Benefits of Camelina sativa Supplementation in Morphine Treatment: Enhanced Analgesia, Delayed Tolerance and Reduced Gut Side Effects Through PPAR-α Receptor Engagement
Abstract
1. Introduction
2. Results
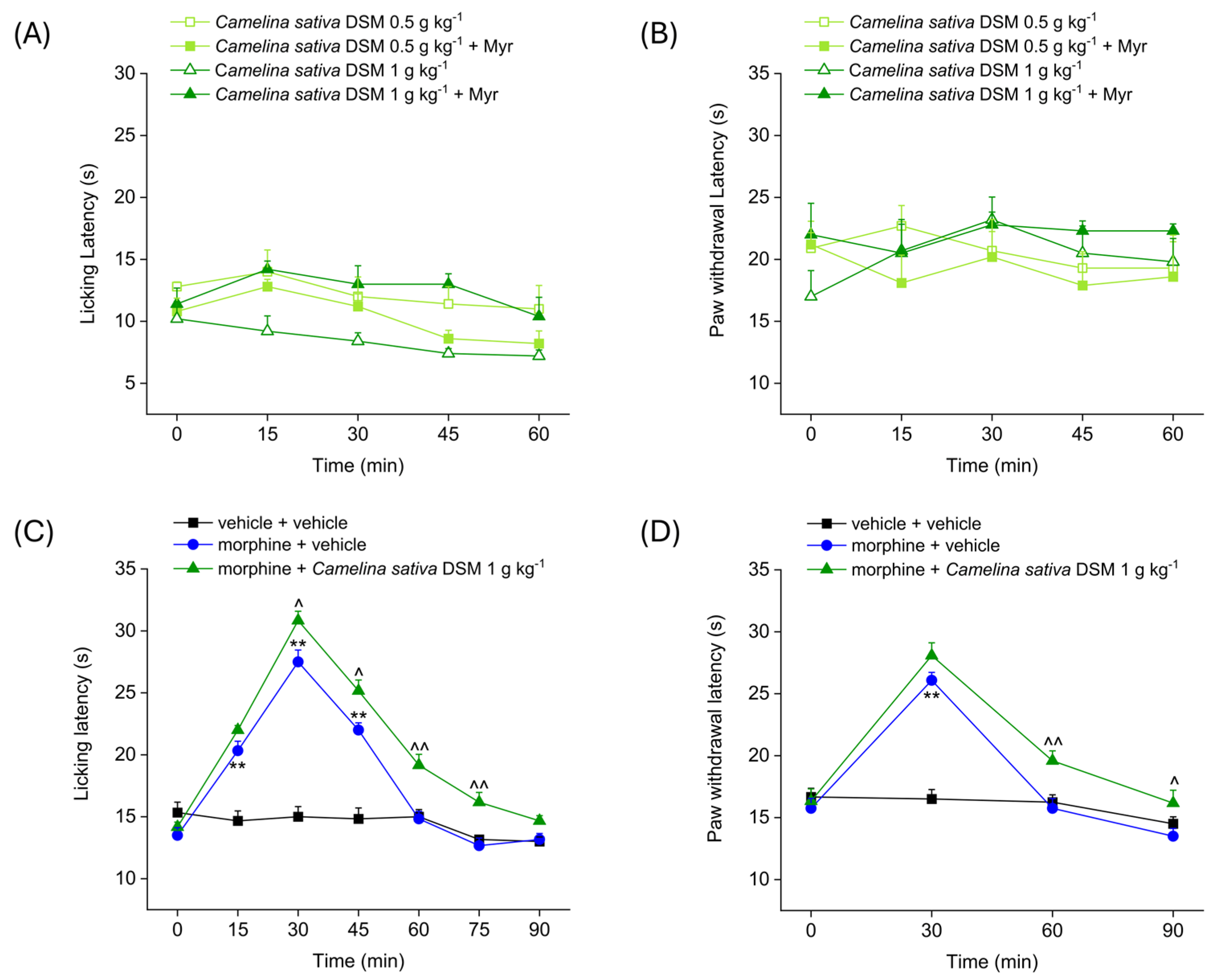
2.1. Evaluation of the Effect of Camelina sativa DSM Acute Administration on Mice’ Pain Threshold and Morphine-Induced Antinociception
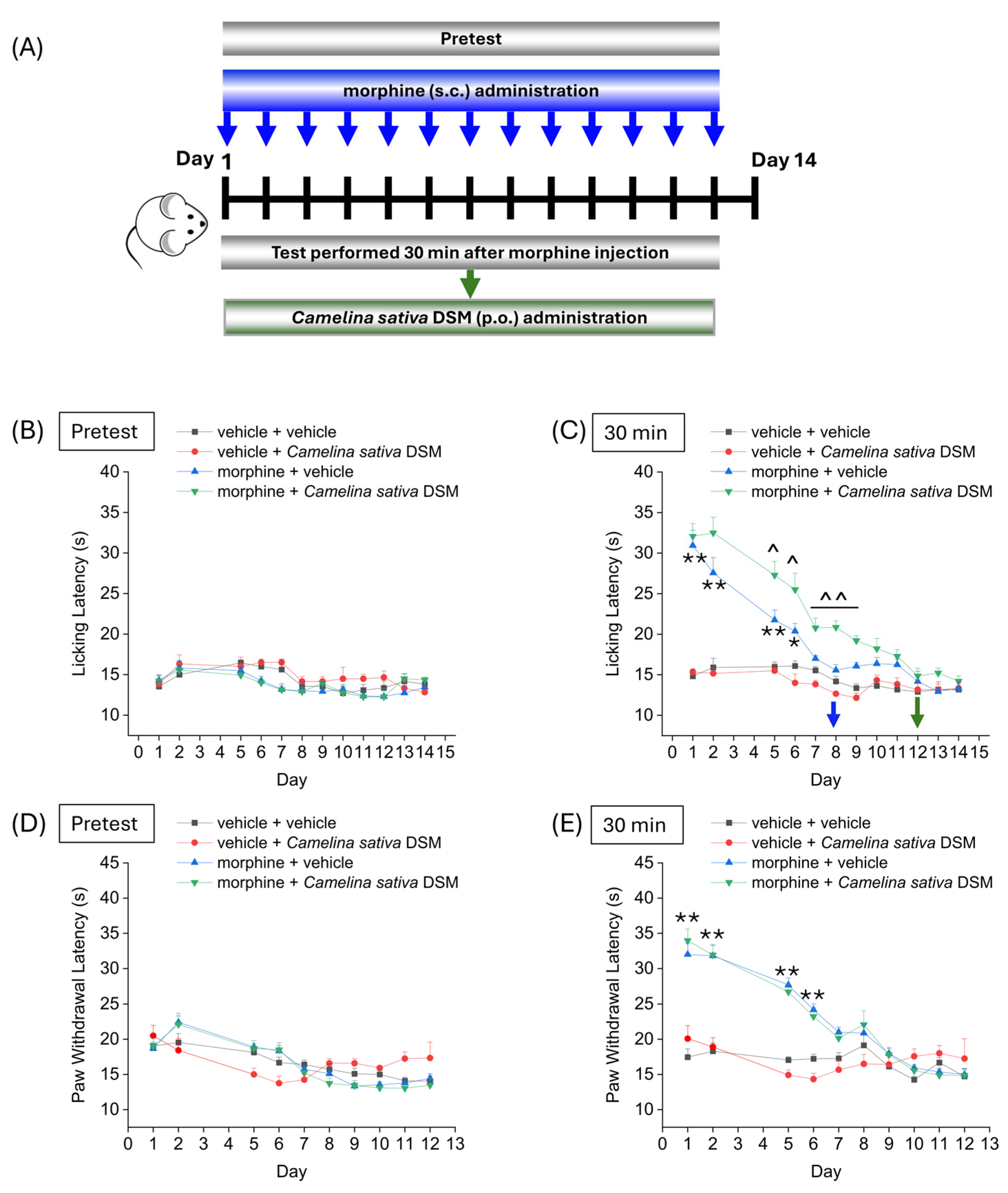
2.2. Evaluation of the Effect of Camelina sativa DSM Supplementation on the Development of Tolerance to Analgesic Effect of Morphine
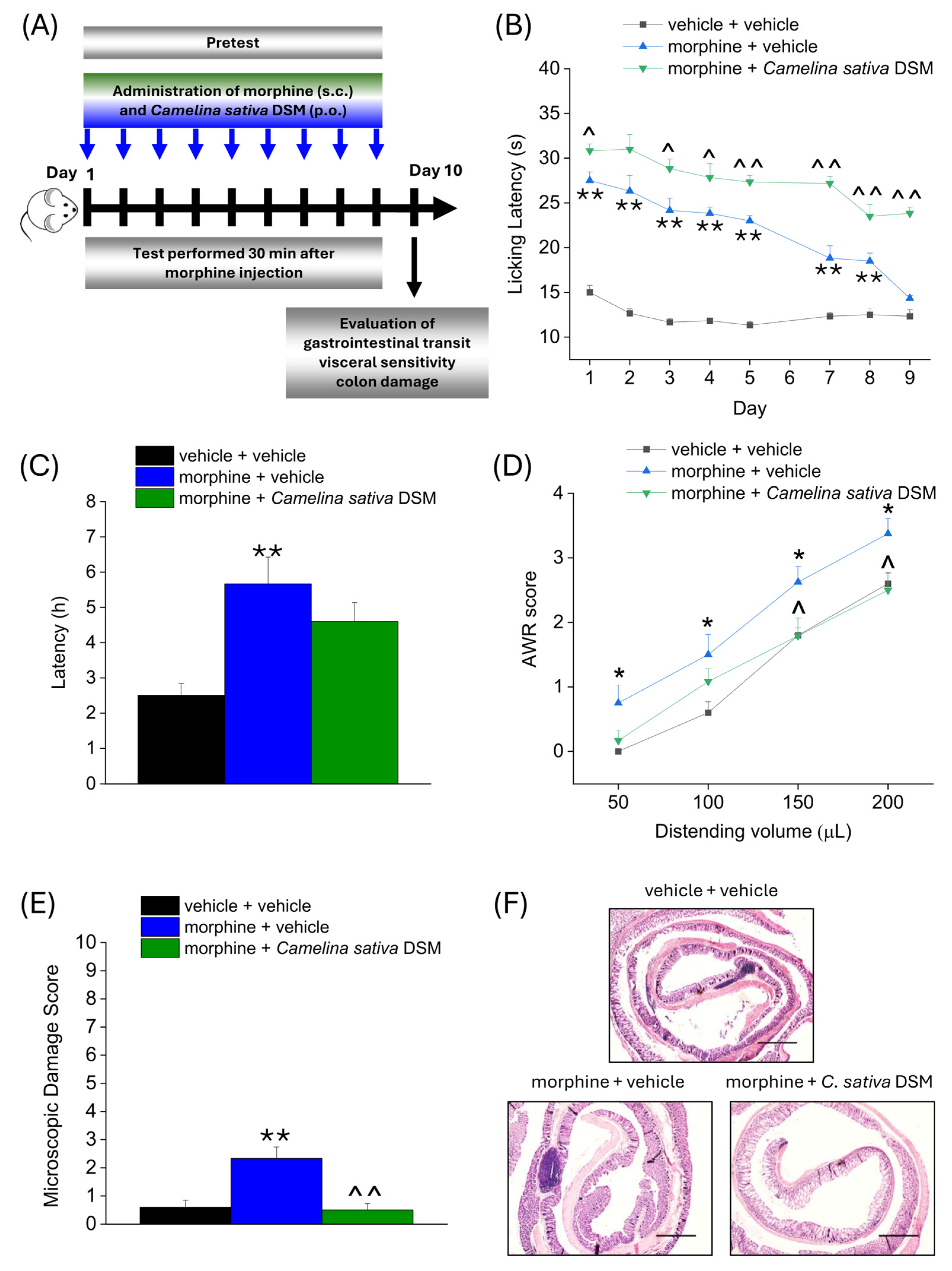
2.3. Effect of Camelina sativa DSM and Morphine Co-Administration on the Development of Opioid-Related Analgesic Tolerance and Side Effects
2.4. Involvement of PPAR-α in Enhancing Antinociception and Reducing Gastrointestinal Side Effects of Morphine Related to Camelina sativa DSM Supplementation
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Treatments
4.3. Hot Plate Test
4.4. Paw Pressure Test
4.5. Assessment of Intestinal Transit Time
4.6. Assessment of Visceral Sensitivity by Abdominal Withdrawal Reflex
4.7. Histological Analysis of Colon
4.8. Withdrawal Paradigms
4.9. Acute Itch
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, J. The WHO guidelines: The new and the old. Curr. Opin. Support. Palliat. Care 2024, 18, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Morlion, B.; Vowles, K.E.; Bannister, K.; Buchser, E.; Casale, R.; Chenot, J.-F.; Chumbley, G.; Drewes, A.M.; Dom, G.; et al. European clinical practice recommendations on opioids for chronic noncancer pain—Part 1: Role of opioids in the management of chronic noncancer pain. Eur. J. Pain 2021, 25, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.K.; Smith, C.M.; Rahmatullah, M.; Nissapatorn, V.; Wilairatana, P.; Spetea, M.; Gueven, N.; Dietis, N. Opioid Analgesia and Opioid-Induced Adverse Effects: A Review. Pharmaceuticals 2021, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.M.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Squeo, F.; Celiberto, F.; Ierardi, E.; Russo, F.; Riezzo, G.; D’attoma, B.; Di Leo, A.; Losurdo, G. Opioid-induced Constipation: Old and New Concepts in Diagnosis and Treatment. J. Neurogastroenterol. Motil. 2024, 30, 131–142. [Google Scholar] [CrossRef]
- De Giorgio, R.; Zucco, F.M.; Chiarioni, G.; Mercadante, S.; Corazziari, E.S.; Caraceni, A.; Odetti, P.; Giusti, R.; Marinangeli, F.; Pinto, C. Management of Opioid-Induced Constipation and Bowel Dysfunction: Expert Opinion of an Italian Multidisciplinary Panel. Adv. Ther. 2021, 38, 3589–3621. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Rosenblatt, M.H. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef]
- Shipton, E.A.; Shipton, E.E.; Shipton, A.J. A review of the opioid epidemic: What do we do about it? Pain Ther. 2018, 7, 23–36. [Google Scholar] [CrossRef]
- Strang, J.; Volkow, N.D.; Degenhardt, L.; Hickman, M.; Johnson, K.; Koob, G.F.; Marshall, B.D.; Tyndall, M.; Walsh, S.L. Opioid use disorder. Nat. Rev. Dis. Primers 2020, 6, 3. [Google Scholar] [CrossRef]
- Griffin, M.L.; Bennett, H.E.; Fitzmaurice, G.M.; Hill, K.P.; Provost, S.E.; Weiss, R.D. Health-related quality of life among prescription opioid-dependent patients: Results from a multi-site study. Am. J. Addict. 2015, 24, 308–314. [Google Scholar] [CrossRef]
- Chou, R.; Turner, J.A.; Devine, E.B.; Hansen, R.N.; Sullivan, S.D.; Blazina, I.; Dana, T.; Bougatsos, C.; Deyo, R.A. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann. Intern. Med. 2015, 162, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Vowles, K.E.; McEntee, M.L.; Julnes, P.S.; Frohe, T.; Ney, J.P.; van der Goes, D.N. Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 2015, 156, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Treillet, E.; Laurent, S.; Hadjiat, Y. Practical management of opioid rotation and equianalgesia. J. Pain Res. 2018, 11, 2587–2601. [Google Scholar] [CrossRef] [PubMed]
- Veldman, S.; van Beek, M.; van Rijswijk, S.; Ellerbroek, H.; Timmerman, H.; van der Wal, S.; Steegers, M.; Schellekens, A. Effects of opioid rotation to buprenorphine/naloxone on pain, pain thresholds, pain tolerance, and quality of life in patients with chronic pain and opioid use disorder. Pain 2022, 163, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Mehan, A. Addressing opioid tolerance and opioid-induced hypersensitivity: Recent developments and future therapeutic strategies. Pharmacol. Res. Perspect. 2021, 9, e00789. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Di Cesare Mannelli, L.; Ghelardini, C. Naturally occurring glucosinolates and isothiocyanates as a weapon against chronic pain: Potentials and limits. Phytochem. Rev. 2022, 21, 647–665. [Google Scholar] [CrossRef]
- Cojocariu, R.O.; Balmus, I.M.; Lefter, R.; Hritcu, L.; Ababei, D.C.; Ciobica, A.; Copaci, S.; Mot, S.E.L.; Copolovici, L.; Copolovici, D.M.; et al. Camelina sativa Methanolic and Ethanolic Extract Potential in Alleviating Oxidative Stress, Memory Deficits, and Affective Impairments in Stress Exposure-Based Irritable Bowel Syndrome Mouse Models. Oxidative Med. Cell. Longev. 2020, 2020, 9510305. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Toti, A.; Ferrara, V.; Ciampi, C.; Margiotta, F.; Martelli, A.; Testai, L.; Calderone, V.; et al. The Efficacy of Camelina sativa Defatted Seed Meal against Colitis-Induced Persistent Visceral Hypersensitivity: The Relevance of PPAR α Receptor Activation in Pain Relief. Nutrients 2022, 14, 3137. [Google Scholar] [CrossRef]
- Nobili, S.; Micheli, L.; Lucarini, E.; Toti, A.; Ghelardini, C.; Di Cesare Mannelli, L. Ultramicronized N-palmitoylethanolamine associated with analgesics: Effects against persistent pain. Pharmacol. Ther. 2024, 258, 108649. [Google Scholar] [CrossRef]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef]
- Chambers, C.T.; Dol, J.; Tutelman, P.R.; Langley, C.L.; Parker, J.A.; Cormier, B.T.; Macfarlane, G.J.; Jones, G.T.; Chapman, D.; Proudfoot, N.; et al. The prevalence of chronic pain in children and adolescents: A systematic review update and meta-analysis. Pain 2024, 165, 2215–2234. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; López, C.J.; Simal-Gandara, J. Glucosinolates: Molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv. Food Nutr. Res. 2019, 90, 305–350. [Google Scholar]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional regulation of human and rat hepatic lipid metabolism by the grapefruit flavonoid naringenin: Role of PPARα, PPARγ and LXRα. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, N.; Woodard, K.; Ramaraju, R.; Greenway, F.L.; Coulter, A.A.; Rebello, C.J. Naringenin increases insulin sensitivity and metabolic rate: A case study. J. Med. Food 2020, 23, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Qiu, H.; Huang, J.; Ding, S.; Huang, B.; Zhou, P.; Jiang, Q. EETs/PPARs activation together mediates the preventive effect of naringenin in high glucose-induced cardiomyocyte hypertrophy. Biomed. Pharmacother. 2019, 109, 1498–1505. [Google Scholar] [CrossRef]
- Sampath, H.; Ntambi, J.M. Polyunsaturated fatty acid regulation of gene expression. Nutr. Rev. 2004, 62, 333–339. [Google Scholar] [CrossRef]
- Zhao, W.; Iskandar, S.; Kooshki, M.; Sharpe, J.G.; Payne, V.; Robbins, M.E. Knocking out peroxisome proliferator-activated receptor (PPAR) α inhibits radiation-induced apoptosis in the mouse kidney through activation of NF-κB and increased expression of IAPs. Radiat. Res. 2007, 167, 581–591. [Google Scholar] [CrossRef]
- Zhou, G.; Fu, X.; Wang, L.; Cao, Y.; Zhuang, J.; Hu, J.; Li, Y.; Xu, C.; Gao, S.; Shao, A. Palmitoylethanolamide ameliorates neuroinflammation via modulating PPAR-α to promote the functional outcome after intracerebral hemorrhage. Neurosci. Lett. 2022, 781, 136648. [Google Scholar] [CrossRef]
- Ruiz-Medina, J.; Flores, J.A.; Tasset, I.; Tunez, I.; Valverde, O.; Fernandez-Espejo, E. Alteration of neuropathic and visceral pain in female C57BL/6J mice lacking the PPAR-α gene. Psychopharmacology 2012, 222, 477–488. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C. Anti-nociceptive and anti-inflammatory actions of sulforaphane in chronic constriction injury-induced neuropathic pain mice. Inflammopharmacology 2017, 25, 99–106. [Google Scholar] [CrossRef]
- Ferreira-Chamorro, P.; Redondo, A.; Riego, G.; Leánez, S.; Pol, O. Sulforaphane inhibited the nociceptive responses, anxiety-and depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Redondo, A.; Chamorro, P.A.F.; Riego, G.; Leánez, S.; Pol, O. Treatment with sulforaphane produces antinociception and improves morphine effects during inflammatory pain in mice. J. Pharmacol. Exp. Ther. 2017, 363, 293–302. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Pagnotta, E.; Matteo, R.; Parisio, C.; Toti, A.; Ferrara, V.; Ciampi, C.; Martelli, A.; Testai, L.; et al. Beneficial Effects of Eruca sativa Defatted Seed Meal on Visceral Pain and Intestinal Damage Resulting from Colitis in Rats. Foods 2022, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Güleç, G. Role of K (v) 7 potassium channels in the morphine-induced antinociception in acute and neuropathic pain. J. Neurol. Sci. 2011, 28, 440–452. [Google Scholar]
- Borghi, S.M.; Carvalho, T.T.; Staurengo-Ferrari, L.; Hohmann, M.S.; Pinge-Filho, P.; Casagrande, R.; Verri, W.A., Jr. Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013, 76, 1141–1149. [Google Scholar] [CrossRef]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and analgesic effects of naringin through selective inhibition of transient receptor potential vanilloid member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef]
- Özkay, Ü.D.; Can, Ö.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol. Biochem. Behav. 2013, 109, 23–30. [Google Scholar] [CrossRef]
- Zhu, Q.; Mao, L.-N.; Liu, C.-P.; Sun, Y.-H.; Jiang, B.; Zhang, W.; Li, J.-X. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci. Rep. 2016, 6, 19266. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, R.; Jin, Y.; Fang, J.; Du, J.; Shao, X.; Liang, Y.; Fang, J. Molecular mechanisms of opioid tolerance: From opioid receptors to inflammatory mediators. Exp. Ther. Med. 2021, 22, 1004. [Google Scholar] [CrossRef]
- Gledhill, L.J.; Babey, A.-M. Synthesis of the mechanisms of opioid tolerance: Do we still say NO? Cell. Mol. Neurobiol. 2021, 41, 927–948. [Google Scholar] [CrossRef]
- Mondor, M.; Hernández-Álvarez, A.J. Camelina sativa composition, attributes, and applications: A review. Eur. J. Lipid Sci. Technol. 2022, 124, 2100035. [Google Scholar] [CrossRef]
- Hartwig, S.; Burron, S.; Richards, T.; Rankovic, A.; Ma, D.W.; Pearson, W.; Ellis, J.; Trevizan, L.; Seymour, D.J.; Shoveller, A.K. The effect of dietary camelina, flaxseed, and canola oil supplementation on skin fatty acid profile and immune and inflammatory responses in healthy adult horses. J. Anim. Sci. 2025. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Rajpurohit, B.; Singha, P. Camelina (Camelina sativa) seed. In Oilseeds: Health Attributes and Food Applications; Springer: Singapore, 2021; pp. 455–471. [Google Scholar]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane Inhibits Lipopolysaccharide-Induced Inflammation, Cytotoxicity, Oxidative Stress, and miR-155 Expression and Switches to Mox Phenotype through Activating Extracellular Signal-Regulated Kinase 1/2-Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element Pathway in Murine Microglial Cells. Front. Immunol. 2018, 9, 36. [Google Scholar] [CrossRef]
- Qin, S.; Yang, C.; Huang, W.; Du, S.; Mai, H.; Xiao, J.; Lü, T. Sulforaphane attenuates microglia-mediated neuronal necroptosis through down-regulation of MAPK/NF-κB signaling pathways in LPS-activated BV-2 microglia. Pharmacol. Res. 2018, 133, 218–235. [Google Scholar] [CrossRef]
- Alifarsangi, A.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M. The citrus flavanone naringenin prevents the development of morphine analgesic tolerance and conditioned place preference in male rats. Am. J. Drug Alcohol. Abus. 2021, 47, 43–51. [Google Scholar] [CrossRef]
- Lucarini, E.; Seguella, L.; Vincenzi, M.; Parisio, C.; Micheli, L.; Toti, A.; Corpetti, C.; Del Re, A.; Squillace, S.; Maftei, D.; et al. Role of Enteric Glia as Bridging Element between Gut Inflammation and Visceral Pain Consolidation during Acute Colitis in Rats. Biomedicines 2021, 9, 1671. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of in vitro metabolism of a broccoli leachate, glucosinolates and S-methylcysteine sulphoxide on the human faecal microbiome. Eur. J. Nutr. 2021, 60, 2141–2154. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral sensitivity modulation by faecal microbiota transplantation: The active role of gut bacteria in pain persistence. Pain 2022, 163, 861–877. [Google Scholar] [CrossRef]
- Zanetti, F.; Alberghini, B.; Marjanović Jeromela, A.; Grahovac, N.; Rajković, D.; Kiprovski, B.; Monti, A. Camelina, an ancient oilseed crop actively contributing to the rural renaissance in Europe. A review. Agron. Sustain. Dev. 2021, 41, 2. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Taboun, Z.S.; Sadeghi, J. The bidirectional relationship between opioids and the gut microbiome: Implications for opioid tolerance and clinical interventions. Int. Immunopharmacol. 2023, 125, 111142. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Tang, Y.; Fu, Y.; Hegde, S.; Shi, D.W.; Huang, L.-Y.M.; Shi, X.-Z. An opioid receptor-independent mechanism underlies motility dysfunction and visceral hyperalgesia in opioid-induced bowel dysfunction. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G1093–G1104. [Google Scholar] [CrossRef]
- Barker, P.A.; Mantyh, P.; Arendt-Nielsen, L.; Viktrup, L.; Tive, L. Nerve Growth Factor Signaling and Its Contribution to Pain. J. Pain Res. 2020, 13, 1223–1241. [Google Scholar] [CrossRef]
- McGrath, J.C.; Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. Br. J. Pharmacol. 2015, 172, 3189–3193. [Google Scholar] [CrossRef]
- Lucarini, E.; Pagnotta, E.; Micheli, L.; Parisio, C.; Testai, L.; Martelli, A.; Calderone, V.; Matteo, R.; Lazzeri, L.; Di Cesare Mannelli, L.; et al. Eruca sativa Meal against Diabetic Neuropathic Pain: An H2S-Mediated Effect of Glucoerucin. Molecules 2019, 24, 3006. [Google Scholar] [CrossRef]
- Pessina, A.; Thomas, R.M.; Palmieri, S.; Luisi, P.L. An improved method for the purification of myrosinase and its physicochemical characterization. Arch. Biochem. Biophys. 1990, 280, 383–389. [Google Scholar] [CrossRef]
- Micheli, L.; Vasarri, M.; Barletta, E.; Lucarini, E.; Ghelardini, C.; Degl’Innocenti, D.; Di Cesare Mannelli, L. Efficacy of Posidonia oceanica Extract against Inflammatory Pain: In Vivo Studies in Mice. Mar. Drugs 2021, 19, 48. [Google Scholar] [CrossRef]
- Russo, R.; D’Agostino, G.; Mattace Raso, G.; Avagliano, C.; Cristiano, C.; Meli, R.; Calignano, A. Central administration of oxytocin reduces hyperalgesia in mice: Implication for cannabinoid and opioid systems. Peptides 2012, 38, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Koester, S.T.; Li, N.; Lachance, D.M.; Dey, N. Marker-based assays for studying gut transit in gnotobiotic and conventional mouse models. STAR Protoc. 2021, 2, 100938. [Google Scholar] [CrossRef] [PubMed]
- Delprete, C.; Rimondini Giorgini, R.; Lucarini, E.; Bastiaanssen, T.F.S.; Scicchitano, D.; Interino, N.; Formaggio, F.; Uhlig, F.; Ghelardini, C.; Hyland, N.P.; et al. Disruption of the microbiota-gut-brain axis is a defining characteristic of the α-Gal A (-/0) mouse model of Fabry disease. Gut Microbes 2023, 15, 2256045. [Google Scholar] [CrossRef]
- Lucarini, E.; Parisio, C.; Branca, J.J.V.; Segnani, C.; Ippolito, C.; Pellegrini, C.; Antonioli, L.; Fornai, M.; Micheli, L.; Pacini, A.; et al. Deepening the Mechanisms of Visceral Pain Persistence: An Evaluation of the Gut-Spinal Cord Relationship. Cells 2020, 9, 1772. [Google Scholar] [CrossRef]
- Uddin, O.; Jenne, C.; Fox, M.E.; Arakawa, K.; Keller, A.; Cramer, N. Divergent profiles of fentanyl withdrawal and associated pain in mice and rats. Pharmacol. Biochem. Behav. 2021, 200, 173077. [Google Scholar] [CrossRef]
- Angeli, A.; Micheli, L.; Turnaturi, R.; Pasquinucci, L.; Parenti, C.; Alterio, V.; Di Fiore, A.; De Simone, G.; Monti, S.M.; Carta, F. Discovery of a novel series of potent carbonic anhydrase inhibitors with selective affinity for μ Opioid receptor for Safer and long-lasting analgesia. Eur. J. Med. Chem. 2023, 260, 115783. [Google Scholar] [CrossRef]
- Jing, P.-B.; Cao, D.-L.; Li, S.-S.; Zhu, M.; Bai, X.-Q.; Wu, X.-B.; Gao, Y.-J. Chemokine receptor CXCR3 in the spinal cord contributes to chronic itch in mice. Neurosci. Bull. 2018, 34, 54–63. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, E.; Pagnotta, E.; Micheli, L.; Trisolini, S.; Matteo, R.; Righetti, L.; Martelli, A.; Testai, L.; Calderone, V.; Di Cesare Mannelli, L.; et al. Benefits of Camelina sativa Supplementation in Morphine Treatment: Enhanced Analgesia, Delayed Tolerance and Reduced Gut Side Effects Through PPAR-α Receptor Engagement. Int. J. Mol. Sci. 2025, 26, 2519. https://doi.org/10.3390/ijms26062519
Lucarini E, Pagnotta E, Micheli L, Trisolini S, Matteo R, Righetti L, Martelli A, Testai L, Calderone V, Di Cesare Mannelli L, et al. Benefits of Camelina sativa Supplementation in Morphine Treatment: Enhanced Analgesia, Delayed Tolerance and Reduced Gut Side Effects Through PPAR-α Receptor Engagement. International Journal of Molecular Sciences. 2025; 26(6):2519. https://doi.org/10.3390/ijms26062519
Chicago/Turabian StyleLucarini, Elena, Eleonora Pagnotta, Laura Micheli, Samuele Trisolini, Roberto Matteo, Laura Righetti, Alma Martelli, Lara Testai, Vincenzo Calderone, Lorenzo Di Cesare Mannelli, and et al. 2025. "Benefits of Camelina sativa Supplementation in Morphine Treatment: Enhanced Analgesia, Delayed Tolerance and Reduced Gut Side Effects Through PPAR-α Receptor Engagement" International Journal of Molecular Sciences 26, no. 6: 2519. https://doi.org/10.3390/ijms26062519
APA StyleLucarini, E., Pagnotta, E., Micheli, L., Trisolini, S., Matteo, R., Righetti, L., Martelli, A., Testai, L., Calderone, V., Di Cesare Mannelli, L., & Ghelardini, C. (2025). Benefits of Camelina sativa Supplementation in Morphine Treatment: Enhanced Analgesia, Delayed Tolerance and Reduced Gut Side Effects Through PPAR-α Receptor Engagement. International Journal of Molecular Sciences, 26(6), 2519. https://doi.org/10.3390/ijms26062519









