Label-Free Proteomics Reveals the Response of Oat (Avena sativa L.) Seedling Root Respiratory Metabolism to Salt Stress
Abstract
1. Introduction
2. Results
2.1. Growth of Oat Seedlings Under Salt Stress
2.2. Effects of Salt Stress on Potassium, Sodium, Calcium, and Magnesium Ion Contents in Oat Root at Seedling Stage
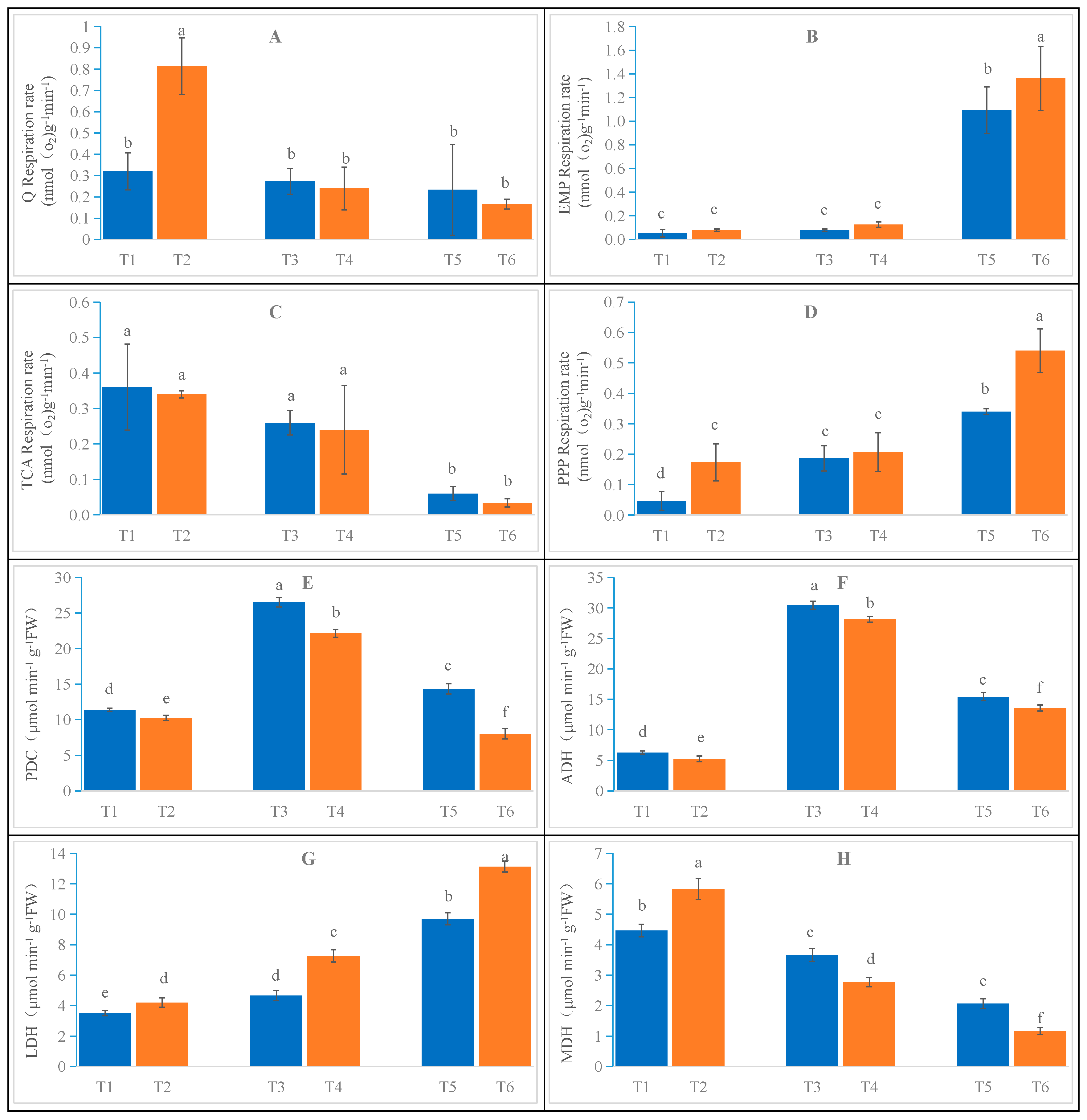
2.3. Effects of Salt Stress on Oat Root Respiratory Metabolism
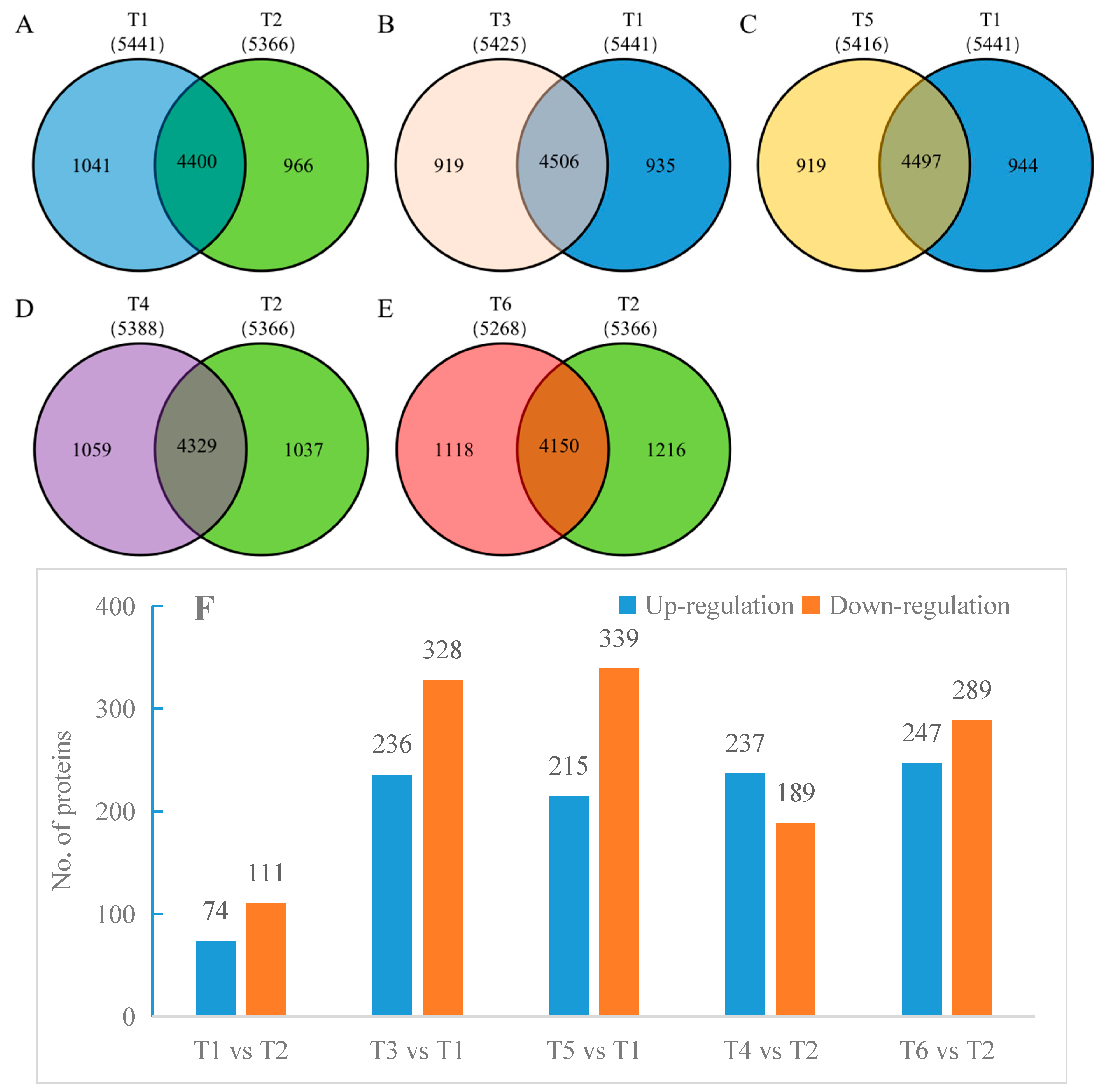
2.4. Effects of Salt Stress on Oat Root Proteomics
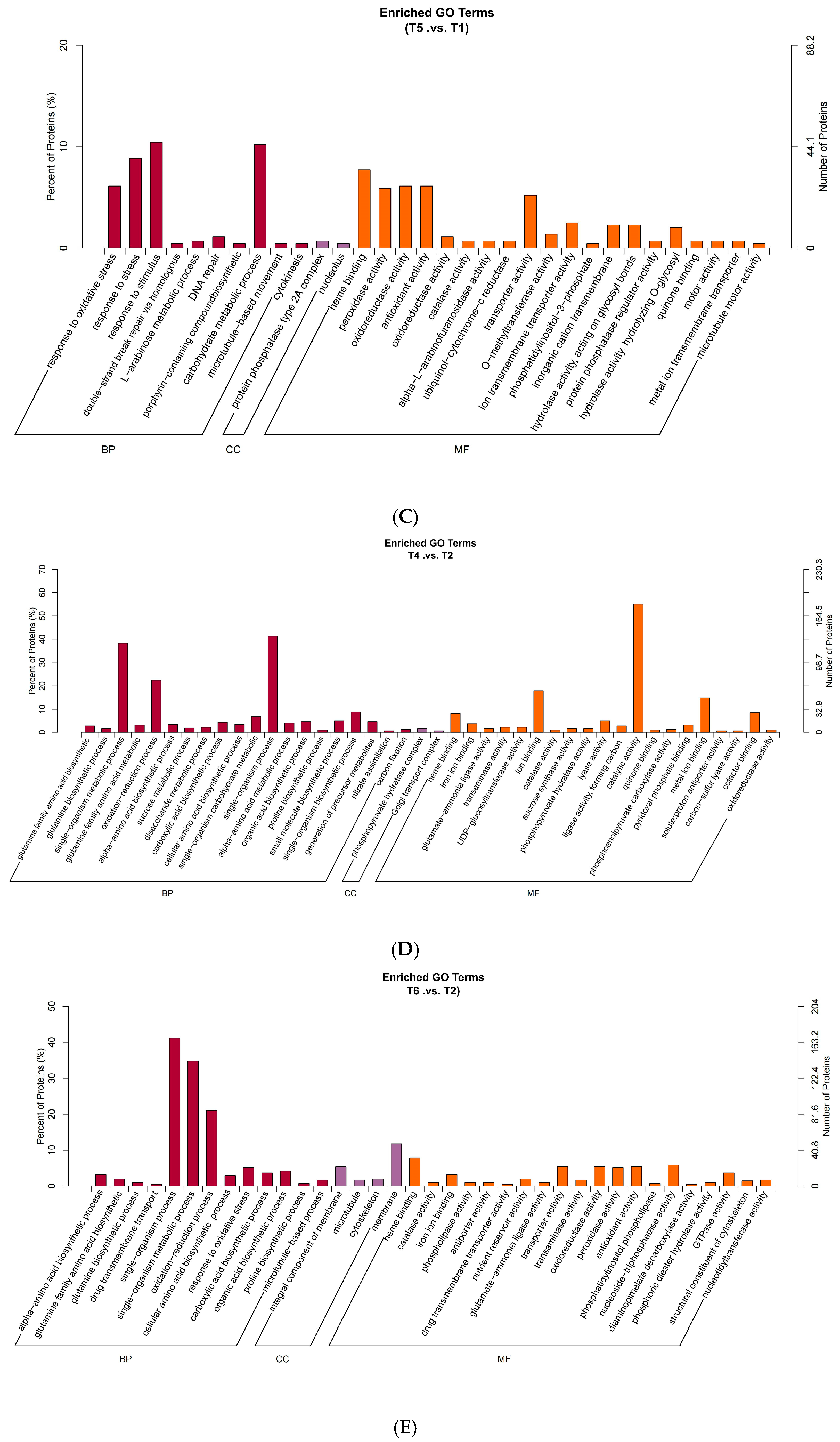
2.5. GO Functional Annotation and Enrichment Analysis of Oat Root Proteome Under Salt Stress
2.6. KEGG Functional Annotation and Enrichment Analysis of Oat Root Proteome Under Salt Stress
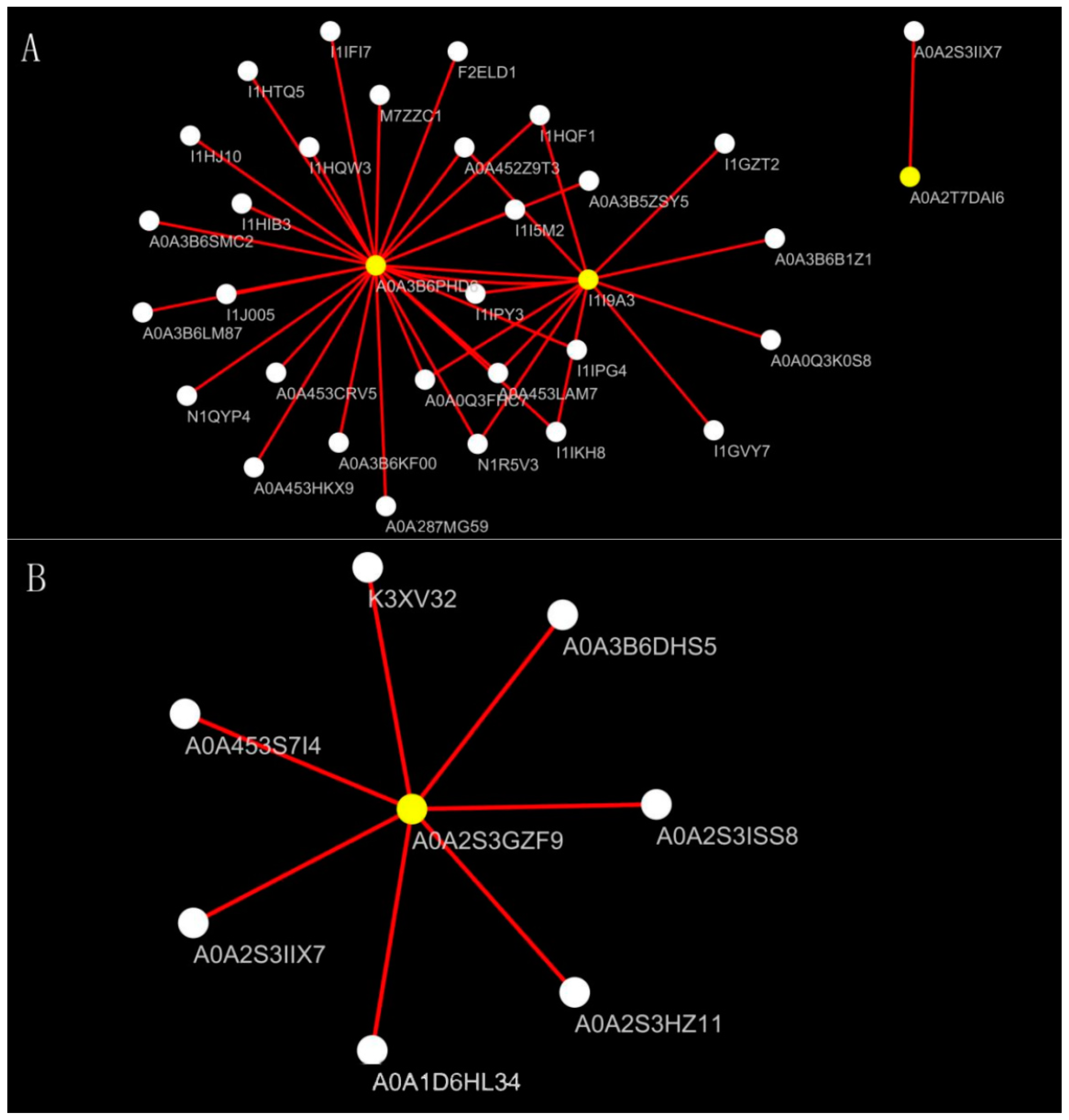
2.7. Screening Differential Proteins
2.8. Fluorescence Quantitative PCR Analysis
3. Discussion
3.1. Effects of Salt Stress on Physiological Growth Indicators of Oat
3.2. Effects of Salt Stress on Oat Root Respiratory Metabolism
3.3. Effects of Salt Stress on Oat Root Proteomics
4. Materials and Methods
4.1. Overview of Experimental Site
4.2. Test Materials
4.3. Experimental Design
4.4. Determination Indexes and Methods
4.4.1. Determination of Biomass and Root–Shoot Ratio of Oat Seedlings
4.4.2. Determination of Root Morphological Indexes
4.4.3. Determination of Root Ion Content
4.4.4. Determination of Root Respiration Rate
4.4.5. Measurement of Enzyme Activity Related to Respiratory Metabolism
- Pyruvate decarboxylase activity determination
- 2.
- Determination of alcohol dehydrogenase activity
- 3.
- Determination of lactate dehydrogenase (LDH) activity
- 4.
- Determination of malate dehydrogenase (NAD-MDH) activity
4.4.6. Root Proteome Determination and Methods
- Root pretreatment
- 2.
- Extraction of total protein from oat root
- 3.
- Protein quantification
- 4.
- Filter-aided sample preparation (FASP)
- 5.
- Nanoflow reversed-phase chromatography-Q Exactive for protein analysis
- 6.
- Mass spectrum data analysis
4.5. Data Processing and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mondal, S.; Borromeo, T.H. Screening of Salinity Tolerance of Rice at Early Seedling Stage. J. Biosci. Agric. Res. 2016, 10, 843–847. [Google Scholar] [CrossRef]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of Salt-induced Land Degradation and Restoration. In Proceedings of the Natural Resources Forum; Wiley Online Library: Hoboken, NJ, USA, 2014; Volume 38, pp. 282–295. [Google Scholar]
- Pessarakli, M.; Szabolcs, I. Soil Salinity and Sodicity as Particular Plant/Crop Stress Factors. In Handbook of Plant and Crop Stress, 4th ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 3–21. [Google Scholar]
- Nam, T.N.; Thia, L.H.; Mai, D.S.; Tuan, N.V. Overexpression of NbWRKY79 Enhances Salt Stress Tolerance in Nicotiana Benthamiana. Acta Physiol. Plant. 2017, 39, 121. [Google Scholar] [CrossRef]
- Wang, B.; Song, F.B. Physiological Responses and Adaptive Capacity of Oats to Saline-Alkali Stress. Ecol. Environ. 2006, 15, 625–629. [Google Scholar]
- Jia, X.; Wang, H.; Svetla, S.; Zhu, Y.; Hu, Y.; Cheng, L.; Zhao, T.; Wang, Y. Comparative Physiological Responses and Adaptive Strategies of Apple Malus Halliana to Salt, Alkali and Saline-Alkali Stress. Sci. Hortic. 2019, 245, 154–162. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, Metabolomics, and Ionomics Perspectives of Salinity Tolerance in Halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zhang, J.; Zhong, C.; Zhu, L.; Cao, X.; Yu, S.; Bohr, J.A.; Hu, J.; Jin, Q. Effects of Salt Stress on Rice Growth, Development Characteristics, and the Regulating Ways: A Review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B. Redefining Fine Roots Improves Understanding of Below-ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Van Dongen, J.T.; Gupta, K.J.; Ramírez-Aguilar, S.J.; Araújo, W.L.; Nunes-Nesi, A.; Fernie, A.R. Regulation of Respiration in Plants: A Role for Alternative Metabolic Pathways. J. Plant Physiol. 2011, 168, 1434–1443. [Google Scholar] [CrossRef]
- Li, L.; Lv, F.; Guo, Y.; Wang, Z. Respiratory Pathway Metabolism and Energy Metabolism Associated with Senescence in Postharvest Broccoli (Brassica oleracea L. Var. Italic style) Florets in Response to O2/CO2 Controlled Atmospheres. Postharvest Biol. Technol. 2016, 111, 330–336. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Balmer, Y. Redox Regulation: A Broadening Horizon. Annu. Rev. Plant Biol. 2005, 56, 187–220. [Google Scholar] [CrossRef]
- Tao, S.; Zhu, Y.; Pan, Y.; Zhang, Z.; Huang, L. Enhancement of Respiratory Metabolism of the Pentose Phosphate Pathway (PPP) Strengthens the Chilling Tolerance of Postharvest Papaya Fruit Stored at 1 °C. Postharvest Biol. Technol. 2022, 191, 111988. [Google Scholar] [CrossRef]
- Shu, L.; Lou, Q.; Ma, C.; Ding, W.; Zhou, J.; Wu, J.; Feng, F.; Lu, X.; Luo, L.; Xu, G. Genetic, Proteomic and Metabolic Analysis of the Regulation of Energy Storage in Rice Seedlings in Response to Drought. Proteomics 2011, 11, 4122–4138. [Google Scholar] [CrossRef]
- Fukao, T.; Xu, K.; Ronald, P.C.; Bailey-Serres, J. A Variable Cluster of Ethylene Response Factor–like Genes Regulates Metabolic and Developmental Acclimation Responses to Submergence in Rice. Plant Cell 2006, 18, 2021–2034. [Google Scholar] [CrossRef]
- Mustroph, A.; Barding, G.A., Jr.; Kaiser, K.A.; Larive, C.K.; Bailey-Serres, J. Characterization of Distinct Root and Shoot Responses to Low-oxygen Stress in A Rabidopsis with a Focus on Primary C-and N-metabolism. Plant Cell Environ. 2014, 37, 2366–2380. [Google Scholar] [CrossRef]
- Weits, D.A.; Kunkowska, A.B.; Kamps, N.C.W.; Portz, K.M.S.; Packbier, N.K.; Nemec Venza, Z.; Gaillochet, C.; Lohmann, J.U.; Pedersen, O.; van Dongen, J.T. An Apical Hypoxic Niche Sets the Pace of Shoot Meristem Activity. Nature 2019, 569, 714–717. [Google Scholar] [CrossRef]
- Rocha, M.; Licausi, F.; Araújo, W.L.; Nunes-Nesi, A.; Sodek, L.; Fernie, A.R.; Van Dongen, J.T. Glycolysis and the Tricarboxylic Acid Cycle Are Linked by Alanine Aminotransferase during Hypoxia Induced by Waterlogging of Lotus Japonicus. Plant Physiol. 2010, 152, 1501–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.; Yuan, Y.; Shu, S.; Sun, J.; Guo, S.; Yuan, R.; Tang, Y. Effects of Exogenous Putrescine on Glycolysis and Krebs Cycle Metabolism in Cucumber Leaves Subjected to Salt Stress. Plant Growth Regul. 2016, 79, 319–330. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The Role of Mitochondrial Respiration in Salinity Tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef]
- Li, L.; Xing, W.; Shao, Q.; Shu, S.; Sun, J.; Guo, S. The Effects of Grafting on Glycolysis and the Tricarboxylic Acid Cycle in Ca (NO3)2-Stressed Cucumber Seedlings with Pumpkin as Rootstock. Acta Physiol. Plant. 2015, 37, 259. [Google Scholar] [CrossRef]
- Che-Othman, M.H.; Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Wheat Mitochondrial Respiration Shifts from the Tricarboxylic Acid Cycle to the GABA Shunt under Salt Stress. New Phytol. 2020, 225, 1166–1180. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Morokuma, M. Ethanolic Fermentation and Anoxia Tolerance in Four Rice Cultivars. J. Plant Physiol. 2007, 164, 168–173. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Rumpho, M.E.; Fox, T.C. Anaerobic Metabolism in Plants. Plant Physiol. 1992, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Grover, A.; Peacock, W.J.; Dennis, E.S.; Ellis, M.H. Effects of Manipulation of Pyruvate Decarboxylase and Alcohol Dehydrogenase Levels on the Submergence Tolerance of Rice. Funct. Plant Biol. 2001, 28, 1231–1241. [Google Scholar] [CrossRef]
- Ismond, K.P.; Dolferus, R.; De Pauw, M.; Dennis, E.S.; Good, A.G. Enhanced Low Oxygen Survival in Arabidopsis through Increased Metabolic Flux in the Fermentative Pathway. Plant Physiol. 2003, 132, 1292–1302. [Google Scholar] [CrossRef] [PubMed]
- Kursteiner, O.; Dupuis, I.; Kuhlemeier, C. The Pyruvate Decarboxylase1 Gene of Arabidopsis Is Required during Anoxia but Not Other Environmental Stresses. Plant Physiol. 2003, 132, 968–978. [Google Scholar] [CrossRef]
- Richardson, S.G.; McCree, K.J. Carbon Balance and Water Relations of Sorghum Exposed to Salt and Water Stress. Plant Physiol. 1985, 79, 1015–1020. [Google Scholar] [CrossRef]
- Hwang, Y.; Morris, J.T. Whole-plant Gas Exchange Responses of Spartina alterniflora (Poaceae) to a Range of Constant and Transient Salinities. Am. J. Bot. 1994, 81, 659–665. [Google Scholar] [CrossRef]
- Moud, A.M.; Maghsoudi, K. Salt Stress Effects on Respiration and Growth of Germinated Seeds of Different Wheat (Triticum aestivum L.) Cultivars. World J. Agric. Sci 2008, 4, 351–358. [Google Scholar]
- Zou, Y.; Zhang, Y.; Testerink, C. Root Dynamic Growth Strategies in Response to Salinity. Plant Cell Environ. 2022, 45, 695–704. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity Tolerance in Halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Kernodle, S.P.; Margis-Pinheiro, M.; Scandalios, J.G. Salt-Induced Antioxidant Metabolism Defenses in Maize (Zea mays L.) Seedlings. Redox Rep. 2004, 9, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, K.; Eskandari, H. Effects of Salt Stress on Germination and Early Seedling Growth of Rice (Oryza sativa) Cultivars in Iran. Afr. J. Biotechnol. 2011, 10, 17789–17792. [Google Scholar] [CrossRef]
- Shimizu, K.; Matsuoka, Y. Redox Rebalance against Genetic Perturbations and Modulation of Central Carbon Metabolism by the Oxidative Stress Regulation. Biotechnol. Adv. 2019, 37, 107441. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Fahad, S.; Tanveer, M.; Saud, S.; Khan, I.A. Plant Responses and Tolerance to Salt Stress. In Approaches for Enhancing Abiotic Stress Tolerance in Plants; CRC Press: Boca Raton, FL, USA, 2019; pp. 61–78. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Shabala, S.; Wu, H.; Bose, J. Salt Stress Sensing and Early Signalling Events in Plant Roots: Current Knowledge and Hypothesis. Plant Sci. 2015, 241, 109–119. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, H.; Shabala, S.; Li, H.; Yang, X.; Zhang, H. Tissue Tolerance Mechanisms Conferring Salinity Tolerance in a Halophytic Perennial Species Nitraria Sibirica Pall. Tree Physiol. 2021, 41, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin Induces Tolerance to Salt/Osmotic Stress through the Regulation of Nitrogen Metabolism, Cellular Redox and ROS Scavenging Capacity in Bean Plants. Plant Physiol. Biochem. 2020, 157, 264–275. [Google Scholar] [CrossRef]
- Dai, L.; Li, P.; Li, Q.; Leng, Y.; Zeng, D.; Qian, Q. Integrated Multi-Omics Perspective to Strengthen the Understanding of Salt Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 5236. [Google Scholar] [CrossRef]
- Jin, T.; An, J.; Xu, H.; Chen, J.; Pan, L.; Zhao, R.; Wang, N.; Gai, J.; Li, Y. A Soybean Sodium/Hydrogen Exchanger GmNHX6 Confers Plant Alkaline Salt Tolerance by Regulating Na+/K+ Homeostasis. Front. Plant Sci. 2022, 13, 938635. [Google Scholar] [CrossRef]
- Wang, C.-F.; Han, G.-L.; Qiao, Z.-Q.; Li, Y.-X.; Yang, Z.-R.; Wang, B.-S. Root Na+ Content Negatively Correlated to Salt Tolerance Determines the Salt Tolerance of Brassica napus L. Inbred Seedlings. Plants 2022, 11, 906. [Google Scholar] [CrossRef]
- Xiao, L.; Shi, Y.; Wang, R.; Feng, Y.; Wang, L.; Zhang, H.; Shi, X.; Jing, G.; Deng, P.; Song, T. The Transcription Factor OsMYBc and an E3 Ligase Regulate Expression of a K+ Transporter during Salt Stress. Plant Physiol. 2022, 190, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, G.; Shen, Z.; Li, J.; Hao, D.; Kong, W.; Wang, H.; Zhang, L.; Chen, J.; Guo, H. Na+ and K+ Homeostasis in Different Organs of Contrasting Zoysia Japonica Accessions under Salt Stress. Environ. Exp. Bot. 2023, 214, 105455. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Biochar-Based Metal Oxide Nanocomposites of Magnesium and Manganese Improved Root Development and Productivity of Safflower (Carthamus tinctorius L.) under Salt Stress. Rhizosphere 2021, 19, 100416. [Google Scholar] [CrossRef]
- Gong, W.; Wang, B.C. The Role of Ca2+ in Plant Response to Abiotic Stress. Chem. Life 2011, 31, 107–111. [Google Scholar]
- Mustroph, A.; Albrecht, G. Tolerance of Crop Plants to Oxygen Deficiency Stress: Fermentative Activity and Photosynthetic Capacity of Entire Seedlings under Hypoxia and Anoxia. Physiol. Plant. 2003, 117, 508–520. [Google Scholar] [CrossRef]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C. Water and Salinity Stress in Grapevines: Early and Late Changes in Transcript and Metabolite Profiles. Funct. Integr. Genom. 2007, 7, 111–134. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S.; Lambers, H.; Oliveira, R.S. Photosynthesis, Respiration, and Long-Distance Transport: Photosynthesis. In Plant Physiological Ecology; Springer: Cham, Switzerland, 2019; pp. 11–114. [Google Scholar]
- Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Investigating the Role of Respiration in Plant Salinity Tolerance by Analyzing Mitochondrial Proteomes from Wheat and a Salinity-Tolerant Amphiploid (Wheat × Lophopyrum elongatum). J. Proteome Res. 2013, 12, 4807–4829. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Gao, Y.; He, X.; Zhang, D.; Liu, B.; Li, Q. Effects of CO2 Enrichment on Non-Structural Carbohydrate Metabolism in Leaves of Cucumber Seedlings under Salt Stress. Sci. Hortic. 2020, 265, 109275. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; Wang, J.; Yang, J. Molecular Cloning and Characterization of Rice 6-Phosphogluconate Dehydrogenase Gene That Is up-Regulated by Salt Stress A. Mol. Biol. Rep. 2003, 30, 223–227. [Google Scholar] [CrossRef]
- Yuxi, Z.; Dan, Y.; Chunying, L.; Shupeng, G. Dynamic of Carbohydrate Metabolism and the Related Genes Highlights PPP Pathway Activation during Chilling Induced Bud Dormancy Release in Tree Peony (Paeonia suffruticosa). Sci. Hortic. 2018, 242, 36–43. [Google Scholar] [CrossRef]
- Guo, S.R.; Nada, K.; Katoh, H.; Tachibana, S. Differences between Tomato (Lycopersicon esuculentum Mill.) and Cucumber (Cucumis sativus L.) in Ethanol, Lactate and Malate Metabolisms and Cell Sap PH of Roots under Hypoxia. J. Jpn. Soc. Hortic. Sci. 1999, 68, 152–159. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-Aeration Following Hypoxia or Anoxia Leads to Activation of the Antioxidative Defense System in Roots of Wheat Seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Muench, D.G.; Archibold, O.W.; Good, A.G. Hypoxic Metabolism in Wild Rice (Zizania palustris): Enzyme Induction and Metabolite Production. Physiol. Plant. 1993, 89, 165–171. [Google Scholar] [CrossRef]
- Kidrič, M.; Kos, J.; Sabotič, J. Proteases and Their Endogenous Inhibitors in the Plant Response to Abiotic Stress. Bot. Serbica 2014, 38, 139–158. [Google Scholar]
- Zadražnik, T.; Hollung, K.; Egge-Jacobsen, W.; Meglič, V.; Šuštar-Vozlič, J. Differential Proteomic Analysis of Drought Stress Response in Leaves of Common Bean (Phaseolus vulgaris L.). J. Proteom. 2013, 78, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Xie, C.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root Proteome of Rice Studied by ITRAQ Provides Integrated Insight into Aluminum Stress Tolerance Mechanisms in Plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef]
- Raessler, M.; Wissuwa, B.; Breul, A.; Unger, W.; Grimm, T. Chromatographic Analysis of Major Non-Structural Carbohydrates in Several Wood Species–an Analytical Approach for Higher Accuracy of Data. Anal. Methods 2010, 2, 532–538. [Google Scholar] [CrossRef]
- Cate, R.L.; Roche, T.E.; Davis, L.C. Rapid Intersite Transfer of Acetyl Groups and Movement of Pyruvate Dehydrogenase Component in the Kidney Pyruvate Dehydrogenase Complex. J. Biol. Chem. 1980, 255, 7556–7562. [Google Scholar] [CrossRef]
- Abbasi, G.H.; Akhtar, J.; Anwar-ul-Haq, M.; Ali, S.; Chen, Z.; Malik, W. Exogenous Potassium Differentially Mitigates Salt Stress in Tolerant and Sensitive Maize Hybrids. Pak. J. Bot. 2014, 46, 135–146. [Google Scholar]
- Büyük, İ.; Soydam-Aydin, S.; Aras, S. Bitkilerin Stres Koşullarına Verdiği Moleküler Cevaplar. Turk. Bull. Hyg. Exp. Biol. Hij. Deney. Biyol. 2012, 69, 97–110. [Google Scholar]
- Kang, G.Z.; Li, G.Z.; Liu, G.Q.; Xu, W.; Peng, X.Q.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Exogenous Salicylic Acid Enhances Wheat Drought Tolerance by Influence on the Expression of Genes Related to Ascorbate-Glutathione Cycle. Biol. Plant. 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Koh, J.; Chen, G.; Yoo, M.-J.; Zhu, N.; Dufresne, D.; Erickson, J.E.; Shao, H.; Chen, S. Comparative Proteomic Analysis of Brassica Napus in Response to Drought Stress. J. Proteome Res. 2015, 14, 3068–3081. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.K.; Vikram, P.; Yadaw, R.B.; Swamy, B.P.M.; Dixit, S.; Cruz, M.T.S.; Maturan, P.; Marker, S.; Kumar, A. QDTY 12. 1: A Locus with a Consistent Effect on Grain Yield under Drought in Rice. BMC Genet. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Lu, X.; Zhao, B.; Yang, Y.; Liu, J. Integrative Analysis of Transcriptome and Metabolome Reveal Mechanism of Tolerance to Salt Stress in Oat (Avena sativa L.). Plant Physiol. Biochem. 2021, 160, 315–328. [Google Scholar] [CrossRef]







| Treatment | 0 mM | 100 mM | 150 mM | |
|---|---|---|---|---|
| Cultivar | ||||
| Bai2 | T1 | T3 | T5 | |
| Bai5 | T2 | T4 | T6 | |
| Treatment | Fresh Weight (g) | Dry Weight (g) | Root–Shoot Ratio | ||
|---|---|---|---|---|---|
| Aboveground | Underground | Aboveground | Underground | ||
| T1 | 0.445 ± 0.09 ab | 0.110 ± 0.02 ab | 0.047 ± 0.01 ab | 0.008 ± 0.00 b | 0.182 ± 0.03 ab |
| T2 | 0.502 ± 0.07 a | 0.166 ± 0.08 a | 0.053 ± 0.01 a | 0.014 ± 0.01 a | 0.257 ± 0.10 ab |
| T3 | 0.373 ± 0.11 abc | 0.060 ± 0.03 bc | 0.048 ± 0.01 ab | 0.004 ± 0.00 b | 0.083 ± 0.03 c |
| T4 | 0.323 ± 0.05 bc | 0.042 ± 0.01 c | 0.046 ± 0.01 ab | 0.005 ± 0.00 b | 0.108 ± 0.00 bc |
| T5 | 0.343 ± 0.02 bc | 0.055 ± 0.01 bc | 0.044 ± 0.00 ab | 0.005 ± 0.00 b | 0.124 ± 0.04 bc |
| T6 | 0.268 ± 0.08 c | 0.020 ± 0.01 c | 0.036 ± 0.00 b | 0.004 ± 0.00 b | 0.112 ± 0.02 bc |
| Treatment | Length (cm) | Surface Area (cm2) | Volume (cm3) | Average Diameter (mm) |
|---|---|---|---|---|
| T1 | 224.53 ± 6.46 a | 41.43 ± 1.20 a | 1.47 ± 0.06 a | 0.62 ± 0.00 a |
| T2 | 209.17 ± 2.85 b | 36.92 ± 0.44 b | 1.27 ± 0.03 b | 0.61 ± 0.00 ab |
| T3 | 184.97 ± 4.90 c | 31.57 ± 1.58 c | 1.06 ± 0.07 c | 0.60 ± 0.02 b |
| T4 | 162.20 ± 4.03 d | 26.90 ± 0.48 d | 0.77 ± 0.04 d | 0.57 ± 0.00 c |
| T5 | 142.33 ± 5.22 e | 23.69 ± 1.36 e | 0.56 ± 0.04 e | 0.56 ± 0.00 c |
| T6 | 124.20 ± 9.00 f | 20.45 ± 0.71 f | 0.32 ± 0.01 f | 0.54 ± 0.00 d |
| Soil Type | pH | Salt Content (g·kg−1) | Organic Matter (g·kg−1) | Alkaline Hydrolysis of Nitrogen (mg·kg−1) | Available Phosphorus (mg·kg−1) | Quick-Acting Potassium (mg·kg−1) |
|---|---|---|---|---|---|---|
| Clay | 7.81 | 0.82 | 20.60 | 55.18 | 1.61 | 145.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Zhao, B.; Mi, J.; Xu, Z.; Liu, J. Label-Free Proteomics Reveals the Response of Oat (Avena sativa L.) Seedling Root Respiratory Metabolism to Salt Stress. Int. J. Mol. Sci. 2025, 26, 2630. https://doi.org/10.3390/ijms26062630
Chen X, Zhao B, Mi J, Xu Z, Liu J. Label-Free Proteomics Reveals the Response of Oat (Avena sativa L.) Seedling Root Respiratory Metabolism to Salt Stress. International Journal of Molecular Sciences. 2025; 26(6):2630. https://doi.org/10.3390/ijms26062630
Chicago/Turabian StyleChen, Xiaojing, Baoping Zhao, Junzhen Mi, Zhongshan Xu, and Jinghui Liu. 2025. "Label-Free Proteomics Reveals the Response of Oat (Avena sativa L.) Seedling Root Respiratory Metabolism to Salt Stress" International Journal of Molecular Sciences 26, no. 6: 2630. https://doi.org/10.3390/ijms26062630
APA StyleChen, X., Zhao, B., Mi, J., Xu, Z., & Liu, J. (2025). Label-Free Proteomics Reveals the Response of Oat (Avena sativa L.) Seedling Root Respiratory Metabolism to Salt Stress. International Journal of Molecular Sciences, 26(6), 2630. https://doi.org/10.3390/ijms26062630




