Notch Is Required for Neural Progenitor Proliferation During Embryonic Eye Regrowth
Abstract
1. Introduction
2. Results
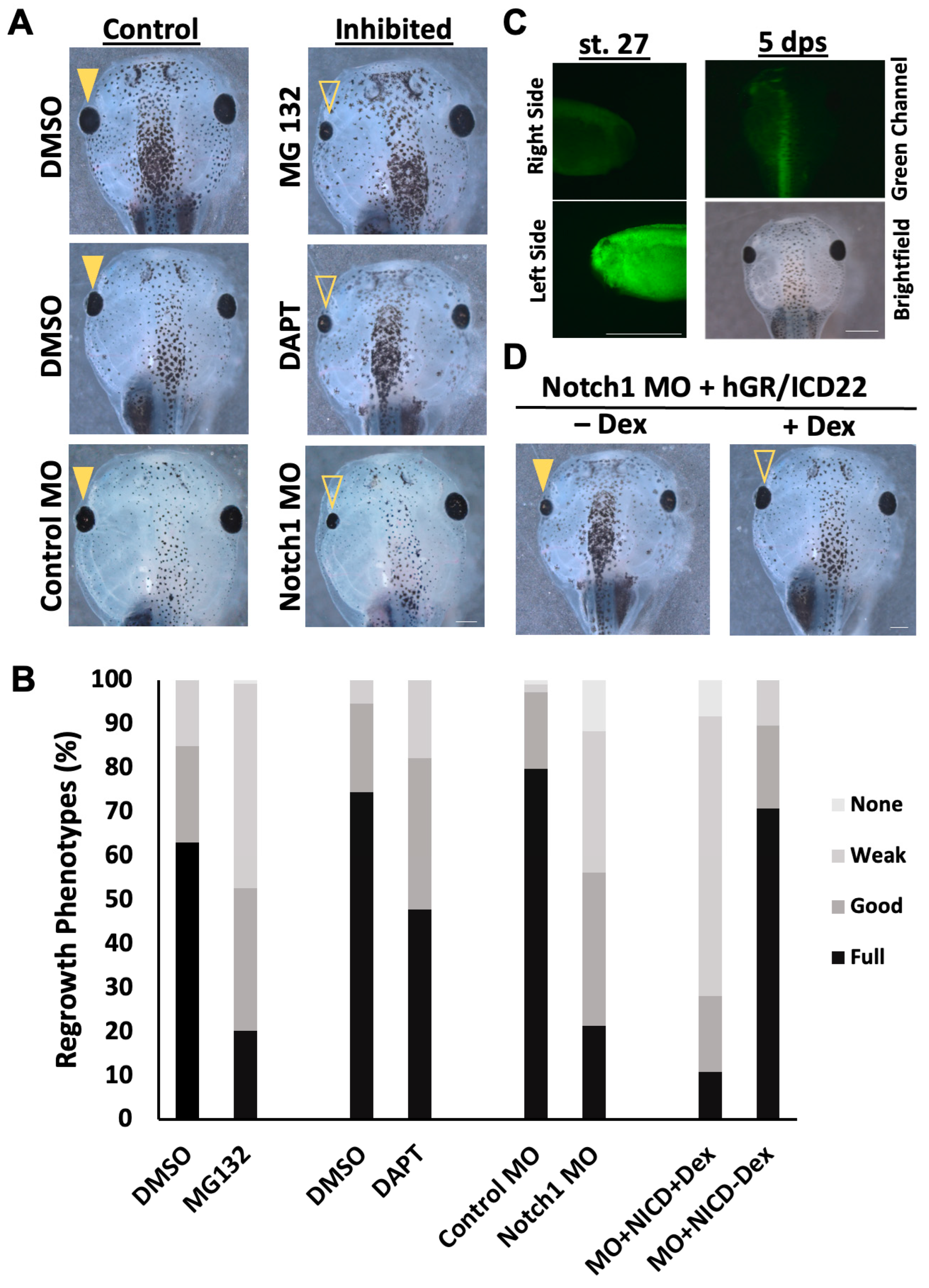
2.1. Reduction in Notch Signaling Following Eye Ablation Inhibits Regrowth
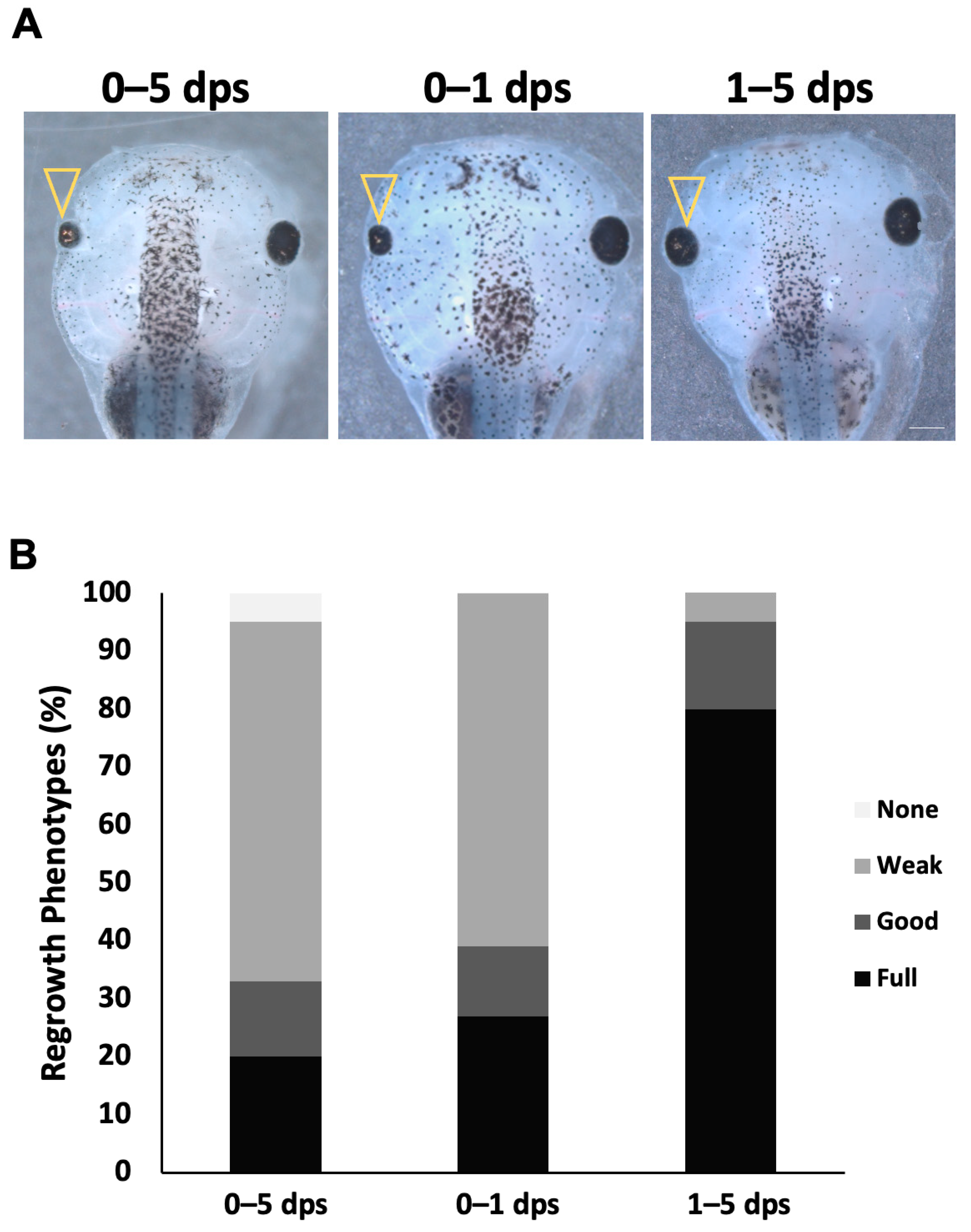
2.2. Notch Is Required During the First Day of Regrowth
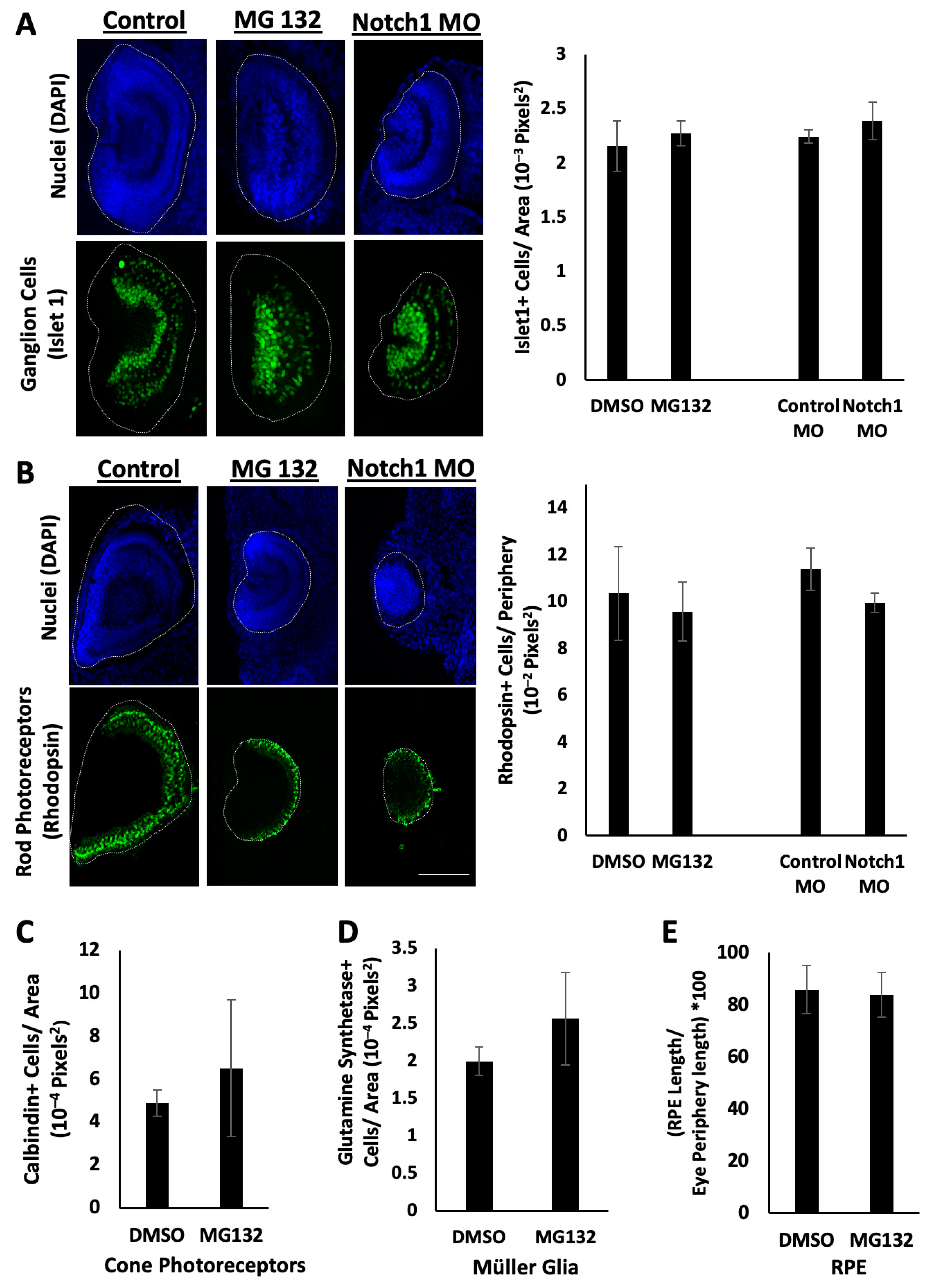
2.3. Retinal Differentiation Occurs During Notch Inhibition
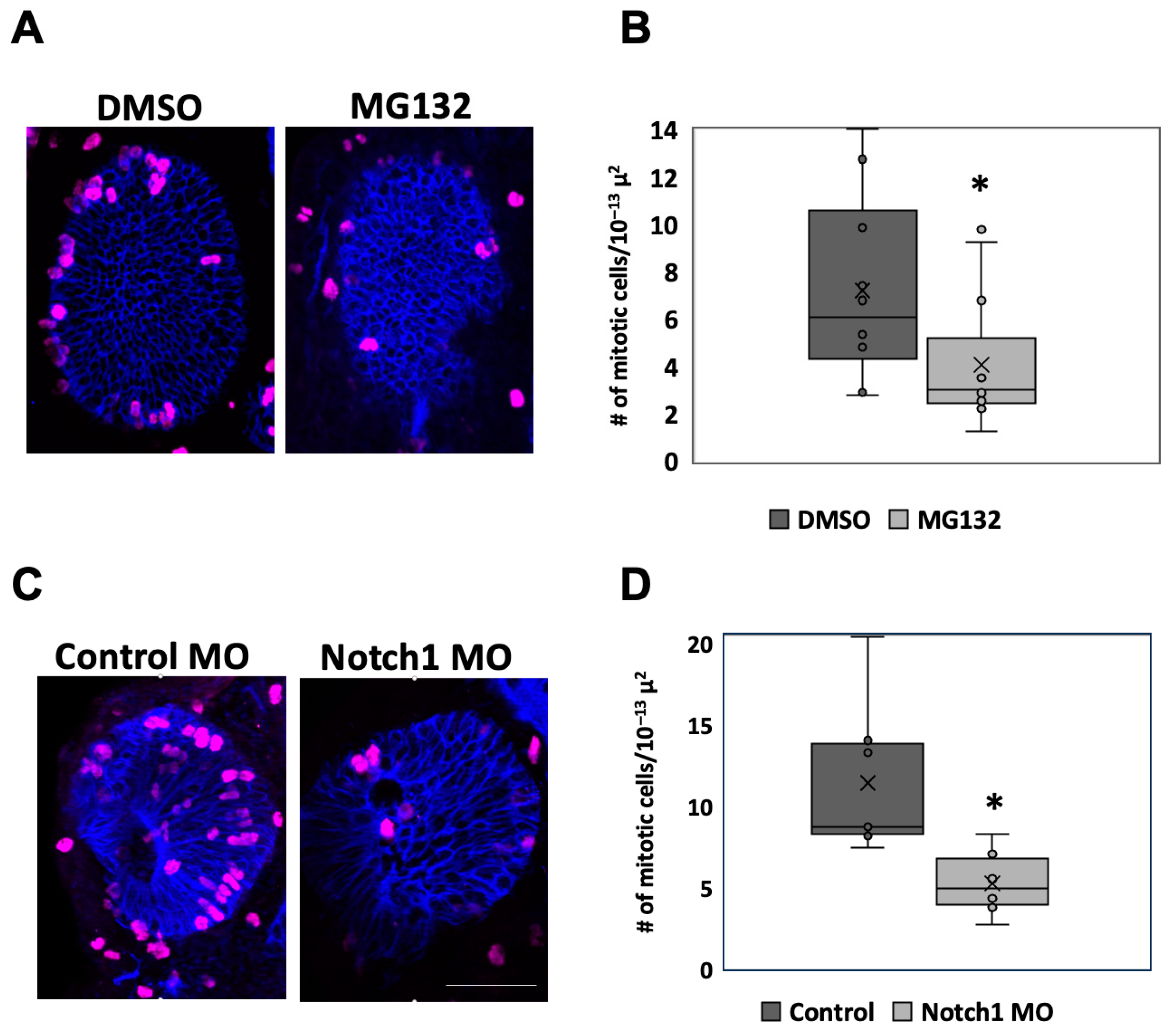
2.4. Inhibition of Notch Function Downregulates Retinal Proliferation
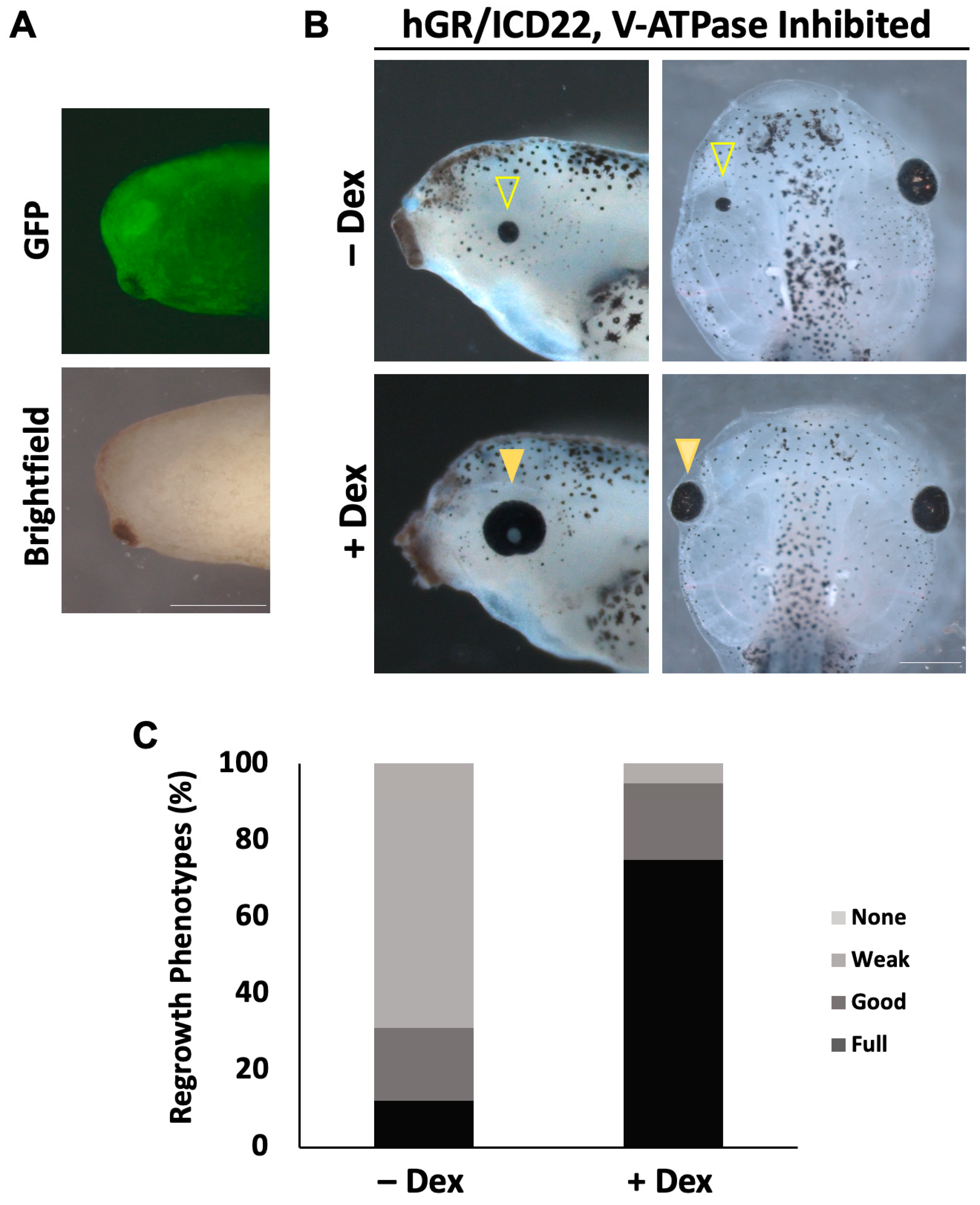
2.5. Notch1 Overexpression Restores Eye Regrowth During V-ATPase Inhibition
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illingworth, C.M. Trapped fingers and amputated finger tips in children. J. Pediatr. Surg. 1974, 9, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, G.K.; DrFrances, M.C. Liver Regeneration. Science 1997, 276, 60–66. [Google Scholar] [CrossRef]
- Slack, J.M.W.; Lin, G.; Chen, Y. The Xenopus tadpole: A new model for regeneration research. Cell. Mol. Life Sci. 2007, 65, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Reddien, P. The cellular basis for animal regeneration. Dev. Cell 2011, 21, 172–185. [Google Scholar] [CrossRef]
- Seifert, A.W.; Kiama, S.G.; Seifert, M.G.; Goheen, J.R.; Palmer, T.M.; Maden, M. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature 2012, 489, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Inflamation in cardiac injury, repair and regeneration. Curr. Opin. Cardiol. 2016, 30, 240–245. [Google Scholar] [CrossRef]
- Tanaka, E.M. The molecular and cellular choreography of appendage regeneration. Cell 2016, 165, 1598–1608. [Google Scholar] [CrossRef]
- Tseng, A.S. Seeing the future: Using Xenopus to understand eye regeneration. Genesis 2017, 55, e23003. [Google Scholar] [CrossRef] [PubMed]
- Joven, A.; Simon, A. Homeostatic and regenerative neurogenesis in salamanders. Prog. Neurobiol. 2018, 170, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Kha, C.X.; Son, P.H.; Lauper, J.; Tseng, K.A. A model for investigating developmental eye repair in Xenopus laevis. Exp. Eye Res. 2018, 169, 38–47. [Google Scholar] [CrossRef]
- Gurdon, J.B.; Hopwood, N. The introduction of Xenopus laevis into developmental biology: Of empire, pregnancy testing and ribosomal genes. Int. J. Dev. Biol. 2000, 44, 43–50. [Google Scholar] [PubMed]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Early Development of Xenopus laevis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2000. [Google Scholar]
- Wlizla, M.; McNamara, S.; Horb, M.E. Generation and Care of Xenopus laevis and Xenopus tropicalis Embryos. Methods Mol. Biol. 2018, 1865, 19–32. [Google Scholar]
- Dent, J.N. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J. Morphol. 1962, 110, 61–77. [Google Scholar] [CrossRef]
- Endo, T.; Tamura, K.; Ide, H. Analysis of gene expressions during Xenopus forelimb regeneration. Dev. Biol. 2000, 220, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yakushiji, N.; Nakada, Y.; Satoh, A.; Ide, H.; Tamura, K. Limb regeneration in Xenopus laevis froglet. Sci. J. 2006, 6 (Suppl. S1), 26–37. [Google Scholar] [CrossRef]
- Vergara, M.N.; Del Rio-Tsonis, K. Retinal regeneration in the Xenopus laevis tadpole: A new model system. Mol. Vis. 2009, 15, 1000–1013. [Google Scholar]
- Mitogawa, K.; Makanae, A.; Satoh, A. Hyperinnervation improves Xenopus laevis limb regeneration. Dev. Biol. 2018, 433, 276–286. [Google Scholar] [CrossRef]
- Slack, J.M.; Beck, C.W.; Gargioli, C.; Christen, B. Cellular and molecular mechanisms of regeneration in Xenopus. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2004, 359, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.W.; Izpisúa Belmonte, J.C.; Christen, B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2009, 238, 1226–1248. [Google Scholar] [CrossRef]
- Kha, C.X.; Tseng, K.A. Developmental dependence for functional eye regrowth in Xenopus laevis. Neural Regen. Res. 2018, 13, 1735–1737. [Google Scholar]
- Kha, C.X.; Guerin, D.J.; Tseng, K.A. Using the Xenopus Developmental Eye Regrowth System to Distinguish the Role of Developmental Versus Regenerative Mechanisms. Front. Physiol. 2019, 10, 502. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.M.; Anderson, R.M. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch. Pathol. 1931, 12, 186. [Google Scholar]
- Eguchi, G.; Shingai, R. Cellular analysis on localization of lens forming potency in the newt iris epithelium. Dev. Growth Differ. 1971, 13, 337–349. [Google Scholar] [CrossRef]
- Yamada, T. Control mechanisms in cell-type conversion in newt lens regeneration. Monogr. Dev. Biol. 1977, 13, 1–126. [Google Scholar] [PubMed]
- Yoshii, C.; Ueda, Y.; Okamoto, M.; Araki, M. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: Transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol. 2007, 303, 45–56. [Google Scholar] [CrossRef]
- Del Rio-Tsonis, K.; Jung, J.C.; Chiu, I.M.; Tsonis, P.A. Conservation of fibroblast growth factor function in lens regeneration. Proc. Natl. Acad. Sci. USA 1997, 94, 13701–13706. [Google Scholar] [CrossRef]
- Suzuki, M.; Satoh, A.; Ide, H.; Tamura, K. Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3K/AKT pathways in blastema formation during limb regeneration. Dev. Biol. 2007, 304, 675–686. [Google Scholar] [CrossRef]
- Bray, S.J. Notch signalling in context. Nature reviews. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar]
- Coffman, C.R.; Skoglund, P.; Harris, W.A.; Kintner, C.R. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell 1993, 73, 659–671. [Google Scholar] [CrossRef]
- Henrique, D.; Hirsinger, E.; Adam, J.; Le Roux, I.; Pourquié, O.; Ish-Horowicz, D.; Lewis, J. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr. Biol. 1997, 7, 661–670. [Google Scholar] [CrossRef]
- Hitoshi, S.; Alexson, T.; Tropepe, V.; Donoviel, D.; Elia, A.J.; Nye, J.S.; Conlon, R.A.; Mak, T.W.; Bernstein, A.; van der Kooy, D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002, 16, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Borggrefe, T.; Oswald, F. The Notch signaling pathway: Transcriptional regulation at Notch target genes. Cell. Mol. Life Sci. 2009, 66, 1631–1646. [Google Scholar] [CrossRef]
- Reddy, B.V.; Rauskolb, C.; Irvine, K.D. Influence of fat-hippo and notch signaling on the proliferation and differentiation of Drosophila optic neuroepithelia. Development 2010, 137, 2397–2408. [Google Scholar] [CrossRef]
- Baonza, A.; Garcia-Bellido, A. Notch signaling directly controls cell proliferation in the Drosophila wing disc. Proc. Natl. Acad. Sci. USA 2000, 97, 2609–2614. [Google Scholar] [CrossRef]
- VanDussen, K.L.; Carulli, A.J.; Keeley, T.M.; Patel, S.R.; Puthoff, B.J.; Magness, S.T.; Tran, I.T.; Maillard, I.; Siebel, C.; Kolterud, Å.; et al. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development 2012, 139, 488–497. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zou, L. Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Exp. Ther. Med. 2019, 18, 1884–1890. [Google Scholar] [CrossRef]
- Beck, C.W.; Christen, B.; Slack, J.M. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev. Cell 2003, 5, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Dorsky, R.I.; Rapaport, D.H.; Harris, W.A. Xotch inhibits cell differentiation in the Xenopus retina. Neuron 1995, 14, 487–496. [Google Scholar] [CrossRef]
- Furukawa, T.; Mukherjee, S.; Bao, Z.Z.; Morrow, E.M.; Cepko, C.L. rax, Hes1, and notch1 promote the formation of Müller glia by postnatal retinal progenitor cells. Neuron 2000, 26, 383–394. [Google Scholar] [CrossRef]
- Kha, C.X.; Nava, I.; Tseng, K.A. V-ATPase Regulates Retinal Progenitor Cell Proliferation During Eye Regrowth in Xenopus. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2023, 39, 499–508. [Google Scholar] [CrossRef]
- Blair, S.S. Eye development: Notch lends a handedness. Curr. Biol. 1999, 9, R356–R360. [Google Scholar] [CrossRef]
- Mills, E.A.; Goldman, D. The Regulation of Notch Signaling in Retinal Development and Regeneration. Curr. Pathobiol. Rep. 2017, 5, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Reichrath, J.; Reichrath, S. Notch Signaling and Embryonic Development: An Ancient Friend, Revisited. Adv. Exp. Med. Biol. 2020, 1218, 9–37. [Google Scholar]
- Zaghloul, N.A.; Moody, S.A. Alterations of rx1 and pax6 expression levels at neural plate stages differentially affect the production of retinal cell types and maintenance of retinal stem cell qualities. Dev. Biol. 2007, 306, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.W.; Slack, J.M. Notch is required for outgrowth of the Xenopus tail bud. Int. J. Dev. Biol. 2002, 46, 255–258. [Google Scholar]
- Geling, A.; Steiner, H.; Willem, M.; Bally-Cuif, L.; Haass, C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002, 3, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Liu, L.; Sahlgren, C.; Dahlqvist, C.; Lendahl, U. High levels of Notch signaling down-regulate Numb and Numblike. J. Cell Biol. 2006, 175, 535–540. [Google Scholar] [CrossRef]
- Zhou, W.; He, Q.; Zhang, C.; He, X.; Cui, Z.; Liu, F.; Li, W. BLOS2 negatively regulates Notch signaling during neural and hematopoietic stem and progenitor cell development. eLife 2016, 5, e18108. [Google Scholar] [CrossRef]
- Dong, Z.; Huo, J.; Liang, A.; Chen, J.; Chen, G.; Liu, D. Gamma-Secretase Inhibitor (DAPT), a potential therapeutic target drug, caused neurotoxicity in planarian regeneration by inhibiting Notch signaling pathway. Sci. Total Environ. 2021, 781, 146735. [Google Scholar] [CrossRef]
- Xu, X.; Wu, Y.; Li, H.; Xie, J.; Cao, D.; Huang, X. Notch pathway inhibitor DAPT accelerates in vitro proliferation and adipogenesis in infantile hemangioma stem cells. Oncol. Lett. 2021, 22, 854. [Google Scholar] [CrossRef]
- Vladar, E.K.; Kunimoto, K.; Rojas-Hernandez, L.S.; Spano, J.M.; Sellers, Z.M.; Joo, N.S.; Cooney, R.A.; Axelrod, J.D.; Milla, C.E. Notch signaling inactivation by small molecule γ-secretase inhibitors restores the multiciliated cell population in the airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L771–L782. [Google Scholar] [CrossRef]
- López, S.L.; Paganelli, A.R.; Siri, M.V.; Ocaña, O.H.; Franco, P.G.; Carrasco, A.E. Notch activates sonic hedgehog and both are involved in the specification of dorsal midline cell-fates in Xenopus. Development 2003, 130, 2225–2238. [Google Scholar] [CrossRef]
- Revinski, D.R.; Paganelli, A.R.; Carrasco, A.E.; López, S.L. Delta-Notch signaling is involved in the segregation of the three germ layers in Xenopus laevis. Dev. Biol. 2010, 339, 477–492. [Google Scholar] [CrossRef]
- Castro Colabianchi, A.M.; Revinski, D.R.; Encinas, P.I.; Baez, M.V.; Monti, R.J.; Rodríguez Abinal, M.; Kodjabachian, L.; Franchini, L.F.; López, S.L. Notch1 is asymmetrically distributed from the beginning of embryogenesis and controls the ventral center. Development 2018, 145, dev159368. [Google Scholar] [CrossRef]
- Pallavi, S.K.; Ho, D.M.; Hicks, C.; Miele, L.; Artavanis-Tsakonas, S. Notch and Mef2 synergize to promote proliferation and metastasis through JNK signal activation in Drosophila. EMBO J. 2012, 31, 2895–2907. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.A.; Hartenstein, V. Neuronal determination without cell division in Xenopus embryos. Neuron 1991, 6, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Go, M.J.; Eastman, D.S.; Artavanis-Tsakonas, S. Cell proliferation control by Notch signaling in Drosophila development. Development 1998, 125, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Masi, A.; Levin, M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development 2007, 134, 1323–1335. [Google Scholar] [CrossRef]
- Miller, K.E.; Cadart, C.; Heald, R. Dodecaploid Xenopus longipes provides insight into the emergence of size scaling relationships during development. Curr. Biol. 2023, 33, 1327–1336.e4. [Google Scholar] [CrossRef]
- Lange, C.; Prenninger, S.; Knuckles, P.; Taylor, V.; Levin, M.; Calegari, F. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011, 20, 843–850. [Google Scholar] [CrossRef]
- Huss, M.; Ingenhorst, G.; König, S.; Gassel, M.; Dröse, S.; Zeeck, A.; Altendorf, K.; Wieczorek, H. Concanamycin A, the specific inhibitor of V-ATPases, binds to the V(o) subunit c. J. Biol. Chem. 2002, 277, 40544–40548. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Matsumoto, G.; Hanyu, Y. Pax-6 expression during retinal regeneration in the adult newt. Dev. Growth Differ. 1999, 41, 723–729. [Google Scholar] [CrossRef]
- Osakada, F.; Ooto, S.; Akagi, T.; Mandai, M.; Akaike, A.; Takahashi, M. Wnt signaling promotes regeneration in the retina of adult mammals. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 4210–4219. [Google Scholar] [CrossRef]
- Spence, J.R.; Aycinena, J.C.; Del Rio-Tsonis, K. Fibroblast growth factor-hedgehog interdependence during retina regeneration. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2007, 236, 1161–1174. [Google Scholar] [CrossRef]
- Hochmann, S.; Kaslin, J.; Hans, S.; Weber, A.; Machate, A.; Geffarth, M.; Funk, R.H.; Brand, M. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS ONE 2012, 7, e30365. [Google Scholar] [CrossRef]
- Todd, L.; Squires, N.; Suarez, L.; Fischer, A.J. Jak/Stat signaling regulates the proliferation and neurogenic potential of Müller glia-derived progenitor cells in the avian retina. Sci. Rep. 2016, 6, 35703. [Google Scholar] [CrossRef]
- Todd, L.; Suarez, L.; Quinn, C.; Fischer, A.J. Retinoic Acid-Signaling Regulates the Proliferative and Neurogenic Capacity of Müller Glia-Derived Progenitor Cells in the Avian Retina. Stem Cells 2018, 36, 392–405. [Google Scholar] [CrossRef]
- Gao, H.A.; Huang, X.; Chen, X.; Xu, H. Müller Glia-Mediated Retinal Regeneration. Mol. Neurobiol. 2021, 58, 2342–2361. [Google Scholar] [CrossRef]
- Perron, M.; Kanekar, S.; Vetter, M.L.; Harris, W.A. The genetic sequence of retinal development in the ciliary margin of the Xenopus eye. Dev. Biol. 1998, 199, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, D. Retinal neurogenesis. In Retinal Development; Sernagor, E., Eglen, S., Harris, B., Wong, R., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 30–58. [Google Scholar]
- Borghese, L.; Dolezalova, D.; Opitz, T.; Haupt, S.; Leinhaas, A.; Steinfarz, B.; Koch, P.; Edenhofer, F.; Hampl, A.; Brüstle, O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 2010, 28, 955–964. [Google Scholar] [CrossRef]
- Alhashem, Z.; Feldner-Busztin, D.; Revell, C.; Alvarez-Garcillan Portillo, M.; Camargo-Sosa, K.; Richardson, J.; Rocha, M.; Gauert, A.; Corbeaux, T.; Milanetto, M.; et al. Notch controls the cell cycle to define leader versus follower identities during collective cell migration. eLife 2022, 11, e73550. [Google Scholar] [CrossRef]
- Fausett, B.V.; Goldman, D. A role for alpha1 tubulin-expressing Müller glia in regeneration of the injured zebrafish retina. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 6303–6313. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef]
- Fimbel, S.M.; Montgomery, J.E.; Burket, C.T.; Hyde, D.R. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Thummel, R.; Kassen, S.C.; Montgomery, J.E.; Enright, J.M.; Hyde, D.R. Inhibition of Müller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev. Neurobiol. 2008, 68, 392–408. [Google Scholar] [CrossRef]
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012, 22, 334–347. [Google Scholar] [CrossRef]
- Hayes, S.; Nelson, B.R.; Buckingham, B.; Reh, T.A. Notch signaling regulates regeneration in the avian retina. Dev. Biol. 2007, 312, 300–311. [Google Scholar] [CrossRef]
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef]
- Del Debbio, C.B.; Balasubramanian, S.; Parameswaran, S.; Chaudhuri, A.; Qiu, F.; Ahmad, I. Notch and Wnt signaling mediated rod photoreceptor regeneration by Müller cells in adult mammalian retina. PLoS ONE 2010, 5, e12425. [Google Scholar] [CrossRef]
- Murato, Y.; Hashimoto, C. Xhairy2 functions in Xenopus lens development by regulating p27(xic1) expression. Dev. Dyn. 2009, 238, 2179–2192. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.A.; Barthel, L.K.; Largent, B.L.; Raymond, P.A. A goldfish Notch-3 homologue is expressed in neurogenic regions of embryonic, adult, and regenerating brain and retina. Dev. Genet 1997, 20, 208–223. [Google Scholar] [CrossRef]
- Yan, Y.; Denef, N.; Schüpbach, T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev. Cell 2009, 17, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Tseng, A.S.; Beane, W.S.; Lemire, J.M.; Masi, A.; Levin, M. Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 13192–13200. [Google Scholar] [CrossRef]
- Wissel, S.; Harzer, H.; Bonnay, F.; Burkard, T.R.; Neumüller, R.A.; Knoblich, J.A. Time-resolved transcriptomics in neural stem cells identifies a v-ATPase/Notch regulatory loop. J. Cell Biol. 2018, 217, 3285–3300. [Google Scholar] [CrossRef]
- Wall, D.S.; Mears, A.J.; McNeill, B.; Mazerolle, C.; Thurig, S.; Wang, Y.; Kageyama, R.; Wallace, V.A. Progenitor cell proliferation in the retina is dependent on Notch-independent Sonic hedgehog/Hes1 activity. J. Cell Biol. 2009, 184, 101–112. [Google Scholar] [CrossRef]
- Schouwey, K.; Aydin, I.T.; Radtke, F.; Beermann, F. RBP-Jκ-dependent Notch signaling enhances retinal pigment epithelial cell proliferation in transgenic mice. Oncogene 2011, 30, 313–322. [Google Scholar] [CrossRef]
- Hack, S.J.; Petereit, J.; Tseng, K.A.-S. Temporal Transcriptomic Profiling of the Developing Xenopus laevis Eye. Cells 2024, 13, 1390. [Google Scholar] [CrossRef]
- Kha, C.X.; Guerin, D.J.; Tseng, K.A.-S. Studying In Vivo Retinal Progenitor Cell Proliferation in Xenopus laevis. In Retinal Development: Methods in Molecular Biology; Mao, C.-A., Ed.; Methods in Molecular Biology: New York, NY, USA, 2020; pp. 19–33. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerin, D.J.; Gutierrez, B.; Zhang, B.; Tseng, K.A.-S. Notch Is Required for Neural Progenitor Proliferation During Embryonic Eye Regrowth. Int. J. Mol. Sci. 2025, 26, 2637. https://doi.org/10.3390/ijms26062637
Guerin DJ, Gutierrez B, Zhang B, Tseng KA-S. Notch Is Required for Neural Progenitor Proliferation During Embryonic Eye Regrowth. International Journal of Molecular Sciences. 2025; 26(6):2637. https://doi.org/10.3390/ijms26062637
Chicago/Turabian StyleGuerin, Dylan J., Belen Gutierrez, Baoyi Zhang, and Kelly Ai-Sun Tseng. 2025. "Notch Is Required for Neural Progenitor Proliferation During Embryonic Eye Regrowth" International Journal of Molecular Sciences 26, no. 6: 2637. https://doi.org/10.3390/ijms26062637
APA StyleGuerin, D. J., Gutierrez, B., Zhang, B., & Tseng, K. A.-S. (2025). Notch Is Required for Neural Progenitor Proliferation During Embryonic Eye Regrowth. International Journal of Molecular Sciences, 26(6), 2637. https://doi.org/10.3390/ijms26062637





