Abstract
Even though in mid-2023 the World Health Organization declared the end of the public health emergency of international concern status for COVID-19, many areas of uncertainty about SARS-CoV-2 infection pathophysiology remain. Although in the last 4 years pharmaceutical industries widely invested in the development of effective antiviral treatments and vaccines, large disparities in their availability worldwide still exist, thus fostering the investigation of nutritional supplements as adjuvant therapeutic approaches for disease management, especially in resource-limited settings. During the COVID-19 pandemic, vitamin D has been widely used as an over-the-counter solution to improve disease evolution, thanks to its known immunomodulatory and anti-inflammatory actions. Ecological and observational studies support a relationship between hypovitaminosis D and COVID-19 negative outcomes and, according to this evidence, several research groups investigated the role of vitamin D supplementation in protecting from SARS-CoV-2 infection and/or improving disease evolution. This narrative review is intended to offer insights into the existing data on vitamin D’s biological effects in respiratory infections, especially in COVID-19. Furthermore, it will also offer a brief overview of the complex interplay between vitamin D and vaccine-elicited immune response, with special attention to anti-COVID-19 vaccines.
Keywords:
vitamin D; COVID-19; SARS-CoV-2; respiratory infections; clinical trials; immunomodulation 1. Introduction
At the end of 2019, a cluster of pneumonia cases of unknown origin was first described in Wuhan (China), representing the beginning of one of the deadliest pandemics in the world’s history. Early in the pandemic course, the etiological agent of the coronavirus disease 2019 (COVID-19) was identified as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 is an enveloped, single-stranded, RNA virus from the Coronaviridae family, characterized by a high rate of mutation, accounting for the different viral strains being dominant during the consecutive waves of disease outbreaks [1,2,3].
After more than 4 years since the first patient identification, it is well accepted that COVID-19 is a very heterogeneous disease, whose clinical manifestations could range from an asymptomatic or paucisymptomatic manifestation to severe and life-threatening conditions, mainly characterized by interstitial pneumonia, acute respiratory distress syndrome (ARDS), hypercytokinemia associated to a dysregulated immune response, impaired coagulation, and severe multiorgan failure [3,4,5,6,7,8].
Due to the high socioeconomic impact of the COVID-19 pandemic, since the beginning of the emergency huge efforts have been made to develop effective and selective treatments as well as vaccines allowing preventive immunization of the global population. To date, several types of vaccines have been developed, and those based on mRNA technology (i.e., Pfizer–BioNTech BNT132b2 and Moderna mRNA-1273 vaccines) were the most commonly used to sustain different immunization campaigns worldwide [9,10,11,12]. Moreover, a large number of pharmacological agents have been evaluated in terms of anti-SARS-CoV-2 activity, such as monoclonal antibodies, immunomodulators (i.e., drugs targeting the main players of the COVID-19-associated cytokine storm), and repurposed as well as newly developed antivirals, finally resulting in several preclinical and clinical studies, allowing the emergency use authorization for many of them [3,10,13].
In spite of this immense effort of the pharmaceutical industry, at the time of writing, only a part of the world population is fully vaccinated, and only a few specific anti-SARS-CoV-2 antiviral agents have been marketed. Moreover, these drugs showed a limited treatment window, with different efficacy depending on the phase of the infection at the time of pharmacological treatment, as well as on the circulating viral variant [3,9,10,14].
Considering that newly emerging variants are characterized by increased transmissibility associated with an increased ability to escape from natural and vaccine-induced immunity and by a reduced response to some pharmacological treatments, it is clear that SARS-CoV-2 management is still challenging. To date, effective therapeutic options to manage the clinical manifestations of the disease are still limited: only a few anti-COVID-19 drugs, such as nirmatrelvir+ritonavir (Paxlovid) and molnupiravir, are available for outpatient management, while the majority of the targeted pharmacological approaches are generally available only in hospital context (i.e., remdesivir, corticosteroids, tocilizumab, baricitinib, anakinra), thus limiting their use in outpatient and resource-limited settings [4,9,10,13,14].
In this scenario, it is not surprising that over-the-counter solutions, as well as traditional remedies coming from traditional Chinese and Indian medicine, still raise interest both in the general population and in the scientific community, as they can represent add-on interventions intended to prevent and/or mitigate COVID-19 clinical manifestations [13,15,16,17,18,19,20].
It is well known that a balanced diet is essential for human well-being, as malnourished individuals are more prone to infectious diseases [7,21,22,23], especially taking into consideration that malnutrition hardly affects immune function, as immune cells rely on nutritive substrate availability to quickly respond to noxious threats [7,21,22,24,25,26]. Among the most important nutrients known to play an important role in assuring immune system efficiency, there are vitamins (i.e., vitamin A, vitamin C, and vitamin D), as well as oligoelements (i.e., zinc, selenium), prebiotics and probiotics [15,21,26,27,28,29].
Even though, especially during the initial phases of the pandemic, several clinical trials and observational studies have focused on nutritional supplementation to identify a potential supportive approach able to prevent or mitigate COVID-19 outcomes, the obtained results were inconclusive, due to the large heterogeneity in the study design, as well as, in some cases, to the small number of patients involved, thus preventing the definition of a clear consensus around their clinical use [21,30]. Among the over-the-counter solutions that were investigated with conflicting results (summarized in Table 1), the only one that underwent a great in-depth investigation was represented by vitamin D, a chemical compound showing a wide range of biological activities, among which the immunomodulatory one seems to be strongly related, not only to immune protection in general, but also specifically to respiratory system protection against pathogen invasion.

Table 1.
Summary of selected recent clinical studies investigating nutraceutical over-the-counter solutions’ effectiveness in preventing or mitigating COVID-19 outcomes.
This narrative review summarizes the available evidence about vitamin D’s biological effects on respiratory infections, especially in COVID-19 (considering both disease evolution and vaccine-elicited immune responses). A literature search was conducted by screening the PubMed, Scopus, and Google Scholar repositories up to 15 December 2024 using the following keywords “vitamin D”, “COVID-19”, “SARS-CoV-2”, “respiratory infections”, “nutraceuticals”, “dietary supplements”, “respiratory system”, “immune function”, and “vaccination response”, alone or in combination. In addition, to avoid missing relevant papers focused on the topic, the references of the original articles, as well as the related results suggested by the different search engines were also considered. Furthermore, when the bibliographic search resulted in an extensive quantity of results, the selection was limited to clinical trials and to the most recent clinical results. Only papers published in English, French, and Spanish with complete full text available were considered.
2. Vitamin D: A Natural Compound with Pleiotropic Actions in the Human Body
Vitamin D has long been known as an essential nutrient in all vertebrates, where it is necessary to maintain calcium and phosphorus homeostasis. In the last decades, several studies highlighted that such compound exerts also several extra-skeletal functions, thus representing a key element in assuring whole-body well-being [4,43,44].
Humans can obtain the vitamin D they need from different sources: this lipophilic vitamin is naturally present in some foods, can be added artificially to some others (the so-called vitamin D fortified foods, such as infant milk), is available as an over-the-counter nutritional supplement and, among all, it can be directly synthesized by the human organism. In particular, skin exposure to sunlight represents the main source of vitamin D production in humans, accounting for nearly 80–90% of the vitamin D needs coverage, while dietary intake covers the remaining 10–20% [4,43,45,46].
At the skin level, UVB radiation promotes the conversion of 7-dehydrocholesterol to cholecalciferol, an inactive precursor that needs to undergo two subsequent hydroxylation steps to become biologically active. The first hydroxylation step, catalyzed by the CYP27A1 enzyme, occurs in the liver and results in 25-hydroxyvitamin D (also known as calcidiol), which is the main circulating form of vitamin D and, thanks to its half-life of approximately two weeks, represents the most commonly evaluated marker to assess vitamin D status. The second hydroxylation step, catalyzed by the CYP27B1 enzyme, occurs in renal tubular cells and results in 1,25-dihydroxyvitamin D (also known as calcitriol), the biologically active form of vitamin D [4,43,44,45,46,47,48].
Once released in the bloodstream at the renal level, the biologically active form of vitamin D functions as a steroid hormone and can interact with specific vitamin D receptors (VDRs). These VDRs could have different cellular locations, accounting for different biological responses following their activation. Some VDRs (nuclear VDR) are located inside the cell: after binding to vitamin D, they form a complex with the retinoid X acid receptor (RXR) which finally binds vitamin D response elements (VDREs). Nuclear VDR thus mediates genomic responses, by regulating, in a direct or indirect way, roughly 3% of the human genome, finally resulting in biological effects ranging from calcium and phosphorous homeostasis maintenance to energy metabolism and immune function control [43,47,49,50]. Instead, other VDRs are located on the plasma membrane (membrane VDR): after binding to vitamin D, these receptors interact with other membrane-resident proteins, finally resulting in the generation of secondary messengers (e.g., cAMP, phosphatidylinositol 3,4,5 triphosphate, Ca++) and in the activation of several downstream signaling pathways (e.g., PKA, PKC, MAPK) [49,51,52].
Considering the wide role of vitamin D in assuring human well-being, it has been estimated that it should be present in a sufficient concentration, corresponding to at least 20–30 ng/mL, while blood concentrations below 20 ng/mL indicate hypovitaminosis or a deficiency state. Since vitamin D levels below the sufficiency range (aka hypovitaminosis D) are associated with several negative health effects, affecting not only the musculoskeletal system, but also the immune domain, it is not surprising that several international agencies, such as the WHO, issued recommendations about the daily doses of vitamin D needed by different population groups [43,50,53].
Despite such efforts to ensure sufficient vitamin D intake, suboptimal levels of this nutrient still affect over 50% of the world population, regardless of age and ethnicity, thus representing a significant health problem worldwide [43,48,54]. To date, more than one billion individuals suffer to various extents of hypovitaminosis D, which clinically manifests as vitamin D insufficiency (when serum vitamin D levels range between 12 and 20 ng/mL) and vitamin D deficiency (when serum vitamin D levels are <12 ng/mL) [55,56,57]. In addition, it is worth noting that the world population is progressively aging, and the elderly represent a population at increased risk of hypovitaminosis D, due to a decrease in their skin’s ability to synthesize it, as well as to a poor dietary intake [43,46]. According to epidemiological data highlighting vitamin D deficiency prevalence on a global scale, it is thus not surprising the growing interest in vitamin D’s role in promoting the maintenance of healthy conditions, as it is recognized that vitamin D deficiency is positively associated with impaired immunity (e.g., autoimmune diseases manifestations, increased susceptibility to infections, poor outcomes from infectious diseases) as well as to a higher susceptibility for all-cause mortality [43,44,47,48,50,53,58,59,60,61].
3. Vitamin D and Immune Function
Biologically active vitamin D (1,25(OH)2D) is known to exert important immunomodulatory actions. All immune cell populations are known to express both the 1α-hydroxylase CYP27B1 and the vitamin D receptor (VDR), so their functions depend on their own ability to synthesize it. Locally produced vitamin D will thus mediate both genomic (e.g., gene transcription regulation following vitamin D-VDR complex translocation to the nucleus) and non-genomic (e.g., autocrine and paracrine signaling pathways activation, strengthening of epithelial and endothelial gap junction complexes) responses [43,47,58]. Furthermore, vitamin D’s immunomodulatory actions rely on its ability to down-regulate inflammatory responses, by suppressing pro-inflammatory cytokines production and by reducing oxidative stress, while increasing anti-microbial peptides (e.g., cathelicidin, β-defensin 2) and neutralizing antibodies production [44,46,47,53,58,62,63,64,65,66]. Moreover, vitamin D supports the host’s ability to fight infections by activating T and B lymphocytes and macrophages, which represent important immune cell populations actively involved in neutralizing infections [43,44,47,58,62,63,64,65,66].
In particular, it is known that specific components of pathogens such as bacteria, viruses, and fungi are recognized by toll-like receptors (TLRs) expressed by monocytes and macrophages: this binding results in the activation of intracellular signaling pathways leading to an increased expression of both VDR and CYP27B1 inside the cell, fostering cathelicidin and β-defensin 2 production [44,48,58,63,65,67,68].
Furthermore, VDR and CYP27B1, the key players in the immunomodulatory effects of vitamin D, are not exclusively expressed by monocytes/macrophages but are expressed in a tightly regulated manner also by lymphocytes. In particular, T cells are known to show an activation-state-dependent expression of VDR, while the biologically active vitamin D regulates their differentiation (Figure 1). Vitamin D is also known to modulate B cell functions in both an indirect and direct manner: while vitamin D-stimulated T helper cells suppress B cell differentiation and proliferation as well as antibody production, the biologically active form of the vitamin is also able to directly suppress naïve B lymphocyte differentiation towards plasma cells and memory B cells [21,44,58,62,63,64,65,66,68,69,70,71].
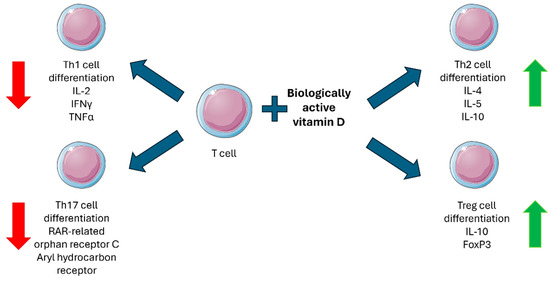
Figure 1.
Biologically active vitamin D effects on T cell differentiation. Red arrows indicate a suppressive effect, and green arrows indicate a stimulatory effect. Figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 4.0 unported license (https://creativecommons.org/licenses/by/4.0/).
When considering immune defenses, the important role played by epithelial barriers should be highlighted, with the epithelium representing the first line of defense in many body districts, where it forms a physical barrier protecting the underlying visceral space from pathogen invasion. For this reason, epithelial barrier leakage due to direct pathogen interactions or the damaging effects secondary to pro-inflammatory cytokine production represents a critical event that could climax in multiorgan failure, especially in severely ill patients. Interestingly, epithelial cells express VDRs, thus representing a target for vitamin D endocrine signaling. Considering that epithelial barrier function relies on the junctional complexes connecting epithelial cells (mainly tight and adherent junctions) and that among the non-genomic biological effects of vitamin D, there is its ability to improve cell-to-cell junction’s functionality, it is not surprising that this vitamin could play an important role in improving host immunity also by acting at epithelial level [72,73,74]. Existing evidence supports the existence of a direct correlation between vitamin D deficiency and an increased risk of developing specific pathological conditions characterized by epithelial barrier compromise, especially at gastrointestinal (e.g., inflammatory bowel disease, celiac disease, ulcerative colitis, and Crohn’s disease) and pulmonary (e.g., chronic obstructive pulmonary disease, asthma, cystic fibrosis, and acute respiratory distress syndrome) level [73,74,75].
4. Vitamin D and Respiratory System Protection
Airways’ lumen is covered by a thin epithelial layer composed of specialized cells that closely interact with the immune cells harbored in the underlying connective tissue. Schematically, the airways’ epithelium is composed of a pseudostratified epithelial layer composed of different classes of cells connected to each other by different junctional complexes (tight and adherent junctions), forming a physical barrier between the inhaled air and the visceral space. The first line of defense assured by this physical barrier is assisted by the activity of ciliated and secretory primary cells in the epithelium, whose physiological role is to sustain mucociliary clearance of inhaled particulate and pathogens. Moreover, respiratory epithelial cells are also able to directly recognize invading pathogens through the toll-like receptor pathway and to respond accordingly by releasing antimicrobial peptides (e.g., defensins, cathelicidins, lysozyme, and lactoferrin) into the airway lumen as well as by releasing chemokines and cytokines (e.g., IL-1, TNF-α, IL-6, IL-8, eotaxin) into the submucosal layer to start an inflammatory response, which will activate immune response [76,77,78,79,80,81,82].
Interestingly, as previously stated, not only immune cells, but also respiratory epithelial cells express both VDR and CYP27B1 enzyme, making this anatomical district sensitive to vitamin D’s immunomodulatory activities [46,48,63,67,71,76,83]. Focusing on the respiratory system, it is known that airway epithelial cells are able to constitutively produce the active form of vitamin D and to increase its synthetic rate in response to viral infections. On the contrary, alveolar macrophages and dendritic cells only produce active vitamin D following pathogenic stimulation. In all cases, active vitamin D, by acting in an autocrine manner, plays a crucial role in fighting infectious agents [83,84].
From a mechanistic point of view, in vitro studies demonstrated that vitamin D is able, following an infection, to downregulate proinflammatory chemokine production in respiratory epithelium, as well as to support lymphocytes’ anti-inflammatory responses by modulating NF-κB signaling pathway [51,69,70,85]. Furthermore, in vitro studies also showed vitamin D’s ability to improve lung epithelial tight junctions’ functionality, by acting both on the proinflammatory milieu (mainly by reducing TNF-α levels) and on the junctional complex itself (mainly by reducing claudin-2 levels), thus representing a promising prophylactic measure against respiratory pathogens [86].
Since vitamin D exerts its biological roles by interacting with a specific receptor, also VDRs’ role in maintaining respiratory epithelial barrier integrity has been investigated. Animal studies in murine models demonstrated that VDR partial knockout resulted in a more severe LPS-induced pulmonary epithelial barrier damage, associated with reduced occludin and zonula occludens protein-1 expression. As already observed in in-vitro models, also in this case vitamin D was able to partially revert the damage, improving epithelial barrier function [73]. The key role of VDR in assuring pulmonary barrier functionality is even more evident when analyzing the effect of its complete knockout: VDR null mice showed an altered expression of tight and adherent junction-specific molecules, such as claudins, occludin, and zonula occludens protein-1, resulting in increased leakage of the epithelial barrier in pulmonary diseases [87,88].
According to both in vitro and animal models-derived evidence, the mechanisms leading to respiratory barrier dysfunction following infection and/or inflammation represent a multifactorial process, finally resulting in lung damage. Epithelial barrier damage is not only the direct result of pathogen interactions but can also be secondary to proinflammatory cytokines directly secreted by epithelial and endothelial cells. In both cases, vitamin D emerges as a potential resource to improve airway defense mechanisms [89].
The available literature on vitamin D’s involvement in pulmonary pathology highlights the correlation between hypovitaminosis D and inflammatory airway diseases, supporting its immunomodulatory and protective role, thus fostering its use as an add-on to the standard of care therapy to improve patient’s quality of life. In particular, recent intervention studies demonstrated vitamin D supplementation’s ability to reduce chronic obstructive pulmonary disease and asthma exacerbations, as well as viral and bacterial infections secondary to these pathological conditions, by improving respiratory epithelial barrier integrity, promoting antimicrobial peptides production, and modulating inflammatory and immune responses [85,90,91].
Furthermore, considering vitamin D’s involvement in lung physiology and the fact that the peak season of respiratory infections generally coincides with the lower circulating vitamin D levels, due to reduced sun exposure in winter months, it is not surprising that available literature shows an increased rate in respiratory infections in people with suboptimal vitamin D levels [45,46,53,62,66,70,89,92,93].
Recent studies revealed a relationship between hypovitaminosis D and tuberculosis prevalence, as well as the likelihood of disease progression from dormant to aggressive form [69,90]. Moreover, vitamin D supplementation in patients affected by tubercular infection has been shown to activate innate antibacterial as well as anti-inflammatory responses, thus representing a promising prophylactic approach in patients with latent infection [69,90,94].
Vitamin D is also known to protect against other respiratory infections. By improving cellular natural as well as adaptive immunity and epithelial barrier functions, it also protects individuals (and especially those already deficient of this nutrient) from the common cold and upper respiratory tract infections [90,95,96,97] as well as from more severe conditions such as acute lung injury and pneumonia [85].
However, existing meta-analyses and literature reviews of currently available controlled trials aimed to investigate vitamin D’s therapeutic role against airway infections yielded limited or inconclusive results [65,92,96,98,99,100,101,102], mainly because of the high heterogeneity in trial design, patients’ selection, vitamin D baseline levels, vitamin D supplementation posology, and endpoints definition. Nevertheless, the hypothesized prophylactic and protective role of this key nutrient in fighting respiratory infections is supported by some in vitro and animal studies, which cast new light on the still poorly understood underlying molecular mechanisms involved [49,65,70,75,89,100,102,103,104,105,106].
Considering these promising results against respiratory infections, it is not surprising that during the COVID-19 pandemic, vitamin D raised increasing interest as an add-on intervention to prevent or mitigate disease evolution.
5. Vitamin D and SARS-CoV-2 Infection
Despite the well-known extrapulmonary manifestations and complications of COVID-19, the disease is still primarily a respiratory infection. Respiratory epithelium lining the airways has been described as the primary infection site of SARS-CoV-2, a feature maintained by all the subsequent viral variants that have emerged so far, and which has evolved to improve viral fitness in such specific milieu in the more recent variants, such as Omicron BA.5 [107,108].
Since the beginning of the COVID-19 pandemic, several researchers have investigated SARS-CoV-2 infection and replication mechanisms, especially in the respiratory environment, revealing that viral spread and disease severity were strictly correlated with epithelium and immune cell interactions and that the most critical respiratory outcomes depend on hyperactivated immune responses finally resulting in exacerbated epithelial barrier destruction [109,110,111,112].
In the context of COVID-19 infection, respiratory epithelium plays a critical role, as it represents the primary SARS-CoV-2 target, due to the surface expression of ACE-2 and TMPRSS2, two key actors involved in viral entry, while acting as an immune organ involved in the neutralization of inhaled pathogens. Such a dual role of the airway epithelium accounts for its role in defining disease evolution, with the most severe clinical outcomes being generally associated with deep lung involvement [109,113].
Several research works cast new light on SARS-CoV-2 pathophysiologic mechanisms, further supporting the pivotal role of respiratory epithelium in viral systemic dissemination [114,115,116]. The first observation of a direct SARS-CoV-2 ability to damage epithelial barrier dates back to 2020 when Hao and coworkers [116] reported a decrease in transepithelial electrical resistance value along with the disappearance in zonula occludens-1 protein in a polarized human airway epithelium grown in an air–liquid interface system. More recently these results were confirmed and expanded by the work of Xu and colleagues [114], who described the role of viral protein E in airway barrier damage, highlighting its ability to down-regulate tight junctions’ integrity, by specifically targeting zonula occludens-1 protein, along with its ability to exacerbate inflammatory responses by increasing intracellular chloride concentrations. Furthermore, it should be considered that after airway infection, leukocytes, and particularly neutrophils, represent the first immune cells recruited to the site, where they start releasing pro-inflammatory mediators responsible for innate immune responses. Respiratory neutrophilia is a common feature of many chronic inflammatory lung diseases, and in the case of COVID-19, it has been correlated to disease severity. To better understand neutrophils’ role in fighting SARS-CoV-2 infection, Calvert and coworkers [115] developed an in vitro model of neutrophilic airways, showing that these granulocytes behave differently in the apical and basolateral compartment of the infected respiratory epithelium, as demonstrated by the specific pro-inflammatory cytokine profile observed, and that neutrophilia is responsible for the reduction in epithelial barrier integrity, thus supporting an increased viral spreading, due to an augmented translocation of the infecting virus from the apical to basolateral compartment of the epithelium.
The above-summarized evidence about SARS-CoV-2 pathophysiology highlights the critical role of airway epithelium and immune system crosstalk in supporting viral spread and dissemination. Furthermore, as respiratory epithelium activation can result in both type 1 and type 2 cytokine production and considering that these immune responses appear to be differently regulated according to disease prognosis [109], it is not surprising that vitamin D, which is known to exert an immunomodulatory action both at systemic and pulmonary level, has been investigated as a potential therapeutic and/or supportive intervention able to improve COVID-19 clinical outcomes.
Based on the previous observation that reduced vitamin D is a clinical condition that could result in increased susceptibility to upper respiratory tract infections [47,57,102,117], similar evaluations have been carried out also in the context of COVID-19 and, even if some conflicting results emerged, many studies highlighted a relationship between baseline vitamin D status and SARS-CoV-2 infection risk and/or severe disease outcomes [4,43,47,55,56,58,118,119,120,121,122,123]. Furthermore, an inverse relationship has been observed between subsequent disease wave peaks and solar irradiation, a key event in determining vitamin D production in the human body [30,124,125].
From a pathobiological point of view, it is well accepted that SARS-CoV-2 infection elicits proinflammatory cytokine release, which could heavily impact disease evolution. Given that vitamin D is known to counteract, at least partially, such phenomenon and that it is involved both directly and indirectly in the regulation of different thrombotic pathways, it is conceivable that great attention has been granted to vitamin D supplementation in order, not only to prevent viral infection, but also to reduce both ARDS risk and coagulation abnormalities, especially in severely ill patients [55,118,126,127].
The growing interest in the vitamin D’s supportive role in fighting COVID-19 is based on the increasing knowledge about its molecular actions and mechanistic interactions with ACE-2, one of the key players in disease evolution. ACE-2, which is known as the primary entry receptor for SARS-CoV-2, is one of the components of the renin-angiotensin-aldosterone (RAAS) system, an important endocrine axis involved in homeostasis maintenance. In particular, ACE-2 is responsible for the conversion of angiotensin II (a proinflammatory and pro-coagulant mediator) into angiotensin(1–7) (an anti-inflammatory mediator). SARS-CoV-2 infection thus results in a depletion of ACE-2 with consequent increase of angiotensinogen II levels, which contribute to the observed sustained inflammation associated with COVID-19 evolution [126,128]. Among vitamin D’s biological effects, it is worth noting its ability to increase both the expression and bioavailability of ACE-2, both in its membrane-associated and soluble form, thus dampening the RAAS system, whose up-regulation is involved in cytokine storm generation [47,118,126]. From a mechanistic point of view, vitamin D’s protective effects mainly depend on its ability to inhibit renin, to increase angiotensin(1–7) levels, and to increase ACE-2 bioavailability. In particular, vitamin D inhibits renin gene transcription, thus reducing RAAS axis activity and angiotensin II levels, while increasing angiotensin(1–7), which has an important role in counterbalancing proinflammatory and pro-thrombogenic angiotensin II effect, by reducing TNF-α and IL6 expression and stimulating nitric oxide release from platelets, finally resulting in inflammation and coagulation abnormalities mitigation [47,58,126,128,129,130]. Furthermore, as previously stated, vitamin D is able to increase soluble ACE-2 that, contrary to the membrane-located form, has been demonstrated to act as a decoy viral receptor in early-stage infection, thus being involved in viral neutralization. In fact, soluble ACE-2 can bind SARS-CoV-2 viral particles, thus preventing them from reaching the membrane-bound form, and the resulting soluble ACE-2/SARS-CoV-2 complex are transported to natural killer cells and macrophages for destruction [128,131,132].
The large amount of possible positive effects of vitamin D in the contest of SARS-CoV-2 infection has fostered the design of several clinical trials aimed at the definition of clear clinical guidelines about its supplementation in COVID-19 patients. Unfortunately, as for many other dietary supplements tested as adjuvants in disease management, clinical research on vitamin D supplementation in COVID-19 patients led to mixed results, with some studies reporting beneficial effects, while others did not show any significant improvement in clinical outcomes [133,134,135,136,137]. As summarized in Table 2 [138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158], several studies reported an improvement in patients’ conditions after vitamin D supplementation [139,140,143,144,145,146,148,149,150,152,153,154,156,157,158], while others did not support the clinical adoption of such supportive intervention [138,141,142,147,151,155]. These inconclusive results mainly depend on the study design, showing a large heterogeneity in terms of vitamin D dose, formulation (biologically active vs. inactive metabolite), frequency and duration of the intervention (single bolus vs. continuous administration over longer periods) as well as in terms of population (i.e., age and disease severity, comorbidities, vitamin D basal status, hospital or outpatient setting).

Table 2.
Summary of the most relevant clinical trials investigating vitamin D (considering all the available pharmaceutical formulations) supplementation effectiveness in preventing or mitigating COVID-19 outcomes in adult patients.
In spite of the large literature evidence about the use of vitamin D supplementation in the context of COVID-19, the vast heterogeneity of the obtained results limited their generalizability. As shown in Table 2, several groups obtained promising results by integrating vitamin D into their standard-of-care routine approaches. Nevertheless, each group adopted different selection criteria, endpoints, doses, formulations, and posology, preventing the definition of clear guidelines about its clinical use. The increasing interest in such cheap and easy-to-use intervention, which could be of great support, especially in those situations where the available prophylactic and/or therapeutic resources are limited, highlights the need for continuous research on the topic. In particular, it is evident that novel clinical trials, designed adopting more standardized selection criteria and clinical as well as laboratory endpoints, showing sufficient statistical power to reach reliable results, will be essential to support national and international agencies in defining clear instructions, in terms of dose, formulation, and administration schedule to support clinicians in the choice of the most effective therapeutic approach for patients suffering from COVID-19 and, potentially, other related respiratory infections.
6. Vitamin D and Anti-SARS-CoV-2 Vaccination Response
The COVID-19 pandemic resulted in a wide effort from the pharmacological industry in developing new vaccines and, since 2020, more than 50 vaccines have been developed and tested in clinical trials (phase II and phase III). Thanks to this quickly evolving scenario, at the beginning of the worldwide mass vaccination campaign, several newly developed vaccines were approved by the competent international authorities [13,159,160,161].
In western countries, the vaccination campaign initially relied on two different vaccine platforms: the one based on recombinant messenger ribonucleic acid (mRNA), represented by the BNT162b2 (Pfizer-BioNTech, Mainz, Germany) and the mRNA-1273 (Moderna, Cambridge, MA, USA) vaccines, and the other based on partially inactivated adenovirus vectors, represented by the ChAdOx1-S (Astra-Zeneca, University of Oxford, UK) and the Ad.26.COV2.S (Janssen/Johnson & Johnson, New Brunswick, NJ, USA) [12,161]. Even if in recent years newly developed vaccine platforms became available, the most widely used worldwide is still represented by the mRNA-based one [11,159,162].
Although the initial clinical trials resulted in a high level of effectiveness of these vaccines [13,162,163,164,165,166], variations in responsiveness between recipients emerged from real-world data [160,167], thus fostering research focused on the understanding of the factors affecting vaccine efficacy, in order to improve world population protection against COVID-19 and reduce SARS-CoV-2 spread.
Considering its well-accepted immunomodulatory role and its easy availability as a nutritional supplement, vitamin D has gained attention also in this field. Previous evidence highlighted the effectiveness of vitamin D supplementation in inducing higher titers of specific IgG antibodies and improving mucosal immunity in response to different vaccines, such as those against tetanus, hepatitis B, and polio, both in animal models and humans [168,169,170,171,172].
To date, the investigation of vitamin D’s role in modulating immune response to the COVID-19 vaccine led to mixed results [159,167,173,174,175,176,177,178,179,180,181,182]. These conflicting results mainly depend on the vaccine platform (mRNA vs. adenoviral vectors), number of doses of the vaccine received, basal vitamin D status, vitamin D supplementation, comorbidities, previous exposure to the virus, and timing of IgG titer evaluation (peak response vs. specific time points) [159,175]. The most relevant clinical studies dealing with vitamin D’s effect on immune response after COVID-19 vaccination are summarized in Table 3.

Table 3.
Summary of the most relevant clinical studies investigating vitamin D (considering all available pharmaceutical formulations) effects on immune response after COVID-19 vaccination (different vaccine platforms).
As summarized in Table 3, results about vitamin D’s effects on anti-SARS-CoV-2 vaccine-elicited immune protection are conflicting, thus fostering further studies on the topic. From a mechanistic point of view, it could be hypothesized that, thanks to its well-known immunomodulatory activities, vitamin D could influence vaccine effectiveness by activating specific genetic pathways resulting in an enhanced T cell activation, proliferation, and conversion in memory T cells. Even if the main role of vitamin D in improving vaccine response seems to be related to its effects on T cell-mediated immunity, it can not be excluded also a possible role of this nutrient also in regulating B cell-mediated responses. Considering that an improvement of vaccine-elicited immune response could be important for all vaccinations, elucidating its mechanism of action could help to define clinical guidelines regarding vitamin D repletion before the beginning of the vaccination schedule, in order to obtain the maximum protection of the recipient.
7. Conclusions
Bibliographic research about the role of vitamin D in the context of COVID-19 supports the constant interest in the topic. Since the beginning of the pandemic, evidence accumulated regarding the possible relationship between vitamin D status and disease evolution. Ecological studies highlighted some interesting correlations between vitamin D levels and COVID-19: subsequent disease wave peaks have been shown to inversely correlate with solar radiation intensity and consequent vitamin D production, both in terms of timing and latitude. Moreover, disease morbidity and mortality have been shown to be related to the mean vitamin D status in different ethnic groups as, according to different authors, hypovitaminosis D differently affects populations with different skin pigmentation. Lastly, COVID-19’s most severe manifestations depend on hypercytokinemia and dysregulated immune response, two clinical features that could be influenced by vitamin D status. Considering the still accumulating evidence about the role of vitamin D as a modifiable risk factor for COVID-19 infection and/or negative outcomes, it is conceivable that the correction of hypovitaminosis D might act synergistically with the available therapies to improve clinical evolution, as well as with the anti-SARS-CoV-2 vaccines to improve immunological protection against infection.
To date, the mechanism by which vitamin D modulates COVID-19 evolution is still unknown: it appears to be reasonable that vitamin D’s protective effects might rely on different biological responses, such as antimicrobial peptides production, reduction in inflammatory mediator levels, and modulation of the renin-angiotensin pathway. For this reason, well-designed clinical trials are still needed to improve the understanding of vitamin D’s role in COVID-19 disease evolution and to establish evidence-based clinical guidelines for vitamin D supplementation, especially in populations at increased risk of developing more severe disease manifestations. Finally, a significant increase in vitamin D levels has been observed compared to the pre-pandemic period, mainly as a result of an uncontrolled, self-prescribed use of vitamin supplements. In particular, such rise in basal vitamin D status in the general population has been described to be temporarily associated with the emergence of widespread reports, often lacking rigorous scientific verification, claiming vitamin D’s benefits in COVID-19 management [183], thus further supporting the role of evidence-based information dissemination in ensuring patient safety, especially during public health emergencies.
Author Contributions
All authors contributed to the literature review and manuscript drafting. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, J.; Wang, R.; Hozumi, Y.; Liu, G.; Qiu, Y.; Wei, X.; Wei, G.W. Emerging dominant SARS-CoV-2 variants. J. Chem. Inf. Model. 2023, 63, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Koelle, K.; Martin, M.A.; Antia, R.; Lopman, B.; Dean, N.E. The changing epidemiology of SARS-CoV-2. Science 2022, 375, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Salasc, F.; Lahlali, T.; Laurent, E.; Rosa-Calatrava, M.; Pizzorno, A. Treatments for COVID-19: Lessons from 2020 and new therapeutic options. Curr. Opin. Pharmacol. 2022, 62, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Abdel-Ghany, S.; Abdallah, M.S.; Abul-Maaty, O.; Khoder, A.I.; Shoman, N.A.; Farrag, M.S.; Martasek, P.; Noreddin, A.M.; Nazih, M. Vitamin D: A key player in COVID-19 immunity and lessons from the pandemic to combat immune-evasive variants. Inflammopharmacology 2024, 32, 3631–3652. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Wi, Y.M.; Park, H.; Lee, J.E.; Kim, S.H.; Lee, K.S. Current and emerging knowledge in COVID-19. Radiology 2023, 306, e222462. [Google Scholar] [CrossRef]
- Rizzi, M.; D’Onghia, D.; Tonello, S.; Minisini, R.; Colangelo, D.; Bellan, M.; Castello, L.M.; Gavelli, F.; Avanzi, G.C.; Pirisi, M.; et al. COVID-19 biomarkers at the crossroad between patient stratification and targeted therapy: The role of validated and proposed parameters. Int. J. Mol. Sci. 2023, 24, 7099. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Frias-Toral, E.; Laudisio, D.; Pugliese, G.; Castellucci, B.; Garcia-Velasquez, E.; Savastano, S.; Colao, A. Nutrition and immune system: From the Mediterranean diet to dietary supplementary through the microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 3066–3090. [Google Scholar] [CrossRef]
- Rizzi, M.; Patrucco, F.; Trevisan, M.; Faolotto, G.; Mercandino, A.; Strola, C.; Ravanini, P.; Costanzo, M.; Tonello, S.; Matino, E.; et al. Baseline plasma SARS-CoV-2 RNA detection predicts an adverse COVID-19 evolution in moderate to severe hospitalized patients. Panminerva Med. 2022, 64, 465–471. [Google Scholar] [CrossRef]
- Gong, W.; Parkkila, S.; Wu, X.; Aspatwar, A. SARS-CoV-2 variants and COVID-19 vaccines: Current challenges and future strategies. Int. Rev. Immunol. 2023, 42, 393–414. [Google Scholar] [CrossRef]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef]
- Rizzi, M.; Tonello, S.; Brinno, C.; Zecca, E.; Matino, E.; Cittone, M.; Rizzi, E.; Casciaro, G.F.; D’Onghia, D.; Colangelo, D.; et al. SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: Results of the extended follow up of the RIVALSA prospective cohort. Front. Immunol. 2023, 14, 1185278. [Google Scholar] [CrossRef]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.E.; Howlader, M.; Akter, F.; Hossain, M.M. Preclinical and clinical investigations of potential drugs and vaccines for COVID-19 therapy: A comprehensive review with recent update. Clin. Pathol. 2024, 17, 2632010X241263054. [Google Scholar] [CrossRef]
- Atluri, K.; Aimlin, I.; Arora, S. Current effective therapeutics in management of COVID-19. J. Clin. Med. 2022, 11, 3838. [Google Scholar] [CrossRef]
- Zaman, R.; Ravichandran, V.; Tan, C.K. Role of dietary supplements in the continuous battle against COVID-19. Phytother. Res. 2024, 38, 1071–1088. [Google Scholar] [CrossRef]
- Arora, I.; White, S.; Mathews, R. Global dietary and herbal supplement use during COVID-19-A scoping review. Nutrients 2023, 15, 771. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Fakhrany, O.M.; Elekhnawy, E.; Al-Gareeb, A.I.; Alorabi, M.; De Waard, M.; Albogami, S.M.; Batiha, G.E. Traditional herbs against COVID-19: Back to old weapons to combat the new pandemic. Eur. J. Med. Res. 2022, 27, 186. [Google Scholar] [CrossRef]
- Corrao, S.; Mallaci Bocchio, R.; Lo Monaco, M.; Natoli, G.; Cavezzi, A.; Troiani, E.; Argano, C. Does evidence exist to blunt inflammatory response by nutraceutical supplementation during COVID-19 pandemic? An overview of systematic reviews of vitamin D, vitamin C, melatonin, and zinc. Nutrients 2021, 13, 1261. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal remedies, nutraceuticals, and dietary supplements for COVID-19 management: An update. Clin. Complement. Med. Pharmacol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- Mrityunjaya, M.; Pavithra, V.; Neelam, R.; Janhavi, P.; Halami, P.M.; Ravindra, P.V. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020, 11, 570122. [Google Scholar] [CrossRef]
- Pavlidou, E.; Poulios, E.; Papadopoulou, S.K.; Fasoulas, A.; Dakanalis, A.; Giaginis, C. Clinical evidence on the potential beneficial effects of diet and dietary supplements against COVID-19 infection risk and symptoms’ severity. Med. Sci. 2024, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Burr, A.H.P.; Bhattacharjee, A.; Hand, T.W. Nutritional modulation of the microbiome and immune response. J. Immunol. 2020, 205, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Rytter, M.J.; Kolte, L.; Briend, A.; Friis, H.; Christensen, V.B. The immune system in children with malnutrition-a systematic review. PLoS ONE 2014, 9, e105017. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vera, D.; Salazar, J.R.; Soriano-Ursúa, M.A.; Guzmán-Pérez, J.; Vergara-Castañeda, A.; Muñoz-Durán, H.; Ramírez-Velez, G.L.; Vivar-Sierra, A.; Naranjo-Navarro, C.R.; Meza-Meneses, P.A.; et al. Effectiveness of omega-3 fatty acid supplementation in improving the metabolic and inflammatory profiles of Mexican adults hospitalized with COVID-19. Diseases 2024, 12, 28. [Google Scholar] [CrossRef]
- Doaei, S.; Gholami, S.; Rastgoo, S.; Gholamalizadeh, M.; Bourbour, F.; Bagheri, S.E.; Samipoor, F.; Akbari, M.E.; Shadnoush, M.; Ghorat, F.; et al. The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: A randomized clinical trial. J. Transl. Med. 2021, 19, 128. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Triantos, C.; Mouzaki, A. The influence of nutritional factors on immunological outcomes. Front. Immunol. 2021, 12, 665968. [Google Scholar] [CrossRef]
- Manzoni, P.; Messina, A.; Germano, C.; Picone, S.; Masturzo, B.; Sainaghi, P.P.; Sola, D.; Rizzi, M. Lactoferrin supplementation in preventing and protecting from SARS-CoV-2 infection: Is there any role in general and special populations? an updated review of literature. Int. J. Mol. Sci. 2024, 25, 10248. [Google Scholar] [CrossRef]
- Polak, E.; Stępień, A.E.; Gol, O.; Tabarkiewicz, J. Potential immunomodulatory effects from consumption of nutrients in whole foods and supplements on the frequency and course of infection: Preliminary results. Nutrients 2021, 13, 1157. [Google Scholar] [CrossRef]
- Scarpellini, E.; Rinninella, E.; Basilico, M.; Colomier, E.; Rasetti, C.; Larussa, T.; Santori, P.; Abenavoli, L. From pre- and probiotics to post-biotics: A narrative review. Int. J. Environ. Res. Public Health 2021, 19, 37. [Google Scholar] [CrossRef]
- Rizzi, M.; Avellis, V.; Messina, A.; Germano, C.; Tavella, E.; Dodaro, V.; Vitale, R.; Revelli, A.; Zola, P.; Picone, S.; et al. Vitamin D supplementation in neonatal and infant MIS-C following COVID-19 infection. Int. J. Mol. Sci. 2024, 25, 3712. [Google Scholar] [CrossRef]
- Matino, E.; Tavella, E.; Rizzi, M.; Avanzi, G.C.; Azzolina, D.; Battaglia, A.; Becco, P.; Bellan, M.; Bertinieri, G.; Bertoletti, M.; et al. Effect of lactoferrin on clinical outcomes of hospitalized patients with COVID-19: The LAC randomized clinical trial. Nutrients 2023, 15, 1285. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, F.D.; Elabbasy, M.T.; Samak, M.A.; Adeboye, A.A.; Yusuf, R.A.; Ghoniem, M.E. The prospect of lactoferrin use as adjunctive agent in management of SARS-CoV-2 patients: A randomized pilot study. Medicina 2021, 57, 842. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Bhushan, D.; Supriya, S.; Ganapule, A.A.; Lohani, P.; Shyama Pandey, S.; Majhi, P.K.; Anand, U.; Kumar, R.; Bhadani, U.K. Efficacy of intravenous vitamin C in management of moderate and severe COVID-19: A double blind randomized placebo controlled trial. J. Fam. Med. Prim. Care 2022, 11, 4758–4765. [Google Scholar] [CrossRef]
- Labbani-Motlagh, Z.; Amini, S.; Aliannejad, R.; Sadeghi, A.; Shafiee, G.; Heshmat, R.; Jafary, M.; Talaschian, M.; Akhtari, M.; Jamshidi, A.; et al. High-dose intravenous vitamin C in early stages of severe acute respiratory syndrome coronavirus 2 infection: A double-blind, randomized, controlled clinical trial. J. Res. Pharm. Pract. 2022, 11, 64–72. [Google Scholar] [CrossRef]
- Suna, K.; Melahat, U.Ş.; Murat, Y.; Figen, Ö.E.; Ayperi, Ö. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med. Clin. Engl. Ed. 2022, 158, 356–360. [Google Scholar] [CrossRef]
- Gao, D.; Xu, M.; Wang, G.; Lv, J.; Ma, X.; Guo, Y.; Zhang, D.; Yang, H.; Jiang, W.; Deng, F.; et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: A retrospective cohort study. Aging 2021, 13, 7020–7034. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: The COVID A to Z randomized clinical trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Alizadeh, N.; Dianatkhah, M.; Alimohamadi, Y.; Moradi, H.; Akbarpour, S.; Akrami, M.; Mansouri, F.; Faraji, N.; Rezaie, Z.; Alizadeh, M.; et al. High dose melatonin as an adjuvant therapy in intubated patients with COVID-19: A randomized clinical trial. J. Taibah Univ. Med. Sci. 2022, 17, 454–460. [Google Scholar] [CrossRef]
- Farnoosh, G.; Akbariqomi, M.; Badri, T.; Bagheri, M.; Izadi, M.; Saeedi-Boroujeni, A.; Rezaie, E.; Ghaleh, H.E.G.; Aghamollaei, H.; Fasihi-Ramandi, M.; et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: A randomized, double-blind clinical trial. Arch. Med. Res. 2022, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Sun, A.; Xiao, T.; Yao, G.; Sang, L.; Zheng, X.; Zhang, J.; Jin, X.; Xu, L.; Yang, W.; et al. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19). Front. Med. 2022, 8, 566609. [Google Scholar] [CrossRef] [PubMed]
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The power of vitamin D: Is the future in precision nutrition through personalized supplementation plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef] [PubMed]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Tsikopoulos, A.; Tsikopoulos, K.; Fountarlis, A.; Efthymiadis, A.; Festas, C.; Garefis, K. Is there any association between vitamin D deficiency and recurrent tonsillopharyngitis? An updated systematic review. Maedica 2024, 19, 116–128. [Google Scholar] [CrossRef]
- Laaksi, I. Vitamin D and respiratory infection in adults. Proc. Nutr. Soc. 2012, 71, 90–97. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity—The immune system and vitamin D: A systematic review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Gao, N.; Raduka, A.; Rezaee, F. Vitamin D3 protects against respiratory syncytial virus-induced barrier dysfunction in airway epithelial cells via PKA signaling pathway. Eur. J. Cell Biol. 2023, 102, 151336. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.; Thangamuthu, A.; Muthumeenal, S.; Houston, K.; Everton, M.; Gowda, S.; Zhang, J.; Subramanian, R. Vital D: A modifiable occupational risk factor of UK healthcare workers. PLoS ONE 2024, 19, e0296247. [Google Scholar] [CrossRef]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and regional prevalence of vitamin D deficiency in population-based studies from 2000 to 2022: A pooled analysis of 7.9 million participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Hafez, W.; Saleh, H.; Arya, A.; Alzouhbi, M.; Fdl Alla, O.; Lal, K.; Kishk, S.; Ali, S.; Raghu, S.; Elgaili, W.; et al. Vitamin D status in relation to the clinical outcome of hospitalized COVID-19 patients. Front. Med. 2022, 9, 843737. [Google Scholar] [CrossRef]
- De Smet, D.; De Smet, K.; Herroelen, P.; Gryspeerdt, S.; Martens, G.A. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am. J. Clin. Pathol. 2021, 155, 381–388. [Google Scholar] [CrossRef]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4048–4052. [Google Scholar] [CrossRef]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Bellan, M.; Andreoli, L.; Mele, C.; Sainaghi, P.P.; Rigamonti, C.; Piantoni, S.; De Benedittis, C.; Aimaretti, G.; Pirisi, M.; Marzullo, P. Pathophysiological role and therapeutic implications of vitamin D in autoimmunity: Focus on chronic autoimmune diseases. Nutrients 2020, 12, 789. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Nerviani, A.; Sola, D.; Molinari, R.; Cerutti, C.; Pirisi, M. Superiority of a high loading dose of cholecalciferol to correct hypovitaminosis d in patients with inflammatory/autoimmune rheumatic diseases. J. Rheumatol. 2013, 40, 166–172. [Google Scholar] [CrossRef]
- Sainaghi, P.P.; Bellan, M.; Antonini, G.; Bellomo, G.; Pirisi, M. Unsuppressed parathyroid hormone in patients with autoimmune/inflammatory rheumatic diseases: Implications for vitamin D supplementation. Rheumatology 2011, 50, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Simanek, V.; Dedeckova, E.; Topolcan, O.; Kralova, M.; Kucera, R. A case study of vitamin D supplementation therapy and acute respiratory tract infection. In Vivo 2024, 38, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D regulation of immune function. Curr. Osteoporos. Rep. 2022, 20, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Khashim Alswailmi, F.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular mechanisms of vitamin D-mediated immunomodulation. Galen. Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Gruber-Bzura, B.M. Vitamin D and influenza-prevention or therapy? Int. J. Mol. Sci. 2018, 19, 2419. [Google Scholar] [CrossRef]
- Lang, P.O.; Aspinall, R. Vitamin D status and the host resistance to infections: What it is currently (not) understood. Clin. Ther. 2017, 39, 930–945. [Google Scholar] [CrossRef]
- White, J.H. Emerging roles of vitamin D-induced antimicrobial peptides in antiviral innate immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Cantorna, M.T. Vitamin D and lung infection. Infect. Immun. 2016, 84, 3094–3096. [Google Scholar] [CrossRef]
- Selvaraj, P.; Harishankar, M.; Afsal, K. Vitamin D: Immuno-modulation and tuberculosis treatment. Can. J. Physiol. Pharmacol. 2015, 93, 377–384. [Google Scholar] [CrossRef]
- Sundaram, M.E.; Coleman, L.A. Vitamin D and influenza. Adv. Nutr. 2012, 3, 517–525. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Del Rio, E.A.; Harty, R.N.; Mullin, J.M. Micronutrients at supplemental levels, tight junctions and epithelial barrier function: A narrative review. Int. J. Mol. Sci. 2024, 25, 3452. [Google Scholar] [CrossRef] [PubMed]
- DiGuilio, K.M.; Rybakovsky, E.; Abdavies, R.; Chamoun, R.; Flounders, C.A.; Shepley-McTaggart, A.; Harty, R.N.; Mullin, J.M. Micronutrient improvement of epithelial barrier function in various disease states: A case for adjuvant therapy. Int. J. Mol. Sci. 2022, 23, 2995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers 2013, 1, e23118. [Google Scholar] [CrossRef]
- Gatera, V.A.; Abdulah, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B. Updates on the status of vitamin D as a risk factor for respiratory distress syndrome. Adv. Pharmacol. Sci. 2018, 2018, 8494816. [Google Scholar] [CrossRef]
- Myszor, I.T.; Gudmundsson, G.H. Modulation of innate immunity in airway epithelium for host-directed therapy. Front. Immunol. 2023, 14, 1197908. [Google Scholar] [CrossRef]
- Gopallawa, I.; Dehinwal, R.; Bhatia, V.; Gujar, V.; Chirmule, N. A four-part guide to lung immunology: Invasion, inflammation, immunity, and intervention. Front. Immunol. 2023, 14, 1119564. [Google Scholar] [CrossRef]
- Davis, J.D.; Wypych, T.P. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021, 14, 978–990. [Google Scholar] [CrossRef]
- Hewitt, R.J.; Lloyd, C.M. Regulation of immune responses by the airway epithelial cell landscape. Nat. Rev. Immunol. 2021, 21, 347–362. [Google Scholar] [CrossRef]
- Coraux, C.; Hajj, R.; Lesimple, P.; Puchelle, E. Réparation et régénération de l’épithélium respiratoire [Repair and regeneration of the airway epithelium]. Med. Sci. 2005, 21, 1063–1069. [Google Scholar] [CrossRef][Green Version]
- Bals, R.; Hiemstra, P.S. Innate immunity in the lung: How epithelial cells fight against respiratory pathogens. Eur. Respir. J. 2004, 23, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.A.; Holgate, S.T. The airway epithelium: Structural and functional properties in health and disease. Respirology 2003, 8, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Hansdottir, S.; Monick, M.M. Vitamin D effects on lung immunity and respiratory diseases. Vitam. Horm. 2011, 86, 217–237. [Google Scholar] [CrossRef]
- Hansdottir, S.; Monick, M.M.; Hinde, S.L.; Lovan, N.; Look, D.C.; Hunninghake, G.W. Respiratory epithelial cells convert inactive vitamin D to its active form: Potential effects on host defense. J. Immunol. 2008, 181, 7090–7099. [Google Scholar] [CrossRef]
- Ahmad, S.; Arora, S.; Khan, S.; Mohsin, M.; Mohan, A.; Manda, K.; Syed, M.A. Vitamin D and its therapeutic relevance in pulmonary diseases. J. Nutr. Biochem. 2021, 90, 108571. [Google Scholar] [CrossRef]
- Rybakovsky, E.; DiGuilio, K.M.; Valenzano, M.C.; Geagan, S.; Pham, K.; Harty, R.N.; Mullin, J.M. Calcitriol modifies tight junctions, improves barrier function, and reduces TNF-α-induced barrier leak in the human lung-derived epithelial cell culture model, 16HBE 14o. Physiol. Rep. 2023, 11, e15592. [Google Scholar] [CrossRef]
- Chen, H.; Lu, R.; Zhang, Y.G.; Sun, J. Vitamin D receptor deletion leads to the destruction of tight and adherens junctions in lungs. Tissue Barriers 2018, 6, 1540904. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Liu, T.J.; Fu, J.H.; Xu, W.; Wu, L.L.; Hou, A.N.; Xue, X.D. Vitamin D/VDR signaling attenuates lipopolysaccharide-induced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol. Med. Rep. 2016, 13, 1186–1194. [Google Scholar] [CrossRef]
- Xiong, J.; Kaleja, P.; Ückert, L.; Nezaratizadeh, N.; Krantz, S.; Krause, M.F.; Fitschen-Oestern, S.; Seekamp, A.; Cassidy, L.; Tholey, A.; et al. Alveolar-capillary barrier protection in vitro: Lung cell type-specific effects and molecular mechanisms induced by 1α, 25-dihydroxyvitamin D3. Int. J. Mol. Sci. 2023, 24, 7298. [Google Scholar] [CrossRef]
- Afzal, M.; Kazmi, I.; Al-Abbasi, F.A.; Alshehri, S.; Ghoneim, M.M.; Imam, S.S.; Nadeem, M.S.; Al-Zahrani, M.H.; Alzarea, S.I.; Alquraini, A. Current overview on therapeutic potential of vitamin D in inflammatory lung diseases. Biomedicines 2021, 9, 1843. [Google Scholar] [CrossRef]
- Schrumpf, J.A.; van der Does, A.M.; Hiemstra, P.S. Impact of the local inflammatory environment on mucosal vitamin D metabolism and signaling in chronic inflammatory lung diseases. Front. Immunol. 2020, 11, 1433. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo CAJr Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; Dubnov-Raz, G.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tagle, C.; Romero, F.; Naves, R.; Balcells, M.E. Vitamin D and cathelicidin levels and susceptibility to Mycobacterium tuberculosis infection acquisition in household contacts. Enferm. Infecc. Microbiol. Clin Engl. Ed. 2023, 41, 489–493. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Greiller, C.L.; Mein, C.A.; Hoti, M.; Bakhsoliani, E.; Telcian, A.G.; Simpson, A.; Barnes, N.C.; Curtin, J.A.; Custovic, A.; et al. Vitamin D receptor genotype influences risk of upper respiratory infection. Br. J. Nutr. 2018, 120, 891–900. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- de Sa Del Fiol, F.; Barberato-Filho, S.; Lopes, L.C.; de Cassia Bergamaschi, C. Vitamin D and respiratory infections. J. Infect. Dev. Ctries. 2015, 9, 355–361. [Google Scholar] [CrossRef]
- Jia, H.; Sheng, F.; Yan, Y.; Liu, X.; Zeng, B. Vitamin D supplementation for prevention of acute respiratory infections in older adults: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0303495. [Google Scholar] [CrossRef]
- Cho, H.; Myung, S.K.; Cho, H.E. Efficacy of vitamin D supplements in treatment of acute respiratory infection: A meta-analysis for randomized controlled trials. Nutrients 2022, 14, 1144. [Google Scholar] [CrossRef]
- Gaudet, M.; Plesa, M.; Mogas, A.; Jalaleddine, N.; Hamid, Q.; Al Heialy, S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir. Res. 2022, 23, 252. [Google Scholar] [CrossRef]
- Cepeda, S.J.; Zenteno, A.D.; Fuentes, S.C.; Bustos, B.R. Vitamina D y enfermedades respiratorias pediátricas [Vitamin D and pediatrics respiratory diseases]. Rev. Chil. Pediatr. 2019, 90, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, 1909. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Huang, Y.; Zhang, J.; Xiong, Q.; Chi, M.; Yang, L.; Zhang, J.; Li, L.; Fan, Y. Vitamin D promotes epithelial tissue repair and host defense responses against influenza H1N1 virus and Staphylococcus aureus infections. Respir. Res. 2023, 24, 175. [Google Scholar] [CrossRef]
- Gatera, V.A.; Lesmana, R.; Musfiroh, I.; Judistiani, R.T.D.; Setiabudiawan, B.; Abdulah, R. Vitamin D inhibits lipopolysaccharide (LPS)-induced inflammation in A549 cells by downregulating inflammatory cytokines. Med. Sci. Monit. Basic Res. 2021, 27, e931481. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamatsu, M.; Ogasawara, H.; Tsugawa, N.; Isoda, N.; Matsuno, K.; Sakoda, Y. Oral supplementation of the vitamin D metabolite 25(OH)D3 against influenza virus infection in mice. Nutrients 2020, 12, 2000. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Oak, E.; Garcia, J.; Liu, H.; Lorenzini, P.A.; Batra, D.; Chhabra, A.; Salazar, J.C.; Roca, X. Vitamin D modulates human macrophage response to Mycobacterium tuberculosis DNA. Tuberculosis 2019, 116S, S131–S137. [Google Scholar] [CrossRef]
- Li, C.; Huang, J.; Yu, Y.; Wan, Z.; Chiu, M.C.; Liu, X.; Zhang, S.; Cai, J.P.; Chu, H.; Li, G.; et al. Human airway and nasal organoids reveal escalating replicative fitness of SARS-CoV-2 emerging variants. Proc. Natl. Acad. Sci. USA 2023, 120, e2300376120. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Cheng, R.; Lee, I.T.; Nakayama, T.; Jiang, S.; He, W.; Demeter, J.; Knight, M.G.; et al. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 2023, 186, 112–130.e20. [Google Scholar] [CrossRef]
- Guo, T.J.F.; Singhera, G.K.; Leung, J.M.; Dorscheid, D.R. Airway epithelial-derived immune mediators in COVID-19. Viruses 2023, 15, 1655. [Google Scholar] [CrossRef]
- Chua, R.L.; Lukassen, S.; Trump, S.; Hennig, B.P.; Wendisch, D.; Pott, F.; Debnath, O.; Thürmann, L.; Kurth, F.; Völker, M.T.; et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020, 38, 970–979. [Google Scholar] [CrossRef]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K.; Mahanty, S. Respiratory virus infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Pathinayake, P.S.; Awatade, N.T.; Wark, P.A.B. Type 2 immunity and its impact on COVID-19 infection in the airways. Viruses 2023, 15, 402. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.B.; Guan, W.J.; Zhang, Y.L.; Qiu, Z.E.; Chen, L.; Hou, X.C.; Yue, J.; Zhou, Y.Y.; Sheng, J.; Zhao, L.; et al. SARS-CoV-2 envelope protein impairs airway epithelial barrier function and exacerbates airway inflammation via increased intracellular Cl− concentration. Signal Transduct. Target. Ther. 2024, 9, 74. [Google Scholar] [CrossRef]
- Calvert, B.A.; Quiroz, E.J.; Lorenzana, Z.; Doan, N.; Kim, S.; Senger, C.N.; Anders, J.J.; Wallace, W.D.; Salomon, M.P.; Henley, J.; et al. Neutrophilic inflammation promotes SARS-CoV-2 infectivity and augments the inflammatory responses in airway epithelial cells. Front. Immunol. 2023, 14, 1112870. [Google Scholar] [CrossRef]
- Hao, S.; Ning, K.; Kuz, C.A.; Vorhies, K.; Yan, Z.; Qiu, J. Long-term modeling of SARS-CoV-2 infection of in vitro cultured polarized human airway epithelium. mBio 2020, 11, e02852-20. [Google Scholar] [CrossRef]
- Arihiro, S.; Nakashima, A.; Matsuoka, M.; Suto, S.; Uchiyama, K.; Kato, T.; Mitobe, J.; Komoike, N.; Itagaki, M.; Miyakawa, Y.; et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza and upper respiratory infection in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2019, 25, 1088–1095. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Nikolakopoulou, S.; Konstantinou, A.; Mascha, O.; Siarkos, E.; Samaras, C.; Athanassiou, P.; Shoenfeld, Y. Vitamin D levels as a marker of severe SARS-CoV-2 infection. Life 2024, 14, 210. [Google Scholar] [CrossRef]
- Coudray, M.S.; Hansel, S.; Alesci, S.; Meyer WA 3rd Christenson, R.H.; Landry, L.G.; Edwards, C.; Puckrein, G.; Forney, D.J.; Akinboboye, O. Vitamin D levels and SARS-CoV-2 infection among medically underserved populations in the minority and rural coronavirus insights study. Viruses 2024, 16, 639. [Google Scholar] [CrossRef]
- Metonidze, I.; Bostoganashvili, N.; Goderidze, T.; Tananashvili, D. Serum 25-hydroxyvitamin D levels and health outcomes of hospitalization owing to COVID-19: A retrospective cross-sectional study. J. Int. Med. Res. 2024, 52, 3000605241271770. [Google Scholar] [CrossRef]
- Salehi, Z.; Askari, M.; Jafari, A.; Ghosn, B.; Surkan, P.J.; Hosseinzadeh-Attar, M.J.; Pouraram, H.; Azadbakht, L. Dietary patterns and micronutrients in respiratory infections including COVID-19: A narrative review. BMC Public Health 2024, 24, 1661. [Google Scholar] [CrossRef] [PubMed]
- Konikowska, K.; Kiliś-Pstrusińska, K.; Matera-Witkiewicz, A.; Kujawa, K.; Adamik, B.; Doroszko, A.; Kaliszewski, K.; Pomorski, M.; Protasiewicz, M.; Sokołowski, J.; et al. Association of serum vitamin D concentration with the final course of hospitalization in patients with COVID-19. Front. Immunol. 2023, 14, 1231813. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K.A.; Lim Alba, R.; Li, K. Association of vitamin D levels on the clinical outcomes of patients hospitalized for COVID-19 in a tertiary hospital. J. ASEAN Fed. Endocr. Soc. 2023, 38, 81–89. [Google Scholar] [CrossRef]
- Mukherjee, S.B.; Gorohovski, A.; Merzon, E.; Levy, E.; Mukherjee, S.; Frenkel-Morgenstern, M. Seasonal UV exposure and vitamin D: Association with the dynamics of COVID-19 transmission in Europe. FEBS Open Bio 2022, 12, 106–117. [Google Scholar] [CrossRef]
- Mariani, J.; Giménez, V.M.M.; Bergam, I.; Tajer, C.; Antonietti, L.; Inserra, F.; Ferder, L.; Manucha, W. Association between vitamin D deficiency and COVID-19 incidence, complications, and mortality in 46 countries: An ecological study. Health Secur. 2021, 19, 302–308. [Google Scholar] [CrossRef]
- AlNafea, H.M.; Korish, A.A. The interplay between hypovitaminosis D and the immune dysfunction in the arteriovenous thrombotic complications of the sever coronavirus disease 2019 (COVID-19) infection. Blood Coagul. Fibrinolysis 2023, 34, 129–137. [Google Scholar] [CrossRef]
- Sengupta, T.; Majumder, R.; Majumder, S. Role of vitamin D in treating COVID-19-associated coagulopathy: Problems and perspectives. Mol. Cell. Biochem. 2021, 476, 2421–2427. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Unveiling the interplay-vitamin D and ACE-2 molecular interactions in mitigating complications and deaths from SARS-CoV-2. Biology 2024, 13, 831. [Google Scholar] [CrossRef]
- Souza, L.L.; Costa-Neto, C.M. Angiotensin-(1-7) decreases LPS-induced inflammatory response in macrophages. J. Cell. Physiol. 2012, 227, 2117–2222. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Pinheiro, S.V.; Gonçalves, A.C.; Alenina, N.; Bader, M.; Santos, R.A. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol. Med. 2008, 14, 28–35. [Google Scholar] [CrossRef]
- Oudit, G.Y.; Wang, K.; Viveiros, A.; Kellner, M.J.; Penninger, J.M. Angiotensin-converting enzyme 2-at the heart of the COVID-19 pandemic. Cell 2023, 186, 906–922. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkrüys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e7. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Saleem, M.M.; Vijay, S.; Ehsan, M.; Atiq, I.; Anwar, E.; Oduoye, M.O. Efficacy of vitamin D supplementation in the treatment of patients with COVID-19: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. Surg. 2024, 86, 6079–6090. [Google Scholar] [CrossRef] [PubMed]
- Ghoreshi, Z.A.; Charostad, J.; Arefinia, N.; Nakhaie, M.; Rezaei Zadeh Rukerd, M.; Salajegheh, F. Effect of vitamin D supplementation on clinical outcomes in adult patients with COVID-19: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. Perspect. 2024, 12, e70013. [Google Scholar] [CrossRef]
- Sobczak, M.; Pawliczak, R. Effect of vitamin D3 supplementation on severe COVID-19: A meta-analysis of randomized clinical trials. Nutrients 2024, 16, 1402. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.; Dong, H.; Shang, N.; Li, Y.; Zhang, Y.; Guo, S.; Mei, X. The impact of supplementing vitamin D through different methods on the prognosis of COVID-19 patients: A systematic review and meta-analysis. Front. Nutr. 2024, 11, 1441847. [Google Scholar] [CrossRef]
- Sîrbu, A.C.; Sabin, O.; Bocșan, I.C.; Vesa, Ș.C.; Buzoianu, A.D. The effect of vitamin D supplementation on the length of hospitalisation, intensive care unit admission, and mortality in COVID-19-A systematic review and meta-analysis. Nutrients 2023, 15, 3470. [Google Scholar] [CrossRef]
- Wang, H.; Tao, L.; Cui, L.; Chen, Y.; Liu, D.; Xue, L.; Yang, Y.; Lv, Y.; Zhang, F.; Wang, T.; et al. Randomized trial of influence of vitamin D on the prevention and improvement of symptomatic COVID-19. Sci. Rep. 2024, 14, 20519. [Google Scholar] [CrossRef]
- Dilokpattanamongkol, P.; Yan, C.; Jayanama, K.; Ngamjanyaporn, P.; Sungkanuparph, S.; Rotjanapan, P. Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: A single-center randomized controlled trial. BMC Complement. Med. Ther. 2024, 24, 97. [Google Scholar] [CrossRef]
- Singh, A.; Rastogi, A.; Puri, G.D.; Ganesh, V.; Naik, N.B.; Kajal, K.; Kahlon, S.; Soni, S.L.; Kaloria, N.; Saini, K.; et al. Therapeutic high-dose vitamin D for vitamin D-deficient severe COVID-19 disease: Randomized, double-blind, placebo-controlled study (SHADE-S). J. Public Health 2024, 46, 256–266. [Google Scholar] [CrossRef]
- Domazet Bugarin, J.; Dosenovic, S.; Ilic, D.; Delic, N.; Saric, I.; Ugrina, I.; Stojanovic Stipic, S.; Duplancic, B.; Saric, L. Vitamin D supplementation and clinical outcomes in severe COVID-19 patients-randomized controlled trial. Nutrients 2023, 15, 1234. [Google Scholar] [CrossRef] [PubMed]
- Abroug, H.; Maatouk, A.; Bennasrallah, C.; Dhouib, W.; Ben Fredj, M.; Zemni, I.; Kacem, M.; Mhalla, S.; Nouira, S.; Ben Belgacem, M.; et al. Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: A randomized-controlled clinical trial. Trials 2023, 24, 123. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, R.R.; Khorasanchi, Z.; Noor, A.R.; Moghadam, M.S.F.; Esfahani, A.J.; Alyakobi, A.K.M.; Alboresha, M.L.; Sharifan, P.; Bahari, A.; Rezvani, R.; et al. High-dose vitamin D supplementation is related to an improvement in serum alkaline phosphatase in COVID-19 patients; a randomized double-blinded clinical trial. J. Health Popul. Nutr. 2023, 42, 71. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.W.; Ashfaq, A.; Melnick, J.Z.; Vazquez-Escarpanter, E.; Fialkow, J.A.; Strugnell, S.A.; Choe, J.; Kalantar-Zadeh, K.; Federman, N.C.; Ng, D.; et al. REsCue trial: Randomized controlled clinical trial with extended-release calcifediol in symptomatic COVID-19 outpatients. Nutrition 2023, 107, 111899. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougère, B.; Guérin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef]
- Villasis-Keever, M.A.; López-Alarcón, M.G.; Miranda-Novales, G.; Zurita-Cruz, J.N.; Barrada-Vázquez, A.S.; González-Ibarra, J.; Martínez-Reyes, M.; Grajales-Muñiz, C.; Santacruz-Tinoco, C.E.; Martínez-Miguel, B.; et al. Efficacy and safety of vitamin D supplementation to prevent COVID-19 in frontline healthcare workers. A randomized clinical trial. Arch. Med. Res. 2022, 53, 423–430. [Google Scholar] [CrossRef]
- Cannata-Andía, J.B.; Díaz-Sottolano, A.; Fernández, P.; Palomo-Antequera, C.; Herrero-Puente, P.; Mouzo, R.; Carrillo-López, N.; Panizo, S.; Ibañez, G.H.; Cusumano, C.A.; et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Karonova, T.L.; Golovatyuk, K.A.; Kudryavtsev, I.V.; Chernikova, A.T.; Mikhaylova, A.A.; Aquino, A.D.; Lagutina, D.I.; Zaikova, E.K.; Kalinina, O.V.; Golovkin, A.S.; et al. Effect of cholecalciferol supplementation on the clinical features and inflammatory markers in hospitalized COVID-19 patients: A randomized, open-label, single-center study. Nutrients 2022, 14, 2602. [Google Scholar] [CrossRef]
- De Niet, S.; Trémège, M.; Coffiner, M.; Rousseau, A.F.; Calmes, D.; Frix, A.N.; Gester, F.; Delvaux, M.; Dive, A.F.; Guglielmi, E.; et al. Positive effects of vitamin D supplementation in patients hospitalized for COVID-19: A randomized, double-blind, placebo-controlled trial. Nutrients 2022, 14, 3048. [Google Scholar] [CrossRef]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Sanchez Cunto, M.; Brosio, D.; Ross, F.; Zylberman, M.; López, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, N.; Abou Warda, A.E.; Sarhan, R.M.; Boshra, M.S.; Mostafa-Hedeab, G.; ALruwaili, B.F.; Ibrahim, H.S.G.; Schaalan, M.F.; Fathy, S. Evidence for the efficacy of a high dose of vitamin D on the hyperinflammation state in moderate-to-severe COVID-19 patients: A randomized clinical trial. Medicina 2022, 58, 1358. [Google Scholar] [CrossRef] [PubMed]
- Cervero, M.; López-Wolf, D.; Casado, G.; Novella-Mena, M.; Ryan-Murua, P.; Taboada-Martínez, M.L.; Rodríguez-Mora, S.; Vigón, L.; Coiras, M.; Torres, M. Beneficial effect of short-term supplementation of high dose of vitamin D3 in hospitalized patients with COVID-19: A multicenter, single-blinded, prospective randomized pilot clinical trial. Front. Pharmacol. 2022, 13, 863587. [Google Scholar] [CrossRef] [PubMed]
- Elamir, Y.M.; Amir, H.; Lim, S.; Rana, Y.P.; Lopez, C.G.; Feliciano, N.V.; Omar, A.; Grist, W.P.; Via, M.A. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone 2022, 154, 116175. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: A randomized clinical trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Sabico, S.; Enani, M.A.; Sheshah, E.; Aljohani, N.J.; Aldisi, D.A.; Alotaibi, N.H.; Alshingetti, N.; Alomar, S.Y.; Alnaami, A.M.; Amer, O.E.; et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate COVID-19: A randomized clinical trial. Nutrients 2021, 13, 2170. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment with 25-hydroxyvitamin D3 (Calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: A pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcalá Díaz, J.F.; López Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Kofahi, H.M.; Badran, B.R.; Nimer, R.M.; Atoom, A.M.; Al Hersh, S.M. Exploring the effects of vitamin D and vitamin A levels on the response to COVID-19 vaccine. Vaccines 2023, 11, 1509. [Google Scholar] [CrossRef]
- Panahi, Y.; Einollahi, B.; Beiraghdar, F.; Darvishi, M.; Fathi, S.; Javanbakht, M.; Shafiee, S.; Akhavan-Sigari, R. Fully understanding the efficacy profile of the COVID-19 vaccination and its associated factors in multiple real-world settings. Front. Immunol. 2022, 13, 947602. [Google Scholar] [CrossRef]
- Zecca, E.; Rizzi, M.; Tonello, S.; Matino, E.; Costanzo, M.; Rizzi, E.; Casciaro, G.F.; Manfredi, G.F.; Acquaviva, A.; Gagliardi, I.; et al. Ongoing mycophenolate treatment impairs anti-SARS-CoV-2 vaccination response in patients affected by chronic inflammatory autoimmune diseases or liver transplantation recipients: Results of the RIVALSA prospective cohort. Viruses 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Ssentongo, A.E.; Voleti, N.; Groff, D.; Sun, A.; Ba, D.M.; Nunez, J.; Parent, L.J.; Chinchilli, V.M.; Paules, C.I. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: A systematic review and meta-analysis. BMC Infect Dis. 2022, 22, 439. [Google Scholar] [CrossRef]
- Eick-Cost, A.A.; Ying, S.; Wells, N. Effectiveness of mRNA-1273, BNT162b2, and JNJ-78436735 COVID-19 vaccines among US military personnel before and during the predominance of the Delta ariant. JAMA Netw. Open 2022, 5, e228071. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Keshavarz, J.; Bagheri-Jamebozorgi, M.; Nemati, M.; Frootan, R.; Shokri, F. The association of the vitamin D status with the persistence of anti-HBs antibody at 20 years after primary vaccination with recombinant hepatitis B vaccine in infancy. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Heine, G.; Drozdenko, G.; Lahl, A.; Unterwalder, N.; Mei, H.; Volk, H.D.; Dörner, T.; Radbruch, A.; Worm, M. Efficient tetanus toxoid immunization on vitamin D supplementation. Eur. J. Clin. Nutr. 2011, 65, 329–334. [Google Scholar] [CrossRef]
- Ivanov, A.P.; Dragunsky, E.M.; Chumakov, K.M. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J. Infect. Dis. 2006, 193, 598–600. [Google Scholar] [CrossRef]
- Enioutina, E.Y.; Visic, D.; McGee, Z.A.; Daynes, R.A. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: Characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine 1999, 17, 3050–3064. [Google Scholar] [CrossRef] [PubMed]
- Daynes, R.A.; Enioutina, E.Y.; Butler, S.; Mu, H.H.; McGee, Z.A.; Araneo, B.A. Induction of common mucosal immunity by hormonally immunomodulated peripheral immunization. Infect. Immun. 1996, 64, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Saroha, H.S.; Bhat, S.; Das, L.; Dutta, P.; Holick, M.F.; Sachdeva, N.; Marwaha, R.K. Calcifediol boosts efficacy of ChAdOx1 nCoV-19 vaccine by upregulating genes promoting memory T cell responses. NPJ Vaccines 2024, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Lavell, A.H.A.; Schramade, A.E.; Sikkens, J.J.; van der Straten, K.; van Dort, K.A.; Slim, M.A.; Appelman, B.; van Vught, L.A.; Vlaar, A.P.J.; Kootstra, N.A.; et al. 25-Hydroxyvitamin D concentrations do not affect the humoral or cellular immune response following SARS-CoV-2 mRNA vaccinations. Vaccine 2024, 42, 1478–1486. [Google Scholar] [CrossRef]
- di Filippo, L.; Frara, S.; Terenzi, U.; Nannipieri, F.; Locatelli, M.; Ciceri, F.; Giustina, A. Lack of vitamin D predicts impaired long-term immune response to COVID-19 vaccination. Endocrine 2023, 82, 536–541. [Google Scholar] [CrossRef]
- Meyers, E.; De Smet, E.; Vercruysse, H.; Callens, S.; Padalko, E.; Heytens, S.; Vandekerckhove, L.; Cools, P.; Witkowski, W. No significant association between 25-OH vitamin D status and SARS-CoV-2 antibody response after COVID-19 vaccination in nursing home residents and staff. Vaccines 2023, 11, 1343. [Google Scholar] [CrossRef]
- Fateh, H.L.; Kareem, G.; Rezaeian, S.; Moludi, J.; Kamari, N. The effect of Vit-D supplementation on the side effect of BioNTech, Pfizer vaccination and immunoglobulin G response against SARS-CoV-2 in the individuals tested positive for COVID-19: A randomized control trial. Clin. Nutr. Res. 2023, 12, 269–282. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Vivaldi, G.; Chambers, E.S.; Cai, W.; Li, W.; Faustini, S.E.; Gibbons, J.M.; Pade, C.; Coussens, A.K.; Richter, A.G.; et al. Vitamin D supplementation does not influence SARS-CoV-2 vaccine efficacy or immunogenicity: Sub-studies nested within the CORONAVIT randomised controlled trial. Nutrients 2022, 14, 3821. [Google Scholar] [CrossRef]
- Zelini, P.; d’Angelo, P.; Cereda, E.; Klersy, C.; Sabrina, P.; Albertini, R.; Grugnetti, G.; Grugnetti, A.M.; Marena, C.; Cutti, S.; et al. Association between vitamin D serum levels and immune response to the BNT162b2 vaccine for SARS-CoV-2. Biomedicines 2022, 10, 1993. [Google Scholar] [CrossRef]
- Piec, I.; Cook, L.; Dervisevic, S.; Fraser, W.D.; Ruetten, S.; Berman, M.; English, E.; John, W.G. Age and vitamin D affect the magnitude of the antibody response to the first dose of the SARS-CoV-2 BNT162b2 vaccine. Curr. Res. Transl. Med. 2022, 70, 103344. [Google Scholar] [CrossRef]
- Pavel-Tanasa, M.; Constantinescu, D.; Cianga, C.M.; Anisie, E.; Mereuta, A.I.; Tuchilus, C.G.; Cianga, P. Adipokines, and not vitamin D, associate with antibody immune responses following dual BNT162b2 vaccination within individuals younger than 60 years. Front. Immunol. 2022, 13, 1000006. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between vitamin D status and antibody response to COVID-19 mRNA vaccination in healthy adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef] [PubMed]
- Durden, W.; Ezaldin, S.; Amos, J.; Kemper, S.; Campbell, J. Rise in serum 25-Hydroxyvitamin D levels during the COVID-19 pandemic. Nutrients 2024, 16, 2449. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).