A Combined GLP-1/PPARa/CB1-Based Therapy to Restore the Central and Peripheral Metabolic Dysregulation Induced by a High-Fructose High-Fat Diet
Abstract
1. Introduction
2. Results
2.1. Body Weight and Plasma Biochemistry
2.2. Protein Expression
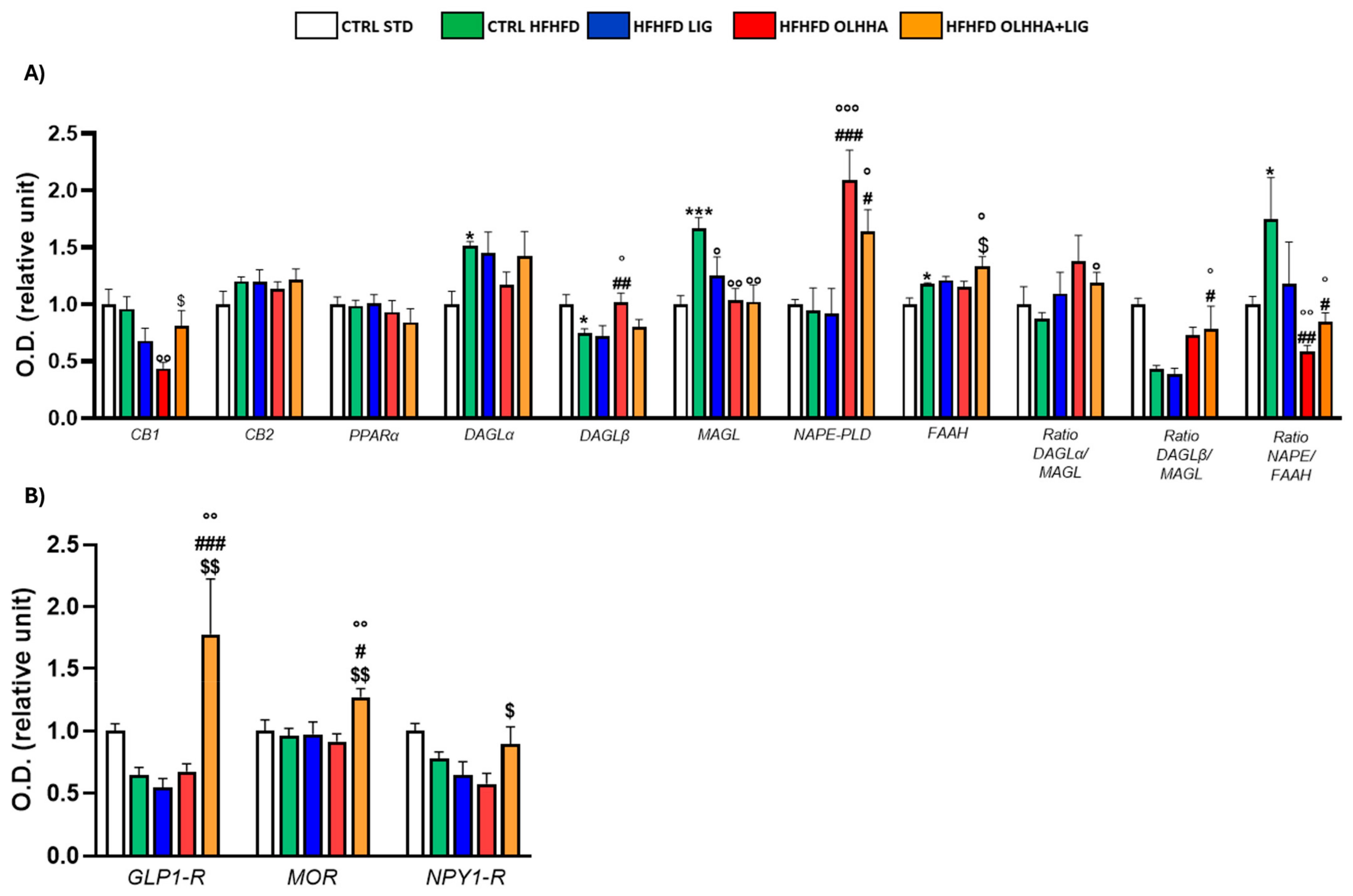
2.2.1. Hypothalamus
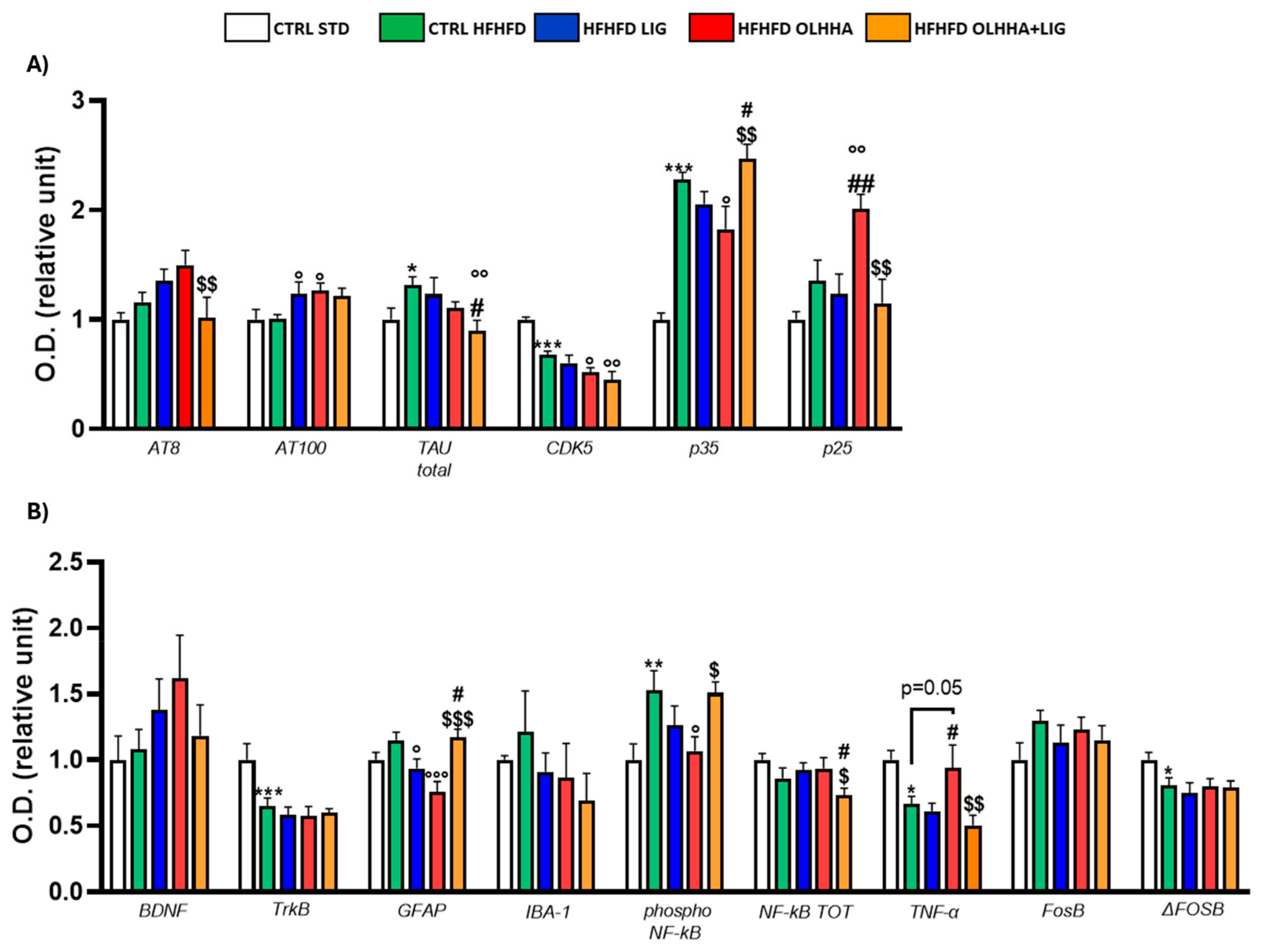
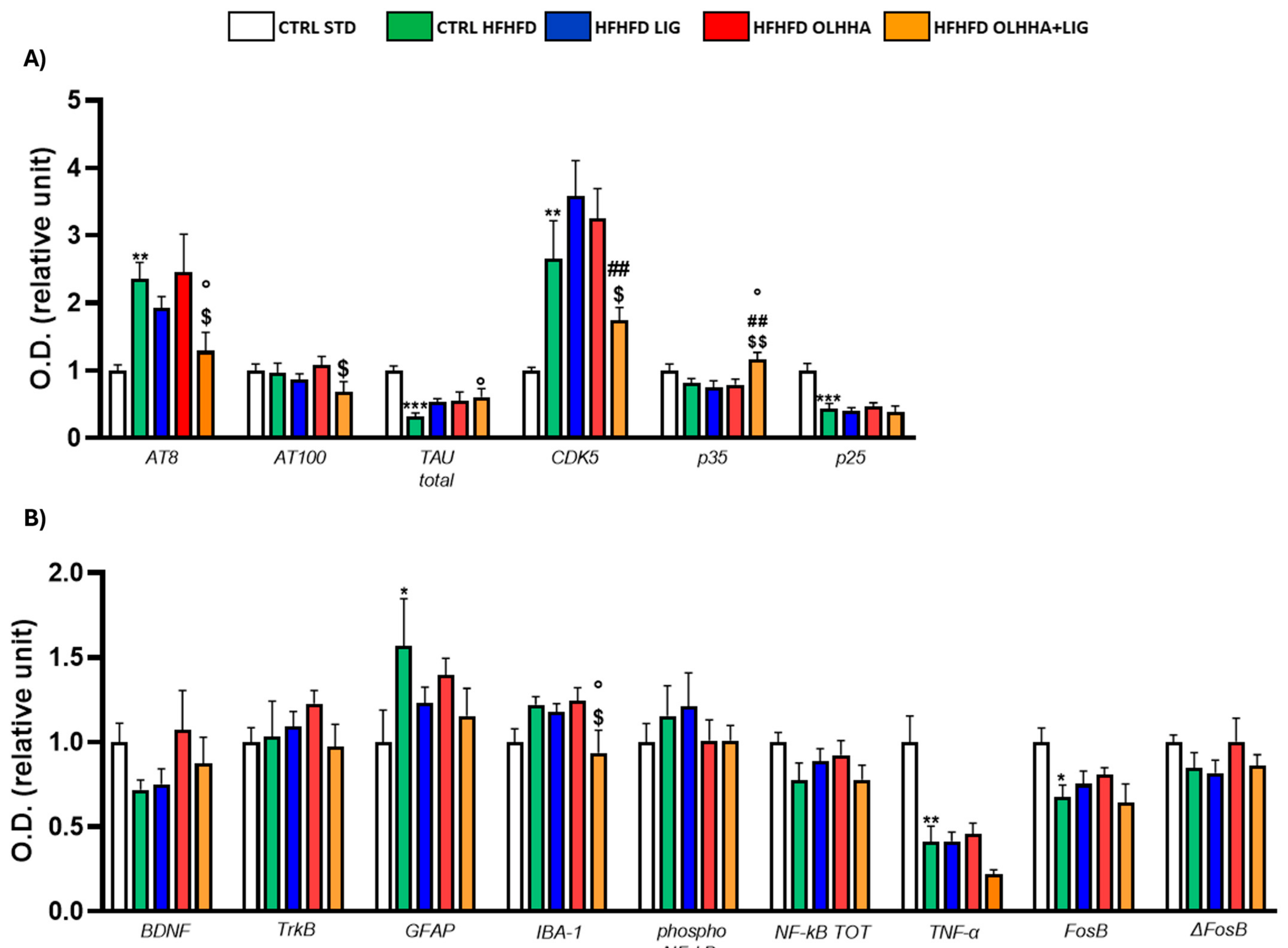
2.2.2. Hippocampus
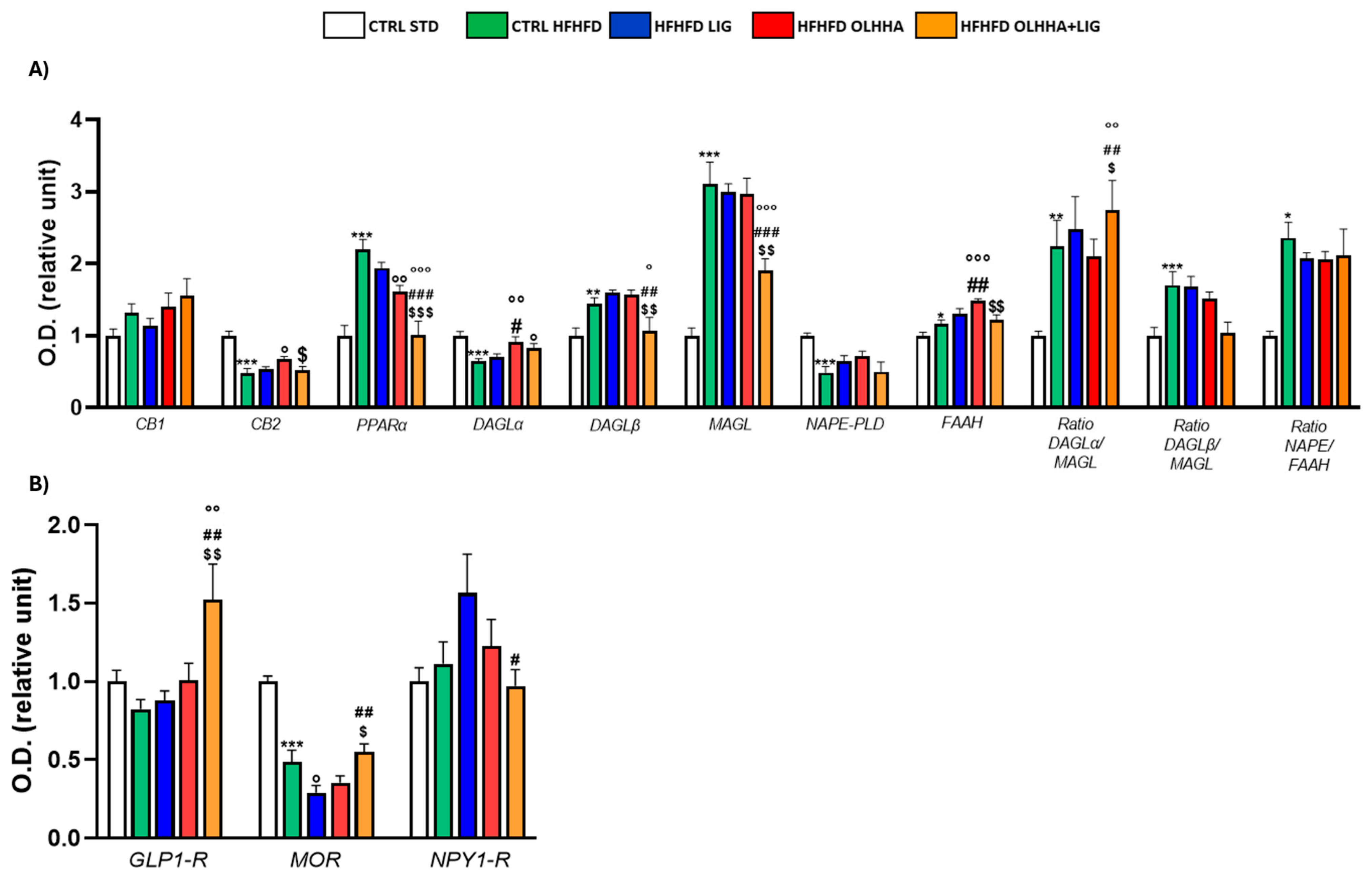
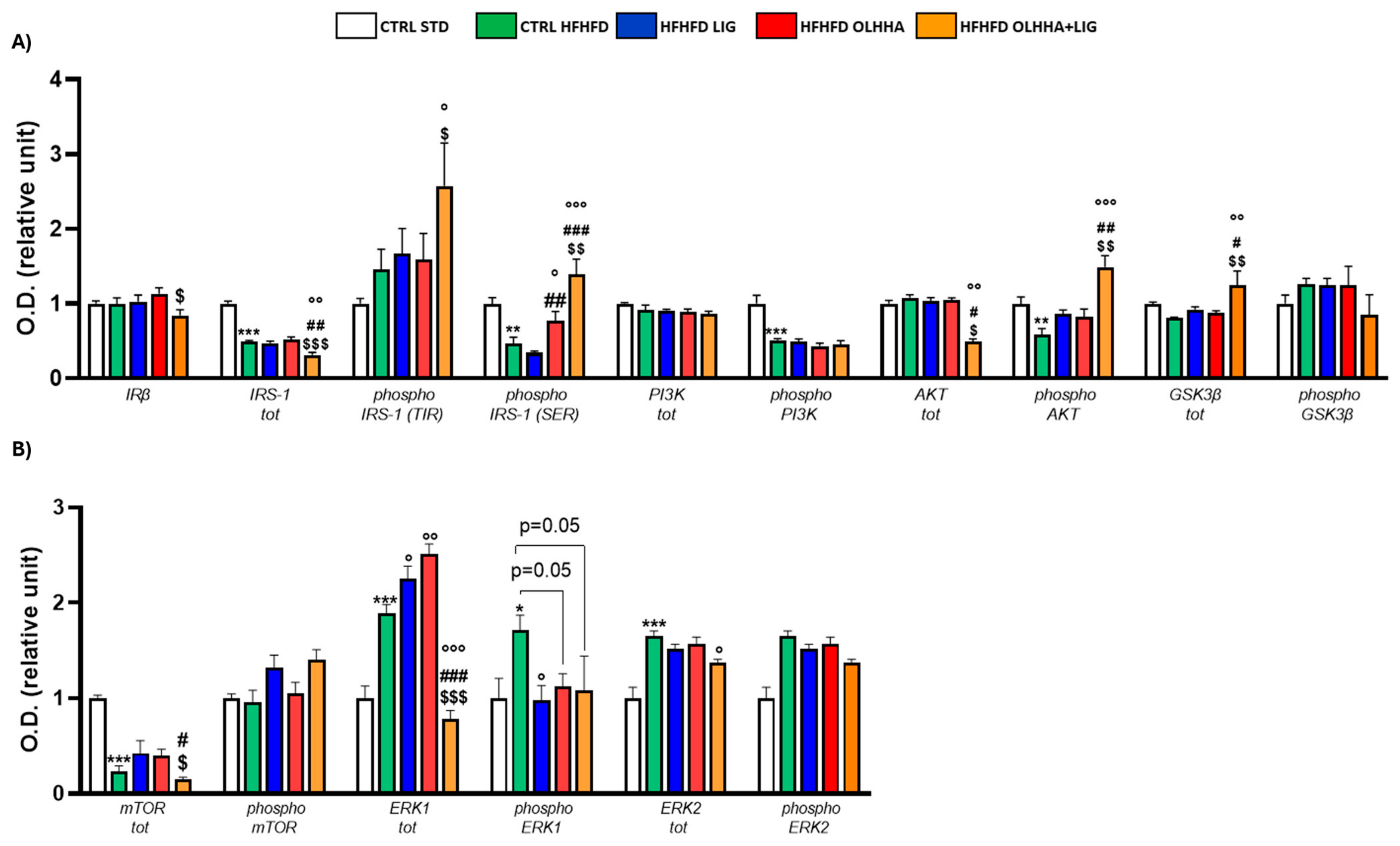
2.2.3. Prefrontal Cortex
2.3. Pearson Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.1.1. Animal Protocol and Ethics Statement
4.1.2. Drugs
4.1.3. Experimental Groups
4.2. Plasma Biochemical Analysis
4.3. Western Blot Analysis
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Obesity Foundation. World Obesity Atlas 2023. Available online: https://data.worldobesity.org/publications/?cat=19 (accessed on 24 August 2023).
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 October 2024).
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22 (Suppl. 7), s176–s185. [Google Scholar] [PubMed]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes. Obes. Metab. 2021, 23 (Suppl. 1), 3–16. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef]
- Neto, A.; Fernandes, A.; Barateiro, A. The complex relationship between obesity and neurodegenerative diseases: An updated review. Front. Cell. Neurosci. 2023, 17, 1294420. [Google Scholar] [CrossRef]
- Medina-Vera, D.; López-Gambero, A.J.; Navarro, J.A.; Sanjuan, C.; Baixeras, E.; Decara, J.; de Fonseca, F.R. Novel insights into D-Pinitol based therapies: A link between tau hyperphosphorylation and insulin resistance. Neural Regen. Res. 2024, 19, 289–295. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- de Ceglia, M.; Decara, J.; Gaetani, S.; Rodríguez de Fonseca, F. Obesity as a Condition Determined by Food Addiction: Should Brain Endocannabinoid System Alterations Be the Cause and Its Modulation the Solution? Pharmaceuticals 2021, 14, 1002. [Google Scholar] [CrossRef]
- Sánchez, A.J.; García-Merino, A. Neuroprotective agents: Cannabinoids. Clin. Immunol. 2012, 142, 57–67. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V.; Petrosino, S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016, 62, 107–128. [Google Scholar] [CrossRef]
- Ghusn, W.; Hurtado, M.D. Glucagon-like Receptor-1 agonists for obesity: Weight loss outcomes, tolerability, side effects, and risks. Obes. Pillars 2024, 12, 100127. [Google Scholar] [CrossRef]
- Knudsen, L.B. Liraglutide: The therapeutic promise from animal models. Int. J. Clin. Pract. 2010, 64, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Olukorode, J.O.; Orimoloye, D.A.; Nwachukwu, N.O.; Onwuzo, C.N.; Oloyede, P.O.; Fayemi, T.; Odunaike, O.S.; Ayobami-Ojo, P.S.; Divine, N.; Alo, D.J.; et al. Recent Advances and Therapeutic Benefits of Glucagon-Like Peptide-1 (GLP-1) Agonists in the Management of Type 2 Diabetes and Associated Metabolic Disorders. Cureus 2024, 16, e72080. [Google Scholar] [CrossRef]
- Tilinca, M.C.; Tiuca, R.A.; Burlacu, A.; Varga, A. A 2021 Update on the Use of Liraglutide in the Modern Treatment of ‘Diabesity’: A Narrative Review. Medicina 2021, 57, 669. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Igarashi, K.; Koeda, T.; Sugimoto, K.; Nakagawa, K.; Hayashi, S.; Yamaji, R.; Inui, H.; Fukusato, T.; Yamanouchi, T. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J. Nutr. 2009, 139, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- Decara, J.M.; Pavón, F.J.; Suárez, J.; Romero-Cuevas, M.; Baixeras, E.; Vázquez, M.; Rivera, P.; Gavito, A.L.; Almeida, B.; Joglar, J.; et al. Treatment with a novel oleic-acid–dihydroxyamphetamine conjugation ameliorates non-alcoholic fatty liver disease in obese Zucker rats. Dis. Models Mech. 2015, 8, 1213–1225. [Google Scholar] [CrossRef]
- Alen, F.; Decara, J.; Brunori, G.; You, Z.B.; Bühler, K.M.; López-Moreno, J.A.; Cippitelli, A.; Pavon, F.J.; Suárez, J.; Gardner, E.L.; et al. PPARα/CB1 receptor dual ligands as a novel therapy for alcohol use disorder: Evaluation of a novel oleic acid conjugate in preclinical rat models. Biochem. Pharmacol. 2018, 157, 235–243. [Google Scholar] [CrossRef]
- Zizzari, P.; He, R.; Falk, S.; Bellocchio, L.; Allard, C.; Clark, S.; Lesté-Lasserre, T.; Marsicano, G.; Clemmensen, C.; Perez-Tilve, D.; et al. CB1 and GLP-1 Receptors Cross Talk Provides New Therapies for Obesity. Diabetes 2021, 70, 415–422. [Google Scholar] [CrossRef]
- Marcondes-de-Castro, I.A.; Oliveira, T.F.; Spezani, R.; Marinho, T.S.; Cardoso, L.E.M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Cotadutide effect in liver and adipose tissue in obese mice. J. Mol. Endocrinol. 2023, 70, e220168. [Google Scholar] [CrossRef]
- Spezani, R.; Marcondes-de-Castro, I.A.; Marinho, T.S.; Reis-Barbosa, P.H.; Cardoso, L.E.M.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Cotadutide improves brown adipose tissue thermogenesis in obese mice. Biochem. Pharmacol. 2023, 217, 115852. [Google Scholar] [CrossRef]
- Peradze, N.; Farr, O.M.; Perakakis, N.; Lázaro, I.; Sala-Vila, A.; Mantzoros, C.S. Short-term treatment with high dose liraglutide improves lipid and lipoprotein profile and changes hormonal mediators of lipid metabolism in obese patients with no overt type 2 diabetes mellitus: A randomized, placebo-controlled, cross-over, double-blind clinical trial. Cardiovasc. Diabetol. 2019, 18, 141. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, R.; Jia, Y.; Zong, J.; Wang, Y.; Dong, Y. The effect of liraglutide on nonalcoholic fatty liver disease in type 2 diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2020, 40, 491–499. [Google Scholar] [CrossRef]
- Hayes, M.R.; Kanoski, S.E.; Alhadeff, A.L.; Grill, H.J. Comparative effects of the long-acting GLP-1 receptor ligands, liraglutide and exendin-4, on food intake and body weight suppression in rats. Obesity 2011, 19, 1342–1349. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Xia, N.; Lai, Y.; Pan, H.; Zhou, S.; Jiang, F.; He, Y. Liraglutide reduces body weight by upregulation of adenylate cyclase 3. Nutr. Diabetes 2017, 7, e265. [Google Scholar] [CrossRef]
- Nagakubo, D.; Shirai, M.; Nakamura, Y.; Kaji, N.; Arisato, C.; Watanabe, S.; Takasugi, A.; Asai, F. Prophylactic effects of the glucagon-like Peptide-1 analog liraglutide on hyperglycemia in a rat model of type 2 diabetes mellitus associated with chronic pancreatitis and obesity. Comp. Med. 2014, 64, 121–127. [Google Scholar]
- Wang, X.; Chen, S.; Lv, D.; Li, Z.; Ren, L.; Zhu, H.; Xie, X.; Liu, Y. Liraglutide suppresses obesity and promotes browning of white fat via miR-27b in vivo and in vitro. J. Int. Med. Res. 2021, 49, 3000605211055059. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Q.; Wang, Y. Brain endocannabinoid control of metabolic and non-metabolic feeding behaviors. Neurochem. Int. 2024, 183, 105921. [Google Scholar] [CrossRef]
- Massa, F.; Mancini, G.; Schmidt, H.; Steindel, F.; Mackie, K.; Angioni, C.; Oliet, S.H.; Geisslinger, G.; Lutz, B. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J. Neurosci. 2010, 30, 6273–6281. [Google Scholar] [CrossRef]
- Alptekin, İ.M.; Çakıroğlu, F.P.; Reçber, T.; Nemutlu, E. Inulin may prevent the high-fat diet induced-obesity via suppressing endocannabinoid system in the prefrontal cortex in Wistar rats. Int. J. Food Sci. Nutr. 2024, 75, 800–811. [Google Scholar] [CrossRef]
- Dazzi, L.; Talani, G.; Biggio, F.; Utzeri, C.; Lallai, V.; Licheri, V.; Lutzu, S.; Mostallino, M.C.; Secci, P.P.; Biggio, G.; et al. Involvement of the cannabinoid CB1 receptor in modulation of dopamine output in the prefrontal cortex associated with food restriction in rats. PLoS ONE 2014, 9, e92224. [Google Scholar] [CrossRef]
- Satta, V.; Scherma, M.; Piscitelli, F.; Usai, P.; Castelli, M.P.; Bisogno, T.; Fratta, W.; Fadda, P. Limited Access to a High Fat Diet Alters Endocannabinoid Tone in Female Rats. Front. Neurosci. 2018, 12, 40. [Google Scholar] [CrossRef]
- de Ceglia, M.; Micioni Di Bonaventura, M.V.; Romano, A.; Friuli, M.; Micioni Di Bonaventura, E.; Gavito, A.L.; Botticelli, L.; Gaetani, S.; de Fonseca, F.R.; Cifani, C. Anxiety associated with palatable food withdrawal is reversed by the selective FAAH inhibitor PF-3845: A regional analysis of the contribution of endocannabinoid signaling machinery. Int. J. Eat. Disord. 2023, 56, 1098–1113. [Google Scholar] [CrossRef] [PubMed]
- de Ceglia, M.; Romano, A.; Micioni Di Bonaventura, M.V.; Gavito, A.; Botticelli, L.; Micioni Di Bonaventura, E.; Friuli, M.; Cifani, C.; Rodríguez de Fonseca, F.; Gaetani, S. Cafeteria Diet Abstinence Induces Depressive Behavior and Disrupts Endocannabinoid Signaling in Dopaminergic Areas: A Preclinical Study. Curr. Neuropharmacol. 2024, 23, 458–474. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, M.; Elezgarai, I.; Mato, S.; Bakiri, Y.; Grandes, P.; Manzoni, O.J. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE 2007, 2, e709. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Trezza, V. The endocannabinoid system: A key modulator of emotions and cognition. Front. Behav. Neurosci. 2012, 6, 73. [Google Scholar] [CrossRef]
- Zhang, C.; Barkholt, P.; Nielsen, J.C.; Thorbek, D.D.; Rigbolt, K.; Vrang, N.; Woldbye, D.P.D.; Jelsing, J. The dorsomedial hypothalamus and nucleus of the solitary tract as key regulators in a rat model of chronic obesity. Brain Res. 2020, 1727, 146538. [Google Scholar] [CrossRef]
- Ip, C.K.; Rezitis, J.; Qi, Y.; Bajaj, N.; Koller, J.; Farzi, A.; Shi, Y.C.; Tasan, R.; Zhang, L.; Herzog, H. Critical role of lateral habenula circuits in the control of stress-induced palatable food consumption. Neuron 2023, 111, 2583–2600.e2586. [Google Scholar] [CrossRef]
- Vucetic, Z.; Kimmel, J.; Reyes, T.M. Chronic high-fat diet drives postnatal epigenetic regulation of μ-opioid receptor in the brain. Neuropsychopharmacology 2011, 36, 1199–1206. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- De Meyts, P. The Insulin Receptor and Its Signal Transduction Network. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, K.W.; Kim, M.S. Leptin signalling pathways in hypothalamic neurons. Cell. Mol. Life Sci. 2016, 73, 1457–1477. [Google Scholar] [CrossRef]
- Benzler, J.; Ganjam, G.K.; Krüger, M.; Pinkenburg, O.; Kutschke, M.; Stöhr, S.; Steger, J.; Koch, C.E.; Ölkrug, R.; Schwartz, M.W.; et al. Hypothalamic glycogen synthase kinase 3β has a central role in the regulation of food intake and glucose metabolism. Biochem. J. 2012, 447, 175–184. [Google Scholar] [CrossRef]
- Ahlawat, A.; Walia, V.; Garg, M. Brain insulin resistance mediated cognitive impairment and neurodegeneration: Type-3 diabetes or Alzheimer’s Disease. Acta Neurol. Belg. 2025, 1–29. [Google Scholar] [CrossRef]
- Alagiakrishnan, K.; Halverson, T. Role of Peripheral and Central Insulin Resistance in Neuropsychiatric Disorders. J. Clin. Med. 2024, 13, 6607. [Google Scholar] [CrossRef]
- Figlewicz, D.P.; Benoit, S.C. Insulin, leptin, and food reward: Update 2008. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R9–R19. [Google Scholar] [CrossRef]
- Lee, S.H.; Zabolotny, J.M.; Huang, H.; Lee, H.; Kim, Y.B. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol. Metab. 2016, 5, 589–601. [Google Scholar] [CrossRef]
- Payant, M.A.; Chee, M.J. Neural mechanisms underlying the role of fructose in overfeeding. Neurosci. Biobehav. Rev. 2021, 128, 346–357. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; Saunders, S.E.; Antunes, V.R.; Boychuk, C.R. Insulin activates parasympathetic hepatic-related neurons of the paraventricular nucleus of the hypothalamus through mTOR signaling. J. Neurophysiol. 2024, 133, 320–332. [Google Scholar] [CrossRef]
- Hu, H.; Lu, X.; He, Y.; Li, J.; Wang, S.; Luo, Z.; Wang, Y.; Wei, J.; Huang, H.; Duan, C.; et al. Sestrin2 in POMC neurons modulates energy balance and obesity related metabolic disorders via mTOR signaling. J. Nutr. Biochem. 2024, 133, 109703. [Google Scholar] [CrossRef]
- Hochmuth, L.; Hirrlinger, J. Physiological and Pathological Role of mTOR Signaling in Astrocytes. Neurochem. Res. 2024, 50, 53. [Google Scholar] [CrossRef]
- Ryskalin, L.; Lazzeri, G.; Flaibani, M.; Biagioni, F.; Gambardella, S.; Frati, A.; Fornai, F. mTOR-Dependent Cell Proliferation in the Brain. Biomed Res. Int. 2017, 2017, 7082696. [Google Scholar] [CrossRef]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef] [PubMed]
- Milstein, J.L.; Ferris, H.A. The brain as an insulin-sensitive metabolic organ. Mol. Metab. 2021, 52, 101234. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, T.M.; Hogg, E.L.; Collingridge, G.L.; Corrêa, S.A. Hippocampal metabotropic glutamate receptor long-term depression in health and disease: Focus on mitogen-activated protein kinase pathways. J. Neurochem. 2016, 139 (Suppl. 2), 200–214. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.Y. Basic roles of key molecules connected with NMDAR signaling pathway on regulating learning and memory and synaptic plasticity. Mil. Med. Res. 2016, 3, 26. [Google Scholar] [CrossRef]
- Kueck, P.J.; Morris, J.K.; Stanford, J.A. Current Perspectives: Obesity and Neurodegeneration—Links and Risks. Degener Neurol. Neuromuscul. Dis 2023, 13, 111–129. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Liang, Z.; Gong, X.; Ye, R.; Zhao, Y.; Yu, J.; Zhao, Y.; Bao, J. Long-Term High-Fat Diet Consumption Induces Cognitive Decline Accompanied by Tau Hyper-Phosphorylation and Microglial Activation in Aging. Nutrients 2023, 15, 250. [Google Scholar] [CrossRef]
- Garemilla, S.; Kumari, R.; Kumar, R. CDK5 as a therapeutic tool for the treatment of Alzheimer’s disease: A review. Eur. J. Pharmacol. 2024, 978, 176760. [Google Scholar] [CrossRef]
- Kopp, K.O.; Glotfelty, E.J.; Li, Y.; Lahiri, D.K.; Greig, N.H. Type 2 diabetes mellitus/obesity drugs: A neurodegenerative disorders savior or a bridge too far? Ageing Res. Rev. 2024, 98, 102343. [Google Scholar] [CrossRef]
- Wilbon, S.S.; Kolonin, M.G. GLP1 Receptor Agonists-Effects beyond Obesity and Diabetes. Cells 2023, 13, 65. [Google Scholar] [CrossRef]
- Firth, W.; Pye, K.R.; Weightman Potter, P.G. Astrocytes at the intersection of ageing, obesity, and neurodegeneration. Clin. Sci. 2024, 138, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, L. Microglia Regulate Neuronal Circuits in Homeostatic and High-Fat Diet-Induced Inflammatory Conditions. Front. Cell. Neurosci. 2021, 15, 722028. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Huang, H.; Dong, S.; Sha, H.; Wei, W.; Liu, C. High mobility group box-1 mediates hippocampal inflammation and contributes to cognitive deficits in high-fat high-fructose diet-induced obese rats. Brain Behav. Immun. 2019, 82, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.; Rios, M. The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update. Biomolecules 2024, 14, 424. [Google Scholar] [CrossRef]
- Melgar-Locatelli, S.; de Ceglia, M.; Mañas-Padilla, M.C.; Rodriguez-Pérez, C.; Castilla-Ortega, E.; Castro-Zavala, A.; Rivera, P. Nutrition and adult neurogenesis in the hippocampus: Does what you eat help you remember? Front. Neurosci. 2023, 17, 1147269. [Google Scholar] [CrossRef]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Kim, W.; Rouse, M.; Egan, J.M. Blockade of cannabinoid 1 receptor improves GLP-1R mediated insulin secretion in mice. Mol. Cell. Endocrinol. 2016, 423, 1–10. [Google Scholar] [CrossRef]
- Patel, K.N.; Joharapurkar, A.A.; Patel, V.; Kshirsagar, S.G.; Bahekar, R.; Srivastava, B.K.; Jain, M.R. Cannabinoid receptor 1 antagonist treatment induces glucagon release and shows an additive therapeutic effect with GLP-1 agonist in diet-induced obese mice. Can. J. Physiol. Pharmacol. 2014, 92, 975–983. [Google Scholar] [CrossRef]
- Radziszewska, E.; Bojanowska, E. Effects of glucagon-like peptide-1 receptor stimulation and blockade on food consumption and body weight in rats treated with a cannabinoid CB1 receptor agonist WIN 55,212-2. Med. Sci. Monit. Basic Res. 2013, 19, 6–11. [Google Scholar] [CrossRef]
- Bojanowska, E.; Radziszewska, E. Combined stimulation of glucagon-like peptide-1 receptor and inhibition of cannabinoid CB1 receptor act synergistically to reduce food intake and body weight in the rat. J. Physiol. Pharmacol. 2011, 62, 395–402. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- Galeano, P.; de Ceglia, M.; Mastrogiovanni, M.; Campanelli, L.; Medina-Vera, D.; Campolo, N.; Novack, G.V.; Rosell-Valle, C.; Suárez, J.; Aicardo, A.; et al. The Effect of Fat Intake with Increased Omega-6-to-Omega-3 Polyunsaturated Fatty Acid Ratio in Animal Models of Early and Late Alzheimer’s Disease-like Pathogenesis. Int. J. Mol. Sci. 2023, 24, 17009. [Google Scholar] [CrossRef]
- López-Gambero, A.J.; Pacheco-Sánchez, B.; Rosell-Valle, C.; Medina-Vera, D.; Navarro, J.A.; Fernández-Arjona, M.D.M.; de Ceglia, M.; Sanjuan, C.; Simon, V.; Cota, D.; et al. Dietary administration of D-chiro-inositol attenuates sex-specific metabolic imbalances in the 5xFAD mouse model of Alzheimer’s disease. Biomed. Pharmacother. 2022, 150, 112994. [Google Scholar] [CrossRef]




| CTRL STD | CTRL HFHFD | HFHFD LIG (25 µg/kg) | HFHFD OLHHA (3 mg/kg) | HFHFD OLHHA+LIG (3 mg/kg + 25 µg/kg) | |
|---|---|---|---|---|---|
| BW gain (mg) | 39.130 ± 3.182 | 165.900 ± 11.160 *** | 146.300 ± 8.277 | 139.400 ± 9.388 ° | 106.500 ± 4.567 °°°##$$ |
| Triglycerides (mg/dL) | 116.9 ± 7.921 | 152.6 ± 9.795 ** | 96.80 ± 5.173 °°° | 131.3 ± 7.610 # | 53.86 ± 6.580 °°°##$$$ |
| Cholesterol (mg/dL) | 55.61 ± 2.058 | 66.88 ± 4.662 | 72.13 ± 5.034 | 70.75 ± 4.439 | 79.75 ± 7.504 |
| HDL (mg/dL) | 22.23 ± 2.045 | 23.60 ± 1.011 | 31.88 ± 4.741 ° | 28.00 ± 1.225 | 23.50 ± 3.246 # |
| LDL (mg/dL) | 44.92 ± 3.102 | 77.06 ± 8.127 ** | 44.58 ± 6.084 °° | 65.12 ± 5.721 # | 36.00 ± 5.672 °°°$$ |
| ALT (IU/L) | 40.80 ± 1.800 | 52.43 ± 1.193 | 52.71 ± 2.801 | 50.86 ± 3.038 | 33.50 ± 6.651 °°##$$ |
| AST (IU/L) | 108.8 ± 3.292 | 123.3 ± 4.448 | 116.7 ± 6.210 | 102.9 ± 4.081 | 73.14 ± 13.27 °°°###$$ |
| Bilirubin (mg/dL) | 0.093 ± 0.007 | 0.188 ± 0.023 ** | 0.213 ± 0.035 | 0.126 ± 0.015 ## | 0.231 ± 0.027 $$ |
| Hypothalamus | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | BW | Triglycerides | Cholesterol | LDL | HDL | ALT | AST | Bilirubin |
| CB1 | + (p < 0.05) | − (p < 0.05) | + (p < 0.0001) | + (p < 0.001) | ||||
| PPARα | + (p < 0.05) | |||||||
| MAGL | + (p < 0.05) | |||||||
| NAPE-PLD | + (p < 0.05) | |||||||
| FAAH | + (p < 0.05) | |||||||
| BDNF | + (p < 0.05) | |||||||
| TrkB | + (p < 0.01) | + (p < 0.05) | + (p < 0.05) | |||||
| GFAP | + (p < 0.05) | |||||||
| AT8 | + (p < 0.01) | − (p < 0.05) | ||||||
| AT100 | + (p < 0.0001) | + (p < 0.05) | − (p < 0.05) | |||||
| TAU | ||||||||
| CDK5 | − (p < 0.05) | + (p < 0.0001) | + (p < 0.01) | − (p < 0.01) | ||||
| P25 | + (p < 0.05) | |||||||
| NF-kB | − (p < 0.01) | − (p < 0.05) | ||||||
| pNF-kB | + (p < 0.05) | + (p < 0.01) | + (p < 0.05) | − (p < 0.05) | ||||
| TNF-α | − (p < 0.05) | |||||||
| mTOR | − (p < 0.05) | − (p < 0.05) | − (p < 0.01) | |||||
| pmTOR | + (p < 0.05) | |||||||
| AKT | + (p < 0.05) | − (p < 0.05) | − (p < 0.05) | − (p < 0.01) | ||||
| pAKT | − (p < 0.05) | |||||||
| pPI3K | − (p < 0.01) | − (p < 0.01) | − (p < 0.01) | |||||
| GSK3β | − (p < 0.05) | − (p < 0.05) | + (p < 0.0001) | + (p < 0.05) | ||||
| pGSK3β | + (p < 0.05) | − (p < 0.0001) | − (p < 0.01) | + (p < 0.05) | ||||
| pERK1 | + (p < 0.05) | |||||||
| pERK2 | ||||||||
| GLP-1R | − (p < 0.01) | − (p < 0.01) | − (p < 0.01) | |||||
| MOR | − (p < 0.001) | |||||||
| NPY1R | + (p < 0.05) | + (p < 0.05) | ||||||
| FOSB | + (p < 0.01) | |||||||
| ΔFOSB | + (p < 0.05) | |||||||
| Hippocampus | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | BW | Triglycerides | Cholesterol | LDL | HDL | ALT | AST | Bilirubin |
| CB1 | − (p < 0.01) | + (p < 0.01) | ||||||
| CB2 | − (p < 0.05) | − (p < 0.05) | + (p < 0.05) | |||||
| PPARα | − (p < 0.05) | + (p < 0.05) | + (p < 0.001) | |||||
| MAGL | + (p < 0.05) | |||||||
| BDNF | + (p < 0.05) | |||||||
| TrkB | + (p < 0.01) | |||||||
| GFAP | − (p < 0.05) | |||||||
| AT8 | + (p < 0.05) | − (p < 0.05) | ||||||
| AT100 | − (p < 0.05) | − (p < 0.05) | − (p < 0.05) | + (p < 0.05) | ||||
| TAU | − (p < 0.05) | + (p < 0.01) | ||||||
| CDK5 | + (p < 0.01) | + (p < 0.001) | ||||||
| P35 | − (p < 0.05) | + (p < 0.01) | − (p < 0.05) | |||||
| P25 | ||||||||
| NF-kB | + (p < 0.01) | + (p < 0.05) | ||||||
| pNF-kB | − (p < 0.05) | |||||||
| IRβ | + (p < 0.01) | + (p < 0.01) | ||||||
| IRS1 | − (p < 0.05) | |||||||
| AKT | + (p < 0.05) | + (p < 0.05) | ||||||
| pAKT | + (p < 0.05) | |||||||
| PI3K | − (p < 0.05) | |||||||
| ERK1 | + (p < 0.05) | + (p < 0.05) | + (p < 0.01) | − (p < 0.05) | ||||
| pERK1 | − (p < 0.0001) | |||||||
| ERK2 | + (p < 0.01) | + (p < 0.01) | − (p < 0.01) | |||||
| pERK2 | − (p < 0.0001) | + (p < 0.01) | ||||||
| GLP-1R | − (p < 0.05) | − (p < 0.01) | ||||||
| MOR | − (p < 0.05) | |||||||
| NPY1R | − (p < 0.05) | − (p < 0.05) | − (p < 0.01) | |||||
| FOSB | − (p < 0.05) | |||||||
| ΔFOSB | − (p < 0.01) | |||||||
| Prefrontal Cortex | ||||||||
|---|---|---|---|---|---|---|---|---|
| Protein | BW | Triglycerides | Cholesterol | LDL | HDL | ALT | AST | Bilirubin |
| CB1 | ||||||||
| CB2 | − (p < 0.05) | + (p < 0.001) | + (p < 0.05) | − (p < 0.01) | ||||
| PPARα | + (p < 0.01) | |||||||
| MAGL | + (p < 0.001) | |||||||
| NAPE−PLD | + (p < 0.05) | − (p < 0.05) | ||||||
| FAAH | + (p < 0.05) | − (p < 0.05) | ||||||
| GFAP | − (p < 0.05) | + (p < 0.01) | ||||||
| TAU | + (p < 0.05) | |||||||
| CDK5 | + (p < 0.01) | |||||||
| P25 | + (p < 0.05) | + (p < 0.001) | + (p < 0.01) | − (p < 0.05) | ||||
| TNF-α | − (p < 0.001) | |||||||
| IRS1 | − (p < 0.05) | + (p < 0.05) | + (p < 0.001) | + (p < 0.01) | ||||
| P(tyr)IRS | − (p < 0.05) | |||||||
| p(ser)IRS1 | − (p < 0.05) | − (p < 0.01) | − (p < 0.05) | |||||
| MTOR | + (p < 0.01) | + (p < 0.01) | + (p < 0.05) | |||||
| pMTOR | − (p < 0.05) | |||||||
| AKT | + (p < 0.01) | +(p < 0.05) | + (p < 0.01) | |||||
| pAKT | − (p < 0.05) | − (p < 0.01) | + (p < 0.05) | + (p < 0.01) | ||||
| pPI3K | − (p < 0.05) | |||||||
| GSK3β | − (p < 0.05) | + (p < 0.01) | + (p < 0.001) | |||||
| ERK1 | + (p < 0.01) | − (p < 0.05) | ||||||
| pERK1 | − (p < 0.05) | + (p < 0.05) | ||||||
| ERK2 | + (p < 0.001) | |||||||
| pERK2 | − (p < 0.01) | + (p < 0.05) | ||||||
| GLP−1R | − (p < 0.05) | − (p < 0.01) | + (p < 0.05) | − (p < 0.01) | − (p < 0.01) | |||
| MOR | + (p < 0.01) | |||||||
| NPY1R | + (p < 0.05) | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Ceglia, M.; Rasheed, N.; Tovar, R.; Pareja-Cerbán, I.; Arias-Sáez, A.; Gavito, A.; Gaetani, S.; Cifani, C.; Rodríguez de Fonseca, F.; Decara, J. A Combined GLP-1/PPARa/CB1-Based Therapy to Restore the Central and Peripheral Metabolic Dysregulation Induced by a High-Fructose High-Fat Diet. Int. J. Mol. Sci. 2025, 26, 2420. https://doi.org/10.3390/ijms26062420
de Ceglia M, Rasheed N, Tovar R, Pareja-Cerbán I, Arias-Sáez A, Gavito A, Gaetani S, Cifani C, Rodríguez de Fonseca F, Decara J. A Combined GLP-1/PPARa/CB1-Based Therapy to Restore the Central and Peripheral Metabolic Dysregulation Induced by a High-Fructose High-Fat Diet. International Journal of Molecular Sciences. 2025; 26(6):2420. https://doi.org/10.3390/ijms26062420
Chicago/Turabian Stylede Ceglia, Marialuisa, Nabila Rasheed, Rubén Tovar, Inés Pareja-Cerbán, Andrea Arias-Sáez, Ana Gavito, Silvana Gaetani, Carlo Cifani, Fernando Rodríguez de Fonseca, and Juan Decara. 2025. "A Combined GLP-1/PPARa/CB1-Based Therapy to Restore the Central and Peripheral Metabolic Dysregulation Induced by a High-Fructose High-Fat Diet" International Journal of Molecular Sciences 26, no. 6: 2420. https://doi.org/10.3390/ijms26062420
APA Stylede Ceglia, M., Rasheed, N., Tovar, R., Pareja-Cerbán, I., Arias-Sáez, A., Gavito, A., Gaetani, S., Cifani, C., Rodríguez de Fonseca, F., & Decara, J. (2025). A Combined GLP-1/PPARa/CB1-Based Therapy to Restore the Central and Peripheral Metabolic Dysregulation Induced by a High-Fructose High-Fat Diet. International Journal of Molecular Sciences, 26(6), 2420. https://doi.org/10.3390/ijms26062420







