Abstract
The pseudo response regulator (PRR) gene is an important component of the core oscillator involved in plant circadian rhythms and plays an important role in regulating plant growth and development and stress responses. In this study, we investigated the function of GmPRR7b by overexpression and gene editing approaches. It was found that GmPRR7b plays a role in delaying flowering. While GmPRR7b overexpressing plants showed significantly delayed flowering compared to untransformed WT, GmPRR7b edited plants flowered earlier than the control WT. On the basis of previous research results and bioinformatics analysis, we re-identified 14 soybean PRR genes and analysed their rhythmic expression. Based on the rhythmic expression pattern, we found that GmPRR5/9a and GmPRR5/9b interacted with GmPRR7b by yeast two-hybrid and bimolecular fluorescence complementation (BiFC) experiments. Combined with the expression regulatory networks of the GmPRR7b, we inferred a possible regulatory mechanism by which GmPRR7b affects flowering through quit rhythm expression. These research elements provide valuable references for understanding growth, development, and circadian regulation in soybean.
1. Introduction
Following the rhythmic phenomenon of the alternation of day and night caused by the rotation of the Earth, plants have a self-regulating mechanism with an approximate 24 h rhythm [1,2]. This endogenous rhythmic regulatory mechanism in plants is called the biological clock or circadian rhythm [3]. The plant circadian system regulates almost all growth and developmental and metabolic processes, such as flowering, leaf movement, and hormone signalling [4,5,6]. The clock consists of three parts: an input pathway, a core oscillator, and an output pathway [7]. The core oscillator is a transcriptional–translational feedback loop consisting mainly of two MYB proteins, LHY and CCA1, and the family of pseudo-response regulators (PRRs) [8].
The first identification of PRRs was in the model plant, Arabidopsis (Arabidopsis thaliana (L.) Heynh.) [9]. Scientists discovered that PRR genes exhibit robust rhythmicity with an expression cycle of approximately 24 h [9,10]. The transcript levels of AtPRR9/AtPRR7/AtPRR5/AtPRR3/AtPRR1 in Arabidopsis accumulate sequentially from morning to evening, reaching a peak within 2–3 h [9,10,11,12,13,14]. Furthermore, AtPRRs have been demonstrated to regulate diverse processes, including photoperiodic regulation of flowering, hypocotyl growth, and seed germination [15,16]. In addition to these functions, AtPRRs have been shown to respond positively to drought stress and cold stress [17,18]. The present research on PRRs in Arabidopsis is predominantly centred on the photoperiodic regulatory pathway of this species [18]. The “GI-CO-FT” pathway, which has been extensively studied, is a notable example of this regulatory mechanism, with PRRs playing a pivotal role in transmitting rhythmic signals [19,20,21,22].
Because of these interesting rhythmic expression patterns, PRRs have quickly become a popular research focus on plants. For example, five PRR genes (OsPRR1, OsPRR37, OsPRR73, OsPRR59, and OsPRR95) have also been identified in rice (Oryza sativa L.) and shown to be involved in the regulation of the biological clock [23,24,25,26,27]. Thirteen PRR genes have been identified in tomato (Solanum lycopersicum L.) [28,29], and Wang et al. [30] identified a total of forty-four PRR genes in four species of cotton (Gossypium hirsutum Linn.). These studies collectively indicate that PRRs are rhythmically expressed.
Research on PRRs in soybean (Glycine max (L.) Merr.) has progressed more slowly compared to model plants such as Arabidopsis and rice. Zhang et al. [31] used a homologous comparison to obtain five homologous genes of PRR5/9, four homologous genes of PRR7, and four homologous genes of TOC1 (PRR1) to study circadian rhythm genes in soybean. This is the first time that PRR genes have been identified in soybeans. Subsequently, Li et al. [32] obtained two homologous genes of APRR3, GmPRR3a, and GmPRR3b, from a recombinant inbred line population of wild soybean (Glycine soja Siebold & Zucc.) and soybean. Li et al. [33] also identified a gene homologous to PRR7 in a population of local and cultivated soybean varieties. Wang et al. [34] also identified GmPRR37 in a population of recombinant inbred lines and developed a mutant suitable for planting in high-latitude areas or under multiple cropping conditions. Similarly, Li et al. [35] also obtained a GmPRR3b gene through a genome-wide association study and found that overexpression of a haplotype of this gene increased the number of main stem nodes and yield. Lu et al. [36] identified two PRR3 homologous genes, Tof11 and Tof12, and found that these two genes can directly bind to the LHY promoter to inhibit its transcription, which adds to the mechanism of photoperiod regulation of flowering in soybeans.
Based on previous laboratory studies [33], this study further studied the function of the identified GmPRR7b gene. Then, by analysing the rhythmic expression of its gene family members, we try to determine the potential interaction proteins of the target gene. These interactions are verified by real-time quantitative PCR (qRT-PCR), yeast two-hybrid detection, and BiFC. It is expected to provide additional evidence for elucidating the role of circadian rhythm signals in regulating soybean photoperiod response.
2. Results
2.1. Phenotypic Characterisation of the Glyma.12G073900
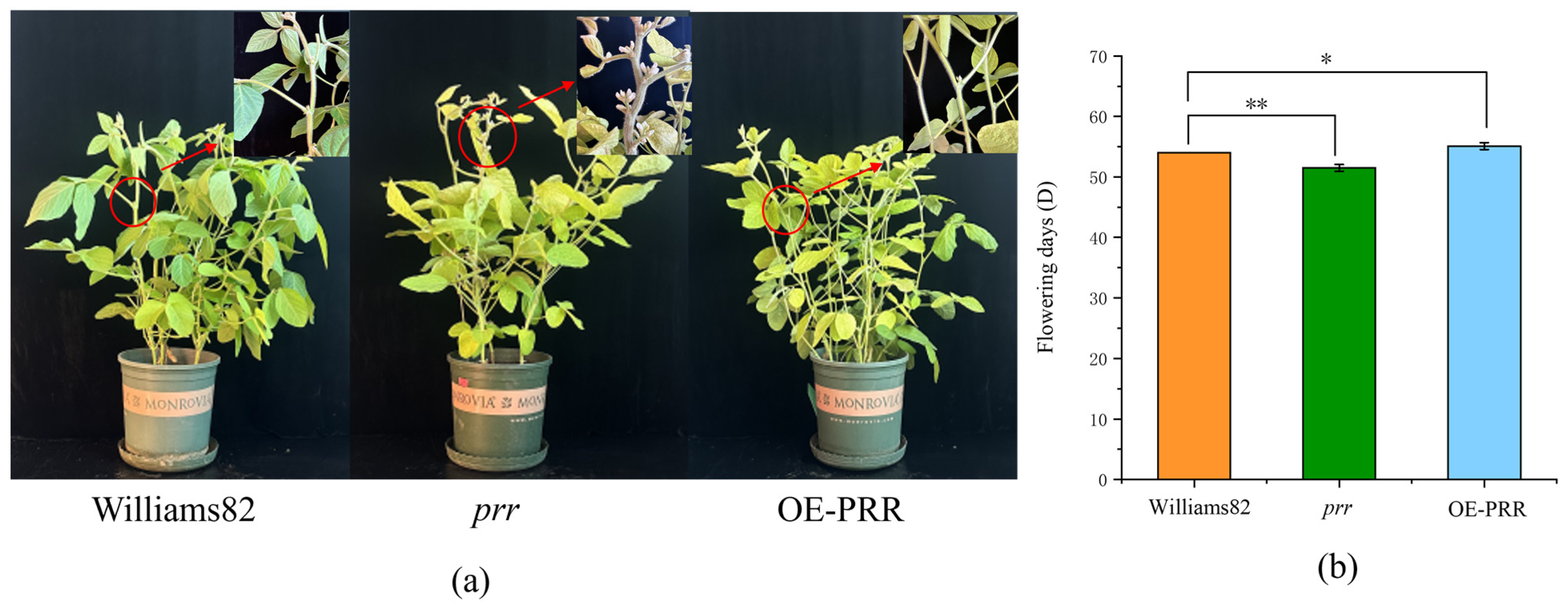
We obtained the Glyma.12G073900 in our preliminary work by obtaining a QTL related to flowering and screening it by localization [33]. To verify the function of Glyma.12G073900, transgentic lines overexpressing (OE-PRR) and gene-edited lines (prr) were obtained by transgenic technology (Figure S1, Table S1). We found that OE-PRR bloomed significantly later than the wild type, while the prr bloomed significantly earlier than the wild type (Figure 1), indicating that Glyma.12G073900 overexpression delayed flowering, while Glyma.12G073900 loss-of-function accelerated flowering time. This result suggests that Glyma.12G073900 functions as a floral repressor in soybean under standard growth conditions.
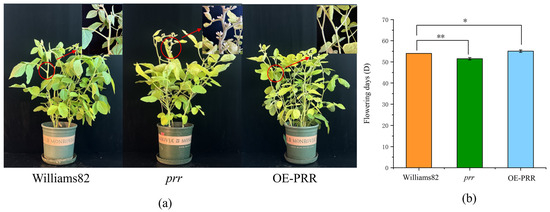
Figure 1.
Mutant, over-expression, and control plants and flowering time. (a), Mutant, overexpression, and control plants; (b) flowering time. * Statistical significance at 0.05 level; ** Statistical significance at 0.01 level.
2.2. Identification and Rhythmic Expression of Gene Family Members
Through a previous study by Li et al. [33], we found that Glyma.12G073900 belongs to the PRR gene family, so we re-identified the PRR gene family members. We searched the soybean genome data and obtained 12 PRR genes that contain both conserved structural domains of the PRR gene family. So Glyma.12G073900 is GmPRR7b. Although GmPRR7a and GmPRR7b do not contain the CCT domain, previous studies [32,33,34,35,36,37,38] have demonstrated their involvement in flowering, and we still consider them to be part of the PRR gene family. In total, we determined that there are 14 members of the PRR gene family in soybean (Table S2).
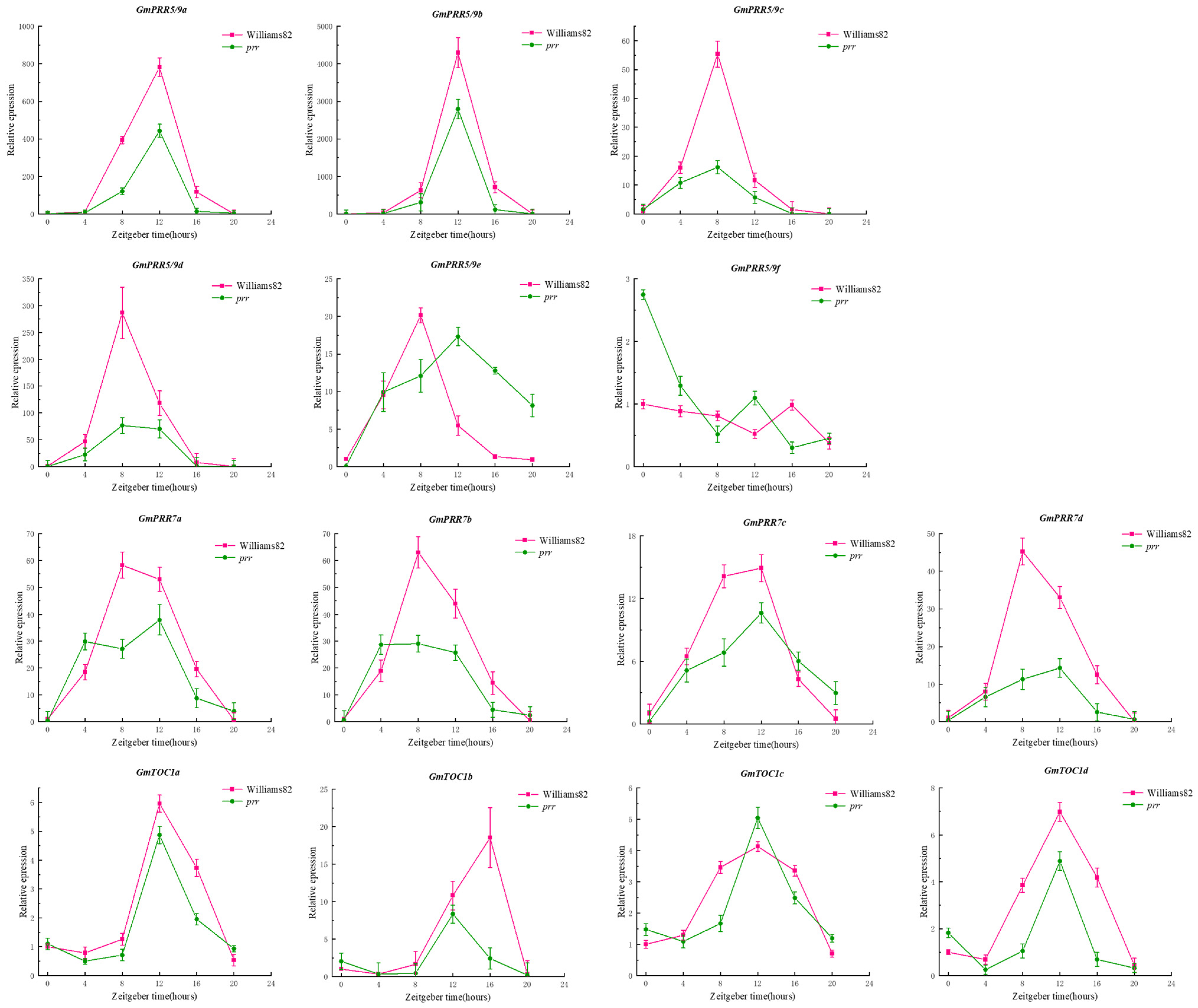
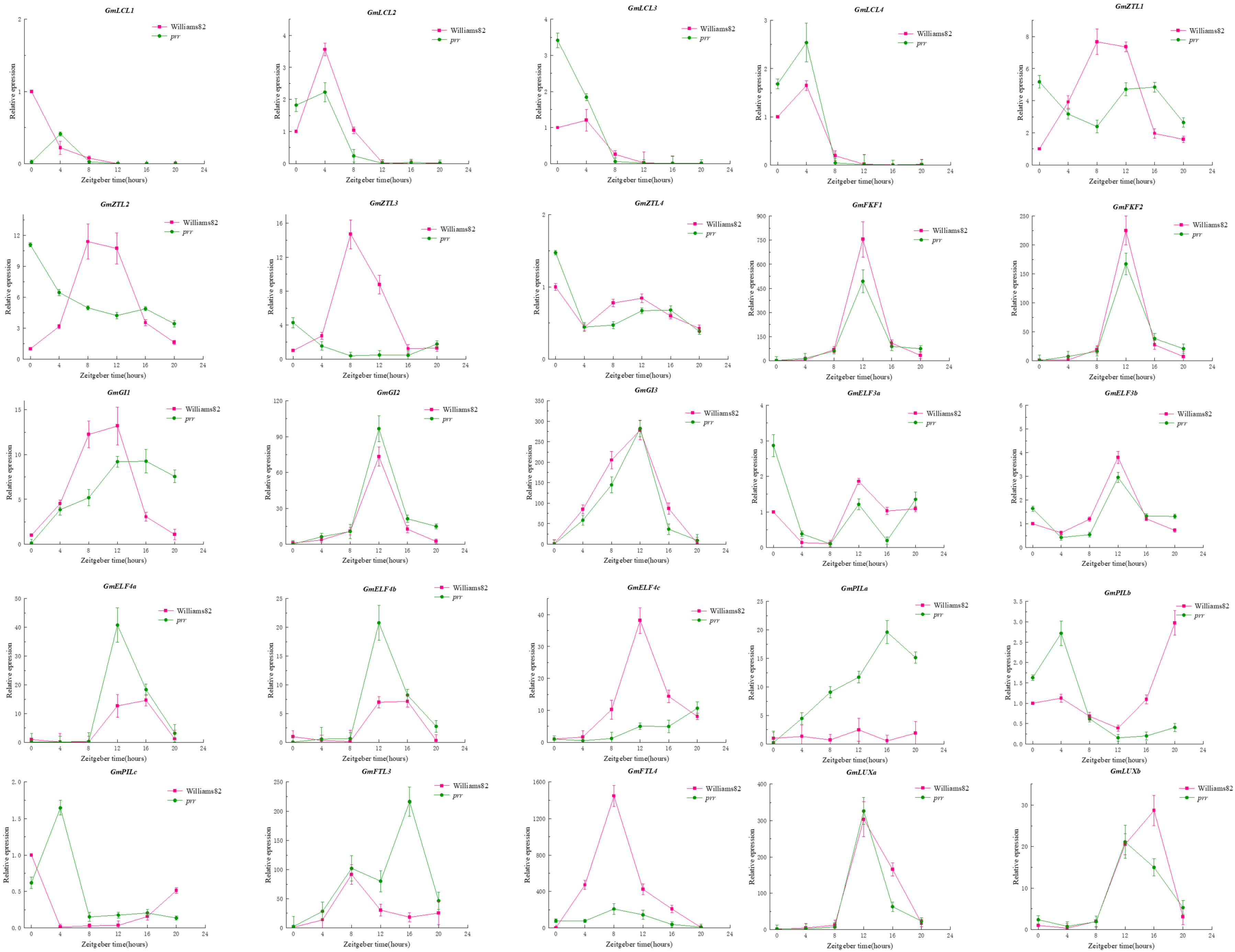
Due to the rhythmic expression characteristics of the PRR gene family, we tried to find the rhythmic expression pattern of GmPRRs as follows (Figure 2): We found that members of the gene family are characterised by rhythmic expression, and GmPRR5/9s appear to be classified into three groups based on the timing of expression, in Williams 82. GmPRR5/9c, GmPRR5/9d, GmPRR5/9e, GmPRR7b, and GmPRR7d were the first to reach peak expression at ZT8, then GmPRR7a; and GmPRR7c showed peak expression at both ZT8 and ZT12. Other PRR genes (GmPRR5/9a, GmPRR5/9b, GmTOC1a, GmTOC1c, GmTOC1d) basically reached peak expression at ZT12, and GmPRR5/9f and GmTOC1b were the latest, reaching peak expression at ZT16. In prr material, GmPRR5/9f was the first to reach peak expression at ZT0; GmPRR7a and GmPRR7b attained high expression at ZT4, followed by GmPRR5/9c, reaching peak expression at ZT10, and GmPRR5/9d reached a high expression, from ZT8 to ZT12, while the remaining genes reached peak expression at ZT12. These results suggest that PRR genes not only exhibit rhythmic expression but also may interact with one another within the gene family to respond to the light signal [9,10,11,12,13,14].
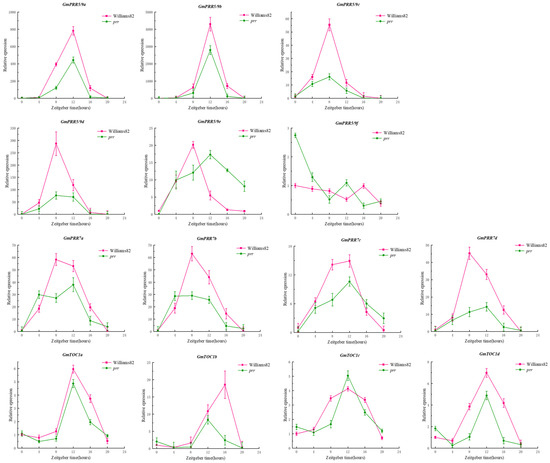
Figure 2.
Rhythmic expression of gene family members.
2.3. Verification of the Interaction Between GmPRR5/9a, GmPRR5/9b, and GmPRR7b
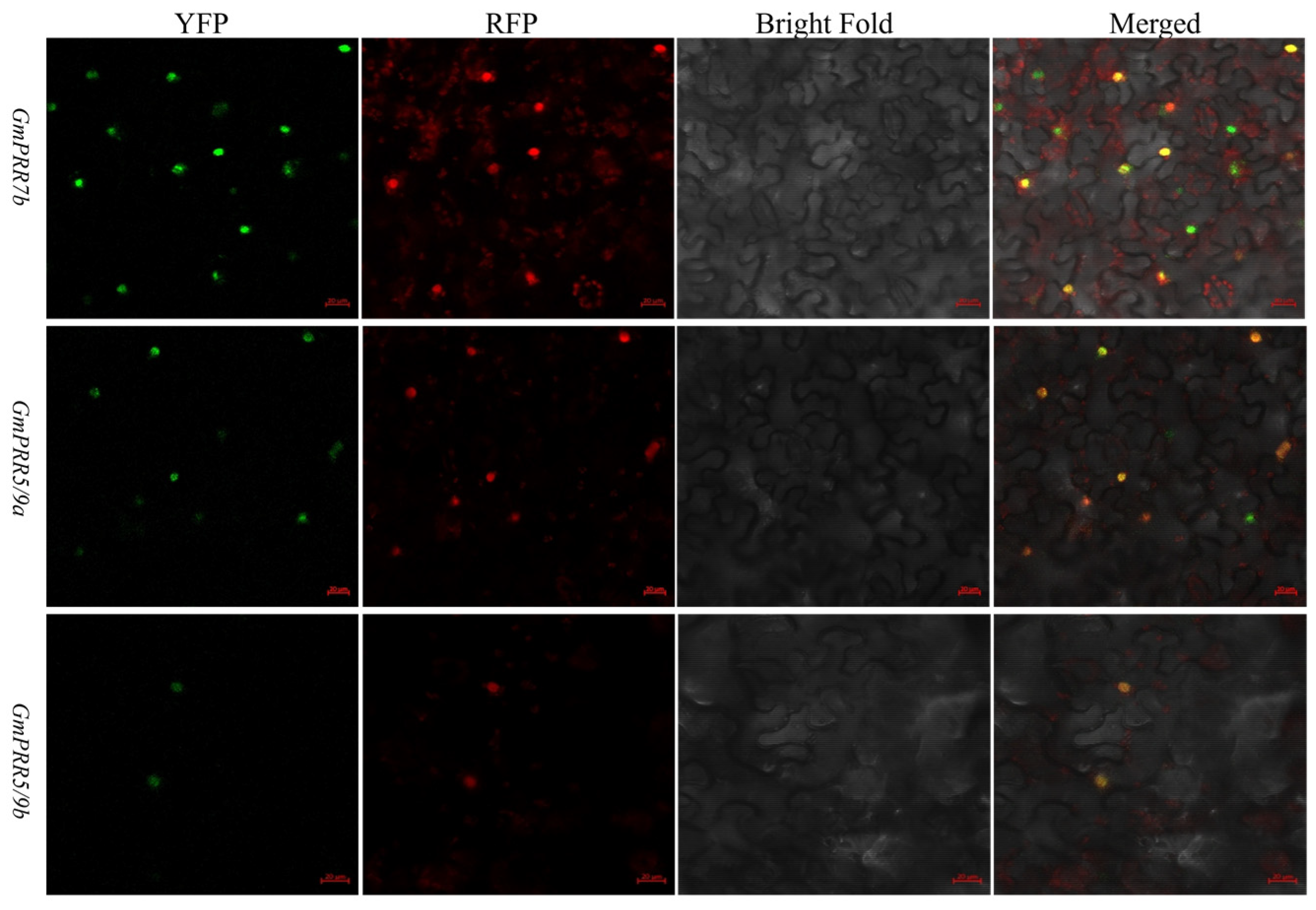
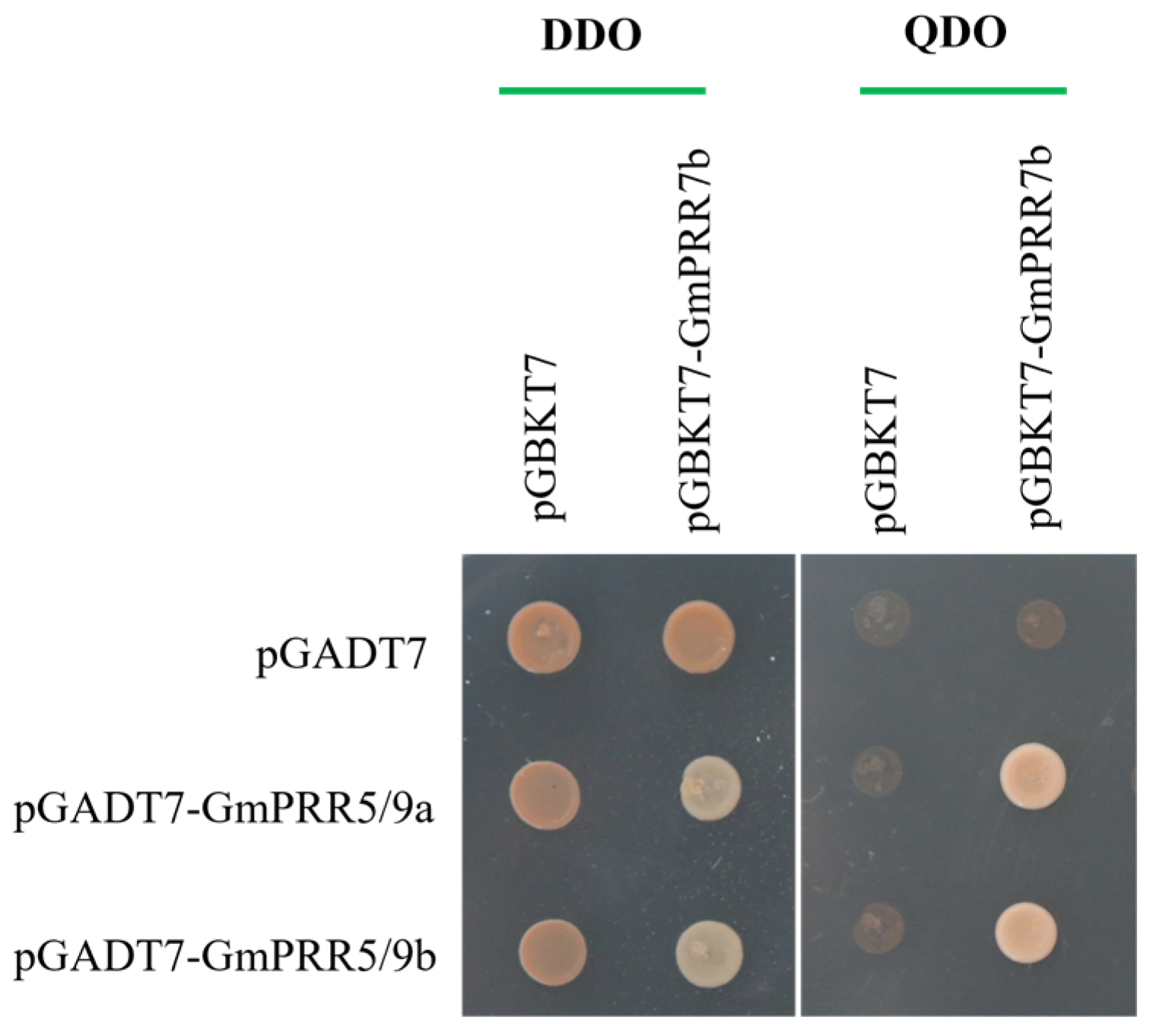
To test the above-stated, we selected GmPRR5/9a and GmPRR5/9b, which peaked after GmPRR7b, for yeast two-hybrid verification based on the order of expression. We first performed subcellular localization verification experiments on these three proteins (Figure 3). The results showed that all three proteins were localized in the nucleus, consistent with the previous prediction. On a high-stringency quadruple drop-out medium (QDO plate), the GmPRR5/9a and GmPRR7b plate grew white colonies, as did the GmPRR5/9b and GmPRR7b plate, indicating that both GmPRR5/9a and GmPRR5/9b interact with GmPRR7b at the protein level in yeast (Figure 4).
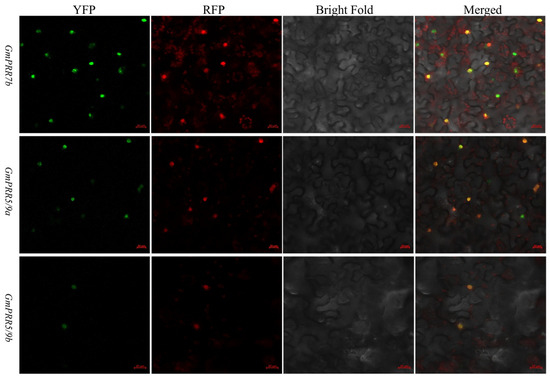
Figure 3.
Subcellular localization. Transient expression of pEarleygate104-GmPRR7b, pEarleygate104-GmPRR5/9a, pEarleygate104-GmPRR5/9b, and PC1302-RFP-PIP2 fusion proteins in tobacco; green indicates the fluorescent colour of YFP, red indicates the fluorescent colour of RFP, and yellow indicates the fluorescent colour of YFP and RFP complexed out (bar = 20 μm).
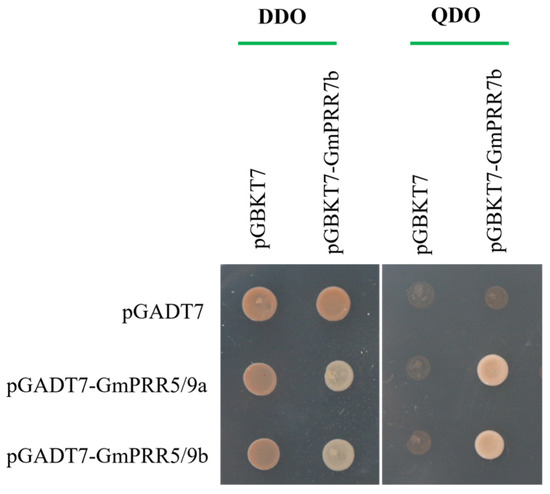
Figure 4.
Interaction protein screening of GmPRR7b.
To further verify the results of the yeast two-hybrid assay, we performed a BiFC assay using the split YFP system to confirm the interaction in living plant cells (Figure 5). Imaging with laser confocal microscopy revealed that there is YFP fluorescence in both construct pairs, indicating that GmPRR7b interacts with both GmPRR5/9a and GmPRR5/9b.
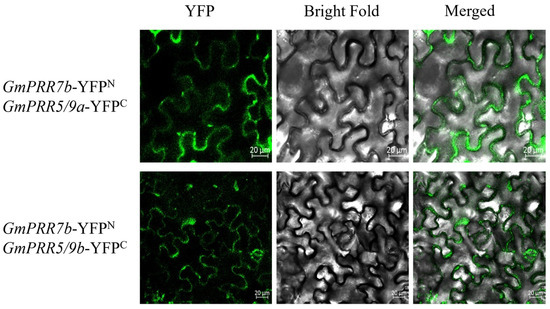
Figure 5.
BIFC for GmPRR7b.
2.4. Gene Regulatory Network Prediction
Based on the fact that circadian rhythm properties are closely linked to photoperiod [36,39] and we have demonstrated that there are interactions between gene family members, we used prr material to perform qRT-PCR on some of the currently known photoperiodic genes in order to be able to resolve the mechanism of GmPRR7b with the photoperiodic regulatory network (Figure 6). The results indicated that GmZTLs (GmZTL1, GmZTL2, GmZTL3), GmELF4s (GmELF4a, GmELF4b, GmELF4c), GmPILs (GmPILa, GmPILb, GmPILc), as well as GmFIL3 and GmFIL4 might be regulated in association with GmPRR7b expression.
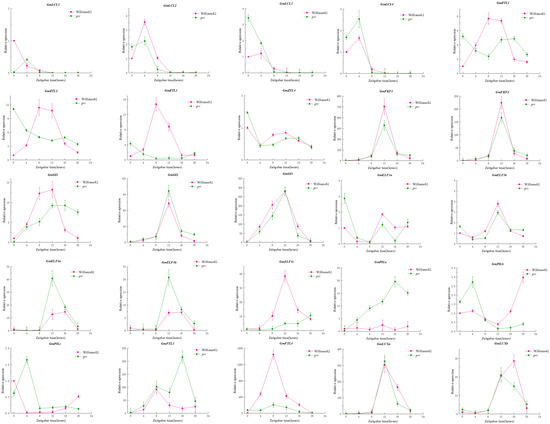
Figure 6.
qRT-PCR validation of gene regulatory networks.
3. Discussion
The two-component signal transduction system (TCS), the main mechanism of extracellular signal transduction, consists of a histidine protein kinase (HK) and a response regulator (RR). The response regulators (RR) are very similar to pseudo-response regulators [40]. In typical TCSs, once the HK senses a stimulus, the His protein kinase self-phosphorylates its conserved His residue to regulate its own signal, transferring the phosphate group to the conserved Asp residue in the RR acceptor domain to stimulate activity and respond accordingly [41]. The RR has an N-terminal receptor domain and a C-terminal output domain. The classical N-terminal receptor domain has a negatively charged amino acid surrounded by an N-terminal aspartic acid (D), a central aspartic acid site (D) that receives a phosphate group, and a C-terminal lysine (K), called the DDK sequence. Several DDK variants that are very similar to the classical DDK sequence have been found in Arabidopsis [42]. In these variants, the aspartic acid at the phosphorylation acceptor site is replaced with glutamic acid, and some amino acid positions differ. This type of protein is called pseudo-response regulatory protein (PRR), while the original classical DDK sequence regulatory protein is called response regulatory protein (RR) [43]. However, because PRR still contains an Asp residue in the conserved motif, it can still be used as the final output of the two-component phosphorelay in plants. Therefore, it is speculated that PRRs may be involved in the signal transduction of the His-to-Asp phosphorelay and the regulation of circadian rhythms.
Our previous work successfully localised the GmPRR7b gene on chromosome 12 [33]. So, we verified the gene function of GmPRR7b by constructing overexpression lines and gene editing lines. We suggest that the GmPRR7b gene is a deterrent to flowering, which is consistent with the findings of Wang et al. [34] and Lu et al. [36]. This could further confirm that GmPRR7b could provide a new target for creating soybean materials.
In Arabidopsis, PRRs are known clock factors involved in rhythmic expression in the core oscillator located in the photoperiod-regulated flowering pathway [44,45]. We re-identified all PRR gene family members in soybean and screened 12 members based on the structure and characteristics of the PRR gene family, which is consistent with the results of Wang et al. [30] and Zhang et al. [31]. There has been a lot of evidence [32,33,34,35,36,37,38] showing that GmPRR7a and GmPRR7b, although they are missing the CCT structural domains, can regulate flowering time. Not only that, in fact, we found that the wild type of GmPRR7b is equipped with the CCT structural domain and exhibits late flowering. However, it is CCT-deficient in bred varieties, such as W82 and CN16, which exhibit early flowering. Therefore, we agree with previous authors [30,31] that GmPRR7a and GmPRR7b belong to the PRR gene family, and the rhythmic expression of the PRR gene family members in prr material shows a different expression pattern from that in W82, which reveals the characteristic of the circadian cycle, ‘A single thread can pull the whole system’. After the change in GmPRR7b, GmPRR5/9a and GmPRR5/9b are the most direct changes. Therefore, we first chose to test the interaction between these two genes, and the yeast two-hybrid and BiFC experiments showed that they interacted with each other, especially in the BiFC experiments; we found that both of them were nuclear localised. GmPRR7b, GmPRR5/9a, and GmPRR5/9b and the interaction in BiFC was shown at the cell membrane, and we believe that when circadian signals are transmitted to GmPRR7b, GmPRR5/9a, and GmPRR5/9b, which are expressed downstream, interact with GmPRR7b at the cell membrane, thus generating a signal that transmits signals and maintains circadian rhythms that occurs.
We identified numerous genes associated with soybean photoperiod through gene regulatory network prediction of PRR gene family members, all of which may be regulated by GmPRR7b. GmLCLs are homologous to CCA1 and LHY and are first expressed in ZT0 and ZT4, in agreement with the results of Wang et al. [46] and Wu et al. [47]. GmZTLs, GmFKFs, and GmGIs have also all been shown to be associated with soybean flowering and photoperiod [48,49]; GmELF3s, GmELF4s, and GmLUXs are members of the EC of the soybean circadian complex [39,50,51]; in our results, GmPRR7b does not seem to have a regulatory relationship with GmELF3s and GmLUXs, but rather GmELF4s shows a strong regulatory relationship, so we infer that GmPRR7b is regulated by GmELF4s and then enters the EC complex, which influences the whole EC complex. Of course, these speculations need to be verified by further experiments, which is also the focus of our research direction in the future.
In summary, we propose a hypothesis that under prolonged sunlight, CCA1 and LHY activate the expression of GmPRR5/9a, GmPRR5/9b, and GmTOCs through activation of GmPRR5/9c, GmPRR5/9d, and GmPRR5/9e, which then affects the expression of GmPRR7s, GmPRR5/9a, GmPRR5/9b, and GmTOCs, and that the GmTOCs then acts, in turn, on CCA1 and LHY, forming a circadian cycle. When GmPRR7b was knocked down, GmPRR5/9a and GmPRR5/9b, which interact with GmPRR7b, showed reduced expression, which in turn affected the GmTOC expression, thereby increasing the expression of CCA1 and LHY and affecting GmELF4s in the evening EC complex, which then affects the entire EC complex and inhibits E1, resulting in the early flowering phenotype.
While we identified other genes that may have a regulatory relationship with each other, more research is needed. Studies on plant PRR genes primarily focus on the PRR gene family of the model plant, Arabidopsis; however, the structure, function, and expression patterns of PRR genes in soybeans require further systematic investigation. Such studies will provide deeper insights into the molecular mechanism underlying the functions of PRR genes more comprehensively, offering a strong scientific basis for future studies on the molecular mechanism underlying soybean growth.
4. Materials and Methods
4.1. Preparation of Plant Material
After amplifying the target gene using the parent CN16 of the group as a template [33], the overexpression vector pCAMBIA3301-GmPRR7b and the gene editing vector pKSE401-GmPRR7b was constructed, and Williams 82 was used as the genetic transformation receptor. The Agrobacterium-mediated method [52] was used to transfer the overexpression vector into Williams 82. The transgenic positive strain was obtained by screening with a concentration (160 mg·L−1) of glufosinate and Bar test strip, verified by sequencing, and then propagated to the F2 generation. After the transgenic plants were genetically stable, the phenotype was identified under long-day conditions (16 h light/8 h dark, LD). The above-related materials were provided by the Jilin Academy of Agricultural Sciences (Changchun, China).
4.2. Identification of Gene Family Members
The genomic data of soybean (Glycine max Wm82.a2. v1) were obtained from Phytozome 13 (https://phytozome.jgi.doe.gov/pz/portal.html, accessed on 27 September 2022). Previous studies have shown that genes belonging to the PRR family contain two conserved structural domains: REC(PF00072) and CCT(PF06203) [28,30]. We downloaded the HMM model from the InterPro website (https://www.ebi.ac.uk/interpro/search/sequence/, accessed on 27 September 2022), used it to perform an HMM search, and obtained the search results. The intersection was considered, and the CDD database (https://www.ncbi.nlm.nih.gov/cdd/, accessed on 27 September 2022), SMART database (http://smart.embl.de/, accessed on 27 September 2022), and Pfam database (http://pfam.xfam.org/, accessed on 27 September 2022) were then used to further identify the conserved domains of the initially screened candidate protein sequences. Following previous studies on PRR gene family members [31], we adopted the naming conventions established in those articles. The reidentified PRR family members in this study retain their original names, while newly identified members were named according to the same rules (Table S1).
4.3. Rhythmic Expression of Members
Williams 82 and prr transgenic materials were grown at room temperature under long-day conditions (16 h light/8 h dark). After the third trifoliate compound leaf was fully expanded, leaf tissue was collected every 4 h for 24 h. Samples were stored at −80 °C [33].
4.4. RNA Isolation and Quantitative Real-Time PCR Analysis
The Plant RNA Extraction Kit (Trans, Beijing, China) was used to extract RNA from the plant samples, and the purity and concentration of the total RNA were determined using the Nanodrop system (Thermo Fisher Scientific, Waltham, MA, USA). According to the kit instructions, cDNA was synthesized using the Prime Script™RT Reagent Kit (Takara Bio, Beijing, China). Real-time fluorescence quantitative PCR (qRT-PCR) was performed on each cDNA template using the TB Green Mix (Takara Bio, Beijing, China). The PCR amplification conditions were as follows: 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s and 60 °C for 30 s in a 10 μL reaction mixture. Three replicates were prepared per sample, and the QuantStudio 6 Flex system (Thermo Fisher Scientific, Waltham, MA, USA) was used to carry out the reactions. This was iterated three times. Relative gene expression was calculated using the 2−ΔΔCt method, with Tubllin serving as the internal reference gene (Table S3).
4.5. Validation of Gene Regulatory Networks
Expression validation was conducted as described in Section 4.3 and Section 4.4.
4.6. Subcellular Localization and Experimental Validation of Interacting Proteins
The target gene vector pEarleygate104-GmPRR7b was constructed for subcellular localization. The marker used was PC1302-RFP-PIP2. Subcellular localization was analysed by transient expression in tobacco leaf epidermal cells, and results were observed using confocal microscopy, following the methods described by Zhu et al. [53].
The target gene CDS sequence was linked to pGADT7 vector, and GmPRR7b was ligated to pGBKT7 vector for yeast two-hybrid interactions validation, and the specific experimental steps were referred to Haobo He [54].
The successful gene verified by yeast two-hybrid experiment was ligated to pSITE-C-EYEP vector, and GmPRR7b was ligated to pSITE-N-EYFP vector, and the results of yeast two-hybrid experiments were verified by tobacco transformation using laser confocal microscopy, and the specific experimental steps were referred to Hongxia Dong [55].
The above-related materials were provided by the Jilin Academy of Agricultural Sciences (Changchun, China).
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26062446/s1.
Author Contributions
Methodology, investigation, writing—original draft, Z.S.; writing, review, and validation, J.L., Z.S., and J.L. contributed equally to this work; review and editing Z.X., B.Z., and M.T.; data curation, X.Q., B.W., N.L., and Z.L.; supervision, Z.Y., Z.D., and C.Z.; project administration, Y.D. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Intergovernmental International Science, Technology, and Innovation Cooperation Key Project of the National Key R&D Programme (NKP) (No. 2022YFE0130300), the National Key R&D Programme (2021YFD1200103), Jilin Provincial Department of Science and Technology Key R&D Project on Agricultural Key Technologies (20240303012NC), the Earmarked Fund for China Agriculture Research System (CARS–04), and Jilin Province Agricultural Science and Technology Innovation Project (CXGC2023RCY01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Further inquiries on data resources can be directed to the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harmer, S.L. The Circadian System in Higher Plants. Annu. Rev. Plant Biol. 2009, 60, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Farré, E.M.; Liu, T. The PRR family of transcriptional regulators reflects the complexity and evolution of plant circadian clocks. Curr. Opin. Plant Biol. 2013, 16, 621–629. [Google Scholar] [CrossRef]
- Mcclung, C.R. Circadian Clock Components Offer Targets for Crop Domestication and Improvemen. Genes 2021, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Bunning, E. Endogenous Rhythms in Plants. Annu. Rev. Plant Physiol. 2003, 7, 71–90. [Google Scholar] [CrossRef]
- Bunning, E. Circadian Leaf Movements in Bean Plants: Earlier Reports. Science 1964, 146, 551. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Yuan, L.; Yang, X.; Zhang, X.; Wang, L.; Xie, Q. Circadian clock in plants: Linking timing to fitness. J. Integr. Plant Biol. 2022, 64, 792–811. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Sec, P.J.; Park, C.M. CCA1 alternative splicing as a way of linking the circadian clock to temperature response in Arabidopsis. Plant Signal. Behav. 2012, 7, 1194–1196. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakamichi, N. Pseudo-Response Regulators (PRRs) or true oscillator components (TOCs). Plant Cell Physiol. 2005, 46, 677–685. [Google Scholar] [CrossRef]
- Millar, A.; Carre, I.; Strayer, C.; Chua, N.; Kay, S. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science 1995, 267, 1161–1163. [Google Scholar] [CrossRef]
- Alabadi, D. Reciprocal Regulation Between TOC1 and LHY/CCA1 Within the Arabidopsis Circadian Clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef]
- Para, A.; Farre, E.M.; Imaizumi, T.; Pruneda-Paz, J.L.; Harmon, F.G.; Kay, S.A. PRR3 Is a vascular regulator of TOC1 stability in the Arabidopsis circadian clock. Plant Cell 2007, 19, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kita, M.; Ito, S.; Sato, E.; Yamashino, T.; Mizuno, T. The Arabidopsis pseudo-response regulators, PRR5 and PRR7, coordinately play essential roles for circadian clock function. Plant Cell Physiol. 2005, 46, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Farre, E.M.; Harmer, S.L.; Harmon, F.G.; Yanovsky, M.J.; Kay, S.A. Overlapping and distinct roles of PRR7 and PRR9 in the Arabidopsis circadian clock. Curr. Biol. 2005, 15, 47–54. [Google Scholar] [CrossRef]
- Matsushika, A.; Makino, S.; Kojima, M.; Mizuno, T. Circadian waves of expression of the APRR1/TOC1 family of pseudo-response regulators in Arabidopsis thaliana: Insight into the plant circadian clock. Plant Cell Physiol. 2000, 41, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kusano, M.; Nakamichi, N.; Kobayashi, M.; Hayashi, N.; Sakakibara, H.; Mizuno, T.; Saito, K. Impact of clock-associated Arabidopsis pseudo-response regulators in metabolic coordination. Proc. Natl. Acad. Sci. USA 2009, 106, 7251–7256. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kita, M.; Niinuma, K.; Ito, S.; Yamashino, T.; Mizoguchi, T.; Mizuno, T. Arabidopsis Clock-Associated Pseudo-Response Regulators PRR9, PRR7 and PRR5 Coordinately and Positively Regulate Flowering Time Through the Canonical CONSTANS-Dependent Photoperiodic Pathway. Plant Cell Physiol. 2007, 48, 822. [Google Scholar] [CrossRef]
- Liu, T.; Carlsson, J.; Takeuchi, T.; Newton, L.; Farre, E.M. Direct regulation of abiotic responses by the Arabidopsis circadian clock component PRR7. Plant J. 2013, 76, 101–114. [Google Scholar] [CrossRef]
- Nakamichi, N. Transcript Profiling of an Arabidopsis PSEUDO RESPONSE REGULATOR Arrhythmic Triple Mutant Reveals a Role for the Circadian Clock in Cold Stress Response. Plant Cell Physiol. 2009, 50, 447–462. [Google Scholar] [CrossRef]
- Nusinow, D.A.; Helfer, A.; Hamilton, E.E.; King, J.J.; Imaizumi, T.; Schultz, T.F.; Farre, E.M.; Kay, S.A. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 2012, 475, 398–402. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Wright, L.; Fujiwara, S.; Cremer, F.; Lee, K.; Onouchi, H.; Mouradov, A.; Fowler, S.; Kamada, H.; Coupland, P.G. Distinct Roles of GIGANTEA in Promoting Flowering and Regulating Circadian Rhythms in Arabidopsis. Plant Cell 2005, 17, 2255–2270. [Google Scholar] [CrossRef]
- Blázquez, M.A. Flower Development Pathways. J. Cell Sci. 2000, 113, 3547–3548. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.H.; Liu, B.H.; Kong, F.J. Regulation of Flowering and Maturation in Soybean. Adv. Bot. Res. 2022, 102, 43–75. [Google Scholar]
- Murakami, M.; Matsushika, A.; Ashikari, M.; Yamashino, T.; Mizuno, T. Circadian-associated rice pseudo response regulators (OsPRRs): Insight into the control of flowering time. Biosci. Biotechnol. Biochem. 2005, 69, 410–414. [Google Scholar] [CrossRef]
- Sun, C.; Chen, D.; Fang, J.; Wang, P.; Deng, X.; Chu, C. Understanding the genetic and epigenetic architecture in complex network of rice flowering pathways. Protein Cell 2014, 5, 889–898. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Qi, F.; Zhang, Z.; Xing, Y. Genetic Interactions Among Ghd7, Ghd8, OsPRR37 and Hd1 Contribute to Large Variation in Heading Date in Rice. Rice 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.H.; Yoo, S.C.; Park, J.W.; Kwon, C.T.; Lee, B.D.; An, G.; Zhang, Z. Natural Variation in OsPRR37 Regulates Heading Date and Contributes to Rice Cultivation at a Wide Range of Latitudes. Mol. Plant 2013, 6, 1877–1888. [Google Scholar] [CrossRef]
- Nakamichi, N.; Kudo, T.; Makita, N.; Kiba, T.; Sakakibara, H. Flowering time control in rice by introducing Arabidopsis clock-associated PSEUDO-RESPONSE REGULATOR 5. Biosci. Biotechnol. Biochem. 2020, 84, 970–979. [Google Scholar] [CrossRef]
- Irum, S.; Rehman, N.; Inam, S.; Khan, M.Z.F.; Khan, M.R. Genome-wide identification and expression profiling of Pseudo-Response Regulator (PRR) gene family in tomato. Environ. Exp. Bot. 2024, 220, 105683. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Wang, Z.; Xie, Z.; Qi, K.; Yue, D.; Li, Y.; Zhang, S.; Wu, J.; Wang, P. Molecular characterization of PSEUDO RESPONSE REGULATOR family in Rosaceae and function of PbPRR59a and PbPRR59b in flowering regulation. BMC Genom. 2024, 25, 794. [Google Scholar] [CrossRef]
- Wang, J.; Du, Z.; Huo, X.; Zhou, J.; Chen, Y.; Zhang, J.; Pan, A.; Wang, X.; Wang, F.; Zhang, J. Genome-wide analysis of PRR gene family uncovers their roles in circadian rhythmic changes and response to drought stress in Gossypium hirsutum L. PeerJ 2020, 8, e9936. [Google Scholar] [CrossRef]
- Zhang, S.R.; Wang, H.; Wang, Z.Y.; Ren, Y.; Niu, L.F. Photoperiodism dynamics during the domestication and improvement of soybea. Sci. China Life Sci. 2017, 60, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Li, M.W.; Liu, W.; Lam, H.M.; Gendron, J.M. Characterization of Two Growth Period QTLs Reveals Modification of PRR3 Genes during Soybean Domestication. Plant Cell Physiol. 2018, 60, 407–420. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Wu, H.; Hu, B.; Xia, Z. Positional Cloning of the Flowering Time QTL qFT12-1 Reveals the Link Between the Clock Related PRR Homolog With Photoperiodic Response in Soybeans. Front. Plant Sci. 2019, 10, 1303. [Google Scholar] [CrossRef]
- Wang, L.W.; Sun, S.; Wu, T.T. Natural variation and CRISPR/Cas9-mediated mutation in GmPRR37 affect photoperiodic flowering and contribute to regional adaptation of soybean. Plant Biotechnol. J. 2020, 18, 1869–1881. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.H.; Li, Y.; Lu, H.; Qiu, L.J. A Domestication-Associated Gene GmPRR3b Regulates Circadian Clock and Flowering Time in Soybean. Mol. Plant 2020, 13, 745–759. [Google Scholar] [CrossRef]
- Lu, S.J.; Dong, L.D.; Fang, C.; Liu, S.L.; Kong, L.P.; Cheng, Q.; Chen, L.Y.; Su, T.; Nan, H.Y.; Zhang, D.; et al. Stepwise selection on homeologous PRR genes controlling flowering and maturity during soybean domestication. Nat. Genet. 2020, 52, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Wang, L.W.; Zhang, L.X.; Wu, T.T.; Sun, B.Q.; Zhang, J.Q.; Sapey, E.; Yuan, S.; Jiang, B.J.; Chen, F.L.; et al. Genomic Dissection and Diurnal Expression Analysis Reveal the Essential Roles of the PRR Gene Family in Geographical Adaptation of Soybean. Int. J. Mol. Sci. 2022, 23, 9970. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Fang, C.; Cheng, Q.; Su, T.; Liu, B. Genetic Basis and Adaptation Trajectory of Soybean from Its Temperate Origin to Tropics. Nat. Commun. 2021, 12, 5445. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.Y.; Zhang, S.R.; Lin, X.Y.; Jia, Z.W.; Zhao, F.; Wei, X.Z.; Jiao, Y.C.; Li, Z.; Niu, Z.Y.; et al. GmEID1 modulates light signaling through the Evening Complex to control flowering time and yield in soybean. Proc. Natl. Acad. Sci. USA 2023, 120, e2212468120. [Google Scholar] [CrossRef]
- Makino, S.; Kiba, T.; Imamura, A.; Hanaki, N.; Nakamura, A.; Suzuki, T.; Taniguchi, M.; Ueguchi, C. Genes Encoding Pseudo-Response Regulators: Insight into His-to-Asp Phosphorelay and Circadian Rhythm in Arabidopsis thalian. Plant Cell Physiol. 2000, 41, 791–803. [Google Scholar] [CrossRef]
- Zhao, S.L.; Jing, Y.F.; Liu, Q.Q.; Yang, M.; Wang, C.L. The role of the pseudo-response regulator protein in the plant photoperiodic regulatory pathway. J. Nucl. Agric. 2018, 32, 1740–1749. [Google Scholar]
- Imamura, A.; Hanaki, N.; Nakamura, A.; Suzuki, T.; Taniguchi, M.; Kiba, T.; Ueguchi, C.; Sugiyama, T.; Mizuno, T. Compilation and Characterization of Arabiopsis thaliana Response Regulators Implicated in His-Asp Phosphorelay Signal Transduction. Plant Cell Physiol. 1999, 40, 733–742. [Google Scholar] [CrossRef]
- Hwang, I.; Chen, H.C.; Sheen, J. Two-Component Signal Transduction Pathways in Arabidopsis. Plant Physiol. 2002, 129, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, T. Arabidopsis Circadian Clock and Photoperiodism: Time to Think about Locatio. Curr. Opin. Plant Biol. 2010, 13, 83–89. [Google Scholar] [CrossRef]
- Pruneda-Paz, J.L.; Kay, S.A. An Expanding Universe of Circadian Networks in Higher Plants. Trends Plant Sci. 2010, 15, 259–265. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, L.; Su, T.; Wang, Q.; Gao, Y.; Zhang, S.Y.; Jia, Q.; Yu, G.L.; Fu, Y.F.; Cheng, Q.; et al. Light- and temperature-entrainable circadian clock in soybean development. Plant Cell Environ. 2020, 43, 637–648. [Google Scholar] [CrossRef]
- Wu, Z.J. Gene Cloning, Expression Pattern and Function Analysis of GmTOC1s and GmLCLs in Soybean. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2010. [Google Scholar]
- Wang, F.; Liu, S.R.; Li, H.Y.; Fang, C.; Fang, S.J.; Wang, J.H.; Li, S.C.; Liu, H.; Du, H.P.; Wang, L.S.; et al. Artificial selection of two antagonistic e3 ubiquitin ligases finetunes soybean photoperiod adaptation and grain yield. Proc. Natl. Acad. Sci. USA 2024, 121, e2321473121. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, X.; Hu, R.; Wu, F.; Fu, Y. Identification and molecular characterization of fkf1 and gi homologous genes in soybean. PLoS ONE 2013, 8, e79036. [Google Scholar] [CrossRef]
- Zhao, X.; Li, H.; Wang, L.; Wang, J.; Huang, Z.; Du, H.; Li, Y.; Yang, J.; He, M.; Cheng, Q. A critical suppression feedback loop determines soybean photoperiod sensitivity. Dev. Cell 2024, 59, 19. [Google Scholar] [CrossRef]
- Liang, Y.M.; Tian, F. E2 family and evening complex identify soybean photoperiod sensitivity. New Crops 2025, 2949–9526. [Google Scholar] [CrossRef]
- Guo, D.Q.; Yang, X.D.; Bao, S.J.; Guo, S.D.; Kang, L.S.; Yi, A.P.; Qian, X.Y.; Zhao, G.L. Obtaining and stably expressing soybean with double-price insect-resistant genes of CryIA and CpTI. Chin. Agric. Sci. 2008, 10, 2957–2962. [Google Scholar]
- Zhu, S.; Chen, M.; Liang, C. Characterization of purple acid phosphatase family and functional analysis of GmPAP7a/7b involved in extracellular ATP utilization in soybean. Front. Plant Sci. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- He, H.B. Functional Analysis of the Soybean MADS-Box Family GmAP3 Gene in Flower Development. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2023; pp. 43–44. [Google Scholar]
- Dong, H.X. Research on the Interaction Between GmNaKR1 and the Soybean Flowering Promoter Gene GmFT2. Master’s Thesis, Harbin Normal University, Harbin, China, 2024; pp. 36–37. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).