2.75 | ||
| ESOL Class | Soluble | ||
| GI absorption | High | ||
| BBB permeant | Yes | ||
| Pgp substrate | Yes | ||
| CYP1A2 inhibitor | No | ||
| CYP2C19 inhibitor | No | ||
| CYP2C9 inhibitor | No | ||
| CYP2D6 inhibitor | Yes | ||
| CYP3A4 inhibitor | No | ||
| log Kp (cm/s) | −7.29 | ||
| Lipinski #violations | 0 | ||
| Synthetic Accessibility | 4.67 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, R.; Burgos, V.; Cabrera-Pardo, J.R.; Ortiz, L.; Camins, A.; Ettcheto, M.; Schmidt, B.; Nchiozem-Ngnitedem, V.-A.; Paz, C. 9-Hydroxyaristoquinolone: A New Indole Alkaloid Isolated from Aristotelia chilensis with Inhibitory Activity of NF-κB in HMC-3 Microglia Cells. Int. J. Mol. Sci. 2025, 26, 2419. https://doi.org/10.3390/ijms26062419
Pérez R, Burgos V, Cabrera-Pardo JR, Ortiz L, Camins A, Ettcheto M, Schmidt B, Nchiozem-Ngnitedem V-A, Paz C. 9-Hydroxyaristoquinolone: A New Indole Alkaloid Isolated from Aristotelia chilensis with Inhibitory Activity of NF-κB in HMC-3 Microglia Cells. International Journal of Molecular Sciences. 2025; 26(6):2419. https://doi.org/10.3390/ijms26062419
Chicago/Turabian StylePérez, Rebeca, Viviana Burgos, Jaime R. Cabrera-Pardo, Leandro Ortiz, Antoni Camins, Miren Ettcheto, Bernd Schmidt, Vaderament-A. Nchiozem-Ngnitedem, and Cristian Paz. 2025. "9-Hydroxyaristoquinolone: A New Indole Alkaloid Isolated from Aristotelia chilensis with Inhibitory Activity of NF-κB in HMC-3 Microglia Cells" International Journal of Molecular Sciences 26, no. 6: 2419. https://doi.org/10.3390/ijms26062419
APA StylePérez, R., Burgos, V., Cabrera-Pardo, J. R., Ortiz, L., Camins, A., Ettcheto, M., Schmidt, B., Nchiozem-Ngnitedem, V.-A., & Paz, C. (2025). 9-Hydroxyaristoquinolone: A New Indole Alkaloid Isolated from Aristotelia chilensis with Inhibitory Activity of NF-κB in HMC-3 Microglia Cells. International Journal of Molecular Sciences, 26(6), 2419. https://doi.org/10.3390/ijms26062419








