Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19
Abstract
:1. Introduction
2. Vitamin D: A Natural Compound with Pleiotropic Actions in the Human Body
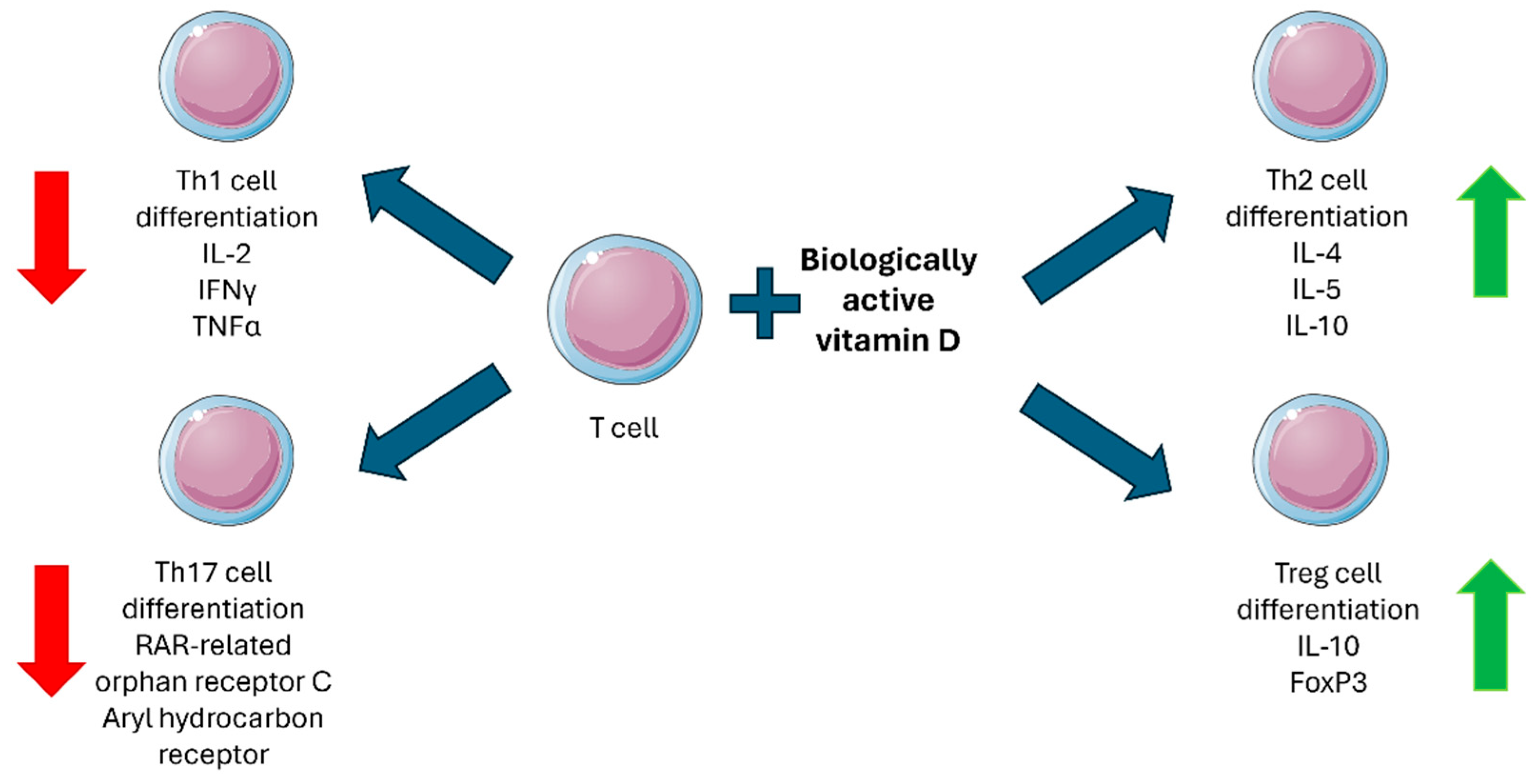
3. Vitamin D and Immune Function
4. Vitamin D and Respiratory System Protection
5. Vitamin D and SARS-CoV-2 Infection
6. Vitamin D and Anti-SARS-CoV-2 Vaccination Response
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, J.; Wang, R.; Hozumi, Y.; Liu, G.; Qiu, Y.; Wei, X.; Wei, G.W. Emerging dominant SARS-CoV-2 variants. J. Chem. Inf. Model. 2023, 63, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Abdel-Ghany, S.; Abdallah, M.S.; Abul-Maaty, O.; Khoder, A.I.; Shoman, N.A.; Farrag, M.S.; Martasek, P.; Noreddin, A.M.; Nazih, M. Vitamin D: A key player in COVID-19 immunity and lessons from the pandemic to combat immune-evasive variants. Inflammopharmacology 2024, 32, 3631–3652. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.H.; Lee, K.S. Current and emerging knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Rizzi, M.; D’Onghia, D.; Tonello, S.; Minisini, R.; Colangelo, D.; Bellan, M.; Castello, L.M.; Gavelli, F.; Avanzi, G.C.; Pirisi, M.; et al. COVID-19 biomarkers at the crossroad between patient stratification and targeted therapy: The role of validated and proposed parameters. Int. J. Mol. Sci. 2023, 24, 7099. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Rizzi, M.; Patrucco, F.; Trevisan, M.; Faolotto, G.; Mercandino, A.; Strola, C.; Ravanini, P.; Costanzo, M.; Tonello, S.; Matino, E.; et al. Baseline plasma SARS-CoV-2 RNA detection predicts an adverse COVID-19 evolution in moderate to severe hospitalized patients. Panminerva Med. 2022, 64, 465–471. [Google Scholar] [CrossRef]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2023, 42, 393–414. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Brinno, C.; Zecca, E.; Matino, E.; Cittone, M.; Rizzi, E.; Casciaro, G.F.; D’Onghia, D.; Colangelo, D.; et al. SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: Results of the extended follow up of the RIVALSA prospective cohort. Front. Immunol. 2023, 14, 1185278. [Google Scholar] [CrossRef]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.E.; Howlader, M.; Akter, F.; Hossain, M.M. Preclinical and clinical investigations of potential drugs and vaccines for COVID-19 therapy: A comprehensive review with recent update. Clin. Pathol. 2024, 17, 2632010X241263054. [Google Scholar] [CrossRef]
- Atluri, K.; Aimlin, I.; Arora, S. Current effective therapeutics in management of COVID-19. J. Clin. Med. 2022, 11, 3838. [Google Scholar] [CrossRef]
- Zaman, R.; Ravichandran, V.; Tan, C.K. Role of dietary supplements in the continuous battle against COVID-19. Phytother. Res. 2024, 38, 1071–1088. [Google Scholar] [CrossRef]
- Arora, I.; White, S.; Mathews, R. Global dietary and herbal supplement use during COVID-19-A scoping review. Nutrients 2023, 15, 771. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Fakhrany, O.M.; Elekhnawy, E.; Al-Gareeb, A.I.; Alorabi, M.; De Waard, M.; Albogami, S.M.; Batiha, G.E. Traditional herbs against COVID-19: Back to old weapons to combat the new pandemic. Eur. J. Med. Res. 2022, 27, 186. [Google Scholar] [CrossRef]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does evidence exist to blunt inflammatory response by nutraceutical supplementation during COVID-19 pandemic? An overview of systematic reviews of vitamin D, vitamin C, melatonin, and zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal remedies, nutraceuticals, and dietary supplements for COVID-19 management: An update. Clin. Complement. Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Pavlidou, E.; Poulios, E.; Papadopoulou, S.K.; Fasoulas, A.; Dakanalis, A.; Giaginis, C. Clinical evidence on the potential beneficial effects of diet and dietary supplements against COVID-19 infection risk and symptoms’ severity. Med. Sci. 2024, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Burr, A.H.P.; Bhattacharjee, A.; Hand, T.W. Nutritional modulation of the microbiome and immune response. J. Immunol. 2020, 205, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition-a systematic review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vera, D.; Salazar, J.R.; Soriano-Ursúa, M.A.; Guzmán-Pérez, J.; Vergara-Castañeda, A.; Muñoz-Durán, H.; Ramírez-Velez, G.L.; Vivar-Sierra, A.; Naranjo-Navarro, C.R.; Meza-Meneses, P.A.; et al. Effectiveness of omega-3 fatty acid supplementation in improving the metabolic and inflammatory profiles of Mexican adults hospitalized with COVID-19. Diseases 2024, 12, 28. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The influence of nutritional factors on immunological outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
- Manzoni, P.; Messina, A.; Germano, C.; Picone, S.; Masturzo, B.; Sainaghi, P.P.; Sola, D.; Rizzi, M. Lactoferrin supplementation in preventing and protecting from SARS-CoV-2 infection: Is there any role in general and special populations? an updated review of literature. Int. J. Mol. Sci. 2024, 25, 10248. [Google Scholar] [CrossRef]
- Polak, E.; Stępień, A.E.; Gol, O.; Tabarkiewicz, J. Potential immunomodulatory effects from consumption of nutrients in whole foods and supplements on the frequency and course of infection: Preliminary results. Nutrients 2021, 13, 1157. [Google Scholar] [CrossRef]
- Scarpellini, E.; Rinninella, E.; Basilico, M.; Colomier, E.; Rasetti, C.; Larussa, T.; Santori, P.; Abenavoli, L. From pre- and probiotics to post-biotics: A narrative review. Int. J. Environ. Res. Public Health 2021, 19, 37. [Google Scholar] [CrossRef]
- Rizzi, M.; Avellis, V.; Messina, A.; Germano, C.; Tavella, E.; Dodaro, V.; Vitale, R.; Revelli, A.; Zola, P.; Picone, S.; et al. Vitamin D supplementation in neonatal and infant MIS-C following COVID-19 infection. Int. J. Mol. Sci. 2024, 25, 3712. [Google Scholar] [CrossRef]
- Matino, E.; Tavella, E.; Rizzi, M.; Avanzi, G.C.; Azzolina, D.; Battaglia, A.; Becco, P.; Bellan, M.; Bertinieri, G.; Bertoletti, M.; et al. Effect of lactoferrin on clinical outcomes of hospitalized patients with COVID-19: The LAC randomized clinical trial. Nutrients 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study. Medicina 2021, 57, 842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bhushan, D.; Supriya, S.; Ganapule, A.A.; Lohani, P.; Shyama Pandey, S.; Majhi, P.K.; Anand, U.; Kumar, R.; Bhadani, U.K. Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial. J. Fam. Med. Prim. Care 2022, 11, 4758–4765. [Google Scholar] [CrossRef]
- Labbani-Motlagh, Z.; Amini, S.; Aliannejad, R.; Sadeghi, A.; Shafiee, G.; Heshmat, R.; Jafary, M.; Talaschian, M.; Akhtari, M.; Jamshidi, A.; et al. High-dose intravenous vitamin C in early stages of severe acute respiratory syndrome coronavirus 2 infection: A double-blind, randomized, controlled clinical trial. J. Res. Pharm. Pract. 2022, 11, 64–72. [Google Scholar] [CrossRef]
- Suna, K.; Melahat, U.Ş.; Murat, Y.; Figen, Ö.E.; Ayperi, Ö. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med. Clin. Engl. Ed. 2022, 158, 356–360. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Alizadeh, N.; Dianatkhah, M.; Alimohamadi, Y.; Moradi, H.; Akbarpour, S.; Akrami, M.; Mansouri, F.; Faraji, N.; Rezaie, Z.; Alizadeh, M.; et al. High dose melatonin as an adjuvant therapy in intubated patients with COVID-19: A randomized clinical trial. J. Taibah Univ. Med. Sci. 2022, 17, 454–460. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial. Arch. Med. Res. 2022, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sun, A.; Xiao, T.; Yao, G.; Sang, L.; Zheng, X.; Zhang, J.; Jin, X.; Xu, L.; Yang, W.; et al. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19). Front. Med. 2022, 8, 566609. [Google Scholar] [CrossRef] [PubMed]
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The power of vitamin D: Is the future in precision nutrition through personalized supplementation plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Tsikopoulos, A.; Tsikopoulos, K.; Fountarlis, A.; Efthymiadis, A.; Festas, C.; Garefis, K. Is there any association between vitamin D deficiency and recurrent tonsillopharyngitis? An updated systematic review. Maedica 2024, 19, 116–128. [Google Scholar] [CrossRef]
- Laaksi, I. Vitamin D and respiratory infection in adults. Proc. Nutr. Soc. 2012, 71, 90–97. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity—The immune system and vitamin D: A systematic review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Gao, N.; Raduka, A.; Rezaee, F. Vitamin D3 protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway. Eur. J. Cell Biol. 2023, 102, 151336. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.; Thangamuthu, A.; Muthumeenal, S.; Houston, K.; Everton, M.; Gowda, S.; Zhang, J.; Subramanian, R. Vital D: A modifiable occupational risk factor of UK healthcare workers. PLoS ONE 2024, 19, e0296247. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Hafez, W.; Saleh, H.; Arya, A.; Alzouhbi, M.; Fdl Alla, O.; Lal, K.; Kishk, S.; Ali, S.; Raghu, S.; Elgaili, W.; et al. Vitamin D status in relation to the clinical outcome of hospitalized COVID-19 patients. Front. Med. 2022, 9, 843737. [Google Scholar] [CrossRef]
- De Smet, D.; De Smet, K.; Herroelen, P.; Gryspeerdt, S.; Martens, G.A. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am. J. Clin. Pathol. 2021, 155, 381–388. [Google Scholar] [CrossRef]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4048–4052. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological role and therapeutic implications of vitamin D in autoimmunity: Focus on chronic autoimmune diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a high loading dose of cholecalciferol to correct hypovitaminosis d in patients with inflammatory/autoimmune rheumatic diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Antonini, G.; Bellomo, G.; Pirisi, M. Unsuppressed parathyroid hormone in patients with autoimmune/inflammatory rheumatic diseases: Implications for vitamin D supplementation. Rheumatology 2011, 50, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Simanek, V.; Dedeckova, E.; Topolcan, O.; Kralova, M.; Kucera, R. A case study of vitamin D supplementation therapy and acute respiratory tract infection. In Vivo 2024, 38, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulation of immune function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Khashim Alswailmi, F.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular mechanisms of vitamin D-mediated immunomodulation. Galen. Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and influenza-prevention or therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef]
- Lang, P.O.; Aspinall, R. Vitamin D status and the host resistance to infections: What it is currently (not) understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef]
- White, J.H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Cantorna, M.T. Vitamin D and lung infection. Infect. Immun. 2016, 84, 3094–3096. [Google Scholar] [CrossRef]
- Selvaraj, P.; Harishankar, M.; Afsal, K. Vitamin D: Immuno-modulation and tuberculosis treatment. Can. J. Physiol. Pharmacol. 2015, 93, 377–384. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Coleman, L.A. Vitamin D and influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at supplemental levels, tight junctions and epithelial barrier function: A narrative review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Rybakovsky, E.; Abdavies, R.; Chamoun, R.; Flounders, C.A.; Shepley-McTaggart, A.; Harty, R.N.; Mullin, J.M. Micronutrient improvement of epithelial barrier function in various disease states: A case for adjuvant therapy. Int. J. Mol. Sci. 2022, 23, 2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers 2013, 1, e23118. [Google Scholar] [CrossRef]
- Gatera, V.A.; Abdulah, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B. Updates on the status of vitamin D as a risk factor for respiratory distress syndrome. Adv. Pharmacol. Sci. 2018, 2018, 8494816. [Google Scholar] [CrossRef]
- Myszor, I.T.; Gudmundsson, G.H. Modulation of innate immunity in airway epithelium for host-directed therapy. Front. Immunol. 2023, 14, 1197908. [Google Scholar] [CrossRef]
- Gopallawa, I.; Dehinwal, R.; Bhatia, V.; Gujar, V.; Chirmule, N. A four-part guide to lung immunology: Invasion, inflammation, immunity, and intervention. Front. Immunol. 2023, 14, 1119564. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Coraux, C.; Hajj, R.; Lesimple, P.; Puchelle, E. Réparation et régénération de l’épithélium respiratoire [Repair and regeneration of the airway epithelium]. Med. Sci. 2005, 21, 1063–1069. [Google Scholar] [CrossRef]
- Bals, R.; Hiemstra, P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004, 23, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.A.; Holgate, S.T. The airway epithelium: Structural and functional properties in health and disease. Respirology 2003, 8, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef]
- Ahmad, S.; Arora, S.; Khan, S.; Mohsin, M.; Mohan, A.; Manda, K.; Syed, M.A. Vitamin D and its therapeutic relevance in pulmonary diseases. J. Nutr. Biochem. 2021, 90, 108571. [Google Scholar] [CrossRef]
- Rybakovsky, E.; DiGuilio, K.M.; Valenzano, M.C.; Geagan, S.; Pham, K.; Harty, R.N.; Mullin, J.M. Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lung-derived epithelial cell culture model, 16HBE 14o. Physiol. Rep. 2023, 11, e15592. [Google Scholar] [CrossRef]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D receptor deletion leads to the destruction of tight and adherens junctions in lungs. Tissue Barriers 2018, 6, 1540904. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Xiong, J.; Kaleja, P.; Ückert, L.; Nezaratizadeh, N.; Krantz, S.; Krause, M.F.; Fitschen-Oestern, S.; Seekamp, A.; Cassidy, L.; Tholey, A.; et al. Alveolar-capillary barrier protection in vitro: Lung cell type-specific effects and molecular mechanisms induced by 1α, 25-dihydroxyvitamin D3. Int. J. Mol. Sci. 2023, 24, 7298. [Google Scholar] [CrossRef]
- Afzal, M.; Kazmi, I.; Al-Abbasi, F.A.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S.; Nadeem, M.S.; Al-Zahrani, M.H.; Alzarea, S.I.; Alquraini, A. Current overview on therapeutic potential of vitamin D in inflammatory lung diseases. Biomedicines 2021, 9, 1843. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; van der Does, A.M.; Hiemstra, P.S. Impact of the local inflammatory environment on mucosal vitamin D metabolism and signaling in chronic inflammatory lung diseases. Front. Immunol. 2020, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo CAJr Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; Dubnov-Raz, G.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tagle, C.; Romero, F.; Naves, R.; Balcells, M.E. Vitamin D and cathelicidin levels and susceptibility to Mycobacterium tuberculosis infection acquisition in household contacts. Enferm. Infecc. Microbiol. Clin Engl. Ed. 2023, 41, 489–493. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- de Sa Del Fiol, F.; Barberato-Filho, S.; Lopes, L.C.; de Cassia Bergamaschi, C. Vitamin D and respiratory infections. J. Infect. Dev. Ctries. 2015, 9, 355–361. [Google Scholar] [CrossRef]
- Jia, H.; Sheng, F.; Yan, Y.; Liu, X.; Zeng, B. Vitamin D supplementation for prevention of acute respiratory infections in older adults: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0303495. [Google Scholar] [CrossRef]
- Cho, H.; Myung, S.K.; Cho, H.E. Efficacy of vitamin D supplements in treatment of acute respiratory infection: A meta-analysis for randomized controlled trials. Nutrients 2022, 14, 1144. [Google Scholar] [CrossRef]
- Gaudet, M.; Plesa, M.; Mogas, A.; Jalaleddine, N.; Hamid, Q.; Al Heialy, S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir. Res. 2022, 23, 252. [Google Scholar] [CrossRef]
- Cepeda, S.J.; Zenteno, A.D.; Fuentes, S.C.; Bustos, B.R. Vitamina D y enfermedades respiratorias pediátricas [Vitamin D and pediatrics respiratory diseases]. Rev. Chil. Pediatr. 2019, 90, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, 1909. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Huang, Y.; Zhang, J.; Xiong, Q.; Chi, M.; Yang, L.; Zhang, J.; Li, L.; Fan, Y. Vitamin D promotes epithelial tissue repair and host defense responses against influenza H1N1 virus and Staphylococcus aureus infections. Respir. Res. 2023, 24, 175. [Google Scholar] [CrossRef]
- Gatera, V.A.; Lesmana, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B.; Abdulah, R. Vitamin D inhibits lipopolysaccharide (LPS)-induced inflammation in A549 cells by downregulating inflammatory cytokines. Med. Sci. Monit. Basic Res. 2021, 27, e931481. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamatsu, M.; Ogasawara, H.; Tsugawa, N.; Isoda, N.; Matsuno, K.; Sakoda, Y. Oral supplementation of the vitamin D metabolite 25(OH)D3 against influenza virus infection in mice. Nutrients 2020, 12, 2000. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Oak, E.; Garcia, J.; Liu, H.; Lorenzini, P.A.; Batra, D.; Chhabra, A.; Salazar, J.C.; Roca, X. Vitamin D modulates human macrophage response to Mycobacterium tuberculosis DNA. Tuberculosis 2019, 116S, S131–S137. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Yu, Y.; Wan, Z.; Chiu, M.C.; Liu, X.; Zhang, S.; Cai, J.P.; Chu, H.; Li, G.; et al. Human airway and nasal organoids reveal escalating replicative fitness of SARS-CoV-2 emerging variants. Proc. Natl. Acad. Sci. USA 2023, 120, e2300376120. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130.e20. [Google Scholar] [CrossRef]
- Guo, T.J.F.; Singhera, G.K.; Leung, J.M.; Dorscheid, D.R. Airway epithelial-derived immune mediators in COVID-19. Viruses 2023, 15, 1655. [Google Scholar] [CrossRef]
- Chua, R.L.; Lukassen, S.; Trump, S.; Hennig, B.P.; Wendisch, D.; Pott, F.; Debnath, O.; Thürmann, L.; Kurth, F.; Völker, M.T.; et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020, 38, 970–979. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Mahanty, S. Respiratory virus infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Pathinayake, P.S.; Awatade, N.T.; Wark, P.A.B. Type 2 immunity and its impact on COVID-19 infection in the airways. Viruses 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Guan, W.J.; Zhang, Y.L.; Qiu, Z.E.; Chen, L.; Hou, X.C.; Yue, J.; Zhou, Y.Y.; Sheng, J.; Zhao, L.; et al. SARS-CoV-2 envelope protein impairs airway epithelial barrier function and exacerbates airway inflammation via increased intracellular Cl− concentration. Signal Transduct. Target. Ther. 2024, 9, 74. [Google Scholar] [CrossRef]
- Calvert, B.A.; Quiroz, E.J.; Lorenzana, Z.; Doan, N.; Kim, S.; Senger, C.N.; Anders, J.J.; Wallace, W.D.; Salomon, M.P.; Henley, J.; et al. Neutrophilic inflammation promotes SARS-CoV-2 infectivity and augments the inflammatory responses in airway epithelial cells. Front. Immunol. 2023, 14, 1112870. [Google Scholar] [CrossRef]
- Hao, S.; Ning, K.; Kuz, C.A.; Vorhies, K.; Yan, Z.; Qiu, J. Long-term modeling of SARS-CoV-2 infection of in vitro cultured polarized human airway epithelium. mBio 2020, 11, e02852-20. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Nikolakopoulou, S.; Konstantinou, A.; Mascha, O.; Siarkos, E.; Samaras, C.; Athanassiou, P.; Shoenfeld, Y. Vitamin D levels as a marker of severe SARS-CoV-2 infection. Life 2024, 14, 210. [Google Scholar] [CrossRef]
- Coudray, M.S.; Hansel, S.; Alesci, S.; Meyer WA 3rd Christenson, R.H.; Landry, L.G.; Edwards, C.; Puckrein, G.; Forney, D.J.; Akinboboye, O. Vitamin D levels and SARS-CoV-2 infection among medically underserved populations in the minority and rural coronavirus insights study. Viruses 2024, 16, 639. [Google Scholar] [CrossRef]
- Metonidze, I.; Bostoganashvili, N.; Goderidze, T.; Tananashvili, D. Serum 25-hydroxyvitamin D levels and health outcomes of hospitalization owing to COVID-19: A retrospective cross-sectional study. J. Int. Med. Res. 2024, 52, 3000605241271770. [Google Scholar] [CrossRef]
- Salehi, Z.; Askari, M.; Jafari, A.; Ghosn, B.; Surkan, P.J.; Hosseinzadeh-Attar, M.J.; Pouraram, H.; Azadbakht, L. Dietary patterns and micronutrients in respiratory infections including COVID-19: A narrative review. BMC Public Health 2024, 24, 1661. [Google Scholar] [CrossRef] [PubMed]
- Konikowska, K.; Kiliś-Pstrusińska, K.; Matera-Witkiewicz, A.; Kujawa, K.; Adamik, B.; Doroszko, A.; Kaliszewski, K.; Pomorski, M.; Protasiewicz, M.; Sokołowski, J.; et al. Association of serum vitamin D concentration with the final course of hospitalization in patients with COVID-19. Front. Immunol. 2023, 14, 1231813. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K.A.; Lim Alba, R.; Li, K. Association of vitamin D levels on the clinical outcomes of patients hospitalized for COVID-19 in a tertiary hospital. J. ASEAN Fed. Endocr. Soc. 2023, 38, 81–89. [Google Scholar] [CrossRef]
- Mukherjee, S.B.; Gorohovski, A.; Merzon, E.; Levy, E.; Mukherjee, S.; Frenkel-Morgenstern, M. Seasonal UV exposure and vitamin D: Association with the dynamics of COVID-19 transmission in Europe. FEBS Open Bio 2022, 12, 106–117. [Google Scholar] [CrossRef]
- Mariani, J.; Giménez, V.M.M.; Bergam, I.; Tajer, C.; Antonietti, L.; Inserra, F.; Ferder, L.; Manucha, W. Association between vitamin D deficiency and COVID-19 incidence, complications, and mortality in 46 countries: An ecological study. Health Secur. 2021, 19, 302–308. [Google Scholar] [CrossRef]
- AlNafea, H.M.; Korish, A.A. The interplay between hypovitaminosis D and the immune dysfunction in the arteriovenous thrombotic complications of the sever coronavirus disease 2019 (COVID-19) infection. Blood Coagul. Fibrinolysis 2023, 34, 129–137. [Google Scholar] [CrossRef]
- Sengupta, T.; Majumder, R.; Majumder, S. Role of vitamin D in treating COVID-19-associated coagulopathy: Problems and perspectives. Mol. Cell. Biochem. 2021, 476, 2421–2427. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Unveiling the interplay-vitamin D and ACE-2 molecular interactions in mitigating complications and deaths from SARS-CoV-2. Biology 2024, 13, 831. [Google Scholar] [CrossRef]
- Souza, L.L.; Costa-Neto, C.M. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J. Cell. Physiol. 2012, 227, 2117–2222. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Pinheiro, S.V.; Gonçalves, A.C.; Alenina, N.; Bader, M.; Santos, R.A. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol. Med. 2008, 14, 28–35. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Wang, K.; Viveiros, A.; Kellner, M.J.; Penninger, J.M. Angiotensin-converting enzyme 2-at the heart of the COVID-19 pandemic. Cell 2023, 186, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Saleem, M.M.; Vijay, S.; Ehsan, M.; Atiq, I.; Anwar, E.; Oduoye, M.O. Efficacy of vitamin D supplementation in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. 2024, 86, 6079–6090. [Google Scholar] [CrossRef] [PubMed]
- Ghoreshi, Z.A.; Charostad, J.; Arefinia, N.; Nakhaie, M.; Rezaei Zadeh Rukerd, M.; Salajegheh, F. Effect of vitamin D supplementation on clinical outcomes in adult patients with COVID-19: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. Perspect. 2024, 12, e70013. [Google Scholar] [CrossRef]
- Sobczak, M.; Pawliczak, R. Effect of vitamin D3 supplementation on severe COVID-19: A meta-analysis of randomized clinical trials. Nutrients 2024, 16, 1402. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Dong, H.; Shang, N.; Li, Y.; Zhang, Y.; Guo, S.; Mei, X. The impact of supplementing vitamin D through different methods on the prognosis of COVID-19 patients: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1441847. [Google Scholar] [CrossRef]
- Sîrbu, A.C.; Sabin, O.; Bocșan, I.C.; Vesa, Ș.C.; Buzoianu, A.D. The effect of vitamin D supplementation on the length of hospitalisation, intensive care unit admission, and mortality in COVID-19-A systematic review and meta-analysis. Nutrients 2023, 15, 3470. [Google Scholar] [CrossRef]
- Wang, H.; Tao, L.; Cui, L.; Chen, Y.; Liu, D.; Xue, L.; Yang, Y.; Lv, Y.; Zhang, F.; Wang, T.; et al. Randomized trial of influence of vitamin D on the prevention and improvement of symptomatic COVID-19. Sci. Rep. 2024, 14, 20519. [Google Scholar] [CrossRef]
- Dilokpattanamongkol, P.; Yan, C.; Jayanama, K.; Ngamjanyaporn, P.; Sungkanuparph, S.; Rotjanapan, P. Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: A single-center randomized controlled trial. BMC Complement. Med. Ther. 2024, 24, 97. [Google Scholar] [CrossRef]
- Singh, A.; Rastogi, A.; Puri, G.D.; Ganesh, V.; Naik, N.B.; Kajal, K.; Kahlon, S.; Soni, S.L.; Kaloria, N.; Saini, K.; et al. Therapeutic high-dose vitamin D for vitamin D-deficient severe COVID-19 disease: Randomized, double-blind, placebo-controlled study (SHADE-S). J. Public Health 2024, 46, 256–266. [Google Scholar] [CrossRef]
- Domazet Bugarin, J.; Dosenovic, S.; Ilic, D.; Delic, N.; Saric, I.; Ugrina, I.; Stojanovic Stipic, S.; Duplancic, B.; Saric, L. Vitamin D supplementation and clinical outcomes in severe COVID-19 patients-randomized controlled trial. Nutrients 2023, 15, 1234. [Google Scholar] [CrossRef] [PubMed]
- Abroug, H.; Maatouk, A.; Bennasrallah, C.; Dhouib, W.; Ben Fredj, M.; Zemni, I.; Kacem, M.; Mhalla, S.; Nouira, S.; Ben Belgacem, M.; et al. Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: A randomized-controlled clinical trial. Trials 2023, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, R.R.; Khorasanchi, Z.; Noor, A.R.; Moghadam, M.S.F.; Esfahani, A.J.; Alyakobi, A.K.M.; Alboresha, M.L.; Sharifan, P.; Bahari, A.; Rezvani, R.; et al. High-dose vitamin D supplementation is related to an improvement in serum alkaline phosphatase in COVID-19 patients; a randomized double-blinded clinical trial. J. Health Popul. Nutr. 2023, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.W.; Ashfaq, A.; Melnick, J.Z.; Vazquez-Escarpanter, E.; Fialkow, J.A.; Strugnell, S.A.; Choe, J.; Kalantar-Zadeh, K.; Federman, N.C.; Ng, D.; et al. REsCue trial: Randomized controlled clinical trial with extended-release calcifediol in symptomatic COVID-19 outpatients. Nutrition 2023, 107, 111899. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; Santacruz-Tinoco, C.E.; Martínez-Miguel, B.; et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Díaz-Sottolano, A.; Fernández, P.; Palomo-Antequera, C.; Herrero-Puente, P.; Mouzo, R.; Carrillo-López, N.; Panizo, S.; Ibañez, G.H.; Cusumano, C.A.; et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID-19 patients: A randomized, open-label, single-center study. Nutrients 2022, 14, 2602. [Google Scholar] [CrossRef]
- De Niet, S.; Trémège, M.; Coffiner, M.; Rousseau, A.F.; Calmes, D.; Frix, A.N.; Gester, F.; Delvaux, M.; Dive, A.F.; Guglielmi, E.; et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: A randomized, double-blind, placebo-controlled trial. Nutrients 2022, 14, 3048. [Google Scholar] [CrossRef]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Sanchez Cunto, M.; Brosio, D.; Ross, F.; Zylberman, M.; López, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, N.; Abou Warda, A.E.; Sarhan, R.M.; Boshra, M.S.; Mostafa-Hedeab, G.; ALruwaili, B.F.; Ibrahim, H.S.G.; Schaalan, M.F.; Fathy, S. Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: A randomized clinical trial. Medicina 2022, 58, 1358. [Google Scholar] [CrossRef] [PubMed]
- Cervero, M.; López-Wolf, D.; Casado, G.; Novella-Mena, M.; Ryan-Murua, P.; Taboada-Martínez, M.L.; Rodríguez-Mora, S.; Vigón, L.; Coiras, M.; Torres, M. Beneficial effect of short-term supplementation of high dose of vitamin D3 in hospitalized patients with COVID-19: A multicenter, single-blinded, prospective randomized pilot clinical trial. Front. Pharmacol. 2022, 13, 863587. [Google Scholar] [CrossRef] [PubMed]
- Elamir, Y.M.; Amir, H.; Lim, S.; Rana, Y.P.; Lopez, C.G.; Feliciano, N.V.; Omar, A.; Grist, W.P.; Via, M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 2022, 154, 116175. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate COVID-19: A randomized clinical trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-hydroxyvitamin D3 (Calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: A pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Kofahi, H.M.; Badran, B.R.; Nimer, R.M.; Atoom, A.M.; Al Hersh, S.M. Exploring the effects of vitamin D and vitamin A levels on the response to COVID-19 vaccine. Vaccines 2023, 11, 1509. [Google Scholar] [CrossRef]
- Panahi, Y.; Einollahi, B.; Beiraghdar, F.; Darvishi, M.; Fathi, S.; Javanbakht, M.; Shafiee, S.; Akhavan-Sigari, R. Fully understanding the efficacy profile of the COVID-19 vaccination and its associated factors in multiple real-world settings. Front. Immunol. 2022, 13, 947602. [Google Scholar] [CrossRef]
- Zecca, E.; Rizzi, M.; Tonello, S.; Matino, E.; Costanzo, M.; Rizzi, E.; Casciaro, G.F.; Manfredi, G.F.; Acquaviva, A.; Gagliardi, I.; et al. Ongoing mycophenolate treatment impairs anti-SARS-CoV-2 vaccination response in patients affected by chronic inflammatory autoimmune diseases or liver transplantation recipients: Results of the RIVALSA prospective cohort. Viruses 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Eick-Cost, A.A.; Ying, S.; Wells, N. Effectiveness of mRNA-1273, BNT162b2, and JNJ-78436735 COVID-19 vaccines among US military personnel before and during the predominance of the Delta ariant. JAMA Netw. Open 2022, 5, e228071. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Keshavarz, J.; Bagheri-Jamebozorgi, M.; Nemati, M.; Frootan, R.; Shokri, F. The association of the vitamin D status with the persistence of anti-HBs antibody at 20 years after primary vaccination with recombinant hepatitis B vaccine in infancy. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Heine, G.; Drozdenko, G.; Lahl, A.; Unterwalder, N.; Mei, H.; Volk, H.D.; Dörner, T.; Radbruch, A.; Worm, M. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur. J. Clin. Nutr. 2011, 65, 329–334. [Google Scholar] [CrossRef]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Visic, D.; McGee, Z.A.; Daynes, R.A. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: Characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine 1999, 17, 3050–3064. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Enioutina, E.Y.; Butler, S.; Mu, H.H.; McGee, Z.A.; Araneo, B.A. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect. Immun. 1996, 64, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Saroha, H.S.; Bhat, S.; Das, L.; Dutta, P.; Holick, M.F.; Sachdeva, N.; Marwaha, R.K. Calcifediol boosts efficacy of ChAdOx1 nCoV-19 vaccine by upregulating genes promoting memory T cell responses. NPJ Vaccines 2024, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Lavell, A.H.A.; Schramade, A.E.; Sikkens, J.J.; van der Straten, K.; van Dort, K.A.; Slim, M.A.; Appelman, B.; van Vught, L.A.; Vlaar, A.P.J.; Kootstra, N.A.; et al. 25-Hydroxyvitamin D concentrations do not affect the humoral or cellular immune response following SARS-CoV-2 mRNA vaccinations. Vaccine 2024, 42, 1478–1486. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Terenzi, U.; Nannipieri, F.; Locatelli, M.; Ciceri, F.; Giustina, A. Lack of vitamin D predicts impaired long-term immune response to COVID-19 vaccination. Endocrine 2023, 82, 536–541. [Google Scholar] [CrossRef]
- Meyers, E.; De Smet, E.; Vercruysse, H.; Callens, S.; Padalko, E.; Heytens, S.; Vandekerckhove, L.; Cools, P.; Witkowski, W. No significant association between 25-OH vitamin D status and SARS-CoV-2 antibody response after COVID-19 vaccination in nursing home residents and staff. Vaccines 2023, 11, 1343. [Google Scholar] [CrossRef]
- Fateh, H.L.; Kareem, G.; Rezaeian, S.; Moludi, J.; Kamari, N. The effect of Vit-D supplementation on the side effect of BioNTech, Pfizer vaccination and immunoglobulin G response against SARS-CoV-2 in the individuals tested positive for COVID-19: A randomized control trial. Clin. Nutr. Res. 2023, 12, 269–282. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, C.; Coussens, A.K.; Richter, A.G.; et al. Vitamin D supplementation does not influence SARS-CoV-2 vaccine efficacy or immunogenicity: Sub-studies nested within the CORONAVIT randomised controlled trial. Nutrients 2022, 14, 3821. [Google Scholar] [CrossRef]
- Zelini, P.; d’Angelo, P.; Cereda, E.; Klersy, C.; Sabrina, P.; Albertini, R.; Grugnetti, G.; Grugnetti, A.M.; Marena, C.; Cutti, S.; et al. Association between vitamin D serum levels and immune response to the BNT162b2 vaccine for SARS-CoV-2. Biomedicines 2022, 10, 1993. [Google Scholar] [CrossRef]
- Piec, I.; Cook, L.; Dervisevic, S.; Fraser, W.D.; Ruetten, S.; Berman, M.; English, E.; John, W.G. Age and vitamin D affect the magnitude of the antibody response to the first dose of the SARS-CoV-2 BNT162b2 vaccine. Curr. Res. Transl. Med. 2022, 70, 103344. [Google Scholar] [CrossRef]
- Pavel-Tanasa, M.; Constantinescu, D.; Cianga, C.M.; Anisie, E.; Mereuta, A.I.; Tuchilus, C.G.; Cianga, P. Adipokines, and not vitamin D, associate with antibody immune responses following dual BNT162b2 vaccination within individuals younger than 60 years. Front. Immunol. 2022, 13, 1000006. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between vitamin D status and antibody response to COVID-19 mRNA vaccination in healthy adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef] [PubMed]
- Durden, W.; Ezaldin, S.; Amos, J.; Kemper, S.; Campbell, J. Rise in serum 25-Hydroxyvitamin D levels during the COVID-19 pandemic. Nutrients 2024, 16, 2449. [Google Scholar] [CrossRef] [PubMed]
| Over-the-Counter Solution | Country | Study Design | Main Findings | References |
|---|---|---|---|---|
| Lactoferrin | Italy | Multicenter, not-for-profit, randomized, double-blind, placebo-controlled, parallel-arm clinical trial | Lactoferrin, used as an adjuvant to the standard of care treatment, did not improve either clinical evolution or laboratory inflammation markers evaluation in moderate-to-severe COVID-19 patients | [31] |
| Egypt | Single-center, randomized, prospective, interventional clinical trial | Lactoferrin, used as an adjuvant to the standard of care treatment, did not result in a statistically significant difference either in clinical resolution of the symptoms or in laboratory parameters | [32] | |
| Vitamin C | India | Single-center, randomized, double-blind, placebo-controlled clinical trial | Vitamin C, used as an adjuvant to the standard of care treatment, reduced in-hospital mortality, need for mechanical ventilation, qSOFA index, inflammatory biomarkers, and need for vasopressors; moreover, it improved respiratory index (pO2/FiO2), even if the statistical significance was not reached due to the limited number of patients enrolled | [33] |
| Iran | Single-center, randomized, double-blind, placebo-controlled clinical trial | High-dose intravenous vitamin C, used as an adjuvant to the standard of care treatment, did not statistically affect SOFA score or 28-day mortality compared to the standard of care alone | [34] | |
| Turkey | Retrospective study | High-dose intravenous vitamin C, used as an adjuvant to the standard of care treatment, did not statistically affect clinical outcomes in patients with severe COVID-19 | [35] | |
| China | Retrospective cohort study | High-dose vitamin C, used as an adjuvant to the standard of care treatment, reduced the mortality and improved oxygen support status | [36] | |
| China | Multicenter, randomized, controlled clinical trial | High-dose intravenous vitamin C did not statistically affect invasive mechanical ventilation-free days in 28 days, but might have a potentially beneficial effect on oxygenation in critically ill patients, by improving PaO2/FiO2 | [37] | |
| Iran | Single-center, randomized, open-label, controlled clinical trial | High-dose intravenous vitamin C, used as an adjuvant to the standard of care treatment, did not statistically affect clinical outcomes in patients with severe COVID-19 | [38] | |
| USA | Multicenter, prospective, randomized, open-label clinical trial | Ascorbic acid, alone or in combination with high-dose zinc gluconate, did not significantly affect the symptoms’ duration compared to the standard of care therapy in an outpatient setting | [39] | |
| Zinc | USA | Multicenter, prospective, randomized, open-label clinical trial | High-dose zinc gluconate, alone or in combination with ascorbic acid, did not significantly affect the symptoms’ duration compared to the standard of care therapy in an outpatient setting | [39] |
| Melatonin | Iran | Single-center, randomized, double-blind, clinical trial | High-dose melatonin, used as an adjuvant to the standard of care treatment for intubated patients reduced C-reactive protein levels but, apparently, had no effects on patient outcomes | [40] |
| Iran | Single-center, randomized, double-blind, clinical trial | Melatonin, used as an adjuvant to the standard of care treatment, improved clinical symptoms (cough, dyspnea, fatigue), C-reactive protein and pulmonary involvement and, also, contributed to a faster return to baseline health | [41] | |
| Omega 3 fatty acids | Mexico | Single-center, randomized, double-blind clinical trial | Omega 3 fatty acid supplementation reduced leukocyte counts and hematocrit levels, low-density lipoproteins and very low-density lipoproteins, glucose, creatinine, and blood urea nitrogen levels, while increasing high-density lipoprotein cholesterol in unvaccinated patients with moderate COVID-19 | [24] |
| China | Single-center, randomized, single-blind, group sequential, active-controlled clinical trial | Omega 3 fatty acid α-lipoic acid, used as an adjuvant to the standard of care treatment, reduced SOFA score and 30-day all-cause mortality with only borderline statistical significance, due to the limited number of patients enrolled | [42] | |
| Iran | Single-center, double-blind, randomized clinical trial | Omega 3 fatty acid enteral supplementation improved 1-month survival as well as several parameters of respiratory and renal functions (arterial pH, bicarbonate, base excess, creatinine, potassium and blood urea nitrogen) in critically ill COVID-19 patients | [25] |
| Country | Study Design | Study Objective | Participants | Intervention | Main Findings | References |
|---|---|---|---|---|---|---|
| China | Multicenter, open-label, randomized controlled trial | Investigation of high-dose vitamin D2 ability to prevent COVID-19 and/or improve disease symptoms | 248 health care workers were assessed for eligibility and 214 underwent randomization. Finally, 99 subjects allocated to the intervention arm received vitamin D2, while 103 subjects allocated to the non-intervention arm did not receive the intervention | 200,000 IU vitamin D2 on the 1st and 14th day of the study (intervention arm) vs. no intervention | The provided intervention did not significantly prevent infection or improve COVID-19 symptoms. Nevertheless, it has been observed that there is a 14.3% difference in positive infection rates between patients with vitamin D levels > 30 ng/mL and patients with vitamin D levels < 20 ng/mL | [138] |
| Thailand | Prospective, open label, randomized controlled trial | Investigation of high-dose alfacalcidiol ability to improve clinical outcomes in patients with COVID-19 pneumonia | 306 COVID-19 patients were assessed for eligibility and 294 underwent randomization. 147 subjects were allocated to the intervention group, and 147 subjects were allocated to the non-intervention group | 2 µg daily or <0.05 µg/kg/day alfacalcidiol in addition to the standard treatment until discharge vs. standard treatment only | Vitamin D supplementation in addition to standard treatment was beneficial for patients with COVID-19 pneumonia who require supplemental oxygen or high-dose corticosteroid therapy or who show CRP levels > 30 mg/L upon treatment initiation | [139] |
| India | Single-center, randomized, double-bind, placebo-controlled clinical trial | Investigation of the effectiveness of a single oral high dose of cholecalciferol in improving sequential organ failure assessment (SOFA) score at 7 days, as well as the total duration of mechanical ventilation, all-cause mortality at 28 days and inflammatory marker levels | 358 COVID-19 patients were assessed for eligibility and 90 underwent randomization. 45 subjects were allocated to the intervention arm, and 45 subjects were allocated to the placebo arm | 60,000 IU cholecalciferol on the day of inclusion (intervention arm) in addition to the standard of care vs. placebo in addition to the standard of care | The administration of a high dose of cholecalciferol at the time of ICU admission improved SOFA score on day 7 and reduced in-hospital mortality in vitamin D-deficient COVID-19 patients | [140] |
| Croatia | Single-center, open-label, randomized clinical trial | Investigation of daily vitamin D supplementation ability to improve clinical outcomes in ICU patients | 292 COVID-19 patients admitted to ICU were assessed for eligibility and 155 underwent randomization. 78 subjects were allocated to the intervention group (75 received vitamin D supplementation according to the study protocol) and 77 subjects were allocated to the non-intervention group | 10,000 IU cholecalciferol administered daily for 14 days in addition to the standard of care vs. standard treatment alone | Vitamin D supplementation did not affect the number of days spent on respiratory support nor the prespecified secondary outcomes | [141] |
| Tunisia | Randomized controlled, parallel-group, blinded, clinical trial | Investigation of the effect of vitamin D supplementation on recovery delay among COVID-19 patients with positive RT-PCR at 14 days post-diagnosis confirmation | 130 COVID-19 patients with positive RT-PCR 14 days after COVID-19 diagnosis confirmation were assessed for eligibility and 117 underwent randomization. 57 subjects were allocated to the intervention arm, and 60 subjects were allocated to the placebo arm | 200,000 IU cholecalciferol vs. placebo | Vitamin D supplementation was not associated with a reduction in recovery delay when administered to patients with positive RT-PCR 14 days after COVID-19 diagnosis; furthermore, the median duration of RNA viral conversion was significantly longer in the intervention group compared to the placebo arm | [142] |
| Iran | Double-blinded, randomized clinical trial | Investigation of the effect of high and low dose vitamin D supplementation on liver function biomarkers in hospitalized COVID-19 patients | 218 COVID-19 patients were assessed for eligibility and 140 underwent randomization. 70 subjects were allocated to the intervention arm (high dose vitamin D), and 70 subjects were allocated to the control arm (low dose vitamin D) | 50,000 IU vitamin D at enrolment followed by 10,000 IU/mL daily till 30 days (high dose vitamin D group) vs. placebo (gelatin soft gel capsule) at enrolment followed by 1000 IU/mL vitamin D daily till 30 days (low dose vitamin D group) | High-dose vitamin D supplementation improved alkaline phosphatase markers in COVID-19 patients | [143] |
| USA | Multicenter, double-blinded, randomized, placebo-controlled phase 2 clinical trial | Investigation of the benefit of raising serum vitamin D to a level ≥ 50 ng/mL with an extended-release calcifediol (ERC) formulation on time to resolution of symptoms in COVID-19 patients in an outpatient setting | 241 COVID-19 patients were assessed for eligibility and 171 underwent randomization. 85 subjects were allocated to the intervention arm, and 86 subjects were allocated to the placebo arm. 80 subjects for each group received at least one dose of the study drug | 300 µg ERC for the first 3 days followed by 60 µg ERC from day 4 to day 27 vs. placebo | ERC treatment effectively increased serum vitamin D levels to the target level in mild-to-moderate COVID-19 patients in an outpatient setting and may have had a role in accelerating the resolution of respiratory symptoms, thus suggesting possible mitigation of COVID-19-related pneumonia risk | [144] |
| France | Multicenter, randomized, controlled, open-label, parallel-group, intent-to-treat, superiority clinical trial | Investigation of the ability of a single oral high dose of vitamin D3 administered within 72 h from COVID-19 diagnosis, compared to a standard vitamin D3 dose, to improve the 14-day overall survival in at-risk individuals | 1027 COVID-19 elderly patients (>65 years) were assessed for eligibility and 260 underwent randomization. 130 subjects were allocated to the high-dose group, and 130 subjects were allocated to the standard-dose group. 126 subjects in the high dose and 127 subjects in the standard dose received the study drug | 400,000 IU cholecalciferol on the day of inclusion (high dose group) vs. 50,000 IU cholecalciferol on the day of inclusion (standard dose group) | The early administration of a high dose of vitamin D3 to at-risk older patients suffering from COVID-19 improved the 14-day overall survival | [145] |
| Mexico | Multicenter, randomized, double-blind, parallel arm clinical trial | Investigation of the efficacy and safety of cholecalciferol supplementation in preventing SARS-CoV-2 infection in highly exposed individuals | 407 healthcare workers were assessed for eligibility and 321 underwent randomization. 160 subjects were allocated to the intervention arm, and 161 subjects were allocated to the placebo arm. 150 (94 analyzed) subjects in the intervention arm and 152 (98 analyzed) subjects in the placebo arm received the study drug | 4000 IU cholecalciferol for 30 days (intervention arm) vs. placebo | Vitamin D supplementation was effective and safe in preventing SARS-CoV-2 infection in highly exposed individuals regardless of the baseline vitamin D status | [146] |
| Spain, Argentina, Guatemala, Chile | Multicenter, randomized, controlled, open-label, international clinical trial | Investigation of the effectiveness of a high dose cholecalciferol administered at the time of hospital admission in modifying clinical outcomes in moderate-to-severe COVID-19 patients | 570 COVID-19 patients were assessed for eligibility and 548 underwent randomization. 277 (274 analyzed) subjects were allocated to the intervention arm, and 271 (269 analyzed) subjects were allocated to the control arm | 100,000 IU cholecalciferol on the day of inclusion (intervention arm) vs. no intervention (control arm) | The proposed intervention did not statistically improve clinical outcomes (reduction in median length of hospital stay, ICU admission rate, mortality rate) | [147] |
| India | Randomized, placebo-controlled clinical trial | Investigation of the effect of a high dose of cholecalciferol supplementation on SARS-CoV-2 viral clearance | 89 COVID-19 patients were assessed for eligibility and 40 underwent randomization. 16 subjects were allocated to the intervention arm, and 24 subjects were allocated to the placebo arm | 60,000 IU cholecalciferol for 7 days (intervention arm) vs. placebo for 7 days | The administration of a high dose of cholecalciferol helped in achieving viral clearance in a greater proportion of asymptomatic vitamin D-deficient individuals along with a significant decrease in fibrinogen | [148] |
| Russia | Randomized, single-center open-label clinical trial | Investigation of the effectiveness of vitamin D supplementation in modifying serum vitamin D level, complete blood count, CRP levels, and B cell subset on the 9th day of hospitalization compared to the first one. | 311 COVID-19 patients were assessed for eligibility and 129 underwent randomization. 65 subjects were allocated to the intervention arm, and 64 subjects were allocated to the no-intervention arm. After serum vitamin D quantification, 56 subjects in the intervention arm received the study drug, while 54 subjects in the no-intervention arm received only the standard-of-care therapy | 50,000 IU vitamin D on the 1st and 8th days of hospitalization in addition to the standard of care (intervention arm) vs. standard of care alone (no-intervention arm) | The proposed intervention resulted in an increase in serum vitamin D levels, neutrophil and lymphocyte counts, and a decrease in CRP levels in vitamin D-deficient patients. Moreover, vitamin D supplementation was associated with a reduction in CD38++CD27 transitional and CD27−CD38+ mature naïve B cells and an increase in CD27−CD38− double negative B cells | [149] |
| Belgium | Single-center, randomized, double-blind, placebo-controlled clinical trial | Investigation of the ability of vitamin D supplementation to improve COVID-19 clinical outcomes in vitamin D deficient hospitalized patients | 69 COVID-19 patients were assessed for eligibility and 50 underwent randomization. 26 subjects (21 analyzed) were allocated to the intervention arm, and 24 (22 analyzed) subjects were allocated to the placebo arm | 25,000 IU cholecalciferol daily for the first 4 days and then once weekly for up to 6 weeks (for a maximum of 36 days; study exit corresponded to hospital discharge) in addition to the standard of care (intervention arm) vs. placebo daily for the first 4 days and then once weekly for up to 6 weeks in addition to the standard of care | The proposed intervention significantly reduced hospitalization length, and oxygen supplementation duration and improved patients’ clinical status (WHO scale) | [150] |
| Argentina | Multicenter, randomized, double-blind, sequential, placebo-controlled clinical trial * * The sequential design consisted of an adaptative design with two stages: stage 1—assessment of the effects of vitamin D supplementation on respiratory sepsis-related organ failure assessment (rSOFA); stage 2—assessment of the effects of vitamin D supplementation on clinical events. As per protocol, after the recruitment of the first 200 participants, a blind analysis was carried out and, based on that, the trial was terminated | Investigation of the ability of a single oral high dose of vitamin D3 to prevent respiratory worsening in hospitalized mild-to-moderate COVID-19 patients with risk factors for disease progression | 256 COVID-19 patients were assessed for eligibility and 218 underwent randomization. 115 subjects were allocated to the intervention arm, and 103 subjects were allocated to the placebo arm | 500,000 IU vitamin D3 on the day of inclusion (intervention arm) vs. placebo on the day of inclusion (placebo arm) | The proposed intervention did not prevent respiratory worsening in mild-to-moderate COVID-19 patients with risk factors for disease progression | [151] |
| Egypt | Single-center, prospective, randomized, controlled clinical trial | Investigation of the ability of a single oral high dose of cholecalciferol compared to a standard alfacalcidiol dose, to improve COVID-19 clinical evolution (improvement of oxygenation parameters, reduction in hospitalization length and mortality rate, variation in inflammatory markers, occurrence of secondary infections and adverse events) in hospitalized moderate-to-severe COVID-19 patients | 116 COVID-19 patients were assessed for eligibility and underwent randomization. 58 subjects were allocated to the high-dose arm, and 58 subjects were allocated to the standard-dose arm | 200,000 IU cholecalciferol on the day of inclusion in addition to the standard of care (high dose group) vs. 1 µg/day alfaclcidiol for at least 5 days in addition to the standard of care (standard dose group) | The high-dose cholecalciferol supplementation resulted in better clinical improvement and fewer adverse outcomes compared to the standard dose supplementation regimen in moderate-to-severe COVID-19 patients | [152] |
| Spain | Multicenter, single-blinded, prospective, randomized, clinical trial | Investigation of the safety, tolerability, and effectiveness of high dose in comparison with moderate dose vitamin D3 supplementation for 14 days in improving COVID-19 clinical evolution in hospitalized patients | 87 COVID-19 patients were assessed for eligibility and 85 underwent randomization. 44 subjects were allocated to the high-dose arm, and 41 subjects were allocated to the moderate-dose arm | 10,000 IU vitamin D3 in addition to the standard of care for 14 days (high dose group) vs. 2000 IU vitamin D3 in addition to the standard of care for 14 days (moderate dose group) | Both interventions were safe and did not cause significant adverse events. The high-dose supplementation was more effective in increasing serum vitamin D levels, especially in overweight and obese individuals. Moreover, the high-dose supplementation in addition to the standard of care was effective in improving oxygen requirements and in reducing hospitalization length in patients developing ARDS | [153] |
| USA | Multicenter, randomized, open-label clinical trial | Investigation of the effectiveness of calcitriol supplementation in improving clinical outcomes (need for oxygen supplementation, hospitalization length, need for ICU admission, mortality and readmission) in COVID-19 patients | 50 consecutive COVID-19 patients underwent randomization. 25 subjects were allocated to the intervention arm, and 25 subjects were allocated to the no-intervention arm | 0.5 µg calcitriol daily for 14 days or till hospital discharge, whichever occurred first, in addition to the standard of care vs. standard of care alone | The proposed intervention resulted in an improvement in oxygenation in COVID-19 patients, but did not result in a significant improvement in the other clinical parameters | [154] |
| Brazil | Multicenter, double blind, randomized, placebo-controlled clinical trial | Investigation of the ability of a single high dose of vitamin D3 to reduce the duration of hospital stay in patients with moderate or severe COVID-19 | 1240 COVID-19 patients were assessed for eligibility and 240 underwent randomization. 120 subjects were allocated to the intervention arm, and 120 subjects were allocated to the placebo arm. 117 subjects in the intervention arm and 118 subjects in the placebo arm received the study drug | 200,000 IU vitamin D3 on the day of inclusion vs. placebo | High-dose vitamin D3 supplementation did not statistically reduce hospital stay length, the in-hospital mortality rate, the ICU admission rate, or the need for mechanical ventilation support | [155] |
| Saudi Arabia | Multicenter, randomized, open-label clinical trial | Investigation of the ability of a daily vitamin D3 supplementation for 2 weeks in reducing the time to symptoms recovery in mild-to-moderate COVID-19 patients | 77 COVID-19 patients were assessed for eligibility and 73 underwent randomization. 35 subjects were allocated to the standard 1000 IU arm, and 38 subjects were allocated to the 5000 IU arm | 5000 IU vitamin D3 daily for 2 weeks vs. 1000 IU vitamin D3 daily for 2 weeks | A 2-week supplementation with 5000 IU vitamin D3 was superior to a 2-week supplementation with 1000 IU vitamin D3 in resolving cough and ageusia in mild-to-moderate COVID-19 patients with sub-optimal vitamin D levels | [156] |
| Iran | Multicenter, randomized, double-blinded, placebo-controlled clinical trial | Investigation of the ability of a daily dose of 25-hydroxyvitamin D3 to increase circulating vitamin D levels in COVID-19 patients and to improve disease evolution (severity, hospitalization length, need for oxygen support, death rate, and lymphocyte count) | 134 COVID-19 patients were assessed for eligibility and 106 underwent randomization. 53 subjects were allocated to the intervention arm, and 53 subjects were allocated to the placebo arm. 24 subjects in the intervention arm and 19 in the placebo arm completed the 2 months follow up | 25 µg 25-hydroxyvitamin D3 daily for 60 days in addition to the standard of care (intervention arm) vs. placebo for 60 days in addition to the standard of care | The proposed intervention was effective in correcting vitamin D deficiency/insufficiency in COVID-19 patients, an effect that was associated with an increase in lymphocyte percentage and a decrease in neutrophil to lymphocyte ratio, a marker of improved clinical prognosis | [157] |
| Spain | Parallel-arm, pilot, open-label, randomized, double-masked clinical trial | Investigation of the effectiveness of early calcifediol administration in reducing ICU admission rate and mortality rate | 76 COVID-19 patients were assessed for eligibility and underwent randomization. 50 subjects were allocated to the intervention arm, and 26 subjects were allocated to the control arm | 0.532 mg calcifediol at admission, followed by 0.266 mg calcifediol on days 3 and 7 and then weekly until study exit (ICU admission, discharge, or death) in addition to the standard of care vs. standard of care alone | The proposed calcifediol supplementation schedule significantly reduced the ICU admission rate in hospitalized COVID-19 patients; furthermore, no deaths were recorded among the patients allocated to the intervention arm | [158] |
| Country | Study Design | Vaccine Type | Main Findings | References |
|---|---|---|---|---|
| India | Open-label, placebo-controlled, interventional trial | ChAdOx1nCoV-19 (adenoviral vaccine) | Calcifediol supplementation improved the efficacy of ChAdOx1nCoV-19 vaccine by augmenting T cell activation, proliferation, and T cell memory responses | [173] |
| The Netherlands | Prospective observational cohort study | BNT162b2 and mRNA1273 vaccines (both mRNA vaccines) | No association was found between vitamin D concentrations and humoral or cellular immune response following vaccination with mRNA vaccine platforms | [174] |
| Jordan | Observational study | BNT162b2 vaccine (mRNA vaccine) | Baseline vitamin D levels had no effect on the short-term response to a single dose of BNT162b2 vaccine | [159] |
| Italy | Retrospective cohort study | BNT162b2 vaccine (mRNA vaccine) | Low baseline vitamin D levels negatively affected long-term humoral response to BNT162b2 dual vaccination | [175] |
| Belgium | Observational study (secondary analysis) | BNT162b2 vaccine (mRNA vaccine) | No significant differences in terms of binding (S1RBD IgG) or neutralizing (50% pseudovirus neutralization titer) response to BNT162b2 dual vaccination were observed neither in subjects with severe vitamin D deficiency (vitamin D levels < 20 ng/mL) compared to vitamin D sufficient subjects, nor in subjects under active vitamin D supplementation compared to non-supplemented subjects | [176] |
| Iraq | Multicenter randomized clinical trial | BNT162b2 vaccine (mRNA vaccine) | A daily 600 IU vitamin D3 supplementation for 14–16 weeks resulted in a significant increase in IgG levels after the second vaccination compared to the first one. Moreover, vitamin D3 supplementation also reduced the vaccine-associated side effects after the second dose administration compared to the first one | [177] |
| United Kingdom | Sub-studies nested within a randomized controlled trial | ChAdOx1nCoV-19 (adenoviral vaccine) and BNT162b2 (mRNA vaccine) vaccines | Vitamin D supplementation (800 IU/day or 3200 IU/day) did not influence IgG, IgA, and IgM anti-spike titers, neutralizing antibodies titers or IFN-γ concentrations in the supernatants of S peptide stimulated whole blood in adults with a sub-optimal vitamin D status at baseline | [178] |
| Italy | Observational, longitudinal, retrospective study | BNT162b2 vaccine (mRNA vaccine) | While there were no significant differences in baseline anti-spike IgG and T-cell responses according to vitamin D status, significant correlations emerged between baseline vitamin D levels and anti-S IgG and neutralizing antibodies titers at six months after the second vaccine dose | [179] |
| United Kingdom | Observational study | BNT162b2 vaccine (mRNA vaccine) | Baseline vitamin D positively affects vaccination response, as demonstrated by an average 29.3% greater peak of anti-spike IgG antibodies in subjects with serum vitamin D levels > 50 nmol/l | [180] |
| Greece | Observational study | BNT162b2 vaccine (mRNA vaccine) | Vitamin D levels showed a trend for a positive association with antibody titer after 3 months from the last vaccination dose (subjects with serum vitamin D in the range of 25.68–32.99 ng/mL showed a positive association with higher antibody titer when compared with subjects with serum vitamin D in the range of 4.1–18.99 ng/mL; no statistically significant results observed in subjects with serum vitamin D in the range of 18.9–25.67 and 33–69.8 ng/mL) | [167] |
| Romania | Observational study | BNT162b2 vaccine (mRNA vaccine) | Baseline vitamin D levels had no effect on antibody responses after BNT162b2 dual vaccination, except for a weak but significant correlation observed only among infection-naïve subjects younger than 60 years | [181] |
| Germany | Observational study | BNT162b2 vaccine (mRNA vaccine) | No significant differences in terms of IgG response to BNT162b2 dual vaccination were observed neither with respect to baseline vitamin D levels nor in relation to self-reported active vitamin D supplementation | [182] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzi, M.; Sainaghi, P.P. Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19. Int. J. Mol. Sci. 2025, 26, 2550. https://doi.org/10.3390/ijms26062550
Rizzi M, Sainaghi PP. Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19. International Journal of Molecular Sciences. 2025; 26(6):2550. https://doi.org/10.3390/ijms26062550
Chicago/Turabian StyleRizzi, Manuela, and Pier Paolo Sainaghi. 2025. "Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19" International Journal of Molecular Sciences 26, no. 6: 2550. https://doi.org/10.3390/ijms26062550
APA StyleRizzi, M., & Sainaghi, P. P. (2025). Vitamin D: A Nutraceutical Supplement at the Crossroad Between Respiratory Infections and COVID-19. International Journal of Molecular Sciences, 26(6), 2550. https://doi.org/10.3390/ijms26062550





