Expression of the RPSA-Containing and 67EBP Laminin Receptors in Relation to the Debatable Nature of the 67 kDa Laminin Receptor 67LR in Colorectal Cancer
Abstract
1. Introduction
2. Results
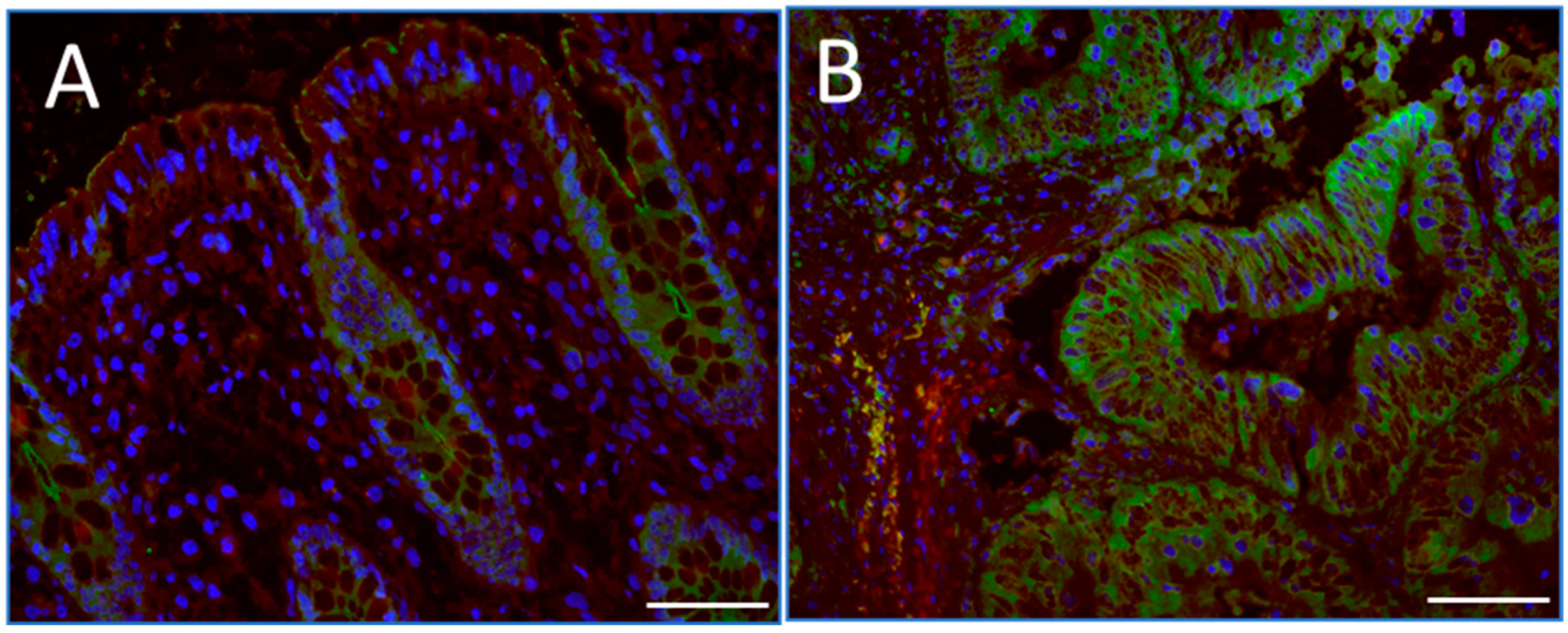
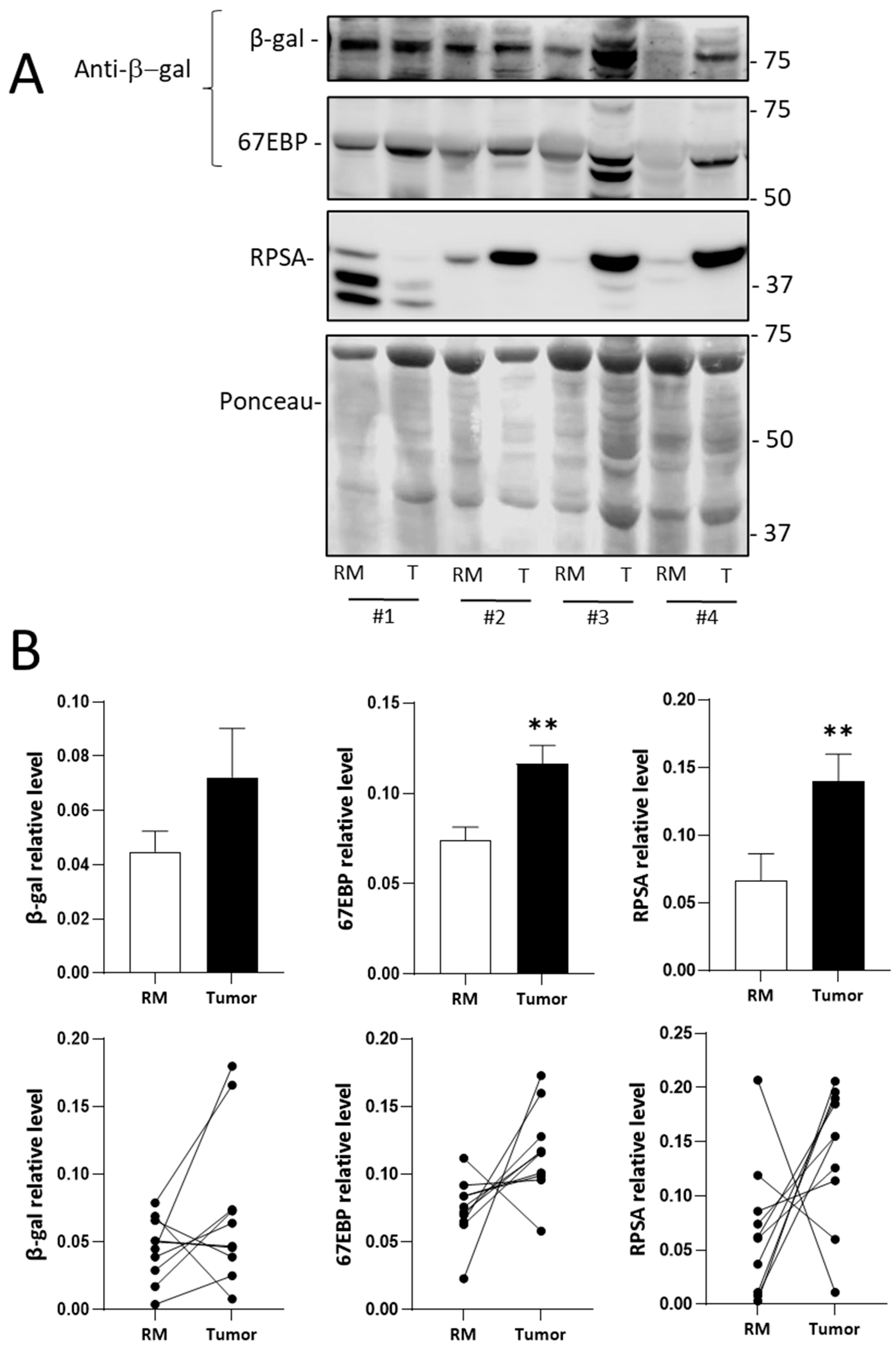
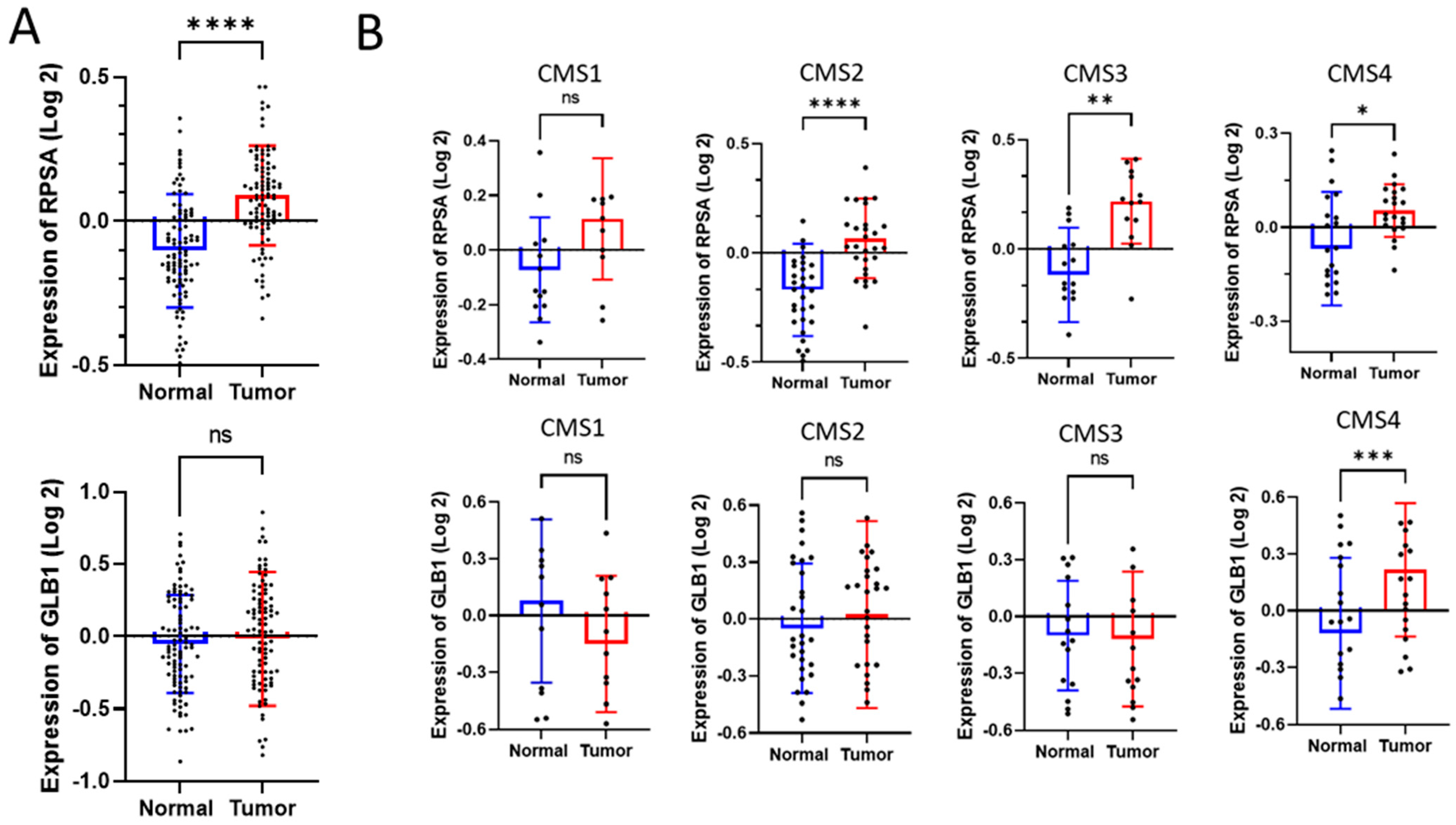
2.1. RPSA and 67EBP Are Overexpressed at the Protein Level in CRC Tissues
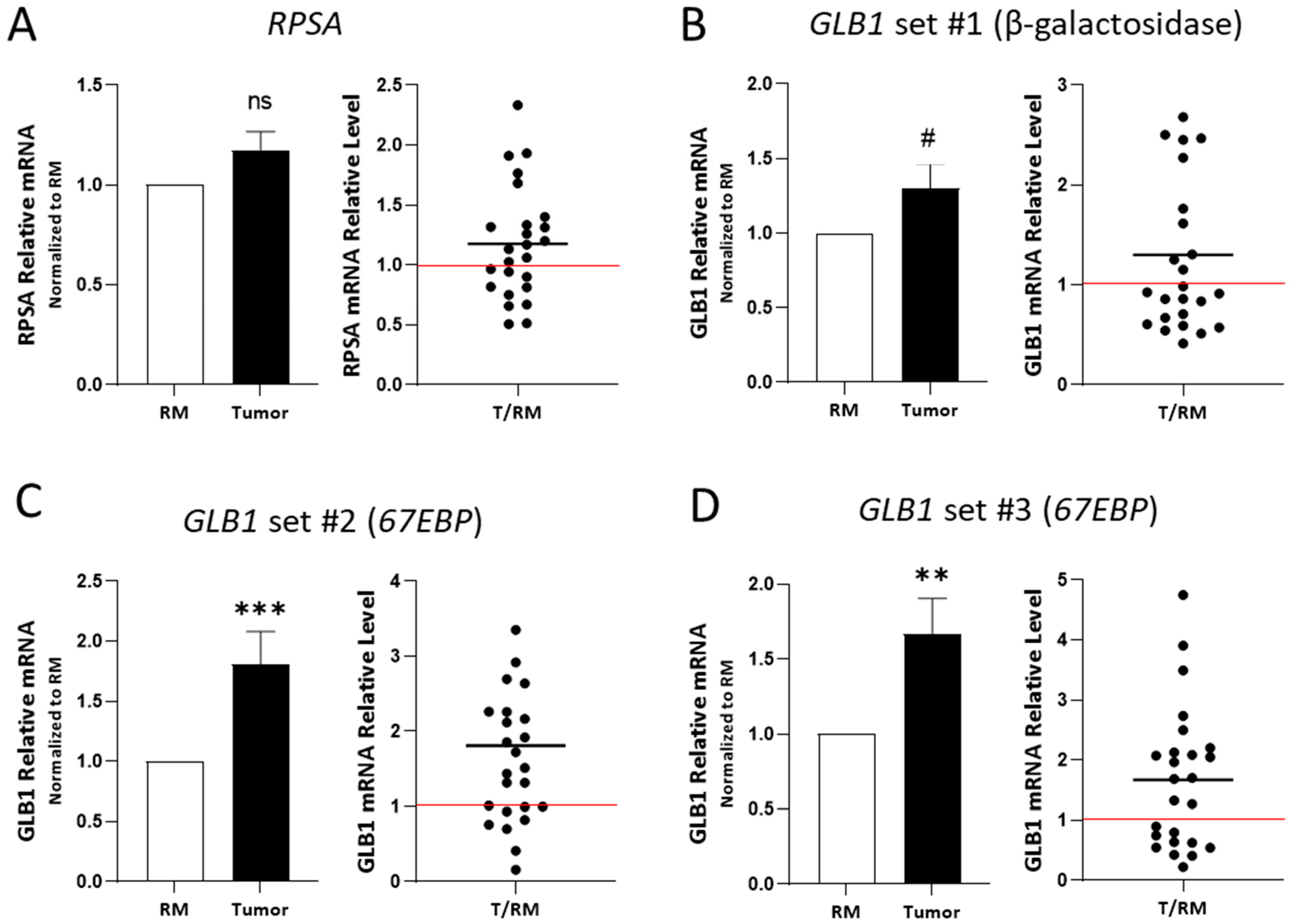
2.2. RPSA and 67EBP Transcript Expression in CRC Tissues
3. Discussion
4. Materials and Methods
4.1. Human Colorectal Tissue Samples
4.2. Expression Analysis Using Publicly Available Datasets of CRC
4.3. Cell Lines
4.4. Immunofluorescence
4.5. RNA Extraction, Reverse Transcriptase, and Quantitative RT-PCR
4.6. Protein Extraction and Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| 67LR | 67 kDa form of the laminin receptor (hypothetical) |
| 67EBP | 67 kDa elastin-binding protein |
| RPSA | Ribosomal protein SA |
| RCLR | RPSA-containing laminin receptor |
| BM | Basement membrane |
| β-gal | β-Galactosidase |
| CPTAC | Clinical Proteomic Tumor Analysis Consortium |
| CMSs | Consensus Molecular Subtypes |
| EMT | Epithelium–mesenchyme transition |
| TNM | Tumour, Node, Metastasis (Staging) |
| GEO | Gene Expression Omnibus |
| qPCR | Quantitative polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| DAPI | 4′,6-Diamidino-2-phenylindole |
References
- Beaulieu, J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997, 31, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Seltana, A.; Cloutier, G.; Reyes Nicolas, V.; Khalfaoui, T.; Teller, I.C.; Perreault, N.; Beaulieu, J.F. Fibrin(ogen) Is Constitutively Expressed by Differentiated Intestinal Epithelial Cells and Mediates Wound Healing. Front. Immunol. 2022, 13, 916187. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrins and human intestinal cell functions. Front. Biosci. 1999, 4, D310–D321. [Google Scholar] [CrossRef] [PubMed]
- Cloutier, G.; Sallenbach-Morrissette, A.; Beaulieu, J.F. Non-integrin laminin receptors in epithelia. Tissue Cell 2019, 56, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Vachon, P.H. Integrin signaling, cell survival, and anoikis: Distinctions, differences, and differentiation. J. Signal Transduct. 2011, 2011, 738137. [Google Scholar] [CrossRef]
- Beaulieu, J.F. Integrin alpha6beta4 in Colorectal Cancer: Expression, Regulation, Functional Alterations and Use as a Biomarker. Cancers 2019, 12, 41. [Google Scholar] [CrossRef]
- Teller, I.C.; Beaulieu, J.F. Interactions between laminin and epithelial cells in intestinal health and disease. Expert Rev. Mol. Med. 2001, 3, 1–18. [Google Scholar] [CrossRef]
- Khalfaoui, T.; Groulx, J.F.; Sabra, G.; GuezGuez, A.; Basora, N.; Vermette, P.; Beaulieu, J.F. Laminin receptor 37/67LR regulates adhesion and proliferation of normal human intestinal epithelial cells. PLoS ONE 2013, 8, e74337. [Google Scholar] [CrossRef]
- Cloutier, G.; Seltana, A.; Fallah, S.; Beaulieu, J.F. Integrin alpha7beta1 represses intestinal absorptive cell differentiation. Exp. Cell Res. 2023, 430, 113723. [Google Scholar] [CrossRef]
- Weiser, M.M.; Sykes, D.E.; Piscatelli, J.J.; Rao, M. The role of the non-integrin 67kDa laminin receptor in enterocyte proliferation, and adhesion, and motility. In The Gut as a Model in Cell and Molecular Biology; Halter, F., Winton, D., Wright, N.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1997; pp. 149–164. [Google Scholar]
- Kim, W.H.; Lee, B.L.; Jun, S.H.; Song, S.Y.; Kleinman, H.K. Expression of 32/67-kDa laminin receptor in laminin adhesion-selected human colon cancer cell lines. Br. J. Cancer 1998, 77, 15–20. [Google Scholar] [CrossRef]
- Mafune, K.; Ravikumar, T.S. Anti-sense RNA of 32-kDa laminin-binding protein inhibits attachment and invasion of a human colon carcinoma cell line. J. Surg. Res. 1992, 52, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Malinoff, H.L.; Wicha, M.S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J. Cell Biol. 1983, 96, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.; Goldstein, I.J.; Liotta, L.A. Lectin-binding domains on laminin. Arch. Biochem. Biophys. 1983, 227, 118–124. [Google Scholar] [CrossRef]
- Liotta, L.A.; Rao, C.N.; Wewer, U.M. Biochemical interactions of tumor cells with the basement membrane. Annu. Rev. Biochem. 1986, 55, 1037–1057. [Google Scholar] [CrossRef]
- Mafune, K.; Ravikumar, T.S.; Wong, J.M.; Yow, H.; Chen, L.B.; Steele, G.D., Jr. Expression of a Mr 32,000 laminin-binding protein messenger RNA in human colon carcinoma correlates with disease progression. Cancer Res. 1990, 50, 3888–3891. [Google Scholar] [PubMed]
- Cioce, V.; Castronovo, V.; Shmookler, B.M.; Garbisa, S.; Grigioni, W.F.; Liotta, L.A.; Sobel, M.E. Increased expression of the laminin receptor in human colon cancer. J. Natl. Cancer Inst. 1991, 83, 29–36. [Google Scholar] [CrossRef]
- Hand, P.H.; Thor, A.; Schlom, J.; Rao, C.N.; Liotta, L. Expression of laminin receptor in normal and carcinomatous human tissues as defined by a monoclonal antibody. Cancer Res. 1985, 45, 2713–2719. [Google Scholar]
- Sanjuan, X.; Fernandez, P.L.; Miquel, R.; Munoz, J.; Castronovo, V.; Menard, S.; Palacin, A.; Cardesa, A.; Campo, E. Overexpression of the 67-kD laminin receptor correlates with tumour progression in human colorectal carcinoma. J. Pathol. 1996, 179, 376–380. [Google Scholar] [CrossRef]
- Liotta, L.A.; Horan Hand, P.; Rao, C.N.; Bryant, G.; Barsky, S.H.; Schlom, J. Monoclonal antibodies to the human laminin receptor recognize structurally distinct sites. Exp. Cell Res. 1985, 156, 117–126. [Google Scholar] [CrossRef]
- Wewer, U.M.; Liotta, L.A.; Jaye, M.; Ricca, G.A.; Drohan, W.N.; Claysmith, A.P.; Rao, C.N.; Wirth, P.; Coligan, J.E.; Albrechtsen, R.; et al. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc. Natl. Acad. Sci. USA 1986, 83, 7137–7141. [Google Scholar] [CrossRef]
- Landowski, T.H.; Uthayakumar, S.; Starkey, J.R. Control pathways of the 67 kDa laminin binding protein: Surface expression and activity of a new ligand binding domain. Clin. Exp. Metastasis 1995, 13, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Buto, S.; Tagliabue, E.; Ardini, E.; Magnifico, A.; Ghirelli, C.; van den Brule, F.; Castronovo, V.; Colnaghi, M.I.; Sobel, M.E.; Menard, S. Formation of the 67-kDa laminin receptor by acylation of the precursor. J. Cell. Biochem. 1998, 69, 244–251. [Google Scholar] [CrossRef]
- DiGiacomo, V.; Meruelo, D. Looking into laminin receptor: Critical discussion regarding the non-integrin 37/67-kDa laminin receptor/RPSA protein. Biol. Rev. Camb. Philos. Soc. 2016, 91, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; McFerran, N.V.; Pivato, G.; Chambers, E.; Doherty, C.; Steele, D.; Timson, D.J. The 67 kDa laminin receptor: Structure, function and role in disease. Biosci. Rep. 2008, 28, 33–48. [Google Scholar] [CrossRef]
- Gauczynski, S.; Peyrin, J.M.; Haik, S.; Leucht, C.; Hundt, C.; Rieger, R.; Krasemann, S.; Deslys, J.P.; Dormont, D.; Lasmezas, C.I.; et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 2001, 20, 5863–5875. [Google Scholar] [CrossRef]
- Cloutier, G.; Beaulieu, J.F. Reconsideration of the laminin receptor 67LR in colorectal cancer cells. Biomol. Biomed. 2024, 24, 1117. [Google Scholar] [CrossRef]
- van den Brule, F.A.; Berchuck, A.; Bast, R.C.; Liu, F.T.; Gillet, C.; Sobel, M.E.; Castronovo, V. Differential expression of the 67-kD laminin receptor and 31-kD human laminin-binding protein in human ovarian carcinomas. Eur. J. Cancer 1994, 30, 1096–1099. [Google Scholar] [CrossRef]
- Fontanini, G.; Vignati, S.; Chine, S.; Lucchi, M.; Mussi, A.; Angeletti, C.A.; Menard, S.; Castronovo, V.; Bevilacqua, G. 67-Kilodalton laminin receptor expression correlates with worse prognostic indicators in non-small cell lung carcinomas. Clin. Cancer Res. 1997, 3, 227–231. [Google Scholar]
- Lee, W.A.; Kim, W.H.; Kim, Y.I.; Yang, H.K.; Kim, J.P.; Kleinman, H.K. Overexpression of the 67 kD laminin receptor correlates with the progression of gastric carcinoma. Pathol. Res. Pract. 1996, 192, 1195–1201. [Google Scholar] [CrossRef]
- Viacava, P.; Naccarato, A.G.; Collecchi, P.; Menard, S.; Castronovo, V.; Bevilacqua, G. The spectrum of 67-kD laminin receptor expression in breast carcinoma progression. J. Pathol. 1997, 182, 36–44. [Google Scholar] [CrossRef]
- Basolo, F.; Pollina, L.; Pacini, F.; Fontanini, G.; Menard, S.; Castronovo, V.; Bevilacqua, G. Expression of the Mr 67,000 laminin receptor is an adverse prognostic indicator in human thyroid cancer: An immunohistochemical study. Clin. Cancer Res. 1996, 2, 1777–1780. [Google Scholar] [PubMed]
- Rao, C.N.; Castronovo, V.; Schmitt, M.C.; Wewer, U.M.; Claysmith, A.P.; Liotta, L.A.; Sobel, M.E. Evidence for a precursor of the high-affinity metastasis-associated murine laminin receptor. Biochemistry 1989, 28, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Hinek, A.; Jung, S.; Rutka, J.T. Cell surface aggregation of elastin receptor molecules caused by suramin amplified signals leading to proliferation of human glioma cells. Acta Neuropathol. 1999, 97, 399–407. [Google Scholar] [CrossRef]
- Timar, J.; Lapis, K.; Fulop, T.; Varga, Z.S.; Tixier, J.M.; Robert, L.; Hornebeck, W. Interaction between elastin and tumor cell lines with different metastatic potential; in vitro and in vivo studies. J. Cancer Res. Clin. Oncol. 1991, 117, 232–238. [Google Scholar] [CrossRef]
- Hinek, A.; Rabinovitch, M.; Keeley, F.; Okamura-Oho, Y.; Callahan, J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J. Clin. Investig. 1993, 91, 1198–1205. [Google Scholar] [CrossRef]
- Stallmach, A.; Orzechowski, H.D.; Feldmann, P.; Riecken, E.O.; Zeitz, M.; Herbst, H. 32/67-kD laminin receptor expression in human colonic neoplasia: Elevated transcript levels correlate with the degree of epithelial dysplasia. Am. J. Gastroenterol. 1999, 94, 3341–3347. [Google Scholar] [CrossRef]
- Xu, T.; Zong, Y.; Peng, L.; Kong, S.; Zhou, M.; Zou, J.; Liu, J.; Miao, R.; Sun, X.; Li, L. Overexpression of eIF4E in colorectal cancer patients is associated with liver metastasis. Onco Targets Ther. 2016, 9, 815–822. [Google Scholar] [CrossRef]
- Coppede, F.; Lopomo, A.; Spisni, R.; Migliore, L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J. Gastroenterol. 2014, 20, 943–956. [Google Scholar] [CrossRef]
- Minnee, E.; Faller, W.J. Translation initiation and its relevance in colorectal cancer. FEBS J. 2021, 288, 6635–6651. [Google Scholar] [CrossRef]
- Roux, P.P.; Topisirovic, I. Signaling Pathways Involved in the Regulation of mRNA Translation. Mol. Cell Biol. 2018, 38, e00070-18. [Google Scholar] [CrossRef]
- Knight, J.R.P.; Alexandrou, C.; Skalka, G.L.; Vlahov, N.; Pennel, K.; Officer, L.; Teodosio, A.; Kanellos, G.; Gay, D.M.; May-Wilson, S.; et al. MNK Inhibition Sensitizes KRAS-Mutant Colorectal Cancer to mTORC1 Inhibition by Reducing eIF4E Phosphorylation and c-MYC Expression. Cancer Discov. 2021, 11, 1228–1247. [Google Scholar] [CrossRef] [PubMed]
- Boudjadi, S.; Beaulieu, J.F. MYC and integrins interplay in colorectal cancer. Oncoscience 2016, 3, 50–51. [Google Scholar] [CrossRef] [PubMed]
- van Riggelen, J.; Yetil, A.; Felsher, D.W. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 2010, 10, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Pal, I.; Safari, M.; Jovanovic, M.; Bates, S.E.; Deng, C. Targeting Translation of mRNA as a Therapeutic Strategy in Cancer. Curr. Hematol. Malig. Rep. 2019, 14, 219–227. [Google Scholar] [CrossRef]
- Lai, M.D.; Xu, J. Ribosomal proteins and colorectal cancer. Curr. Genom. 2007, 8, 43–49. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, J.; Li, X.; Cao, F.; He, J.; Yang, B.; Zhu, X.; Sun, Y.; Pu, Y.D. RPS24 knockdown inhibits colorectal cancer cell migration and proliferation in vitro. Gene 2015, 571, 286–291. [Google Scholar] [CrossRef]
- Dong, Z.; Jiang, H.; Liang, S.; Wang, Y.; Jiang, W.; Zhu, C. Ribosomal Protein L15 is involved in Colon Carcinogenesis. Int. J. Med. Sci. 2019, 16, 1132–1141. [Google Scholar] [CrossRef]
- Reza, A.; Yuan, Y.G. microRNAs Mediated Regulation of the Ribosomal Proteins and its Consequences on the Global Translation of Proteins. Cells 2021, 10, 110. [Google Scholar] [CrossRef]
- Orom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Brassart, B.; Fuchs, P.; Huet, E.; Alix, A.J.; Wallach, J.; Tamburro, A.M.; Delacoux, F.; Haye, B.; Emonard, H.; Hornebeck, W.; et al. Conformational dependence of collagenase (matrix metalloproteinase-1) up-regulation by elastin peptides in cultured fibroblasts. J. Biol. Chem. 2001, 276, 5222–5227. [Google Scholar] [CrossRef]
- Toupance, S.; Brassart, B.; Rabenoelina, F.; Ghoneim, C.; Vallar, L.; Polette, M.; Debelle, L.; Birembaut, P. Elastin-derived peptides increase invasive capacities of lung cancer cells by post-transcriptional regulation of MMP-2 and uPA. Clin. Exp. Metastasis 2012, 29, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xie, M.; Zhou, J.Q.; Tao, L. 67-kDa laminin receptor in human laryngeal squamous cell carcinoma. Laryngoscope 2006, 116, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Damaschke, N.; Yang, B.; Truong, M.; Guenther, C.; McCormick, J.; Huang, W.; Jarrard, D. Overexpression of the novel senescence marker beta-galactosidase (GLB1) in prostate cancer predicts reduced PSA recurrence. PLoS ONE 2015, 10, e0124366. [Google Scholar] [CrossRef]
- Szajda, S.D.; Jankowska, A.; Zwierz, K. Carbohydrate markers in colon carcinoma. Dis. Markers 2008, 25, 233–242. [Google Scholar] [CrossRef]
- Bosmann, H.B.; Hall, T.C. Enzyme activity in invasive tumors of human breast and colon. Proc. Natl. Acad. Sci. USA 1974, 71, 1833–1837. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Da Silva, J.; Lameiras, P.; Beljebbar, A.; Berquand, A.; Villemin, M.; Ramont, L.; Dukic, S.; Nuzillard, J.M.; Molinari, M.; Gautier, M.; et al. Structural characterization and in vivo pro-tumor properties of a highly conserved matrikine. Oncotarget 2018, 9, 17839–17857. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Jiang, Y.; Hansbro, N.G.; Hansbro, P.M.; Xu, J.; Liu, G. Elastin is a key factor of tumor development in colorectal cancer. BMC Cancer 2020, 20, 217. [Google Scholar] [CrossRef]
- Tembely, D.; Henry, A.; Vanalderwiert, L.; Toussaint, K.; Bennasroune, A.; Blaise, S.; Sartelet, H.; Jaisson, S.; Gales, C.; Martiny, L.; et al. The Elastin Receptor Complex: An Emerging Therapeutic Target Against Age-Related Vascular Diseases. Front. Endocrinol. 2022, 13, 815356. [Google Scholar] [CrossRef]
- Scandolera, A.; Odoul, L.; Salesse, S.; Guillot, A.; Blaise, S.; Kawecki, C.; Maurice, P.; El Btaouri, H.; Romier-Crouzet, B.; Martiny, L.; et al. The Elastin Receptor Complex: A Unique Matricellular Receptor with High Anti-tumoral Potential. Front. Pharmacol. 2016, 7, 32. [Google Scholar] [CrossRef]
- Bennasroune, A.; Romier-Crouzet, B.; Blaise, S.; Laffargue, M.; Efremov, R.G.; Martiny, L.; Maurice, P.; Duca, L. Elastic fibers and elastin receptor complex: Neuraminidase-1 takes the center stage. Matrix Biol. 2019, 84, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vania, L.; Morris, G.; Otgaar, T.C.; Bignoux, M.J.; Bernert, M.; Burns, J.; Gabathuse, A.; Singh, E.; Ferreira, E.; Weiss, S.F.T. Patented therapeutic approaches targeting LRP/LR for cancer treatment. Expert Opin. Ther. Pat. 2019, 29, 987–1009. [Google Scholar] [CrossRef] [PubMed]
- Ardini, E.; Sporchia, B.; Pollegioni, L.; Modugno, M.; Ghirelli, C.; Castiglioni, F.; Tagliabue, E.; Menard, S. Identification of a novel function for 67-kDa laminin receptor: Increase in laminin degradation rate and release of motility fragments. Cancer Res. 2002, 62, 1321–1325. [Google Scholar] [PubMed]
- Omar, A.; Jovanovic, K.; Da Costa Dias, B.; Gonsalves, D.; Moodley, K.; Caveney, R.; Mbazima, V.; Weiss, S.F. Patented biological approaches for the therapeutic modulation of the 37 kDa/67 kDa laminin receptor. Expert Opin. Ther. Pat. 2011, 21, 35–53. [Google Scholar] [CrossRef]
- Fujimura, Y.; Kumazoe, M.; Tachibana, H. 67-kDa Laminin Receptor-Mediated Cellular Sensing System of Green Tea Polyphenol EGCG and Functional Food Pairing. Molecules 2022, 27, 5130. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- Pesapane, A.; Di Giovanni, C.; Rossi, F.W.; Alfano, D.; Formisano, L.; Ragno, P.; Selleri, C.; Montuori, N.; Lavecchia, A. Discovery of new small molecules inhibiting 67 kDa laminin receptor interaction with laminin and cancer cell invasion. Oncotarget 2015, 6, 18116–18133. [Google Scholar] [CrossRef]
- Limone, A.; Maggisano, V.; Sarnataro, D.; Bulotta, S. Emerging roles of the cellular prion protein (PrP(C)) and 37/67 kDa laminin receptor (RPSA) interaction in cancer biology. Cell. Mol. Life Sci. 2023, 80, 207. [Google Scholar] [CrossRef]
- Bian, B.; Mongrain, S.; Cagnol, S.; Langlois, M.J.; Boulanger, J.; Bernatchez, G.; Carrier, J.C.; Boudreau, F.; Rivard, N. Cathepsin B promotes colorectal tumorigenesis, cell invasion, and metastasis. Mol. Carcinog. 2016, 55, 671–687. [Google Scholar] [CrossRef]
- Sheffer, M.; Bacolod, M.D.; Zuk, O.; Giardina, S.F.; Pincas, H.; Barany, F.; Paty, P.B.; Gerald, W.L.; Notterman, D.A.; Domany, E. Association of survival and disease progression with chromosomal instability: A genomic exploration of colorectal cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 7131–7136. [Google Scholar] [CrossRef]
- Vasaikar, S.V.; Straub, P.; Wang, J.; Zhang, B. LinkedOmics: Analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018, 46, D956–D963. [Google Scholar] [CrossRef] [PubMed]
- Dydensborg, A.B.; Herring, E.; Auclair, J.; Tremblay, E.; Beaulieu, J.F. Normalizing genes for quantitative RT-PCR in differentiating human intestinal epithelial cells and adenocarcinomas of the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1067–G1074. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]

| Series: | #1-Biobank | #2-Biobank | #3-CPTAC 3 | #4-GSE41258 |
|---|---|---|---|---|
| Use: | Protein expression | mRNA expression | Proteomics | Gene expression microarray |
| Total n: | 10 | 25 | 104 | 43 |
| Age: | 73.6 ± 8.27 | 68.2 ± 13.3 | 65.3 ± 11.6 | 62.6 ± 15.9 |
| Sex (F/M): | 5/5 | 11/14 | 45/65 | 20/23 |
| Stage TNM | ||||
| I | 0 | 3 | 10 | 7 |
| II | 1 | 7 | 42 | 8 |
| III | 7 | 9 | 44 | 10 |
| IV | 2 | 6 | 8 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cloutier, G.; Khalfaoui, T.; Carrier, J.C.; Beaulieu, J.-F. Expression of the RPSA-Containing and 67EBP Laminin Receptors in Relation to the Debatable Nature of the 67 kDa Laminin Receptor 67LR in Colorectal Cancer. Int. J. Mol. Sci. 2025, 26, 2564. https://doi.org/10.3390/ijms26062564
Cloutier G, Khalfaoui T, Carrier JC, Beaulieu J-F. Expression of the RPSA-Containing and 67EBP Laminin Receptors in Relation to the Debatable Nature of the 67 kDa Laminin Receptor 67LR in Colorectal Cancer. International Journal of Molecular Sciences. 2025; 26(6):2564. https://doi.org/10.3390/ijms26062564
Chicago/Turabian StyleCloutier, Gabriel, Taoufik Khalfaoui, Julie C. Carrier, and Jean-François Beaulieu. 2025. "Expression of the RPSA-Containing and 67EBP Laminin Receptors in Relation to the Debatable Nature of the 67 kDa Laminin Receptor 67LR in Colorectal Cancer" International Journal of Molecular Sciences 26, no. 6: 2564. https://doi.org/10.3390/ijms26062564
APA StyleCloutier, G., Khalfaoui, T., Carrier, J. C., & Beaulieu, J.-F. (2025). Expression of the RPSA-Containing and 67EBP Laminin Receptors in Relation to the Debatable Nature of the 67 kDa Laminin Receptor 67LR in Colorectal Cancer. International Journal of Molecular Sciences, 26(6), 2564. https://doi.org/10.3390/ijms26062564





