Tephrosia purpurea, with (-)-Pseudosemiglabrin as the Major Constituent, Alleviates Severe Acute Pancreatitis-Mediated Acute Lung Injury by Modulating HMGB1 and IL-22
Abstract
1. Introduction
2. Results
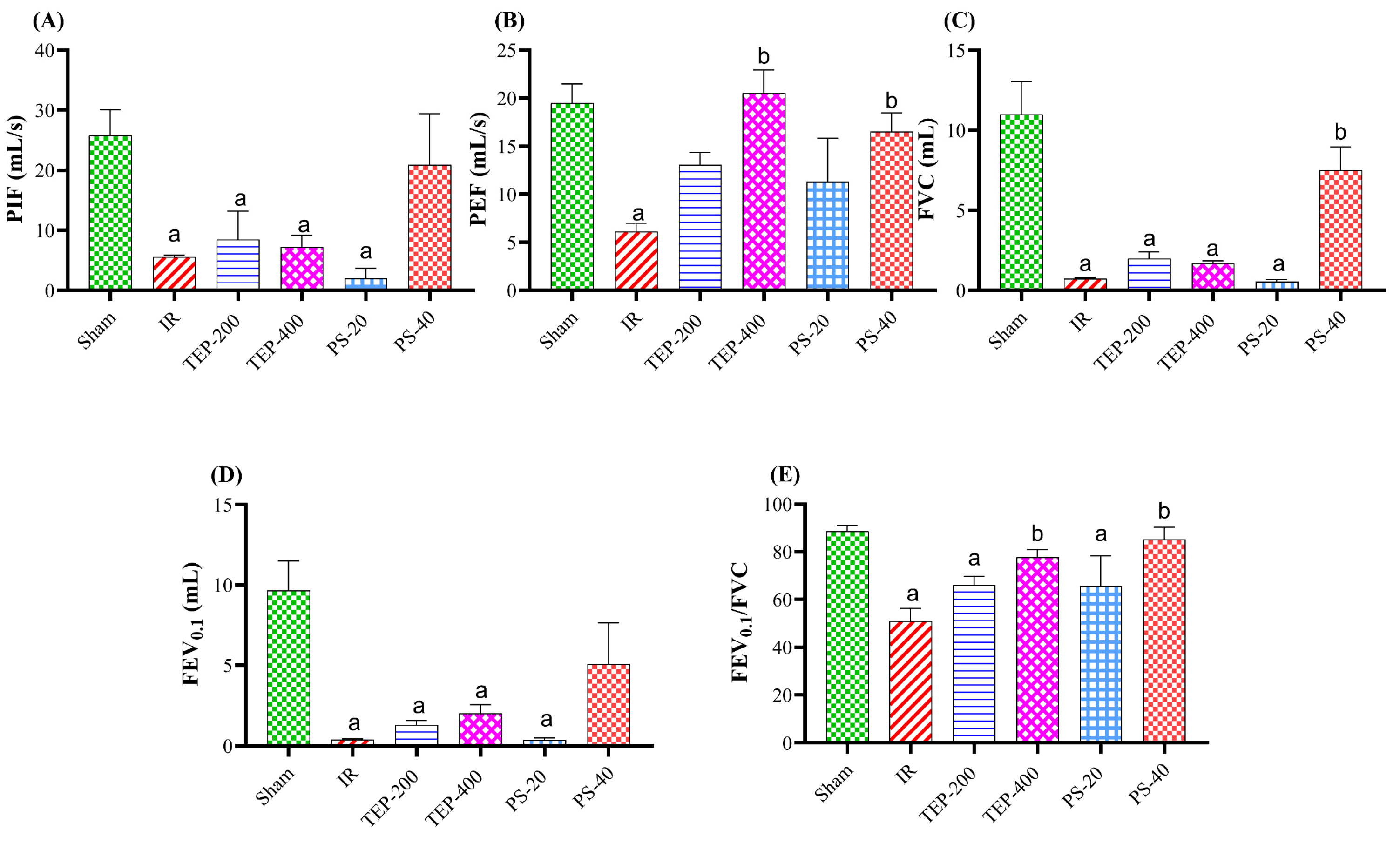
2.1. Respirometer
2.2. Serum Parameters
2.3. Assessment of Inflammatory Markers in Pancreatic and Lung Homogenates
2.4. Assessment of Pancreatic Oxidative Stress Markers
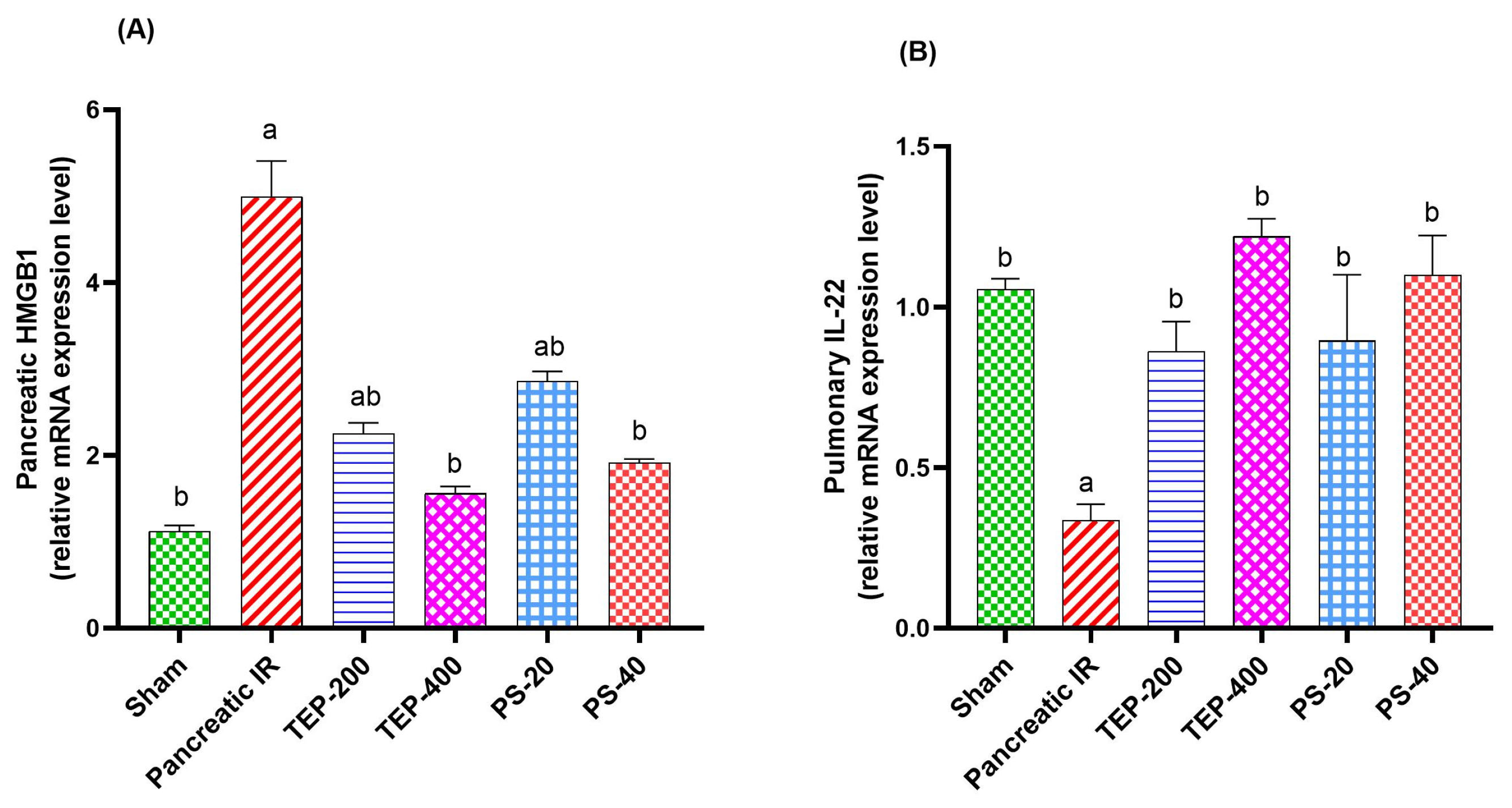
2.5. Gene Expression Analyses
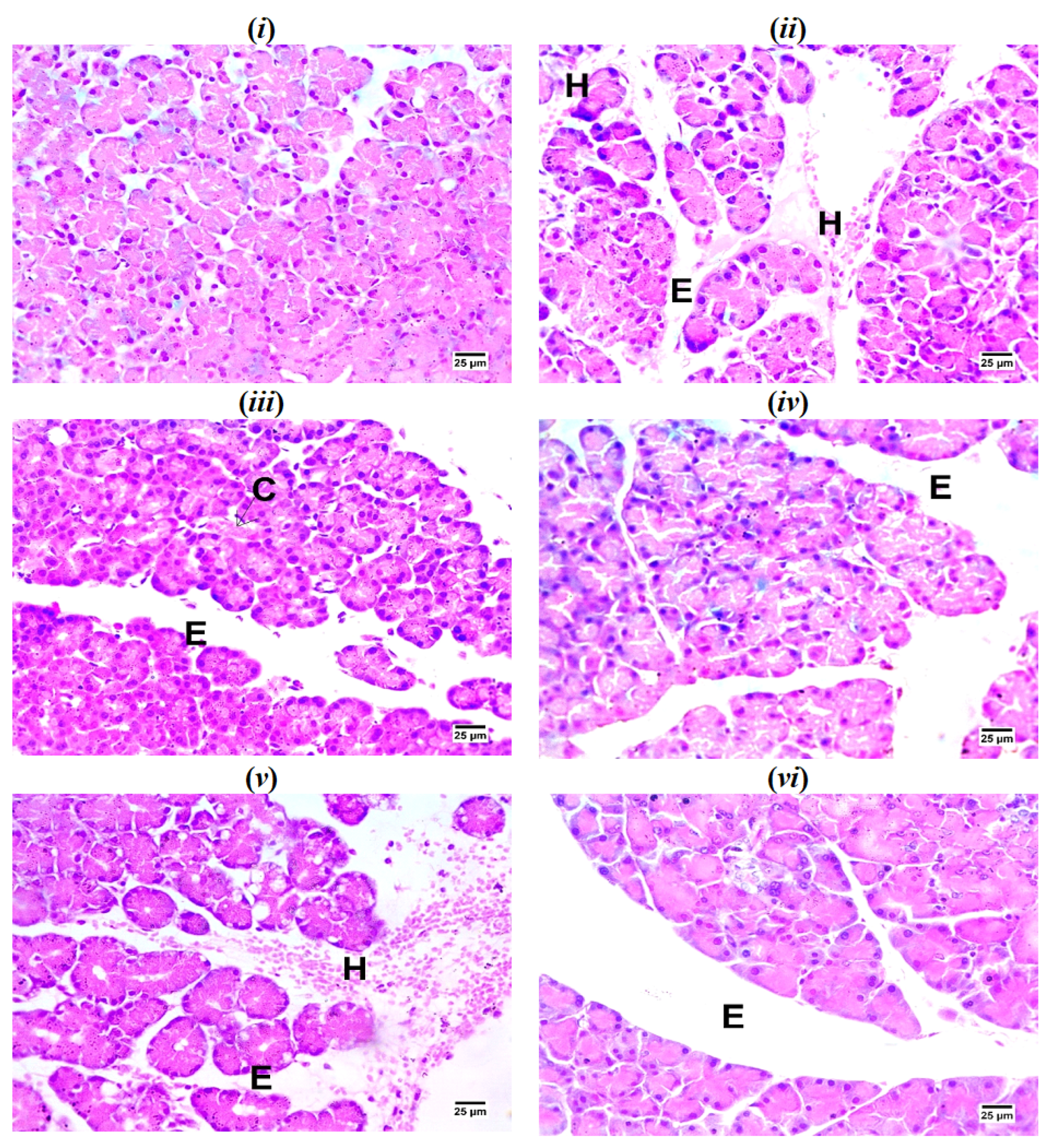
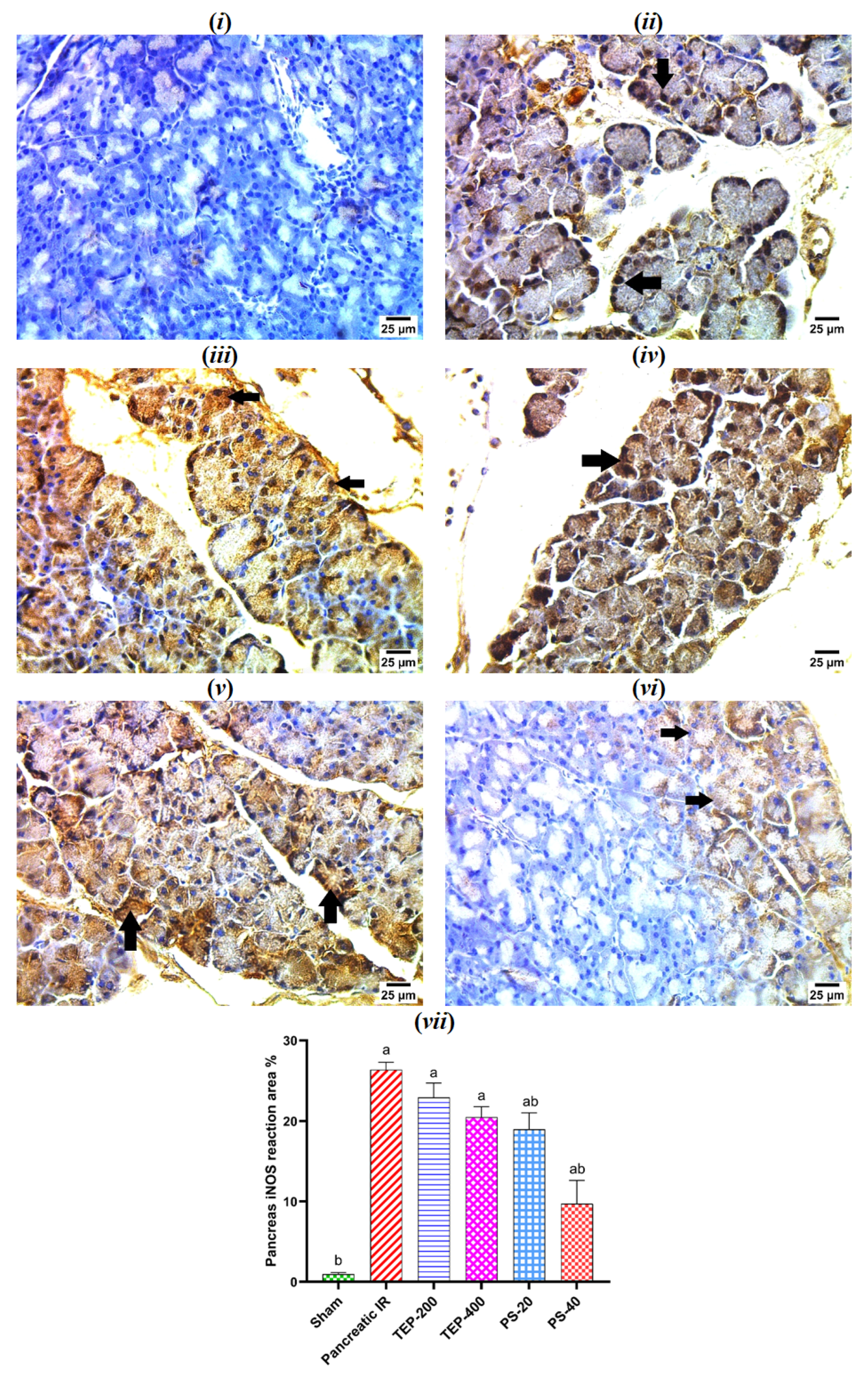
2.6. Histopathological Examination of Pancreatic Tissues
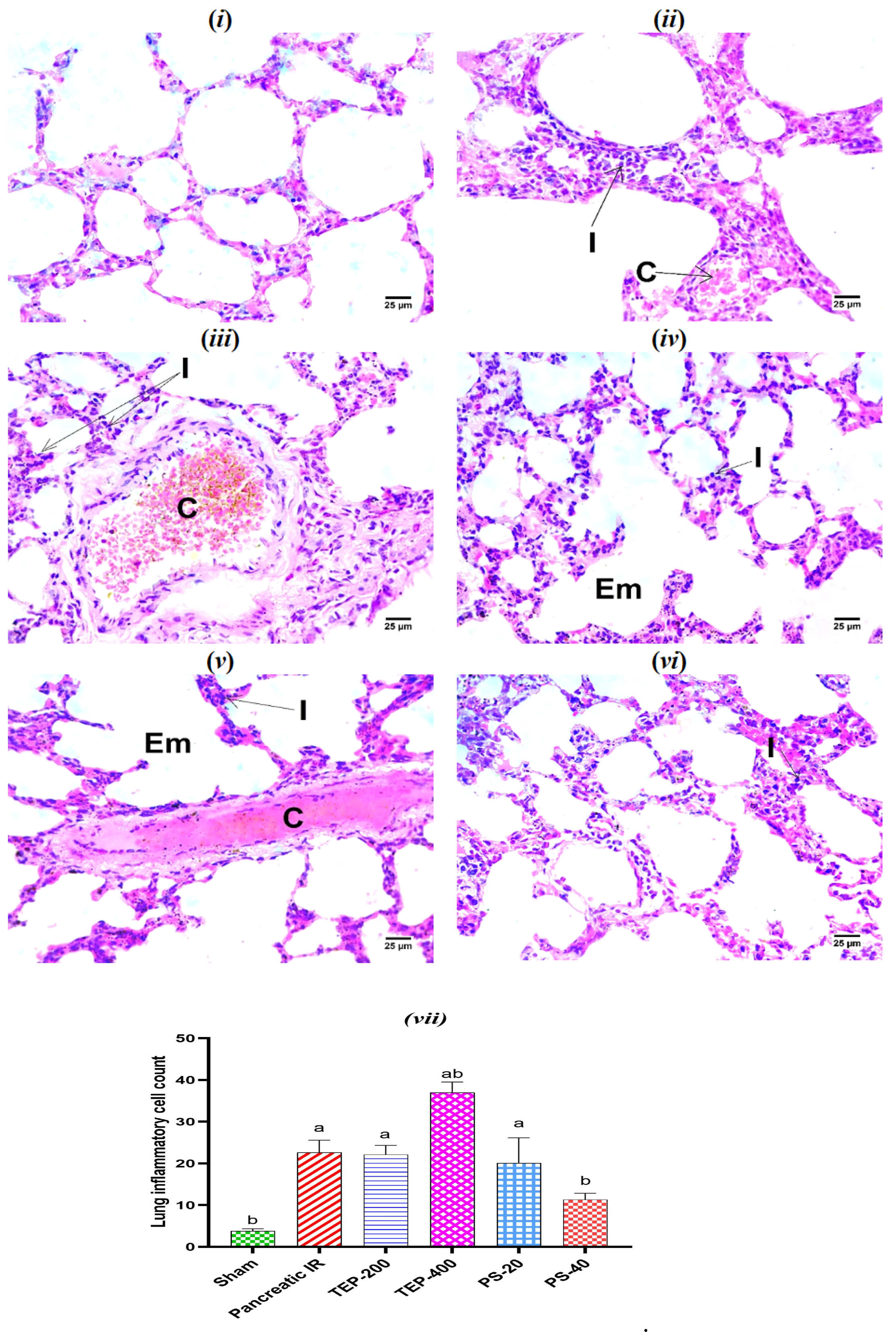
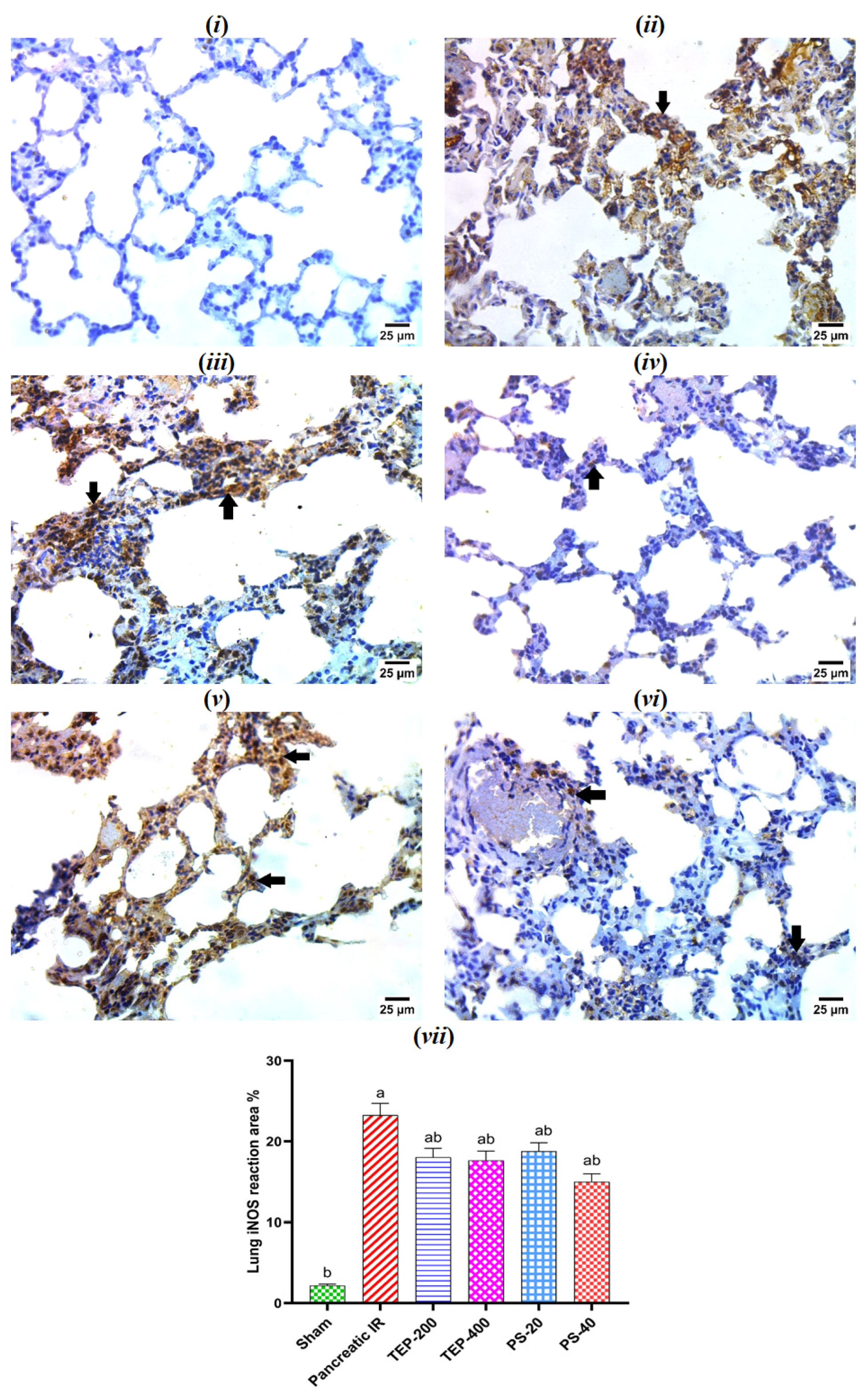
2.7. Histopathological Examination of Lung Tissues
2.8. Immunohistochemical Examination of iNOS in Pancreatic Tissue
2.9. Immunohistochemical Examination of iNOS in Lung Tissue
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Animals
4.3. Experimental Design
- Sham group: Rats received 1 mL of the vehicle (2% Tween 80 in sterile saline).
- IR control group: Rats orally received 1 mL of the vehicle kg.
- TEP-200 group: Rats treated orally with T. purpurea at 200 mg/kg.
- TEP-400 group: Rats treated orally with T. purpurea at 400 mg/kg.
- PS-20 group: Rats treated orally with pseudosemiglabrin at 20 mg/kg.
- PS-40 group: Rats treated orally with pseudosemiglabrin at 40 mg/kg.
4.4. Induction of Pancreatic Ischemia
4.5. Assessment of Respiratory Functions Using Respirometer
4.6. Serum Analysis
4.7. Assessment of Inflammatory Markers in Pancreatic and Lung Homogenate
4.8. Assessment of Pancreatic Oxidative Stress Markers
4.9. Real-Time Polymerase Chain Reaction (RT PCR)
4.10. Histopathological Examination of Pancreatic and Lung Tissues
4.11. Immunohistochemical Examination of iNOS in Pancreatic and Lung Tissues
4.12. Statistical Analysis
5. Conclusions
Limitation of the Current Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IR | Ischemia-reperfusion |
| AP | Acute pancreatitis |
| SIRS | Systemic inflammatory response syndrome |
| ROS | Reactive oxygen species |
| TEP | Tephrosia purpurea |
| PS | Pseudosemiglabrin |
| IL-1 | Interleukin 1 |
| TNF-α | Tumor necrosis factor-α |
| NO | Nitric oxide |
| Ti | Inspiratory time |
| Te | Expiratory time |
| D | Duration |
| RR | Respiratory rate |
| PIF | Peak inspiratory flow |
| FVC | Forced vital capacity |
| FEV0.1 | Forced expiratory volume at 0.1 s |
| HMGB1 | High mobility group box 1 protein |
| IL-22 | Interleukin 22 |
| Th22 | T helper cell 22 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| iNOS | Nitric oxide synthase |
| NF-κB | Nuclear factor kappa B |
| MDA | Malondialdehyde |
| GPx | Glutathione peroxidase |
| MPO | Myeloperoxidase |
| DAMP | Damage- associated molecular pattern family |
| TLRs | Toll-like receptors |
| CD4+ | Cluster of differentiation 4-positive |
| STAT3 | Signal transducer and activator of transcription 3 |
| Bcl-2 | B-cell leukemia/lymphoma 2 protein |
| i.p. | Intraperitoneal |
| TAP | Trypsinogen activation peptide |
| ABC | Avidin biotin peroxidase complex |
| DAB | Diaminobenzidine |
References
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/reperfusion injury revisited: An overview of the latest pharmacological strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.F.; Leiderer, R.; Haeris, A.C.; Messmer, K. Ischemia and reperfusion in pancreas. Microsc. Res. Tech. 1997, 37, 557–571. [Google Scholar] [CrossRef]
- Robert, F.; Matthew, T. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists; University of Adelaide Press: Adelaide, SA, Australia, 2011; Available online: http://www.jstor.org/stable/10.20851/j.ctt1sq5w94 (accessed on 2 March 2025).
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Hoffmann, T.F.; Leiderer, R.; Waldner, H.; Arbogast, S.; Messmer, K. Ischemia reperfusion of the pancreas: A new in vivo model for acute pancreatitis in rats. Res. Exp. Med. 1995, 195, 125–144. [Google Scholar] [CrossRef]
- Rolim, M.F.; Riger, C.J.; Eleutherio, E.C.A.; Colão, C.F.; Pereira, G.C.; Schanaider, A. Colonic healing after portal ischemia and reperfusion: An experimental study with oxidative stress biomarkers. Redox Rep. 2007, 12, 267–274. [Google Scholar] [CrossRef]
- Schanaider, A.; de Carvalho, T.P.; de Oliveira Coelho, S.; Renteria, J.M.; Eleuthério, E.C.A.; Castelo-Branco, M.T.L.; Madi, K.; Baetas-da-Cruz, W.; de Souza, H.S.P. Ischemia–reperfusion rat model of acute pancreatitis: Protein carbonyl as a putative early biomarker of pancreatic injury. Clin. Exp. Med. 2015, 15, 311–320. [Google Scholar] [CrossRef]
- Zhang, X.P.; Li, Z.J.; Zhang, J. Inflammatory mediators and microcirculatory disturbance in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2009, 4, 351–357. [Google Scholar]
- Samanta, J.; Singh, S.; Arora, S.; Muktesh, G.; Aggarwal, A.; Dhaka, N.; Sinha, S.K.; Gupta, V.; Sharma, V.; Kochhar, R. Cytokine profile in prediction of acute lung injury in patients with acute pancreatitis. Pancreatology 2018, 18, 878–884. [Google Scholar] [CrossRef]
- Ge, P.; Luo, Y.; Okoye, C.S.; Chen, H.; Liu, J.; Zhang, G.; Xu, C.; Chen, H. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: A troublesome trio for acute pancreatitis. Biomed. Pharmacother. 2020, 132, 110770. [Google Scholar] [CrossRef]
- Arora, S.K.; Verma, P.R.; Itankar, P.R.; Prasad, S.K.; Nakhate, K.T. Evaluation of pancreatic regeneration activity of Tephrosia purpurea leaves in rats with streptozotocin-induced diabetes. J. Tradit. Complement. Med. 2021, 11, 435–445. [Google Scholar] [CrossRef]
- Shenoy, S.; Shwetha, K.; Prabhu, K.; Maradi, R.; Bairy, K.L.; Shanbhag, T. Evaluation of antiinflammatory activity of Tephrosia purpurea in rats. Asian Pac. J. Trop. Med. 2010, 3, 193–195. [Google Scholar] [CrossRef]
- Khatri, A.; Garg, A.; Agrawal, S.S. Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharmacol. 2009, 122, 1–5. [Google Scholar] [CrossRef]
- Damre, A.S.; Gokhale, A.B.; Phadke, A.S.; Kulkarni, K.R.; Saraf, M.N. Studies on the immunomodulatory activity of flavonoidal fraction of Tephrosia purpurea. Fitoterapia 2003, 74, 257–261. [Google Scholar] [CrossRef]
- Chinniah, A.; Mohapatra, S.; Goswami, S. On the potential of Tephrosia purpurea as anti-Helicobacter pylori agent. J. Ethnopharmacol. 2009, 124, 642–645. [Google Scholar] [CrossRef]
- Gokhale, A.B.; Saraf, M.N. Tephrosia Purpurea: A review of contemporary literature and medicinal properties. Indian Drugs 2000, 37, 553–560. [Google Scholar]
- Hassan, L.E.A.; Ahamed, M.B.K.; Majid, A.S.A.; Iqbal, M.A.; Suede, F.S.R.A.; Haque, R.A.; Ismail, Z.; Ein, O.C.; Majid, A.M.S.A. Crystal structure elucidation and anticancer studies of (-)-pseudosemiglabrin: A flavanone isolated from the aerial parts of Tephrosia apollinea. PLoS ONE 2014, 9, e90806. [Google Scholar] [CrossRef]
- Balaha, M.F.; Alamer, A.A.; Abdel-Kader, M.S.; Alharthy, K.M. Ameliorative Potential of (-) Pseudosemiglabrin in Mice with Pilocarpine-Induced Epilepsy: Antioxidant, Anti-Inflammatory, Anti-Apoptotic, and Neurotransmission Modulation. Int. J. Mol. Sci. 2023, 24, 10773. [Google Scholar] [CrossRef]
- Hassan, L.E.A.; Dahham, S.S.; Fadul, S.M.; Umar, M.I.; Majid, A.S.A.; Khaw, K.Y.; Majid, A.M.S.A. Evaluation of in vitro and in vivo anti-inflammatory effects of (−)-pseudosemiglabrin, a major phytoconstituent isolated from Tephrosia apollinea (Delile) DC. J. Ethnopharmacol. 2016, 193, 312–320. [Google Scholar] [CrossRef]
- Lodhi, S.; Pawar, R.S.; Jain, A.P.; Singhai, A.K. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. J. Ethnopharmacol. 2006, 108, 204–210. [Google Scholar] [CrossRef]
- Carden, D.; Granger, D. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Raedschelders, K.; Ansley, D.; Chen, D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol. Ther. 2012, 133, 230–255. [Google Scholar] [CrossRef]
- Park, S.W.; Kim, M.; Brown, K.M.; D’Agati, V.D.; Lee, H.T. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology 2011, 53, 1662–1675. [Google Scholar] [CrossRef]
- Nishikata, R.; Kato, N.; Hiraiwa, K. Oxidative stress may be involved in distant organ failure in tourniquet shock model mice. Leg. Med. 2014, 16, 70–75. [Google Scholar] [CrossRef]
- Liu, D.; Wen, L.; Wang, Z.; Hai, Y.; Yang, D.; Zhang, Y.; Bai, M.; Song, B.; Wang, Y. The Mechanism of Lung and Intestinal Injury in Acute Pancreatitis: A Review. Front. Med. 2022, 7, 904078. [Google Scholar] [CrossRef]
- Levy, B.D.; Hickey, L.; Morris, A.J.; Larvie, M.; Keledjian, R.; Petasis, N.A.; Bannenberg, G.; Serhan, C.N. Novel polyisoprenyl phosphates block phospholipase D and human neutrophil activation in vitro and murine peritoneal inflammation in vivo. Br. J. Pharmacol. 2005, 146, 344–351. [Google Scholar] [CrossRef]
- Que, R.S.; Cao, L.P.; Ding, G.P.; Hu, J.A.; Mao, K.J.; Wang, G.F. Correlation of nitric oxide and other free radicals with the severity of acute pancreatitis and complicated systemic inflammatory response syndrome. Pancreas 2010, 39, 536–540. [Google Scholar] [CrossRef]
- Nakanishi, N.; Oto, J.; Ueno, Y.; Nakataki, E.; Itagaki, T.; Nishimura, M. Change in diaphragm and intercostal muscle thickness in mechanically ventilated patients: A prospective observational ultrasonography study. J. Intensive Care 2019, 7, 56. [Google Scholar] [CrossRef]
- Zhou, M.T.; Chen, C.S.; Chen, B.C.; Zhang, Q.Y.; Andersson, R. Acute lung injury and ARDS in acute pancreatitis: Mechanisms and potential intervention. World J. Gastroenterol. 2010, 16, 2094–2099. [Google Scholar] [CrossRef]
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Matull, W.R.; Pereira, S.P.; O’Donohue, J.W. Biochemical markers of acute pancreatitis. J. Clin. Pathol. 2006, 59, 340–344. [Google Scholar] [CrossRef]
- Sah, R.P.; Dawra, R.K.; Saluja, A.K. New insights into the pathogenesis of pancreatitis. Curr. Opin. Gastroenterol. 2013, 29, 523–530. [Google Scholar] [CrossRef]
- Dinarello, C.A. The Role of the Interleukin-1–Receptor Antagonist in Blocking Inflammation Mediated by Interleukin-1. N. Engl. J. Med. 2000, 343, 732–734. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and interleukin-1 antagonism. Blood 1991, 77, 1627–1652. [Google Scholar] [CrossRef]
- Kingsnorth, A. Role of cytokines and their inhibitors in acute pancreatitis. Gut 1997, 40, 1–4. [Google Scholar] [CrossRef]
- Norman, J.; Franz, M.; Messina, J.; Riker, A.; Fabri, P.J.; Rosemurgy, A.S.; Gower, W.R., Jr. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995, 117, 648–655. [Google Scholar] [CrossRef]
- Li, B.F.; Liu, Y.F.; Cheng, Y.; Zhang, K.Z.; Li, T.M.; Zhao, N. Protective effect of inducible nitric oxide synthase inhibitor on pancreas transplantation in rats. World J. Gastroenterol. 2007, 13, 6066–6071. [Google Scholar] [CrossRef]
- Sakorafas, G.; Tsiotos, G.; Sarr, M. Ischemia/Reperfusion-Induced Pancreatitis. Dig. Surg. 2000, 17, 3–14. [Google Scholar] [CrossRef]
- Muñoz-Casares, F.; Padillo, F.; Briceño, J.; Collado, J.; Muñoz-Castañeda, J.; Ortega, R.; Cruz, A.; Túnez, I.; Montilla, P.; Pera, C.; et al. Melatonin Reduces Apoptosis and Necrosis Induced by Ischemia/Reperfusion Injury of the Pancreas. J. Pineal Res. 2006, 40, 195–203. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Halladin, N.L.; Ekeløf, S.; Jensen, S.E.; Aarøe, J.; Kjærgaard, B.; Heegaard, P.M.H.; Lykkesfeldt, J.; Rosenberg, J.; Gögenur, I. Melatonin does not affect oxidative and inflammatory biomarkers in a closed-chest porcine model of acute myocardial infarction. In Vivo 2014, 28, 483–488. [Google Scholar]
- Valenzuela, A. The biological significance of malondialdehyde determination in the assessment of tissue oxidative stress. Life Sci. 1991, 48, 301–309. [Google Scholar] [CrossRef]
- Lips, J.; de Haan, P.; Bodewits, P.; Vanicky, I.; Dzoljic, M.; Jacobs, M.J.; Kalkman, C.J. Neuroprotective effects of riluzole and ketamine during transient spinal cord ischemia in the rabbit. Anesthesiology 2000, 93, 1303–1311. [Google Scholar] [CrossRef]
- Kryl’skii, E.D.; Popova, T.N.; Safonova, O.A.; Stolyarova, A.O.; Razuvaev, G.A.; de Carvalho, M.A.P. Transcriptional regulation of antioxidant enzymes activity and modulation of oxidative stress by melatonin in rats under cerebral ischemia/reperfusion conditions. Neuroscience 2019, 406, 653–666. [Google Scholar] [CrossRef]
- Chooklin, S.; Pereyaslov, A.; Bihalskyy, I. Pathogenic role of myeloperoxidase in acute pancreatitis. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 627–631. [Google Scholar]
- Bukowczan, J.; Warzecha, Z.; Ceranowicz, P.; Kuśnierz-Cabala, B.; Tomaszewska, R. Obestatin Accelerates the Recovery in the Course of Ischemia/Reperfusion-Induced Acute Pancreatitis in Rats. PLoS ONE 2015, 10, e0134380. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Bonham, M.; Buss, H.; Abu-Zidan, F.; Windsor, J.A. Elevated Protein Carbonyls as Plasma Markers of Oxidative Stress in Acute Pancreatitis. Pancreatology 2003, 3, 375–382. [Google Scholar] [CrossRef]
- Hernández, V.; Miranda, M.; Pascual, I.; Sanchiz, V.; Almela, P.; Añón, R.; Cuadrado, E.; Sanz, M.I.; Mínguez, M.; Mora, F.; et al. Malondialdehyde in Early Phase of Acute Pancreatitis. Rev. Esp. Enferm. Dig. 2011, 103, 563–569. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Febriza, A.; Idrus, H.H. Linking Interaction between Antimicrobial Peptide and High Motility Group Box-1 (HMBG-1) in Bacterial Infection. Open Biochem. J. 2024, 18, e1874091X277312. [Google Scholar] [CrossRef]
- Wulandari, S.; Nuryastuti, T.; Oktoviani, F.N.; Daniwijaya, M.E.W.; Supriyati, E.; Arguni, E.; Wibawa, T. The association between high mobility group box 1 (HMGB1) and Interleukin-18 (IL-18) serum concentrations in COVID-19 inpatients. Heliyon 2024, 10, e26619. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Abdel-Rahman, R.F.; Soliman, G.A.; Ogaly, H.A.; Alamri, M.A.; Alharbi, A.G. Oleuropein Relieves Pancreatic Ischemia Reperfusion Injury in Rats by Suppressing Inflammation and Oxidative Stress through HMGB1/NF-κB Pathway. Int. J. Mol. Sci. 2024, 25, 10171. [Google Scholar] [CrossRef]
- Liu, J.Q.; Hao, W.A.; Liu, Y.L.; Yang, D.; Wang, H.L.; Zhao, L.; Chen, H.; Li, L.; Jiang, C.L.; Zhou, X.; et al. The efficacy and active compounds of Chaihuang Qingyi Huoxue granule to Ameliorate intestinal mucosal barrier injury in rats with severe acute pancreatitis by suppressing the HMGB1/TLR4/NF-κB signaling pathway. Int. Immunopharmacol. 2025, 144, 113632. [Google Scholar] [CrossRef]
- Tsung, A.; Sahai, R.; Tanaka, H.; Nakao, A.; Fink, M.P.; Lotze, M.T.; Yang, H.; Li, J.; Tracey, K.J.; Geller, D.A.; et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J. Exp. Med. 2005, 201, 1135–1143. [Google Scholar] [CrossRef]
- Albaqami, F.F.; Abdel-Rahman, R.F.; Althurwi, H.N.; Alharthy, K.M.; Soliman, G.A.; Aljarba, T.M.; Ogaly, H.A.; Abdel-Kader, M.S. Targeting inflammation and oxidative stress for protection against ischemic brain injury in rats using cupressuflavone. Saudi Pharm. J. 2024, 32, 101933. [Google Scholar] [CrossRef]
- Zhu, J. T helper cell differentiation, heterogeneity, and plasticity. Cold Spring Harb. Perspect. Biol. 2018, 10, a030338. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, X.; Guo, S. From pancreas to lungs: The role of immune cells in severe acute pancreatitis and acute lung injury. Immun. Inflamm. Dis. 2024, 12, e1351. [Google Scholar] [CrossRef]
- Li, W.; Jiang, H.; Bai, C.; Yu, S.; Pan, Y.; Wang, C.; Li, H.; Li, M.; Sheng, Y.; Chu, F.; et al. Ac2-26 attenuates hepatic ischemia-reperfusion injury in mice via regulating IL-22/IL-22R1/STAT3 signaling. PeerJ 2022, 10, e14086. [Google Scholar] [CrossRef]
- Feng, D.; Park, O.; Radaeva, S.; Wang, H.; Yin, S.; Kong, X.; Zheng, M.; Zakhari, S.; Kolls, J.K.; Gao, B. Interleukin-22 ameliorates cerulein-induced pancreatitis in mice by inhibiting the autophagic pathway. Int. J. Biol. Sci. 2012, 8, 249–257. [Google Scholar] [CrossRef]
- Stojanovic, B.; Jovanovic, I.P.; Stojanovic, M.D.; Jovanovic, M.; Vekic, B.; Milosevic, B.; Cvetkovic, A.; Spasic, M.; Stojanovic, B.S. The emerging roles of the adaptive immune response in acute pancreatitis. Cells 2023, 12, 1495. [Google Scholar] [CrossRef]
- Huai, J.P.; Sun, X.C.; Chen, M.J.; Jin, Y.; Ye, X.; Wu, J.S.; Huang, Z.M. Melatonin attenuates acute pancreatitis-associated lung injury in rats by modulating interleukin 22. World J. Gastroenterol. 2012, 18, 5122–5128. [Google Scholar] [CrossRef]
- Pociask, D.A.; Scheller, E.V.; Mandalapu, S.; McHugh, K.J.; Enelow, R.I.; Fattman, C.L.; Kolls, J.K.; Alcorn, J.F. IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 2013, 182, 1286–1296. [Google Scholar] [CrossRef]
- Qiao, Y.Y.; Liu, X.Q.; Xu, C.Q.; Zhang, Z.; Xu, H.W. Interleukin-22 ameliorates acute severe pancreatitis-associated lung injury in mice. World J. Gastroenterol. 2016, 22, 5023. [Google Scholar] [CrossRef]
- Pickert, G.; Neufert, C.; Leppkes, M.; Zheng, Y.; Wittkopf, N.; Warntjen, M.; Lehr, H.A.; Hirth, S.; Weigmann, B.; Wirtz, S.; et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 2009, 206, 1465–1472. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Saeedan, A.S.; Rehman, N.U.; Faqihi, H.M.; Soliman, G.A. New flavonoids with multiple bronchodilator activity pathways from Tephrosia purpurea L. (Pers.) growing in Saudi Arabia. Saudi Pharm. J. 2024, 32, 101992. [Google Scholar] [CrossRef]
- Hussain, T.; Fareed, S.; Siddiqui, H.H.; Vijaykumar, M.; Rao, C.V. Acute and subacute oral toxicity evaluation of Tephrosia purpurea extract in rodents. Asian Pac. J. Trop. Dis. 2012, 2, 129–132. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Churchill Livingstone: London, UK, 2008. [Google Scholar]

| Group | Amylase (ng/mL) | Lipase (pg/mL) | TAP (ng/mL) |
|---|---|---|---|
| Sham | 5.0 b ± 0.46 | 32.4 b ± 1.57 | 0.4 b ± 0.04 |
| IR control | 21.9 a ± 1.11 | 144.4 a ± 6.23 | 3.1 a ± 0.09 |
| TEP-200 | 14.5 ab ± 0.66 | 91.4 ab ± 4.64 | 2.0 ab ± 0.05 |
| TEP-400 | 7.6 b ± 0.44 | 45.5 b ± 1.85 | 1.0 ab ± 0.07 |
| PS-20 | 14.1 ab ± 0.55 | 90.4 ab ± 4.15 | 2.2 ab ± 0.10 |
| PS-40 | 7.3 b ± 0.40 | 48.1 b ± 1.87 | 1.4 ab ± 0.06 |
| Group | Pancreatic TNF-α (pg/mg Protein) | Pancreatic IL-1β (pg/mg Protein) | Pancreatic NF-κB (ng/mg Protein) |
|---|---|---|---|
| Sham | 30.3 b ± 1.00 | 40.5 b ± 1.56 | 29.6 b ± 1.18 |
| IR control | 324.5 a ± 5.37 | 212.9 a ± 4.71 | 287.7 a ± 15.34 |
| TEP-200 | 194.6 ab ± 2.47 | 163.3 ab ± 3.98 | 156.1 ab ± 9.85 |
| TEP-400 | 72.2 ab ± 6.50 | 56.5 b ± 4.83 | 72.9 ab ± 2.45 |
| PS-20 | 248.6 ab ± 11.03 | 159.5 ab ± 8.22 | 155.6 ab ± 3.44 |
| PS-40 | 100.5 ab ± 7.22 | 59.2 b ± 3.60 | 73.6 ab ± 3.84 |
| Group | Lung TNF-α (pg/mg Protein) | Lung IL-1β (pg/mg Protein) | Lung NF-κB (ng/mg Protein) |
|---|---|---|---|
| Sham | 33.5 b ± 2.77 | 31.5 b ± 1.03 | 18.4 b ± 0.95 |
| IR control | 177.3 a ± 5.48 | 205.6 a ± 3.88 | 90.6 a ± 3.47 |
| TEP-200 | 72.1 ab ± 4.39 | 136.6 ab ± 3.43 | 50.9 ab ± 2.18 |
| TEP-400 | 31.2 b ± 1.28 | 51.7 ab ± 4.11 | 33.1 ab ± 2.77 |
| PS-20 | 96.0 ab ± 4.98 | 148.6 ab ± 3.73 | 62.0 ab ± 2.12 |
| PS-40 | 70.3 ab ± 2.67 | 53.8 ab ± 3.69 | 34.3 ab ± 1.71 |
| Group | Pancreatic MDA (nmol/mg Protein) | Pancreatic GPx (nmol/mg Protein) | Pancreatic MPO (ng/mg Protein) |
|---|---|---|---|
| Sham | 0.3 b ± 0.01 | 3.0 b ± 0.23 | 0.9 b ± 0.09 |
| IR control | 1.8 a ± 0.03 | 0.7 a ± 0.03 | 6.4 a ± 0.31 |
| TEP-200 | 1.1 ab ± 0.05 | 1.9 ab ± 0.12 | 4.9 ab ± 0.18 |
| TEP-400 | 0.7 ab ± 0.03 | 3.0 b ± 0.09 | 2.9 ab ± 0.08 |
| PS-20 | 1.3 ab ± 0.05 | 1.9 ab ± 0.09 | 5.2 ab ± 0.27 |
| PS-40 | 0.8 ab ± 0.03 | 2.6 b ± 0.11 | 3.0 ab ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, G.A.; Alamri, M.A.; Abdel-Rahman, R.F.; Elbaset, M.A.; Ogaly, H.A.; Abdel-Kader, M.S. Tephrosia purpurea, with (-)-Pseudosemiglabrin as the Major Constituent, Alleviates Severe Acute Pancreatitis-Mediated Acute Lung Injury by Modulating HMGB1 and IL-22. Int. J. Mol. Sci. 2025, 26, 2572. https://doi.org/10.3390/ijms26062572
Soliman GA, Alamri MA, Abdel-Rahman RF, Elbaset MA, Ogaly HA, Abdel-Kader MS. Tephrosia purpurea, with (-)-Pseudosemiglabrin as the Major Constituent, Alleviates Severe Acute Pancreatitis-Mediated Acute Lung Injury by Modulating HMGB1 and IL-22. International Journal of Molecular Sciences. 2025; 26(6):2572. https://doi.org/10.3390/ijms26062572
Chicago/Turabian StyleSoliman, Gamal A., Mohammed A. Alamri, Rehab F. Abdel-Rahman, Marawan A. Elbaset, Hanan A. Ogaly, and Maged S. Abdel-Kader. 2025. "Tephrosia purpurea, with (-)-Pseudosemiglabrin as the Major Constituent, Alleviates Severe Acute Pancreatitis-Mediated Acute Lung Injury by Modulating HMGB1 and IL-22" International Journal of Molecular Sciences 26, no. 6: 2572. https://doi.org/10.3390/ijms26062572
APA StyleSoliman, G. A., Alamri, M. A., Abdel-Rahman, R. F., Elbaset, M. A., Ogaly, H. A., & Abdel-Kader, M. S. (2025). Tephrosia purpurea, with (-)-Pseudosemiglabrin as the Major Constituent, Alleviates Severe Acute Pancreatitis-Mediated Acute Lung Injury by Modulating HMGB1 and IL-22. International Journal of Molecular Sciences, 26(6), 2572. https://doi.org/10.3390/ijms26062572









