Exploring a Nitric Oxide-Releasing Celecoxib Derivative as a Potential Modulator of Bone Healing: Insights from Ex Vivo and In Vivo Imaging Experiments
Abstract
1. Introduction
2. Results
2.1. First In Vivo Application of NO-Coxib
2.2. Imaging of Inflammatory Activity by [18F]FDG PET/CT
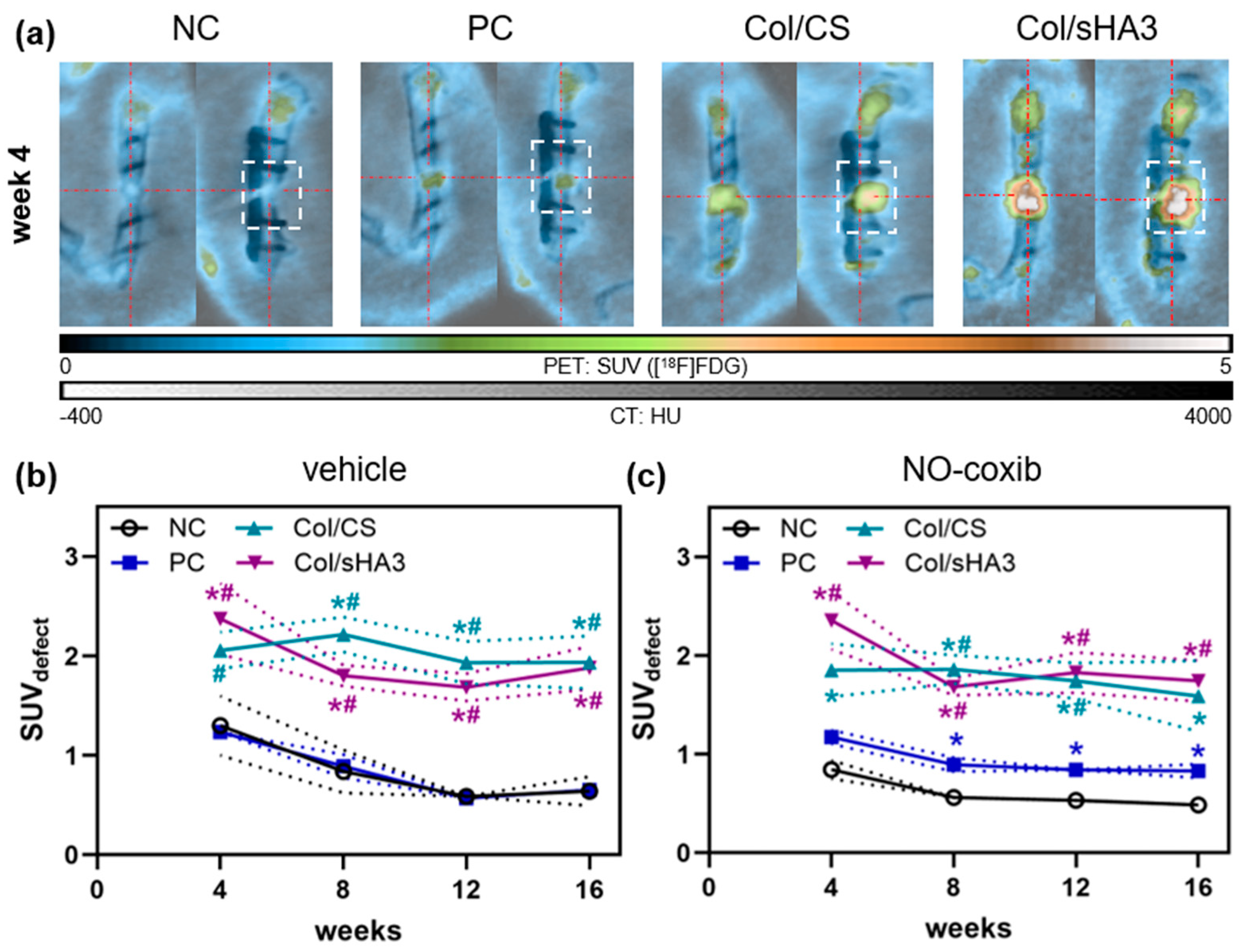
2.3. Imaging of Bone Mineralization by [18F]Fluoride PET/CT
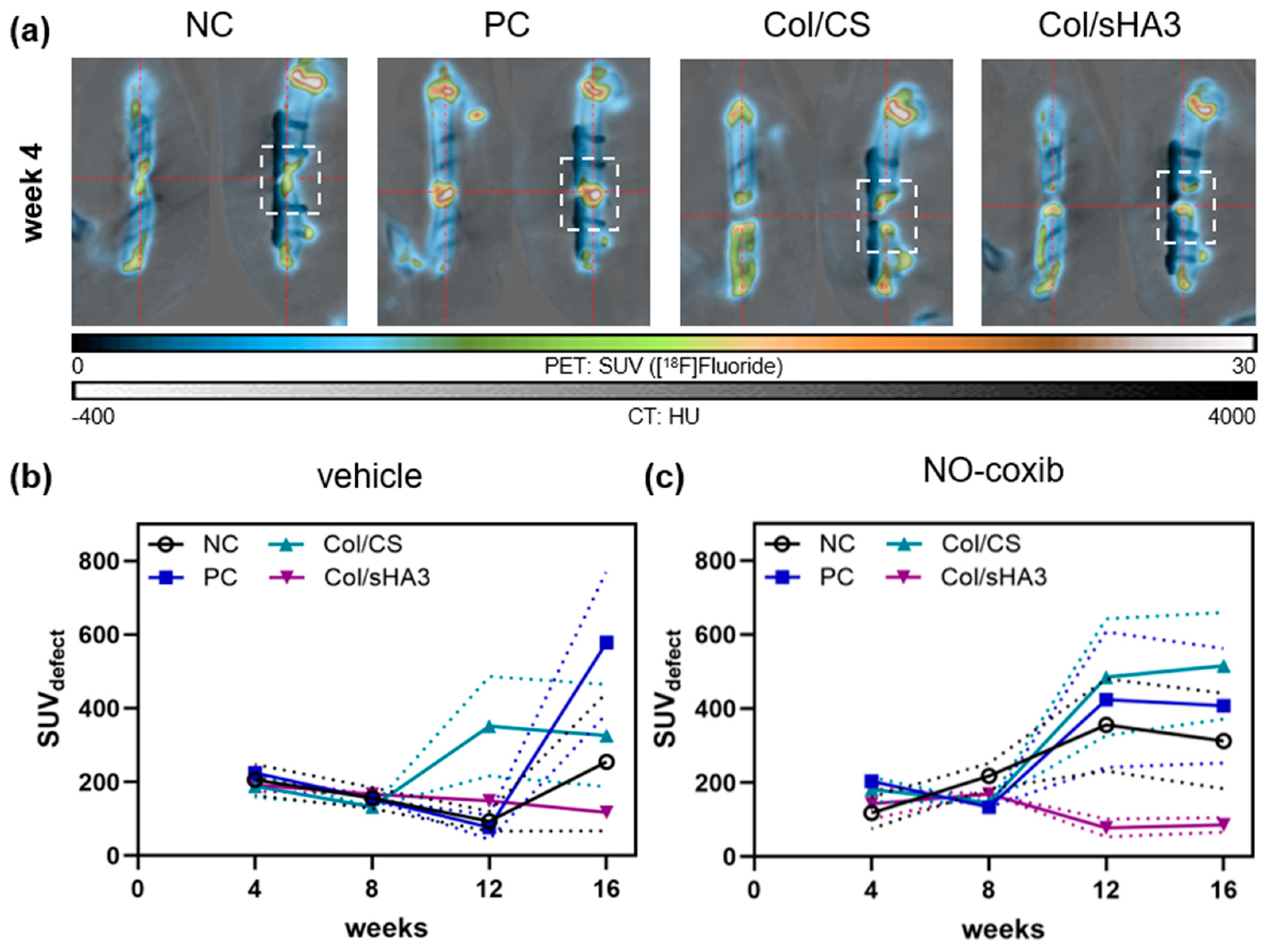
2.4. Quantitative Analysis of Bone Defect Closure by High-Resolution CT and µCT Imaging
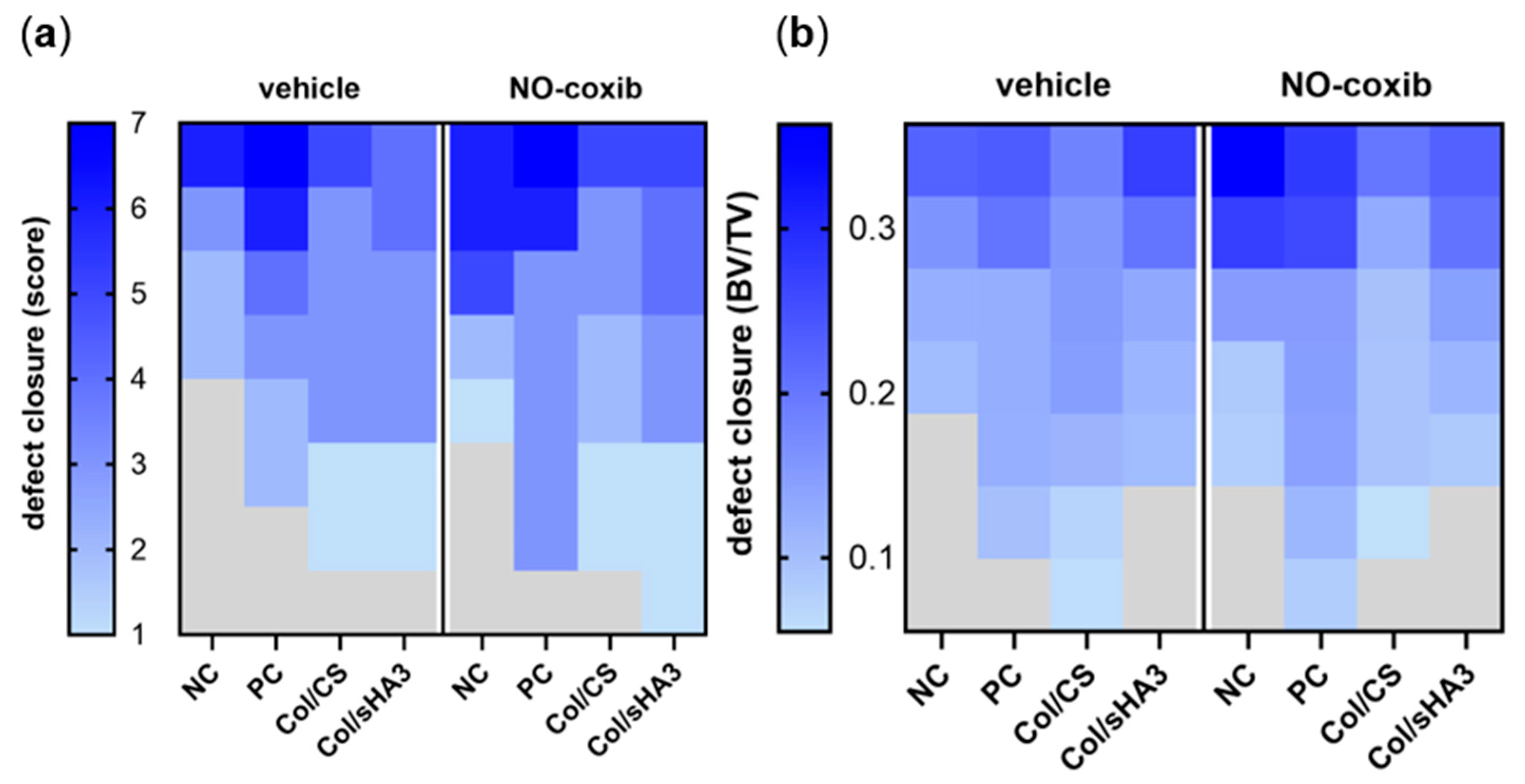
2.5. Qualitative Characterization of Bone Defect Closure by Histology
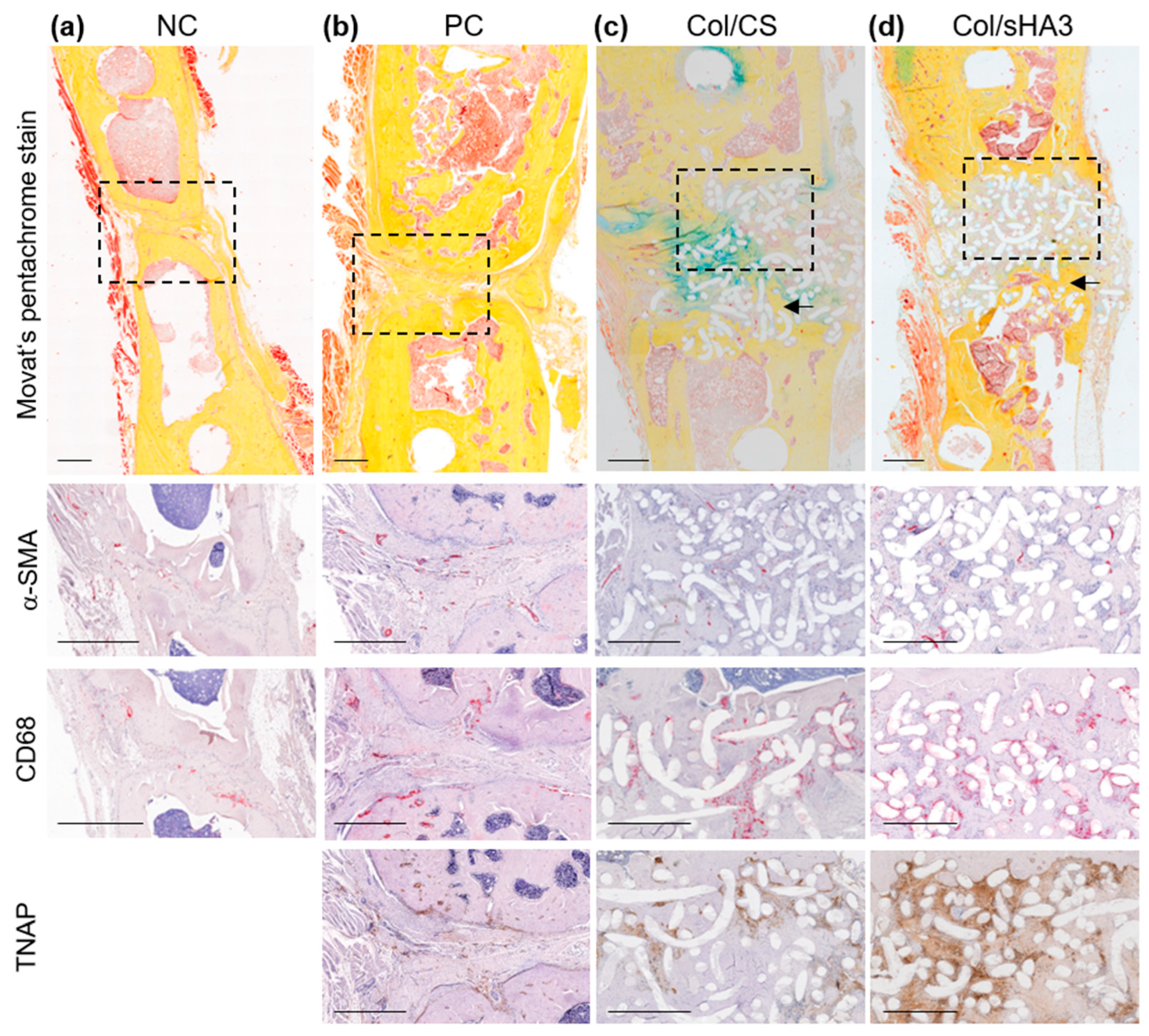
2.6. Qualitative Characterization of Bone Defect Closure by Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Synthesis of NO-Coxib
4.2. Rat Femur Defect Model and Adjuvant Therapy with NO-Coxib
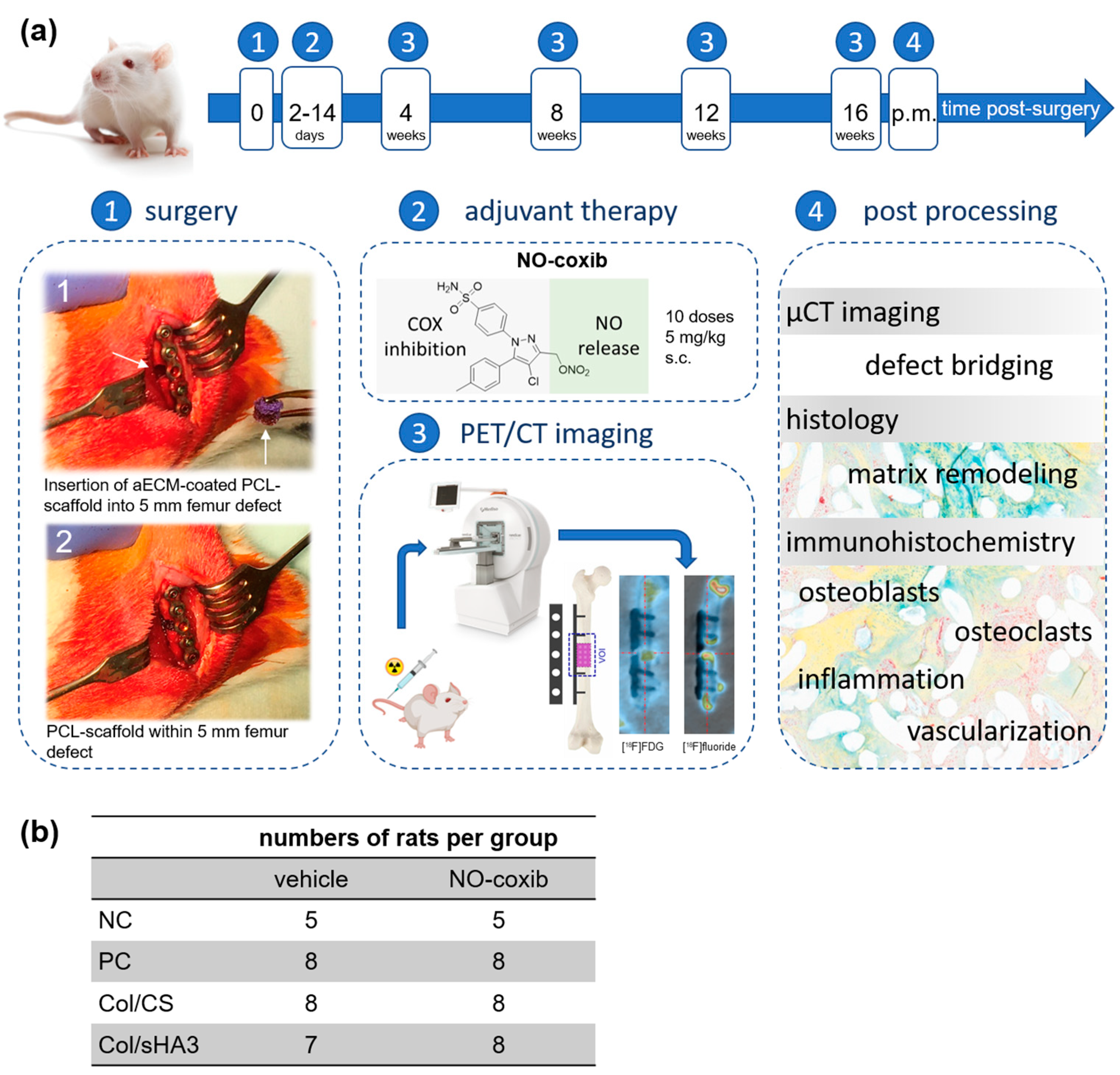
4.3. In Vivo PET/CT Imaging and Data Processing
4.4. High-Resolution CT and µCT Imaging
4.5. Histology and Immunohistochemistry
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, T.W.; Muschler, G.F. Bone graft materials. An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. S2), S96–S101. [Google Scholar] [CrossRef] [PubMed]
- Buttner, M.; Moller, S.; Keller, M.; Huster, D.; Schiller, J.; Schnabelrauch, M.; Dieter, P.; Hempel, U. Over-sulfated chondroitin sulfate derivatives induce osteogenic differentiation of hMSC independent of BMP-2 and TGF-beta1 signalling. J. Cell. Physiol. 2013, 228, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Bernhardt, R.; Hintze, V.; Moller, S.; Schnabelrauch, M.; Scharnweber, D.; Rammelt, S. Collagen/glycosaminoglycan coatings enhance new bone formation in a critical size bone defect—A pilot study in rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 84–92. [Google Scholar] [CrossRef]
- Neuber, C.; Schulze, S.; Förster, Y.; Hofheinz, F.; Wodke, J.; Moller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; Rammelt, S.; et al. Biomaterials in repairing rat femoral defects: In vivo insights from small animal positron emission tomography/computed tomography (PET/CT) studies. Clin. Hemorheol. Microcirc. 2019, 73, 177–194. [Google Scholar] [CrossRef]
- Rammelt, S.; Illert, T.; Bierbaum, S.; Scharnweber, D.; Zwipp, H.; Schneiders, W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials 2006, 27, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Förster, Y.; Rentsch, C.; Schneiders, W.; Bernhardt, R.; Simon, J.C.; Worch, H.; Rammelt, S. Surface modification of implants in long bone. Biomatter 2012, 2, 149–157. [Google Scholar] [CrossRef]
- Bierbaum, S.; Douglas, T.; Hanke, T.; Scharnweber, D.; Tippelt, S.; Monsees, T.K.; Funk, R.H.; Worch, H. Collageneous matrix coatings on titanium implants modified with decorin and chondroitin sulfate: Characterization and influence on osteoblastic cells. J. Biomed. Mater. Res. A 2006, 77, 551–562. [Google Scholar] [CrossRef]
- Rentsch, B.; Hofmann, A.; Breier, A.; Rentsch, C.; Scharnweber, D. Embroidered and surface modified polycaprolactone-co-lactide scaffolds as bone substitute: In vitro characterization. Ann. Biomed. Eng. 2009, 37, 2118–2128. [Google Scholar] [CrossRef]
- Hempel, U.; Matthaus, C.; Preissler, C.; Moller, S.; Hintze, V.; Dieter, P. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J. Cell. Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Hempel, U.; Preissler, C.; Vogel, S.; Moller, S.; Hintze, V.; Becher, J.; Schnabelrauch, M.; Rauner, M.; Hofbauer, L.C.; Dieter, P. Artificial extracellular matrices with oversulfated glycosaminoglycan derivatives promote the differentiation of osteoblast-precursor cells and premature osteoblasts. BioMed Res. Int. 2014, 2014, 938368. [Google Scholar] [CrossRef]
- Stadlinger, B.; Bierbaum, S.; Grimmer, S.; Schulz, M.C.; Kuhlisch, E.; Scharnweber, D.; Eckelt, U.; Mai, R. Increased bone formation around coated implants. J. Clin. Periodontol. 2009, 36, 698–704. [Google Scholar] [CrossRef]
- Rentsch, C.; Rentsch, B.; Breier, A.; Spekl, K.; Jung, R.; Manthey, S.; Scharnweber, D.; Zwipp, H.; Biewener, A. Long-bone critical-size defects treated with tissue-engineered polycaprolactone-co-lactide scaffolds: A pilot study on rats. J. Biomed. Mater. Res. A 2010, 95, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Schulze, S.; Neuber, C.; Moller, S.; Pietzsch, J.; Schaser, K.D.; Rammelt, S. Microdialysis Reveals Anti-Inflammatory Effects of Sulfated Glycosaminoglycanes in the Early Phase of Bone Healing. Int. J. Mol. Sci. 2023, 24, 2077. [Google Scholar] [CrossRef] [PubMed]
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Part I—Modulation of inflammation. Clin. Hemorheol. Microcirc. 2019, 73, 381–408. [Google Scholar] [CrossRef]
- Zhang, X.P.; Schwarz, E.M.; Young, D.A.; Puzas, J.E.; Rosier, R.N.; O’Keefe, R.J. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Investig. 2002, 110, 1211. [Google Scholar] [CrossRef]
- Lin, H.N.; O’Connor, J.P. Immunohistochemical localization of key arachidonic acid metabolism enzymes during fracture healing in mice. PLoS ONE 2014, 9, e88423. [Google Scholar] [CrossRef]
- Lu, L.Y.; Loi, F.; Nathan, K.; Lin, T.H.; Pajarinen, J.; Gibon, E.; Nabeshima, A.; Cordova, L.; Jamsen, E.; Yao, Z.; et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J. Orthop. Res. 2017, 35, 2378–2385. [Google Scholar] [CrossRef]
- Anastasio, A.T.; Paniagua, A.; Diamond, C.; Ferlauto, H.R.; Fernandez-Moure, J.S. Nanomaterial Nitric Oxide Delivery in Traumatic Orthopedic Regenerative Medicine. Front. Bioeng. Biotechnol. 2020, 8, 592008. [Google Scholar] [CrossRef]
- Rothe, R.; Schulze, S.; Neuber, C.; Hauser, S.; Rammelt, S.; Pietzsch, J. Adjuvant drug-assisted bone healing: Part II—Modulation of angiogenesis. Clin. Hemorheol. Microcirc. 2019, 73, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, R.E.; Shoghi, K.I.; Silva, M.J. Nitric oxide-mediated vasodilation increases blood flow during the early stages of stress fracture healing. J. Appl. Physiol. 2014, 116, 416–424. [Google Scholar] [CrossRef]
- Ding, Z.C.; Lin, Y.K.; Gan, Y.K.; Tang, T.T. Molecular pathogenesis of fracture nonunion. J. Orthop. Transl. 2018, 14, 45–56. [Google Scholar] [CrossRef]
- Corbett, S.A.; McCarthy, I.D.; Batten, J.; Hukkanen, M.; Polak, J.M.; Hughes, S.P. Nitric oxide mediated vasoreactivity during fracture repair. Clin. Orthop. Relat. Res. 1999, 365, 247–253. [Google Scholar] [CrossRef]
- Meesters, D.M.; Neubert, S.; Wijnands, K.A.P.; Heyer, F.L.; Zeiter, S.; Ito, K.; Brink, P.R.G.; Poeze, M. Deficiency of inducible and endothelial nitric oxide synthase results in diminished bone formation and delayed union and nonunion development. Bone 2016, 83, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Corbett, S.A.; Hukkanen, M.; Batten, J.; McCarthy, I.D.; Polak, J.M.; Hughes, S.P. Nitric oxide in fracture repair. Differential localisation, expression and activity of nitric oxide synthases. J. Bone Jt. Surg. Br. 1999, 81, 531–537. [Google Scholar] [CrossRef]
- Brandt, F.; Ullrich, M.; Seifert, V.; Haase-Kohn, C.; Richter, S.; Kniess, T.; Pietzsch, J.; Laube, M. Exploring Nitric Oxide (NO)-Releasing Celecoxib Derivatives as Modulators of Radioresponse in Pheochromocytoma Cells. Molecules 2022, 27, 6587. [Google Scholar] [CrossRef]
- Bechmann, N.; Kniess, T.; Kockerling, M.; Pigorsch, A.; Steinbach, J.; Pietzsch, J. Novel (pyrazolyl)benzenesulfonamides with a nitric oxide-releasing moiety as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 3295–3300. [Google Scholar] [CrossRef]
- Phelps, M.E. PET: A biological imaging technique. Neurochem. Res. 1991, 16, 929–940. [Google Scholar] [CrossRef]
- van den Hoff, J. Principles of quantitative positron emission tomography. Amino Acids 2005, 29, 341–353. [Google Scholar] [CrossRef]
- Rogasch, J.M.M.; Hofheinz, F.; van Heek, L.; Voltin, C.A.; Boellaard, R.; Kobe, C. Influences on PET Quantification and Interpretation. Diagnostics 2022, 12, 451. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. On the metabolism of carcinoma cells. Biochem. Z. 1924, 152, 309–344. [Google Scholar] [CrossRef]
- Sambuceti, G.; Cossu, V.; Bauckneht, M.; Morbelli, S.; Orengo, A.; Carta, S.; Ravera, S.; Bruno, S.; Marini, C. 18F-fluoro-2-deoxy-d-glucose (FDG) uptake. What are we looking at? Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Li, F.; Niu, G.; Chen, X. PET imaging of inflammation biomarkers. Theranostics 2013, 3, 448–466. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Choi, Y.; Huang, S.C.; Hoh, C.K.; Dahlbom, M.; Schiepers, C.; Satyamurthy, N.; Barrio, J.R.; Phelps, M.E. Evaluation of the Skeletal Kinetics of Fluorine-18-Fluoride Ion with PET. J. Nucl. Med. 1992, 33, 633–642. [Google Scholar] [PubMed]
- Mathavan, N.; Koopman, J.; Raina, D.B.; Turkiewicz, A.; Tagil, M.; Isaksson, H. 18F-fluoride as a prognostic indicator of bone regeneration. Acta Biomater. 2019, 90, 403–411. [Google Scholar] [CrossRef]
- Rentsch, C.; Schneiders, W.; Manthey, S.; Rentsch, B.; Rammelt, S. Comprehensive histological evaluation of bone implants. Biomatter 2014, 4, e27993. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Seidenberg, A.B.; An, Y.H. Is there an inhibitory effect of COX-2 inhibitors on bone healing? Pharmacol. Res. 2004, 50, 151–156. [Google Scholar] [CrossRef]
- Saura, M.; Tarin, C.; Zaragoza, C. Recent insights into the implication of nitric oxide in osteoblast differentiation and proliferation during bone development. Sci. World J. 2010, 10, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Baldik, Y.; Talu, U.; Altinel, L.; Bilge, H.; Demiryont, M.; Aykac-Toker, G. Bone healing regulated by nitric oxide: An experimental study in rats. Clin. Orthop. Relat. Res. 2002, 404, 343–352. [Google Scholar] [CrossRef]
- Aisa, M.C.; Datti, A.; Orlacchio, A.; Di Renzo, G.C. COX inhibitors and bone: A safer impact on osteoblasts by NO-releasing NSAIDs. Life Sci. 2018, 208, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.A.; Mirsky, N.A.; Silva, B.L.G.; Shinde, A.R.; Arakelians, A.R.L.; Nayak, V.V.; Marcantonio, R.A.C.; Gupta, N.; Witek, L.; Coelho, P.G. Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions. J. Funct. Biomater. 2024, 15, 280. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Gómez-Cerezo, N.; Alneairi, A.; Luqman, M.; Pant, H.R.; Ivanovski, S. A review of protein adsorption and bioactivity characteristics of poly ε-caprolactone scaffolds in regenerative medicine. Eur. Polym. J. 2022, 162, 110892. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Yamaguchi, Y. Proteoglycans as modulators of growth factor activities. Cell 1991, 64, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Ruoslahti, E. Proteoglycans in cell regulation. J. Biol. Chem. 1989, 264, 13369–13372. [Google Scholar] [CrossRef]
- Brighton, C.T.; Albelda, S.M. Identification of integrin cell-substratum adhesion receptors on cultured rat bone cells. J. Orthop. Res. 1992, 10, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Salasznyk, R.M.; Williams, W.A.; Boskey, A.; Batorsky, A.; Plopper, G.E. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. BioMed Res. Int. 2004, 2004, 24–34. [Google Scholar] [CrossRef]
- Salbach, J.; Kliemt, S.; Rauner, M.; Rachner, T.D.; Goettsch, C.; Kalkhof, S.; von Bergen, M.; Moller, S.; Schnabelrauch, M.; Hintze, V.; et al. The effect of the degree of sulfation of glycosaminoglycans on osteoclast function and signaling pathways. Biomaterials 2012, 33, 8418–8429. [Google Scholar] [CrossRef]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.C.; Hofbauer, L.C. Regenerative potential of glycosaminoglycans for skin and bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Toegel, S.; Hoffmann, O.; Wadsak, W.; Ettlinger, D.; Mien, L.K.; Wiesner, K.; Nguemo, J.; Viernstein, H.; Kletter, K.; Dudczak, R.; et al. Uptake of bone-seekers is solely associated with mineralisation! A study with Tc-99m-MDP, Sm-153-EDTMP and F-18-fluoride on osteoblasts. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Ullmark, G. PET scanning for evaluation of bone metabolism. Acta Orthop. 2009, 80, 738–739. [Google Scholar] [CrossRef] [PubMed]
- Volaric, D.; Zauhar, G.; Chen, J.; Jerbic Radetic, A.T.; Omrcen, H.; Raic, A.; Pirovic, R.; Cvijanovic Peloza, O. The Effect of Low-Intensity Pulsed Ultrasound on Bone Regeneration and the Expression of Osterix and Cyclooxygenase-2 during Critical-Size Bone Defect Repair. Int. J. Mol. Sci. 2024, 25, 3882. [Google Scholar] [CrossRef]
- Cottrell, J.; O’Connor, J.P. Effect of Non-Steroidal Anti-Inflammatory Drugs on Bone Healing. Pharmaceuticals 2010, 3, 1668–1693. [Google Scholar] [CrossRef]
- Tremoleda, J.L.; Khalil, M.; Gompels, L.L.; Wylezinska-Arridge, M.; Vincent, T.; Gsell, W. Imaging technologies for preclinical models of bone and joint disorders. EJNMMI Res. 2011, 1, 11. [Google Scholar] [CrossRef]
- Ventura, M.; Boerman, O.C.; de Korte, C.; Rijpkema, M.; Heerschap, A.; Oosterwijk, E.; Jansen, J.A.; Walboomers, X.F. Preclinical imaging in bone tissue engineering. Tissue Eng. Part. B Rev. 2014, 20, 578–595. [Google Scholar] [CrossRef]
- Ventura, M.; Franssen, G.M.; Oosterwijk, E.; Boerman, O.C.; Jansen, J.A.; Walboomers, X.F. SPECT vs. PET monitoring of bone defect healing and biomaterial performance in vivo. J. Tissue Eng. Regen. Med. 2016, 10, 843–854. [Google Scholar] [CrossRef]
- Cheng, C.; Alt, V.; Dimitrakopoulou-Strauss, A.; Pan, L.; Thormann, U.; Schnettler, R.; Weber, K.; Strauss, L.G. Evaluation of new bone formation in normal and osteoporotic rats with a 3-mm femur defect: Functional assessment with dynamic PET-CT (dPET-CT) using 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) and 18F-fluoride. Mol. Imaging Biol. 2013, 15, 336–344. [Google Scholar] [CrossRef]
- Cheng, C.; Alt, V.; Pan, L.; Thormann, U.; Schnettler, R.; Strauss, L.G.; Schumacher, M.; Gelinsky, M.; Dimitrakopoulou-Strauss, A. Preliminary evaluation of different biomaterials for defect healing in an experimental osteoporotic rat model with dynamic PET-CT (dPET-CT) using F-18-sodium fluoride (NaF). Injury 2014, 45, 501–505. [Google Scholar] [CrossRef]
- Cheng, C.; Alt, V.; Pan, L.; Thormann, U.; Schnettler, R.; Strauss, L.G.; Heinemann, S.; Schumacher, M.; Gelinsky, M.; Nies, B.; et al. Application of F-18-sodium fluoride (NaF) dynamic PET-CT (dPET-CT) for defect healing: A comparison of biomaterials in an experimental osteoporotic rat model. Med. Sci. Monit. 2014, 20, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Schar, M.O.; Ma, R.; Demange, M.; Morgan, M.; Chen, T.; Ballon, D.J.; Dyke, J.P.; Deng, X.H.; Rodeo, S.A. Use of small animal PET-CT imaging for in vivo assessment of tendon-to-bone healing: A pilot study. J. Orthop. Surg. 2022, 30, 23094990221076654. [Google Scholar] [CrossRef]
- Schulze, S.; Rothe, R.; Neuber, C.; Hauser, S.; Ullrich, M.; Pietzsch, J.; Rammelt, S. Men who stare at bone: Multimodal monitoring of bone healing. Biol. Chem. 2021, 402, 1397–1413. [Google Scholar] [CrossRef]
- Petri, M.; Namazian, A.; Wilke, F.; Ettinger, M.; Stubig, T.; Brand, S.; Bengel, F.; Krettek, C.; Berding, G.; Jagodzinski, M. Repair of segmental long-bone defects by stem cell concentrate augmented scaffolds: A clinical and positron emission tomography--computed tomography analysis. Int. Orthop. 2013, 37, 2231–2237. [Google Scholar] [CrossRef][Green Version]
- Lundblad, H.; Karlsson-Thur, C.; Maguire, G.Q., Jr.; Jonsson, C.; Noz, M.E.; Zeleznik, M.P.; Weidenhielm, L. Can Spatiotemporal Fluoride (18F−) Uptake be Used to Assess Bone Formation in the Tibia? A Longitudinal Study Using PET/CT. Clin. Orthop. Relat. Res. 2017, 475, 1486–1498. [Google Scholar] [CrossRef]
- Sinibaldi, R.; Conti, A.; Sinjari, B.; Spadone, S.; Pecci, R.; Palombo, M.; Komlev, V.S.; Ortore, M.G.; Tromba, G.; Capuani, S.; et al. Multimodal-3D imaging based on muMRI and muCT techniques bridges the gap with histology in visualization of the bone regeneration process. J. Tissue Eng. Regen. Med. 2018, 12, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Hofheinz, F.; Hoff, J.; Steffen, I.G.; Lougovski, A.; Ego, K.; Amthauer, H.; Apostolova, I. Comparative evaluation of SUV, tumor-to-blood standard uptake ratio (SUR), and dual time point measurements for assessment of the metabolic uptake rate in FDG PET. EJNMMI Res. 2016, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Patlak, C.S.; Blasberg, R.G. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data-Generalizations. J. Cereb. Blood Flow Metab. 1985, 5, 584–590. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuber, C.; Niedenzu, L.; Schulze, S.; Laube, M.; Hofheinz, F.; Rammelt, S.; Pietzsch, J. Exploring a Nitric Oxide-Releasing Celecoxib Derivative as a Potential Modulator of Bone Healing: Insights from Ex Vivo and In Vivo Imaging Experiments. Int. J. Mol. Sci. 2025, 26, 2582. https://doi.org/10.3390/ijms26062582
Neuber C, Niedenzu L, Schulze S, Laube M, Hofheinz F, Rammelt S, Pietzsch J. Exploring a Nitric Oxide-Releasing Celecoxib Derivative as a Potential Modulator of Bone Healing: Insights from Ex Vivo and In Vivo Imaging Experiments. International Journal of Molecular Sciences. 2025; 26(6):2582. https://doi.org/10.3390/ijms26062582
Chicago/Turabian StyleNeuber, Christin, Luisa Niedenzu, Sabine Schulze, Markus Laube, Frank Hofheinz, Stefan Rammelt, and Jens Pietzsch. 2025. "Exploring a Nitric Oxide-Releasing Celecoxib Derivative as a Potential Modulator of Bone Healing: Insights from Ex Vivo and In Vivo Imaging Experiments" International Journal of Molecular Sciences 26, no. 6: 2582. https://doi.org/10.3390/ijms26062582
APA StyleNeuber, C., Niedenzu, L., Schulze, S., Laube, M., Hofheinz, F., Rammelt, S., & Pietzsch, J. (2025). Exploring a Nitric Oxide-Releasing Celecoxib Derivative as a Potential Modulator of Bone Healing: Insights from Ex Vivo and In Vivo Imaging Experiments. International Journal of Molecular Sciences, 26(6), 2582. https://doi.org/10.3390/ijms26062582





