State of the Art of Immune Checkpoint Inhibitors in Unresectable Pancreatic Cancer: A Comprehensive Systematic Review
Abstract
1. Introduction
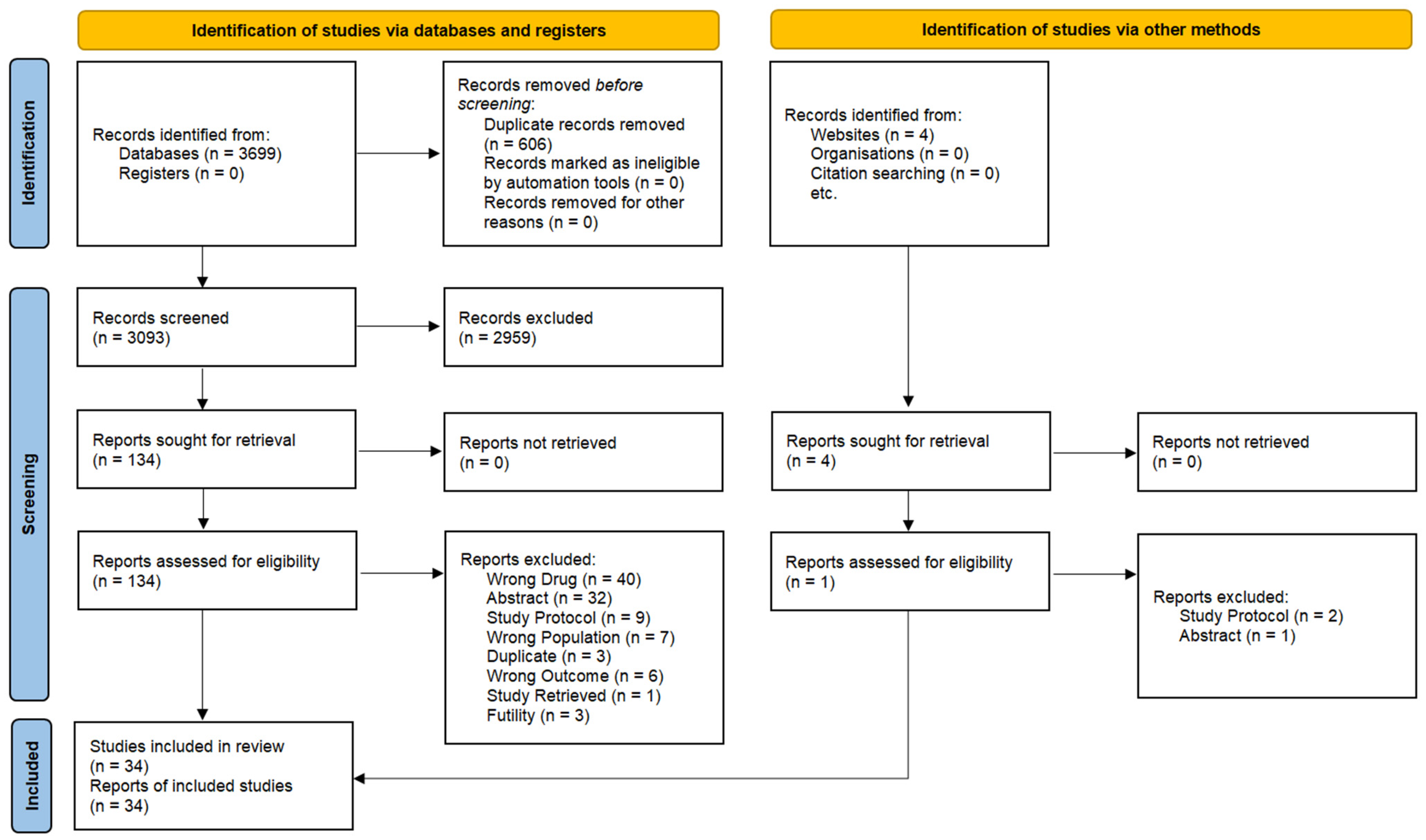
2. Materials and Methods
2.1. Information Sources
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Study Quality Assessment
2.5. Statistical Analysis
2.6. Assessment of Heterogeneity
2.7. Publication Bias
2.8. Sensitivity Analysis
3. Results and Discussion
| Author; Year | Country | Study Type | RTC Phase | N Interv Group | N Control Group | Line of Therapy | Age (±SD) | Sex M (% M) | PS (ECOG) | Stage | ICI Type | Combined Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2023 [23] | Asia | RO | 27 | 0 | I | 64 (46–77) | 18 (66.7%) | 0–1 | IV | Anti-PD-1 (Pembrolizumab; Nivolumab; Toripalimab; Sintilimab; Tislelizumab; Camrelizumab) | GnP | |
| Cheng 2023 [9] | USA, Asia | RO | 27 | 26 | I | 64 (55–79) | 16 (53.3%) | 0–1 | III, IV | Anti-PD-1 (Sintilimab; Camrelizumab; Tislelizumab; Pembrolizumab) | GnP | |
| Gong 2022 [24] | Asia | RO | 104 | 0 | ≥I | 63 (30–80) | 62 (59.6%) | 0–2 | III, IV | Anti-PD-1 (Pembrolizumab; Camrelizumab; Toripalimab; Sintilimab; Tislelizumab) | CT, targeted therapy, neoantigen vaccine therapy, with or without RT | |
| Lemech 2023 [25] | Australia | RCT | I | 18 | 0 | I, II | 62 (45–71) | 8 (44.4%) | 0–1 | IV | Anti-PD1 (nivolumab) | Pixatimod |
| Ma 2019 [26] | Asia | RO | 22 | 36 | ≥I | 56 (34–73) | 13 (59.1%) | 0–1–2–3 | IV | Anti-PD1 (Pembrolizumab; Nivolumab) or anti-PDL1 (Atezolizumab) | CT | |
| Melisi 2021 [27] | Europe, USA, Asia | RCT | Ib | 42 | 0 | I, II, III | 57 (38–81) | 17 (40.5%) | NA | IV | Anti-PDL1 (durvalumab) | (TGFβ) receptor inhibitor; galunisertib |
| Reiss 2022 [28] | USA | RCT | Ib, II | 44 | 0 | ≥I | 65 (54–73) | 29 (66%) | 0–1 | III, IV | Anti-PD1 (nivolumab 240 mg q3w) | Niraparib |
| 40 | 0 | 63 (58–69) | 20 (50%) | anti-PD1 (nivolumab 3 mg/kg) | ||||||||
| Renouf 2022 [29] | USA, Canada | RCT | II | 119 | 61 | I | 64 (29–81) | 67 (56.3%) | 0–1 | IV | antiCTLA4 (tremelimumab) + antiPDL1 (durvalumab) | GnP |
| Royal 2010 [30] | USA | RCT | II | 27 | 0 | ≥I | 55 (27–68) | 15 (55.5%) | 0–1–2 | III, IV | AntiCTLA4 (ipilimumab) | No |
| Sun 2018 [31] | Asia | RO | 43 | 0 | ≥I | 56 (35–85) | 25 (58.1%) | 0–1–2 | IV | AntiPD1 or anti PDL1 or antiCTLA4 | CT, targeted therapy, ipilimumab | |
| Tsujikawa 2020 [32] | USA | RCT | II | 51 | 42 | II | 64 (58–69) | 37 (73%) | 0–1 | IV | Anti-PD1 (nivolumab) | GVAX pancreas vaccine, cyclophosphamide and CRS-207 |
| Wang 2024 [33] | Asia | RCT | II | 23 | 0 | ≥I | 65,3 (53–80) | 11 (47.8%) | NA | IV | AntiPD1 (sintilimab, toripalimab) | Oxaliplatin + S1 |
| Xie 2020 [34] | USA | RCT | I | 14 | 0 | >I | 62 (43–80) | 7 (50%) | 0–1 | IV | AntiPDL1 (durvalumab) | SBRT 8 Gy 1 fr |
| 19 | 0 | 60 (43–85) | 10 (53%) | AntiPDL1 (durvalumab) + antiCTLA4 (tremelimumab) | SBRT 8 Gy 1 fr | |||||||
| 16 | 0 | 60.5 (44–79) | 15 (94%) | AntiPDL1 (durvalumab) + antiCTLA4 (tremelimumab) | SBRT 25 Gy 5 fr | |||||||
| Yang 2024 [35] | Asia | RO | 43 | 301 | 65 (37–78) | 30 (69.8%) | 0–1–2–3 | IV | Anti-PD1 (nivolumab) | CT add-on | ||
| 33 | 301 | 62 (46–81) | 20 (60.6%) | CT concurrent | ||||||||
| 16 | 301 | 62 (52–73) | 7 (43.75%) | No | ||||||||
| Zhang 2022 [36] | Asia | RO | 17 | 31 | ≥I | ≤60: 20 (64.5%); >60: 7 (41.2%) | 10 (58.8%) | 0–1–2 | IV | Anti-PD1 (Toripalimab; Camrelizumab; Tislelizumab; Sintilimab) | GnP | |
| Bockorny 2020 [37] | USA, Europe, Asia | RCT | IIa | 29 | 0 | ≥I | 63.9 (46–86) | 18 (48.6%) | 0–1 | IV | Anti-PD1 (pembrolizumab) | BL8040 |
| 22 | II | 68 (50–83) | 13 (59%) | BL8040 + NALIRI + 5FU + leucovorin | ||||||||
| Liu 2022 [38] | Asia | RO | 32 | 34 | I | 61.5 (49.3–66.0) | 20 (62.5%) | 0–1 | IV | Anti-PD1 (sintilimab) | Nab-paclitaxel plus S1 | |
| Liu 2024 [39] | Asia | RO | 25 | 27 | I | <60: 16 (66.7%); ≥60: 8 (33.3%) | 16 (64%) | 0–1–2 | IV | Anti-PD1 (camrelizumab and sintilimab) | Gemcitabine/nab-paclitaxel + anlotinib | |
| Parikh 2021 [40] | USA | RCT | II | 25 | 0 | ≥I | 60 (32–75) | 18 (72%) | 0–1 | III, IV | Anti-PD1 (nivolumab) + anti-CTLA4 (ipilimumab) | Radiation |
| Hong 2022 [41] | USA | RCT | II | 17 | 0 | ≥I | 62.5 (32–80) | 61 (53.5%) | 0–1 | IV | Anti-PD1 (nivolumab) | Mogamulizumab |
| Callahan 2023 [42] | USA | RCT | II | 18 | 0 | ≥II | 66.5 (35–76) | 13 (72%) | 0–1 | IV | Anti-PD1 (nivolumab) | no |
| 21 | 0 | 63.0 (47–79) | 11 (52%) | 0–1 | Anti-PD1 (nivolumab) + anti-CTLA4 (ipilimumab) | no | ||||||
| 30 | 0 | 65.0 (31–78) | 18 (60%) | 0–1–2 | Anti-PD1 (nivolumab) + anti-CTLA4 (ipilimumab) | Cobimetinib |
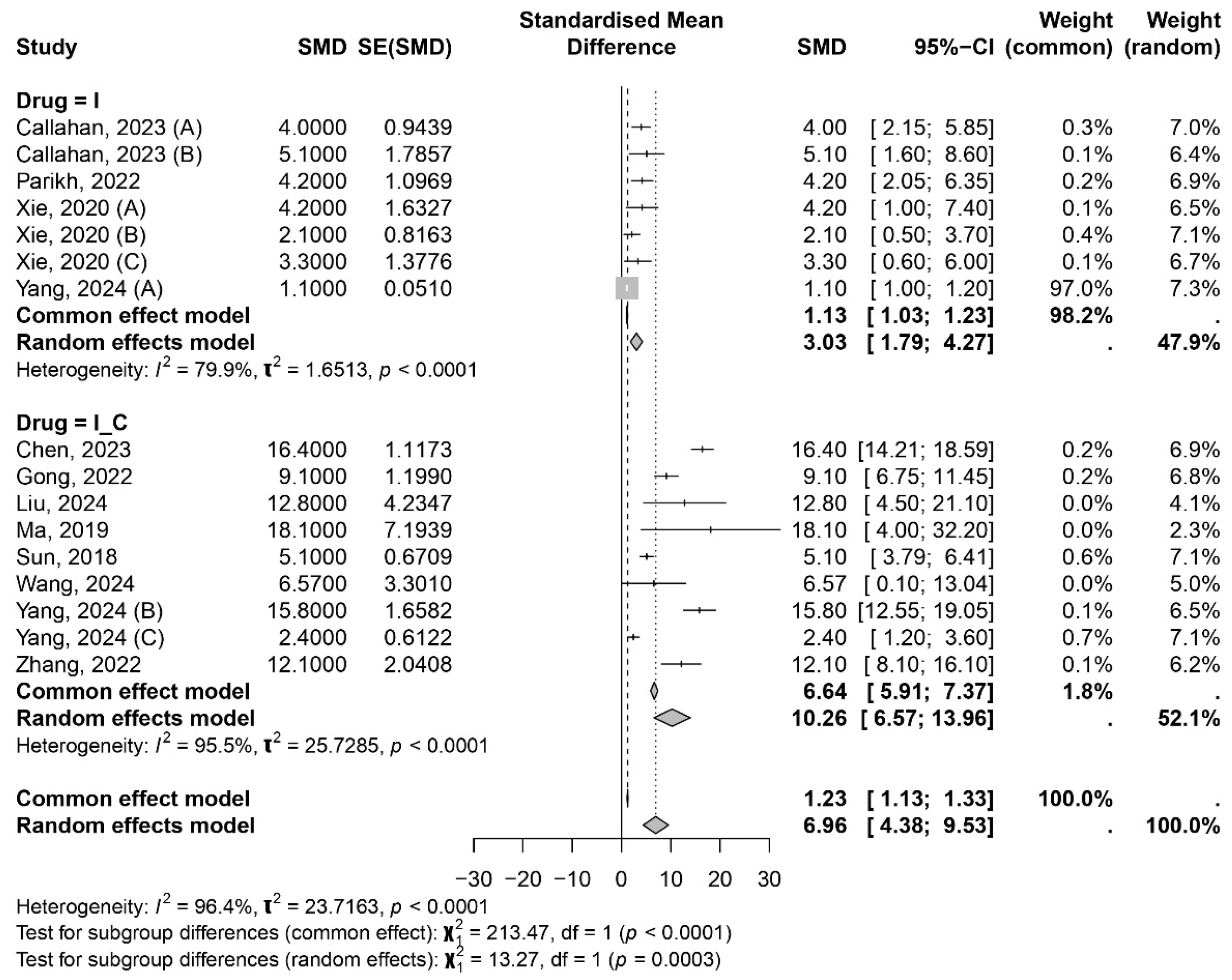
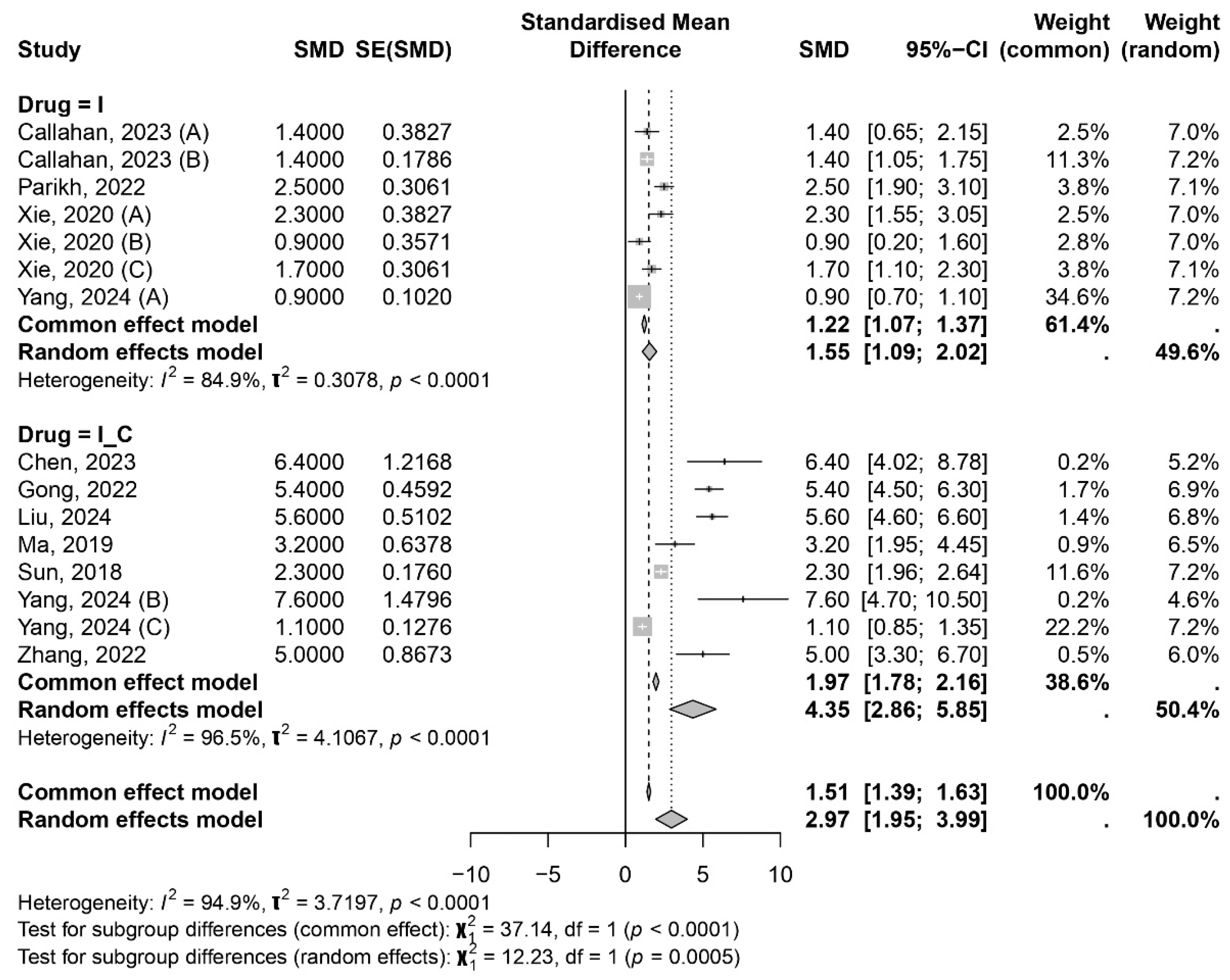
3.1. Meta-Analysis
3.2. MSI-Driven Studies
3.3. Phase I-II/II Multi-Tumor Studies
| Author; Year | Country | Study Type | RTC Phase | N Interv Group | N Control Group | Age (±SD) | Sex M (% M) | ICI Type | Combined Treatment | Biomarker Driven |
|---|---|---|---|---|---|---|---|---|---|---|
| Taieb 2024 [53] | Europe | RO | 31 | 0 | 62.1 (37–82) | 17 (54.8%) | AntiPD1 or antiPDL1 | CT, ipilimumab | MSI/ dMMR | |
| Marabelle 2020 [10] | Asia, USA, Europe | RCT | II | 22 | 0 | 60.0 (20–87) | 96 (41.2%) | Anti-PD1 (pembrolizumab) | no | dMMR and MSI-H |
| André 2023 [54] | Europe, Canada | RCT | II | 12 | 0 | 63 (24–85) | 106 (30.5%) | Anti-PD1 (dostarlimab) | no | dMMR and MSI-H and/or POLE-altered |
| Author; Year | Country | Stage | ICI Type | Combined Treatment | Total Population | Pancreas-Specific Subgroup (n, %) | Outcomes (Global) |
|---|---|---|---|---|---|---|---|
| Curigliano 2021 [43] | USA, Europe | IV | Anti-PD1 (spartalizumab) | Sabatolimab | 86 | 2 (2.3%) | Response: 6%; lasting 12–27 months |
| Hedge 2021 [44] | USA | IV | Anti CTLA4 (ipilimumab) | Evofosfamide | 22 | 7 (31.8%) | ORR: 16.7%; DCR: 83.3% |
| Kitano 2020 [45] | Asia | IV | Anti-PD1 (cemiplimab) | no | 13 | 1 (14.3%) | ORR: 30.8%; DCR: 46.2%; grade ≥ 3 TEAEs: 30.8% |
| Muik 2022 [46] | Europe | IV | DuoBody-PD-L1×4-1BB | no | 61 | 6 (9.8%) | DCR: 65.6%; DLT: 9.8% |
| Naing 2023 [47] | USA | IV | Anti-PDL1 (durvalumab) | IDO1 inhibitor epacadostat (25–300 mg twice daily) | 34 | 15 (44.1%) | ORR: 12.0%; Grade ≥ 3 TRAEs: 20.6% |
| Papadopoulos 2022 [48] | USA, Australia, Asia | IV | Anti-PD1 (pembrolizumab) | MK-4166 1.1 to 900 mg Q3W | 65 | 8 (12.3%) | ORR: 61.5% (95% CI, 31.6–86.1); DCR: 61.5% (95% CI, 31.6–86.1); Grade ≥ 3 TRAEs: 10.8% |
| Yamamoto 2024 [50] | Asia | IV | Anti-PDL1 (atezolizumab) | AMY109 14/45 mg/kg Q3W | 20 | 7 (35%) | ORR: 5% (95% CI, 0.1–24.9); DCR: 30% (95% CI, 11.9–54.3); Grade ≥ 3 TRAEs: 15% |
| Zamarin 2020 [51] | USA | IV | Anti-PDL1 (durvalumab) | Mogamulizumab 1 mg/kg | 12 | 12 (100% dose expansion) | ORR: 5.3% (95% CI, 0.1–26.0); mOS: 8.9 (95% CI, 4.3–18.4); mPFS: 1.9 (95% CI; 1.7–4.4) |
| USA | Anti-CTLA4 (tremelimumab) | ORR: 5.3% (95% CI, 0.1–26.0); mOS: 4.4 (95% CI, 2.5–13.4); mPFS: 1.9 (95% CI, 1.4–3.7) | |||||
| Zheng 2022 [52] | Asia, Australia | IV | Anti-PD1 (penpulimab) | no | 60 | 11 (18.3%) | ORR: 20% (95% CI, 10.8–32.3); DCR: 45% (95% CI, 32.1–58.4); TRAE: 95.5% |
| Voisin 2024 [49] | Europe | IV | Anti-PD1 (pembrolizumab) | Xevinapant (100, 150, and 200 mg daily for 14 days on/7 days off) | 41 | 14 (dose expansion) | DCR: 13%; ORR: 3% |
3.4. Quality Assessment
3.5. Discussion
3.6. Study Limitations
4. Conclusions
Clinical Implications and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence Interval |
| CT | Chemotherapy |
| CTLA4 | Cytotoxic T-Lymphocyte Associated Protein 4 |
| DCR | Disease Control Rate |
| DLT | Dose Limiting Toxicity |
| dMMR | Mismatch Repair Deficiency |
| ECOG | Eastern Cooperative Oncology Group |
| EMBASE | Excerpta Medica Database |
| GnP | Gemcitabine nab-Paclitaxel |
| ICIs | Immune checkpoint inhibitors |
| iRAEs | Immune-Related Adverse Event |
| IRE | Irreversible Electroporation |
| MEDLINE | Medical Literature Analysis and Retrieval System Online |
| MeSH | Medical Subject Headings |
| MSI | Microsatellite Instability |
| MSI-H | High Microsatellite Instability |
| MSS | Microsatellite Stable |
| nal-IRI | Nanoliposomal Irinotecan |
| NIH | National Institute of Health |
| NOS | Newcastle-Ottawa Scale |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| PD-1 | Program Death 1 |
| PD-L1 | Program Death Ligand 1 |
| PFS | Progression Free Survival |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PS | Performance Status |
| QoL | Quality of Life |
| RCTs | Randomized Controlled Trials |
| RO | Retrospective Observational |
| SBRT | Stereotactic Body Radiation Therapy |
| SD | Standard Deviation |
| TGFβ | Tumor Growth Factor β |
| TMB | Tumor Mutational Burden |
| TRAEs | Treatment-Related Adverse Events |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Jooste, V.; Bengrine-Lefevre, L.; Manfredi, S.; Quipourt, V.; Grosclaude, P.; Facy, O.; Lepage, C.; Ghiringhelli, F.; Bouvier, A.-M. Management and Outcomes of Pancreatic Cancer in French Real-World Clinical Practice. Cancers 2022, 14, 1675. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel Plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Burris, H.A.; Moore, M.J.; Andersen, J.; Green, M.R.; Rothenberg, M.L.; Modiano, M.R.; Cripps, M.C.; Portenoy, R.K.; Storniolo, A.M.; Tarassoff, P.; et al. Improvements in Survival and Clinical Benefit with Gemcitabine as First-Line Therapy for Patients with Advanced Pancreas Cancer: A Randomized Trial. J. Clin. Oncol. 1997, 15, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid in Metastatic Pancreatic Cancer after Previous Gemcitabine-Based Therapy (NAPOLI-1): A Global, Randomised, Open-Label, Phase 3 Trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef]
- Cartwright, T.H.; Cohn, A.; Varkey, J.A.; Chen, Y.-M.; Szatrowski, T.P.; Cox, J.V.; Schulz, J.J. Phase II Study of Oral Capecitabine in Patients With Advanced or Metastatic Pancreatic Cancer. J. Clin. Oncol. 2002, 20, 160–164. [Google Scholar] [CrossRef]
- Rahma, O.E.; Duffy, A.; Liewehr, D.J.; Steinberg, S.M.; Greten, T.F. Second-Line Treatment in Advanced Pancreatic Cancer: A Comprehensive Analysis of Published Clinical Trials. Ann. Oncol. 2013, 24, 1972–1979. [Google Scholar] [CrossRef]
- Blair, A.B.; Zheng, L. Rational Combinations of Immunotherapy for Pancreatic Ductal Adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 31. [Google Scholar] [CrossRef]
- Cheng, D.; Hu, J.; Wu, X.; Wang, B.; Chen, R.; Zhao, W.; Fang, C.; Ji, M. PD-1 Blockade Combined with Gemcitabine Plus Nab-Paclitaxel Is Superior to Chemotherapy Alone in the Management of Unresectable Stage III/IV Pancreatic Cancer: A Retrospective Real-World Study. Front. Oncol. 2023, 13, 1281545. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive Characterisation of Pancreatic Ductal Adenocarcinoma with Microsatellite Instability: Histology, Molecular Pathology and Clinical Implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Lalani, A.-K.; Robert, C.; Sharma, P.; Peters, S. Defining Unique Clinical Hallmarks for Immune Checkpoint Inhibitor-Based Therapies. J. Immunother. Cancer 2022, 10, e003024. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 November 2024).
- NIH Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 29 January 2025).
- Shamim, M.A.; Gandhi, A.P.; Dwivedi, P.; Padhi, B.K. How to Perform Meta-Analysis in R: A Simple yet Comprehensive Guide. Evid 2023, 1, 93–113. [Google Scholar]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.; Hill, C. A Graphical Method for Exploring Heterogeneity in Meta-analyses: Application to a Meta-analysis of 65 Trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Patsopoulos, N.A.; Evangelou, E.; Ioannidis, J.P. Sensitivity of Between-Study Heterogeneity in Meta-Analysis: Proposed Metrics and Empirical Evaluation. Int. J. Epidemiol. 2008, 37, 1148–1157. [Google Scholar] [CrossRef]
- Chen, Z.; He, Y.; Ding, C.; Chen, J.; Gu, Y.; Xiao, M.; Li, Q. Safety and Efficacy Analysis of PD-1 Inhibitors in Combination with Gemcitabine Plus Nab-Paclitaxel for Advanced Pancreatic Cancer: A Real-World, Single-Center Study. OncoTargets Ther. 2023, 16, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zhu, Y.; Zhang, Q.; Qiu, X.; Lu, C.; Tong, F.; Wang, Q.; Kong, W.; Zhou, H.; Liu, B.; et al. Efficacy and Safety of Immune Checkpoint Inhibitors in Advanced Pancreatic Cancer: A Real World Study in Chinese Cohort. Hum. Vaccines Immunother. 2022, 18, 2143154. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.; Dredge, K.; Bampton, D.; Hammond, E.; Clouston, A.; Waterhouse, N.J.; Stanley, A.C.; Leveque-El Mouttie, L.; Chojnowski, G.M.; Haydon, A.; et al. Phase Ib Open-Label, Multicenter Study of Pixatimod, an Activator of TLR9, in Combination with Nivolumab in Subjects with Microsatellite-Stable Metastatic Colorectal Cancer, Metastatic Pancreatic Ductal Adenocarcinoma and Other Solid Tumors. J. Immunother. Cancer 2023, 11, e006136. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.; Wang, J.; Han, C.; Qian, Y.; Chen, G.; Li, X.; Zhang, J.; Cui, P.; Du, W.; et al. Immune Checkpoint Inhibitors Combined with Chemotherapy for the Treatment of Advanced Pancreatic Cancer Patients. Cancer Immunol. Immunother. 2020, 69, 365–372. [Google Scholar] [CrossRef]
- Melisi, D.; Oh, D.-Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and Activity of the TGFβ Receptor I Kinase Inhibitor Galunisertib Plus the Anti-PD-L1 Antibody Durvalumab in Metastatic Pancreatic Cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef]
- Reiss, K.A.; Mick, R.; Teitelbaum, U.; O’Hara, M.; Schneider, C.; Massa, R.; Karasic, T.; Tondon, R.; Onyiah, C.; Gosselin, M.K.; et al. Niraparib Plus Nivolumab or Niraparib Plus Ipilimumab in Patients with Platinum-Sensitive Advanced Pancreatic Cancer: A Randomised, Phase 1b/2 Trial. Lancet Oncol. 2022, 23, 1009–1020. [Google Scholar] [CrossRef]
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 Phase II Trial of Gemcitabine and Nab-Paclitaxel with or without Durvalumab and Tremelimumab as Initial Therapy in Metastatic Pancreatic Ductal Adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 Trial of Single Agent Ipilimumab (Anti-CTLA-4) for Locally Advanced or Metastatic Pancreatic Adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef]
- Sun, D.; Ma, J.-X.; Wang, J.; Zhang, F.; Wang, L.; Zhang, S.; Chen, G.; Li, X.; Du, W.; Cui, P.-F.; et al. Clinical Observation of Immune Checkpoint Inhibitors in the Treatment of Advanced Pancreatic Cancer: A Real-World Study in Chinese Cohort. Ther. Clin. Risk Manag. 2018, 14, 1691–1700. [Google Scholar] [CrossRef]
- Tsujikawa, T.; Crocenzi, T.; Durham, J.N.; Sugar, E.A.; Wu, A.A.; Onners, B.; Nauroth, J.M.; Anders, R.A.; Fertig, E.J.; Laheru, D.A.; et al. Evaluation of Cyclophosphamide/GVAX Pancreas Followed by Listeria-Mesothelin (CRS-207) with or without Nivolumab in Patients with Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 3578–3588. [Google Scholar] [CrossRef]
- Wang, Q.; Tong, F.; Qiao, L.; Qi, L.; Sun, Y.; Zhu, Y.; Ni, J.; Liu, J.; Kong, W.; Liu, B.; et al. Hypofractionated Radiotherapy Plus PD-1 Antibody and SOX Chemotherapy as Second-Line Therapy in Metastatic Pancreatic Cancer: A Single-Arm, Phase II Clinical Trial. Cancer Immunol. Immunother. 2024, 73, 201. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Duffy, A.G.; Brar, G.; Fioravanti, S.; Mabry-Hrones, D.; Walker, M.; Bonilla, C.M.; Wood, B.J.; Citrin, D.E.; Gil Ramirez, E.M.; et al. Immune Checkpoint Blockade in Combination with Stereotactic Body Radiotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-H.; Kuo, S.-H.; Lee, J.-C.; Chen, B.-B.; Shan, Y.-S.; Tien, Y.-W.; Chiu, S.-C.; Cheng, A.-L.; Yeh, K.-H. Adding-on Nivolumab to Chemotherapy-Stabilized Patients Is Associated with Improved Survival in Advanced Pancreatic Ductal Adenocarcinoma. Cancer Immunol. Immunother. 2024, 73, 227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Yang, F.; Zhang, Y.; Jiang, M.; Zhang, X. The Efficacy and Safety of PD-1 Inhibitors Combined with Nab-Paclitaxel Plus Gemcitabine versus Nab-Paclitaxel Plus Gemcitabine in the First-Line Treatment of Advanced Pancreatic Cancer: A Retrospective Monocentric Study. Cancer Manag. Res. 2022, 14, 535–546. [Google Scholar] [CrossRef]
- Bockorny, B.; Semenisty, V.; Macarulla, T.; Borazanci, E.; Wolpin, B.M.; Stemmer, S.M.; Golan, T.; Geva, R.; Borad, M.J.; Pedersen, K.S.; et al. BL-8040, a CXCR4 Antagonist, in Combination with Pembrolizumab and Chemotherapy for Pancreatic Cancer: The COMBAT Trial. Nat. Med. 2020, 26, 878–885. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, G.; Zhang, X.; Jiang, N.; Zhao, Z.; Wang, Y.; Xu, S.; Zhu, L.; Lau, W.Y.; Dai, G.; et al. Nab-Paclitaxel Plus S-1 with or Without PD-1 Inhibitor in Pancreatic Ductal Adenocarcinoma with Only Hepatic Metastases: A Retrospective Cohort Study. Langenbecks Arch. Surg. 2022, 407, 633–643. [Google Scholar] [CrossRef]
- Liu, H.; Pan, D.; Yao, Z.; Wang, H.; Li, Y.; Qin, X.; Qu, P.; Tang, J.; Han, Z. Efficacy and Safety of Gemcitabine/Nab-Paclitaxel Combined with Anlotinib and PD-1 Inhibitors as a First-Line Treatment for Advanced Pancreatic Cancer. Int. Immunopharmacol. 2024, 139, 112635. [Google Scholar] [CrossRef]
- Parikh, A.R.; Szabolcs, A.; Allen, J.N.; Clark, J.W.; Wo, J.Y.; Raabe, M.; Thel, H.; Hoyos, D.; Mehta, A.; Arshad, S.; et al. Radiation Therapy Enhances Immunotherapy Response in Microsatellite Stable Colorectal and Pancreatic Adenocarcinoma in a Phase II Trial. Nat. Cancer 2021, 2, 1124–1135. [Google Scholar] [CrossRef]
- Hong, D.S.; Rixe, O.; Chiu, V.K.; Forde, P.M.; Dragovich, T.; Lou, Y.; Nayak-Kapoor, A.; Leidner, R.; Atkins, J.N.; Collaku, A.; et al. Mogamulizumab in Combination with Nivolumab in a Phase I/II Study of Patients with Locally Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2022, 28, 479–488. [Google Scholar] [CrossRef]
- Callahan, M.; Amin, A.; Kaye, F.J.; Morse, M.A.; Taylor, M.H.; Peltola, K.J.; Sharma, P.; O’Reilly, E.M.; Meadows Shropshire, S.; O’Brien, S.; et al. Nivolumab Monotherapy or Combination with Ipilimumab with or without Cobimetinib in Previously Treated Patients with Pancreatic Adenocarcinoma (CheckMate 032). J. Immunother. Cancer 2024, 12, e007883. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti–TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti–PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A.; Jayaprakash, P.; Couillault, C.A.; Piha-Paul, S.; Karp, D.; Rodon, J.; Pant, S.; Fu, S.; Dumbrava, E.E.; Yap, T.A.; et al. A Phase I Dose-Escalation Study to Evaluate the Safety and Tolerability of Evofosfamide in Combination with Ipilimumab in Advanced Solid Malignancies. Clin. Cancer Res. 2021, 27, 3050–3060. [Google Scholar] [CrossRef] [PubMed]
- Kitano, S.; Shimizu, T.; Koyama, T.; Ebata, T.; Iwasa, S.; Kondo, S.; Shimomura, A.; Fujiwara, Y.; Yamamoto, N.; Paccaly, A.; et al. Dose Exploration Results from Phase 1 Study of Cemiplimab, a Human Monoclonal Programmed Death (PD)-1 Antibody, in Japanese Patients with Advanced Malignancies. Cancer Chemother. Pharmacol. 2021, 87, 53–64. [Google Scholar] [CrossRef]
- Muik, A.; Garralda, E.; Altintas, I.; Gieseke, F.; Geva, R.; Ben-Ami, E.; Maurice-Dror, C.; Calvo, E.; LoRusso, P.M.; Alonso, G.; et al. Preclinical Characterization and Phase I Trial Results of a Bispecific Antibody Targeting PD-L1 and 4-1BB (GEN1046) in Patients with Advanced Refractory Solid Tumors. Cancer Discov. 2022, 12, 1248–1265. [Google Scholar] [CrossRef]
- Naing, A.; Algazi, A.P.; Falchook, G.S.; Creelan, B.C.; Powderly, J.; Rosen, S.; Barve, M.; Mettu, N.B.; Triozzi, P.L.; Hamm, J.; et al. Phase 1/2 Study of Epacadostat in Combination with Durvalumab in Patients with Metastatic Solid Tumors. Cancer 2023, 129, 71–81. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Autio, K.; Golan, T.; Dobrenkov, K.; Chartash, E.; Chen, Q.; Wnek, R.; Long, G.V. Phase I Study of MK-4166, an Anti-Human Glucocorticoid-Induced TNF Receptor Antibody, Alone or with Pembrolizumab in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 1904–1911. [Google Scholar] [CrossRef]
- Voisin, A.; Terret, C.; Schiffler, C.; Bidaux, A.-S.; Vanacker, H.; Perrin-Niquet, M.; Barbery, M.; Vinceneux, A.; Eberst, L.; Stéphan, P.; et al. Xevinapant Combined with Pembrolizumab in Patients with Advanced, Pretreated, Colorectal and Pancreatic Cancer: Results of the Phase Ib/II CATRIPCA Trial. Clin. Cancer Res. 2024, 30, 2111–2120. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kitano, S.; Koyama, T.; Ikeda, M.; Mizugaki, H.; Narikiyo, T.; Yamaguchi, Y.; Ishida, T.; Takubo, R.; Ogami, C.; et al. Phase I Study of the Safety and Clinical Activity of the Interleukin-8 Inhibitor AMY109 Combined with Atezolizumab in Patients with Advanced Solid Cancers. J. Immunother. Cancer 2024, 12, e009262. [Google Scholar] [CrossRef]
- Zamarin, D.; Hamid, O.; Nayak-Kapoor, A.; Sahebjam, S.; Sznol, M.; Collaku, A.; Fox, F.E.; Marshall, M.A.; Hong, D.S. Mogamulizumab in Combination with Durvalumab or Tremelimumab in Patients with Advanced Solid Tumors: A Phase I Study. Clin. Cancer Res. 2020, 26, 4531–4541. [Google Scholar] [CrossRef]
- Zheng, Y.; Mislang, A.R.A.; Coward, J.; Cosman, R.; Cooper, A.; Underhill, C.; Zhu, J.; Xiong, J.; Jiang, O.; Wang, H.; et al. Penpulimab, an Anti-PD1 IgG1 Antibody in the Treatment of Advanced or Metastatic Upper Gastrointestinal Cancers. Cancer Immunol. Immunother. 2022, 71, 2371–2379. [Google Scholar] [CrossRef]
- Taïeb, J.; Sayah, L.; Heinrich, K.; Kunzmann, V.; Boileve, A.; Cirkel, G.; Lonardi, S.; Chibaudel, B.; Turpin, A.; Beller, T.; et al. Efficacy of Immune Checkpoint Inhibitors in Microsatellite Unstable/Mismatch Repair-Deficient Advanced Pancreatic Adenocarcinoma: An AGEO European Cohort. Eur. J. Cancer 2023, 188, 90–97. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; De Braud, F.; Arkenau, H.-T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Parikh, A.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.; Elez, E.; Shao, S.H.; Deming, D.; Holdridge, R.; et al. Modified FOLFOX6 Plus Bevacizumab with and Without Nivolumab for First-Line Treatment of Metastatic Colorectal Cancer: Phase 2 Results from the CheckMate 9X8 Randomized Clinical Trial. J. Immunother. Cancer 2024, 12, e008409. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Version 1. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1457 (accessed on 29 January 2025).
- Wang, T.; Denman, D.; Bacot, S.M.; Feldman, G.M. Challenges and the Evolving Landscape of Assessing Blood-Based PD-L1 Expression as a Biomarker for Anti-PD-(L)1 Immunotherapy. Biomedicines 2022, 10, 1181. [Google Scholar] [CrossRef]
- Kim, H.; Kim, R.; Jo, H.; Kim, H.R.; Hong, J.; Ha, S.Y.; Park, J.O.; Kim, S.T. Expression of PD-L1 as a Predictive Marker of Sensitivity to Immune Checkpoint Inhibitors in Patients with Advanced Biliary Tract Cancer. Ther. Adv. Gastroenterol. 2022, 15, 17562848221117638. [Google Scholar] [CrossRef]
- Copur, M.S.; Peterson, T.; Doornbos, K.; Tun, S.M.; Springer, C.R.; Robbins, L.; Arbogast, J.; Muske, C.; Buescher, L.; Sukup, J.; et al. Programmed Cell Death Ligand 1 (PD-L1) Expression Landscape and Its Relationship with Tumor Mutational Burden (TMB) in a Community-Based Cancer Center Patient Population in Rural Central Nebraska. J. Clin. Oncol. 2023, 41, e15141. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Z.; Du, X.; Chen, S.; Zhang, W.; Wang, J.; Li, H.; He, X.; Cao, J.; Wang, J. Co-Inhibition of the TGF-β Pathway and the PD-L1 Checkpoint by pH-Responsive Clustered Nanoparticles for Pancreatic Cancer Microenvironment Regulation and Anti-Tumor Immunotherapy. Biomater. Sci. 2020, 8, 5121–5132. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, Z.; Li, T.; Chen, H.; Yuan, Y.; Wang, Y.A.; Hsiao, C.-H.; Chow, D.S.-L.; Overwijk, W.W.; Li, C. Stromal Modulation Reverses Primary Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. ACS Nano 2018, 12, 9881–9893. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, E.; Guasconi, M.; Romboli, A.; Giuffrida, M.; Toscani, I.; Anselmi, E.; Porzio, R.; Madaro, S.; Vecchia, S.; Citterio, C. State of the Art of Immune Checkpoint Inhibitors in Unresectable Pancreatic Cancer: A Comprehensive Systematic Review. Int. J. Mol. Sci. 2025, 26, 2620. https://doi.org/10.3390/ijms26062620
Orlandi E, Guasconi M, Romboli A, Giuffrida M, Toscani I, Anselmi E, Porzio R, Madaro S, Vecchia S, Citterio C. State of the Art of Immune Checkpoint Inhibitors in Unresectable Pancreatic Cancer: A Comprehensive Systematic Review. International Journal of Molecular Sciences. 2025; 26(6):2620. https://doi.org/10.3390/ijms26062620
Chicago/Turabian StyleOrlandi, Elena, Massimo Guasconi, Andrea Romboli, Mario Giuffrida, Ilaria Toscani, Elisa Anselmi, Rosa Porzio, Serena Madaro, Stefano Vecchia, and Chiara Citterio. 2025. "State of the Art of Immune Checkpoint Inhibitors in Unresectable Pancreatic Cancer: A Comprehensive Systematic Review" International Journal of Molecular Sciences 26, no. 6: 2620. https://doi.org/10.3390/ijms26062620
APA StyleOrlandi, E., Guasconi, M., Romboli, A., Giuffrida, M., Toscani, I., Anselmi, E., Porzio, R., Madaro, S., Vecchia, S., & Citterio, C. (2025). State of the Art of Immune Checkpoint Inhibitors in Unresectable Pancreatic Cancer: A Comprehensive Systematic Review. International Journal of Molecular Sciences, 26(6), 2620. https://doi.org/10.3390/ijms26062620






