Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders
Abstract
1. Introduction
2. The Biogenesis of Exosomes
3. Methods for Isolating Exosomes
3.1. Ultracentrifugation
3.2. Density Gradient Ultracentrifugation
3.3. Ultrafiltration
3.4. Size Exclusion Chromatography
3.5. Polymer Precipitation
3.6. Immunoaffinity
3.7. Microfluidics
| Method | Principle | Advantage | Drawback | Purity | Time | Refs. |
|---|---|---|---|---|---|---|
| Ultracentrifugation | Components exhibiting disparities in size and density demonstrate a range of sedimentation velocities. | Gold standard; suiable for large-volume samples; ralatively cheap; mature. | Time-consuming; cumbersome operation; low yield; may damage exosomes. | Medium (with the co-precipitation and non-exosome contaminants). | >4 h. | [47] |
| Density gradient ultra-centrifugation | Based on EV density. | Good to maintain the activity of EVs. | Complexity; time-consuming. | High. | >16 h. | [81] |
| Ultrafiltration | The relative division employing various interception methods utilizes a sub-mass ultrafiltration membrane for the targeted separation of samples. | Low cost; fast speed; portable. | Membrane blockage and exosome loss; exosomes can be deformed or damaged. | High. | ≈2–4 h. | [82] |
| Size exclusion chromatography | Separates exosomes based on hydrodynamic radii. | Preserve the integrity and natural biological activity; economical; good reproducibility. | Special columns and packing are required; lipoprotein. | High. | 0.3 h. | [83] |
| Polymer precipitation | Highly hydrophilic water-separating polymers can alter the solubility of exosomes. | The polymer precipitation method is simple to operate; no need for complex equipment; suitable for handling large sample volumes; preserve the integrity and natural biological activity. | Lead to the wrong quantification; additional steps for higher; purity. | Low. | 0.5–12 h. | [70,84] |
| Immunoaffinity | Based on antigen–antibody-specific recognition and binding. | Highly specific; easy to use; no contamination. | Low efficiency; Not suitable from large quantities; Expensive Nonspecific binding | High. | 2–6 h. | [70,73] |
| Microfluidics | Based on different principles, including immunoaffinity, size, and density. | Highly efficient low cost; portable; easy to automate and integrate with diagnostics. | Low sample volume. | High. | - | [85] |
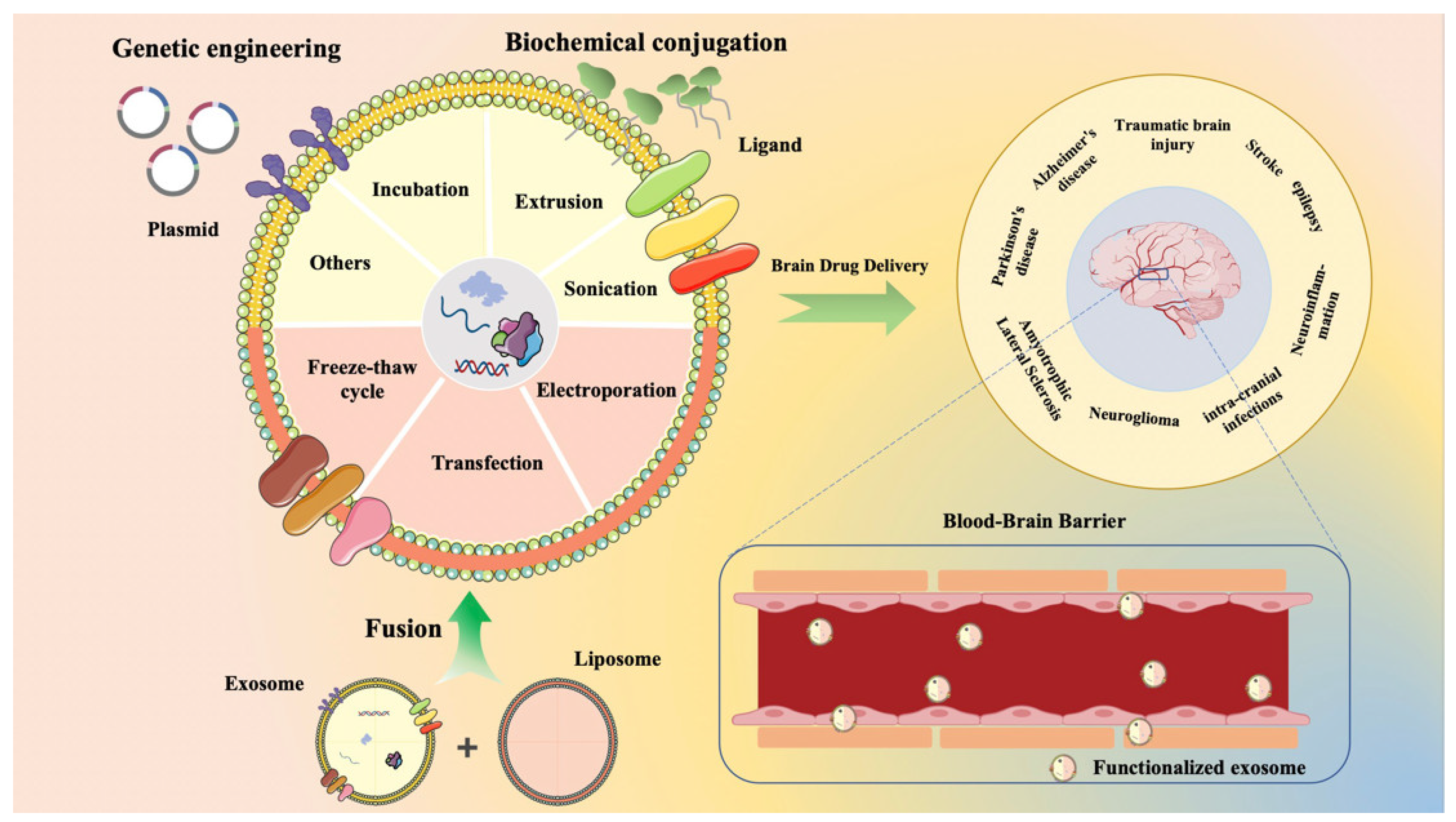
4. Functionalization of Exosomes
4.1. Gene Engineering
4.2. Chemical Modification
4.3. Membrane Fusion
5. Strategies for Drug Loading in Exosomes
5.1. Incubation
5.2. Electroporation
5.3. Sonication
5.4. Extrusion
5.5. Transfection
5.6. Freeze–Thaw Cycle
5.7. Other Methods
| Loading Strategy | Pros | Cons | Refs. |
|---|---|---|---|
| Incubation | The procedure remains straightforward, incurs minimal expenses, and preserves the integrity and biological functionality of exosomes with minimal impact. | Inefficient and will retain unwanted content, subsequent need to purify the removal of unloaded drugs. | [104,125] |
| Electroporation | It has high loading efficiency and can load drugs with large molecular weight. | It will damage the structure and function of exosomes and cause exosome aggregation. | [109,126] |
| Sonication | Simple operation. Drug loading and continuous drug release are highly efficient. | It changes the structure of exosomes and causes exosome aggregation. | [114] |
| Extrusion | The drug loading efficiency is high, and the size of the drug-loaded exosomes is uniform. | Mechanical force may destroy the properties of exosome membrane and affect the integrity of exosome function. | [115] |
| Transfection | Compared with other methods, transfection makes drug loading efficiency and molecular stability higher. | Transfection agents have certain toxicity and safety problems, which may lead to changes in gene expression of donor cells that produce exosomes. | [118,120] |
| Freeze–thaw cycles | The process is mild, maintaining the integrity of the exosome membrane, and maintaining higher cell viability. | It is low in efficiency and easy to cause exosome aggregation. | [121,127] |
| Thermal shock | Load a large number of drugs in a short time | It has a negative impact on the integrity and function of exosome membrane and the stability of drugs. | [122] |
| Chemical penetration | High loading efficiency | It is difficult to completely remove chemical reagents, and may lead to exosome membrane permeability and increase cytotoxicity. | [123] |
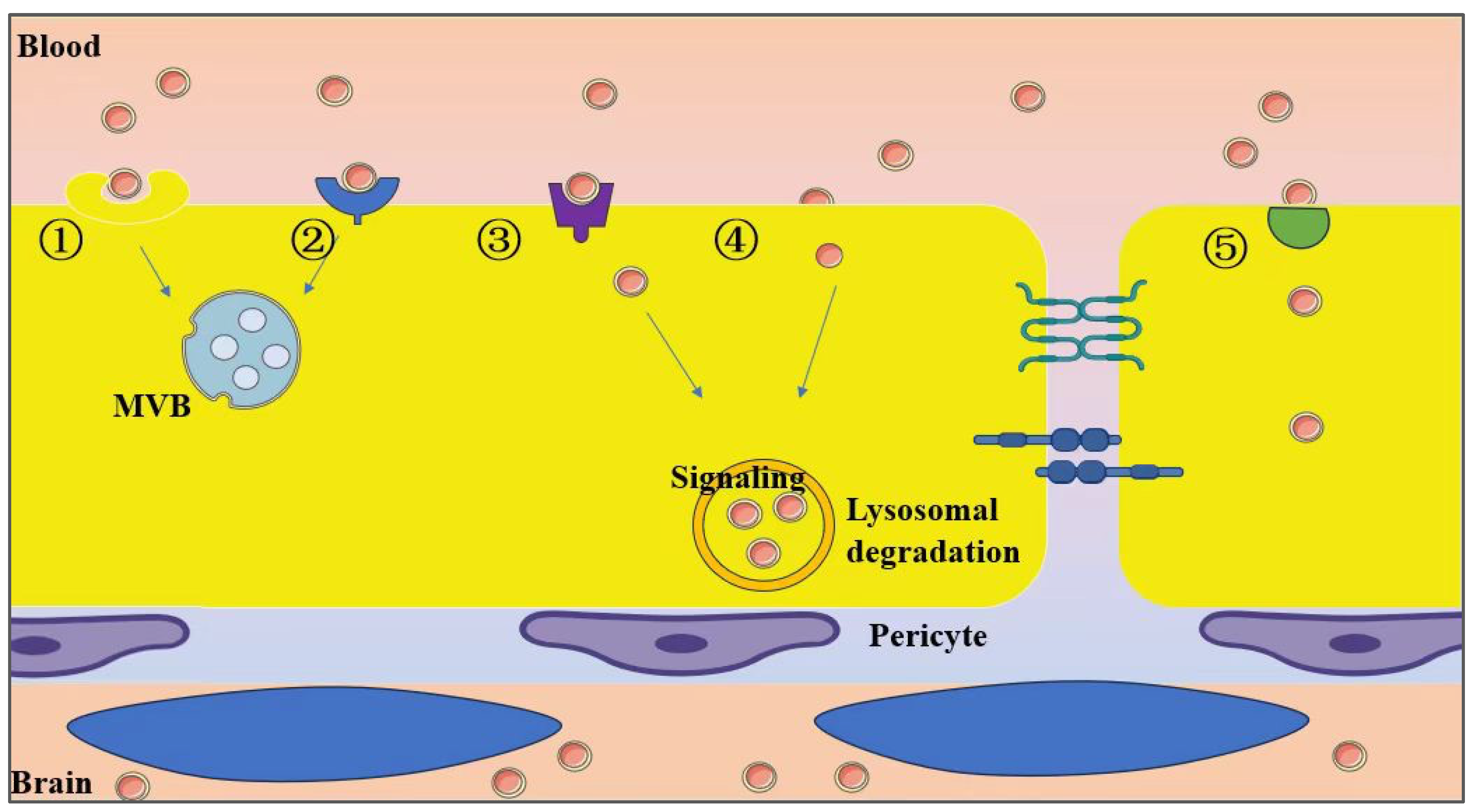
6. Mechanisms of Exosomal Penetration Through the BBB
6.1. Disruption of the BBB Impacts Exosome Penetration Efficiency
6.2. Exosome Trafficking in Brain Metastasis
6.3. Regulation of Transcytosis
7. Exosome-Based Therapies for Brain Disorders
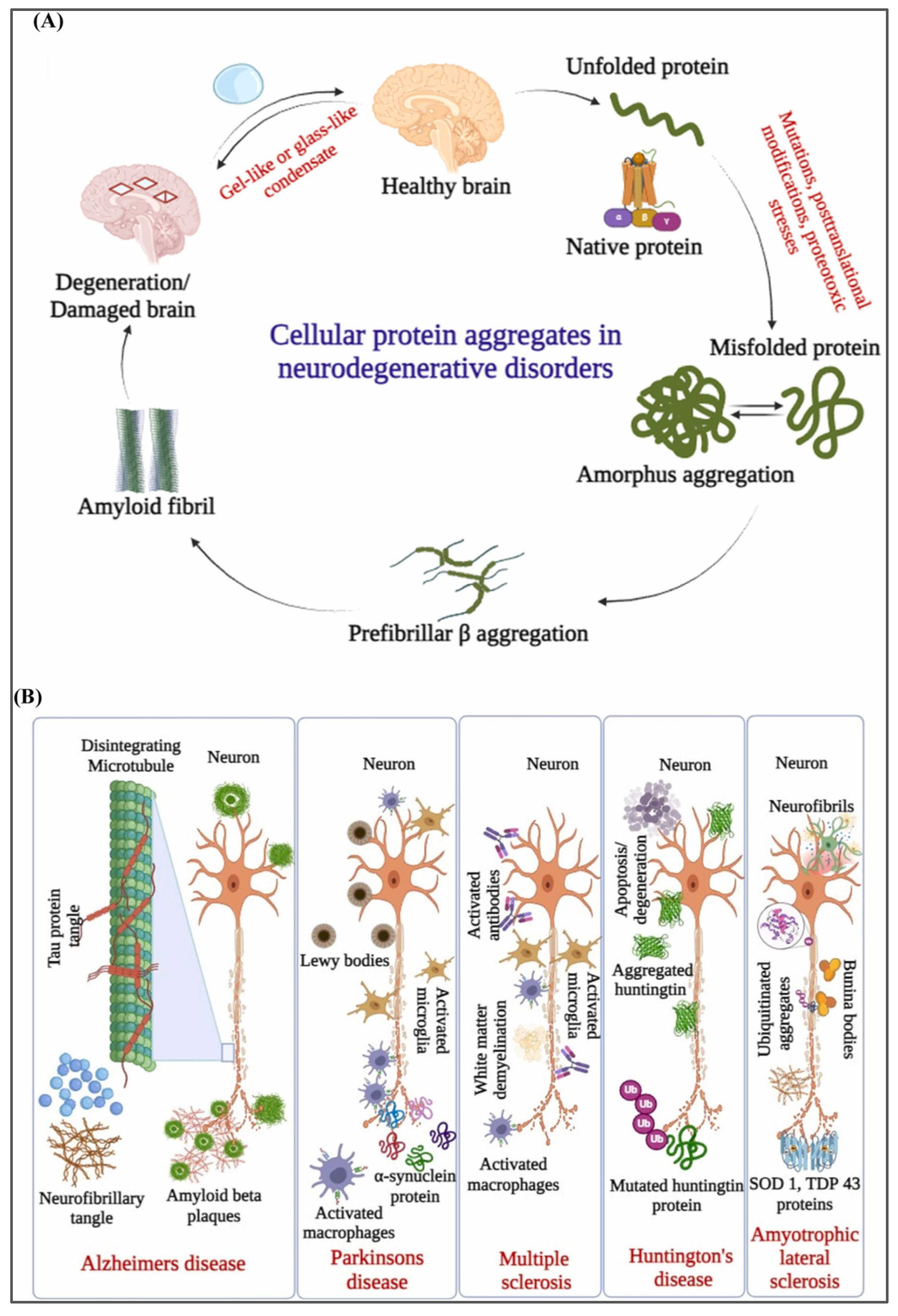
7.1. Exosomes and Acute Neurodegenerative Disorders
7.1.1. TBI
7.1.2. Stroke
7.2. Exosomes and Chronic Neurodegenerative Diseases
7.2.1. Alzheimer’s Disease
7.2.2. Parkinson’s Disease
7.2.3. Amyotrophic Lateral Sclerosis
7.3. Neuroglioma
7.4. Exosomes and Other Brain Disorders
7.4.1. Exosomes and Intra-Cranial Infections
7.4.2. Exosomes and Neuroinflammation
7.4.3. Exosomes and Epilepsy
| Disease | Origin of Exosomes | Exosome Content | In Vitro | In Vivo | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| TBI | Astrocyte | miRNA-873a-5p | LPS-induced primary microglia | Male C57BL/10ScNJ mice | Inhibit the phosphorylation of ERK and the NF-κB signalling pathway | [149] |
| TBI | HucMSC | - | Mouse cortical neurons | Male ICR mice | ↓ Neuron cell death, suppressed apoptosis, pyroptosis, and ferroptosis, ↑ the PINK1/Parkin pathway | [150] |
| TBI | BMSC | - | NSC | Male SD rat | Achieved endogenous NSC recruitment and neuronal differentiation, and promoted angiogenesis | [151] |
| TBI | hNSC | - | - | Male Wistar rat | Improved neurobehavioral performance after TBI | [152] |
| TBI | NSC | LPS-induced Microglial cell (BV2) | Male SD rat | Promoted NSC differentiation and reduced neuroinflammation | [153] | |
| Stroke | NSC | - | - | Male C57BL/6 mice | Improved brain tissue damage such as cerebral infarction, neuronal death, and glial scar formation, and promote motor function recovery | [156] |
| Stroke | hNSC | BDNF | H2O2-induced NSC | Male SD rat | Inhibited the activation of microglia and promoted the differentiation of endogenous NSCs into neurons | [157] |
| Stroke | Astrocyte | miRNA-34c | The mouse neuroblastoma cell (N2a) | Male Wistar rat | Downregulation of NF-κB/MAPK axis and alleviation of I/R-induced nerve damage | [158] |
| Stroke | Panax notoginseng | - | Primary microglia | Male SD rat | Reduced I/R injury and improved behavioral outcomes | [160] |
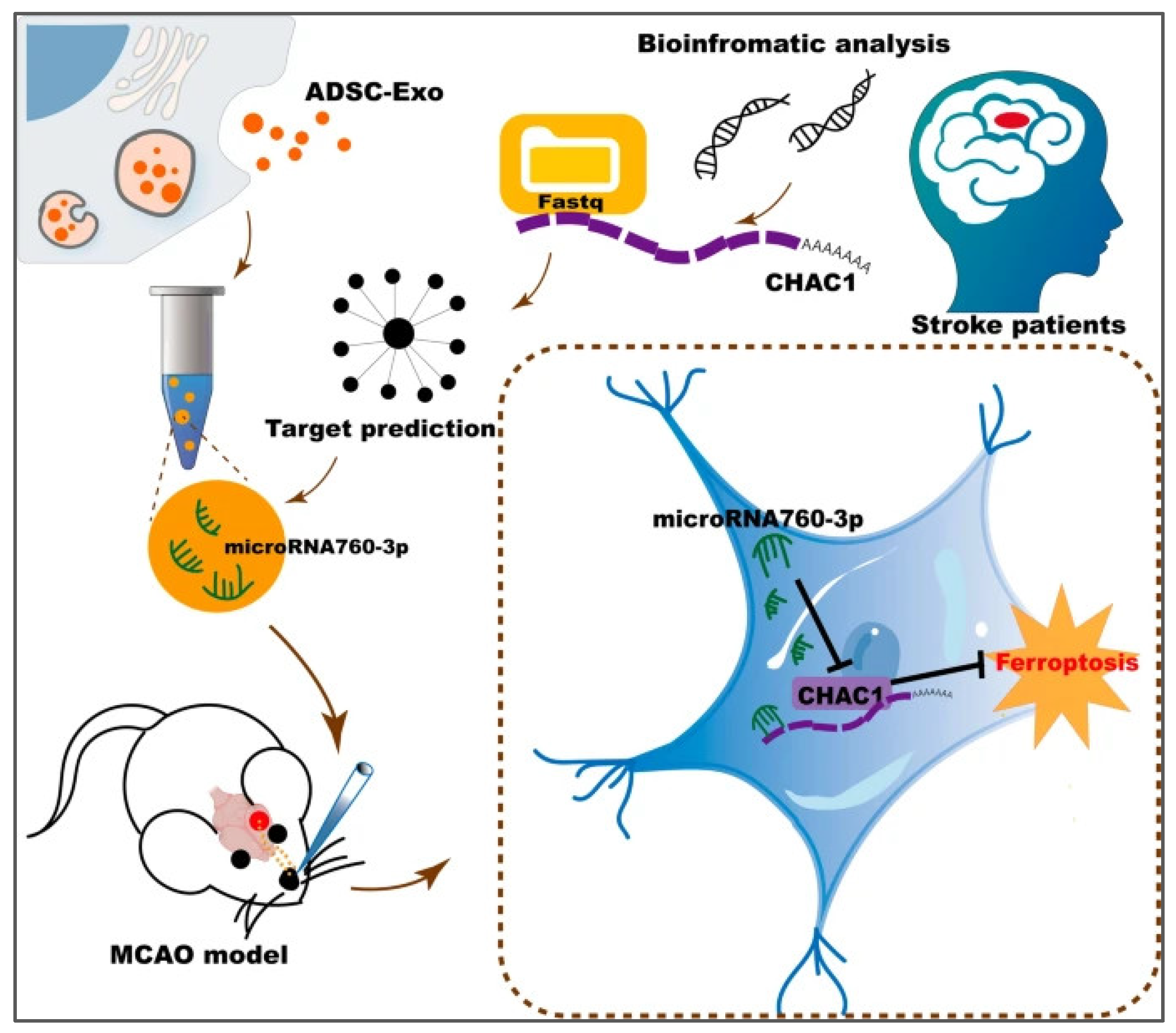
| Stroke | ADSC | miRNA-760-3p | The mouse neuroblastoma cell (N2a) | Male C57/BL6 mice | Improved the neurobehavioral function of mice after I/R and inhibited ferroptosis | [161] |
| Stroke | ADSC | miRNA-19b-3p | - | C57BL/6 mice | Improved the neurological function of mice and inhibited ferroptosis | [162] |
| Stroke | ADSC | - | - | Male C57/BL6 mice | Inhibited M2 microglia ferroptosis and improved neurological function in ischemic stroke mice | [241] |
| AD | Dendritic cells | GAPDH siRNA | Neuro2A and SH-SY5Y | Male C57BL/6 mice | ↓ The BACE1 mRNA ↓ Aβ 1–42 | [172] |
| AD | Mesenchymal stem cell-derived | - | Microglia cells | Female triple-transgenic AD mice | ↓ Neuroinflammation and tau pathology | [173] |
| AD | Serum | fasudil | Hippocampus tissues | Male APP/PS1 mice | The mmu-miR-451a and mmu-miR-19a-3p can enhance cognitive function | [174] |
| AD | HEK-293T | miR-29b | U87 cells | Male Wistar rats | ↓ Amyloid-β (Aβ) peptide | [242] |
| AD | Mesenchymal stem cell-derived | - | FAD human neural cell | AD transgenic mice | ↓ A β expression | [177] |
| PD | MSCs | ASO | SH-SY5Y cells | A53T α-syn transgenic mice | ↓ The expression of α-syn and attenuated its aggregation | [187] |
| PD | hucMSCs | - | SH-SY5Y cells | Male SD rats | Promoting dopaminergic neuron survival in a Parkinson’s disease model, ↓ neuroinflammation, and improve the motor function | [189] |
| PD | UCB | - | MN9D cells and SH-SY5Y cells | Male C57BL/6 mice | Inhibition of hyperphosphorylation of MAPK p38 and ERK 1/2 signaling pathways | [190] |
| PD | HEK293T self-assembly | CA | Neuro-2a, iPC12, HeLa cell | C57BL/6 mice | Improve motor function, ↓ neuronal loss, ↓ α-syn pathological burden | [188] |
| PD | NSC | - | SH-SY5Y, BV2 | Male C57BL/6 mice | ↓ ROS and proinflammatory cytokines | [192] |
| PD | Umbilical cord blood mononuclear cell | MicroRNA-124-3p | N27 dopaminergic cells, NSC | Male C57BL/6 | Protecting the dopaminergic neurons in the nigra and striatal fibers | [193] |
| PD | HEK-293T | Therapeutic catalase mRNA | Neuro2A cell, HEK-293T | Female C57BL/6 J mice | ↓ Neurotoxicity and neuroinflammation | [194] |
| ALS | Schwann cells | - | - | An 81-year-old male patient | No adverse effects | [197] |
| ALS | ASCs from inguinal adipose tissues of C57Bl6/J mice | - | - | Transgenic mice overexpressing human SOD1 carrying a Gly93-Ala mutation and WT mice (B6SJL) | ↓ The glial cells activation | [198] |
| ALS | hBMEPC | - | mBEC | - | ↓ The damage of mBECs | [199] |
| glioma | MSCs | miR-199a | U251 | Female Balb/c nude mice | ↓ AGAP2 | [207] |
| glioma | Raw264.7 cells | SPIONs, Cur, RGE | U251 | Female BALB/c nude mice | Image, synergistic antitumor effect | [92] |
| glioma | Marrow stromal cells | miR-146b | 9L gliosarcoma cells | Male Fischer rats | Anti-tumor effect | [208] |
| glioma | U87MG | Selumetinib | U87MG, A549 | Male Balb/c-nude mice | Specific antitumor effect | [209] |
| GBM | Mouse brain endothelial bEnd.3 cells | NPDOX | glioma GL261 cells | C57BL/6 male mice | Induce apoptosis and ICD | [210] |
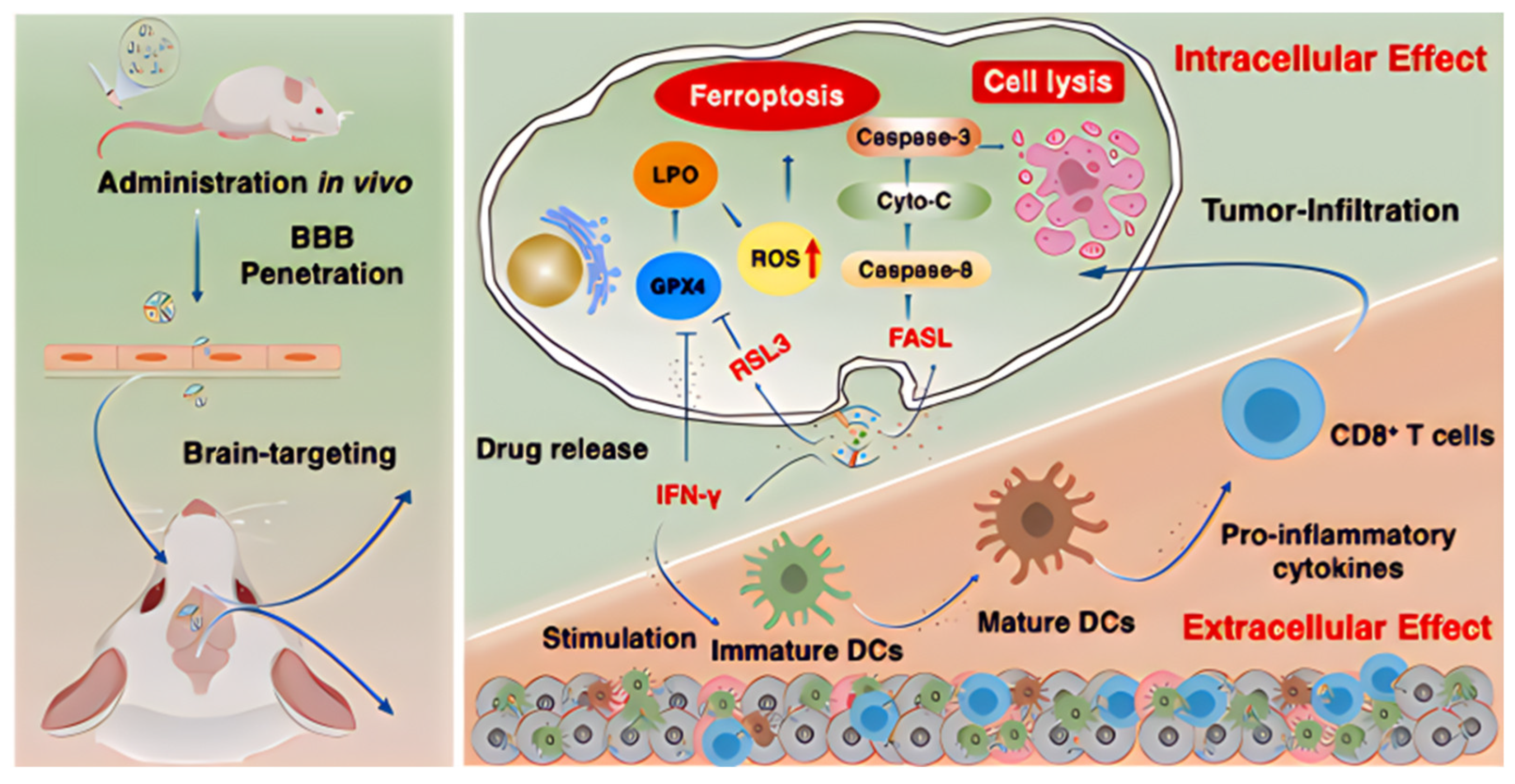
| glioma | NK cells | RSL3 | C6 glioma cells, bEnd.3 cells | Both male and female ICR mice | ↑ Ferroptosis and immune activation | [211] |
| Neuroinflammation | hUC-MSC | - | BV-2 cells, Primary microglia | Male C57BL/6J mice | Inhibit the microglial NRF2/NF-κB/NLRP3 signaling pathway | [236] |
| Status epilepticus | hMSCs from bone marrow | - | - | Male C57BL/6J mice | Neuroprotective and antiinflammatory effects | [240] |
8. Clinical Research on Brain Diseases
| Therapeutic Applications | Exosome Subtypes | ClinicalTrials.gov ID | Ref. |
|---|---|---|---|
| TBI | Blood-derived exosomes | NCT04928534 | [244] |
| Intracerebral Hemorrhage | Circulating exosomes | NCT05035134 | [246] |
| Post-Stroke Dementia | - | NCT05326724 | [249] |
| Stroke | Serum exosomes | NCT05370105 | [247] |
| Stroke | Mesenchymal stem cell | NCT03384433 | [250] |
| Acute Ischemic Stroke | Human-induced pluripotent stem cell (GD-iExo-003) | NCT06138210 | [251] |
| Stroke | Blood-derived exosomes | NCT06319742 | [245] |
| AD | Allogenic adipose mesenchymal stem cells (MSCs-Exos) | NCT04388982 | [253] |
| PD | - | NCT01860118 | [248] |
| ALS | Human umbilical cord blood mesenchymal stem cells (hUC-MSC-sEV-001) | NCT06598202 | [252] |
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Olesen, J.; Leonardi, M. The burden of brain diseases in Europe. Eur. J. Neurol. 2003, 10, 471–477. [Google Scholar] [CrossRef] [PubMed]
- DiLuca, M.; Olesen, J. The cost of brain diseases: A burden or a challenge? Neuron 2014, 82, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Ray Dorsey, E.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and projected future economic burden of Parkinson’s disease in the U.S. npj Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.L.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Nance, E.; Pun, S.H.; Saigal, R.; Sellers, D.L. Drug delivery to the central nervous system. Nat. Rev. Mater. 2022, 7, 314–331. [Google Scholar] [CrossRef]
- Stockwell, J.; Abdi, N.; Lu, X.; Maheshwari, O.; Taghibiglou, C. Novel central nervous system drug delivery systems. Chem. Biol. Drug Des. 2014, 83, 507–520. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef]
- Bechmann, I.; Galea, I.; Perry, V.H. What is the blood–brain barrier (not)? Trends Immunol. 2007, 28, 5–11. [Google Scholar] [CrossRef]
- Banks, W.A. Drug delivery to the brain in Alzheimer’s disease: Consideration of the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 629–639. [Google Scholar] [CrossRef]
- Tsou, Y.H.; Zhang, X.Q.; Zhu, H.; Syed, S.; Xu, X. Drug delivery to the brain across the blood–brain barrier using nanomaterials. Small 2017, 13, 1701921. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood–brain barrier to blood–brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood–brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef]
- Gaurav, I.; Thakur, A.; Iyaswamy, A.; Wang, X.; Chen, X.; Yang, Z. Factors Affecting Extracellular Vesicles Based Drug Delivery Systems. Molecules 2021, 26, 1544. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control. Release 2015, 219, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Karami, N.; Taheri-Anganeh, M.; Taghvimi, S.; Tondro, G.; Khorsand, M.; Soltani Fard, E.; Sedighimehr, N.; Kazemi, M.; Rahimi Jaberi, K. Exosomes: Promising delivery tools for overcoming blood-brain barrier and glioblastoma therapy. Mol. Neurobiol. 2023, 60, 4659–4678. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood–brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, S.; Wen, Y.; Zhao, W.; Huang, Y.; Liu, J. Overcoming the blood-brain barrier: Exosomes as theranostic nanocarriers for precision neuroimaging. J. Control. Release 2022, 349, 902–916. [Google Scholar] [CrossRef]
- Zhai, K.; Duan, H.; Wang, W.; Zhao, S.; Khan, G.J.; Wang, M.; Zhang, Y.; Thakur, K.; Fang, X.; Wu, C. Ginsenoside Rg1 ameliorates blood–brain barrier disruption and traumatic brain injury via attenuating macrophages derived exosomes miR-21 release. Acta Pharm. Sin. B 2021, 11, 3493–3507. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, L.; Zeng, X.; Schwarz, H.; Nanda, H.S.; Peng, X.; Zhou, Y. Exosomes, a New Star for Targeted Delivery. Front. Cell Dev. Biol. 2021, 9, 751079. [Google Scholar] [CrossRef]
- Familtseva, A.; Jeremic, N.; Tyagi, S.C. Exosomes: Cell-created drug delivery systems. Mol. Cell. Biochem. 2019, 459, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Brown, B.A.; Siegel, A.P.; El Masry, M.S.; Zeng, X.; Song, W.; Das, A.; Khandelwal, P.; Clark, A.; Singh, K.; et al. Exosome-Mediated Crosstalk between Keratinocytes and Macrophages in Cutaneous Wound Healing. ACS Nano 2020, 14, 12732–12748. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, X.; Sun, M.; Xiong, S.; Xiao, N.; Li, J.; He, X.; Xie, J. Recent Progress in Extracellular Vesicle-Based Carriers for Targeted Drug Delivery in Cancer Therapy. Pharmaceutics 2023, 15, 1902. [Google Scholar] [CrossRef]
- Bashyal, S.; Thapa, C.; Lee, S. Recent progresses in exosome-based systems for targeted drug delivery to the brain. J. Control. Release 2022, 348, 723–744. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Spencer, W.; Gupta, R.C. Exosomes as an emerging plasmid delivery vehicle for gene therapy. Pharmaceutics 2023, 15, 1832. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, J.N.; Rodrigues, L.R. New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol./Hematol. 2022, 172, 103628. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Das, C.K.; Jena, B.C.; Banerjee, I.; Das, S.; Parekh, A.; Bhutia, S.K.; Mandal, M. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol. Pharm. 2018, 16, 24–40. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Tang, Y.; Cheng, Y.C. Exosomes as targeted delivery drug system: Advances in exosome loading, surface functionalization and potential for clinical application. Curr. Drug Deliv. 2024, 21, 473–487. [Google Scholar] [CrossRef]
- Palakurthi, S.S.; Shah, B.; Kapre, S.; Charbe, N.; Immanuel, S.; Pasham, S.; Thalla, M.; Jain, A.; Palakurthi, S. A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 2024, 6, 5803–5826. [Google Scholar] [CrossRef] [PubMed]
- Thind, A.; Wilson, C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J. Extracell. Vesicles 2016, 5, 31292. [Google Scholar] [CrossRef]
- Jiang, L.; Gu, Y.; Du, Y.; Liu, J. Exosomes: Diagnostic biomarkers and therapeutic delivery vehicles for cancer. Mol. Pharm. 2019, 16, 3333–3349. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183. [Google Scholar] [CrossRef]
- Rehman, F.U.; Liu, Y.; Zheng, M.; Shi, B. Exosomes based strategies for brain drug delivery. Biomaterials 2023, 293, 121949. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Williamson, C.D.; Jarnik, M.; Bonifacino, J.S. Inhibition of endolysosome fusion increases exosome secretion. J. Cell Biol. 2023, 222, e202209084. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zhan, W.; Gao, Y.; Huang, L.; Gong, R.; Wang, W.; Zhang, R.; Wu, Y.; Gao, S.; Kang, T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021, 31, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, F.; Ma, Y.; Wang, J.; Li, H.; Zhang, J. Isolation and Detection Technologies of Extracellular Vesicles and Application on Cancer Diagnostic. Dose Response 2019, 17, 1559325819891004. [Google Scholar] [CrossRef]
- Lin, S.; Yu, Z.; Chen, D.; Wang, Z.; Miao, J.; Li, Q.; Zhang, D.; Song, J.; Cui, D. Progress in Microfluidics-Based Exosome Separation and Detection Technologies for Diagnostic Applications. Small 2020, 16, e1903916. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Cheng, H.; Fang, H.; Xu, R.D.; Fu, M.Q.; Chen, L.; Song, X.Y.; Qian, J.Y.; Zou, Y.Z.; Ma, J.Y.; Ge, J.B. Development of a rinsing separation method for exosome isolation and comparison to conventional methods. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5074–5083. [Google Scholar] [CrossRef]
- Zhang, P.; Yeo, J.C.; Lim, C.T. Advances in Technologies for Purification and Enrichment of Extracellular Vesicles. SLAS Technol. 2019, 24, 477–488. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Li, Y.; Yan, H.; Wen, Y.; Zhou, X.; Friedman, L.; Zeng, Y. Advances in microfluidic extracellular vesicle analysis for cancer diagnostics. Lab Chip 2021, 21, 3219–3243. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Nguyen, T.N.G.; Lee, B.J.; Duan, W. Aspirin-loaded nanoexosomes as cancer therapeutics. Int. J. Pharm. 2019, 572, 118786. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Pei, F.; Zeng, C.; Yao, Y.; Liao, W.; Zhao, Z. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. BioMed Res. Int. 2021, 2021, 6611244. [Google Scholar] [CrossRef] [PubMed]
- Street, J.M.; Koritzinsky, E.H.; Glispie, D.M.; Yuen, P.S.T. Urine Exosome Isolation and Characterization. Methods Mol. Biol. 2017, 1641, 413–423. [Google Scholar] [CrossRef]
- Pérez-González, R.; Gauthier, S.A.; Kumar, A.; Saito, M.; Saito, M.; Levy, E. A Method for Isolation of Extracellular Vesicles and Characterization of Exosomes from Brain Extracellular Space. Methods Mol. Biol. 2017, 1545, 139–151. [Google Scholar] [CrossRef]
- Onódi, Z.; Pelyhe, C.; Terézia Nagy, C.; Brenner, G.B.; Almási, L.; Kittel, Á.; Manček-Keber, M.; Ferdinandy, P.; Buzás, E.I.; Giricz, Z. Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography from Blood Plasma. Front. Physiol. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Lim, Y.J.; Lee, S.J. Are exosomes the vehicle for protein aggregate propagation in neurodegenerative diseases? Acta Neuropathol. Commun. 2017, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.W.; Bergenstal, R.M.; Laffel, L.M.; Pickup, J.C. Advances in technology for management of type 1 diabetes. Lancet 2019, 394, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Simpson, R.J.; Greening, D.W. A Protocol for Isolation and Proteomic Characterization of Distinct Extracellular Vesicle Subtypes by Sequential Centrifugal Ultrafiltration. Methods Mol. Biol. 2017, 1545, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Zhu, J.; Liu, J.X.; Jiang, F.; Ni, W.K.; Qu, L.S.; Ni, R.Z.; Lu, C.H.; Xiao, M.B. A Comparison of Traditional and Novel Methods for the Separation of Exosomes from Human Samples. BioMed Res. Int. 2018, 2018, 3634563. [Google Scholar] [CrossRef]
- Soda, N.; Rehm, B.H.A.; Sonar, P.; Nguyen, N.T.; Shiddiky, M.J.A. Advanced liquid biopsy technologies for circulating biomarker detection. J. Mater. Chem. B 2019, 7, 6670–6704. [Google Scholar] [CrossRef]
- Popović, M.; de Marco, A. Canonical and selective approaches in exosome purification and their implications for diagnostic accuracy. Transl. Cancer Res. 2018, 7 (Suppl. S2), S209–S225. [Google Scholar] [CrossRef]
- An, M.; Wu, J.; Zhu, J.; Lubman, D.M. Comparison of an Optimized Ultracentrifugation Method versus Size-Exclusion Chromatography for Isolation of Exosomes from Human Serum. J. Proteome Res. 2018, 17, 3599–3605. [Google Scholar] [CrossRef]
- Hong, C.S.; Funk, S.; Whiteside, T.L. Isolation of Biologically Active Exosomes from Plasma of Patients with Cancer. Methods Mol. Biol. 2017, 1633, 257–265. [Google Scholar] [CrossRef]
- Soares Martins, T.; Catita, J.; Martins Rosa, I.; AB da Cruz e Silva, O.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017, 77, 6480–6488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jin, K.; Gao, L.; Zhang, Z.; Li, F.; Zhou, F.; Zhang, L. Methods and technologies for exosome isolation and characterization. Small Methods 2018, 2, 1800021. [Google Scholar] [CrossRef]
- Visan, K.S.; Wu, L.Y.; Voss, S.; Wuethrich, A.; Möller, A. Status quo of Extracellular Vesicle isolation and detection methods for clinical utility. Semin. Cancer Biol. 2023, 88, 157–171. [Google Scholar] [CrossRef]
- Jiang, X.; Jing, W.; Zheng, L.; Liu, S.; Wu, W.; Sui, G. A continuous-flow high-throughput microfluidic device for airborne bacteria PCR detection. Lab Chip 2014, 14, 671–676. [Google Scholar] [CrossRef]
- Pang, B.; Zhu, Y.; Ni, J.; Thompson, J.; Malouf, D.; Bucci, J.; Graham, P.; Li, Y. Extracellular vesicles: The next generation of biomarkers for liquid biopsy-based prostate cancer diagnosis. Theranostics 2020, 10, 2309–2326. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, W.; Yang, F. Cancer Liquid Biopsy Using Integrated Microfluidic Exosome Analysis Platforms. Biotechnol. J. 2020, 15, e1900225. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; He, M.; Shang, Y.; Tetlow, A.L.; Godwin, A.K.; Zeng, Y. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat. Biomed. Eng. 2019, 3, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes—Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano 2022, 16, 17802–17846. [Google Scholar] [CrossRef]
- Kobiela, A.; Hovhannisyan, L.; Jurkowska, P.; de la Serna, J.B.; Bogucka, A.; Deptuła, M.; Paul, A.A.; Panek, K.; Czechowska, E.; Rychłowski, M. Excess filaggrin in keratinocytes is removed by extracellular vesicles to prevent premature death and this mechanism can be hijacked by Staphylococcus aureus in a TLR2-dependent fashion. J. Extracell. Vesicles 2023, 12, 12335. [Google Scholar] [CrossRef]
- André-Grégoire, G.; Roux, Q.; Gavard, J. Isolating plasma extracellular vesicles from mouse blood using size-exclusion chromatography, density gradient, and ultracentrifugation. STAR Protoc. 2023, 4, 102740. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, X.; Gao, Z.; Effah, C.Y.; Zhang, X.; Wu, Y.; Qu, L. A Holistic Review of the State-of-the-Art Microfluidics for Exosome Separation: An Overview of the Current Status, Existing Obstacles, and Future Outlook. Small 2021, 17, e2007174. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Suwatthanarak, T.; Thiodorus, I.A.; Tanaka, M.; Shimada, T.; Takeshita, D.; Yasui, T.; Baba, Y.; Okochi, M. Microfluidic-based capture and release of cancer-derived exosomes via peptide-nanowire hybrid interface. Lab Chip 2021, 21, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Fitzner, D.; Schnaars, M.; van Rossum, D.; Krishnamoorthy, G.; Dibaj, P.; Bakhti, M.; Regen, T.; Hanisch, U.K.; Simons, M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011, 124, 447–458. [Google Scholar] [CrossRef]
- Sarkar, S.N.; Corbin, D.; Simpkins, J.W. Brain-Wide Transgene Expression in Mice by Systemic Injection of Genetically Engineered Exosomes: CAP-Exosomes. Pharmaceuticals 2024, 17, 270. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of miR-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Bai, J.; Duan, J.; Liu, R.; Du, Y.; Luo, Q.; Cui, Y.; Su, Z.; Xu, J.; Xie, Y.; Lu, W. Engineered targeting tLyp-1 exosomes as gene therapy vectors for efficient delivery of siRNA into lung cancer cells. Asian J. Pharm. Sci. 2020, 15, 461–471. [Google Scholar] [CrossRef]
- Iyaswamy, A.; Thakur, A.; Guan, X.J.; Krishnamoorthi, S.; Fung, T.Y.; Lu, K.; Gaurav, I.; Yang, Z.; Su, C.F.; Lau, K.F.; et al. Fe65-engineered neuronal exosomes encapsulating corynoxine-B ameliorate cognition and pathology of Alzheimer’s disease. Signal Transduct. Target. Ther. 2023, 8, 404. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.X.; He, C.P.; Fan, S.; Zhu, Y.L.; Qi, C.; Huang, N.P.; Xiao, Z.D.; Lu, Z.H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Guo, H.D.; Li, H.; Zhai, Y.; Gong, Z.B.; Wu, J.; Liu, J.S.; Dong, Y.R.; Hou, S.X.; Liu, J.R. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer’s disease. Immun. Ageing 2019, 16, 10. [Google Scholar] [CrossRef]
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153–3170. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, J.; Gu, W.; Huang, Y.; Tong, Z.; Huang, L.; Tan, J. Exosome-Liposome Hybrid Nanoparticles Deliver CRISPR/Cas9 System in MSCs. Adv. Sci. 2018, 5, 1700611. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhi, S.; Nguyen, T.D.T.; Marasini, R.; Aryal, S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019, 94, 482–494. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Ding, F.; Fan, H.; Shi, L.; Zhu, L.; Li, J.; Feng, J.; Zhu, X.; Zhang, C. Rapid Detection of Exosomal MicroRNAs Using Virus-Mimicking Fusogenic Vesicles. Angew. Chem. Int. Ed. Engl. 2019, 58, 8719–8723. [Google Scholar] [CrossRef]
- Takeda, A.; Tachibana, A.; Nagumo, H.; Sakai-Kato, K. An in vitro lipid-mixing assay to investigate the fusion between small extracellular vesicles and endosome. Anal. Biochem. 2023, 669, 115130. [Google Scholar] [CrossRef]
- Mukherjee, D.; Paul, D.; Sarker, S.; Hasan, M.N.; Ghosh, R.; Prasad, S.E.; Vemula, P.K.; Das, R.; Adhikary, A.; Pal, S.K.; et al. Polyethylene Glycol-Mediated Fusion of Extracellular Vesicles with Cationic Liposomes for the Design of Hybrid Delivery Systems. ACS Appl. Bio Mater. 2021, 4, 8259–8266. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Jiang, G.; Huang, X. Sequential Targeting Hybrid Nanovesicles Composed of Chimeric Antigen Receptor T-Cell-Derived Exosomes and Liposomes for Enhanced Cancer Immunochemotherapy. ACS Nano 2023, 17, 16770–16786. [Google Scholar] [CrossRef]
- Lv, Q.; Cheng, L.; Lu, Y.; Zhang, X.; Wang, Y.; Deng, J.; Zhou, J.; Liu, B.; Liu, J. Thermosensitive Exosome-Liposome Hybrid Nanoparticle-Mediated Chemoimmunotherapy for Improved Treatment of Metastatic Peritoneal Cancer. Adv. Sci. 2020, 7, 2000515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mukhopadhyay, C.D. Exosome as drug delivery system: Current advancements. Extracell. Vesicle 2024, 3, 100032. [Google Scholar] [CrossRef]
- Kibria, G.; Ramos, E.K.; Wan, Y.; Gius, D.R.; Liu, H. Exosomes as a drug delivery system in cancer therapy: Potential and challenges. Mol. Pharm. 2018, 15, 3625–3633. [Google Scholar] [CrossRef]
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X. Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12, 1416. [Google Scholar] [CrossRef]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Li, X.Q.; Liu, J.T.; Fan, L.L.; Liu, Y.; Cheng, L.; Wang, F.; Yu, H.Q.; Gao, J.; Wei, W.; Wang, H.; et al. Exosomes derived from gefitinib-treated EGFR-mutant lung cancer cells alter cisplatin sensitivity via up-regulating autophagy. Oncotarget 2016, 7, 24585–24595. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, G.; Guo, J.; Wang, C.; He, Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 8095–8104. [Google Scholar] [CrossRef]
- Al Faruque, H.; Choi, E.S.; Kim, J.H.; Kim, E. Enhanced effect of autologous EVs delivering paclitaxel in pancreatic cancer. J. Control. Release 2022, 347, 330–346. [Google Scholar] [CrossRef]
- Pan, S.; Pei, L.; Zhang, A.; Zhang, Y.; Zhang, C.; Huang, M.; Huang, Z.; Liu, B.; Wang, L.; Ma, L.; et al. Passion fruit-like exosome-PMA/Au-BSA@Ce6 nanovehicles for real-time fluorescence imaging and enhanced targeted photodynamic therapy with deep penetration and superior retention behavior in tumor. Biomaterials 2020, 230, 119606. [Google Scholar] [CrossRef]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control. Release 2020, 317, 273–281. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, J.; Zhao, Q.; Zhuang, W.; Ding, J.; Zhang, C.; Gao, H.; Pang, D.W.; Pu, K.; Xie, H.Y. Molecularly Engineered Macrophage-Derived Exosomes with Inflammation Tropism and Intrinsic Heme Biosynthesis for Atherosclerosis Treatment. Angew. Chem. Int. Ed. Engl. 2020, 59, 4068–4074. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, S.; Aarrass, H.; Lai-Kee-Him, J.; Bron, P.; Armengaud, J.; Miotello, G.; Bertrand-Michel, J.; Dubois, E.; George, S.; Faklaris, O.; et al. Post-production modifications of murine mesenchymal stem cell (mMSC) derived extracellular vesicles (EVs) and impact on their cellular interaction. Biomaterials 2020, 231, 119675. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to Lysosomes with Extracellular Vesicles and their Enhanced Brain Distribution in the Animal Model of Batten Disease. Adv. Healthc. Mater. 2019, 8, e1801271. [Google Scholar] [CrossRef]
- Sun, L.; Fan, M.; Huang, D.; Li, B.; Xu, R.; Gao, F.; Chen, Y. Clodronate-loaded liposomal and fibroblast-derived exosomal hybrid system for enhanced drug delivery to pulmonary fibrosis. Biomaterials 2021, 271, 120761. [Google Scholar] [CrossRef]
- Khosravi, N.; Pishavar, E.; Baradaran, B.; Oroojalian, F.; Mokhtarzadeh, A. Stem cell membrane, stem cell-derived exosomes and hybrid stem cell camouflaged nanoparticles: A promising biomimetic nanoplatforms for cancer theranostics. J. Control. Release 2022, 348, 706–722. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, A.; Nandy, A.; Ghatak, S. Insight into the functional dynamics and challenges of exosomes in pharmaceutical innovation and precision medicine. Pharmaceutics 2024, 16, 709. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Chen, R.; Huang, H.; Liu, H.; Xi, J.; Ning, J.; Zeng, W.; Shen, C.; Zhang, T.; Yu, G.; Xu, Q.; et al. Friend or Foe? Evidence Indicates Endogenous Exosomes Can Deliver Functional gRNA and Cas9 Protein. Small 2019, 15, e1902686. [Google Scholar] [CrossRef]
- de Abreu, R.C.; Ramos, C.V.; Becher, C.; Lino, M.; Jesus, C.; da Costa Martins, P.A.; Martins, P.A.T.; Moreno, M.J.; Fernandes, H.; Ferreira, L. Exogenous loading of miRNAs into small extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12111. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Encabo-Berzosa, M.D.M.; Beltrán-Visiedo, M.; Fernández-Messina, L.; Sebastián, V.; Sánchez-Madrid, F.; Arruebo, M.; Santamaría, J.; Martín-Duque, P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 2019, 11, 18825–18836. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Choi, Y.; Yim, H.Y.; Mirzaaghasi, A.; Yoo, J.K.; Choi, C. Biodistribution of Exosomes and Engineering Strategies for Targeted Delivery of Therapeutic Exosomes. Tissue Eng. Regen. Med. 2021, 18, 499–511. [Google Scholar] [CrossRef]
- Rodríguez-Morales, B.; Antunes-Ricardo, M.; González-Valdez, J. Exosome-Mediated Insulin Delivery for the Potential Treatment of Diabetes Mellitus. Pharmaceutics 2021, 13, 1870. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Zlokovic, B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Ségaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell. Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef]
- Saint-Pol, J.; Gosselet, F.; Duban-Deweer, S.; Pottiez, G.; Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 2020, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Thom, G.; Burrell, M.; Delaney, C.E.; Brunette, E.; Baumann, E.; Sodja, C.; Jezierski, A.; Webster, C.; Stanimirovic, D.B. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J. Neurochem. 2018, 146, 735–752. [Google Scholar] [CrossRef]
- Morad, G.; Carman, C.V.; Hagedorn, E.J.; Perlin, J.R.; Zon, L.I.; Mustafaoglu, N.; Park, T.E.; Ingber, D.E.; Daisy, C.C.; Moses, M.A. Tumor-Derived Extracellular Vesicles Breach the Intact Blood-Brain Barrier via Transcytosis. ACS Nano 2019, 13, 13853–13865. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, F.; Liu, L.; Xu, X.; Zhang, B.; Wu, Y.; Yin, D.; Zhou, S.; Sun, D.; Huang, Y.; et al. Endothelial colony-forming cell-derived exosomes restore blood-brain barrier continuity in mice subjected to traumatic brain injury. Exp. Neurol. 2018, 307, 99–108. [Google Scholar] [CrossRef]
- Fang, X.; Gong, R.; Yang, D.; Li, C.; Zhang, Y.; Wang, Y.; Nie, G.; Li, M.; Peng, X.; Zhang, B. NIR-II Light-Driven Genetically Engineered Exosome Nanocatalysts for Efficient Phototherapy against Glioblastoma. J. Am. Chem. Soc. 2024, 146, 15251–15263. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.J.; Choi, W.; Lee, Y.M.; Pyo, J.H.; Lee, J.; Kim, J.; Kim, J.; Kim, J.H.; Kim, C.; et al. Deep brain stimulation by blood-brain-barrier-crossing piezoelectric nanoparticles generating current and nitric oxide under focused ultrasound. Nat. Biomed. Eng. 2023, 7, 149–163. [Google Scholar] [CrossRef]
- Villa, A.; De Mitri, Z.; Vincenti, S.; Crippa, E.; Castiglioni, L.; Gelosa, P.; Rebecchi, M.; Tosi, D.; Brunialti, E.; Oevermann, A.; et al. Canine glioblastoma-derived extracellular vesicles as precise carriers for glioblastoma imaging: Targeting across the blood-brain barrier. Biomed. Pharmacother. 2024, 172, 116201. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, L.; Li, L.; Cao, Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying lncRNA GS1-600G8.5. BioMed Res. Int. 2020, 2020, 7461727. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gürsoy-Özdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kubo, K.; Waguri, S.; Yabashi, A.; Shin, H.W.; Katoh, Y.; Nakayama, K. Rab11 regulates exocytosis of recycling vesicles at the plasma membrane. J. Cell Sci. 2012, 125, 4049–4057. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Killen, M.J.; Giorgi-Coll, S.; Helmy, A.; Hutchinson, P.J.; Carpenter, K.L. Metabolism and inflammation: Implications for traumatic brain injury therapeutics. Expert Rev. Neurother. 2019, 19, 227–242. [Google Scholar] [CrossRef]
- Ghaith, H.S.; Nawar, A.A.; Gabra, M.D.; Abdelrahman, M.E.; Nafady, M.H.; Bahbah, E.I.; Ebada, M.A.; Ashraf, G.M.; Negida, A.; Barreto, G.E. A Literature Review of Traumatic Brain Injury Biomarkers. Mol. Neurobiol. 2022, 59, 4141–4158. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, X.; Shu, K. Cell-Derived Exosomes as Therapeutic Strategies and Exosome-Derived microRNAs as Biomarkers for Traumatic Brain Injury. J. Clin. Med. 2022, 11, 3223. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J. Neuroinflamm. 2020, 17, 89. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Bai, W.; Sun, L.; Tian, M. Human umbilical cord mesenchymal stem cell-derived exosome suppresses programmed cell death in traumatic brain injury via PINK1/Parkin-mediated mitophagy. CNS Neurosci. Ther. 2023, 29, 2236–2258. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Zhang, Y.; Chen, S.; Ding, J.; Chen, Z.; Wu, K.; Wu, X.; Zhou, T.; Zeng, M.; et al. Hyaluronan-based hydrogel integrating exosomes for traumatic brain injury repair by promoting angiogenesis and neurogenesis. Carbohydr. Polym. 2023, 306, 120578. [Google Scholar] [CrossRef] [PubMed]
- Abedi, M.; Hajinejad, M.; Atabi, F.; Sahab-Negah, S. Exosome Derived from Human Neural Stem Cells Improves Motor Activity and Neurogenesis in a Traumatic Brain Injury Model. BioMed Res. Int. 2022, 2022, 6409346. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chang, Z.H.; Yao, B.; Liu, X.Y.; Zhang, X.W.; Liang, J.; Wang, J.J.; Bao, S.Q.; Chen, M.M.; Zhu, P.; et al. 3D printing of interferon γ-preconditioned NSC-derived exosomes/collagen/chitosan biological scaffolds for neurological recovery after TBI. Bioact. Mater. 2024, 39, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, H.; Zhang, Y.; Zeng, R.; Yu, J.; Liu, R.; Jin, X.; Zhao, Y. Plasma Exosome-derived MicroRNAs as Novel Biomarkers of Traumatic Brain Injury in Rats. Int. J. Med. Sci. 2020, 17, 437–448. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, Y.; Xu, S.; Liu, F.; Gao, Y. Exosomal microRNAs as Potential Biomarkers and Therapeutic Agents for Acute Ischemic Stroke: New Expectations. Front. Neurol. 2021, 12, 747380. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, W.; Niu, L.; Bao, W.; Wang, Y.; Wang, Y.; Zhu, Y.; Yang, Z.; Chen, J.; Dong, J.; et al. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. eLife 2023, 12, e84493. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Jia, F.; Ahmed, W.; Zhang, G.L.; Wang, H.; Lin, C.Q.; Chen, W.H.; Chen, L.K. Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regen. Res. 2023, 18, 404–409. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Yang, C.; Xu, Z.; Huang, J.; Lin, J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res. Bull. 2020, 163, 84–94. [Google Scholar] [CrossRef]
- Wang, L.D.; Xu, Z.M.; Liang, X.; Qiu, W.R.; Liu, S.J.; Dai, L.L.; Wang, Y.F.; Guo, C.Y.; Qi, X.H.; Wang, J.; et al. Systematic Review and Meta-Analysis on Randomized Controlled Trials on Efficacy and Safety of Panax Notoginseng Saponins in Treatment of Acute Ischemic Stroke. Evid.-Based Complement. Altern. Med. 2021, 2021, 4694076. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Wang, A.; Li, Y.; Zhang, M.; Kim, J.; Zhu, Y.; Wang, Q.; Zhang, Y.; Wei, Y.; et al. Panax notoginseng: Derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J. Nanobiotechnol. 2023, 21, 416. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnol. 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Tang, X. Exosomes from miR-19b-3p-Modified ADSCs Inhibit Ferroptosis in Intracerebral Hemorrhage Mice. Front. Cell Dev. Biol. 2021, 9, 661317. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative disorders: Mechanisms of degeneration and therapeutic approaches with their clinical relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, S.M.; Heo, Y.; Lee, G.; Kang, J.Y.; Yoon, D.S. Nanoelectrical characterization of individual exosomes secreted by Aβ(42)-ingested cells using electrostatic force microscopy. Nanotechnology 2021, 32, 025705. [Google Scholar] [CrossRef]
- Wang, Y.; Balaji, V.; Kaniyappan, S.; Krüger, L.; Irsen, S.; Tepper, K.; Chandupatla, R.; Maetzler, W.; Schneider, A.; Mandelkow, E.; et al. The release and trans-synaptic transmission of Tau via exosomes. Mol. Neurodegener. 2017, 12, 5. [Google Scholar] [CrossRef]
- Malm, T.; Loppi, S.; Kanninen, K.M. Exosomes in Alzheimer’s disease. Neurochem. Int. 2016, 97, 193–199. [Google Scholar] [CrossRef]
- Rastogi, S.; Rani, K.; Kumar, S. Progression of Cognitive Impairment to Alzheimer’s Disease: Through the Lens of Salivary Extracellular Vesicles. Neurosci. Insights 2021, 16, 26331055211058687. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, Q.; Zhang, H.; Chu, L.; Du, Y.; Zhang, J.; Zhou, C.; Liang, F.; Shi, S.; Wang, S.; et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimer’s Dement. 2019, 15, 1071–1080. [Google Scholar] [CrossRef]
- McKeever, P.M.; Schneider, R.; Taghdiri, F.; Weichert, A.; Multani, N.; Brown, R.A.; Boxer, A.L.; Karydas, A.; Miller, B.; Robertson, J.; et al. MicroRNA Expression Levels Are Altered in the Cerebrospinal Fluid of Patients with Young-Onset Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8826–8841. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, G.; Yang, Z.; Shi, H.; Zeng, H.; Ye, Q.; Hu, Z.; Wang, Z. Biomimetic Nanovesicles as a Dual Gene Delivery System for the Synergistic Gene Therapy of Alzheimer’s Disease. ACS Nano 2024, 18, 11753–11768. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, M.; Pedrazzoli, M.; D’Agostino, C.; Elia, C.A.; Massenzio, F.; Lonati, E.; Mauri, M.; Rizzi, L.; Molteni, L.; Bresciani, E.; et al. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer’s disease. Stem Cells Transl. Med. 2020, 9, 1068–1084. [Google Scholar] [CrossRef]
- Yan, Y.; Gao, Y.; Kumar, G.; Fang, Q.; Yan, H.; Zhang, N.; Zhang, Y.; Song, L.; Li, J.; Zheng, Y.; et al. Exosomal MicroRNAs modulate the cognitive function in fasudil treated APPswe/PSEN1dE9 transgenic (APP/PS1) mice model of Alzheimer’s disease. Metab. Brain Dis. 2024, 39, 1335–1351. [Google Scholar] [CrossRef]
- Šála, M.; Hollinger, K.R.; Thomas, A.G.; Dash, R.P.; Tallon, C.; Veeravalli, V.; Lovell, L.; Kögler, M.; Hřebabecký, H.; Procházková, E.; et al. Novel Human Neutral Sphingomyelinase 2 Inhibitors as Potential Therapeutics for Alzheimer’s Disease. J. Med. Chem. 2020, 63, 6028–6056. [Google Scholar] [CrossRef]
- Kim, E.; Otgontenger, U.; Jamsranjav, A.; Kim, S.S. Deleterious Alteration of Glia in the Brain of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6676. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Singh, P.K.; Muqit, M.M.K. Parkinson’s: A Disease of Aberrant Vesicle Trafficking. Annu. Rev. Cell Dev. Biol. 2020, 36, 237–264. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.; Mukherjee, R.; Singh, E.; Kumar, S.; Sharma, V.; Vishwakarma, P.; Bharti, P.S.; Nikolajeff, F.; Dinda, A.K.; Goyal, V.; et al. Neuronal exosomes in saliva of Parkinson’s disease patients: A pilot study. Park. Relat. Disord. 2019, 67, 21–23. [Google Scholar] [CrossRef]
- Park, G.; Kim, B.S.; Kim, E. A novel function of FAF1, which induces dopaminergic neuronal death through cell-to-cell transmission. Cell Commun. Signal. 2020, 18, 133. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, G. Potential of extracellular vesicles in the Parkinson’s disease—Pathological mediators and biomarkers. Neurochem. Int. 2021, 144, 104974. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Trojanowski, J.Q.; Lee, V.M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 2020, 16, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Hopfner, F.; Katsikoudi, A.; Hein, R.; Catli, C.; Evetts, S.; Huang, Y.; Wang, H.; Ryder, J.W.; Kuhlenbaeumer, G.; et al. Serum neuronal exosomes predict and differentiate Parkinson’s disease from atypical parkinsonism. J. Neurol. Neurosurg. Psychiatry 2020, 91, 720–729. [Google Scholar] [CrossRef]
- Chang, C.W.; Yang, S.Y.; Yang, C.C.; Chang, C.W.; Wu, Y.R. Plasma and Serum Alpha-Synuclein as a Biomarker of Diagnosis in Patients with Parkinson’s Disease. Front. Neurol. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Wang, S.; Kelly, K.; Brotchie, J.M.; Koprich, J.B.; West, A.B. Exosome markers of LRRK2 kinase inhibition. npj Park. Dis. 2020, 6, 32. [Google Scholar] [CrossRef]
- Yang, J.; Luo, S.; Zhang, J.; Yu, T.; Fu, Z.; Zheng, Y.; Xu, X.; Liu, C.; Fan, M.; Zhang, Z. Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2021, 148, 105218. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Zhang, J.; Zhang, Y.; Liu, K.; Song, J.X.; Sreenivasmurthy, S.G.; Wang, Z.; Shi, Y.; Chu, C.; et al. A Self-Assembled α-Synuclein Nanoscavenger for Parkinson’s Disease. ACS Nano 2020, 14, 1533–1549. [Google Scholar] [CrossRef]
- Chen, H.X.; Liang, F.C.; Gu, P.; Xu, B.L.; Xu, H.J.; Wang, W.T.; Hou, J.Y.; Xie, D.X.; Chai, X.Q.; An, S.J. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020, 11, 288. [Google Scholar] [CrossRef]
- Ye, J.; Sun, X.; Jiang, Q.; Gui, J.; Feng, S.; Qin, B.; Xie, L.; Guo, A.; Dong, J.; Sang, M. Umbilical cord blood-derived exosomes attenuate dopaminergic neuron damage of Parkinson’s disease mouse model. J. Nanobiotechnol. 2024, 22, 567. [Google Scholar] [CrossRef]
- Shukla, S.; Currim, F.; Singh, J.; Goyani, S.; Saranga, M.V.; Shinde, A.; Mane, M.; Chandak, N.; Kishore, S.; Singh, R. hsa-miR-320a mediated exosome release under PD stress conditions rescue mitochondrial ROS and cell death in the recipient neuronal and glial cells. Int. J. Biochem. Cell Biol. 2023, 162, 106439. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Choi, Y.; Lee, H.J.; Hwang, D.W.; Lee, D.S. Human neural stem cell-derived extracellular vesicles protect against Parkinson’s disease pathologies. J. Nanobiotechnol. 2022, 20, 198. [Google Scholar] [CrossRef]
- Esteves, M.; Abreu, R.; Fernandes, H.; Serra-Almeida, C.; Martins, P.A.T.; Barão, M.; Cristóvão, A.C.; Saraiva, C.; Ferreira, R.; Ferreira, L.; et al. MicroRNA-124-3p-enriched small extracellular vesicles as a therapeutic approach for Parkinson’s disease. Mol. Ther. 2022, 30, 3176–3192. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Ren, C.; Wang, Y.; He, W.; Jiang, Y. Extracellular Vesicles as Innovative Treatment Strategy for Amyotrophic Lateral Sclerosis. Front. Cell Dev. Biol. 2021, 9, 754630. [Google Scholar] [CrossRef]
- Ferrara, D.; Pasetto, L.; Bonetto, V.; Basso, M. Role of Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2018, 12, 574. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Khan, A.; Jimsheleishvili, G.; Graham, P.; Brooks, A.; Silvera, R.; Goldschmidt, A.J.P.; Pearse, D.D.; Dietrich, W.D.; Levi, A.D.; et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell-derived exosomal vesicles: A case report. Neural. Regen Res. 2025, 20, 1207–1216. [Google Scholar] [CrossRef]
- Bonafede, R.; Turano, E.; Scambi, I.; Busato, A.; Bontempi, P.; Virla, F.; Schiaffino, L.; Marzola, P.; Bonetti, B.; Mariotti, R. ASC-Exosomes Ameliorate the Disease Progression in SOD1(G93A) Murine Model Underlining Their Potential Therapeutic Use in Human ALS. Int. J. Mol. Sci. 2020, 21, 3651. [Google Scholar] [CrossRef]
- Garbuzova-Davis, S.; Borlongan, C.V. Stem cell-derived extracellular vesicles as potential mechanism for repair of microvascular damage within and outside of the central nervous system in amyotrophic lateral sclerosis: Perspective schema. Neural Regen. Res. 2021, 16, 680–681. [Google Scholar] [CrossRef]
- Weller, M.; Wen, P.Y.; Chang, S.M.; Dirven, L.; Lim, M.; Monje, M.; Reifenberger, G. Glioma. Nat. Rev. Dis. Primers 2024, 10, 33. [Google Scholar] [CrossRef]
- He, X.; Qi, Y.; Zhang, X.; Liu, X.; Li, X.; Li, S.; Wu, Y.; Zhang, Q. Current landscape of tumor-derived exosomal ncRNAs in glioma progression, detection, and drug resistance. Cell Death Dis. 2021, 12, 1145. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Luan, X.; Jiang, G.; Yang, L.; Yan, K.; Li, S.; Xiang, W.; Zhou, J. The Dual Effects of Exosomes on Glioma: A Comprehensive Review. J. Cancer 2023, 14, 2707–2719. [Google Scholar] [CrossRef]
- Xu, H.; Li, M.; Pan, Z.; Zhang, Z.; Gao, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; et al. miR-3184-3p enriched in cerebrospinal fluid exosomes contributes to progression of glioma and promotes M2-like macrophage polarization. Cancer Sci. 2022, 113, 2668–2680. [Google Scholar] [CrossRef]
- Lu, G.F.; You, C.Y.; Chen, Y.S.; Jiang, H.; Zheng, X.; Tang, W.W.; Wang, X.Y.; Xu, H.Y.; Geng, F. MicroRNA-671-3p promotes proliferation and migration of glioma cells via targeting CKAP4. Onco Targets Ther. 2018, 11, 6217–6226. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, X.; Chen, W.; Shi, P.; Lian, M.; Wang, H.; Wang, K.; Qian, D.; Xiao, D.; Long, H. Silencing of microRNA-708 promotes cell growth and epithelial-to-mesenchymal transition by activating the SPHK2/AKT/β-catenin pathway in glioma. Cell Death Dis. 2019, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Wei, Z.; Yan, W.; Yin, J.; Huang, X.; Zhou, X.; Li, R.; Shen, F.; Wu, W.; Wang, X.; et al. Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett. 2018, 436, 10–21. [Google Scholar] [CrossRef]
- Yu, L.; Gui, S.; Liu, Y.; Qiu, X.; Zhang, G.; Zhang, X.; Pan, J.; Fan, J.; Qi, S.; Qiu, B. Exosomes derived from microRNA-199a-overexpressing mesenchymal stem cells inhibit glioma progression by down-regulating AGAP2. Aging 2019, 11, 5300–5318. [Google Scholar] [CrossRef] [PubMed]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Lee, H.; Bae, K.; Baek, A.R.; Kwon, E.B.; Kim, Y.H.; Nam, S.W.; Lee, G.H.; Chang, Y. Glioblastoma-Derived Exosomes as Nanopharmaceutics for Improved Glioma Treatment. Pharmaceutics 2022, 14, 1002. [Google Scholar] [CrossRef]
- Zhang, C.; Song, J.; Lou, L.; Qi, X.; Zhao, L.; Fan, B.; Sun, G.; Lv, Z.; Fan, Z.; Jiao, B.; et al. Doxorubicin-loaded nanoparticle coated with endothelial cells-derived exosomes for immunogenic chemotherapy of glioblastoma. Bioeng. Transl. Med. 2021, 6, e10203. [Google Scholar] [CrossRef]
- Hao, W.; Sun, N.; Fan, Y.; Chen, M.; Liu, Q.; Yang, M.; Yang, Y.; Gao, C. Targeted Ferroptosis-Immunotherapy Synergy: Enhanced Antiglioma Efficacy with Hybrid Nanovesicles Comprising NK Cell-Derived Exosomes and RSL3-Loaded Liposomes. ACS Appl. Mater. Interfaces 2024, 16, 28193–28208. [Google Scholar] [CrossRef]
- Moholkar, D.N.; Kandimalla, R.; Gupta, R.C.; Aqil, F. Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes. Cancer Lett. 2023, 565, 216220. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Zhou, L.; Luo, Z.; Liu, G.; Hang, Z.; Li, H.; Li, F.; Wen, Y. Emerging Delivery Systems for Enabling Precision Nucleic Acid Therapeutics. ACS Nano 2025, 19, 4039–4083. [Google Scholar] [CrossRef] [PubMed]
- Suthar, R.; Sankhyan, N. Bacterial Infections of the Central Nervous System. Indian J. Pediatr. 2019, 86, 60–69. [Google Scholar] [CrossRef]
- Carod Artal, F.J. Clinical management of infectious cerebral vasculitides. Expert Rev. Neurother. 2016, 16, 205–221. [Google Scholar] [CrossRef]
- Banerjee, A.; Tripathi, A. Recent advances in understanding Japanese encephalitis. F1000Research 2019, 8, F1000 Faculty Rev-1915. [Google Scholar] [CrossRef]
- Aleyas, A.G.; Han, Y.W.; George, J.A.; Kim, B.; Kim, K.; Lee, C.K.; Eo, S.K. Multifront assault on antigen presentation by Japanese encephalitis virus subverts CD8+ T cell responses. J. Immunol. 2010, 185, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Aleyas, A.G.; Han, Y.W.; Patil, A.M.; Kim, S.B.; Kim, K.; Eo, S.K. Impaired cross-presentation of CD8α+ CD11c+ dendritic cells by Japanese encephalitis virus in a TLR2/MyD88 signal pathway-dependent manner. Eur. J. Immunol. 2012, 42, 2655–2666. [Google Scholar] [CrossRef]
- Adhya, D.; Dutta, K.; Kundu, K.; Basu, A. Histone deacetylase inhibition by Japanese encephalitis virus in monocyte/macrophages: A novel viral immune evasion strategy. Immunobiology 2013, 218, 1235–1247. [Google Scholar] [CrossRef]
- Manocha, G.D.; Mishra, R.; Sharma, N.; Kumawat, K.L.; Basu, A.; Singh, S.K. Regulatory role of TRIM21 in the type-I interferon pathway in Japanese encephalitis virus-infected human microglial cells. J. Neuroinflamm. 2014, 11, 24. [Google Scholar] [CrossRef]
- Patabendige, A.; Michael, B.D.; Craig, A.G.; Solomon, T. Brain microvascular endothelial-astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood-brain barrier model. Mol. Cell. Neurosci. 2018, 89, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Akbar, I.; Kumari, B.; Vrati, S.; Basu, A.; Banerjee, A. Japanese Encephalitis Virus-induced let-7a/b interacted with the NOTCH-TLR7 pathway in microglia and facilitated neuronal death via caspase activation. J. Neurochem. 2019, 149, 518–534. [Google Scholar] [CrossRef]
- Bian, P.; Ye, C.; Zheng, X.; Yang, J.; Ye, W.; Wang, Y.; Zhou, Y.; Ma, H.; Han, P.; Zhang, H.; et al. Mesenchymal stem cells alleviate Japanese encephalitis virus-induced neuroinflammation and mortality. Stem Cell Res. Ther. 2017, 8, 38. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick-Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kovalev, S.Y.; Mukhacheva, T.A. Reconsidering the classification of tick-borne encephalitis virus within the Siberian subtype gives new insights into its evolutionary history. Infect. Genet. Evol. 2017, 55, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 2018, 7, 74. [Google Scholar] [CrossRef]
- Süss, J.; Kahl, O.; Aspöck, H.; Hartelt, K.; Vaheri, A.; Oehme, R.; Hasle, G.; Dautel, H.; Kunz, C.; Kupreviciene, N.; et al. Tick-borne encephalitis in the age of general mobility. Wien. Med. Wochenschr. 2010, 160, 94–100. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-borne encephalitis virus—A review of an emerging zoonosis. J. Gen. Virol. 2009, 90, 1781–1794. [Google Scholar] [CrossRef]
- Růžek, D.; Salát, J.; Singh, S.K.; Kopecký, J. Breakdown of the blood-brain barrier during tick-borne encephalitis in mice is not dependent on CD8+ T-cells. PLoS ONE 2011, 6, e20472. [Google Scholar] [CrossRef]
- Palus, M.; Vancova, M.; Sirmarova, J.; Elsterova, J.; Perner, J.; Ruzek, D. Tick-borne encephalitis virus infects human brain microvascular endothelial cells without compromising blood-brain barrier integrity. Virology 2017, 507, 110–122. [Google Scholar] [CrossRef]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Ritzel, R.M.; Harris, N.M.; Lee, J.; Kim, T.; Pandi, G.; Vemuganti, R.; McCullough, L.D. Inhibition of miR-141-3p Ameliorates the Negative Effects of Poststroke Social Isolation in Aged Mice. Stroke 2018, 49, 1701–1707. [Google Scholar] [CrossRef]
- Gayen, M.; Bhomia, M.; Balakathiresan, N.; Knollmann-Ritschel, B. Exosomal MicroRNAs Released by Activated Astrocytes as Potential Neuroinflammatory Biomarkers. Int. J. Mol. Sci. 2020, 21, 2312. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Wang, H.; Dong, J.; Wu, Y.; Zhang, H.; Fu, L.; Zhang, J. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate neuroinflammation and oxidative stress through the NRF2/NF-κB/NLRP3 pathway. CNS Neurosci. Ther. 2024, 30, e14454. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Liu, Y.; Zhang, X.; Wang, Q.; Liu, Y.; Ma, X.; Long, X.; Ruan, Y.; Lei, H.; et al. Astrocyte-derived exosomal lncRNA 4933431K23Rik modulates microglial phenotype and improves post-traumatic recovery via SMAD7 regulation. Mol. Ther. 2023, 31, 1313–1331. [Google Scholar] [CrossRef]
- Khalyfa, A.; Sanz-Rubio, D. Genetics and Extracellular Vesicles of Pediatrics Sleep Disordered Breathing and Epilepsy. Int. J. Mol. Sci. 2019, 20, 5483. [Google Scholar] [CrossRef]
- Hastuti, S.; Idroes, R.; Imran, I.; Ramli, Y.; Abas, A.H.; Tallei, T.E. hUMSC vs. hUMSC-Exosome: Which One Is Better for Epilepsy? Pharmaceuticals 2022, 15, 1247. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, L.; Zhang, Z.; Zhang, K.; Chu, M.; Liu, Y.; Mao, X.; Wu, D.; Xu, D.; et al. Anti-ferroptosis exosomes engineered for targeting M2 microglia to improve neurological function in ischemic stroke. J. Nanobiotechnol. 2024, 22, 291. [Google Scholar] [CrossRef] [PubMed]
- Jahangard, Y.; Monfared, H.; Moradi, A.; Zare, M.; Mirnajafi-Zadeh, J.; Mowla, S.J. Therapeutic Effects of Transplanted Exosomes Containing miR-29b to a Rat Model of Alzheimer’s Disease. Front. Neurosci. 2020, 14, 564. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04928534 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06319742 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05035134 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05370105 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT01860118 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05326724 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03384433 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06138210 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06598202 (accessed on 8 February 2025).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT04388982 (accessed on 8 February 2025).
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical applications of stem cell-derived exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer 2022, 21, 179. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, H.; Cho, H.; Choi, J.; Hwang, K.Y.; Choi, Y.; Kim, S.H.; Yang, Y. Recent Advances in Exosome-Based Drug Delivery for Cancer Therapy. Cancers 2021, 13, 4435. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Yadav, A.; Xuan, Y.; Sen, C.K.; Ghatak, S. Standardized reporting of research on exosomes to ensure rigor and reproducibility. Adv. Wound Care 2024, 13, 584–599. [Google Scholar] [CrossRef]
- You, Q.; Liang, F.; Wu, G.; Cao, F.; Liu, J.; He, Z.; Wang, C.; Zhu, L.; Chen, X.; Yang, Y. The landscape of biomimetic nanovesicles in brain diseases. Adv. Mater. 2024, 36, 2306583. [Google Scholar] [CrossRef]
- Wang, C.K.; Tsai, T.H.; Lee, C.H. Regulation of exosomes as biologic medicines: Regulatory challenges faced in exosome development and manufacturing processes. Clin. Transl. Sci. 2024, 17, e13904. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Yu, L.; Ran, M.; Zhong, X.; Sun, M.; Xu, M.; Wang, Y.; Yan, X.; Lee, R.J.; Tang, Y.; et al. Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders. Int. J. Mol. Sci. 2025, 26, 2491. https://doi.org/10.3390/ijms26062491
Bai L, Yu L, Ran M, Zhong X, Sun M, Xu M, Wang Y, Yan X, Lee RJ, Tang Y, et al. Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders. International Journal of Molecular Sciences. 2025; 26(6):2491. https://doi.org/10.3390/ijms26062491
Chicago/Turabian StyleBai, Lu, Leijie Yu, Mengqiong Ran, Xing Zhong, Meng Sun, Minhao Xu, Yu Wang, Xinlei Yan, Robert J. Lee, Yaqin Tang, and et al. 2025. "Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders" International Journal of Molecular Sciences 26, no. 6: 2491. https://doi.org/10.3390/ijms26062491
APA StyleBai, L., Yu, L., Ran, M., Zhong, X., Sun, M., Xu, M., Wang, Y., Yan, X., Lee, R. J., Tang, Y., & Xie, J. (2025). Harnessing the Potential of Exosomes in Therapeutic Interventions for Brain Disorders. International Journal of Molecular Sciences, 26(6), 2491. https://doi.org/10.3390/ijms26062491






