Synergistic Activity of Second Mitochondrial-Derived Activator of Caspases Mimetic with Toll-like Receptor 8 Agonist Reverses HIV-1-Latency and Enhances Antiviral Immunity
Abstract
1. Introduction
2. Results
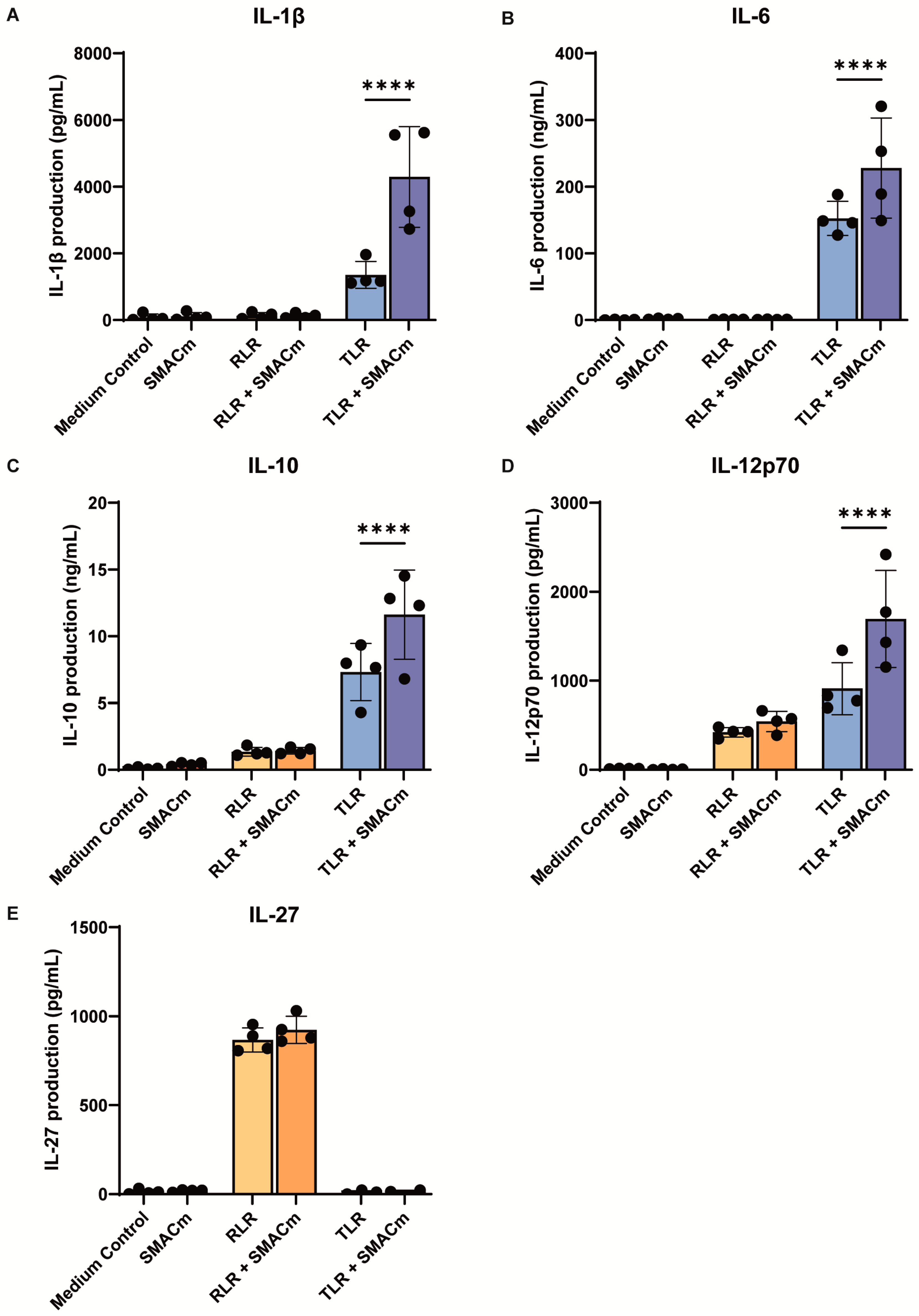
2.1. SMAC Mimetic Enhances TLR8-Induced Immune Responses
2.2. SMACm Enhances TLR8-Induced Cytotoxic T Cell Activity
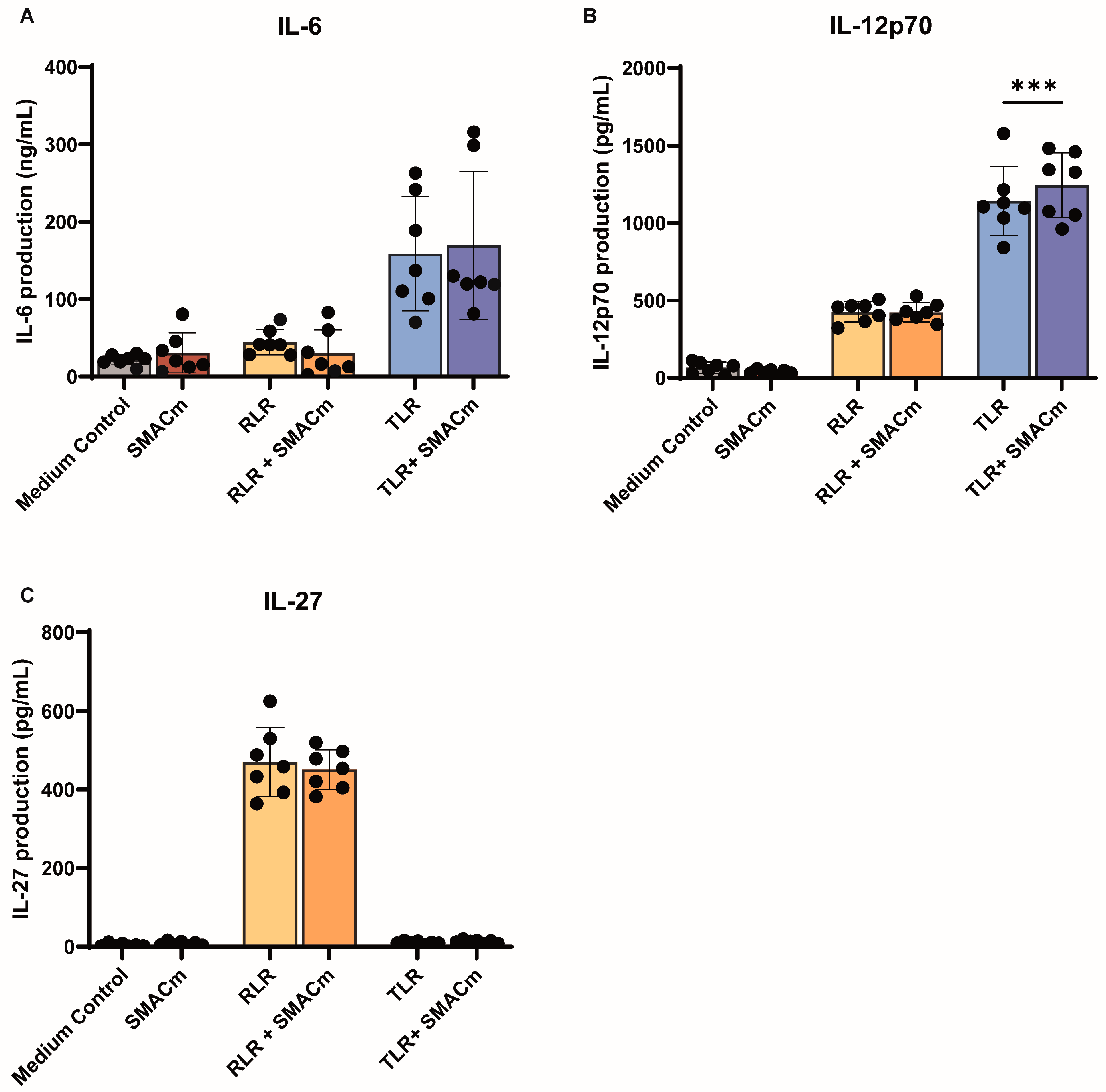
2.3. SMAC Mimetic Modulates TLR8 Mediated Immune Activation in PWH
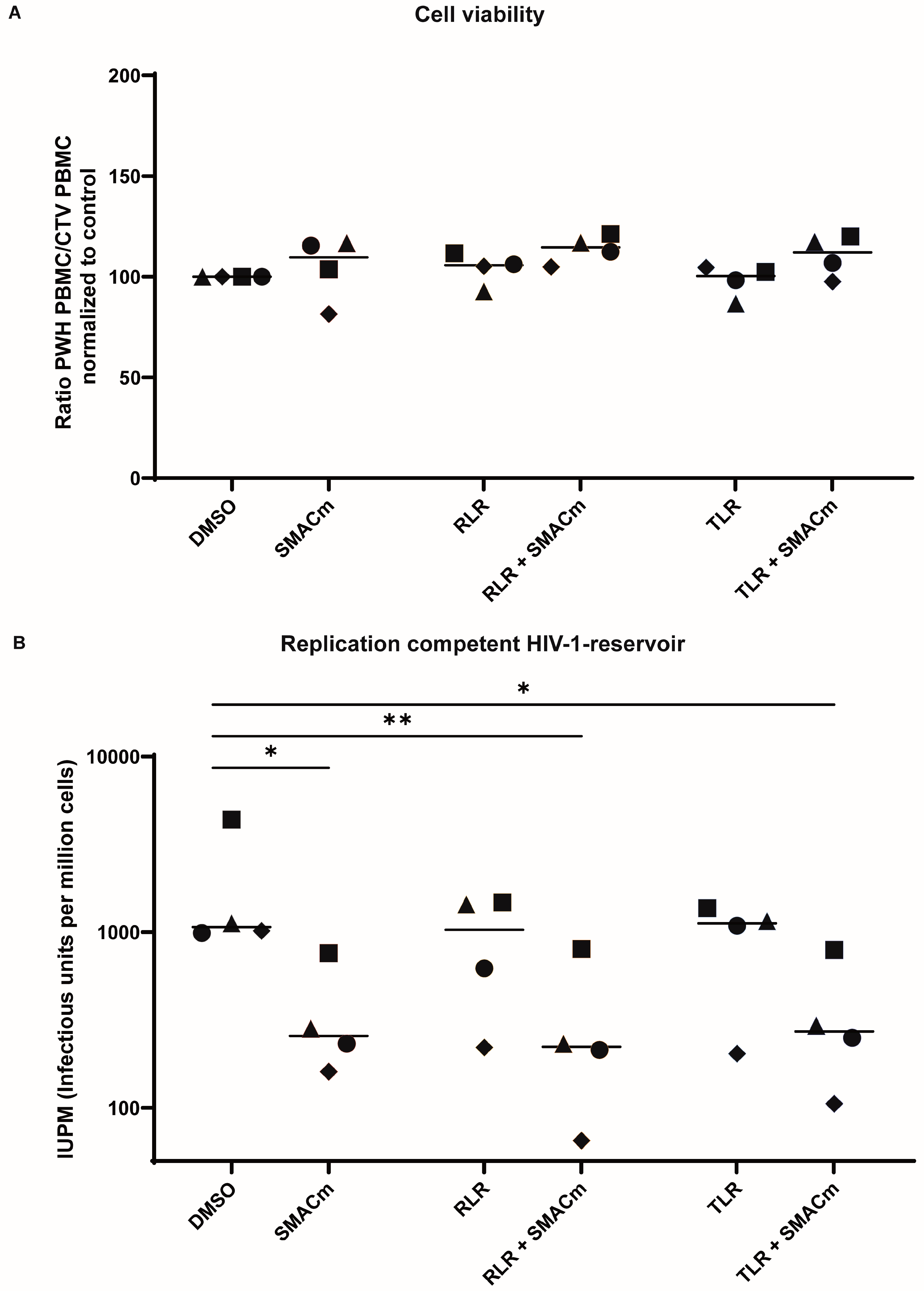
2.4. TLR8 and RLR Agonists Do Not Alter SMACm-Induced Reservoir Reduction
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Compounds
4.3. Cytokine Secretion
4.4. Inducible HIV-1 Reservoir Reduction Assay (HIVRRA)
4.5. Flowcytometry
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HIV-1 | Human Immunodeficiency Virus 1 |
| SMACm | Second mitochondrial-derived activator of caspases mimetics |
| LRA | Latency reversal agent |
| TLR8 | Toll-like receptor 8 |
| RLR | Rig-I-like receptor |
| PBMC | Peripheral blood mononuclear cells |
| ART | Antiretroviral therapy |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| PRR | Pattern recognition receptor |
| PAMP | Pathogen-associated molecular patterns |
| RIG-I | Retinoic acid-inducible gene I |
| MDA5 | Melanoma differentiation-associated protein 5 |
| CTL | Cytotoxic T lymphocytes |
| MAVS | Mitochondrial antiviral-signaling protein |
| IRF | Interferon regulatory factor |
| PWH | People living with HIV |
| ACS | The Amsterdam Cohort Studies |
| LTR | Long terminal repeat |
| FCS | Fetal calf serum |
| IFNγ | Interferon gamma |
| RLU | Relative light units |
| CTV | Cell trace violet |
| HIVRRA | Inducible HIV-1 reservoir reduction assay |
| DMSO | Dimethylsulfoxide |
| IUPM | Infectious units per million cells |
| NK | Natural killer cells |
| TNF-α | Tumor necrosis factor |
| LPS | Lipopolysaccharides |
| AIDS | Acquired immunodeficiency syndrome |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| RPMI | Roswell Park Memorial Institute |
References
- Chun, T.W.; Stuyver, L.; Mizell, S.B.; Ehler, L.A.; Mican, J.A.; Baseler, M.; Lloyd, A.L.; Nowak, M.A.; Fauci, A.S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 13193–13197. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Kajdas, J.; Finzi, D.; Quinn, T.C.; Chadwick, K.; Margolick, J.B.; Kovacs, C.; Gange, S.J.; Siliciano, R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003, 9, 727–728. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G. Shock and kill. Nature 2012, 487, 439–440. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Zerbato, J.M.; Purves, H.V.; Lewin, S.R.; Rasmussen, T.A. Between a shock and a hard place: Challenges and developments in HIV latency reversal. Curr. Opin. Virol. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Landovitz, R.J.; Scott, H.; Deeks, S.G. Prevention, treatment and cure of HIV infection. Nat. Rev. Microbiol. 2023, 21, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Molyer, B.; Kumar, A.; Angel, J.B. SMAC Mimetics as Therapeutic Agents in HIV Infection. Front. Immunol. 2021, 12, 780400. [Google Scholar] [CrossRef]
- Jansen, J.; Kroeze, S.; Man, S.; Andreini, M.; Bakker, J.W.; Zamperini, C.; Tarditi, A.; Kootstra, N.A.; Geijtenbeek, T.B. Noncanonical-NF-κB activation and DDX3 inhibition reduces the HIV-1 reservoir by elimination of latently infected cells ex-vivo. Microbiol. Spectr. 2024, 12, e03180-23. [Google Scholar] [CrossRef]
- Pache, L.; Dutra, M.S.; Spivak, A.M.; Marlett, J.M.; Murry, J.P.; Hwang, Y.; Maestre, A.M.; Manganaro, L.; Vamos, M.; Teriete, P.; et al. BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host Microbe 2015, 18, 345–353. [Google Scholar] [CrossRef]
- Pache, L.; Marsden, M.D.; Teriete, P.; Portillo, A.J.; Heimann, D.; Kim, J.T.; Soliman, M.S.; Dimapasoc, M.; Carmona, C.; Celeridad, M.; et al. Pharmacological Activation of Non-canonical NF-κB Signaling Activates Latent HIV-1 Reservoirs In Vivo. Cell Rep. Med. 2020, 1, 100037. [Google Scholar]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.A.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Pham, T.N.; Begum, S.; Romero-Medina, M.C.; Bellini, N.; Li, Y.; Dallaire, F.; Béland, K.; Patey, N.; Guimond, J.V.; et al. Bivalent SMAC mimetic APG-1387 reduces HIV reservoirs and limits viral rebound in humanized mice. iScience 2024, 27, 111470. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappaB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Gorden, K.B.; Gorski, K.S.; Gibson, S.J.; Kedl, R.M.; Kieper, W.C.; Qiu, X.; Tomai, M.A.; Alkan, S.S.; Vasilakos, J.P. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 2005, 174, 1259–1268. [Google Scholar] [CrossRef]
- Martínez-Espinoza, I.; Guerrero-Plata, A. The Relevance of TLR8 in Viral Infections. Pathogens 2022, 11, 134. [Google Scholar] [CrossRef]
- Saruta, M.; Michelsen, K.S.; Thomas, L.S.; Yu, Q.T.; Landers, C.J.; Targan, S.R. TLR8-mediated activation of human monocytes inhibits TL1A expression. Eur. J. Immunol. 2009, 39, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Onomoto, K.; Jogi, M.; Akaboshi, T.; Fujita, T. Viral RNA detection by RIG-I-like receptors. Curr. Opin. Immunol. 2015, 32, 48–53. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell. Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef]
- Kanzler, H.; Barrat, F.J.; Hessel, E.M.; Coffman, R.L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 2007, 13, 552–559. [Google Scholar] [CrossRef]
- Bai, L.; Smith, D.C.; Wang, S. Small-molecule SMAC mimetics as new cancer therapeutics. Pharmacol. Ther. 2014, 144, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Onomoto, K.; Onoguchi, K.; Yoneyama, M. Regulation of RIG-I-like receptor-mediated signaling: Interaction between host and viral factors. Cell Mol. Immunol. 2021, 18, 539–555. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Desnues, B.; Demaria, O. Toll-like receptor 8: The awkward TLR. Med. Sci. 2012, 28, 96–102. [Google Scholar]
- Henry, C.J.; Ornelles, D.A.; Mitchell, L.M.; Brzoza-Lewis, K.L.; Hiltbold, E.M. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. 2008, 181, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- de Groot, R.; van Beelen, A.J.; Bakdash, G.; Taanman-Kueter, E.W.; de Jong, E.C.; Kapsenberg, M.L. Viral dsRNA-activated human dendritic cells produce IL-27, which selectively promotes cytotoxicity in naive CD8+ T cells. J. Leukoc. Biol. 2012, 92, 605–610. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; Kaptein, T.M.; Wevers, B.A.; van der Vlist, M.; Klaver, E.J.; van Die, I.; Vriend, L.E.M.; de Jong, M.A.W.P.; Geijtenbeek, T.B.H. Fucose-based PAMPs prime dendritic cells for follicular T helper cell polarization via DC-SIGN-dependent IL-27 production. Nat. Commun. 2014, 5, 5074. [Google Scholar] [CrossRef]
- Gringhuis, S.I.; Den Dunnen, J.; Litjens, M.; Van Der Vlist, M.; Wevers, B.; Bruijns, S.C.; Geijtenbeek, T.B. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat. Immunol. 2009, 10, 203–213. [Google Scholar] [CrossRef]
- Jacque, E.; Tchenio, T.; Piton, G.; Romeo, P.-H.; Baud, V. RelA repression of RelB activity induces selective gene activation downstream of TNF receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 14635–14640. [Google Scholar] [CrossRef]
- Tau, G.; Rothman, P. Biologic functions of the IFN-gamma receptors. Allergy 1999, 54, 1233–1251. [Google Scholar] [CrossRef] [PubMed]
- Kieper, W.C.; Prlic, M.; Schmidt, C.S.; Mescher, M.F.; Jameson, S.C. IL-12 Enhances CD8 T Cell Homeostatic Expansion. J. Immunol. 2001, 166, 5515–5521. [Google Scholar] [CrossRef]
- Starbeck-Miller, G.R.; Harty, J.T. The Role of Il-12 and Type I Interferon in Governing the Magnitude of CD8 T Cell Responses. In Crossroads Between Innate and Adaptive Immunity V; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Van Den Eeckhout, B.; Tavernier, J.; Gerlo, S. Interleukin-1 as Innate Mediator of T Cell Immunity. Front. Immunol. 2020, 11, 621931. [Google Scholar] [CrossRef] [PubMed]
- Querec, T.; Bennouna, S.; Alkan, S.; Laouar, Y.; Gorden, K.; Flavell, R.; Akira, S.; Ahmed, R.; Pulendran, B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 2006, 203, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Patankar, V.; Kitchen, S.; Zhen, A. Examining Chronic Inflammation, Immune Metabolism, and T Cell Dysfunction in HIV Infection. Viruses 2024, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Price, P.; Keane, N.; Murray, R.; French, M. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002, 3, 21–27. [Google Scholar] [CrossRef]
- Neuhaus, J.; Jacobs, D.R., Jr.; Baker, J.V.; Calmy, A.; Duprez, D.; La Rosa, A.; Kuller, L.H.; Pett, S.L.; Ristola, M.; Ross, M.J.; et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J. Infect. Dis. 2010, 201, 1788–1795. [Google Scholar] [CrossRef]
- Burdo, T.H.; Lentz, M.R.; Autissier, P.; Krishnan, A.; Halpern, E.; Letendre, S.; Rosenberg, E.S.; Ellis, R.J.; Williams, K.C. Soluble CD163 made by monocyte/macrophages is a novel marker of HIV activity in early and chronic infection prior to and after anti-retroviral therapy. J. Infect. Dis. 2011, 204, 154–163. [Google Scholar] [CrossRef]
- Taiwo, B.; Barcena, L.; Tressler, R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr. HIV/AIDS Rep. 2013, 10, 21–32. [Google Scholar] [CrossRef]
- Paiardini, M.; Muller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef]
- Wilson, E.M.P.; Singh, A.; Hullsiek, K.H.; Gibson, D.; Henry, W.K.; Lichtenstein, K.; Önen, N.F.; Kojic, E.; Patel, P.; Brooks, J.T.; et al. Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J. Infect. Dis. 2014, 210, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.I.; Jacobson, L.P.; Margolick, J.B.; Breen, E.C.; Macatangay, B.; Penugonda, S.; Martínez-Maza, O.; Bream, J.H. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015, 29, 463–471. [Google Scholar] [CrossRef]
- Novelli, S.; Lécuroux, C.; Goujard, C.; Reynes, J.; Villemant, A.; Blum, L.; Essat, A.; Avettand-Fenoël, V.; Launay, O.; Molina, J.-M.; et al. Persistence of monocyte activation under treatment in people followed since acute HIV-1 infection relative to participants at high or low risk of HIV infection. EBioMedicine 2020, 62, 103129. [Google Scholar] [CrossRef]
- Corley, M.J.; Sacdalan, C.; Pang, A.P.S.; Chomchey, N.; Ratnaratorn, N.; Valcour, V.; Kroon, E.; Cho, K.S.; Belden, A.C.; Colby, D.; et al. Abrupt and altered cell-type specific DNA methylation profiles in blood during acute HIV infection persists despite prompt initiation of ART. PLOS Pathog. 2021, 17, e1009785. [Google Scholar] [CrossRef]
- Cai, C.W.; Sereti, I. Residual immune dysfunction under antiretroviral therapy. Semin. Immunol. 2021, 51, 101471. [Google Scholar] [CrossRef]
- van der Heijden, W.A.; Van de Wijer, L.; Keramati, F.; Trypsteen, W.; Rutsaert, S.; Ter Horst, R.; Jaeger, M.; Koenen, H.J.; Stunnenberg, H.G.; Joosten, I.; et al. Chronic HIV infection induces transcriptional and functional reprogramming of innate immune cells. JCI Insight 2021, 6, e145928. [Google Scholar] [CrossRef]
- Campbell, G.R.; Spector, S.A. Current strategies to induce selective killing of HIV-1-infected cells. J. Leukoc. Biol. 2022, 112, 1273–1284. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Schank, M.; El Gazzar, M.; Moorman, J.P.; Yao, Z.Q. HIV-1 Latency and Viral Reservoirs: Existing Reversal Approaches and Potential Technologies, Targets, and Pathways Involved in HIV Latency Studies. Cells 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.R.; Shinn, P.; Chen, H.; Berry, C.; Ecker, J.R.; Bushman, F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 2002, 110, 521–529. [Google Scholar] [CrossRef]
- Marini, B.; Kertesz-Farkas, A.; Ali, H.; Lucic, B.; Lisek, K.; Manganaro, L.; Pongor, S.; Luzzati, R.; Recchia, A.; Mavilio, F.; et al. Nuclear architecture dictates HIV-1 integration site selection. Nature 2015, 521, 227–231. [Google Scholar] [CrossRef]
- Cohn, L.B.; Silva, I.T.; Oliveira, T.Y.; Rosales, R.A.; Parrish, E.H.; Learn, G.H.; Hahn, B.H.; Czartoski, J.L.; McElrath, M.J.; Lehmann, C.; et al. HIV-1 integration landscape during latent and active infection. Cell 2015, 160, 420–432. [Google Scholar] [CrossRef]
- Symons, J.; Cameron, P.U.; Lewin, S.R. HIV integration sites and implications for maintenance of the reservoir. Curr. Opin. HIVAIDS 2018, 13, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Jütte, B.B.; Love, L.; Svensson, J.P. Molecular Mechanisms of HIV-1 Latency from a Chromatin and Epigenetic Perspective. Curr. Clin. Microbiol. Rep. 2023, 10, 246–254. [Google Scholar] [CrossRef]
- Hsieh, E.; Janssens, D.H.; Paddison, P.J.; Browne, E.P.; Henikoff, S.; OhAinle, M.; Emerman, M. A modular CRISPR screen identifies individual and combination pathways contributing to HIV-1 latency. PLoS Pathog. 2023, 19, e1011101. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.J.; Dunbar, P.R.; Brooks, A.E.; Zhang, F.; Chen, D.; Wallin, J.J.; van Buuren, N.; Arora, P.; Fletcher, S.P.; Tan, S.K.; et al. Safety and efficacy of the oral TLR8 agonist selgantolimod in individuals with chronic hepatitis B under viral suppression. J. Hepatol. 2023, 78, 513–523. [Google Scholar] [CrossRef]
- Morrish, E.; Brumatti, G.; Silke, J. Future Therapeutic Directions for Smac-Mimetics. Cells 2020, 9, 406. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. HIV cure: The daunting scale of the problem. Science 2024, 383, 703–705. [Google Scholar] [CrossRef]
- De Wolf, F.; Goudsmit, J.; Paul, D.A.; Lange, J.M.; Hooijkaas, C.; Schellekens, P.; Coutinho, R.A.; van der Noordaa, J. Risk of AIDS related complex and AIDS in homosexual men with persistent HIV antigenaemia. Br. Med. J. 1987, 295, 569–572. [Google Scholar] [CrossRef]
- Platt, E.J.; Wehrly, K.; Kuhmann, S.E.; Chesebro, B.; Kabat, D. Effects of CCR5 and CD4 Cell Surface Concentrations on Infections by Macrophagetropic Isolates of Human Immunodeficiency Virus Type 1. J. Virol. 1998, 72, 2855–2864. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Total participants | 7 |
| Gender: male | 100% |
| Age at time of sampling (years, range) | 38 (27–52) |
| CD4+T cell count (cells/mm3, range) | 521 (180–800) |
| Plasma viral load (copies/mL, range) | 21,012 (2650–34,000) |
| Condition | Median IUPM | Mean IUPM | Range |
|---|---|---|---|
| Medium control | 1071 | 1879 | 989–4383 |
| SMACm | 257 | 359 | 161–761 |
| TLR | 1089 | 955 | 204–1371 |
| TLR + SMACm | 272 | 361 | 106–793 |
| RLR | 1033 | 941 | 221–1480 |
| RLR + SMACm | 223 | 328 | 65–801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlaming, K.E.; Jansen, J.; de Bree, G.J.; Kootstra, N.A.; Geijtenbeek, T.B.H. Synergistic Activity of Second Mitochondrial-Derived Activator of Caspases Mimetic with Toll-like Receptor 8 Agonist Reverses HIV-1-Latency and Enhances Antiviral Immunity. Int. J. Mol. Sci. 2025, 26, 2575. https://doi.org/10.3390/ijms26062575
Vlaming KE, Jansen J, de Bree GJ, Kootstra NA, Geijtenbeek TBH. Synergistic Activity of Second Mitochondrial-Derived Activator of Caspases Mimetic with Toll-like Receptor 8 Agonist Reverses HIV-1-Latency and Enhances Antiviral Immunity. International Journal of Molecular Sciences. 2025; 26(6):2575. https://doi.org/10.3390/ijms26062575
Chicago/Turabian StyleVlaming, Killian E., Jade Jansen, Godelieve J. de Bree, Neeltje A. Kootstra, and Teunis B. H. Geijtenbeek. 2025. "Synergistic Activity of Second Mitochondrial-Derived Activator of Caspases Mimetic with Toll-like Receptor 8 Agonist Reverses HIV-1-Latency and Enhances Antiviral Immunity" International Journal of Molecular Sciences 26, no. 6: 2575. https://doi.org/10.3390/ijms26062575
APA StyleVlaming, K. E., Jansen, J., de Bree, G. J., Kootstra, N. A., & Geijtenbeek, T. B. H. (2025). Synergistic Activity of Second Mitochondrial-Derived Activator of Caspases Mimetic with Toll-like Receptor 8 Agonist Reverses HIV-1-Latency and Enhances Antiviral Immunity. International Journal of Molecular Sciences, 26(6), 2575. https://doi.org/10.3390/ijms26062575






