The Insulin-like Growth Factor Family as a Potential Peripheral Biomarker in Psychiatric Disorders: A Systematic Review
Abstract
1. Introduction
1.1. Psychiatric Disorders and the Need for Peripheral Biomarkers
1.2. Rationale and Objectives
1.3. The Insulin-like Growth Factor (IGF) Family
1.3.1. IGF-1
1.3.2. IGF-2
1.3.3. IGFBPs
2. Searching Strategy
3. Results
4. IGF Peripheral Levels in Schizophrenia Patients
4.1. Schizophrenia (SZ)
4.2. IGF-1 in SZ Patients
4.2.1. First Studies and the Potential Influence of Antipsychotics in Weight Gain
4.2.2. IGF-1 Deficiency Hypothesis in SZ Patients
4.2.3. IGF-1 and Metabolic Dysregulations in the Insulin–Glucose Homeostasis in SZ Patients
4.2.4. IGF-1 and the Hypothalamic–Pituitary–Adrenal (HPA) Axis in SZ Patients
4.2.5. IGF-1 and Inflammation in SZ Patients
4.2.6. IGF-1 in FE: Differences Between SZ and BD Patients
4.2.7. IGF-1 and Cognitive Alterations in SZ Patients
4.2.8. IGF-1 in Remitted, Treatment Resistant, and Chronic SZ Patients
4.2.9. IGF-1 Meta-Analysis in SZ Patients
4.3. IGF-2 in SZ Patients
4.3.1. IGF-2 First Study and the Atherogenic Profile in SZ Patients
4.3.2. IGF-2 and Cognition in SZ Patients
4.3.3. IGF-2 Elevation Through Ap Actions over C/EBPβ
4.3.4. IGF-2, IGFBP-3, and IGFBP-7 in FE SZ Patients
4.4. Other IGFBPs Studies in SZ Patients
4.4.1. IGFBP-3 and Exercise in SZ Patients
4.4.2. IGFBP-1 in Cognition and Treatment-Resistant SZ
4.4.3. Still Unexplored IGFBPs in SZ Patients
| Ref. | DSM | Group | Sample Size | Age (Years) | Sex (F/M) | Treatment | Sample Source | Techn | Country | IGF Ligands | Statistics | IGFBPs | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [35] | III-R | SZ | 28 | 44 ± 12 | 12/16 | Class Aps | >6 m | Fasting Serum | RIA | Sweden | IGF-1 (ng/mL) | 211 ± 75 * | ns (↓) | p = 0.246 | IGFBP-1 (ng/mL) | 20 ± 15 | ns | p = 0.157 |
| SZ | 13 | 36 ± 6 | 6/7 | Cloz | 181 ± 79 | 16 ± 8 | ||||||||||||
| [36] | III-R | SZ | 14 | 43 ± 10 | 7/7 | Olanz | >2 m | Fasting Serum | RIA | Sweden | IGF-1 (ng/mL) | 0.18 ± 1.10 | N/A | IGFBP-1 (ng/mL) | 14.7 ± 7.8 | ↓ | p < 0.05 | |
| [37] | III-R | SZ | 47 | 42 (27–80) | 26/21 | Classical APs | >6 m | Fasting Serum | RIA | Sweden | IGF-1 (ng/mL) | 207.5 ± 66.9 * | “Normal Range” | |||||
| [38] | IV | SZ | 18 | 46.9 ± 14.8 | 5/13 | Cloz | >6 m | Fasting Serum | RIA | Sweden | IGF-1 (ng/mL) | 0.1 (−2.0–2.4) | “Normal Range” | IGFBP-1 (ng/mL) | 12 (5–40) | ↓ | p < 0.05 | |
| SZ | 16 | 39 (23–52) | 6/10 | Olanz | 0.2 (−1.3–1.3) | 14 (6–27) | ||||||||||||
| [39] | IV | SZ0 | 19 | 31 ± 5.8 | 10/9 | Baseline | 2.5 m | Blood | CLIA, ELISA | UK + Afrikaan | IGF-1 (nmol/L) | 25.5 ± 8.7 | ns | p = 0.57 | IGFBP-1 (µg/L) | 3.1 ± 3.7 | ns | p= 0.24 |
| SZ1 | Cloz | 24.7 ± 9.1 | 2.4 ± 2.8 | |||||||||||||||
| [44] | IV | SZ | 44 | 33 ± 7.7 | 21/23 | DN | Fasting Plasma | AU400 | India | IGF-1 (ng/mL) | 123.7 ± 50.0 | ↓ | p < 0.006 | |||||
| HC | 44 | 32.5 ± 7.6 | 21/23 | 159.1 ± 67.9 | ||||||||||||||
| [87] | IV | SZ | 53 | 40.5 (22.0–69.0) | All M | Halo | “Stable” | Serum | ELISA | Kuwait | IGF-1 (ng/mL) | 178.46 | ns | p = 0.15 | IGFBP-3 (ng/mL) | 5300 | ns | p= 0.21 |
| HC | 52 | 41.0 (27.0–60.0) | 152.31 | 5157.14 | ||||||||||||||
| IGF-2 (ng/mL) | 1445.38 | ↑ | p = 0.02 | |||||||||||||||
| 1228.46 | ||||||||||||||||||
| [47] | IV | SZ OB | 71 | 40.4 ± 7.5 | All M | Cloz | Fasting Plasma | ELISA | China | IGF-1 (ng/mL) | 134.7 ± 10.4 | ↓ | p < 0.01 | IGFBP-3 (ng/mL) | 4496 ± 124 | ns | p > 0.05 | |
| HC OB | 50 | 39.7 ± 1.17 | 201.0 ± 14.4 | 4874 ± 166 | ||||||||||||||
| HC | 51 | 40.9 ± 1.14 | 232.8 ± 12.4 | 4696 ± 134 | ||||||||||||||
| [55] | IV | SZ0 | 33 | 33.8 ± 8.2 | 13/20 | DN | Fasting Serum | EAIC | India | IGF-1 (ng/mL) | 113.9 ± 44.7 | ↓ HC | p < 0.0001 | |||||
| SZ1 | 33 | 33.8 ± 8.2 | 13/20 | A-Aps | 3 m | 141.5 ± 58.8 | ↑ sz0 | p < 0.0001 | ||||||||||
| HC | 33 | 32.2 ± 8.0 | 13/20 | 175.2 ± 63.0 | ||||||||||||||
| [95] | IV | SZ | 71 | 31 ± 10 | 29/42 | DF | Serum | HM MAIA | USA and Europe | IGFBP-2 (ng/mL) | 52.9 ± 27 | ↑ | p = 0.045 | |||||
| HC | 59 | 30 ± 8 | 28/31 | 43.29 ± 19.51 | ||||||||||||||
| [49] | IV | Offs SZ | 32 | 27.6 ± 6.4 | 15/17 | Fasting Plasma | RIA | China | IGF-1 (ng/mL) | 168.6 ± 53.5 | ↓ | p = 0.028 | ||||||
| HC | 37 | 26.6 ± 3.4 | 17/20 | 195.1 ± 44.8 | ||||||||||||||
| [73] | IV | SZ_FE | 27 | 24.7 | 7/20 | DN | Fasting Plasma | ELISA | Spain | IGF-1 (ng/mL) | 182.42 ± 96.13 | ns | p = 0.659 | |||||
| SZ1m | 21 | N/A | A-Aps + MS | 1 m | 237.60 ± 122.39 | ↑ | p = 0.039 | |||||||||||
| SZ6m | 19 | 6 m | 193.92 ± 74.41 | ns | p = 0.216 | |||||||||||||
| SZ12m | 16 | 12 m | 178.02 ± 97.63 | ns | p = 0.88 | |||||||||||||
| HC | 27 | 25.7 | 13/30 | 171.60 ± 96.13 | ||||||||||||||
| [50] | IV | SZ | 50 | 36.5 ± 11.2 | 13/37 | A-Aps | Fasting Plasma | RIA | Turkey | IGF-1 (ng/mL) | 176.06 ± 81.65 | ns | p = 1.0 | |||||
| Sibl SZ | 50 | 35.7 ± 11.1 | 19/31 | 175.04 ± 72.14 | ||||||||||||||
| HC | 50 | 35.5 ± 9.2 | 18/32 | 175.04 ± 64.01 | ||||||||||||||
| [93] | IV | SZ Re0 | 12 | 32.9 ± 2.3 | All M | Treated | base | Fasting Serum | ELISA, CLIA | Brazil | IGF-1 (ng/mL) | 173.70 ± 34.53 | ns | IGFBP-3 (ng/mL) | 3290.97 ± 440.38 | ns | ||
| SZ Re1 | 10 w | 143.65 ± 24.23 | 3388.79 ± 434.08 | |||||||||||||||
| SZ Re2 | 20 w | 151.34 ± 26.69 | 3666.98 ± 500.35 | |||||||||||||||
| SZ Co0 | 9 | 33.5 ± 2.6 | base | 220.33 ± 33.74 | 3939.81 ± 404.66 | |||||||||||||
| SZ Co1 | 10 w | 174.96 ± 23.67 | 4424.55 ± 398.88 | |||||||||||||||
| SZ Co2 | 20 w | 195.06 ± 26.08 | 4211.39 ± 459.77 | |||||||||||||||
| SZ CT0 | 13 | 33.4 ± 12.2 | base | 197.22 ± 35.39 | 4278.81 ± 447.21 | |||||||||||||
| SZ CT1 | 10 w | 181.11 ± 24.83 | 4322.47 ± 440.81 | |||||||||||||||
| SZ CT2 | 20 w | 170.56 ± 27.35 | 4153.81 ± 508.11 | |||||||||||||||
| [67] | IV-TR | FE | 25 | 25.48 ± 5.4 | All M | A-Aps (8) | 1.75 ± 0.8 | Serum | ELISA | Greece | IGF-1 (ng/mL) | 5.57 (5.29, 5.84) | ns | p = 0.82 | ||||
| UHR | 12 | 24.5 ± 3.1 | DN | 5.68 (5.4, 6.02) | p = 0.259 | |||||||||||||
| HC | 23 | 27.04 ± 2.9 | 5.56 (5.37, 5.72) | |||||||||||||||
| [62] | IV | PE | 40 | 32.4 ± 9.8 | 13/27 | DN | Fasting Serum | ELISA | Greece | IGF-1 (ng/mL) | 109.66 (15.22–313.48) | ↑ | p = 0.039 | |||||
| HC | 40 | 31.9 ± 8.3 | 15/25 | 86.96 (18.76–160.36) | ||||||||||||||
| [89] | IV | SZ | 30 | 30.5 ± 8.5 | 14/16 | WO | 3 m | Fasting Serum | ELISA | China | IGF-1 (ng/mL) | 114.96 ± 65.85 | ↓ | p = 0.001 | ||||
| HC | 26 | 34.4 ± 9.9 | 14/12 | 183.43 ± 86.42 | ||||||||||||||
| [88] | IV | SZ | 32 | 30.0 ± 8.5 | 16/17 | DN + WO | 3 m | Fasting Serum | ELISA | China | IGF-2 (ng/mL) | 199.2 ± 67.2 | ↓ | p = 0.04 | IGFBP-3 (ng/mL) | 849.7 ± 227.1 | ↓ | p = 0.049 |
| HC | 30 | 33.5 ± 9.1 | 16/14 | 355.7 ± 70.4 | 1034.5 ± 390.7 | |||||||||||||
| IGFBP-7 (ng/mL) | 26.8 ± 9.8 | ↓ | p = 0.041 | |||||||||||||||
| 33.0 ± 12.9 | ||||||||||||||||||
| [77] | IV | SZ0 | 30 | 30.3 ± 8.5 | 17/13 | Fasting Serum | ELISA | China | IGF-2 (ng/mL) | 203.13 ± 64.62 | ↓ HC | p = 0.002 | ||||||
| SZ1 | 30 | A-Aps | 8 w | 426.99 ± 124.26 | ↑ SZ0 | p < 0.001 | ||||||||||||
| HC | 31 | 34.2 ± 9.3 | 15/16 | 442.34 ± 105.33 | ||||||||||||||
| [69] | IV | SZ0 | 113 | 29.2 ± 9.3 | 68/45 | DF | 2 w | Fasting Plasma | ELISA | China | IGF-1 (ng/mL) | 214.45 ± 33.42 | ↑ HC | p = 0.017 | ||||
| SZ1 | 89 | N/A | Risper | 10 w | 202.29 ± 32.40 | ↓ SZ0 | p < 0.01 | |||||||||||
| HC | 58 | 30.2 ± 6.4 | N/A | N/A | ||||||||||||||
| [83] | V | SZ | 65 | 49 ± 10 | 32/33 | Aps | “C” | Fasting Serum | IRMA | Japan | IGF-1 (ng/mL) | 109 ± 38 | ns | p = 0.27 | ||||
| HC | 20 | 46 ± 7.4 | 12/8 | 120 ± 39 | ||||||||||||||
| [82] | V | SZ R | 55 | 36.6 ± 8.5 | 18/37 | Aps | Fasting Plasma | RIA | Turkey | IGF-1 (ng/mL) | 137.54 ± 40.28 | ↓ HC, TR | p < 0.001 | |||||
| SZ TR | 62 | 33.9 ± 8.6 | 16/46 | 165.11 ± 40.95 | ||||||||||||||
| HC | 60 | 33.9 ± 7.9 | 20/40 | 173.37 ± 38.85 | ||||||||||||||
| [92] | V | SZ_FE | 15 | 31.7 ± 15.5 | 4/11 | DN | Fasting Plasma | ELISA | Spain | IGF-2 (ng/mL) | 66.93 ± 39.99 | ↓ HC, ME | HCp = 0.0017 | IGFBP-7 (ng/mL) | 54.04 ± 23.49 | ↓ ME | MEp = 0.0017 | |
| SZ_FE0 | 11 | 32.4 ± 16.2 | 3/8 | DN | MEp < 0.0001 | |||||||||||||
| SZ_FE1 | A-Aps | 153.45 ± 30.98 | ↑ FE0 | p = 0.0078 | 82.47 ± 27.04 | ↑ FE0 | p = 0.0137 | |||||||||||
| SZ_ME | 40 | 39.9 ± 11.3 | 13/27 | Treated | 144.06 ± 30.84 | ↑ FE | FEp = 0.0065 | 78.28 ± 18.55 | ↑ HC | HCp = 0.0214 | ||||||||
| SZ_MER | 19 | 40.0 ± 12.1 | 5/14 | Treated | 148.64 ± 33.41 | ↑ HC | p = 0.0192 | 81.98 ± 15.61 | ↑ HC | p = 0.0185 | ||||||||
| SZ_MENR | 21 | 39.7 ± 10.7 | 8/13 | Treated | 140.95 ± 28.64 | nsHC, MER | p > 0.05 | 74.93 ± 20.67 | nsHC, MER | p > 0.05 | ||||||||
| HC | 45 | 41.2 ± 10.6 | 20/25 | 114.24 ± 55.00 | 64.9174 ± 25.36 | |||||||||||||
| [58] | V | SZ | 71 | 38.2 ± 9.9 | 33/38 | Treated | Fasting Serum | ELISA | Japan | IGF-1 (ng/mL) | 159.7 ± 49.6 | ↑ | p = 0.01 | |||||
| HC | 71 | 41.4 ± 9.3 | 33/38 | 137.9 ± 41.3 | ||||||||||||||
| [94] | IV | SZ TRS | 31 | 40.6 ± 9.2 | All M | Treated | >6 m | Fasting Serum | LSCD | China | Log IGFBP-1 (pg/mL) | 4.49 ± 0.27 | ||||||
| SZ CM | 49 | 40.6 ± 10.3 | 6 m | 4.27 ± 0.38 | ↓ TRS | p = 0.048 | ||||||||||||
| HC | 53 | 40.0 ± 9.3 | 3.78 ± 0.46 | ↓ TRS, CM | p < 0.001 | |||||||||||||
5. IGF Peripheral Levels in Depression
5.1. Major Depressive Disorder (MDD)
5.2. IGF-1 in Depression
5.2.1. IGF-1 First Studies in MDD Patients: GH Challenges and Dexamethasone Bias
5.2.2. Saliva Cortisol and Serum IGF-1/IGFBP-3 in MDD Patients
5.2.3. IGF-1 and Cortisol in MDD Patients: A Counter-Regulatory Neuroprotective Mechanism
5.2.4. IGF-1 and Duman’s Neurotrophic Hypothesis in MDD Patients
5.2.5. IGF-1 Potential Diagnostic Value and Cognitive Assessment in MDD Patients
5.2.6. IGF-1 and Relaxin-3 in MDD Patients
5.2.7. IGF-1 in Relation to Hormones, HPA Axis and Insulin Resistance in MDD Patients
5.2.8. IGF-1 in Drug-Naïve First Episode (FE) MDD Patients
5.2.9. IGF-1 in Women Under Different Conditions in MDD Patients
5.2.10. IGF-1 Rhythm in FE MDD
5.2.11. IGF-1 Meta-Analysis in MDD Patients
5.2.12. IGF-1 in the Cerebrospinal Fluid (CSF) in MDD Patients
5.2.13. IGF-1 in Alternative Therapies and Exercise in MDD Patients
5.2.14. IGF-1 Population-Based Studies and Depressive Symptoms
5.2.15. IGF-1, IGFBP-3, Depressive Symptoms and Ageing
5.2.16. IGF-1 and Depressive Symptoms in Concomitant Diseases
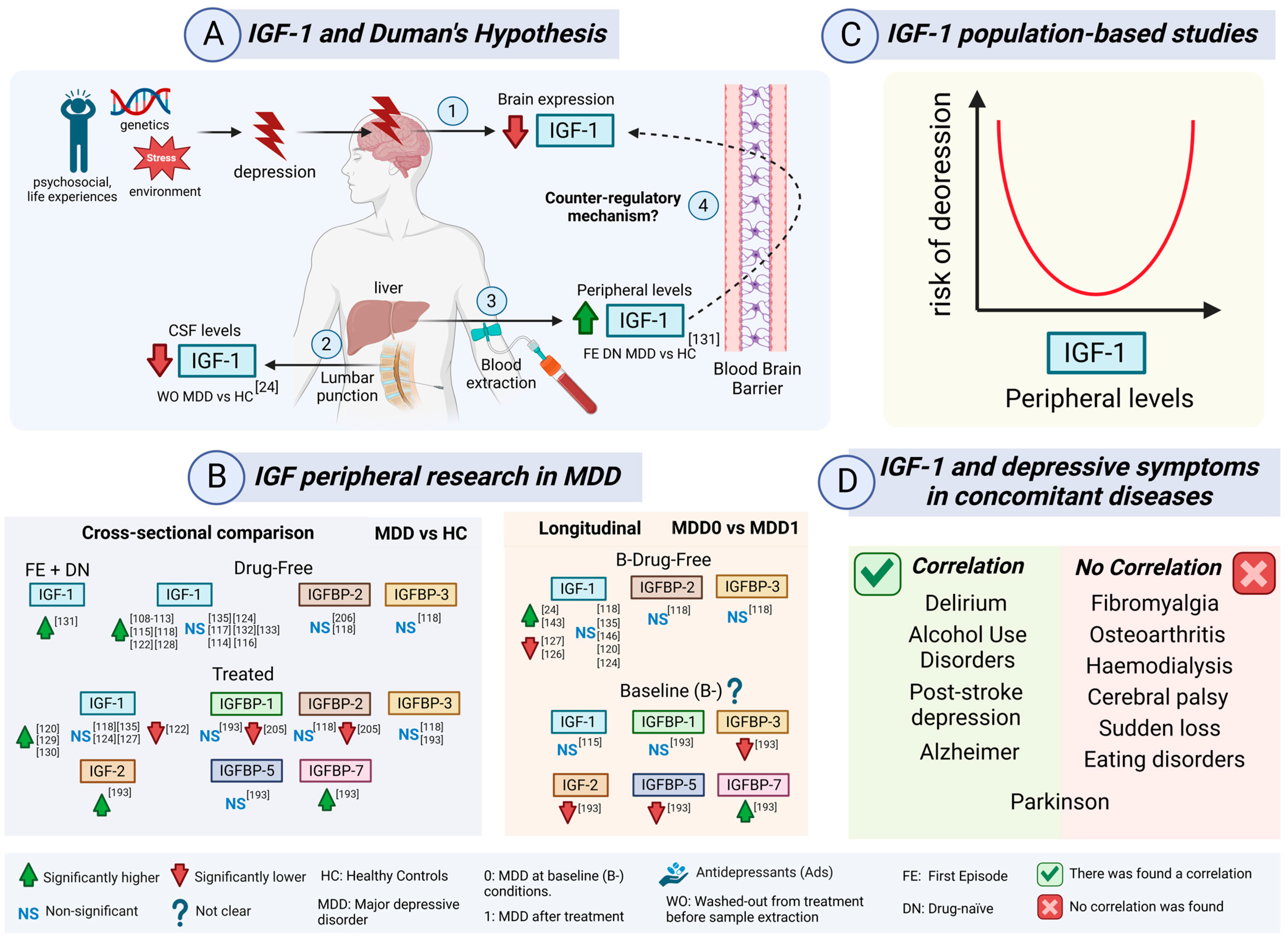
5.2.17. IGF-1 in Post-Stroke Depression (PSD) Compared to MDD Patients
5.3. IGF-2 as a Promising Candidate in MDD Patients
5.4. IGFBPs in MDD Patients
5.4.1. IGFBP-1, IGFBP-3, IGFBP-5, and IGFBP-7 in MDD Patients
5.4.2. IGFBP-2 in MDD Patients
5.4.3. Still Unexplored IGFBP-4 and IGFBP-6 in MDD Patients
| Ref. | DSM | Group | Sample Size | Age (Years) | Sex (F/M) | Treatment | Time | Scale | Sample Source | Techn | Country | IGFs | Statistics | IGFBPs | Statistics | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [108] * | III | MDD | 11 | 53.2 ± 11.6 | 7/4 | DF | >3 d | 28.5 ± 6.3 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.1 ± 0.2 | ↑ | p < 0.05 | ||||
| HC | 11 | 49.2 ± 11.3 | 7/4 | 0.6 ± 0.1 | |||||||||||||||
| [109] * | III | MDD | 34 | 48.2 ± 12.2 | 23/11 | DF | 14 d | 26.9 ± 5.4 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.41 ± 0.79 | ↑ | p < 0.001 | ||||
| HC | 34 | 44.7 ± 11.9 | 7/6 | 0.81 ± 0.31 | |||||||||||||||
| [110] * | III | MDD | 12 | 53.1 ± 1.2 | 7/5 | DF | 7 d | 29.1 ± 1.7 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.18 ± 0.16 | ↑ | p < 0.05 | ||||
| HC | 12 | 51.0 ± 3.5 | 7/5 | 0.65 ± 0.07 | |||||||||||||||
| [111] * | III | MDD | 10 | 52.9 ± 12.2 | 7/3 | DF | 7 d | 28 ± 5.9 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.09 ± 0.55 | ↑ | p < 0.05 | ||||
| HC | 10 | 51.2 ± 12 | 7/3 | 0.64 ± 0.23 | |||||||||||||||
| [112] * | III-R | MDD | 15 | 49.6 ± 9.2 | 7/8 | DF | 14 d | 28.3 ± 6 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.05 ± 0.16 | ↑ | p < 0.06 | ||||
| HC | 15 | 51.1 ± 8.8 | 7/8 | 0.63 ± 0.06 | |||||||||||||||
| [113] * | III | MDD | 10 | 48.1 ± 7 | 7/3 | DF | 14 d | 26.4 ± 5.1 a | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.08 ± 0.39 | ↑ | p < 0.05 | ||||
| HC | 10 | 49.6 ± 10.2 | 7/3 | 0.57 ± 0.16 | |||||||||||||||
| [114] * | III-R | MDD0 | 16 | 38 ± 15.1 | 11/5 | 23 ± 7.8 a | Plasma | RIA | Germany | IGF-1 (U/mL) | 1.34 ± 0.45 | ||||||||
| PreDex | N/A | N/A | DF | 4 w | ~0.73 *2 | ↑ | p < 0.01 | ||||||||||||
| PostDex | N/A | N/A | ~0.92 *2 | ||||||||||||||||
| MDD1 | 16 | 38 ± 15.1 | 11/5 | Treated | 6 ± 3.7 a | 1.12 ± 0.18 | |||||||||||||
| PreDex | N/A | N/A | DF | 4 w | ~0.73 *2 | ↑ | p < 0.05 | ||||||||||||
| PostDex | N/A | N/A | ~0.83 *2 | ||||||||||||||||
| HC | 28 | 33 ± 8.7 | 13/15 | 2 ± 3.1 a | 1.06 ± 0.29 | ||||||||||||||
| PreDex | N/A | N/A | ~0.92 *2 | ns | N/A | ||||||||||||||
| PostDex | N/A | N/A | ~0.96 *2 | ||||||||||||||||
| PreDex-CS | 30 | N/A | N/A | DF | 4 w | ~0.72 *2 | ns | p = 0.06 | |||||||||||
| PostDex-CS | N/A | N/A | ~0.75 *2 | ||||||||||||||||
| PreDex-NS | 10 | N/A | N/A | DF | 4 w | ~0.96 *2 | ↑ | p < 0.02 | |||||||||||
| Post-Dex-NS | N/A | N/A | ~1.27 *2 | ||||||||||||||||
| [115] | III-R | MDD0 | 10 | 45 ± 13.3 | 8/2 | MOC | 300 mg/d | 26 ± 2.2 H | Fasting Plasma | RIA | Germany | IGF-1 (U/mL) | 1.39 ± 0.4 | ↑ HC | p < 0.05 | ||||
| MDD0 | 13 | 48.4 ± 14.7 | 9/4 | MAP | 150 mg/d | 28.9 ± 1.4 H | 1.31 ± 0.3 | ↑ HC | p < 0.05 | ||||||||||
| HC | 15 | N/A | N/A | 0.76 ± 0.31 | |||||||||||||||
| MDD1 | 10 | 45 ± 13.3 | 8/2 | MOC | 4 w | 10.3 ± 1.9 H | 1.37 ± 0.17 | ns MDD0 | N/A | ||||||||||
| MDD1 | 13 | 48.4 ± 14.7 | 9/4 | MAP | 11.3 ± 2 H | 1.73 ± 0.47 | ns MDD0 | ||||||||||||
| [116] | III-R | MDD-C | 9 | 13 ± 3 | 2/7 | DN or DF | >3 m | 57.2 ± 10.3 b | Fasting Plasma | RIA | Italy | IGF-1 (ng/mL) | 338.8 ± 155.7 | ns | N/A | ||||
| HC-C | 9 | 14 ± 5 | 2/7 | 247.3 ± 171.6 | |||||||||||||||
| MDD-GHRH | 9 | 13 ± 3 | 2/7 | DN or DF | >3 m | 57.2 ± 10.3 b | 310.1 ± 113.9 | ns | N/A | ||||||||||
| HC-GHRH | 9 | 14 ± 5 | 2/7 | 378.4 ± 200 | |||||||||||||||
| [117] | III-R | MDD-C | 12 | 43 ± 12 | 8/4 | DF | >7 d | 25.2 ± 1.3 a | Fasting Plasma | RIA | Germany | IGF-1 (ng/mL) | 193 ± 43 | ns | p = 0.757 | ||||
| HC-C | 42 ± 11 | 200 ± 61 | |||||||||||||||||
| MDD-GHRH | 43 ± 12 | DF | >7 d | 25.2 ± 1.3 a | 186 ± 49 | ns | p = 0.206 | ||||||||||||
| HC-GHRH | 42 ± 11 | 216 ± 62 | |||||||||||||||||
| [132] | III | MDD | 10 | 41.0 ± 8.0 | All F | DF | 14 d | N/A | Serum | RIA | US | IGF-1 (ng/mL) | 189 ± 86 | ns | p = 0.98 | ||||
| HC | 10 | 41.0 ± 7.0 | 189 ± 37 | ||||||||||||||||
| [118] | III | MDD | 24 | 47.2 ± 16.4 | 11/13 | WO | 6 d | 31.8 ± 5.8 a | Plasma | RIA | Germany | IGF-1 (ng/mL) | 157 ± 40 | ↑ | p < 0.001 | IGFBP-2 (ng/mL) | 286 ± 220 | ns | N/A |
| HC | 33 | 51.4 ± 19.2 | 11/22 | 120 ± 33 | 236 ± 134 | ||||||||||||||
| MDD0 | 15 | 41.4 ± 15.4 | 6/9 | WO | 6 d | 32.1 ± 5.6 a | 168 ± 41 | ns | N/A | 338 ± 252 | |||||||||
| MDD1 | Treated | 24–55 d | 11.8 ± 8.3 a | 152 ± 33 | 320 ± 168 | ||||||||||||||
| MDD-R0 | 9 | 42.9 ± 17 | 3/6 | WO | 6 d | 30.1 ± 4.5 a | 174 ± 46 | ↑ | p < 0.05 | 332 ± 310 | |||||||||
| MDD-R1 | Treated | 24–55 d | 5.9 ± 2.6 a | 147 ± 33 | 301 ± 209 | ||||||||||||||
| MDD-NR0 | 6 | 39.2 ± 13.8 | 3/3 | WO | 6 d | 35 ± 6.3 a | 158 ± 33 | ns | N/A | 347 ± 153 | |||||||||
| MDD-NR1 | Treated | 24–55 d | 20.7 ± 4.8 a | 161 ± 34 | 350 ± 90 | ||||||||||||||
| IGFBP-3 (ng/mL) | 2325 ± 329 | ns | N/A | ||||||||||||||||
| 2203 ± 391 | |||||||||||||||||||
| 2371 ± 327 | |||||||||||||||||||
| 2334 ± 358 | |||||||||||||||||||
| 2372 ± 284 | |||||||||||||||||||
| 2348 ± 315 | |||||||||||||||||||
| 2371 ± 413 | |||||||||||||||||||
| 2563 ± 409 | |||||||||||||||||||
| [133] | III | MDD | 19 | 34.7 ± 8.8 | All F | DF | 18.8 ± 3.9 c | Serum | N/A | USA | IGF-1 (ng/mL) | 289 ± 108 | ns | p = 0.07 | |||||
| HC | 16 | 36.1 ± 6.6 | 228 ± 58 | ||||||||||||||||
| [142] | N/A | MDD0 | 37 | 40.0 ± 11.4 | 28/9 | Fluox | 5 d | 1.46 ± 5.36 c | Plasma | N/A | USA | IGF-1 (ng/mL) | 161.2 ± 63.8 | ns | p = 0.732 | ||||
| MDD1 | Plac | 0.95 ± 4.4 c | 162.5 ± 58.7 | ||||||||||||||||
| MDD0 | 34 | 38.7 ± 14.5 | 26/8 | Sert | 1.56 ± 5.66 c | 170.2 ± 73.9 | ns | p = 0.61 | |||||||||||
| MDD1 | Plac | 3.59 ± 5.86 c | 174.2 ± 67.6 | ||||||||||||||||
| MDD0 | 36 | 39.9 ± 11.1 | 22/14 | Parox | 0.68 ± 5.42 c | 163 ± 63.9 | ↑ | p = 0.007 | |||||||||||
| MDD1 | Plac | 6.22± 6.63 c | 186.1 ± 74.0 | ||||||||||||||||
| [119] | IV | MDDR | 25 | 51 ± 17 | 18/7 | WO-6d | base | 23.9 ± 5.2 H | Serum | RIA | Germany | IGF-1 (ng/mL) | 175 ± 40 | ↓ | p < 0.01 | IGFBP-3 (ng/mL) | 3.07 ± 0.55 | ↓ | p < 0.01 |
| Ami | 14 d | N/A | 162 ± 49 | 3.09 ± 0.59 | |||||||||||||||
| 35 d | 6.8 ± 3.6 H | 144 ± 45 | 2.87 ± 0.56 | ||||||||||||||||
| MDDNR | 9 | 46 ± 16 | 8/1 | WO-6d | base | 22.1 ± 3.9 H | 170 ± 47 | ns | 2.96 ± 0.24 | ns | N/A | ||||||||
| Ami | 14 d | N/A | 173 ± 51 | 3.17 ± 0.33 | |||||||||||||||
| 35 d | 18.4 ± 5.6 H | 174 ± 49 | 3.07 ± 0.33 | ||||||||||||||||
| MDDR | 27 | 58 ± 16 | 17/10 | WO-6d | base | 23.0 ± 3.2 H | 164 ± 52 | ↓ | p < 0.01 | 2.92 ± 0.39 | ns | N/A | |||||||
| Paro | 14 d | N/A | 147 ± 57 | 2.89 ± 0.46 | |||||||||||||||
| 35 d | 6.0 ± 2.9 H | 148 ± 57 | 2.80 ± 0.55 | ||||||||||||||||
| MDDNR | 16 | 57 ± 14 | 12/4 | WO-6d | base | 23.7 ± 3.5 H | 152 ± 79 | ns | 2.99 ± 0.84 | ns | N/A | ||||||||
| Paro | 15 d | N/A | 142 ± 70 | 2.92 ± 0.70 | |||||||||||||||
| 35 d | 19.2 ± 5.2 H | 142 ± 70 | 3.00 ± 0.99 | ||||||||||||||||
| [24] | DSM N/A | MDD0 | 12 | 59.1 ± 10 | 5/7 | WO | >6 d | 24.4 ± 5.3 a | Fasting CSF | RIA | Germany | IGF-1 (µg/L) | 0.235 ± 0.135 | ↑ | p < 0.05 | ||||
| MDD1 | Venla, Fluox, Doxe, Ami | 17.1 ± 9.4 a | 0.305 ± 0.096 | ||||||||||||||||
| [143] | IV-R | MDD0 | 8 | 52.9 ± 8.8 | 6/2 | DF | >2 w | 26.9 ± 6.9 H | Serum | CLIA | Europe | IGF-1 (f.c.) | 1.59 | ↑ | p= 0.0156 | IGFBP-2 (f.c.) | 1.06 | ↑ | p= 0.0156 |
| MDD1 | ECT | 19.7 ± 8 H | |||||||||||||||||
| [135] | IV | MDD | 15 | 32.25 ± 7.65 | All M | base | Serum | RIA | China | IGF-1 (ng/mL) | 167.3 ± 6.6 | ns | p > 0.05 | ||||||
| MDD1 | 12 | N/A | Esci | 8 w | <22 M | 175.11 ± 8.59 | |||||||||||||
| HC | 12 | 31.15 ± 10.19 | 159.6 ± 11.8 | ||||||||||||||||
| [146] | IV | MDD0 | 41 | 38.9 ± 11.7 | 30/11 | 19.0 ± 3.9 c | Serum | ELISA | Denmark | IGF-1 (ng/mL) | 86.6 ± 110 | ns | p > 0.05 | ||||||
| MDD1 | A-Exer | 3 m | N/A | 81.7 ± 114.6 | |||||||||||||||
| MDD0 | 38 | 43.8 ± 12.2 | 23/15 | 18.9 ± 4.6 c | 88.6 ± 104 | ||||||||||||||
| MDD1 | C-Exer | 3 m | N/A | 67.4 ± 110.3 | |||||||||||||||
| [120] | IV | MDD0 | 78 | 48.64 ± 13.88 | 35/34 | Treated | 26.37 ± 6.73 a | Serum | ELISA | Germany | IGF-1 (ng/mL) | 189.6 ± 79.7 | ↑ HC | p = 3.29 × 10−4 | |||||
| MDD1 | 6 w | 9.67 ± 6.54 a | 184.90 ± 87.29 | ↑ HC | p = 0.002 | ||||||||||||||
| MDDR0 | 39 | 49.95 ± 11.91 | 18/21 | 24.46 ± 6.63 a | 169.02 ± 60.58 | ns | N/A | ||||||||||||
| MDDR1 | 6 w | 4.44 ± 3.04 a | 167.52 ± 67.24 | ||||||||||||||||
| MDDNR0 | 39 | 47.33 ± 15.64 | 17/22 | 28.28 ± 6.34 a | 210.17 ± 91.34 | ↑ MDDNR1 | p = 0.046 | ||||||||||||
| MDDNR1 | 6 w | 14.9 ± 24.57 a | 202.26 ± 101.49 | ns | p = 0.11 | ||||||||||||||
| HC | 92 | 48.13 ± 13.7 | 42/50 | 155.6 ± 60.0 | |||||||||||||||
| [122] | IV | Re D/A | 502 | 44.2 ± 13.1 | 348/154 | DF | 12 (7–19) I | Fasting Plasma | CIA | Holland | IGF-1 (nmol/L) | 26.2 ± 6.9 | ns | p = 0.09 | |||||
| Cu D/A | 963 | 40.3 ± 12.8 | 654/309 | DF | 27 (18–35) I | 26.5 ± 6.8 | ↑ HC | p = 0.006 | |||||||||||
| MDD | 647 | 42.6 ± 11.5 | 429/218 | Treated | 30 (19–40) I | 24.9 ± 6.9 | ↓ HC | p = 0.028 | |||||||||||
| HC | 602 | 41.0 ± 14.6 | 370/232 | 6 (3–12) I | 25.8 ± 6.9 | ||||||||||||||
| [124] | IV | MDD0 | 37 | 42.4 ± 11.9 | 29/8 | DF | 3 m | 19.7 ± 2.6 c | Serum | ELISA | Italy | IGF-1 (ng/mL) | 128.1 ± 48.3 | ns | |||||
| MDD1 | SRIs | 1 w | N/A | 122 ± 46.8 | ns | p = 0.42 | |||||||||||||
| HC | 43 | 42.3 ± 11.3 | 28/15 | 121.2 ± 51.6 | |||||||||||||||
| [184] | IV | MDD | 40 | 58.7 ± 9.92 | 31/9 | N/A | 19.45 ± 4.75 H | Serum | ELISA | China | IGF-1 (ng/mL) | 136.6 ± 39.02 | ↑ N-PSD | p < 0.05 | |||||
| N-PSD | 42 | 61.1 ± 6.58 | 17/25 | 3.36 ± 2.02 H | 109.62 ± 34.54 | ns | N/A | ||||||||||||
| PSD | 39 | 62.44 ± 10.34 | 19/20 | 16 ± 5.45 H | 113.68 ± 51.46 | ns | N/A | ||||||||||||
| HC | 38 | 57.58 ± 5.28 | 16/22 | 2.18 ± 1.98 H | 124.29 ± 49.48 | ||||||||||||||
| [205] | IV | MDD Me | 231 | 41.7 ± 12 | 68% F | Treated | 38.6 (9.7) I | Fasting Serum | CLIA | Holland | IGFBP-1 (β.c.) | 0.062 | ns HC | p = 0.135 | |||||
| MDD At | 128 | 40.7 ± 11.7 | 71.9% F | Treated | 39.1 (8.8) I | −0.256 | ↓ Me | p = 1.398 × 10−6 | |||||||||||
| HC | 414 | 39 ± 14.8 | 60.6% F | 8.0 (7.1) I | − 0.194 | ↑ At | p = 7.296 × 10−5 | ||||||||||||
| IGFBP-2 (β.c.) | 0.023 | ns HC | p = 0.198 | ||||||||||||||||
| − 0.094 | ↓ Me | 3.246 × 10−5 | |||||||||||||||||
| − 0.072 | ↑ At | p = 0.001 | |||||||||||||||||
| [206] | IV | MDD | 43 | 52.18 ± 12.5 | 35/8 | DN or DF | 23 ± 3.52 c | Fasting Serum | ELISA | Italy | IGFBP-2 (ng/mL) | 225.82 ± 129.11 | ns | p = 0.09 | |||||
| HC | 93 | 49.56 ± 12.9 | 41/52 | 232.9 ± 125.48 | |||||||||||||||
| [121] | IV | MDD | 91 | 44.1 ± 13.1 | 32/59 | Treated | 13.9 ± 9 H | Fasting Serum | N/A | Japan | IGF-1 (ng/mL) | 152.0 ± 50.0 | IGF-1-HAMD (R = 0.349, p = 0.001). No correlation with cortisol. | ||||||
| [128] | V | MDD | 86 | 32.64 ± 8.89 | 52/34 | N/A | 19.02 ± 2.41 H | Serum | ELISA | Bangladesh | IGF-1 (ng/mL) | 3.43 ± 4.66 | ↑ | p = 0.006 | |||||
| HC | 85 | 31.13 ± 8.72 | 51/34 | 2.08 ± 0.86 | |||||||||||||||
| [127] | V | MDD | 41 | 36.4 ± 12.8 | 27/14 | DF | 28(21.5,31.5) M | Serum | ELISA | Ukraine | IGF-1 (ng/mL) | 289.2 ± 125.3 | ↑ HC | p < 0.0001 | |||||
| MDD0 | 30 | 35.1 ± 12.9 | 20/10 | 29 (24.5–33) M | 288.2 ± 132.6 | ||||||||||||||
| MDD1 | 30 | Vortio | 8 w | 5 (2.5–10) M | 173.4 ± 71.2 | ↓ MDD0 | p < 0.0001 | ||||||||||||
| HC | 32 | 38.0 ± 12.2 | 20/12 | 2 (0–3.5) M | 170.2 ± 58.2 | nsMDD1 | p = 0.18 | ||||||||||||
| [126] | V | MDD | 78 | 38.2 ± 11.9 | 48/30 | DF | 29 (22–33) M | Fasting Serum | ELISA | Ukraine | IGF-1 (ng/mL) | 228 (183–312) | ↑ HC | p < 0.0001 | |||||
| MDD0 | 48 | N/A | N/A | 29 (22–33) M | 236 (184–316) | ||||||||||||||
| MDD1 | Vortio | 8 w | 6 (3–11) M | 170 (132–210) | ↓ MDD0 | p < 0.0001 | |||||||||||||
| HC | 47 | 37.8 ± 12.3 | 27/20 | 2 (0–4) M | 153 (129–186) | ||||||||||||||
| [129] | V | MDD | 54 | 42.4 ± 14.7 | All M | Treated | 19.3 ± 7 H | Fasting Serum | N/A | Japan | IGF-1 (ng/mL) | 171.5 ± 61.8 | ↑ | p = 0.011 | |||||
| HC | 37 | 39.4 ± 7.0 | 144.1 ± 39.2 | ||||||||||||||||
| [193] | V | MDD | 51 | 52.71 ± 14.57 | 30/21 | Treated | Fasting Plasma | ELISA | Spain | IGF-2 (ng/mL) | 249.86 ± 119.57 | ↑ HC | p < 0.001 | IGFBP-1 (ng/mL) | 12.84 ±14.31 | ns | p = 0.061 | ||
| MDD0 | 15 | 61.60 ± 10.60 | 7/8 | 24.93 ± 6.18 a | 241.29 ± 99.86 | 10.02 ± 6.61 | |||||||||||||
| MDD1 | 15 ± 4.11 a | 156.71 ± 35.64 | ↓ MDD0 | p < 0.01 | 7.35 ± 4.16 | ns | p = 0.258 | ||||||||||||
| HC | 48 | 42.58 ± 11.54 | 22/26 | 116.69 ± 53.40 | p < 0.01 | 7.57 ± 5.08 | |||||||||||||
| MDD ^ | IGFBP-3 (ng/mL) | 506.12 ± 139.91 | ns | p = 0.809 | |||||||||||||||
| MDD0 ^ | 456.49 ± 118.11 | ||||||||||||||||||
| MDD1 ^ | 270.14 ± 39.86 | ↓ MDD0 | p < 0.001 | ||||||||||||||||
| HC ^ | 498.26 ± 89.57 | ||||||||||||||||||
| MDD ^ | IGFBP-5 (ng/mL) | 86.95 ± 20.69 | ns | p = 0.904 | |||||||||||||||
| MDD0 ^ | 84.24 ± 13.56 | ||||||||||||||||||
| MDD1 ^ | 42.78 ± 6.14 | ↓ MDD0 | p < 0.001 | ||||||||||||||||
| HC ^ | 86.14 ± 15.12 | ||||||||||||||||||
| MDD ^ | IGFBP-7 (ng/mL) | 82.90 ± 26.44 | ↑ HC | p < 0.01 | |||||||||||||||
| MDD0 ^ | 82.36 ± 25.50 | ||||||||||||||||||
| MDD1 ^ | 116.19 ± 29.85 | ↑ MDD0 | p < 0.05 | ||||||||||||||||
| HC ^ | 67.01 ± 25.14 | ||||||||||||||||||
| [58] | V | MDD | 129 | 40.5 ± 12.8 | 69/60 | Treated | 13.9 ± 8.4 c | Fasting Serum | RIA | Japan | IGF-1 (ng/mL) | 160.0 ± 54.3 | ↑ | p < 0.01 | |||||
| HC | 71 | 41.4 ± 9.3 | 38/33 | 137.9 ± 41.3 | |||||||||||||||
| [130] | V | MDD NR | 84 | 38.6 ± 13.2 | 38/46 | Treated | 18.1 ± 6.5 c | Fasting Serum | RIA | Japan | IGF-1 (ng/mL) | 166.9 ± 54.9 | ↑ HC, R | p = 0.001 | |||||
| MDD R | 36 | 44.2 ± 10.7 | 17/19 | 4.3 ± 2.2 c | 138.8 ± 40.0 | p = 0.007 | |||||||||||||
| HC | 99 | 40.8 ± 9.3 | 44/55 | 139.9 ± 42.4 | |||||||||||||||
| [131] | ICD-10 | MDD FE | 60 | 35.48 ± 11.77 | 37/23 | DN | 23.32 ± 5.69 c | Fasting Serum | ELISA | China | IGF-1 (ng/mL) | 149.81 ± 34.35 | ↑ | p = 0.000 | |||||
| HC | 60 | 34.63 ± 14.05 | 39/21 | 128.23 ± 25.83 | |||||||||||||||
6. IGF Peripheral Levels in Bipolar Disorder
6.1. Bipolar Disorder
6.2. IGF-1 in BD Patients
6.2.1. IGF-1 and IGFBP-1 and Weight Gain Induced by Mood Stabilizers in BD Women
6.2.2. IGF-1 in FE BD Patients
6.2.3. IGF-1 and Other Neurotrophins in BD-I Patients
6.2.4. IGF-1 and Inflammation in BD Euthymic Patients
6.2.5. IGF-1 and the Glutamatergic System in BD Patients
6.2.6. IGF-1 Meta-Analysis in BD Patients
6.2.7. IGF-1 in Children with Double Diagnosis of ASD and BD
6.3. First Studies on IGF-2 in BD Patients
6.4. IGFBPs in BD Patients
6.4.1. IGFBP-1, IGFBP-3, IGFBP-5, and IGFBP-7 in BD Patients
6.4.2. IGFBP-2 Research in BD Patients
6.4.3. IGFBP-4 and IGFBP-6 Still Unexplored in BD Patients
| Ref. | DSM | Group | Sample Size | Age (Years) | Sex (F/M) | Treatment | Sample Source | Techn | Country | IGFs | Statistics | IGFBPs | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [217] | IV | BD0 All | 18 | 31.5 | All F | Val | 4–136 m | Fasting Serum | ELISA | Europe *2 | IGF-1 (ng/mL) | 124.4 ± 13.89 | ↑ | p = 0.001 | IGFBP-1 (ng/mL) | 21.76 ± 5.68 | ↑ | p = 0.034 |
| BD1 All | 20 | 33.2 | Lit | 3–156 m | 232.8 ± 41.13 | 34.91 ± 8.42 | ||||||||||||
| [73] | IV | BD0 | 23 | 27 | 8/15 | DN | Fasting Plasma | ELISA | Spain | IGF-1 (ng/mL) | 126.15 ± 66.09 | ns HC | p = 0.143 | |||||
| BD1m | 15 | N/A | N/A | Treated | 1 m | 194.98 ± 87.19 | p = 0.123 | |||||||||||
| BD6m | 14 | N/A | N/A | Treated | 6 m | 173.11 ± 78.44 | p = 0.47 | |||||||||||
| BD12m | 11 | N/A | N/A | Treated | 12 m | 149.54 ± 60.82 | p = 0.807 | |||||||||||
| HC | 23 | 25.7 | 13/30 | 155.41 ± 67.03 | ||||||||||||||
| [222] | IV | BD M | 116 | 35.9 ± 11.8 | 74/42 | DN (n = 79) Treated (n = 12) | Blood | ELISA | Korea | IGF-1 (pg/mL) | 514.6 ± 259.8 | ↑ | p < 0.0001 | |||||
| DF (n = 25) | >2 m | |||||||||||||||||
| HC | 123 | 35.5 ± 10.4 | 67/56 | 316.8 ± 270.0 | ||||||||||||||
| [224] | IV | BD M | 70 | 37.9 ± 14.5 | 29/41 | DN (n = 64) + DF (n = 6) | Fasting Serum | ELISA | China | IGF-1 (ng/mL) | 162.0 ± 72.0 | ↑ | p = 0.029 | |||||
| HC | 50 | 36.8 ± 11.2 | 20/30 | 138.9 ± 80.1 | ||||||||||||||
| [242] | IV | BD | 31 | 48 ± 13.09 | 21/10 | Treated | Serum | bb-Ls | Europe | IGFBP-2 (ng/mL) | 123.84 ± 67.22 | Correlation with BMI | ||||||
| [243] | IV | Offs BD NMD | 96 | 16.4 ± 2.65 | N/A | N/A | Serum | bb-Ls | Holland | IGFBP-2 (ng/mL) | 175.5(147.5–199.9) | ↑ HC | p < 0.05 | |||||
| Offs BD MD | 150.1(110.8–209.4) | ns | p > 0.05 | |||||||||||||||
| HC | 50 | 15.0 ± 1.88 ↓ | 133.2(117.5–159) | |||||||||||||||
| [206] *1 | IV | BD All | 41 | 46.76 ± 14.5 | 27/14 | Treated | Fasting Serum | ELISA | Italy | IGFBP-2 (ng/mL) | 173.24 ± 77.95 | p = 0.003 | ||||||
| HC | 93 | 49.56 ± 12.9 | 41/52 | 232.90 ± 125.48 | ||||||||||||||
| [231] | IV | BD E | 31 | 41.7 ± 11.8 | 25/6 | Treated | Fasting Serum | CLIA | Brazil | IGF-1 (ng/mL) | 248.8 ± 104.9 | ↑ | p = 0.001 | |||||
| HC | 33 | 41.0 ± 11.9 | 27/6 | 169.2 ± 74.2 | ||||||||||||||
| [233] | IV | BD a M | 19 | 42 ± 14 | 10/9 | 12 y of illness | Fasting Serum | ELISA | Poland | IGF-1 (ng/mL) | 143 ± 51 | ↑ BD Lit | p = 0.033 | |||||
| BD ra M | 13 | Treated | 40 ± 20 d | 175 ± 63 | ↑ BD Lit | p = 0.019 | ||||||||||||
| BD a D | 17 | 50 ± 14 | 11/6 | 18 y of illness | 129 ± 56 | ns | p > 0.05 | |||||||||||
| BD ra D | 12 | Treated | 46 ± 17 d | 120 ± 52 | ns | p > 0.05 | ||||||||||||
| BD Lit | 18 | 61 ± 4 | 9/9 | Lit | 22 y | 117 ± 37 | ||||||||||||
| [225] *1 | IV | BD All | 45 | 34.9 ± 10.8 | 22/23 | Treated | Fasting Serum | ELISA | Turkey | IGF-1 (ng/mL) | 279.3 ± 139.5 | ↑ | p = 0.0001 | |||||
| HC | 45 | 34.9 ± 10.8 | 22/23 | 190.7 ± 56.6 | ||||||||||||||
| [235] | IV | BD | 78 | N/A | 44/34 | DN + WO | > 3 m | Fasting Serum | ELISA | China | IGF-2 (ng/mL) | 66.08 ± 21.22 | ↓ | p < 0.001 | ||||
| BD M | 55 | 30.49 ± 8.44 | 34/21 | 67.19 ± 21.52 | ↓ | p < 0.001 | ||||||||||||
| BD D | 23 | 24.56 ± 7.24 | 10/13 | 63.43 ± 20.67 | ↓ | p < 0.001 | ||||||||||||
| HC | 50 | 29.28 ± 3.83 | 24/26 | 88.72 ± 31.55 | ||||||||||||||
| [236] | V | BD M | 20 | 51.10 ± 9.85 | 10/10 | Treated | Fasting Plasma | ELISA | Spain | IGF-2 (ng/mL) | 135.25 ± 64.02 | ns | p = 0.478 | IGFBP-1 (ng/mL) | 8.92 ± 4.06 | ns | p = 0.597 | |
| HC | 20 | 48.80 ± 7.34 | 10/10 | 114.53 ± 50.59 | 8.17 ± 4.78 | |||||||||||||
| BD0 | 10 | 53.10 ± 10.18 | 5/5 | P treated | 17 d | 168.91 ± 51.27 | ns | p = 0.548 | 10.12 ± 4.66 | ↓ BD0 | p < 0.01 | |||||||
| BD1 | 10 | 5/5 | Treated | 162.97 ± 50.06 | 6.96 ± 3.35 | |||||||||||||
| BD M ^ | IGFBP-3 (ng/mL) | 264.11 ± 60.22 | ↓ HC | p < 0.0001 | ||||||||||||||
| HC | 474.33 ± 82.24 | |||||||||||||||||
| BD0 | 257.83 ± 38.98 | ns | p = 0.429 | |||||||||||||||
| BD1 | 249.93 ± 39.63 | |||||||||||||||||
| BD M | IGFBP-5 (ng/mL) | 33.35 ± 13.06 | ↓ HC | p < 0.0001 | ||||||||||||||
| HC | 82.46 ± 14.77 | |||||||||||||||||
| BD0 | 43.09 ± 7.94 | ns | p = 0.169 | |||||||||||||||
| BD1 | 39.81 ± 5.51 | |||||||||||||||||
| BD M | IGFBP-7 (ng/mL) | 143.21 ± 130.69 | ns | p = 0.165 | ||||||||||||||
| HC | 70.09 ± 28.89 | |||||||||||||||||
| BD0 | 205.08 ± 148.79 | ns | p = 0.652 | |||||||||||||||
| BD1 | 154.86 ± 90.42 | |||||||||||||||||
| [234] | V | ASD + BD | 40 | 14.03 ± 2.97 | N/A | Treated | Fasting Serum | ELISA | Turkey | IGF-1 (ng/mL) | 9.10 (4.51–77.13) | ns | p = 0.855 | |||||
| ASD | 40 | 13.08 ± 3.06 | treated (n = 34) | 10.87 (3.96–58.83) | ||||||||||||||
7. IGF Peripheral Levels in Borderline Personality Disorder and Obsessive—Compulsive Disorder
7.1. IGF-1 in Borderline Personality Disorder (BPD)
7.2. IGF-1 in Obsessive–Compulsive Disorder (OCD)
| Ref. | DSM | Group | Sample Size | Age (Years) | Sex (F/M) | Treatment | Sample Source | Techn | Country | IGFs | Statistics | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [245] | IV | BDP | 16 | 26.1 ± 5.1 | All F | SSRIs (n = 3) | Fasting Serum | ELISA | German | IGF-1 (ng/mL) | 189 ± 63 | ns | N/A | |
| BPD + MDD 1 | 12 | 31.8 ± 6.5 | SSRIs (n = 3) | 161 ± 62 | ||||||||||
| BPD + MDD 2 | 10 | 25.9 ± 5 | SSRIs (n = 5) | 164 ± 62 | ||||||||||
| HC | 20 | 24.2 ± 5.9 | 176 ± 32 | |||||||||||
| [246] | III-R | CB ADex | 25 | ~60 *1 | All M | Treated | Dex Test | Serum | RIA | Sweden | IGF-1 (ng/mL) | 174.9 ± 33.8 | ↓ HC ADex | p = 0.007 |
| CA ADex | 42 | 196.4 ± 60.5 | ns | p > 0.20 | ||||||||||
| HC ADex | 223 | 212.8 ± 66.1 | ||||||||||||
| CB BDex | 25 | Treated | Dex Test | 182.6 ± 44.0 | ns | p = 0.062 | ||||||||
| CA BDex | 42 | 193.3 ± 67.9 | ↓ HC BDex | p = 0.017 | ||||||||||
| HC BDex | 223 | 210.3 ± 63.1 | ||||||||||||
| [124] | IV | OCD | 40 | 38.7 ± 13.3 | 22/18 | Treated | Fasting Serum | ELISA | Italy | IGF-1 (ng/mL) | 149.9 ± 60.2 | ↑ HC | p = 0.04 | |
| HC | 43 | 42.3 ± 11.3 | 15/28 | 121.2 ± 51.6 | ||||||||||
| OCD0 | 18 | N/A | N/A | 131.4 ± 50.3 | ns | p = 0.215 | ||||||||
| OCD1 | SSRIs | 1 w | 126.6 ± 50.0 | |||||||||||
| [251] | IV | OCD0 | 16 | 28.1 ± 6.2 | 11/5 | DN | Fasting Plasma | ELISA | India | IGF-1 (ng/mL) | 129.7 ± 62.1 | Y-BOCs (% red) *2 (r = 0.6; p = 0.02) | ||
| OCD1 | SSRIs | 3 m | N/A | |||||||||||
8. IGF Peripheral Levels in Autism Spectrum Disorder
8.1. Autism Spectrum Disorder
8.2. First Studies and IGF Cerebrospinal Fluid Levels of Members in ASD Children
8.3. Relation Between IGFs and Growth in ASD Children
8.4. IGF-1 in ASD Children
8.4.1. IGF-1 in Reduced Bone Mineral Density in ASD Children
8.4.2. IGF-1 and the Neurotrophic Approach in ASD Children
8.5. IGF Peripheral Levels in Different Stages of ASD Severity
| Ref. | DSM | Groups | Sample Size | Age (Years) | Sex (F/M) | Treatment | Sample Source | Techn | Country | IGFs | Statistics | IGFBPs | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [257] | III | ASD | 11 | 3.8 ± 1.1 | 4/7 | No treatment | CSF | RIA | Finland | IGF-1 (µg/L) | 0.34 ± 0.08 | ↓ *2 | p = 0.03 | |||||
| C | 11 | 3.8 ± 1.3 | 6/5 | 0.40 ± 0.15 | ||||||||||||||
| [25] | IV | ASD | 6 | 5.5 ± 2.5 | 2/4 | N/A | CSF | ELISA | USA | IGF-1 was not measured. Data was not given for CSF IGFBPs but fold changes and p-values. | IGFBP-1 *4 | 0.4 | ↓ | p = 0.036 | ||||
| C | 9 | 33.9 ± 10.8 | 6/3 | IGFBP-3 *4 | 26.3 | ↑ | p < 0.001 | |||||||||||
| IGFBP-4 *4 | 13.3 | ↑ | p = 0.003 | |||||||||||||||
| [259] | III | ASD | 25 | 5y5m 1y11m–15y10m | 5/25 | N/A | CSF | RIA | Finland | IGF-1 (µg/L) | 0.41 ± 0.18 | ↓ | p = 0.02 | |||||
| C | 16 | 7y4m 1y11m–15y10m | 8/8 | 0.58 ± 0.27 | ||||||||||||||
| IGF-2 (µg/L) | 19.1 ± 3.10 | ns | p = 0.33 | |||||||||||||||
| 20.5 ± 4.40 | ||||||||||||||||||
| [261] | N/A | ASDR0 | 13 | 5–16 | 1/12 | Fluox | 2 m | CSF | N/A | Finland | IGF-1 (µg/L) | 0.55 ± 0.16 | ns | p = 0.069 | ||||
| ASDR1 | 0.69 ± 0.21 | |||||||||||||||||
| ASDPR0 | 0.51 ± 0.2 | ↑ | p = 0.001 | |||||||||||||||
| ASDPR1 | 0.67 ± 0.2 | |||||||||||||||||
| [262] | IV | ASD | 71 | 6.6 ± 1.5 | All M | treated (n = 8) | Plasma | RIA | England | IGF-1 (ng/mL) | 149.0 ± 58.3 | ↑ | p < 0.0001 | IGFBP-3 (mg/L) | 2.5 ± 0.6 | ↑ | p < 0.0001 | |
| HC | 59 | 6.5 ± 1.2 | 113.7 ± 45.0 | 2.1 ± 0.4 | ||||||||||||||
| IGF-2 (ng/mL) | 397.2 ± 99.5 | ↑ | p < 0.0001 | |||||||||||||||
| 306.1 ± 76.0 | ||||||||||||||||||
| [263] | IV | ASD | 34 | 3.1 ± 0.9 | 8/26 | N/A | Urinary | ELISA | Turkey | IGF-1 (µg/d) | 0.7 ± 0.08 | ↓ | p = 0.03 | IGFBP-3 (mg/d) | 2.2 ± 0.32 | ns/↓ | p = 0.05 | |
| HC | 29 | 3.3 ± 1.2 | 4/25 | 1.5 ± 0.34 | 3.3 ± 0.37 | |||||||||||||
| [264] | IV | ASD | 20 | 13.6 ± 0.53 | All M | N/A | Serum | CMS | USA | IGF-1 (Z-scores) | 0.18 ± 0.13 | ↑ | p < 0.001 | |||||
| HC | 20 | 14.2 ± 0.56 | −0.73 ± 0.16 | |||||||||||||||
| [265] | V | ASD | 40 | 6.98 ± 2.58 | 3/37 | treated (yes/no) (19/21) | Serum | ELISA | Turkey | IGF-1 (ng/mL) | 250.1 ± 131.5 | ↑ | p < 0.001 | |||||
| HC | 40 | 7.79 ± 2.05 | 3/37 | 141.9 ± 62.36 | ||||||||||||||
| [266] | V | ASD0 | 16 | 9.38 ± 2.63 | 3/13 | NIBS (n = 13) | Serum | ELISA | Cuba | IGF-1 (ng/mL) | 168.00 ± 85.40 | ns | p = 0.669 | |||||
| ASD1 | 162.00 ± 81.40 | |||||||||||||||||
| [267] | V | ASD | 22 | 9.45 ± 2.94 | 5/17 | treated (n = 14) | Serum | ELISA | Cuba | IGF-1 (ng/mL) | 153.09 ± 77.69 | ↑ | p = 0.037 | |||||
| HC | 29 | 8.68 ± 2.82 | 8/21 | 115.31 ± 50.50 | ||||||||||||||
| [268] | V | ASD | 200 | 6.6 ± 4.1 | N/A | N/A | Serum | ELISA | Iran | IGF-1 (ng/mL) | 31.45 ± 9.84 | ↓ | p = 0.001 | |||||
| HC | 198 | 6.8 ± 3.2 | 54.62 ± 11.63 | |||||||||||||||
| [269] | V | ASD | 150 | 4.17 ± 1.67 | 37/113 | DN | Fasting Serum | CL | China | IGF-1 (ng/mL) | 106 (80,139) | ↓ | p = 0.021 | IGFBP-3 (µg/mL) | 3.6 ± 0.9 | ns | p = 0.108 | |
| HC | 165 | 4 ± 1.33 | 41/124 | 113 (90,152) | 3.7 ± 0.8 | |||||||||||||
| [26] | V | ASD *1 | 180 | 8 ± 3.8 | 34/146 | N/A | Serum | ELISA | Iran | IGF-1 (ng/mL) | 39.06 ± 14.76 | ↓ lev ^ ↓ HC | N/A | IGFBP-3 (ng/mL) | 2867.33 ± 496.49 | ↓ lev ^ ↓ HC | N/A | |
| ASDmi | 69 | N/A | N/A | 35.46 ± 14.92 | 2883.46 ± 472.37 | |||||||||||||
| ASDmo | 58 | 26.46 ± 13.77 | 2214.86 ± 543.20 | |||||||||||||||
| ASDs | 53 | 45.2 ± 17.69 | 3185.73 ± 559.67 | |||||||||||||||
| HC | 118 | 7.3 ± 3.7 | 23/95 | IGF-2 (ng/mL) | 1490.6 ± 159.9 | IGFBP-4 (ng/mL) | 397.86 ± 91.64 | |||||||||||
| 1455.93 ± 142.56 | 387.8 ± 77.96 | |||||||||||||||||
| 1380.13 ± 178.55 | 357.13 ± 75.05 | |||||||||||||||||
| 1546.07 ± 166.88 | 409.6 ± 102.06 | |||||||||||||||||
| IGFBP-1 *3 (ng/mL) | 9.8 ± 4.2 | IGFBP-5 (ng/mL) | 216.8 ± 61.36 | |||||||||||||||
| 9.4 ± 6.5 | 182.2 ± 45.18 | |||||||||||||||||
| 9.1 ± 3.4 | 161.26 ± 45.60 | |||||||||||||||||
| 11.8 ± 4.6 | 224.86 ± 70.99 | |||||||||||||||||
| IGFBP-2 *3 (ng/mL) | 175.13 ± 47.40 | IGFBP-6 (ng/mL) | 189 ± 60 | |||||||||||||||
| 167.53 ± 51.27 | 176.93 ± 50.15 | |||||||||||||||||
| 150.8 ± 40.28 | 165.06 ± 47.74 | |||||||||||||||||
| 178.6 ± 60.43 | 200.53 ± 64.72 | |||||||||||||||||
| [234] ^ | V | ASD + BD | 40 | 14.03 ± 2.97 | N/A | treated | Fasting Serum | ELISA | Turkey | IGF-1 (ng/mL) | 9.10 (4.51–77.13) | ns | p = 0.855 | |||||
| ASD | 40 | 13.08 ± 3.06 | treated (n = 34) | 10.87 (3.96–58.83) | ||||||||||||||
8.6. Animal Models Supporting IGF-2 Research in ASD Children
9. IGF Peripheral Levels in Attention Deficit/Hyperactive Disorder
9.1. Attention-Deficit/Hyperactive Disorder
9.2. IGF-1 in ADHD Patients
9.2.1. IGF-1 in the Context of Potential Growth Restriction by Methylphenidate (MPH) in ADHD Children
9.2.2. Potential Influence of Systemic Inflammation and IGF-1 Levels with ADHD Increased Risk
9.2.3. Influence of Atomoxetine and MPH in IGF-1/IGFBP-3 Levels in ADHD Children
9.2.4. IGF-1 in Drug-Naïve ADHD Children and Urine Levels of Phthalates
9.2.5. IGF-1 and Melatonin Treatment in ADHD Adult Patients with Delayed Sleep Phase Syndrome (DSPS)
9.3. Supporting IGF-2 and Other IGFBPs in the Context of ADHD
| Ref. | DSM | Group | Sample Size | Age (Years) | Sex (F/M) | Treatment | Sample Source | Techn | Country | IGFs | Statistics | IGFBPs | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [293] | N/A | T ADHD | 8 | 10.99 ± 1.69 | 2/6 | MPH | 15.4 ± 14.4 m | Fasting Serum | RIA | USA | IGF-1 (U/mL) | 0.76 ± 0.3 | ns | p = 0.28 † | ||||
| NT ADHD | 9 | 11.14 ± 1.64 | 2/7 | Off-MPH treatment | 0.9 ± 0.24 * | |||||||||||||
| [294] | III-R | NT ADHD | 21 | 10.1 ± 2.4 | All M | No treated | Serum | RIA | USA | IGF-1 (nmol/L) | 19.4 ± 11.5 | ns | p > 0.05 | |||||
| T ADHD | 21 | MPH | 7.2 ± 7.4 m | 24.1 ± 9.5 | ||||||||||||||
| HC | 30 | 10.08 ± 2.06 | 21.7 ± 8.5 | |||||||||||||||
| [295] | IV | ADHD0 | 14 | 8.12 ± 1.8 | 4/10 | DN | Serum | IRMA | Turkey | IGF-1 (ng/mL) | 163 ± 107 | IGFBP-3 (mg/L) | 4.87 ± 0.8 | |||||
| ADHD4m | MPH | 4 m | 97 ± 32 | ↓ 0 | p < 0.05 | 4.13 ± 0.9 | ↓ 0 | p < 0.05 | ||||||||||
| ADHD8m | MPH | 8 m | 134 ± 45 | ns | N/A | 4.30 ± 0.5 | ns | N/A | ||||||||||
| ADHH14m | MPH | 12 m | 178 ± 92 | ns | N/A | 4.81 ± 0.6 | ns | N/A | ||||||||||
| [296] | IV | NT ADHD | 41 | 8.81 ± 1.52 | All M | DN | Serum | CLIA | Korea | IGF-1 (ng/mL) | 198.49 ± 105.42 | ns | p = 0.75 | |||||
| T ADHD | 31 | 9.48 ± 1.44 | MPH | 1.79 ± 1.11 y | 213.72 ± 137.09 | |||||||||||||
| HC | 79 | 9.20 ± 1.65 | 189.85 ± 92.97 | |||||||||||||||
| NT ADHD | 23 | 9.22 ± 1.51 | All F | DN | 316.25 ± 169.19 | ns | p = 0.45 | |||||||||||
| T ADHD | 8 | 9.89 ± 1.45 | MPH | 1.79 ± 1.11 y | 289.56 ± 174.99 | |||||||||||||
| HC | 33 | 9.36 ± 1.44 | 257.22 ± 131.28 | |||||||||||||||
| [299] | V | ADHD0 | 149 | 8.9 ± 2.78 | 56/93 | DN | Serum | CLIA | China | IGF-1 (ng/mL) | 170.43 ± 37.27 | ns | p = 0.28 | IGFBP-3 (µg/mL) | 4.22 ± 0.87 | ns | p = 0.51 | |
| ADHD1 | ATO | 12 w | 209.71 ± 83.53 | 4.62 ± 0.98 | ||||||||||||||
| [300] | V | NT ADHD0 | 22 | 8.8 ± 1.9 | 6/16 | DN | Serum | ELISA | Taiwan | IGF-1 (ng/mL) | 134.61 ± 54.04 | ↑ 0,MPH12m | p < 0.05 | IGFBP-3 (ng/mL) | 2746.83 ± 702.44 | ns | N/A | |
| NT ADHD12m | 174.94 ± 63.54 | 3099.68 ± 663.76 | ||||||||||||||||
| ADHD0 | 39 | 9.0 ± 2.3 | 9/30 | MPH | 12 m *2 | 121.43 ± 56.01 | ↑ 0 | p < 0.05 | 2511.93 ± 790.25 | ns | N/A | |||||||
| ADHD12m | 161.59 ± 72.31 | 2653.39 ± 631.24 | ||||||||||||||||
| ADHD0 | 40 | 9.5 ± 2.5 | 7/33 | O-MPH | 138.89 ± 73.44 | ↑ 0,NT12m | p < 0.05 | 2377.14 ± 690.58 | ↑ 0 | p < 0.05 | ||||||||
| ADHD12m | 189.11 ± 83.77 | 2643.85 ± 539.67 | ||||||||||||||||
| ADHD0 | 17 | 9.1 ± 2.3 | 3/14 | ATO | 144.11 ± 76.27 | ns | N/A | 2636.61 ± 444.64 | ↑ 0,O-MPH | p < 0.05 | ||||||||
| ADHD12m | 181.57 ± 86.14 | 2962.45 ± 391.60 | ||||||||||||||||
| [301] | V | ADHD | 144 | 8.9 ± 2.2 | 34/110 | DN | Fasting Serum | ELISA | Taiwan | IGF-1 (ng/mL) | 141.5 ± 69.5 | ↓ | p = 0.003 | IGFBP-3 (mg/L) | N/A | ns | p > 0.05 | |
| HC | 70 | 9.2 ± 2.2 | 24/46 | 182.7 ± 100.8 | N/A | |||||||||||||
| [302] | V | ADHD | 40 | 9.87 ± 1.32 | 16/24 | DN | Fasting Serum | ELISA | Turkey | IGF-1 (ng/mL) | 7.42 ± 4.79 | ns | p = 0.074 | |||||
| HC | 40 | 9.94 ± 1.58 | 16/24 | 6.60 ± 6.45 | ||||||||||||||
| [303] | IV | ADHD + DSPS | 12 | 29.75 ± 9.03 | 6/6 | MEL | 3 w | Fasting Serum | ECLIA | Holland | IGF-1 (nmol/L) | 30.30 ± 7.69 | nsPLAC | p = 0.938 | ||||
| 13 | 28.77 ± 10.99 | 9/4 | MEL + BLT | 31.28 ± 9.54 | nsPLAC | p = 0.595 | ||||||||||||
| 12 | 31.25 ± 6.70 | 7/5 | Plac | 26.69 ± 8.54 | ||||||||||||||
| [297] | N/A | ADHD | 51 | 11.91 ± 2.21 | 6 | GH provocative test Treated | Serum | RIA | Australia | IGF-1 (ng/mL) | 29.1 ± 11.8 | ↑ | p = 0.018 | IGFBP-3 (nmol/L) | 140.75 ± 34.61 | ns | p = 0.298 | |
| 13 ± 2.37 | 45 | 20 ± 8.1 | no | |||||||||||||||
10. Heterogeneity and Potential Source of Bias Among Studies
11. Limitations and Future Perspectives
12. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Arias, D.; Saxena, S.; Verguet, S. Quantifying the global burden of mental disorders and their economic value. eClinicalMedicine 2022, 54, 101675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Sourav, M.S.U.; Yang, M.; Zhang, J. Classifying mental disorders through clinicians’ subjective approach based on three-way decisions. Front. Psychol. 2023, 14, 1144826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; López, M.; Spuch, C.; Olivares, J.M.; Agís-Balboa, R.C. Schizophrenia: A review of potential biomarkers. J. Psychiatr. Res. 2017, 93, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, D.; Penedo, M.A.; Rivera-Baltanás, T.; Peña-Centeno, T.; Burkhardt, S.; Fischer, A.; Prieto-González, J.M.; Olivares, J.M.; López-Fernández, H.; Agís-Balboa, R.C. MiRNA Differences Related to Treatment-Resistant Schizophrenia. Int. J. Mol. Sci. 2023, 24, 1891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, Z.; Min, J.; Tan, Q.; Si, K.; Yang, H.; Xu, C. Circulating insulin-like growth factor-1 and brain health: Evidence from 369,711 participants in the UK Biobank. Alzheimers Res. Ther. 2023, 15, 140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- LeRoith, D.; Holly, J.M.P.; Forbes, B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021, 52, 101245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwa, V.; Oh, Y.; Rosenfeld, R.G. Insulin-like growth factor binding proteins: A proposed superfamily. Acta Paediatr. Suppl. 1999, 88, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Chowen, J.A.; Martín-Rivada, Á.; Guerra-Cantera, S.; Pozo, J.; Yakar, S.; Rosenfeld, R.G.; Pérez-Jurado, L.A.; Suárez, J.; Argente, J. Pregnancy-Associated Plasma Protein (PAPP)-A2 in Physiology and Disease. Cells 2021, 10, 3576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailes, J.; Soloviev, M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules 2021, 11, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Werner, H.; LeRoith, D. Insulin and insulin-like growth factor receptors in the brain: Physiological and pathological aspects. Eur. Neuropsychopharmacol. 2014, 24, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Newhouse, A.; Chemali, Z. Neuroendocrine Disturbances in Neurodegenerative Disorders: A Scoping Review. Psychosomatics 2020, 61, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Puche, J.E.; Castilla-Cortázar, I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. J. Transl. Med. 2012, 10, 224. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holly, J.M.P.; Biernacka, K.; Perks, C.M. The Neglected Insulin: IGF-II, a Metabolic Regulator with Implications for Diabetes, Obesity, and Cancer. Cells 2019, 8, 1207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardo, M.; Cheng, Y.; Sitbon, Y.H.; Lowell, J.A.; Grieco, S.F.; Worthen, R.J.; Desse, S.; Barreda-Diaz, A. Insulin growth factor 2 (IGF2) as an emergent target in psychiatric and neurological disorders. Review. Neurosci. Res. 2019, 149, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Agis-Balboa, R.C.; Fischer, A. Generating new neurons to circumvent your fears: The role of IGF signaling. Cell Mol. Life Sci. 2014, 71, 21–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nordin, M.; Bergman, D.; Halje, M.; Engström, W.; Ward, A. Epigenetic regulation of the Igf2/H19 gene cluster. Cell Prolif. 2014, 47, 189–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bohnsack, R.N.; Misra, S.K.; Liu, J.; Ishihara-Aoki, M.; Pereckas, M.; Aoki, K.; Ren, G.; Sharp, J.S.; Dahms, N.M. Lysosomal enzyme binding to the cation-independent mannose 6-phosphate receptor is regulated allosterically by insulin-like growth factor 2. Sci. Rep. 2024, 14, 26875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agis-Balboa, R.C.; Arcos-Diaz, D.; Wittnam, J.; Govindarajan, N.; Blom, K.; Burkhardt, S.; Haladyniak, U.; Agbemenyah, H.Y.; Zovoilis, A.; Salinas-Riester, G.; et al. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011, 30, 4071–4083. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clemmons, D.R. Role of IGF Binding Proteins in Regulating Metabolism. Trends Endocrinol. Metab. 2016, 27, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bach, L.A. IGF-binding proteins. J. Mol. Endocrinol. 2018, 61, T11–T28. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin Growth Factor Binding Protein 7 (IGFBP7)-Related Cancer and IGFBP3 and IGFBP7 Crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baxter, R.C. Signaling Pathways of the Insulin-like Growth Factor Binding Proteins. Endocr. Rev. 2023, 44, 753–778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schilling, C.; Blum, W.F.; Heuser, I.; Paslakis, G.; Wudy, S.A.; Deuschle, M. Treatment with antidepressants increases insulin-like growth factor-I in cerebrospinal fluid. J. Clin. Psychopharmacol. 2011, 31, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.L.; Nascimbene, C.; Krishnan, C.; Zimmerman, A.W.; Pardo, C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 2005, 57, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, F.; Shabani, S.; Salehi, Z. Comparison of serum IGF1, IGF2 and IGFBP1-6 concentration in the children with different stages of autism spectrum disorder. Arch. Psychiatry Psychother. 2022, 24, 20–24. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Li, X.; Wei, N.; Song, J.; Liu, J.; Yuan, J.; Song, R.; Liu, L.; Mei, L.; Yan, S.; Wu, Y.; et al. The global burden of schizophrenia and the impact of urbanization during 1990–2019: An analysis of the global burden of disease study 2019. Environ. Res. 2023, 232, 116305. [Google Scholar] [CrossRef] [PubMed]
- Penedo, M.A.; Rivera-Baltanás, T.; Pérez-Rodríguez, D.; Allen, J.; Borrajo, A.; Alonso-Crespo, D.; Fernández-Pereira, C.; Nieto-Araujo, M.; Ramos-García, S.; Barreiro-Villar, C.; et al. The role of dopamine receptors in lymphocytes and their changes in schizophrenia. Brain Behav. Immun. Health 2021, 12, 100199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Correll, C.U. Pharmacologic treatment of schizophrenia. Dialogues Clin. Neurosci. 2010, 12, 345–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haddad, P.M.; Correll, C.U. The acute efficacy of antipsychotics in schizophrenia: A review of recent meta-analyses. Ther. Adv. Psychopharmacol. 2018, 8, 303–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Chen, K.P.; Chiu, C.C.; Tai, M.H.; Lung, F.W. Early predictors of poor treatment response in patients with schizophrenia treated with atypical antipsychotics. BMC Psychiatry 2018, 18, 376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melkersson, K.I.; Hulting, A.L.; Brismar, K.E. Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychoses. J. Clin. Psychiatry 1999, 60, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Melkersson, K.I.; Hulting, A.L.; Brismar, K.E. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J. Clin. Psychiatry 2000, 61, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Melkersson, K.I.; Hulting, A.L.; Rane, A.J. Dose requirement and prolactin elevation of antipsychotics in male and female patients with schizophrenia or related psychoses. Br. J. Clin. Pharmacol. 2001, 51, 317–324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melkersson, K.I.; Dahl, M.L. Relationship between levels of insulin or triglycerides and serum concentrations of the atypical antipsychotics clozapine and olanzapine in patients on treatment with therapeutic doses. Psychopharmacology 2003, 170, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Gaughran, F.P.; Amiel, S.A.; Murray, R.M.; Pilowsky, L.S. The effect of clozapine on factors controlling glucose homeostasis. J. Clin. Psychiatry 2004, 65, 1352–1355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gunnell, D.; Holly, J.M. Hypothesis: Do insulin-like growth factors underlie associations of birth complications, fetal and pre-adult growth with schizophrenia? Schizophr. Res. 2004, 71, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H. Resistance to insulin in mentally disturbed soldiers. Arch. Neurol. Psychiatry 1946, 56, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Collins, P.; Thakore, J.H. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am. J. Psychiatry 2003, 160, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.I.; Simpson, H.L.; Sönksen, P.H. The role of the growth hormone-insulin-like growth factor axis in glucose homeostasis. Diabet. Med. 2003, 20, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, G.; Chittiprol, S.; Neelakantachar, N.; Naveen, M.N.; Thirthall, J.; Gangadhar, B.N.; Shetty, K.T. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am. J. Psychiatry 2007, 164, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Doré, S.; Kar, S.; Quirion, R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc. Natl. Acad. Sci. USA 1997, 94, 4772–4777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gisabella, B.; Bolshakov, V.Y.; Benes, F.M. Regulation of synaptic plasticity in a schizophrenia model. Proc. Natl. Acad. Sci. USA 2005, 102, 13301–13306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.K.; Huang, C.Y.; Liou, Y.J.; Wang, C.K.; Lee, S.D. Glucose-insulin homeostasis, lipid profiles and GH-IGF-IGFBP axis in clozapine-treated schizophrenic obesity versus non-psychiatric obesity. Int J Obes. 2008, 32, 436–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haj-Ahmad, L.M.; Mahmoud, M.M.; Sweis, N.W.G.; Bsisu, I.; Alghrabli, A.M.; Ibrahim, A.M.; Zayed, A.A. Serum IGF-1 to IGFBP-3 Molar Ratio: A Promising Diagnostic Tool for Growth Hormone Deficiency in Children. J. Clin. Endocrinol. Metab. 2023, 108, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Liu, X.; Wang, L.; Lv, H.; Yu, J.; Xun, Z.; Yang, G. Abnormality of glycometabolism related factors in non-psychotic offspring of schizophrenic patients. Psychiatry Res. 2012, 198, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Demirel, A.; Demirel, O.F.; Emül, M.; Duran, A.; Uğur, M. Relationships between IGF-1, schizophrenia, and treatment of metabolic syndrome. Compr. Psychiatry 2014, 55, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Forhead, A.J.; Dauncey, M.J.; Gilmour, R.S.; Fowden, A.L. Control of growth hormone receptor and insulin-like growth factor-I expression by cortisol in ovine fetal skeletal muscle. J. Physiol. 2002, 541 Pt 2, 581–589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, P.; Spelman, L.; Sharifi, N.; Thakore, J.H. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology 2005, 30, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Rossbach, W.; Müller, M.J.; Müller-Siecheneder, F.; Pott, T.; Linde, I.; Dittmann, R.W.; Hiemke, C. Nocturnal hormone profiles in patients with schizophrenia treated with olanzapine. Psychoneuroendocrinology 2006, 31, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, G.; Chittiprol, S.; Neelakantachar, N.; Shetty, T.; Gangadhar, B.N. Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: A longitudinal study. Schizophr. Res. 2010, 119, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Girshkin, L.; Matheson, S.L.; Shepherd, A.M.; Green, M.J. Morning cortisol levels in schizophrenia and bipolar disorder: A meta-analysis. Psychoneuroendocrinology 2014, 49, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, D.B.; Miller, B.J. Meta-analysis of blood cortisol levels in individuals with first-episode psychosis. Psychoneuroendocrinology 2019, 104, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Arinami, H.; Watanabe, Y.; Suzuki, Y.; Tajiri, M.; Tsuneyama, N.; Someya, T. Serum cortisol and insulin-like growth factor 1 levels in major depressive disorder and schizophrenia. Sci. Rep. 2023, 13, 1148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.J.; Goldsmith, D.R. Towards an Immunophenotype of Schizophrenia: Progress, Potential Mechanisms, and Future Directions. Neuropsychopharmacology 2017, 42, 299–317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pillinger, T.; Osimo, E.F.; Brugger, S.; Mondelli, V.; McCutcheon, R.A.; Howes, O.D. A Meta-analysis of Immune Parameters, Variability, and Assessment of Modal Distribution in Psychosis and Test of the Immune Subgroup Hypothesis. Schizophr. Bull. 2019, 45, 1120–1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lesh, T.A.; Careaga, M.; Rose, D.R.; McAllister, A.K.; Van de Water, J.; Carter, C.S.; Ashwood, P. Cytokine alterations in first-episode schizophrenia and bipolar disorder: Relationships to brain structure and symptoms. J. Neuroinflamm. 2018, 15, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrikis, P.; Boumba, V.A.; Tzallas, A.T.; Voulgari, P.V.; Archimandriti, D.T.; Skapinakis, P.; Mavreas, V. Elevated levels of Insulin-like Growth Factor-1 (IGF-1) in drug-naïve patients with psychosis. Psychiatry Res. 2016, 246, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vardy, E.R.; Rice, P.J.; Bowie, P.C.; Holmes, J.D.; Grant, P.J.; Hooper, N.M. Increased circulating insulin-like growth factor-1 in late-onset Alzheimer’s disease. J. Alzheimers Dis. 2007, 12, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, F.; Mirzajani, E.; Naji, M.; Azari, M. Expression of insulin-like growth factor-1 and insulin-like growth factor binding proteins in the serum and cerebrospinal fluid of patients with Parkinson’s disease. J. Clin. Neurosci. 2010, 17, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Torres-Aleman, I. The role of insulin and insulin-like growth factor I in the molecular and cellular mechanisms underlying the pathology of Alzheimer’s disease. Eur. J. Pharmacol. 2004, 490, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Karanikas, E.; Manganaris, S.; Ntouros, E.; Floros, G.; Antoniadis, D.; Garyfallos, G. Cytokines, cortisol and IGF-1 in first episode psychosis and ultra high risk males. Evidence for TNF-α, IFN-γ, ΤNF-β, IL-4 deviation. Asian J. Psychiatr. 2017, 26, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Berk, M. Th17 pathway-mediated immunopathogenesis of schizophrenia: Mechanisms and implications. Schizophr. Bull. 2014, 40, 1412–1421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Li, H.; Zhao, Q.; Song, J.; Lin, C.; Yu, J. Effect of risperidone treatment on insulin-like growth factor-1 and interleukin-17 in drug naïve first-episode schizophrenia. Psychiatry Res. 2021, 297, 113717. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.H.; Lee, S.; Yantis, J.; Valdez, C.; Paredes, R.M.; Braida, N.; Velligan, D.; Walss-Bass, C. Differential correlations between inflammatory cytokines and psychopathology in veterans with schizophrenia: Potential role for IL-17 pathway. Schizophr. Res. 2013, 151, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Borovcanin, M.; Jovanovic, I.; Radosavljevic, G.; Djukic Dejanovic, S.; Bankovic, D.; Arsenijevic, N.; Lukic, M.L. Elevated serum level of type-2 cytokine and low IL-17 in first episode psychosis and schizophrenia in relapse. J. Psychiatr. Res. 2012, 46, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Schmitt Junior, A.A.; Primo de Carvalho Alves, L.; Padilha, B.L.; da Rocha, N.S. Serum cytokine variations among inpatients with major depression, bipolar disorder, and schizophrenia versus healthy controls: A prospective ‘true-to-life’ study. Ther. Adv. Psychopharmacol. 2023, 13, 20451253221135463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palomino, A.; González-Pinto, A.; Martinez-Cengotitabengoa, M.; Ruiz de Azua, S.; Alberich, S.; Mosquera, F.; Matute, C. Relationship between negative symptoms and plasma levels of insulin-like growth factor 1 in first-episode schizophrenia and bipolar disorder patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Saleh, N.; Moutereau, S.; Azulay, J.P.; Verny, C.; Simonin, C.; Tranchant, C.; El Hawajri, N.; Bachoud-Lévi, A.C.; Maison, P.; Huntington French Speaking Group. High insulinlike growth factor I is associated with cognitive decline in Huntington disease. Neurology 2010, 75, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Torres-Alemán, I. Circulating insulin-like growth factor I and cognitive function: Neuromodulation throughout the lifespan. Prog. Neurobiol. 2009, 89, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.; Eyermann, C.; Colognato, H. Dystroglycan modulates the ability of insulin-like growth factor-1 to promote oligodendrocyte differentiation. J. Neurosci. Res. 2010, 88, 3295–3307. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, X.L.; Jiang, S.Z.; Xiong, J.W.; Zhan, J.Q.; Yan, K.; Yang, Y.J.; Jiang, L.P. The association between serum insulin-like growth factor 1 and cognitive impairments in patients with schizophrenia. Psychiatry Res. 2020, 285, 112731. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Abraham, P.M.; Paulose, C.S. Striatal dopamine receptors modulate the expression of insulin receptor, IGF-1 and GLUT-3 in diabetic rats: Effect of pyridoxine treatment. Eur. J. Pharmacol. 2012, 696, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F.; Hallberg, M. Growth hormone and cognitive function. Nat. Rev. Endocrinol. 2013, 9, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Picillo, M.; Pivonello, R.; Santangelo, G.; Pivonello, C.; Savastano, R.; Auriemma, R.; Amboni, M.; Scannapieco, S.; Pierro, A.; Colao, A.; et al. Serum IGF-1 is associated with cognitive functions in early, drug-naïve Parkinson’s disease. PLoS ONE 2017, 12, e0186508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frater, J.; Lie, D.; Bartlett, P.; McGrath, J.J. Insulin-like Growth Factor 1 (IGF-1) as a marker of cognitive decline in normal ageing: A review. Ageing Res. Rev. 2018, 42, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Yesilkaya, U.H.; Gica, S.; Ilnem, M.C.; Sen, M.; Ipekcioglu, D. Evaluation of IGF-1 as a novel theranostic biomarker for schizophrenia. J. Psychiatr. Res. 2021, 140, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Yoshino, K.; Kitagawa, S.; Fujii, R.; Hamada, S.; Ikenouchi, A.; Konishi, Y.; Ueda, N.; Eto, Y.; Tsutsumi, Y.; et al. Association Between Serum Insulin-Like Growth Factor 1 Levels and the Clinical Symptoms of Chronic Schizophrenia: Preliminary Findings. Front. Psychiatry 2021, 12, 653802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teja, V.S.K. Insulin-like Growth Factor-1 in First-Episode Schizophrenia: A Cross-Sectional Study. Master’s Thesis, Ranchi University, Central Institute of Psychiatry, Kanke, India, 2018. [Google Scholar]
- Pejcic, A.V.; Jankovic, S.M.; Janjic, V.; Djordjic, M.; Milosavljevic, J.Z.; Milosavljevic, M.N. Meta-analysis of peripheral insulin-like growth factor 1 levels in schizophrenia. Brain Behav. 2023, 13, e2819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiong, J.; Ding, Y.; Wu, X.; Zhan, J.; Wan, Q.; Wan, H.; Wei, B.; Chen, H.; Yang, Y. Association between serum insulin-like growth factor 1 levels and the improvements of cognitive impairments in a subgroup of schizophrenia: Preliminary findings. Schizophr. Res. 2024, 264, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Akanji, A.O.; Ohaeri, J.U.; Al-Shammri, S.A.; Fatania, H.R. Associations of blood levels of insulin-like growth factor (IGF)-I, IGF-II and IGF binding protein (IGFBP)-3 in schizophrenic Arab subjects. Clin. Chem. Lab. Med. 2007, 45, 1229–1231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.J.; Luo, T.; Zhao, Y.; Jiang, S.Z.; Xiong, J.W.; Zhan, J.Q.; Yu, B.; Yan, K.; Wei, B. Altered insulin-like growth factor-2 signaling is associated with psychopathology and cognitive deficits in patients with schizophrenia. PLoS ONE 2020, 15, e0226688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, X.L.; Jiang, S.Z.; Xiong, J.W.; Zhan, J.Q.; Wei, B.; Chen, C.N.; Yang, Y.J. Changes of Serum Insulin-like Growth Factor-2 Response to Negative Symptom Improvements in Schizophrenia Patients Treated with Atypical Antipsychotics. Curr. Med. Sci. 2020, 40, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Shamblott, M.J.; Leung, S.; Greene, M.W.; Chen, T.T. Characterization of a teleost insulin-like growth factor II (IGF-II) gene: Evidence for promoter CCAAT/enhancer-binding protein (C/EBP) sites, and the presence of hepatic C/EBP. Mol. Mar. Biol. Biotechnol. 1998, 7, 181–190. [Google Scholar] [PubMed]
- Chase, K.A.; Rosen, C.; Gin, H.; Bjorkquist, O.; Feiner, B.; Marvin, R.; Conrin, S.; Sharma, R.P. Metabolic and inflammatory genes in schizophrenia. Psychiatry Res. 2015, 225, 208–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Pereira, C.; Penedo, M.A.; Rivera-Baltanas, T.; Fernández-Martínez, R.; Ortolano, S.; Olivares, J.M.; Agís-Balboa, R.C. Insulin-like Growth Factor 2 (IGF-2) and Insulin-like Growth Factor Binding Protein 7 (IGFBP-7) Are Upregulated after Atypical Antipsychotics in Spanish Schizophrenia Patients. Int. J. Mol. Sci. 2022, 23, 9591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silva, B.A.; Cassilhas, R.C.; Attux, C.; Cordeiro, Q.; Gadelha, A.L.; Telles, B.A.; Bressan, R.A.; Ferreira, F.N.; Rodstein, P.H.; Daltio, C.S.; et al. A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: Results of a blind, randomized controlled trial. Braz. J. Psychiatry 2015, 37, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, M.; Zhang, Y.; Shi, Z.; Zhang, X.; Zhang, C. Elevated serum IGFBP-1 levels correlate with cognitive deficits in treatment-resistant and chronic medicated schizophrenia patients. Cytokine 2024, 182, 156728. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.; Guest, P.C.; Rahmoune, H.; Harris, L.W.; Wang, L.; Leweke, F.M.; Rothermundt, M.; Bogerts, B.; Koethe, D.; Kranaster, L.; et al. Identification of a biological signature for schizophrenia in serum. Mol. Psychiatry 2012, 17, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, C.; Webster, M.J.; Barry, G.; Shannon Weickert, C. Reduced Insulin-Like Growth Factor Family Member Expression Predicts Neurogenesis Marker Expression in the Subependymal Zone in Schizophrenia and Bipolar Disorder. Schizophr. Bull. 2021, 47, 1168–1178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, Y.; Kim, J.; Shin, J.Y.; Kim, J.I.; Seo, J.S.; Webster, M.J.; Lee, D.; Kim, S. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl. Psychiatry 2013, 3, e321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, S.; Guan, F.; Ma, M.; Zhang, L.; Cheng, B.; Qi, X.; Liang, C.; Li, P.; Kafle, O.P.; Wen, Y.; et al. An atlas of genetic correlations between psychiatric disorders and human blood plasma proteome. Eur. Psychiatry 2020, 63, e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primers 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.B.; Mors, O.; Bertelsen, A.; Waltoft, B.L.; Agerbo, E.; McGrath, J.J.; Mortensen, P.B.; Eaton, W.W. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry 2014, 71, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.; Hicks, B.M.; Foster, K.T.; McGue, M.; Iacono, W.G. Age of onset and course of major depressive disorder: Associations with psychosocial functioning outcomes in adulthood. Psychol. Med. 2015, 45, 505–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karrouri, R.; Hammani, Z.; Benjelloun, R.; Otheman, Y. Major depressive disorder: Validated treatments and future challenges. World J. Clin. Cases 2021, 9, 9350–9367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol. Med. 2004, 5, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; Réus, G.Z.; de Moura, A.B.; Quevedo, J.; Kim, Y.K. The Role of Neurotrophic Factors in Pathophysiology of Major Depressive Disorder. Adv. Exp. Med. Biol. 2021, 1305, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Mendlewicz, J.; Linkowski, P.; Kerkhofs, M.; Desmedt, D.; Golstein, J.; Copinschi, G.; Van Cauter, E. Diurnal hypersecretion of growth hormone in depression. J. Clin. Endocrinol. Metab. 1985, 60, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Laux, G.; Pfüller, H.; Erb, A.; Beckmann, H. Growth hormone (GH) response to GH-releasing hormone in depression. J. Clin. Endocrinol. Metab. 1987, 65, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Rupprecht, R.; Müller, U.; Pfüller, H.; Beckmann, H. Insulin-like growth factor I in depressed patients and controls. Acta Psychiatr. Scand. 1988, 78, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Laux, G.; Erb, A.; Pfüller, H.; Beckmann, H. Growth hormone (GH) and prolactin responses after GH-releasing hormone in major depressive disorder: Relationship to somatomedin C levels and dexamethasone suppressibility of cortisol. Psychoneuroendocrinology 1988, 13, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Laux, G.; Erb, A.; Pfüller, H.; Beckmann, H. Growth hormone (GH) responses to GH-releasing hormone in depression: Correlation with GH release following clonidine. Psychiatry Res. 1988, 25, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Rupprecht, R.; Müller, U.; Pfüller, H. Comparison of GH responses after human GHRH-44 amide administration and TRH-induced TSH release in depressed patients. Biol. Psychiatry 1989, 25, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Müller, U.; Rupprecht, R.; Kruse, K.; Schulte, H.M. Endocrine responses to growth hormone-releasing hormone, thyrotropin-releasing hormone and corticotropin-releasing hormone in depression. Acta Psychiatr. Scand. 1989, 79, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, R.; Rupprecht, C.; Rupprecht, M.; Noder, M.; Lesch, K.P.; Mössner, J. Effects of glucocorticoids on the regulation of the hypothalamic-pituitary-somatotropic system in depression. J. Affect. Disord. 1989, 17, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Laux, G.; Mueller, T. Alpha 2-adrenoceptor responsivity in depression: Effect of chronic treatment with moclobemide, a selective MAO-A-inhibitor, versus maprotiline. J. Neural Transm. Suppl. 1990, 32, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, F.; Guareschi-Cazzullo, A.; Tacchini, C.; Gerra, G.; Musetti, C. Growth hormone response to growth hormone releasing hormone and to clonidine stimulation in peripubertal patients with major depressive disorder. Biol. Psychiatry 1994, 36, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Gann, H.; Riemann, D.; Stoll, S.; Berger, M.; Müller, W.E. Growth hormone response to growth hormone-releasing hormone and clonidine in depression. Biol. Psychiatry 1995, 38, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Deuschle, M.; Blum, W.F.; Strasburger, C.J.; Schweiger, U.; Weber, B.; Körner, A.; Standhardt, H.; Gotthardt, U.; Schmider, J.; Pflaum, C.D.; et al. Insulin-like growth factor-I (IGF-I) plasma concentrations are increased in depressed patients. Psychoneuroendocrinology 1997, 22, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Weber-Hamann, B.; Blum, W.F.; Kratzsch, J.; Gilles, M.; Heuser, I.; Deuschle, M. Insulin-like growth factor-I (IGF-I) serum concentrations in depressed patients: Relationship to saliva cortisol and changes during antidepressant treatment. Pharmacopsychiatry 2009, 42, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Kopczak, A.; Stalla, G.K.; Uhr, M.; Lucae, S.; Hennings, J.; Ising, M.; Holsboer, F.; Kloiber, S. IGF-I in major depression and antidepressant treatment response. Eur. Neuropsychopharmacol. 2015, 25, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, M.; Suzuki, Y.; Tsuneyama, N.; Arinami, H.; Someya, T. Hormonal Dynamics Effect of Serum Insulin-Like Growth Factor I and Cortisol/Dehydroepiandrosterone Sulfate Ratio on Symptom Severity of Major Depressive Disorder. J. Clin. Psychopharmacol. 2019, 39, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Bot, M.; Milaneschi, Y.; Penninx, B.W.; Drent, M.L. Plasma insulin-like growth factor I levels are higher in depressive and anxiety disorders, but lower in antidepressant medication users. Psychoneuroendocrinology 2016, 68, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, 24, 14810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosso, G.; Zanardini, R.; Chiodelli, D.F.; Ferrari, C.; Gennarelli, M.; Bocchio-Chiavetto, L. Serum Levels of Insulin-Like Growth Factor-1 and Obsessive-Compulsive Disorder: A Case-Control Study. Neuropsychobiology 2016, 74, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Levada, O.A.; Troyan, A.S. Insulin-like growth factor-1: A possible marker for emotional and cognitive disturbances, and treatment effectiveness in major depressive disorder. Ann. Gen. Psychiatry 2017, 16, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levada, O.A.; Troyan, A.S.; Pinchuk, I.Y. Serum insulin-like growth factor-1 as a potential marker for MDD diagnosis, its clinical characteristics, and treatment efficacy validation: Data from an open-label vortioxetine study. BMC Psychiatry 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Troyan, A.S.; Levada, O.A. The Diagnostic Value of the Combination of Serum Brain-Derived Neurotrophic Factor and Insulin-Like Growth Factor-1 for Major Depressive Disorder Diagnosis and Treatment Efficacy. Front. Psychiatry 2020, 11, 800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ali, S.; Nahar, Z.; Rahman, M.R.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Serum insulin-like growth factor-1 and relaxin-3 are linked with major depressive disorder. Asian J. Psychiatr. 2020, 53, 102164. [Google Scholar] [CrossRef] [PubMed]
- Arinami, H.; Suzuki, Y.; Tajiri, M.; Tsuneyama, N.; Someya, T. Role of insulin-like growth factor 1, sex and corticosteroid hormones in male major depressive disorder. BMC Psychiatry 2021, 21, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arinami, H.; Suzuki, Y.; Watanabe, Y.; Tajiri, M.; Tsuneyama, N.; Someya, T. Association between insulin resistance and serum insulin-like growth factor 1 levels in patients with non-remitting major depressive disorder. J. Affect. Disord. 2024, 344, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Yan, J.; Zang, Z.; Xi, L.; Zhu, W.; Zhang, E.; Wu, L. Association between IGF-1 levels and MDD: A case-control and meta-analysis. Front Psychiatry 2024, 15, 1396938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michelson, D.; Stratakis, C.; Hill, L.; Reynolds, J.; Galliven, E.; Chrousos, G.; Gold, P. Bone mineral density in women with depression. N. Engl. J. Med. 1996, 335, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Franz, B.; Buysse, D.J.; Cherry, C.R.; Gray, N.S.; Grochocinski, V.J.; Frank, E.; Kupfer, D.J. Insulin-like growth factor 1 and growth hormone binding protein in depression: A preliminary communication. J. Psychiatr. Res. 1999, 33, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Tokuda, N.; Kobayashi, Y.; Tanaka, H.; Sawai, H.; Shibahara, H.; Takeshima, Y.; Shima, M.; Japan Environment and Children’s Study Group. Association between the serum insulin-like growth factor-1 concentration in the first trimester of pregnancy and postpartum depression. Psychiatry Clin. Neurosci. 2021, 75, 159–165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.X.; Liu, L.J.; Xu, L.Z.; Gao, L.; Wang, X.F.; Zhang, J.T.; Lu, L. Diurnal alterations in circadian genes and peptides in major depressive disorder before and after escitalopram treatment. Psychoneuroendocrinology 2013, 38, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.Y.; Wu, M.K.; Chen, Y.W.; Lin, P.Y.; Wang, H.Y.; Wu, C.K.; Tseng, P.T. Significantly Higher Peripheral Insulin-Like Growth Factor-1 Levels in Patients with Major Depressive Disorder or Bipolar Disorder Than in Healthy Controls: A Meta-Analysis and Review Under Guideline of PRISMA. Medicine 2016, 95, e2411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quan, J.J.; Yang, Z. Correlation between serum insulin-like growth factor (IGF-1) concentration and depression. Gansu Sci. Technol. 2016, 32, 133–135. [Google Scholar]
- Li, Q.; Guo, Q. The application value of serum IGF-1, AVP, BDNF level combined detection in the assessment of depressive patients. J. Med. Forum 2017, 38, 143–145. [Google Scholar]
- Chen, M.; Zhang, L.; Jiang, Q. Peripheral IGF-1 in bipolar disorder and major depressive disorder: A systematic review and meta-analysis. Ann. Palliat. Med. 2020, 9, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Luan, D.; Song, R.; Zhang, Z. Value of peripheral neurotrophin levels for the diagnosis of depression and response to treatment: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 41, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Mosiołek, A.; Mosiołek, J.; Jakima, S.; Pięta, A.; Szulc, A. Effects of Antidepressant Treatment on Neurotrophic Factors (BDNF and IGF-1) in Patients with Major Depressive Disorder (MDD). J. Clin. Med. 2021, 10, 3377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michelson, D.; Amsterdam, J.; Apter, J.; Fava, M.; Londborg, P.; Tamura, R.; Pagh, L. Hormonal markers of stress response following interruption of selective serotonin reuptake inhibitor treatment. Psychoneuroendocrinology 2000, 25, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Stelzhammer, V.; Guest, P.C.; Rothermundt, M.; Sondermann, C.; Michael, N.; Schwarz, E.; Rahmoune, H.; Bahn, S. Electroconvulsive therapy exerts mainly acute molecular changes in serum of major depressive disorder patients. Eur. Neuropsychopharmacol. 2013, 23, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Oberste, M.; Großheinrich, N.; Wunram, H.L.; Graf, J.L.; Ziemendorff, A.; Meinhardt, A.; Fricke, O.; Mahabir, E.; Bender, S. Effects of a 6-week, whole-body vibration strength-training on depression symptoms, endocrinological and neurobiological parameters in adolescent inpatients experiencing a major depressive episode (the “Balancing Vibrations Study”): Study protocol for a randomized placebo-controlled trial. Trials 2018, 19, 347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Sousa, R.A.L.; Rocha-Dias, I.; de Oliveira, L.R.S.; Improta-Caria, A.C.; Monteiro-Junior, R.S.; Cassilhas, R.C. Molecular mechanisms of physical exercise on depression in the elderly: A systematic review. Mol. Biol. Rep. 2021, 48, 3853–3862. [Google Scholar] [CrossRef] [PubMed]
- Krogh, J.; Rostrup, E.; Thomsen, C.; Elfving, B.; Videbech, P.; Nordentoft, M. The effect of exercise on hippocampal volume and neurotrophines in patients with major depression--a randomized clinical trial. J. Affect. Disord. 2014, 165, 24–30. [Google Scholar] [CrossRef] [PubMed]
- de Alcantara Borba, D.; da Silva Alves, E.; Rosa, J.P.P.; Facundo, L.A.; Costa, C.M.A.; Silva, A.C.; Narciso, F.V.; Silva, A.; de Mello, M.T. Can IGF-1 Serum Levels Really be Changed by Acute Physical Exercise? A Systematic Review and Meta-Analysis. J. Phys. Act Health 2020, 17, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Wunram, H.L.; Oberste, M.; Ziemendorff, A.; Hamacher, S.; Kapanci, T.; Heller, R.; Blick, S.; Bloch, W.; Clajus, T.C.; Schönau, E.; et al. Differential effects of ergometer-cycling and Whole-Body-Vibration training on serological BDNF and IGF-1 in the treatment of adolescent depression—Is there an impact of BDNFp.Val66Met variants? Physiol. Behav. 2021, 241, 113596. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Meyer, J.; Stielow, C.; Heinzel, S.; Heissel, A. Effects of an acute maximal exercise bout on serum insulin-like growth factor-1 in adults with MDD. Psychoneuroendocrinology 2025, 171, 107215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sievers, C.; Auer, M.K.; Klotsche, J.; Athanasoulia, A.P.; Schneider, H.J.; Nauck, M.; Völzke, H.; John, U.; Schulz, A.; Freyberger, H.J.; et al. IGF-I levels and depressive disorders: Results from the Study of Health in Pomerania (SHIP). Eur. Neuropsychopharmacol. 2014, 24, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Emeny, R.T.; Bidlingmaier, M.; Lacruz, M.E.; Linkohr, B.; Peters, A.; Reincke, M.; Ladwig, K.H. Mind over hormones: Sex differences in associations of well-being with IGF-I, IGFBP-3 and physical activity in the KORA-Age study. Exp. Gerontol. 2014, 59, 58–64. [Google Scholar] [CrossRef] [PubMed]
- van Varsseveld, N.C.; van Bunderen, C.C.; Sohl, E.; Comijs, H.C.; Penninx, B.W.; Lips, P.; Drent, M.L. Serum insulin-like growth factor 1 and late-life depression: A population-based study. Psychoneuroendocrinology 2015, 54, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Chigogora, S.; Zaninotto, P.; Kivimaki, M.; Steptoe, A.; Batty, G.D. Insulin-like growth factor 1 and risk of depression in older people: The English Longitudinal Study of Ageing. Transl. Psychiatry 2016, 6, e898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raynaud-Simon, A.; Lafont, S.; Berr, C.; Dartigues, J.F.; Baulieu, E.E.; Le Bouc, Y. Plasma insulin-like growth factor I levels in the elderly: Relation to plasma dehydroepiandrosterone sulfate levels, nutritional status, health and mortality. Gerontology 2001, 47, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Capoluongo, E.; Russo, A.; Onder, G.; Cesari, M.; Lulli, P.; Minucci, A.; Pahor, M.; Zuppi, C.; Bernabei, R. Free insulin-like growth factor-I and cognitive function in older persons living in community. Growth Horm. IGF Res. 2007, 17, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Rueda Alfaro, S.; Serra-Prat, M.; Palomera, E.; Falcón, I.; Cadenas, I.; Boquet, X.; Burdoy, E.; Mussoll, J.; Serra, P.; Puig Domingo, M.; et al. Hormonal determinants of depression and cognitive function in independently-living elders. Endocrinol. Nutr. 2008, 55, 396–401, English, Spanish. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Suhr, J.; Diebold, S.; Heffner, K.L. Associations between depressive symptoms and memory deficits vary as a function of insulin-like growth factor (IGF-1) levels in healthy older adults. Psychoneuroendocrinology 2014, 42, 118–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, E.; Ploubidis, G.; Fancourt, D. Social engagement and loneliness are differentially associated with neuro-immune markers in older age: Time-varying associations from the English Longitudinal Study of Ageing. Brain Behav. Immun. 2019, 82, 224–229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bennett, R.M.; Cook, D.M.; Clark, S.R.; Burckhardt, C.S.; Campbell, S.M. Hypothalamic-pituitary-insulin-like growth factor-I axis dysfunction in patients with fibromyalgia. J. Rheumatol. 1997, 24, 1384–1389. [Google Scholar] [PubMed]
- Jones, K.D.; Deodhar, A.A.; Burckhardt, C.S.; Perrin, N.A.; Hanson, G.C.; Bennett, R.M. A combination of 6 months of treatment with pyridostigmine and triweekly exercise fails to improve insulin-like growth factor-I levels in fibromyalgia, despite improvement in the acute growth hormone response to exercise. J. Rheumatol. 2007, 34, 1103–1111. [Google Scholar] [PubMed]
- Tander, B.; Atmaca, A.; Aliyazicioglu, Y.; Canturk, F. Serum ghrelin levels but not GH, IGF-1 and IGFBP-3 levels are altered in patients with fibromyalgia syndrome. Jt. Bone Spine 2007, 74, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xiong, Y.; Wang, B.; Zhou, Y.; Wang, Z.; Shi, J.; Li, C.; Lu, X.; Chen, G. Potential value of serum brain-derived neurotrophic factor, vascular endothelial growth factor, and S100B for identifying major depressive disorder in knee osteoarthritis patients. Front. Psychiatry 2022, 13, 1019367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zare, S.; Hasani, M.; Estêvão, M.D.; Tahmasebi, R.; Azadbakht, L.; Shidfar, F.; Heshmati, J.; Ziaei, S. Muscle Strength and Biochemical Markers as Predictors of Depression in Hemodialysis Patients: A Cross-Sectional Study. Clin. Nutr. Res. 2023, 12, 293–303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, T.K.S.; Heyn, P.C.; Tagawa, A.; Coughlan, C.; Carollo, J.J. Associations of Circulating Insulin-Growth Factor-1 with Cognitive Functions and Quality of Life Domains in Ambulatory Young Adults with Cerebral Palsy: A Pilot Study. Front. Neurol. 2022, 13, 748015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cankaya, B.; Chapman, B.P.; Talbot, N.L.; Moynihan, J.; Duberstein, P.R. History of sudden unexpected loss is associated with elevated interleukin-6 and decreased insulin-like growth factor-1 in women in an urban primary care setting. Psychosom. Med. 2009, 71, 914–919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brambilla, F.; Santonastaso, P.; Caregaro, L.; Favaro, A. Growth hormone and insulin-like growth factor 1 secretions in eating disorders: Correlations with psychopathological aspects of the disorders. Psychiatry Res. 2018, 263, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Broadhurst, C.; Diver, M.; Jackson, M.; Mottram, P. Plasma insulin growth factor-1 and incident delirium in older people. Int. J. Geriatr. Psychiatry 2005, 20, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Ahn, D.; Hahm, W.; Nam, J.; Park, Y.; Lim, S.; Kim, D.J. Serum Levels of Growth Factors in Alcohol-dependent Patients according to Comorbid Depressive Symptoms. Clin. Psychopharmacol. Neurosci. 2016, 14, 43–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- García-Marchena, N.; Silva-Peña, D.; Martín-Velasco, A.I.; Villanúa, M.Á.; Araos, P.; Pedraz, M.; Maza-Quiroga, R.; Romero-Sanchiz, P.; Rubio, G.; Castilla-Ortega, E.; et al. Decreased plasma concentrations of BDNF and IGF-1 in abstinent patients with alcohol use disorders. PLoS ONE 2017, 12, e0187634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kelly, D.F.; McArthur, D.L.; Levin, H.; Swimmer, S.; Dusick, J.R.; Cohan, P.; Wang, C.; Swerdloff, R. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J. Neurotrauma 2006, 23, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Kreber, L.A.; Griesbach, G.S.; Ashley, M.J. Detection of Growth Hormone Deficiency in Adults with Chronic Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1607–1613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sung, C.W.; Chen, K.Y.; Chiang, Y.H.; Chiu, W.T.; Ou, J.C.; Lee, H.C.; Tsai, S.H.; Lin, J.W.; Yang, C.M.; Tsai, Y.R.; et al. Heart rate variability and serum level of insulin-like growth factor-1 are correlated with symptoms of emotional disorders in patients suffering a mild traumatic brain injury. Clin. Neurophysiol. 2016, 127, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; Frölich, M.; Van Dulken, H. Somatomedin-C levels in treated and untreated patients with acromegaly. Clin. Endocrinol. 1987, 26, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kepicoglu, H.; Hatipoglu, E.; Bulut, I.; Darici, E.; Hizli, N.; Kadioglu, P. Impact of treatment satisfaction on quality of life of patients with acromegaly. Pituitary 2014, 17, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Algahtany, M.; Sharma, S.; Fahoum, K.; Jing, R.; Zhang, S.; Kovacs, K.; Rotondo, F.; Lee, J.; Vanek, I.; Cusimano, M.D. The Role of Growth Hormone in Depression: A Human Model. Front. Neurosci. 2021, 15, 661819. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wexler, T.; Gunnell, L.; Omer, Z.; Kuhlthau, K.; Beauregard, C.; Graham, G.; Utz, A.L.; Biller, B.; Nachtigall, L.; Loeffler, J.; et al. Growth hormone deficiency is associated with decreased quality of life in patients with prior acromegaly. J. Clin. Endocrinol. Metab. 2009, 94, 2471–2477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhard, F.P.; Heinzel, S.; Binder, G.; Weber, K.; Apel, A.; Roeben, B.; Deuschle, C.; Maechtel, M.; Heger, T.; Nussbaum, S.; et al. Insulin-Like Growth Factor 1 (IGF-1) in Parkinson’s Disease: Potential as Trait-, Progression- and Prediction Marker and Confounding Factors. PLoS ONE 2016, 11, e0150552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seifried, C.; Boehncke, S.; Heinzmann, J.; Baudrexel, S.; Weise, L.; Gasser, T.; Eggert, K.; Fogel, W.; Baas, H.; Badenhoop, K.; et al. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson’s disease. Neuroendocrinology 2013, 97, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Stuckenschneider, T.; Abeln, V.; Foitschik, T.; Abel, T.; Polidori, M.C.; Strüder, H.K. Disease-inclusive exercise classes improve physical fitness and reduce depressive symptoms in individuals with and without Parkinson’s disease-A feasibility study. Brain Behav. 2021, 11, e2352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shi, X.; Zheng, J.; Ma, J.; Wang, Z.; Sun, W.; Li, M.; Huang, S.; Hu, S. Insulin-like growth factor in Parkinson’s disease is related to nonmotor symptoms and the volume of specific brain areas. Neurosci. Lett. 2022, 783, 136735. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zheng, J.; Ma, J.; Li, D.; Gu, Q.; Chen, S.; Wang, Z.; Sun, W.; Li, M. Correlation between serum IGF-1 and EGF levels and neuropsychiatric and cognitive in Parkinson’s disease patients. Neurol. Sci. 2023, 44, 881–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Royall, D.R.; Al-Rubaye, S.; Bishnoi, R.; Palmer, R.F. Serum proteins mediate depression’s association with dementia. PLoS ONE 2017, 12, e0175790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stein, A.M.; da Silva, T.M.V.; Coelho, F.G.M.; Rueda, A.V.; Camarini, R.; Galduróz, R.F.S. Acute exercise increases circulating IGF-1 in Alzheimer’s disease patients, but not in older adults without dementia. Behav. Brain Res. 2021, 396, 112903. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Jiang, H.; Liu, R.; Yin, Y.; Zhang, Y.; Liang, J.; Li, S.; Wang, J.; Lu, J.; Geng, D.; et al. Towards a multi protein and mRNA expression of biological predictive and distinguish model for post stroke depression. Oncotarget 2016, 7, 54329–54338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Wang, W.; Kuang, L. The relation between insulin-like growth factor 1 levels and risk of depression in ischemic stroke. Int. J. Geriatr. Psychiatry 2018, 33, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, L.; Cichoń, N.; Karbownik, M.S.; Saso, L.; Saluk, J.; Miller, E. Circulating Serum VEGF, IGF-1 and MMP-9 and Expression of Their Genes as Potential Prognostic Markers of Recovery in Post-Stroke Rehabilitation-A Prospective Observational Study. Brain Sci. 2023, 13, 846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Jiang, T.; Li, R.; Zheng, T.; Han, Q.; Wang, M. Whether serum leptin and insulin-like growth factor-1 are predictive biomarkers for post-stroke depression: A meta-analysis and systematic review. J. Psychiatr. Res. 2024, 169, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Xu, Y.; Liu, Z.; Wang, Y.; Xu, X.; Li, C.; Li, S.; Zhang, J.; Xiong, T.; Cao, W.; et al. IGF2 inhibits hippocampal over-activated microglia and alleviates depression-like behavior in LPS- treated male mice. Brain Res. Bull. 2023, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Pryzbyl, K.J.; Bigio, E.H.; Weintraub, S.; Mesulam, M.M.; Redei, E.E. Reduced Hippocampal and Anterior Cingulate Expression of Antioxidant Enzymes and Membrane Progesterone Receptors in Alzheimer’s Disease with Depression. J. Alzheimers Dis. 2022, 89, 309–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Chen, Y.; Gao, X.; Zhang, Z. The behavioral deficits and cognitive impairment are correlated with decreased IGF-II and ERK in depressed mice induced by chronic unpredictable stress. Int. J. Neurosci. 2017, 127, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, Y.; Kim, G.; Son, H.; Lee, D.H.; Roh, G.S.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Kim, H.J. Decreased expression of extracellular matrix proteins and trophic factors in the amygdala complex of depressed mice after chronic immobilization stress. BMC Neurosci. 2012, 13, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grieco, S.F.; Cheng, Y.; Eldar-Finkelman, H.; Jope, R.S.; Beurel, E. Up-regulation of insulin-like growth factor 2 by ketamine requires glycogen synthase kinase-3 inhibition. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 72, 49–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Pereira, C.; Penedo, M.A.; Rivera-Baltanás, T.; Pérez-Márquez, T.; Alves-Villar, M.; Fernández-Martínez, R.; Veiga, C.; Salgado-Barreira, Á.; Prieto-González, J.M.; Ortolano, S.; et al. Protein Plasma Levels of the IGF Signalling System Are Altered in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 15254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agís-Balboa, R.C.; Pinheiro, P.S.; Rebola, N.; Kerimoglu, C.; Benito, E.; Gertig, M.; Bahari-Javan, S.; Jain, G.; Burkhardt, S.; Delalle, I.; et al. Formin 2 links neuropsychiatric phenotypes at young age to an increased risk for dementia. EMBO J. 2017, 36, 2815–2828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sáiz-Vázquez, O.; Gracia-García, P.; Ubillos-Landa, S.; Puente-Martínez, A.; Casado-Yusta, S.; Olaya, B.; Santabárbara, J. Depression as a Risk Factor for Alzheimer’s Disease: A Systematic Review of Longitudinal Meta-Analyses. J. Clin. Med. 2021, 10, 1809. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rivera, E.J.; Goldin, A.; Fulmer, N.; Tavares, R.; Wands, J.R.; de la Monte, S.M. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: Link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005, 8, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Lucas, M.; Viana da Silva, S.; Di Scala, M.; Garcia-Barroso, C.; González-Aseguinolaza, G.; Mulle, C.; Alberini, C.M.; Cuadrado-Tejedor, M.; Garcia-Osta, A. Insulin-like growth factor 2 reverses memory and synaptic deficits in APP transgenic mice. EMBO Mol. Med. 2014, 6, 1246–1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hertze, J.; Nägga, K.; Minthon, L.; Hansson, O. Changes in cerebrospinal fluid and blood plasma levels of IGF-II and its binding proteins in Alzheimer’s disease: An observational study. BMC Neurol. 2014, 14, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tham, A.; Nordberg, A.; Grissom, F.E.; Carlsson-Skwirut, C.; Viitanen, M.; Sara, V.R. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 5, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Åberg, D.; Johansson, P.; Isgaard, J.; Wallin, A.; Johansson, J.O.; Andreasson, U.; Blennow, K.; Zetterberg, H.; Åberg, N.D.; Svensson, J. Increased Cerebrospinal Fluid Level of Insulin-like Growth Factor-II in Male Patients with Alzheimer’s Disease. J. Alzheimers Dis. 2015, 48, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Heywood, W.E.; Galimberti, D.; Bliss, E.; Sirka, E.; Paterson, R.W.; Magdalinou, N.K.; Carecchio, M.; Reid, E.; Heslegrave, A.; Fenoglio, C.; et al. Identification of novel CSF biomarkers for neurodegeneration and their validation by a high-throughput multiplexed targeted proteomic assay. Mol. Neurodegener. 2015, 10, 64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, J.; Zhang, Y.; Su, C.; Zheng, Q.; Guo, J. Insulin-Like Growth Factor Signaling in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurobiol. 2024. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Agbemenyah, H.Y.; Agis-Balboa, R.C.; Burkhardt, S.; Delalle, I.; Fischer, A. Insulin growth factor binding protein 7 is a novel target to treat dementia. Neurobiol. Dis. 2014, 62, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.K.; Cooper, J.D.; Bot, M.; Birkenhager, T.K.; Bergink, V.; Drexhage, H.A.; Steiner, J.; Rothermundt, M.; Penninx, B.W.; Bahn, S. Blood-based immune-endocrine biomarkers of treatment response in depression. J. Psychiatr. Res. 2016, 83, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Bot, M.; Jansen, R.; Chan, M.K.; Cooper, J.D.; Bahn, S.; Penninx, B.W. Serum proteomic profiles of depressive subtypes. Transl. Psychiatry 2016, 6, e851. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milanesi, E.; Zanardini, R.; Rosso, G.; Maina, G.; Barbon, A.; Mora, C.; Minelli, A.; Gennarelli, M.; Bocchio-Chiavetto, L. Insulin-like growth factor binding protein 2 in bipolar disorder: An expression study in peripheral tissues. World J. Biol. Psychiatry 2018, 19, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Szczęsny, E.; Slusarczyk, J.; Głombik, K.; Budziszewska, B.; Kubera, M.; Lasoń, W.; Basta-Kaim, A. Possible contribution of IGF-1 to depressive disorder. Pharmacol. Rep. 2013, 65, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Basta-Kaim, A.; Szczesny, E.; Glombik, K.; Slusarczyk, J.; Trojan, E.; Tomaszewski, K.A.; Budziszewska, B.; Kubera, M.; Lason, W. Prenatal stress leads to changes in IGF-1 binding proteins network in the hippocampus and frontal cortex of adult male rat. Neuroscience 2014, 274, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hjortebjerg, R. IGFBP-4 and PAPP-A in normal physiology and disease. Growth Horm. IGF Res. 2018, 41, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Geddes, J.R.; Tunbridge, E.M. The Emerging Neurobiology of Bipolar Disorder. Trends Neurosci. 2018, 41, 18–30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonnín, C.D.M.; Reinares, M.; Martínez-Arán, A.; Jiménez, E.; Sánchez-Moreno, J.; Solé, B.; Montejo, L.; Vieta, E. Improving Functioning, Quality of Life, and Well-being in Patients with Bipolar Disorder. Int. J. Neuropsychopharmacol. 2019, 22, 467–477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Young, A.H.; Juruena, M.F. The Neurobiology of Bipolar Disorder. Curr. Top. Behav. Neurosci. 2021, 48, 1–20. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.R.; de Araujo Andrade, T.; de Figueiredo, E.C. Biomarkers in psychiatric disorders. Adv. Clin. Chem. 2023, 116, 183–208. [Google Scholar] [CrossRef] [PubMed]
- Carli, M.; Weiss, F.; Grenno, G.; Ponzini, S.; Kolachalam, S.; Vaglini, F.; Viaggi, C.; Pardini, C.; Tidona, S.; Longoni, B.; et al. Pharmacological Strategies for Bipolar Disorders in Acute Phases and Chronic Management with a Special Focus on Lithium, Valproic Acid, and Atypical Antipsychotics. Curr. Neuropharmacol. 2023, 21, 935–950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bobo, W.V. The Diagnosis and Management of Bipolar I and II Disorders: Clinical Practice Update. Mayo Clin. Proc. 2017, 92, 1532–1551. [Google Scholar] [CrossRef] [PubMed]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Mancini, D.A.; McCann, S.; Srinivasan, J.; Kennedy, S.H. Valproate, bipolar disorder and polycystic ovarian syndrome. Bipolar Disord. 2003, 5, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Liu, C.; Chi, J.; Wang, Y.; Xu, L. The role of C-peptide in diabetes and its complications: An updated review. Front. Endocrinol. 2023, 14, 1256093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, P.D.; Giudice, L.C.; Conover, C.A.; Powell, D.R. Insulin-like growth factor binding protein-1: Recent findings and new directions. Proc. Soc. Exp. Biol. Med. 1997, 216, 319–357. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; McQuillin, A.; Puri, V.; Anjorin, A.; Bass, N.; Kandaswamy, R.; Lawrence, J.; Curtis, D.; Sklar, P.; Purcell, S.M.; et al. Genetic association and sequencing of the insulin-like growth factor 1 gene in bipolar affective disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011, 156, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Squassina, A.; Costa, M.; Congiu, D.; Manchia, M.; Angius, A.; Deiana, V.; Ardau, R.; Chillotti, C.; Severino, G.; Calza, S.; et al. Insulin-like growth factor 1 (IGF-1) expression is up-regulated in lymphoblastoid cell lines of lithium responsive bipolar disorder patients. Pharmacol. Res. 2013, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Na, K.S.; Hwang, J.A.; Yoon, H.K.; Lee, H.J.; Hahn, S.W.; Lee, B.H.; Jung, H.Y. High insulin-like growth factor-1 in patients with bipolar I disorder: A trait marker? J Affect Disord 2013, 151, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Carro, E.; Garcia-Galloway, E.; Torres-Aleman, I. Role of insulin-like growth factor I signaling in neurodegenerative diseases. J. Mol. Med. 2004, 82, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, T.; He, S.; Hong, B.; Chen, Z.; Peng, D.; Wu, Y.; Wen, H.; Lin, Z.; Fang, Y.; et al. Elevated serum levels of FGF-2, NGF and IGF-1 in patients with manic episode of bipolar disorder. Psychiatry Res. 2014, 218, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Tunçel, Ö.K.; Sarisoy, G.; Çetin, E.; Kaynar Tunçel, E.; Bilgici, B.; Karaustaoğlu, A. Neurotrophic factors in bipolar disorders patients with manic episode. Turk. J. Med. Sci. 2020, 50, 985–993. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sayana, P.; Colpo, G.D.; Simões, L.R.; Giridharan, V.V.; Teixeira, A.L.; Quevedo, J.; Barichello, T. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J. Psychiatr. Res. 2017, 92, 160–182. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Taslimi, S.; Brietzke, E.; Ashrafi, M. Cytokine alterations in bipolar disorder: A meta-analysis of 30 studies. Biol. Psychiatry 2013, 74, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Munkholm, K.; Jacoby, A.S.; Lenskjold, T.; Bruunsgaard, H.; Vinberg, M.; Kessing, L.V. Leukocytes in peripheral blood in patients with bipolar disorder—Trait and state alterations and association with levels of cytokines and C-reactive protein. Psychiatry Res. 2018, 261, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.; Perry, B.I.; Upthegrove, R.; Barnes, N.; Chatterjee, J.; Gallacher, D.; Marwaha, S. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: Systematic review and meta-analyses. Br. J. Psychiatry 2018, 213, 514–525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solmi, M.; Suresh Sharma, M.; Osimo, E.F.; Fornaro, M.; Bortolato, B.; Croatto, G.; Miola, A.; Vieta, E.; Pariante, C.M.; Smith, L.; et al. Peripheral levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and interleukin-1β across the mood spectrum in bipolar disorder: A meta-analysis of mean differences and variability. Brain Behav. Immun. 2021, 97, 193–203. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.G.; Pfaffenseller, B.; Walz, J.; Stertz, L.; Fries, G.; Rosa, A.R.; Magalhães, P.V. Peripheral insulin-like growth factor 1 in bipolar disorder. Psychiatry Res. 2017, 250, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferensztajn-Rochowiak, E.; Kaczmarek, M.; Wójcicka, M.; Kaufman-Szukalska, E.; Dziuda, S.; Remlinger-Molenda, A.; Szeliga-Neymann, A.; Losy, J.; Rybakowski, J.K. Glutamate-Related Antibodies and Peripheral Insulin-Like Growth Factor in Bipolar Disorder and Lithium Prophylaxis. Neuropsychobiology 2019, 77, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Guldiken, G.; Karayagmurlu, A.; Kucukgergin, C.; Coskun, M. VEGF, IGF-1 and FGF-2 Serum Levels in Children and Adolescents with Autism Spectrum Disorder with and without Bipolar Disorder. J. Autism Dev. Disord. 2024, 54, 3854–3862. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.Y.; Zhao, Y.; Liu, Z.B.; Luo, C.P.; Xiong, J.W.; Zhan, J.Q.; Li, Y.H.; Wei, B.; Chen, C.N.; Yang, Y.J. Corrigendum: Lower serum insulin-like growth factor 2 level in patients with bipolar disorder is associated with the severity of manic symptoms during manic episodes. Front. Psychiatry 2024, 15, 1408899, Erratum in Front. Psychiatry 2024, 15, 1354999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Pereira, C.; Penedo, M.A.; Alonso-Núñez, A.; Rivera-Baltanás, T.; Viéitez, I.; Prieto-González, J.M.; Vilariño-Vilariño, M.I.; Olivares, J.M.; Ortolano, S.; Agís-Balboa, R.C. Plasma IGFBP-3 and IGFBP-5 levels are decreased during acute manic episodes in bipolar disorder patients. Front. Pharmacol. 2024, 15, 1384198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chesik, D.; De Keyser, J.; Wilczak, N. Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: Implications in multiple sclerosis. Cytokine Growth Factor. Rev. 2007, 18, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Bezchlibnyk, Y.B.; Xu, L.; Wang, J.F.; Young, L.T. Decreased expression of insulin-like growth factor binding protein 2 in the prefrontal cortex of subjects with bipolar disorder and its regulation by lithium treatment. Brain Res. 2007, 1147, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Blum, W.F.; Horn, N.; Kratzsch, J.; Jørgensen, J.O.; Juul, A.; Teale, D.; Mohnike, K.; Ranke, M.B. Clinical studies of IGFBP-2 by radioimmunoassay. Growth Regul. 1993, 3, 100–104. [Google Scholar] [PubMed]
- Aizenman, Y.; de Vellis, J. Brain neurons develop in a serum and glial free environment: Effects of transferrin, insulin, insulin-like growth factor-I and thyroid hormone on neuronal survival, growth and differentiation. Brain Res. 1987, 406, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Collett-Solberg, P.F.; Cohen, P. The role of the insulin-like growth factor binding proteins and the IGFBP proteases in modulating IGF action. Endocrinol. Metab. Clin. N. Am. 1996, 25, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Poletti, S.; Hoogenboezem, T.A.; Locatelli, C.; Ambrée, O.; de Wit, H.; Wijkhuijs, A.J.; Mazza, E.; Bulgarelli, C.; Vai, B.; et al. Stem Cell Factor (SCF) is a putative biomarker of antidepressant response. J. Neuroimmune Pharmacol. 2016, 11, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Snijders, G.; Mesman, E.; de Wit, H.; Wijkhuijs, A.; Nolen, W.A.; Drexhage, H.A.; Hillegers, M.H.J. Immune dysregulation in offspring of a bipolar parent. Altered serum levels of immune growth factors at adolescent age. Brain Behav. Immun. 2017, 64, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Miller, M.; Naccarato, J.; Radico, J.A. Borderline Personality Disorder. Am. Fam. Physician 2022, 105, 156–161. [Google Scholar] [PubMed]
- Kahl, K.G.; Rudolf, S.; Stoeckelhuber, B.M.; Dibbelt, L.; Gehl, H.B.; Markhof, K.; Hohagen, F.; Schweiger, U. Bone mineral density, markers of bone turnover, and cytokines in young women with borderline personality disorder with and without comorbid major depressive disorder. Am. J. Psychiatry 2005, 162, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Rosmond, R.; Eriksson, E.; Björntorp, P. Personality disorders in relation to anthropometric, endocrine and metabolic factors. J. Endocrinol. Investig. 1999, 22, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Savaheli, S.; Ahmadiani, A. Obsessive-compulsive disorder and growth factors: A comparative review. Behav. Brain Res. 2019, 372, 111967. [Google Scholar] [CrossRef] [PubMed]
- Sultania, A.; Venkatesan, S.; Batra, D.R.; Rajesh, K.; Vashishth, R.; Ravi, S.; Ahmad, F. Potential biomarkers and therapeutic targets for obsessive compulsive disorder: Evidences from clinical studies. Biochem. Med. 2024, 34, 010503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brambilla, F.; Bellodi, L.; Perna, G.; Arancio, C.; Bertani, A. Growth hormone response to growth hormone-releasing hormone stimulation in obsessive-compulsive disorder. Psychiatry Res. 1998, 81, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Schüssler, P.; Weikel, J.; Dresler, M.; Zuber, V.; Querfurt, F.; Yassouridis, A.; Steiger, A. Altered nocturnal growth hormone (GH) secretion in obsessive compulsive disorder. Psychoneuroendocrinology 2006, 31, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, J.C.; Jose, D.; Shivakumar, V.; Shrinivasa, B.; Kaur, M.; Kalmady, S.V.; Venkatasubramanian, G.; Reddy, Y.C.J. Plasma insulin-like growth factor-1 levels and response to selective serotonin reuptake inhibitor treatment: A prospective study of medication-naïve OCD patients. Asian J. Psychiatr. 2017, 28, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Mehan, S.; Khan, Z.; Gupta, G.D.; Narula, A.S. Melatonin-mediated IGF-1/GLP-1 activation in experimental OCD rats: Evidence from CSF, blood plasma, brain and in-silico investigations. Biochem. Pharmacol. 2023, 217, 115831. [Google Scholar] [CrossRef] [PubMed]
- Hodges, H.; Fealko, C.; Soares, N. Autism spectrum disorder: Definition, epidemiology, causes, and clinical evaluation. Transl. Pediatr. 2020, 9 (Suppl. S1), S55–S65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCrossin, R. Finding the True Number of Females with Autistic Spectrum Disorder by Estimating the Biases in Initial Recognition and Clinical Diagnosis. Children 2022, 9, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, X.; Wang, G.; Shen, S.; Zhan, J. Advances in the Diagnosis and Treatment of Autism Spectrum Disorders in Children. Altern. Ther. Health Med. 2024, 30, 170–175. [Google Scholar] [PubMed]
- Hong, J.S.; Perrin, J.; Singh, V.; Kalb, L.; Cross, E.A.; Wodka, E.; Richter, C.; Landa, R. Psychometric Evaluation of the Autism Spectrum Rating Scales (6–18 Years Parent Report) in a Clinical Sample. J. Autism Dev. Disord. 2024, 54, 1024–1035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanhala, R.; Turpeinen, U.; Riikonen, R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child. Neurol. 2001, 43, 614–616. [Google Scholar] [CrossRef] [PubMed]
- Cioana, M.; Michalski, B.; Fahnestock, M. Insulin-Like Growth Factor and Insulin-Like Growth Factor Receptor Expression in Human Idiopathic Autism Fusiform Gyrus Tissue. Autism Res. 2020, 13, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R.; Makkonen, I.; Vanhala, R.; Turpeinen, U.; Kuikka, J.; Kokki, H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev. Med. Child. Neurol. 2006, 48, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R. Insulin-like growth factor delivery across the blood-brain barrier. Potential use of IGF-1 as a drug in child neurology. Chemotherapy 2006, 52, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Makkonen, I.; Kokki, H.; Kuikka, J.; Turpeinen, U.; Riikonen, R. Effects of fluoxetine treatment on striatal dopamine transporter binding and cerebrospinal fluid insulin-like growth factor-1 in children with autism. Neuropediatrics 2011, 42, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.L.; Hediger, M.L.; Molloy, C.A.; Chrousos, G.P.; Manning-Courtney, P.; Yu, K.F.; Brasington, M.; England, L.J. Elevated levels of growth-related hormones in autism and autism spectrum disorder. Clin. Endocrinol. 2007, 67, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Anlar, B.; Oktem, F.; Bakkaloglu, B.; Haliloglu, M.; Oguz, H.; Unal, F.; Pehlivanturk, B.; Gokler, B.; Ozbesler, C.; Yordam, N. Urinary epidermal and insulin-like growth factor excretion in autistic children. Neuropediatrics 2007, 38, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Neumeyer, A.M.; Cano Sokoloff, N.; McDonnell, E.; Macklin, E.A.; McDougle, C.J.; Misra, M. Bone microarchitecture in adolescent boys with autism spectrum disorder. Bone 2017, 97, 139–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Şimşek, F.; Işık, Ü.; Aktepe, E.; Kılıç, F.; Şirin, F.B.; Bozkurt, M. Comparison of Serum VEGF, IGF-1, and HIF-1α Levels in Children with Autism Spectrum Disorder and Healthy Controls. J. Autism Dev. Disord. 2021, 51, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Robinson-Agramonte, M.L.A.; Michalski, B.; Vidal-Martinez, B.; Hernández, L.R.; Santiesteban, M.W.; Fahnestock, M. BDNF, proBDNF and IGF-1 serum levels in naïve and medicated subjects with autism. Sci. Rep. 2022, 12, 13768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson-Agramonte, M.L.A.; Michalski, B.; Fernández, L.G.; Vidal-Martinez, B.; Cuesta, H.V.; Rizo, C.M.; Fahnestock, M. Effect of non-invasive brain stimulation on behavior and serum brain-derived neurotrophic factor and insulin-like growth factor-1 levels in autistic patients. Drug Dev. Res. 2021, 82, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Abedini, M.; Mashayekhi, F.; Salehi, Z. Analysis of Insulin-like growth factor-1 serum levels and promoter (rs12579108) polymorphism in the children with autism spectrum disorders. J. Clin. Neurosci. 2022, 99, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, G.Y.; He, C.Y.; Liu, X.; Fan, X.; Zhao, Y.; Wang, N.R. Serum levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in children with autism spectrum disorder. Zhongguo Dang Dai Er Ke Za Zhi 2022, 24, 186–191, (In English and Chinese). [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bibollet-Bahena, O.; Cui, Q.L.; Almazan, G. The insulin-like growth factor-1 axis and its potential as a therapeutic target in central nervous system (CNS) disorders. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Steinman, G.; Mankuta, D. Insulin-like growth factor and the etiology of autism. Med. Hypotheses 2013, 80, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alberts, I.; Li, X. Dysregulation of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum disorders. Int. J. Dev. Neurosci. 2014, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chadman, K.K. Making progress in autism drug discovery. Expert. Opin. Drug Discov. 2014, 9, 1389–1391. [Google Scholar] [CrossRef] [PubMed]
- Canitano, R. New experimental treatments for core social domain in autism spectrum disorders. Front. Pediatr. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebrahimi-Fakhari, D.; Sahin, M. Autism and the synapse: Emerging mechanisms and mechanism-based therapies. Curr. Opin. Neurol. 2015, 28, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Riikonen, R. Treatment of autistic spectrum disorder with insulin-like growth factors. Eur. J. Paediatr. Neurol. 2016, 20, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Costales, J.; Kolevzon, A. The therapeutic potential of insulin-like growth factor-1 in central nervous system disorders. Neurosci. Biobehav. Rev. 2016, 63, 207–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vahdatpour, C.; Dyer, A.H.; Tropea, D. Insulin-Like Growth Factor 1 and Related Compounds in the Treatment of Childhood-Onset Neurodevelopmental Disorders. Front. Neurosci. 2016, 10, 450. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zegarra-Valdivia, J.A. Insulin-like growth factor type 1 and its relation with neuropsychiatric disorders. Medwave 2017, 17, e7031, Spanish, English. [Google Scholar] [CrossRef] [PubMed]
- Bou Khalil, R. Is insulin growth factor-1 the future for treating autism spectrum disorder and/or schizophrenia? Med. Hypotheses 2017, 99, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Steinman, G. IGF—Autism prevention/amelioration. Med. Hypotheses 2019, 122, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Bou Khalil, R. Insulin-growth-factor-1 (IGF-1): Just a few steps behind the evidence in treating schizophrenia and/or autism. CNS Spectr. 2019, 24, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Bose, M.; Farias Quipildor, G.; Ehrlich, M.E.; Salton, S.R. Intranasal Peptide Therapeutics: A Promising Avenue for Overcoming the Challenges of Traditional CNS Drug Development. Cells 2022, 11, 3629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, J.; Liu, L.; Yang, Y.; Zhou, M.; Xu, S.; Zhang, W.; Zhang, C. Insulin-Like Growth Factor 1 Has the Potential to Be Used as a Diagnostic Tool and Treatment Target for Autism Spectrum Disorders. Cureus 2024, 16, e65393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinmetz, A.B.; Stern, S.A.; Kohtz, A.S.; Descalzi, G.; Alberini, C.M. Insulin-Like Growth Factor II Targets the mTOR Pathway to Reverse Autism-Like Phenotypes in Mice. J. Neurosci. 2018, 38, 1015–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizzarelli, R.; Pimpinella, D.; Jacobs, C.; Tartacca, A.; Kullolli, U.; Monyer, H.; Alberini, C.M.; Griguoli, M. Insulin-like growth factor 2 (IGF-2) rescues social deficits in NLG3-/y mouse model of ASDs. Front. Cell Neurosci. 2024, 17, 1332179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cruz, E.; Descalzi, G.; Steinmetz, A.; Scharfman, H.E.; Katzman, A.; Alberini, C.M. CIM6P/IGF-2 Receptor Ligands Reverse Deficits in Angelman Syndrome Model Mice. Autism Res. 2021, 14, 29–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berg, E.L.; Petkova, S.P.; Born, H.A.; Adhikari, A.; Anderson, A.E.; Silverman, J.L. Insulin-like growth factor-2 does not improve behavioral deficits in mouse and rat models of Angelman Syndrome. Mol. Autism 2021, 12, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayano, G.; Demelash, S.; Gizachew, Y.; Tsegay, L.; Alati, R. The global prevalence of attention deficit hyperactivity disorder in children and adolescents: An umbrella review of meta-analyses. J. Affect. Disord. 2023, 339, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Pulay, A.J.; Réthelyi, J.M. Genetics in the ADHD Clinic: How Can Genetic Testing Support the Current Clinical Practice? Front. Psychol. 2022, 13, 751041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Austerman, J. ADHD and behavioral disorders: Assessment, management, and an update from DSM-5. Cleve Clin. J. Med. 2015, 82 (Suppl. S1), S2–S7. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Chen, W.; Chen, S.; Hu, Y.; Dai, X.; Liu, X. Evaluation of the Relationship Between BDNF Val66Met Gene Polymorphism and Attention Deficit Hyperactivity Disorder: A Meta-Analysis. Front. Psychiatry 2022, 13, 888774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schultz, F.R.; Hayford, J.T.; Wolraich, M.L.; Hintz, R.L.; Thompson, R.G. Methylphenidate treatment of hyperactive children: Effects on the hypothalamic-pituitary-somatomedin axis. Pediatrics 1982, 70, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Silbergeld, A.; Eldar, S.; Laor, N.; Wolmer, L.; Koren, S.; Weitz, R.; Inbar, D.; Reiss, A.; Eshet, R.; et al. Lack of effect of methylphenidate on serum growth hormone (GH), GH-binding protein, and insulin-like growth factor I. Clin. Neuropharmacol. 1997, 20, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Bereket, A.; Turan, S.; Karaman, M.G.; Haklar, G.; Ozbay, F.; Yazgan, M.Y. Height, weight, IGF-I, IGFBP-3 and thyroid functions in prepubertal children with attention deficit hyperactivity disorder: Effect of methylphenidate treatment. Horm. Res. 2005, 63, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Bang, Y.R.; Kang, J.W.; Yoo, J.H.; Kim, S.H.; Park, J.H. Preliminary Investigation of Association between Methylphenidate and Serum Growth Markers in Children with Attention-Deficit/Hyperactivity Disorder: A Cross-Sectional Case-Control Study. Soa Chongsonyon Chongsin Uihak 2020, 31, 154–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velayutham, V.; Chakrabarty, S.; Greer, R.; Cotterill, A.M.; Leong, G.M. Slow growth and short stature in children with attention deficit hyperactivity disorder (ADHD): A retrospective study of 493 children who underwent growth hormone provocation testing at one tertiary paediatric endocrine centre. J. Pediatr. Endocrinol. Metab. 2024, 37, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Allred, E.N.; Dammann, O.; Fichorova, R.N.; Hooper, S.R.; Hunter, S.J.; Joseph, R.M.; Kuban, K.; Leviton, A.; O’Shea, T.M.; Scott, M.N.; et al. Systemic Inflammation during the First Postnatal Month and the Risk of Attention Deficit Hyperactivity Disorder Characteristics among 10 year-old Children Born Extremely Preterm. J. Neuroimmune Pharmacol. 2017, 12, 531–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, H.; Xie, R.; Li, T.; Chen, Z.; Liu, Y.; Sun, C. Effect of Atomoxetine on Behavioral Difficulties and Growth Development of Primary School Children with Attention-Deficit/Hyperactivity Disorder: A Prospective Study. Children 2022, 9, 212. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.J.; Huang, Y.H.; Chou, W.J.; Lee, S.Y. Growth Hormone and Thyroid Function in Children with Attention Deficit Hyperactivity Disorder Undergoing Drug Therapy. J. Clin. Endocrinol. Metab. 2022, 107, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Huang, Y.H.; Chou, W.J.; Lee, S.Y.; Chang, H.Y.; Chen, C.C.; Chao, H.R. Interrelationships among growth hormone, thyroid function, and endocrine-disrupting chemicals on the susceptibility to attention-deficit/hyperactivity disorder. Eur. Child. Adolesc. Psychiatry 2023, 32, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, E.; Işık, Ü.; Şirin, F.B. Analysis of Serum VEGF, IGF-1, and HIF-1α Levels in ADHD. J. Atten. Disord. 2024, 28, 58–65. [Google Scholar] [CrossRef] [PubMed]
- van Andel, E.; Vogel, S.W.N.; Bijlenga, D.; Kalsbeek, A.; Beekman, A.T.F.; Kooij, J.J.S. Effects of Chronotherapeutic Interventions in Adults with ADHD and Delayed Sleep Phase Syndrome (DSPS) on Regulation of Appetite and Glucose Metabolism. J. Atten. Disord. 2024, 28, 1653–1667. [Google Scholar] [CrossRef] [PubMed]
- Rijlaarsdam, J.; Cecil, C.A.; Walton, E.; Mesirow, M.S.; Relton, C.L.; Gaunt, T.R.; McArdle, W.; Barker, E.D. Prenatal unhealthy diet, insulin-like growth factor 2 gene (IGF2) methylation, and attention deficit hyperactivity disorder symptoms in youth with early-onset conduct problems. J. Child. Psychol. Psychiatry 2017, 58, 19–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cecil, C.A.; Walton, E.; Barker, E.D. Prenatal diet and childhood ADHD: Exploring the potential role of IGF2 methylation. Epigenomics 2016, 8, 1573–1576. [Google Scholar] [CrossRef] [PubMed]



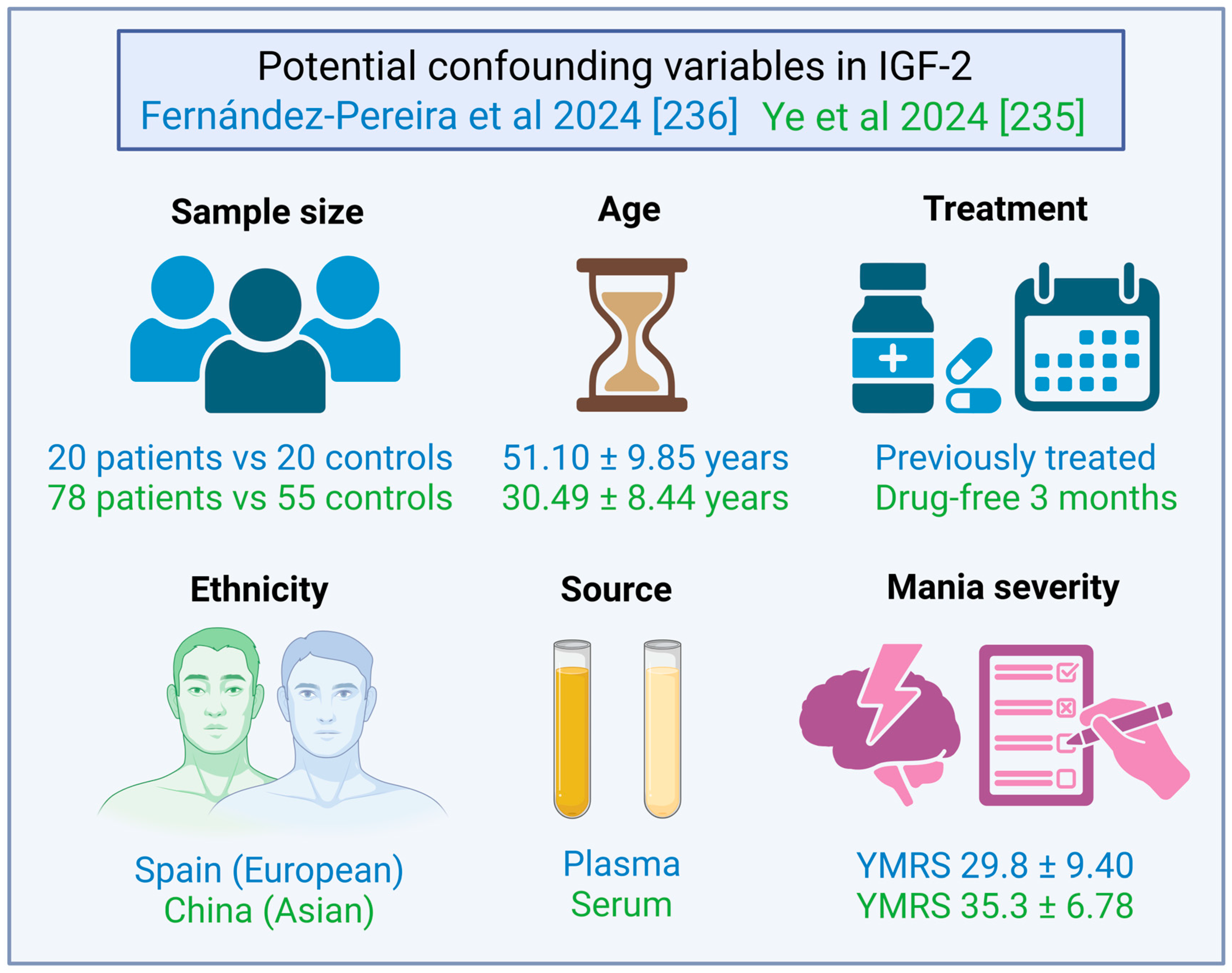

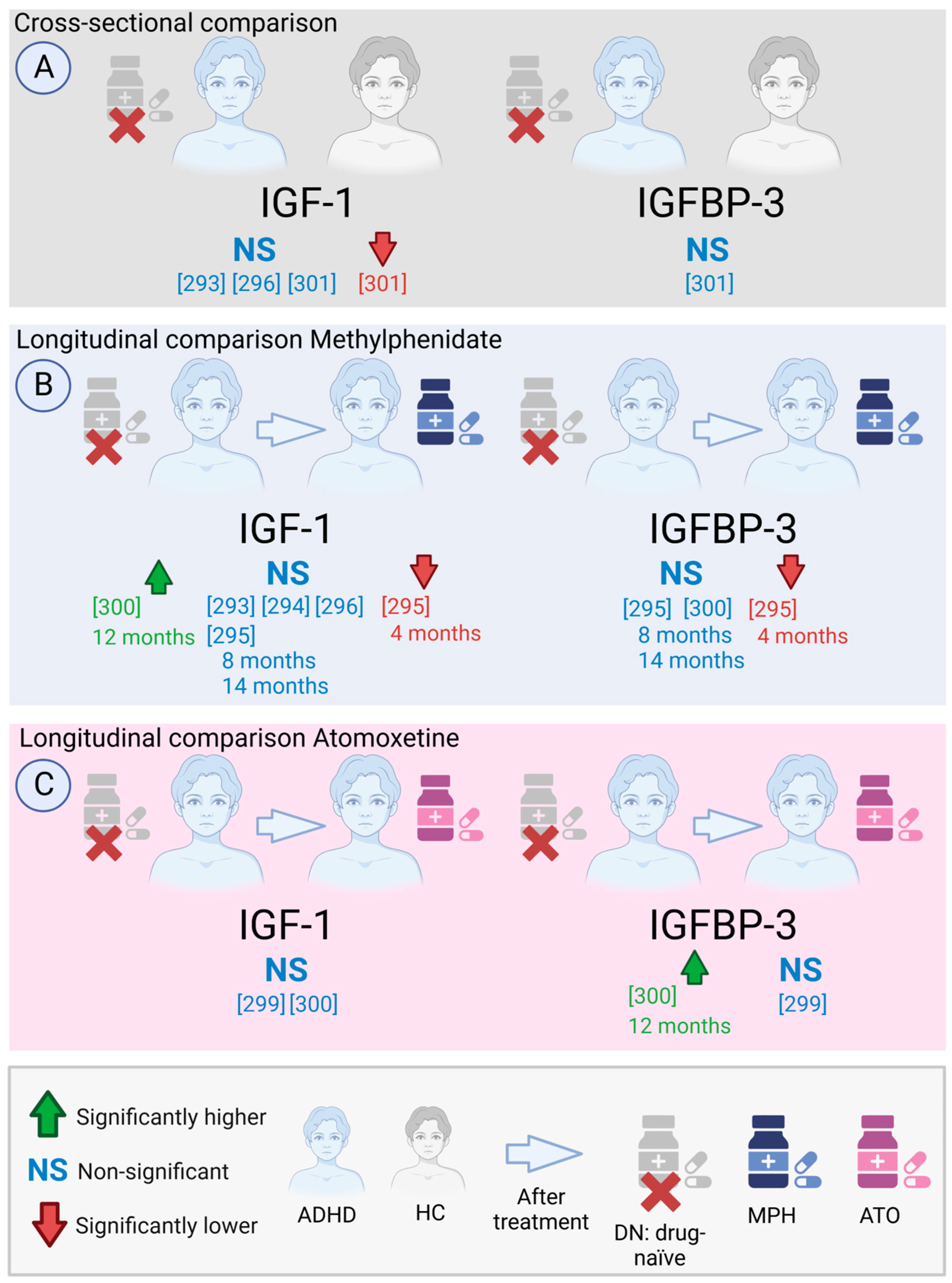

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Pereira, C.; Agís-Balboa, R.C. The Insulin-like Growth Factor Family as a Potential Peripheral Biomarker in Psychiatric Disorders: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2561. https://doi.org/10.3390/ijms26062561
Fernández-Pereira C, Agís-Balboa RC. The Insulin-like Growth Factor Family as a Potential Peripheral Biomarker in Psychiatric Disorders: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(6):2561. https://doi.org/10.3390/ijms26062561
Chicago/Turabian StyleFernández-Pereira, Carlos, and Roberto Carlos Agís-Balboa. 2025. "The Insulin-like Growth Factor Family as a Potential Peripheral Biomarker in Psychiatric Disorders: A Systematic Review" International Journal of Molecular Sciences 26, no. 6: 2561. https://doi.org/10.3390/ijms26062561
APA StyleFernández-Pereira, C., & Agís-Balboa, R. C. (2025). The Insulin-like Growth Factor Family as a Potential Peripheral Biomarker in Psychiatric Disorders: A Systematic Review. International Journal of Molecular Sciences, 26(6), 2561. https://doi.org/10.3390/ijms26062561






