The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research
Abstract
1. Background
2. Podocyte Injury in the Continuum of MCD/FSGS
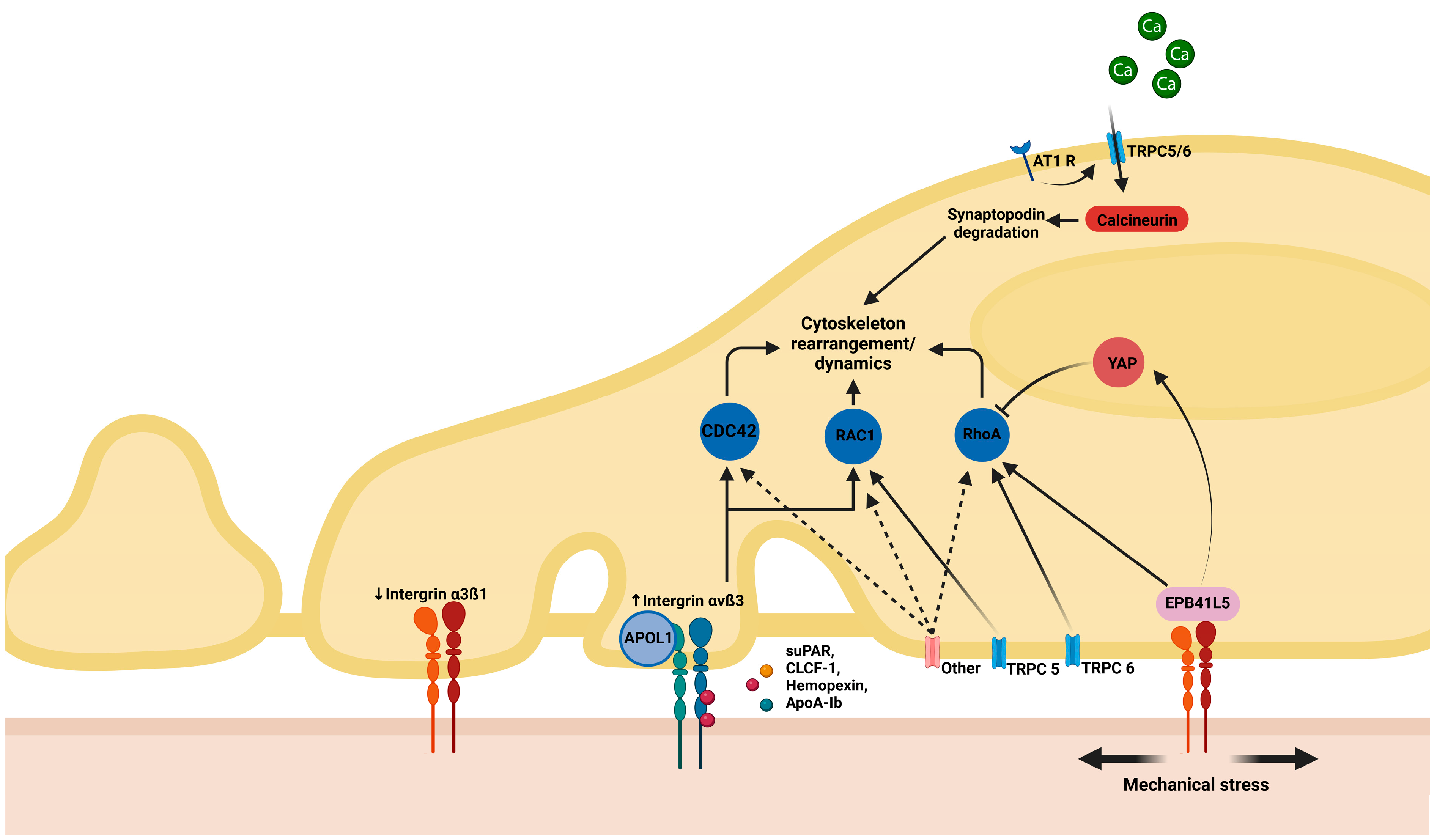
2.1. Structural Deficiencies
2.2. Virus-Induced Lesions
2.3. Permeability Factors
2.4. Immune-Mediated Injury
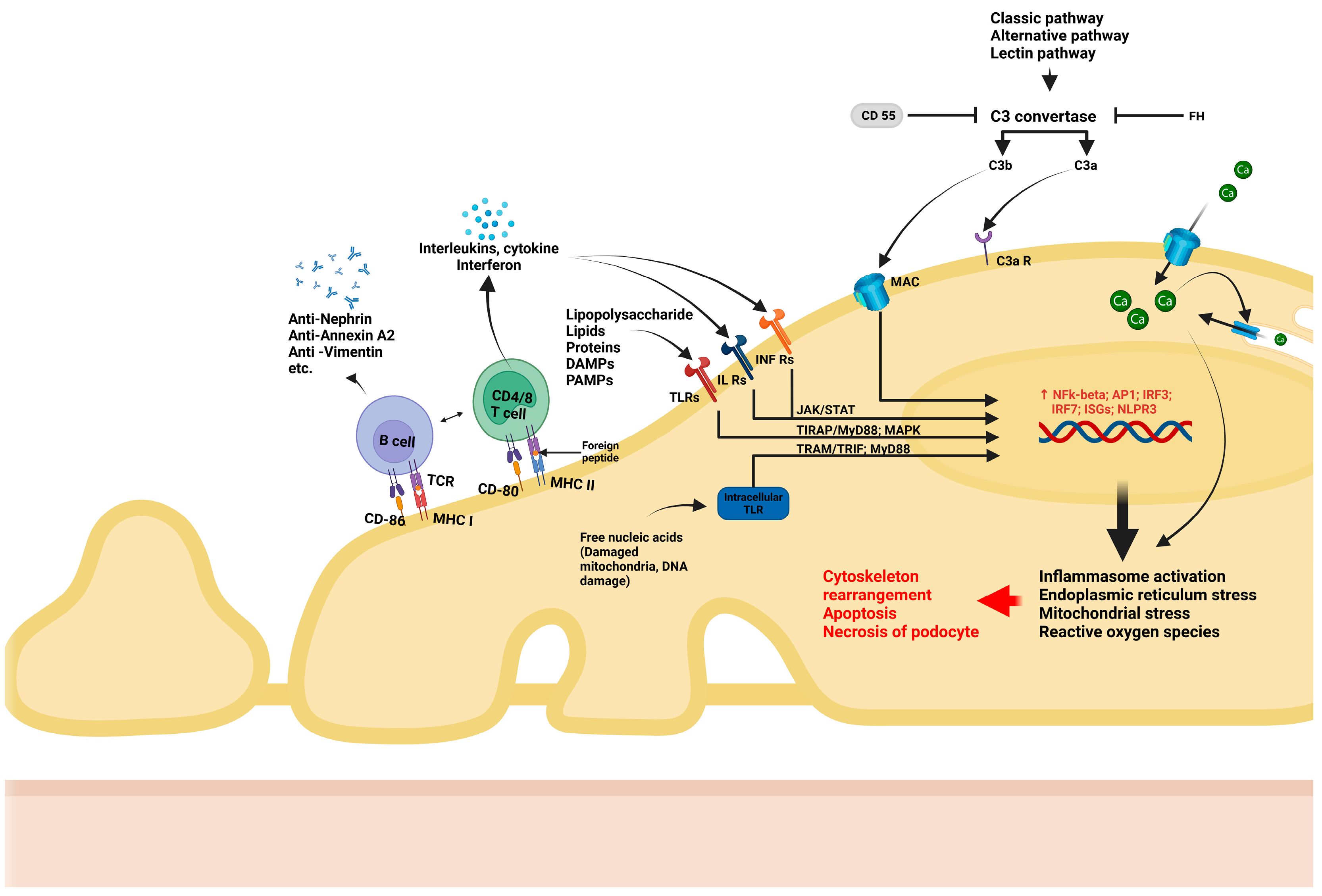
- T lymphocytes have long been suspected of being involved in podocyte dysfunction, particularly in MCD. Podocytes express major histocompatibility factor I/II proteins which are involved in immune recognition, as well as B7-1 (CD80), which are T cell co-stimulatory molecules. These influence the activation of lymphocytes, trigger imbalance between effector and regulatory T lymphocytes, as well as interleukin (IL) and other cytokine synthesis by T lymphocytes. For example, IL-17 synthesis by CD17 lymphocytes, with subsequent TNF-α-induced inflammatory cascade [32] and inflammatory responses [33], are all consequences of podocyte/immune cell interactions. More recently, the role of B lymphocyte-driven mechanisms in podocyte injury has gained attention. Podocytes also express B7-2 (CD86), a co-stimulatory molecule that plays a key role in the activation of B lymphocytes and antigen-presenting cells [34]. Recent studies have convincingly documented antibody-mediated podocyte injury, providing evidence for the pathogenic role of nephrin antibodies in steroid-responsive MCD [35]. In addition to nephrin, several antibodies, such as those against annexin A2 [36], or other components of the cytoskeleton [37] have been suggested as etiological factors in certain subsets of podocyte diseases.
- Podocytes also express recognition receptors such as TLRs, which can recognize pathogen-associated molecular patterns (PAMPs), including DNA/RNA fragments. Upon recognition, TLRs activate intracellular pathways that ultimately lead to generation of reactive oxygen species (ROS), mitochondrial stress, and endoplasmic reticulum stress [38]. Additionally, podocytes can respond to metabolic stimuli (e.g., hyperglycemia) and toxic environment stimuli (e.g., puromycin aminoxide toxicity). Such stimuli lead to the activation of intracellular pathways such as NF-κB or MAPK, triggering the production of inflammatory cytokines (IL-1 and IL-18) and TGF-ß, increase oxidative stress, and activate the inflammasome, ultimately resulting in podocyte damage and apoptosis [34,39,40].
- Podocytes are increasingly recognized for their role in regulating the complement system. They are able to synthetize components of the complement system including C1q, C1r, C2, C3, C3a receptor (C3aR), C5a receptor (C5aR), and C7, as well as their inhibitors such as CD47, CD55, CD59, soluble complement factor I (CFI), and complement factor H (CFH). This enables podocytes to precisely regulate inflammatory responses triggered by complement, thereby supporting glomerular homeostasis and reducing damage caused by complement activation. However, podocytes can also become targets of complement-mediated injury under certain instances. For example, in an elegant experimental model of adriamycin-induced podocyte injury, the lack of a C3 convertase inhibitor (CD55) exacerbated podocyte injury and proteinuria, suggesting that complement regulation is essential for podocyte protection [41]. Moreover, podocytes are heavily dependent on factor H protection against excessive complement activation, as complement factors can leak into the GBM, especially under proteinuric conditions. Factor H is able to inactivate them by N terminal binding while being anchored through the C terminal fragment to components of the GBM. Additionally, studies have shown that puromycin/immunotoxin-induced podocyte injury leads to increased factor H expression in podocytes, which correlates with the clearance of subendothelial immune complex deposits [42]. Finally, recent evidence suggests that the activation of the lectin pathway of the complement system may contribute to the pathogenesis of FSGS [43].
- Recent studies have increasingly recognized that sub-lytic levels of the membrane attack complex (MAC) can induce podocyte injury without causing cell death. When internalized, excess MAC can influence calcium (Ca2+) entry into the cell, leading to a cascade of downstream effects. One major consequence is the activation of the NF-κB-mediated inflammatory response, which can play a critical role in podocyte dysfunction. Additionally, excessive calcium influx can dysregulate oxidative stress pathways and cause endoplasmic reticulum and mitochondrial stress [44].
2.5. Toxic-Induced Podocyte Damage
2.6. Mechanical Stress
3. From Pathogenesis to Therapeutic Approach
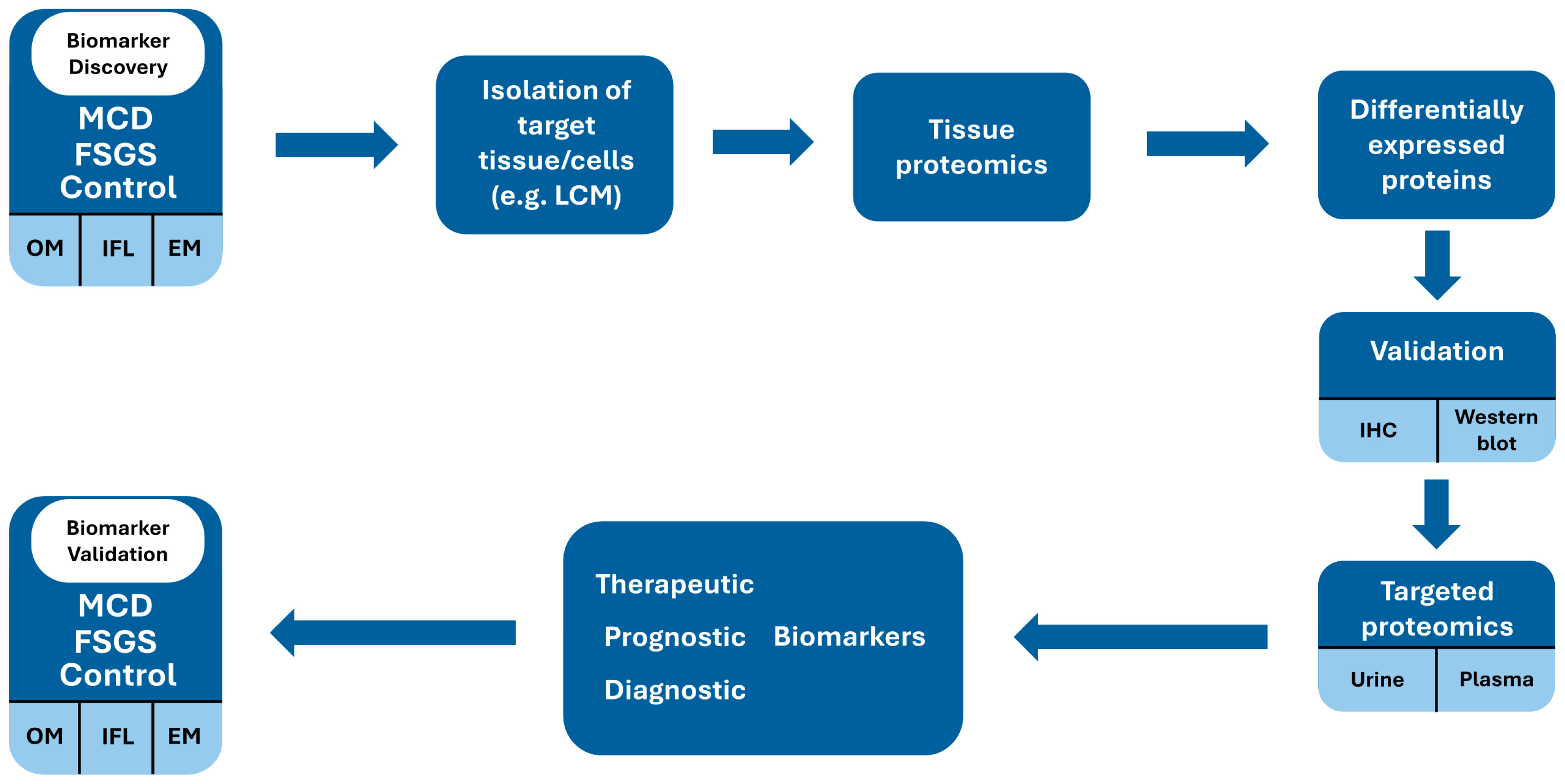
4. Advancements in Biomarker Discovery: Insights from Proteomic Approaches
4.1. MCD/FSGS Versus Other Nephrotic Syndromes/Healthy Controls
4.2. MCD Versus FSGS
4.3. Primary Versus Secondary FSGS
4.4. Prognosis and Response to Treatment
5. Proteomic Biomarkers: The Way Forward?
- Complement activation has emerged as an important mechanism in the pathogenesis of FSGS and is strongly associated with more severe morphological injury and poorer prognosis. Decreased plasma C3 levels were associated with loss of kidney function and, more importantly, proteinuria and tubulointerstitial injury, as well as a progressive renal disease in FSGS [82]. Furthermore, both plasma and urine complement proteins, including MAC, correlate inversely with kidney function and directly with proteinuria and histologic findings in FSGS [83,84]. Recent reports have indicated that urinary C5a and MAC may serve as useful biomarkers for distinguishing FSGS from MCD [85].
- T lymphocyte-mediated mechanisms have been identified through the increased CD80 levels in the urine of nephrotic MCD patients, a marker not observed in patients in remission from MCD or in FSGS patients [86]. This suggests that CD80 may serve as a potential biomarker for active disease and could play a role in the pathogenesis of nephrotic syndrome in certain patient subsets.
- Proinflammatory and profibrotic pathways can be reflected by elevated urinary TGF-ß levels, which are reported to be higher in FSGS compared to MCD [87].
6. Concluding Remarks/Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MCD | Minimal change disease |
| FSGS | Focal segmental glomerulosclerosis |
| FPE | Foot process effacement |
| EM | Electron microscopy |
| LM | Light microscopy |
| FP | Foot process |
| SD | Slit diaphragm |
| FA | Focal adhesion |
| ECM | Extracellular matrix |
| GBM | Glomerular basement membrane |
| TLRs | Toll-like receptors |
| ILs | Interleukins |
| MAC | Membrane attack complex |
| IgAN | IgA nephropathy |
| MN | Membranous nephropathy |
| rFSGS | Recurrent focal segmental glomerulosclerosis |
| MS | Mass spectrometry |
References
- Caster, D.J.; Hobeika, L.; Klein, J.B.; Powell, D.W.; McLeish, K.R. Changing the Concepts of Immune-mediated Glomerular Diseases through Proteomics. Proteom. Clin. Appl. 2015, 9, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Smoyer, W.E.; Schachter, A.D. Minimal Change Disease and FSGS Are a Spectrum of a Single Disease within Immune-Mediated Nephrotic Syndrome. Kidney360 2024, 5, 1197–1199. [Google Scholar] [CrossRef]
- Ravaglia, F.; Melica, M.E.; Angelotti, M.L.; De Chiara, L.; Romagnani, P.; Lasagni, L. The Pathology Lesion Patterns of Podocytopathies: How and Why? Front. Cell Dev. Biol. 2022, 10, 838272. [Google Scholar] [CrossRef]
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- Ishizuka, K.; Miura, K.; Hashimoto, T.; Kaneko, N.; Harita, Y.; Yabuuchi, T.; Hisano, M.; Fujinaga, S.; Omori, T.; Yamaguchi, Y.; et al. Degree of Foot Process Effacement in Patients with Genetic Focal Segmental Glomerulosclerosis: A Single-Center Analysis and Review of the Literature. Sci. Rep. 2021, 11, 12008. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef]
- Maas, R.J.; Deegens, J.K.; Beukhof, J.R.; Reichert, L.J.; ten Dam, M.A.; Beutler, J.J.; van den Wall Bake, A.W.L.; Rensma, P.L.; Konings, C.J.; Geerse, D.A.; et al. The Clinical Course of Minimal Change Nephrotic Syndrome with Onset in Adulthood or Late Adolescence: A Case Series. Am. J. Kidney Dis. 2017, 69, 637–646. [Google Scholar] [CrossRef]
- Husain, S. Role of Podocyte in Kidney Disease. Front. Biosci.-Landmark 2024, 29, 250. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef]
- Haydak, J.; Azeloglu, E.U. Role of Biophysics and Mechanobiology in Podocyte Physiology. Nat. Rev. Nephrol. 2024, 20, 371–385. [Google Scholar] [CrossRef]
- Kriz, W.; Shirato, I.; Nagata, M.; LeHir, M.; Lemley, K.V. The Podocyte’s Response to Stress: The Enigma of Foot Process Effacement. Am. J. Physiol.-Ren. Physiol. 2013, 304, F333–F347. [Google Scholar] [CrossRef] [PubMed]
- Bărar, A.A.; Pralea, I.-E.; Maslyennikov, Y.; Munteanu, R.; Berindan-Neagoe, I.; Pîrlog, R.; Rusu, I.; Nuțu, A.; Rusu, C.C.; Moldovan, D.T.; et al. Minimal Change Disease: Pathogenetic Insights from Glomerular Proteomics. Int. J. Mol. Sci. 2024, 25, 5613. [Google Scholar] [CrossRef]
- Somlo, S.; Mundel, P. Getting a Foothold in Nephrotic Syndrome. Nat. Genet. 2000, 24, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.B.; Benzing, T. The Slit Diaphragm: A Signaling Platform to Regulate Podocyte Function. Curr. Opin. Nephrol. Hypertens. 2005, 14, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.; Sun, H.; Majmundar, A.J. Mechanisms of Podocyte Injury in Genetic Kidney Disease. Pediatr. Nephrol. 2024. [Google Scholar] [CrossRef]
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef]
- Yamashita, M.; Millward, C.A.; Inoshita, H.; Saikia, P.; Chattopadhyay, S.; Sen, G.C.; Emancipator, S.N. Antiviral Innate Immunity Disturbs Podocyte Cell Function. J. Innate Immun. 2013, 5, 231–241. [Google Scholar] [CrossRef]
- Migliorini, A.; Angelotti, M.L.; Mulay, S.R.; Kulkarni, O.O.; Demleitner, J.; Dietrich, A.; Sagrinati, C.; Ballerini, L.; Peired, A.; Shankland, S.J.; et al. The Antiviral Cytokines IFN-α and IFN-β Modulate Parietal Epithelial Cells and Promote Podocyte Loss. Am. J. Pathol. 2013, 183, 431–440. [Google Scholar] [CrossRef]
- Li, J.; Das, J.R.; Tang, P.; Han, Z.; Jaiswal, J.K.; Ray, P.E. Transmembrane TNF-α Facilitates HIV-1 Infection of Podocytes Cultured from Children with HIV-Associated Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 862–875. [Google Scholar] [CrossRef]
- Gbadegesin, R.A.; Ulasi, I.; Ajayi, S.; Raji, Y.; Olanrewaju, T.; Osafo, C.; Ademola, A.D.; Asinobi, A.; Winkler, C.A.; Burke, D.; et al. APOL1 Bi- and Monoallelic Variants and Chronic Kidney Disease in West Africans. N. Engl. J. Med. 2025, 392, 228–238. [Google Scholar] [CrossRef]
- Kopp, J.B.; Nelson, G.W.; Sampath, K.; Johnson, R.C.; Genovese, G.; An, P.; Friedman, D.; Briggs, W.; Dart, R.; Korbet, S.; et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J. Am. Soc. Nephrol. 2011, 22, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Daneshpajouhnejad, P.; Kopp, J.B.; Winkler, C.A.; Rosenberg, A.Z. The Evolving Story of Apolipoprotein L1 Nephropathy: The End of the Beginning. Nat. Rev. Nephrol. 2022, 18, 307–320. [Google Scholar] [CrossRef]
- Wada, T.; Nangaku, M. A Circulating Permeability Factor in Focal Segmental Glomerulosclerosis: The Hunt Continues. Clin. Kidney J. 2015, 8, 708–715. [Google Scholar] [CrossRef]
- Bakker, W.W.; Borghuis, T.; Harmsen, M.C.; Van den Berg, A.; Kema, I.P.; Niezen, K.E.; Kapojos, J.J. Protease Activity of Plasma Hemopexin. Kidney Int. 2005, 68, 603–610. [Google Scholar] [CrossRef]
- Clement, L.C.; Macé, C.; Avila-Casado, C.; Joles, J.A.; Kersten, S.; Chugh, S.S. Circulating Angiopoietin-like 4 Links Proteinuria with Hypertriglyceridemia in Nephrotic Syndrome. Nat. Med. 2014, 20, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; El Hindi, S.; Li, J.; Fornoni, A.; Goes, N.; Sageshima, J.; Maiguel, D.; Karumanchi, S.A.; Yap, H.-K.; Saleem, M.; et al. Circulating Urokinase Receptor as a Cause of Focal Segmental Glomerulosclerosis. Nat. Med. 2011, 17, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Nast, C.C.; Alachkar, N. Permeability Factors in Focal and Segmental Glomerulosclerosis. Adv. Chronic Kidney Dis. 2014, 21, 417–421. [Google Scholar] [CrossRef]
- Chhuon, C.; Herrera-Marcos, L.V.; Zhang, S.-Y.; Charrière-Bertrand, C.; Jung, V.; Lipecka, J.; Savas, B.; Nasser, N.; Pawlak, A.; Boulmerka, H.; et al. Proteomics of Plasma and Plasma-Treated Podocytes: Application to Focal and Segmental Glomerulosclerosis. Int. J. Mol. Sci. 2023, 24, 12124. [Google Scholar] [CrossRef]
- Lopez-Hellin, J.; Cantarell, C.; Jimeno, L.; Sanchez-Fructuoso, A.; Puig-Gay, N.; Guirado, L.; Vilariño, N.; Gonzalez-Roncero, F.M.; Mazuecos, A.; Lauzurica, R.; et al. A Form of Apolipoprotein A-I Is Found Specifically in Relapses of Focal Segmental Glomerulosclerosis Following Transplantation. Am. J. Transplant. 2013, 13, 493–500. [Google Scholar] [CrossRef]
- Taherkhani, A.; Farrokhi Yekta, R.; Mohseni, M.; Saidijam, M.; Arefi Oskouie, A. Chronic Kidney Disease: A Review of Proteomic and Metabolomic Approaches to Membranous Glomerulonephritis, Focal Segmental Glomerulosclerosis, and IgA Nephropathy Biomarkers. Proteome Sci. 2019, 17, 7. [Google Scholar] [CrossRef]
- Jacobs-Cachá, C.; Puig-Gay, N.; Helm, D.; Rettel, M.; Sellarès, J.; Meseguer, A.; Savitski, M.M.; Moreso, F.J.; Soler, M.J.; Seron, D.; et al. A Misprocessed Form of Apolipoprotein A-I Is Specifically Associated with Recurrent Focal Segmental Glomerulosclerosis. Sci. Rep. 2020, 10, 1159. [Google Scholar] [CrossRef]
- Zhai, S.; Sun, B.; Zhang, Y.; Zhao, L.; Zhang, L. IL-17 Aggravates Renal Injury by Promoting Podocyte Injury in Children with Primary Nephrotic Syndrome. Exp. Ther. Med. 2020, 20, 409–417. [Google Scholar] [CrossRef]
- Bhargava, R.; Tsokos, G.C. The Immune Podocyte. Curr. Opin. Rheumatol. 2019, 31, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wen, X.; Peng, X.; Zhao, M.; Mi, L.; Lei, J.; Xu, K. Immune Podocytes in the Immune Microenvironment of Lupus Nephritis (Review). Mol. Med. Rep. 2023, 28, 204. [Google Scholar] [CrossRef] [PubMed]
- Hengel, F.E.; Dehde, S.; Lassé, M.; Zahner, G.; Seifert, L.; Schnarre, A.; Kretz, O.; Demir, F.; Pinnschmidt, H.O.; Grahammer, F.; et al. Autoantibodies Targeting Nephrin in Podocytopathies. N. Engl. J. Med. 2024, 391, 422–433. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Y.; Zhuang, J.; Bi, Y.; Xu, H.; Shen, Q.; Liu, J.; Fu, H.; Wang, J.; Feng, C.; et al. The Important Roles and Molecular Mechanisms of Annexin A2 Autoantibody in Children with Nephrotic Syndrome. Ann. Transl. Med. 2021, 9, 1452. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhou, C.; Wang, D.; Fu, H.; Wang, J.; Mao, J. Seven Novel Podocyte Autoantibodies Were Identified to Diagnosis a New Disease Subgroup-Autoimmune Podocytopathies. Clin. Immunol. 2021, 232, 108869. [Google Scholar] [CrossRef]
- Chang, A.; Ko, K.; Clark, M.R. The Emerging Role of the Inflammasome in Kidney Diseases. Curr. Opin. Nephrol. Hypertens. 2014, 23, 204–210. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. Biomed. Res. Int. 2021, 2021. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Srivastava, A.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Mulay, S.R.; Scholze, A. Involvement of Inflammasome Components in Kidney Disease. Antioxidants 2022, 11, 246. [Google Scholar] [CrossRef]
- Angeletti, A.; Cantarelli, C.; Petrosyan, A.; Andrighetto, S.; Budge, K.; D’Agati, V.D.; Hartzell, S.; Malvi, D.; Donadei, C.; Thurman, J.M.; et al. Loss of Decay-Accelerating Factor Triggers Podocyte Injury and Glomerulosclerosis. J. Exp. Med. 2020, 217, e20191699. [Google Scholar] [CrossRef]
- Zoshima, T.; Hara, S.; Yamagishi, M.; Pastan, I.; Matsusaka, T.; Kawano, M.; Nagata, M. Possible Role of Complement Factor H in Podocytes in Clearing Glomerular Subendothelial Immune Complex Deposits. Sci. Rep. 2019, 9, 7857. [Google Scholar] [CrossRef] [PubMed]
- Zagorec, N.; Horvatić, I.; Šenjug, P.; Sović, S.; Galešić, K.; Galešić Ljubanović, D. The Lectin Pathway–A Dominant Pattern of the Complement System Activation in Primary Focal Segmental Glomerulosclerosis? Kidney Int. Rep. 2024, 9, 1925–1926. [Google Scholar] [CrossRef]
- Nagata, M. Podocyte Injury and Its Consequences. Kidney Int. 2016, 89, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Herlitz, L.C.; Markowitz, G.S.; Farris, A.B.; Schwimmer, J.A.; Stokes, M.B.; Kunis, C.; Colvin, R.B.; D’Agati, V.D. Development of Focal Segmental Glomerulosclerosis after Anabolic Steroid Abuse. J. Am. Soc. Nephrol. 2010, 21, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Amico, F.; Cocimano, G.; Liberto, A.; Maglietta, F.; Esposito, M.; Rosi, G.L.; Di Nunno, N.; Salerno, M.; Montana, A. Adverse Effects of Anabolic-Androgenic Steroids: A Literature Review. Healthcare 2021, 9, 97. [Google Scholar] [CrossRef]
- Mirioglu, S.; Daniel-Fischer, L.; Berke, I.; Ahmad, S.H.; Bajema, I.M.; Bruchfeld, A.; Fernandez-Juarez, G.M.; Floege, J.; Frangou, E.; Goumenos, D.; et al. Management of Adult Patients with Podocytopathies: An Update from the ERA Immunonephrology Working Group. Nephrol. Dial. Transplant. 2024, 39, 569–580. [Google Scholar] [CrossRef]
- van den Broek, M.; Smeets, B.; Schreuder, M.F.; Jansen, J. The Podocyte as a Direct Target of Glucocorticoids in Nephrotic Syndrome. Nephrol. Dial. Transplant. 2022, 37, 1808–1815. [Google Scholar] [CrossRef]
- Schijvens, A.M.; ter Heine, R.; de Wildt, S.N.; Schreuder, M.F. Pharmacology and Pharmacogenetics of Prednisone and Prednisolone in Patients with Nephrotic Syndrome. Pediatr. Nephrol. 2019, 34, 389–403. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, Z.; Zhuang, J.; Lu, C.; Qu, Y.; Xu, C.; Yang, S.; Tian, X. Understanding the Podocyte Immune Responses in Proteinuric Kidney Diseases: From Pathogenesis to Therapy. Front. Immunol. 2024, 14, 1335936. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Li, X.; Wang, X.; Wang, S.; Ding, J. Cyclosporine A Protects Podocytes via Stabilization of Cofilin-1 Expression in the Unphosphorylated State. Exp. Biol. Med. 2014, 239, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Inoue, C.N. Once-Daily Low-Dose Cyclosporine A Treatment with Angiotensin Blockade for Long-Term Remission of Nephropathy in Frasier Syndrome. Tohoku J. Exp. Med. 2019, 247, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Liu, Q.; Zheng, Z.; Fan, J.; Peng, W.; Kong, Q.; He, H.; Yang, S.; Chen, W.; Tang, X.; et al. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS ONE 2015, 10, e0132724. [Google Scholar] [CrossRef]
- Vivarelli, M.; Barratt, J.; Beck, L.H.; Fakhouri, F.; Gale, D.P.; Goicoechea de Jorge, E.; Mosca, M.; Noris, M.; Pickering, M.C.; Susztak, K.; et al. The Role of Complement in Kidney Disease: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2024, 106, 369–391. [Google Scholar] [CrossRef]
- Shui, H.-A.; Huang, T.-H.; Ka, S.-M.; Chen, P.-H.; Lin, Y.-F.; Chen, A. Urinary Proteome and Potential Biomarkers Associated with Serial Pathogenesis Steps of Focal Segmental Glomerulosclerosis. Nephrol. Dial. Transplant. 2007, 23, 176–185. [Google Scholar] [CrossRef][Green Version]
- Sedic, M.; Gethings, L.A.; Vissers, J.P.C.; Shockcor, J.P.; McDonald, S.; Vasieva, O.; Lemac, M.; Langridge, J.I.; Batinić, D.; Pavelić, S.K. Label-Free Mass Spectrometric Profiling of Urinary Proteins and Metabolites from Paediatric Idiopathic Nephrotic Syndrome. Biochem. Biophys. Res. Commun. 2014, 452, 21–26. [Google Scholar] [CrossRef]
- Candiano, G.; Musante, L.; Bruschi, M.; Petretto, A.; Santucci, L.; Del Boccio, P.; Pavone, B.; Perfumo, F.; Urbani, A.; Scolari, F.; et al. Repetitive Fragmentation Products of Albumin and A1-Antitrypsin in Glomerular Diseases Associated with Nephrotic Syndrome. J. Am. Soc. Nephrol. 2006, 17, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Nafar, M.; Kalantari, S.; Samavat, S.; Rezaei-Tavirani, M.; Rutishuser, D.; Zubarev, R.A. The Novel Diagnostic Biomarkers for Focal Segmental Glomerulosclerosis. Int. J. Nephrol. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Muruve, D.A.; Debiec, H.; Dillon, S.T.; Gu, X.; Plaisier, E.; Can, H.; Otu, H.H.; Libermann, T.A.; Ronco, P. Serum Protein Signatures Using Aptamer-Based Proteomics for Minimal Change Disease and Membranous Nephropathy. Kidney Int. Rep. 2022, 7, 1539–1556. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.G.; Song, M.-Y.; Moon, J.-Y.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G.; Park, K.-S.; Lee, S.-H. Potential Urine Proteomics Biomarkers for Primary Nephrotic Syndrome. Clin. Proteom. 2017, 14, 18. [Google Scholar] [CrossRef]
- Araumi, A.; Osaki, T.; Ichikawa, K.; Kudo, K.; Suzuki, N.; Watanabe, S.; Watanabe, M.; Konta, T. Urinary and Plasma Proteomics to Discover Biomarkers for Diagnosing between Diabetic Nephropathy and Minimal Change Nephrotic Syndrome or Membranous Nephropathy. Biochem. Biophys. Rep. 2021, 27, 101102. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Muñoz, M.; Ibernon, M.; Bonet, J.; Pérez, V.; Pastor, M.C.; Bayés, B.; Casado-Vela, J.; Navarro, M.; Ara, J.; Espinal, A.; et al. Uromodulin and Antitrypsin Urinary Peptide Analysis to Differentiate Glomerular Kidney Diseases. Kidney Blood Press. Res. 2012, 35, 314–325. [Google Scholar] [CrossRef]
- Pérez, V.; Ibernón, M.; López, D.; Pastor, M.C.; Navarro, M.; Navarro-Muñoz, M.; Bonet, J.; Romero, R. Urinary Peptide Profiling to Differentiate between Minimal Change Disease and Focal Segmental Glomerulosclerosis. PLoS ONE 2014, 9, e87731. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.; López, D.; Boixadera, E.; Ibernón, M.; Espinal, A.; Bonet, J.; Romero, R. Comparative Differential Proteomic Analysis of Minimal Change Disease and Focal Segmental Glomerulosclerosis. BMC Nephrol. 2017, 18, 49. [Google Scholar] [CrossRef]
- Chebotareva, N.V.; Vinogradov, A.; Brzhozovskiy, A.G.; Kashirina, D.N.; Indeykina, M.I.; Bugrova, A.E.; Lebedeva, M.; Moiseev, S.; Nikolaev, E.N.; Kononikhin, A.S. Potential Urine Proteomic Biomarkers for Focal Segmental Glomerulosclerosis and Minimal Change Disease. Int. J. Mol. Sci. 2022, 23, 12607. [Google Scholar] [CrossRef]
- Suresh, C.P.; Saha, A.; Kaur, M.; Kumar, R.; Dubey, N.K.; Basak, T.; Tanwar, V.S.; Bhardwaj, G.; Sengupta, S.; Batra, V.V.; et al. Differentially Expressed Urinary Biomarkers in Children with Idiopathic Nephrotic Syndrome. Clin. Exp. Nephrol. 2016, 20, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Catanese, L.; Siwy, J.; Wendt, R.; Amann, K.; Beige, J.; Hendry, B.; Mischak, H.; Mullen, W.; Paterson, I.; Schiffer, M.; et al. Differentiating Primary and Secondary FSGS Using Non-Invasive Urine Biomarkers. Clin. Kidney J. 2024, 17, sfad296. [Google Scholar] [CrossRef] [PubMed]
- Hellin, J.L.; Bech-Serra, J.J.; Moctezuma, E.L.; Chocron, S.; Santin, S.; Madrid, A.; Vilalta, R.; Canals, F.; Torra, R.; Meseguer, A.; et al. Very Low-Molecular-Mass Fragments of Albumin in the Plasma of Patients With Focal Segmental Glomerulosclerosis. Am. J. Kidney Dis. 2009, 54, 871–880. [Google Scholar] [CrossRef]
- Zhao, M.; Li, M.; Li, X.; Shao, C.; Yin, J.; Gao, Y. Dynamic Changes of Urinary Proteins in a Focal Segmental Glomerulosclerosis Rat Model. Proteome Sci. 2014, 12, 42. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, W.; Guo, Q.; Zou, Y. Screening for Urinary Biomarkers of Steroid-Resistant Nephrotic Syndrome in Children. Exp. Ther. Med. 2013, 5, 860–864. [Google Scholar] [CrossRef][Green Version]
- Kalantari, S.; Nafar, M.; Samavat, S.; Rezaei-Tavirani, M.; Rutishauser, D.; Zubarev, R. Urinary Prognostic Biomarkers in Patients With Focal Segmental Glomerulosclerosis. Nephrourol. Mon. 2014, 6, e16806. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.F.; Palmfeldt, J.; Jespersen, B.; Gregersen, N.; Rittig, S. Plasma and Urine Proteomic Profiles in Childhood Idiopathic Nephrotic Syndrome. Proteom. Clin. Appl. 2012, 6, 382–393. [Google Scholar] [CrossRef]
- Piyaphanee, N.; Ma, Q.; Kremen, O.; Czech, K.; Greis, K.; Mitsnefes, M.; Devarajan, P.; Bennett, M.R. Discovery and Initial Validation of α 1-B Glycoprotein Fragmentation as a Differential Urinary Biomarker in Pediatric Steroid-resistant Nephrotic Syndrome. Proteom. Clin. Appl. 2011, 5, 334–342. [Google Scholar] [CrossRef]
- Kalantari, S.; Nafar, M.; Rutishauser, D.; Samavat, S.; Rezaei-Tavirani, M.; Yang, H.; Zubarev, R.A. Predictive Urinary Biomarkers for Steroid-Resistant and Steroid-Sensitive Focal Segmental Glomerulosclerosis Using High Resolution Mass Spectrometry and Multivariate Statistical Analysis. BMC Nephrol. 2014, 15, 141. [Google Scholar] [CrossRef][Green Version]
- Dong, J.; Zheng, F.; Liu, F.; He, J.; Li, S.; Pu, W.; Xu, H.; Luo, Z.; Liu, S.; Yin, L.; et al. Global-Feature of Autoimmune Glomerulonephritis Using Proteomic Analysis of Laser Capture Microdissected Glomeruli. Front. Immunol. 2023, 14, 1131164. [Google Scholar] [CrossRef] [PubMed]
- Bărar, A.A.; Pralea, I.E.; Berindan-Neagoe, I.; Pirlog, R.; Nutu, A.; Maslyennikov, Y.; Potra, A.R.; Iuga, C.A.; Kacso, I.M. Proteomic Patterns in Glomerular Research, a Laser Capture Microdissection and Liquid Chromatography-Tandem Mass Spectrometry Approach. Rev. Rom. Med. Lab. 2023, 31, 263–274. [Google Scholar] [CrossRef]
- Merchant, M.L.; Barati, M.T.; Caster, D.J.; Hata, J.L.; Hobeika, L.; Coventry, S.; Brier, M.E.; Wilkey, D.W.; Li, M.; Rood, I.M.; et al. Proteomic Analysis Identifies Distinct Glomerular Extracellular Matrix in Collapsing Focal Segmental Glomerulosclerosis. J. Am. Soc. Nephrol. 2020, 31, 1883–1904. [Google Scholar] [CrossRef]
- Höhne, M.; Frese, C.K.; Grahammer, F.; Dafinger, C.; Ciarimboli, G.; Butt, L.; Binz, J.; Hackl, M.J.; Rahmatollahi, M.; Kann, M.; et al. Single-Nephron Proteomes Connect Morphology and Function in Proteinuric Kidney Disease. Kidney Int. 2018, 93, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.; Kelly, R.T. What’s New in Single-Cell Proteomics. Curr. Opin. Biotechnol. 2024, 86, 103077. [Google Scholar] [CrossRef]
- Mund, A.; Coscia, F.; Kriston, A.; Hollandi, R.; Kovács, F.; Brunner, A.-D.; Migh, E.; Schweizer, L.; Santos, A.; Bzorek, M.; et al. Deep Visual Proteomics Defines Single-Cell Identity and Heterogeneity. Nat. Biotechnol. 2022, 40, 1231–1240. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Du, M.; Xin, W. Functioning and Mechanisms of PTMs in Renal Diseases. Front. Pharmacol. 2023, 14, 1238706. [Google Scholar] [CrossRef]
- Liu, J.; Xie, J.; Zhang, X.; Tong, J.; Hao, X.; Ren, H.; Wang, W.; Chen, N. Serum C3 and Renal Outcome in Patients with Primary Focal Segmental Glomerulosclerosis. Sci. Rep. 2017, 7, 4095. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J.M.; Wong, M.; Renner, B.; Frazer-Abel, A.; Giclas, P.C.; Joy, M.S.; Jalal, D.; Radeva, M.K.; Gassman, J.; Gipson, D.S.; et al. Complement Activation in Patients with Focal Segmental Glomerulosclerosis. PLoS ONE 2015, 10, e0136558. [Google Scholar] [CrossRef]
- Huang, J.; Cui, Z.; Gu, Q.; Zhang, Y.; Qu, Z.; Wang, X.; Wang, F.; Cheng, X.; Meng, L.; Liu, G.; et al. Complement Activation Profile of Patients with Primary Focal Segmental Glomerulosclerosis. PLoS ONE 2020, 15, e0234934. [Google Scholar] [CrossRef]
- Cambier, A.; Patey, N.; Royal, V.; Gougeon, F.; Genest, D.S.; Brachemi, S.; Bollée, G.; Merlen, C.; Bonnefoy, A.; Lapeyraque, A.-L.; et al. A Prospective Study on Complement Activation Distinguishes Focal Segmental Glomerulosclerosis from Minimal Change Disease. Kidney Int. Rep. 2024, 9, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Garin, E.H.; Mu, W.; Arthur, J.M.; Rivard, C.J.; Araya, C.E.; Shimada, M.; Johnson, R.J. Urinary CD80 Is Elevated in Minimal Change Disease but Not in Focal Segmental Glomerulosclerosis. Kidney Int. 2010, 78, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Woroniecki, R.P.; Shatat, I.F.; Supe, K.; Du, Z.; Kaskel, F.J. Urinary Cytokines and Steroid Responsiveness in Idiopathic Nephrotic Syndrome of Childhood. Am. J. Nephrol. 2008, 28, 83–90. [Google Scholar] [CrossRef]
- Almaani, S.; Madhavan, S.M.; Shapiro, J.P.; Satoskar, A.A.; Ayoub, I.; Rovin, B.H.; Parikh, S.V. Comparison of Glomerular Proteomics Profiles of Healthy Human Kidney and Minimal Change Disease Identifies Distinct Targets and Pathways. J. Am. Soc. Nephrol. 2021, 32, 445. [Google Scholar] [CrossRef]
- Ni, J.; Tian, S.; Bai, L.; Lv, Q.; Liu, J.; Liu, J.; Fang, Y.; Zhai, Y.; Shen, Q.; Rao, J.; et al. Comparative Proteomic Analysis of Children FSGS FFPE Tissues. BMC Pediatr. 2022, 22, 707. [Google Scholar] [CrossRef]
- Bukosza, E.N.; Kornauth, C.; Hummel, K.; Schachner, H.; Huttary, N.; Krieger, S.; Nöbauer, K.; Oszwald, A.; Razzazi Fazeli, E.; Kratochwill, K.; et al. ECM Characterization Reveals a Massive Activation of Acute Phase Response during FSGS. Int. J. Mol. Sci. 2020, 21, 2095. [Google Scholar] [CrossRef]
- Akankwasa, G.; Jianhua, L.; Guixue, C.; Changjuan, A.; Xiaosong, Q. Urine Markers of Podocyte Dysfunction: A Review of Podocalyxin and Nephrin in Selected Glomerular Diseases. Biomark. Med. 2018, 12, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Kostovska, I.; Trajkovska, K.T.; Topuzovska, S.; Cekovska, S.; Labudovic, D.; Kostovski, O.; Spasovski, G. Nephrinuria and Podocytopathies. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–36. [Google Scholar]

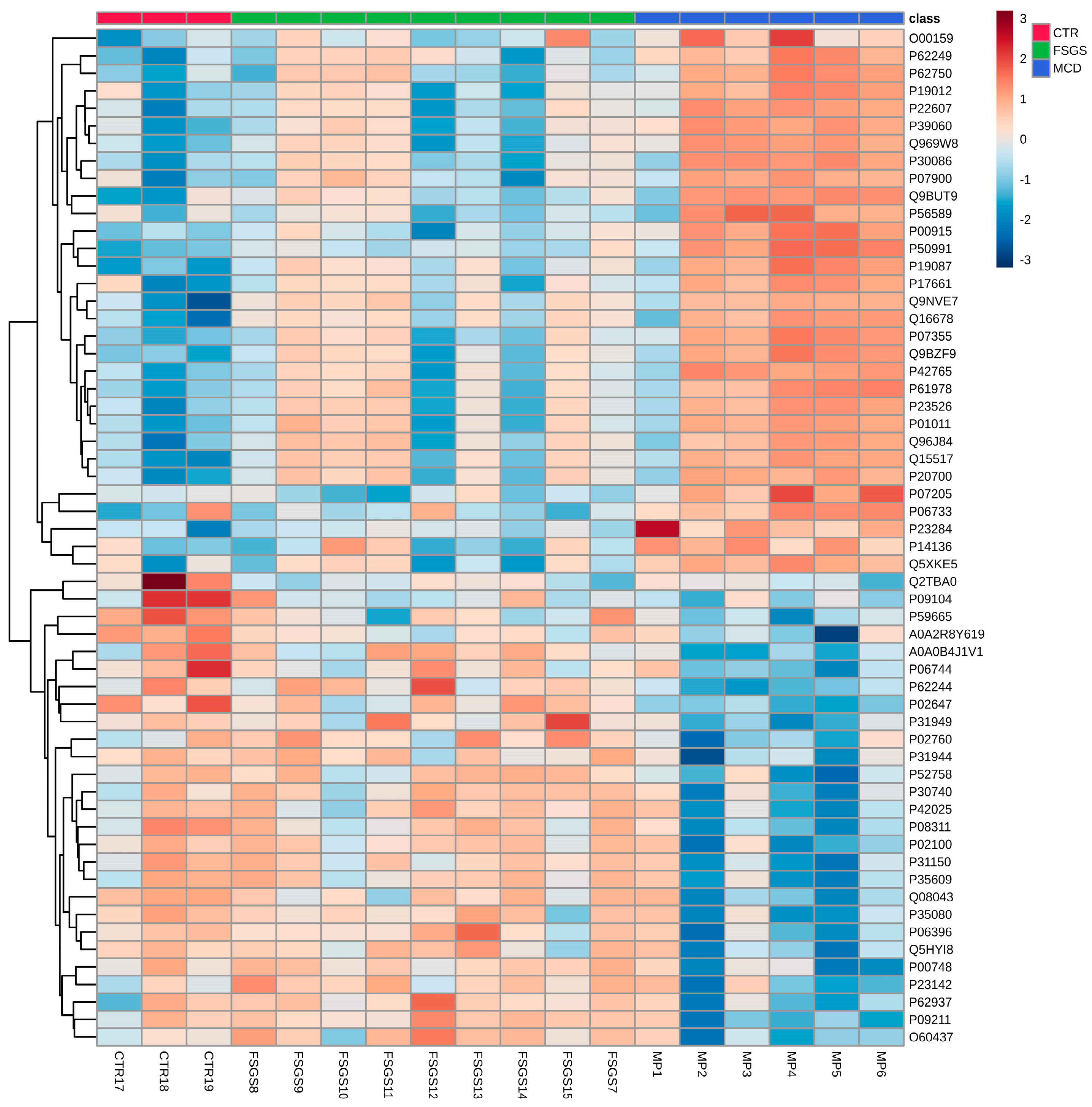
| Study | Groups | Tissue/Biofluid | Method | Significance |
|---|---|---|---|---|
| Shui et al. [55], 2008 | (Murine model) FSGS and healthy controls | Urine | 2DE, MALDI-TOF MS | FSGS—upregulated proteins involved hemodynamic disturbance, apoptosis, ECM protein deposition, and sclerosis (COL4A1, ECM-1, KLK, KNG1 precursor, ANXA1, CDH1, and ADAM32) |
| Sedic et al. [56], 2014 | 12 INS, 12 healthy controls | Urine | LC-MS | INS—74 proteins upregulated, 9 potential biomarkers Oxidative stress may be a pathogenic mechanism |
| Candiano et al. [57], 2006 | 10 MCD, 7 FSGS 6 MN, 10 healthy controls | Urine/Plasma | 2DE, MALDI-TOF/MS | MCD/FSGS/MN—upregulated Albumin fragments and SERPINA1 in urine and plasma compared to healthy controls |
| Nafar et al. [58], 2014 | 11 FSGS,6 IgAN, 8 healthy controls | Urine | nano-LC/MS | FSGS—upregulated CD59, CD44, IBP7, Robo4, and DPEP1 |
| Muruve et al. [59], 2022 | 15 MCD, 37 MN, 20 healthy controls | Plasma | SOMAscan | MCD—70-protein signature; serpin family proteins downregulated compared to MN/healthy controls (SERPINA10, SERPINA4, SERPINC1, SERPINF2, and SERPINF1); complement system and coagulation pathway downregulated MCD—upregulated immune and growth factor signaling proteins (STAT1, STAT3, CD40LG, and FGF16); carbohydrate and lipid metabolism (GAPDH, GSK3A/B, PKM2, HK2, CHST6, LRP1B, APOE, and APOA1) compared to MN/healthy controls |
| Choi et al. [60], 2017 | Discovery cohort: 4 MCD, 4 FSGS, 4 MN, 4 healthy controls Validation cohort: 13 MCD, 5 FSGS, 26 MN, 9 IgAN, 8 healthy controls | Urine | SDS-PAGE, LC-MS | MCD—upregulated CD14, C9, and SERPINA1 FSGS—upregulated CDH26, RNASE1, and DIS3L |
| Araumi et al. [61], 2021 | 14 MCD, 11 DN, 23 MN | Urine/Plasma | nano-LC/MS | DN—upregulated urinary RBP4 and SH3BGRL3 compared to MCD |
| Navarro-Muñoz et al. [62], 2012 | 9 FSGS, 3 MCD, 9 IgAN, 6 MN, 7 healthy controls | Urine | HPLC-MS/MS | MCD/FSGS—upregulated SERPINA1 and downregulated UMOD compared to healthy controls |
| Perez et al. [63], 2014 | 22 FSGS, 22 MCD | Urine | MALDI-TOF MS | UMOD and SERPINA1 can differentiate between FSGS and MCD |
| Perez et al. [64], 2017 | 25 FSGS, 24 MCD | Urine | 2D-DIGE; MALDI-TOF MS | MCD—upregulated SERPINA1, PTAFR, CCNY, TF, HTN3, and MRPL17 FSGS—upregulated CALB2 |
| Chebotareva et al. [65], 2022 | 30 FSGS, 9 MCD | Urine | LC-MS | “Severe” FSGS vs. “mild” FSGS/MCD—upregulated complement activity (C4b, C9, CFB, and CFI); upregulated podocyte damage (VTN, HPX, GSN, and APOA1); upregulated ECM accumulation (CST3, DBP, RBP4, AHSG, SERPING1, LUM, and CLU) |
| Suresh et al. [66], 2016 | 55 INS: 5 SRNS MCD, 5 SRNS FSGS, 2 SRNS MN | Urine | iTRAQ LC/MS | SRNS FSGS vs. SRNS MCD—upregulated A2M and ORM2 |
| Catanese et al. [67], 2023 | 19 primary FSGS, 44 secondary FSGS | Urine | CE-MS | Primary FSGS vs. secondary FSGS—upregulated collagen fragments, SERPINA1, UBE3A, RNF146, complement C3, and PLG; Downregulated fragment of PIGR. |
| Hellin et al. [68], 2009 | 15 idiopathic FSGS, 11 genetic FSGS | Plasma | 2DE, MALDI-TOF MS, Western blot, LC-ES-MS | Three very low-molecular-mass albumin fragments in plasma of patients with genetic FSGS vs. idiopathic FSGS/ healthy controls |
| Zhao et al. [69], 2014 | (Murine model) FSGS | Urine | LC-MS | FSGS—urine protein change pattern in time: upregulation of AFM and CP; downregulation of CDH2 and ACAN; distinct pattern of FETUB and B2M |
| Bai et al. [70], 2012 | 9 SRNS, 32 SSNS | Urine | Chip-MS | SRNS—upregulated SAMDC1, FKBP1A, and rpsK SRNS—downregulated rpmF |
| Kalantari et al. [71], 2014 | 5 mild FSGS, 5 advanced FSGS | Urine | nano-LC/MS | Mild FSGS—upregulated DNASE2 Advanced FSGS—upregulated HP Complement and coagulation pathways activated in FSGS |
| Chhuon et al. [28], 2023 | 4 recurrent FSGS, 4 non-INS controls; post-transplant | Plasma | nano-LC-MS/MS | Recurrent FSGS—upregulated neutrophil degranulation; downregulated platelet degranulation and lipid-binding proteins; dysregulation of mTOR pathway |
| Lopez-Hellin et al. [29], 2012 | 6 recurrent FSGS, 34 non-recurrent FSGS; post-transplant | Urine | 2DE/MALDI-TOF/LC-ESI-MS/MS | Urinary ApoA-Ib associated with recurrent FSGS |
| Andersen et al. [72], 2012 | 4 INS remission/active disease | Plasma/Urine | nano-LC/MS | Active disease—downregulated urinary CDH1, CDH3, KLKB1, HPX |
| Piyaphanee et al. [73], 2011 | 19 SRNS, 15 SSNS, 10 healthy controls | Urine | MALDI-TOF/MS | A1BG is associated with SRNS FSGS |
| Kalantari et al. [74], 2014 | 6 SS FSGS, 4 SR FSGS | Urine | LC-MS | SS—upregulated APOA1 SR—upregulated MXRA8 Acute inflammatory response was the predominant biological process (CLUS, A1AG2, AACT, and TRFE) |
| Dong et al. [75], 2023 | 3 MCD, 11 IgAN, 19 LN. 5 MN, 8 healthy controls | Tissue | LCM + nano-LC-MS/MS | MCD/IgAN vs. LN/MN—downregulated CD59; A2M upregulated in every group but not in MCD; downregulated FLII (regulatory cytoskeleton protein) in glomerular disease versus control |
| Bărar et al. [76], 2023 | 6 MCD, 9 FSGS, 3 healthy controls | Tissue | LC-MS/MS | 58 significant proteins between the 3 groups |
| Bărar et al. [12], 2023 | 5 MCD, 3 healthy controls | Tissue | LC-MS/MS | MCD—upregulated ANXA2 and NID1 MCD—downregulated ZO-1, MYO1C, ITGA3, ACTR3B, and NES |
| Merchant et al. [77], 2020 | Collapsing FSGS 7, NOS-FSGS 6, healthy controls | Tissue/Urine | LC-MS/MS | Collapsing FSGS—distinct pattern of sclerosis compared to other form of FSGS; upregulated cathepsin B and cathepsin C in tissue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslyennikov, Y.; Bărar, A.A.; Rusu, C.C.; Potra, A.R.; Tirinescu, D.; Ticala, M.; Urs, A.; Pralea, I.E.; Iuga, C.A.; Moldovan, D.T.; et al. The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research. Int. J. Mol. Sci. 2025, 26, 2450. https://doi.org/10.3390/ijms26062450
Maslyennikov Y, Bărar AA, Rusu CC, Potra AR, Tirinescu D, Ticala M, Urs A, Pralea IE, Iuga CA, Moldovan DT, et al. The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research. International Journal of Molecular Sciences. 2025; 26(6):2450. https://doi.org/10.3390/ijms26062450
Chicago/Turabian StyleMaslyennikov, Yuriy, Andrada Alina Bărar, Crina Claudia Rusu, Alina Ramona Potra, Dacian Tirinescu, Maria Ticala, Alexandra Urs, Ioana Ecaterina Pralea, Cristina Adela Iuga, Diana Tania Moldovan, and et al. 2025. "The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research" International Journal of Molecular Sciences 26, no. 6: 2450. https://doi.org/10.3390/ijms26062450
APA StyleMaslyennikov, Y., Bărar, A. A., Rusu, C. C., Potra, A. R., Tirinescu, D., Ticala, M., Urs, A., Pralea, I. E., Iuga, C. A., Moldovan, D. T., & Kacso, I. M. (2025). The Spectrum of Minimal Change Disease/Focal Segmental Glomerulosclerosis: From Pathogenesis to Proteomic Biomarker Research. International Journal of Molecular Sciences, 26(6), 2450. https://doi.org/10.3390/ijms26062450











