Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterizations of Materials
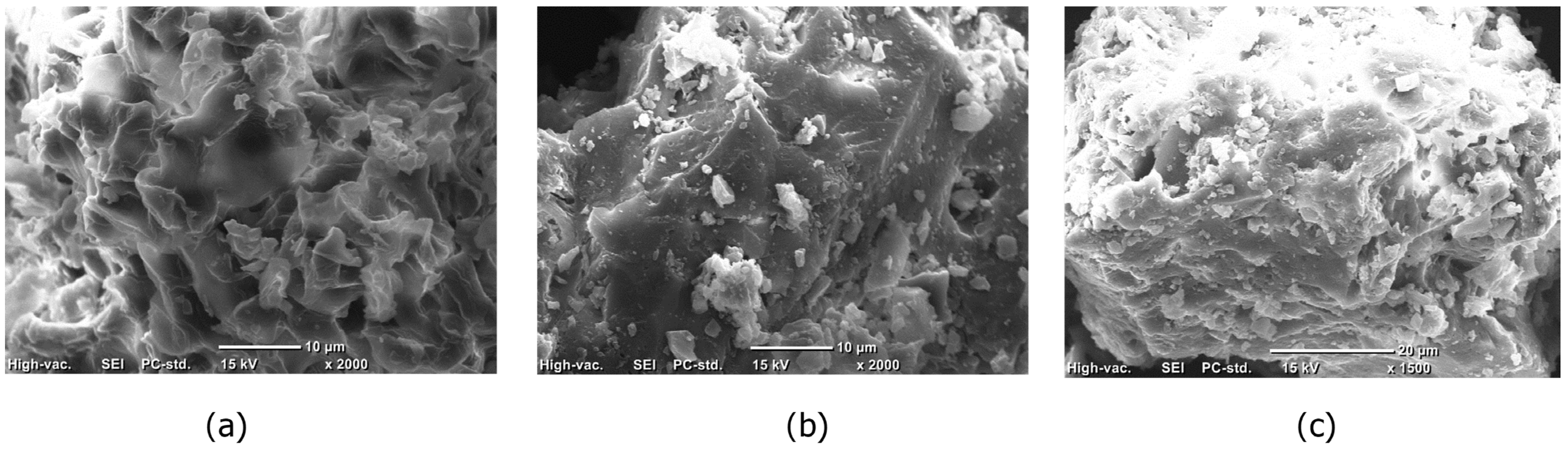
2.1.1. SEM-EDS Analysis
2.1.2. N2-BET Analysis
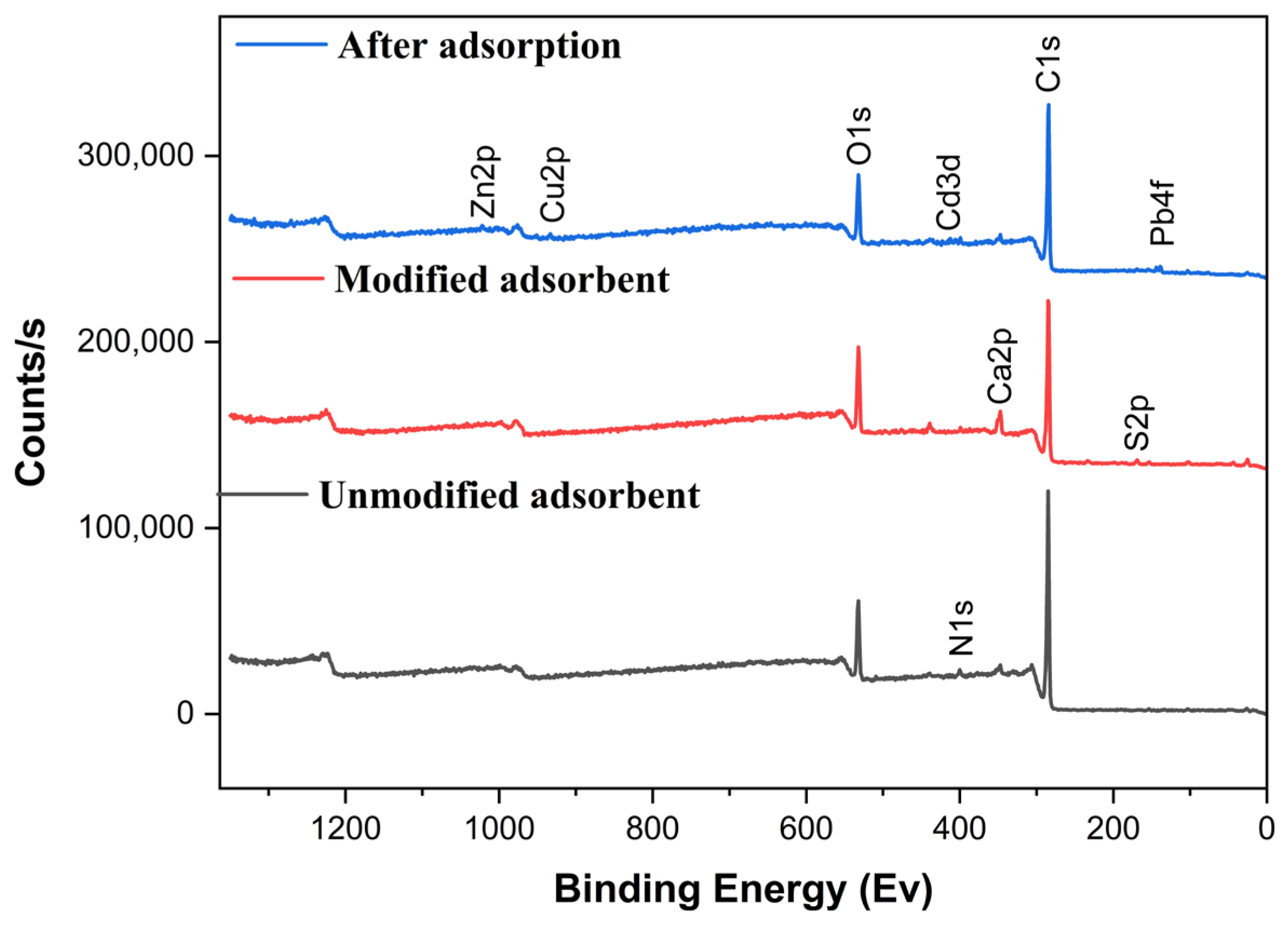
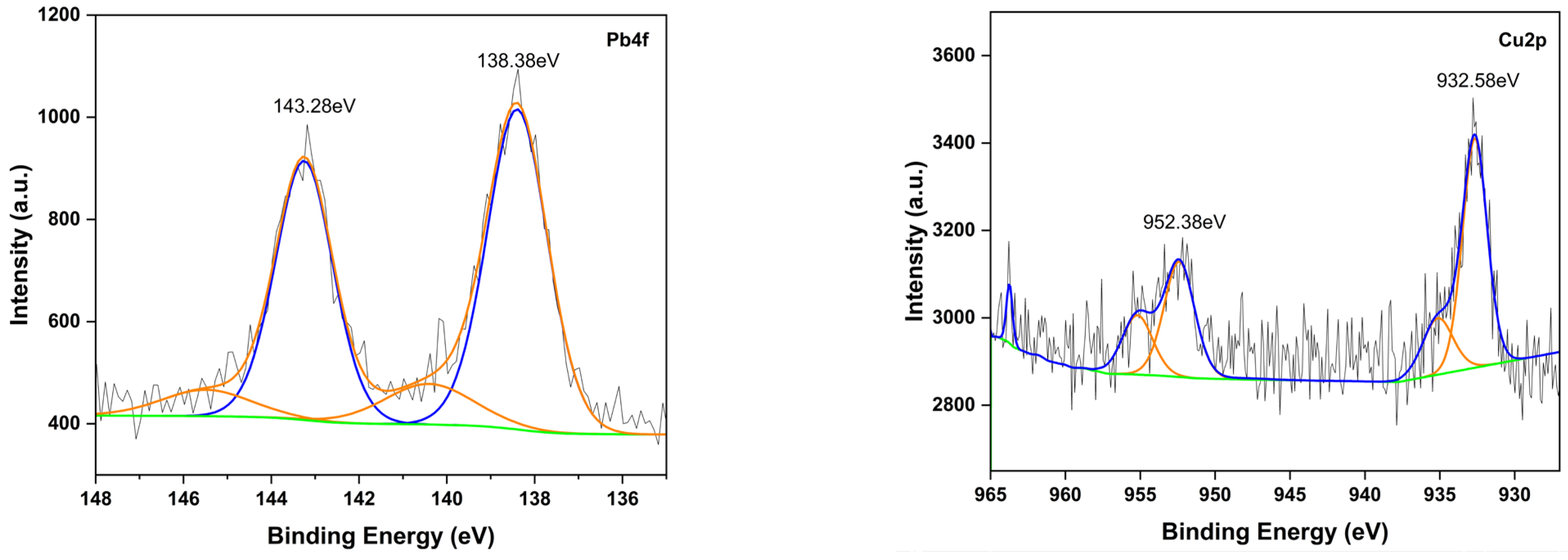
2.1.3. XPS Spectra
2.1.4. FT-IR Spectra
2.2. Evaluation of Multi-Heavy-Metal Adsorption Performance
2.2.1. Effect of Sulfuric Acid Concentration
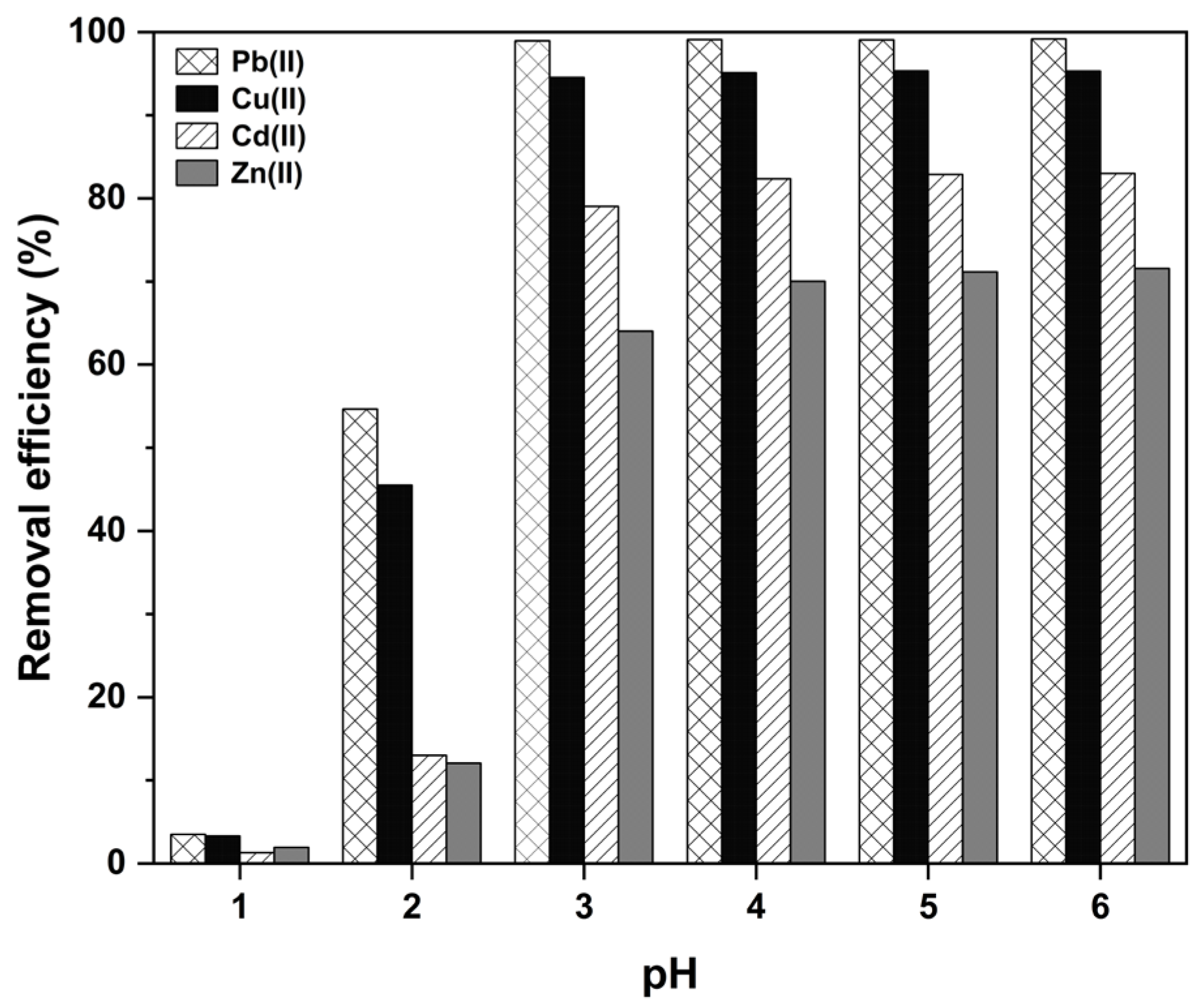
2.2.2. Effect of Initial pH
2.2.3. Effect of Heat Treatment
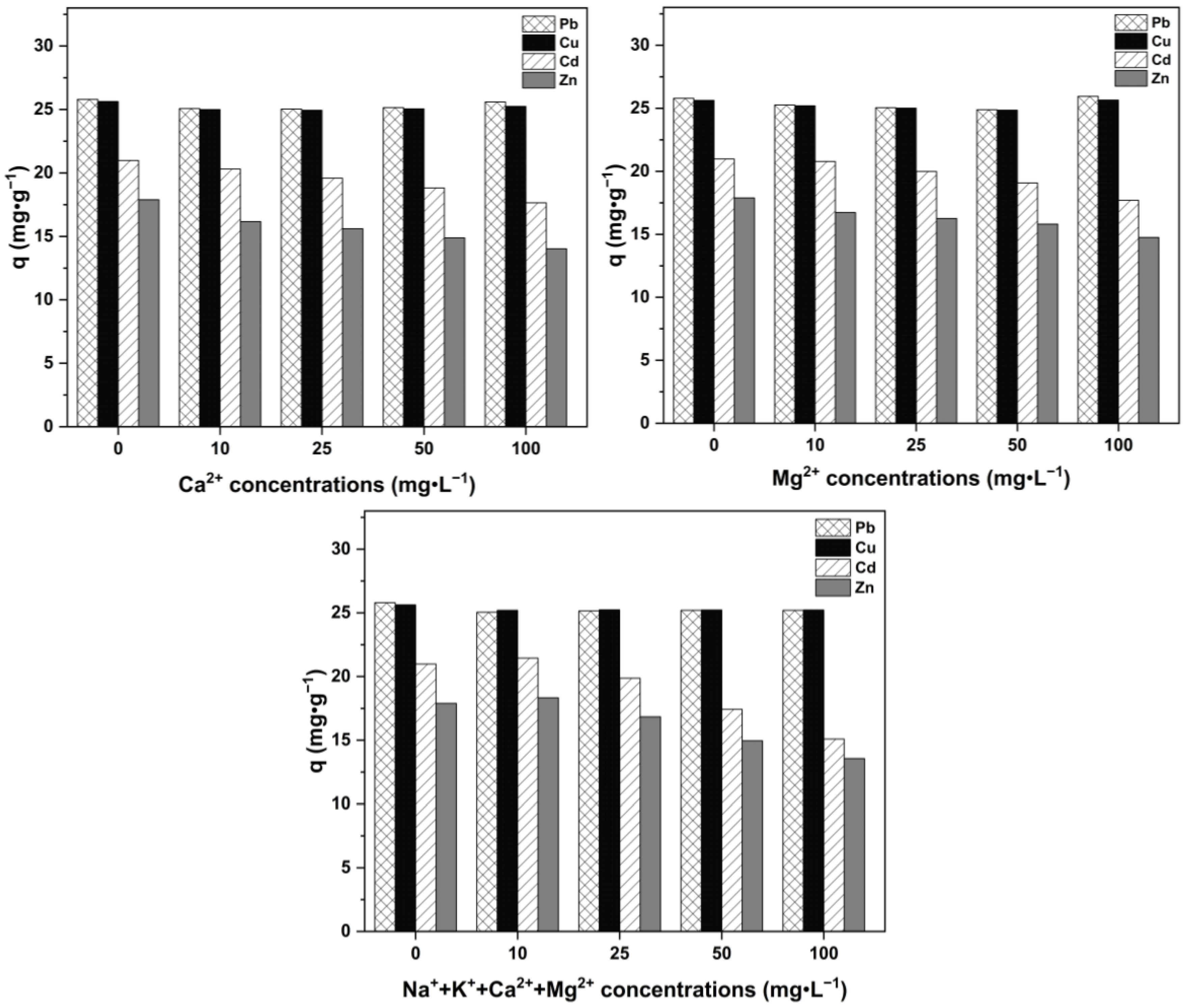
2.2.4. Effect of Competitive Ions
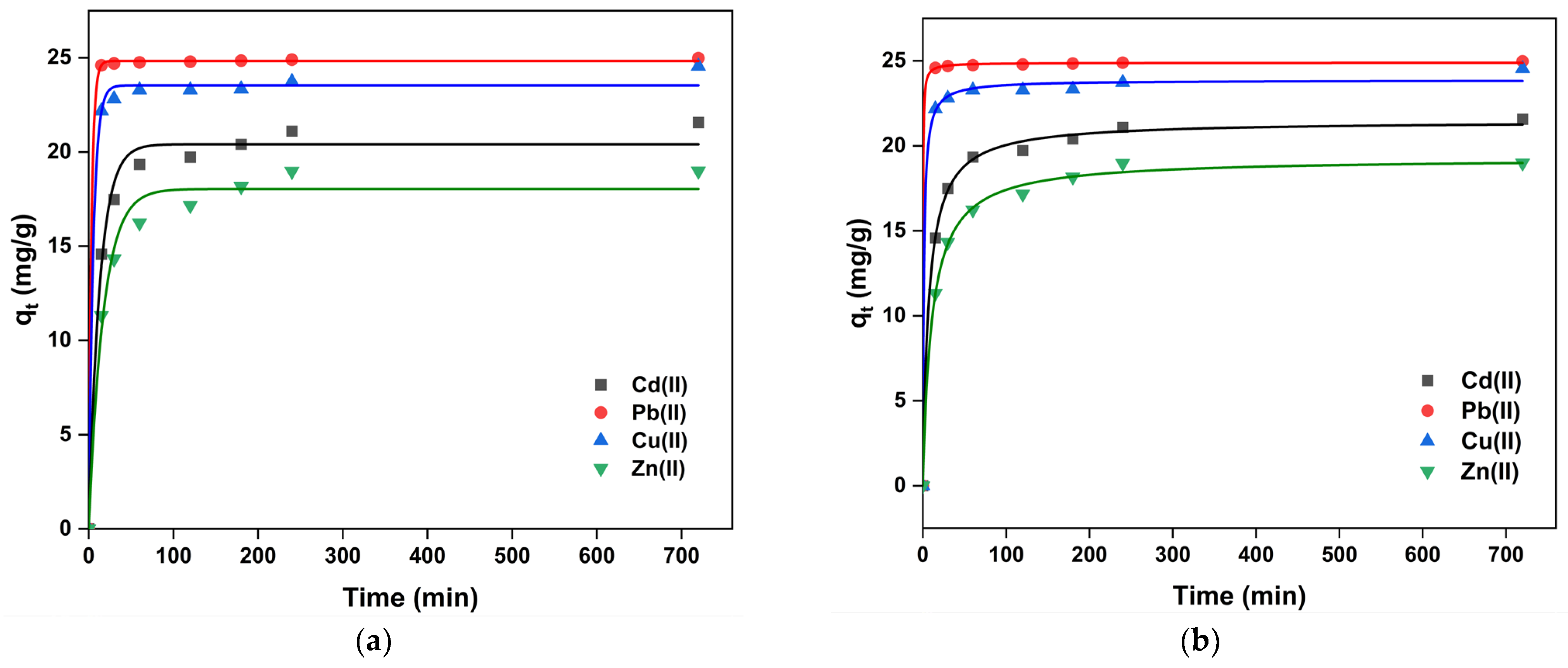
2.3. Adsorption Kinetics Study
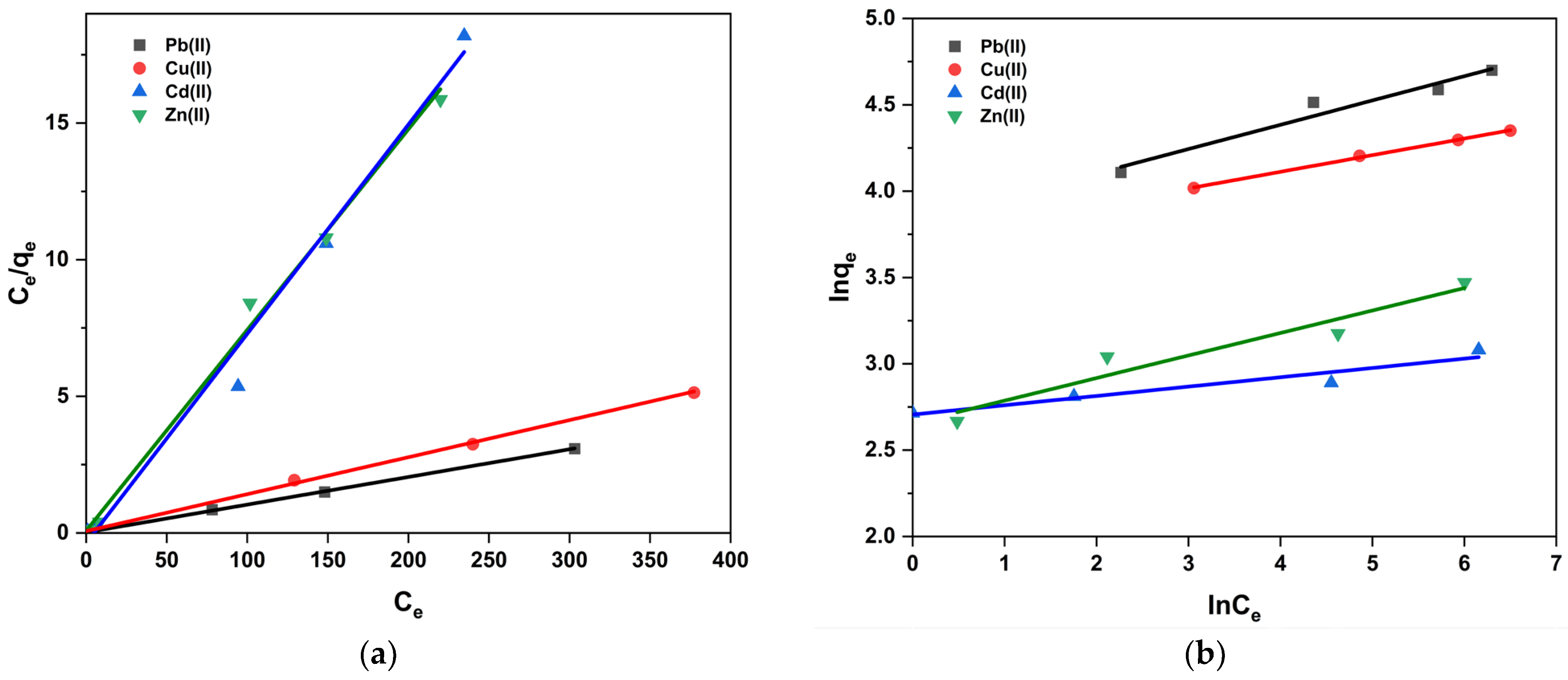
2.4. Adsorption Isotherm Study
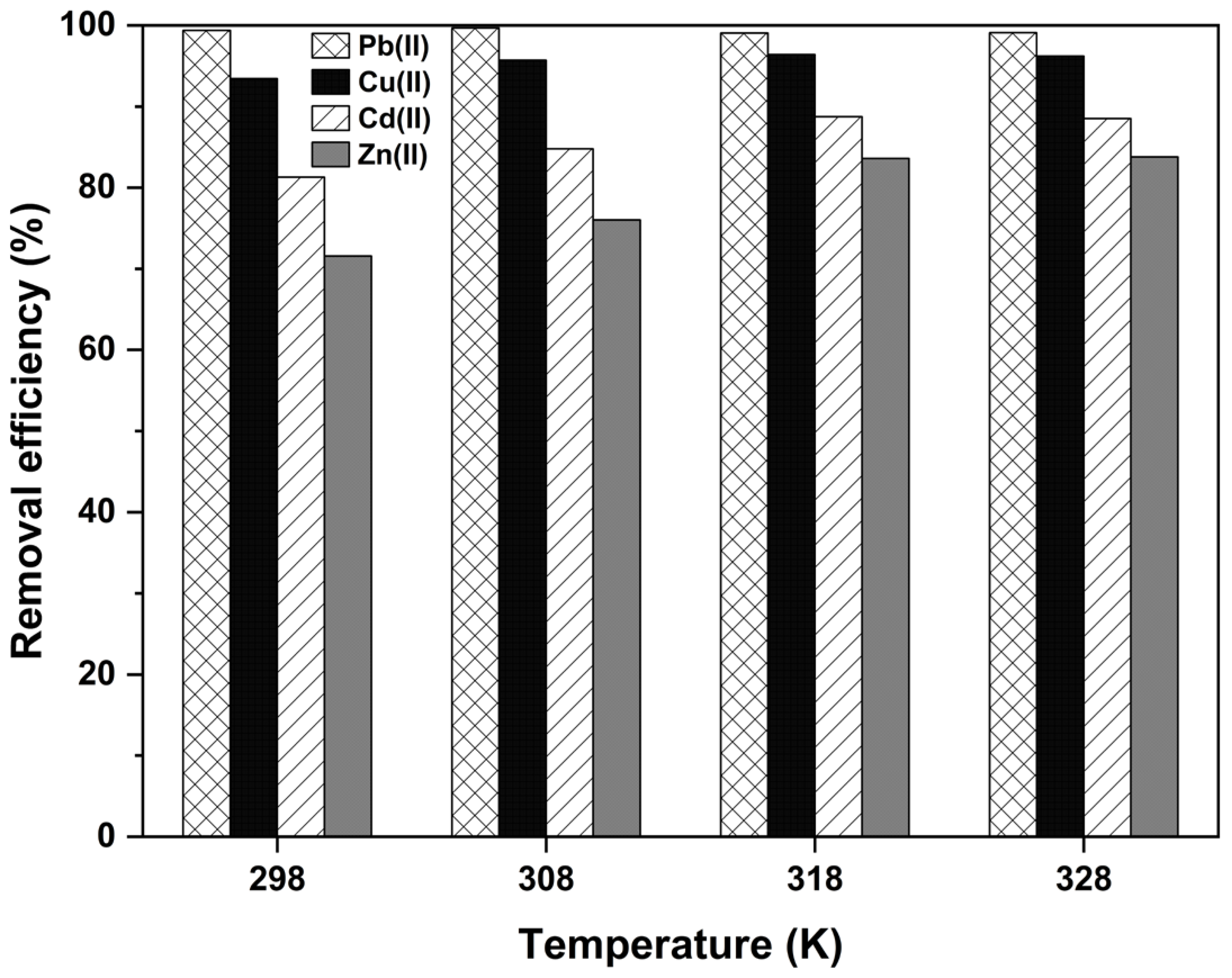
2.5. Adsorption Thermodynamics
2.6. Chemical Mechanism
3. Materials and Methods
3.1. Characterization of the Adsorbent
3.2. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peydayesh, M.; Mezzenga, R. Protein nanofibrils for next generation sustainable water purification. Nat. Commun. 2021, 12, 3248. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. The Sustainable Development Goals Report 2024; United Nations Publications 2024; United Nations Department of Economic and Social Affairs: New York, NY, USA, 2024. [Google Scholar]
- Zhao, M.; Wang, S.; Wang, H.; Qin, P.; Yang, D.; Sun, Y.; Kong, F. Application of sodium titanate nanofibers as constructed wetland fillers for efficient removal of heavy metal ions from wastewater. Environ. Pollut. 2019, 248, 938–946. [Google Scholar] [CrossRef]
- Ijagbemi, C.O.; Baek, M.-H.; Kim, D.-S. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J. Hazard. Mater. 2009, 166, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetics and isotherm models of heavy metals by various adsorbents: An overview. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1837–1865. [Google Scholar] [CrossRef]
- Tran, N.N.; Escribà-Gelonch, M.; Sarafraz, M.M.; Pho, Q.H.; Sagadevan, S.; Hessel, V. Process Technology and Sustainability Assessment of Wastewater Treatment. Ind. Eng. Chem. Res. 2023, 62, 1195–1214. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Sillanpää, M.; Mtei, K.M. Removal of heavy metals from binary and multicomponent adsorption systems using various adsorbents—A systematic review. RSC Adv. 2023, 13, 13052–13093. [Google Scholar] [CrossRef]
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment—A review. Chem. Eng. J. 2010, 157, 277–296. [Google Scholar] [CrossRef]
- Thiebault, T. Raw and modified clays and clay minerals for the removal of pharmaceutical products from aqueous solutions: State of the art and future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1451–1514. [Google Scholar] [CrossRef]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sudiyarmanto; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef]
- Khan, Q.; Zahoor, M.; Salman, S.M.; Wahab, M.; Khan, F.A.; Gulfam, N.; Zekker, I. Removal of Iron(II) from Effluents of Steel Mills Using Chemically Modified Pteris vittata Plant Leaves Utilizing the Idea of Phytoremediation. Water 2022, 14, 2004. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. Biosorption of pollutants by plant leaves: An empirical review. J. Environ. Chem. Eng. 2019, 7, 103100. [Google Scholar] [CrossRef]
- Jayan, N.; Bhatlu M, L.D.; Akbar, S.T. Central Composite Design for Adsorption of Pb(II) and Zn(II) Metals on PKM-2 Moringa oleifera Leaves. ACS Omega 2021, 6, 25277–25298. [Google Scholar] [CrossRef]
- Candido, I.C.M.; Soares, J.M.D.; de Araujo Barros Barbosa, J.; de Oliveira, H.P. Adsorption and identification of traces of dyes in aqueous solutions using chemically modified eggshell membranes. Bioresour. Technol. Rep. 2019, 7, 100267. [Google Scholar] [CrossRef]
- Cholico-González, D.; Ortiz Lara, N.; Fernández Macedo, A.M.; Chavez Salas, J. Adsorption Behavior of Pb(II), Cd(II), and Zn(II) onto Agave Bagasse, Characterization, and Mechanism. ACS Omega 2020, 5, 3302–3314. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Tran, T.V.; Kumar, P.S.; Din, A.T.M.; Jalil, A.A.; Vo, D.N. Invasive plants as biosorbents for environmental remediation: A review. Environ. Chem. Lett. 2022, 20, 1421–1451. [Google Scholar] [CrossRef]
- Arce, C.; Kratky, L. Mechanical pretreatment of lignocellulosic biomass toward enzymatic/fermentative valorization. iScience 2022, 25, 104610. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Sarangi, P.K.; Kovalev, A.A.; Vivekanand, V. Effect of physical and thermal pretreatment of lignocellulosic biomass on biohydrogen production by thermochemical route: A critical review. Bioresour. Technol. 2023, 369, 128458. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Singh, K.; Singh, P.K.; Chowdhury, F.I.; Yahya, M.Z.A.; Yusuf, S.N.F.; Diantoro, M.; Latif, F.A.; Singh, N.B. Recent development on neem (Azadirachta indica) biomass absorbent: Surface modifications and its applications in water remediation. Chem. Phys. Impact 2024, 9, 100773. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Fan, Y.; Liu, T.; Zhang, Y.; Wan, Q. Adsorption and enrichment of Ag(I) from industrial wastewater using woody biomass-based biosorbent. Hydrometallurgy 2023, 219, 106083. [Google Scholar] [CrossRef]
- Feng, S.; Du, X.; Bat-Amgalan, M.; Zhang, H.; Miyamoto, N.; Kano, N. Adsorption of REEs from Aqueous Solution by EDTA-Chitosan Modified with Zeolite Imidazole Framework (ZIF-8). Int. J. Mol. Sci. 2021, 22, 3447. [Google Scholar] [CrossRef]
- Bat-Amgalan, M.; Miyamoto, N.; Kano, N.; Yunden, G.; Kim, H.-J. Preparation and Characterization of Low-Cost Ceramic Membrane Coated with Chitosan: Application to the Ultrafine Filtration of Cr(VI). Membranes 2022, 12, 835. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, H.; Miyamoto, N.; Kano, N.; Okawa, H. Adsorption of an Anionic Surfactant (Sodium Dodecyl Sulfate) from an Aqueous Solution by Modified Cellulose with Quaternary Ammonium. Polymers 2022, 14, 1473. [Google Scholar] [CrossRef] [PubMed]
- Shirendev, N.; Bat-Amgalan, M.; Kano, N.; Kim, H.-J.; Gunchin, B.; Ganbat, B.; Yunden, G. A Natural Zeolite Developed with 3-Aminopropyltriethoxysilane and Adsorption of Cu(II) from Aqueous Media. Appl. Sci. 2022, 12, 11344. [Google Scholar] [CrossRef]
- Du, X.; Kishima, C.; Zhang, H.; Miyamoto, N.; Kano, N. Removal of Chromium(VI) by Chitosan Beads Modified with Sodium Dodecyl Sulfate (SDS). Appl. Sci. 2020, 10, 4745. [Google Scholar] [CrossRef]
- Krajewska, A.; Mietlińska, K. Determining the Parameters of the Stinging Nettle (Urtica dioica L.) Hydrolate Distillation Process. Molecules 2022, 27, 3912. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.-J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, K.; Rahnavard, A.; Saeb, K.; Gholamreza Fahimi, F.; Tavana, A. Ability of Urtica dioica L. to adsorb heavy metals (Pb, Cd, As, and Ni) from contaminated soils. Soil Sediment Contam. 2023, 32, 51–84. [Google Scholar] [CrossRef]
- Dimitrijević, V.D.; Stanković, M.N.; Đorđević, D.M.; Krstić, I.M.; Nikolić, M.G.; Bojić, A.L.; Krstić, N.S. The preliminary adsorption investigation of Urtica dioica L. biomass material as a potential biosorbent for heavy metal ions. Stud. Univ. Babes-Bolyai Chem. 2019, 64, 19–39. [Google Scholar] [CrossRef]
- Tiwari, P.; Vishwakarma, M.; Joshi, S.; Sharma, H.; Bhandari, N. Adsorption of Pb (II), Cu (II), and Zn (II) Ions onto Urtica dioica Leaves (UDL) as a Low Cost Adsorbent: Equilibrium and Thermodynamic Studies. Mod. Chem. 2017, 5, 11–18. [Google Scholar] [CrossRef]
- Isik, B.; Cakar, F.; Cankurtaran, O. Valorization of Urtica dioica roots as a highly-effective and eco-friendly biosorbent for adsorptive removal of hazardous textile dyes. Mater. Sci. Eng. B 2023, 293, 116451. [Google Scholar] [CrossRef]
- Viju, S.; Thilagavathi, G.; Vignesh, B.; Brindha, R. Oil sorption behavior of acetylated nettle fiber. J. Text. Inst. 2019, 110, 1415–1423. [Google Scholar] [CrossRef]
- Wattanakornsiri, A.; Rattanawan, P.; Sanmueng, T.; Satchawan, S.; Jamnongkan, T.; Phuengphai, P. Local fruit peel biosorbents for lead(II) and cadmium(II) ion removal from waste aqueous solution: A kinetic and equilibrium study. S. Afr. J. Chem. Eng. 2022, 42, 306–317. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Küçük, M.; Makarava, I.; Kinnarinen, T.; Häkkinen, A. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar] [CrossRef]
- Arana Juve, J.-M.; Christensen, F.M.S.; Wang, Y.; Wei, Z. Electrodialysis for metal removal and recovery: A review. Chem. Eng. J. 2022, 435, 134857. [Google Scholar] [CrossRef]
- Dagdag, O.; Quadri, T.W.; Haldhar, R.; Kim, S.-C.; Daoudi, W.; Berdimurodov, E.; Akpan, E.D.; Ebenso, E.E. An Overview of Heavy Metal Pollution and Control. In Heavy Metals in the Environment: Management Strategies for Global Pollution; American Chemical Society: Washington, DC, USA, 2023; Volume 1456, pp. 3–24. [Google Scholar]
- Zeng, H.; Su, Y.; Gong, X.; Zheng, L.; Zhang, L.; Meng, P.; Zhou, Q.; Ren, J. Competitive adsorption behavior of typical heavy metal ions from acid mine drainage by multigroup-functionalization cellulose: Qualitative and quantitative mechanism. Environ. Sci. Pollut. Res. 2023, 30, 68191–68205. [Google Scholar] [CrossRef]
- Orabi, A.H. Synthesis of a cellulose derivative for enhanced sorption and selectivity of uranium from phosphate rocks prior to its fluorometric determination. Int. J. Environ. Anal. Chem. 2019, 99, 741–766. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, L.; Hu, F.; Hu, Y. Advanced landfill leachate biochemical effluent treatment using Fe-Mn/AC activates O3/Na2S2O8 process: Process optimization, wastewater quality analysis, and activator characterization. Environ. Sci. Pollut. Res. 2020, 27, 15337–15349. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.; Thenpandiyan, E.; Suresh, G.; Sathishpriya, T.; Sagadevan, S. Enhanced electrochemical behavior of naturally derived Eu3+: PEG/CaCO3 nanocomposite for supercapacitor application. Inorg. Chem. Commun. 2024, 159, 111758. [Google Scholar] [CrossRef]
- Orabi, A.; Atrees, M.; Salem, H. Selective preconcentration of uranium on chitosan stearoyl thiourea prior to its spectrophotometric determination. Sep. Sci. Technol. 2018, 53, 2267–2283. [Google Scholar] [CrossRef]
- Hamed, A.; Orabi, A.; Salem, H.; Ismaiel, D.; Saad, G.; Abdelhamid, I.; Elwahy, A.; Elsabee, M. An effective uranium removal using diversified synthesized cross-linked chitosan bis-aldehyde Schiff base derivatives from aqueous solutions. Environ. Sci. Pollut. Res. 2023, 30, 106790–106811. [Google Scholar] [CrossRef]
- Cho, M.-H.; Song, Y.-J.; Rhu, C.-J.; Go, B.-R. Pyrolysis Process of Mixed Microplastics Using TG-FTIR and TED-GC-MS. Polymers 2023, 15, 241. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, K.; Jiao, Z.; Zhan, W.; Ge, S.; Ning, S.; Fang, S.; Ruan, X. Simultaneous Removal of Cu2+, Cd2+ and Pb2+ by Modified Wheat Straw Biochar from Aqueous Solution: Preparation, Characterization and Adsorption Mechanism. Toxics 2022, 10, 316. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, N.; Feng, C.; Li, M.; Gao, Y. Chromium removal using a magnetic corncob biochar/polypyrrole composite by adsorption combined with reduction: Reaction pathway and contribution degree. Colloids Surf. A Physicochem. Eng. Asp. 2018, 556, 201–209. [Google Scholar] [CrossRef]
- Fouda, I.M.; El-Kady, M.Y.; El-Sheikh, E.M.; Orabi, A.H.; Youssef, A.O. Elimination of Cr (VI), Pb (II), V (V), and Cd (II) ions from Titanium oxide Pigment from Rosetta ilmenite Concentrate Using synthesised Cellulose Phosphorus Oxychloride. Int. J. Environ. Anal. Chem. 2023, 103, 5795–5814. [Google Scholar] [CrossRef]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2022, 12, 925–947. [Google Scholar] [CrossRef]
- Majigsuren, E.; Byambasuren, U.; Bat-Amgalan, M.; Mendsaikhan, E.; Kano, N.; Kim, H.J.; Yunden, G. Adsorption of Chromium (III) and Chromium (VI) Ions from Aqueous Solution Using Chitosan–Clay Composite Materials. Polymers 2024, 16, 1399. [Google Scholar] [CrossRef]
- Fan, X.; Liu, H.; Anang, E.; Ren, D. Effects of Electronegativity and Hydration Energy on the Selective Adsorption of Heavy Metal Ions by Synthetic NaX Zeolite. Materials 2021, 14, 4066. [Google Scholar] [CrossRef]
- Lee, C.-L.; Hsi, H.-C.; Chang, C.M. Linear correlation between electronegativity and adsorption energy of hydrated metal ions by carboxyl-functionalized single-walled carbon nanotubes. J. Nanopart. Res. 2023, 25, 59. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef]
- Mittal, A.; Kaur, D.; Mittal, J. Batch and bulk removal of a triarylmethane dye, Fast Green FCF, from wastewater by adsorption over waste materials. J. Hazard. Mater. 2009, 163, 568–577. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S.; Ibrahim, H.S. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Islam, M.S.; Rahaman, M.S.; Barbeau, B. Removal of Pb (II), Zn (II), Cu (II), and As (III) ions from water using kraft pulp-based carboxymethylated cellulose in a fixed-bed column adsorption process. J. Environ. Chem. Eng. 2023, 11, 111181. [Google Scholar] [CrossRef]






| UDLs | BET Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| Unmodified | 0.4659 | 0.00092 | 7.89508 |
| H2SO4-modified | 1.0157 | 0.00397 | 15.63720 |
| TUDL500 | 451.9304 | 0.252627 | 2.23598 |
| UDLs | C | N | O | S | Ca |
|---|---|---|---|---|---|
| Unmodified (%) | 81.04 | 2.79 | 13.74 | - | 1.59 |
| Modified (%) | 74.97 | 2.02 | 16.47 | 1.4 | 3.38 |
| After adsorption (%) | 80.46 | 2.88 | 14.19 | 0.35 | 0.13 |
| Pb(II) | Cu(II) | Cd(II) | Zn(II) | |
|---|---|---|---|---|
| Pseudo-First-Order Model | ||||
| qe (mg·g−1) | 24.83 | 23.54 | 20.41 | 18.04 |
| Κ1 (min−1) | 0.31 | 0.18 | 0.07 | 0.06 |
| R2 | 0.9999 | 0.9966 | 0.9882 | 0.9820 |
| Pseudo-Second-Order Model | ||||
| qe (mg·g−1) | 24.89 | 23.87 | 21.47 | 19.27 |
| Κ2 (g mg−1 min−1) | 0.20 | 0.04 | 0.007 | 0.005 |
| R2 | 0.9999 | 0.9983 | 0.9985 | 0.9979 |
| qe exp (mg·g−1) | 25.52 | 25.64 | 22.52 | 20.90 |
| Pb(II) | Cu(II) | Cd(II) | Zn(II) | |
|---|---|---|---|---|
| Langmuir Equation | ||||
| qmax (mg·g−1) | 98.72 | 73.85 | 13.06 | 13.61 |
| ΚL (L·mg−1) | 0.0002 | 0.001 | 0.028 | 0.006 |
| R2 | 0.99 | 0.99 | 0.98 | 0.99 |
| χ2 | 0.03 | 0.26 | 0.01 | 0.02 |
| MPSD | 0.007 | 0.05 | 0.12 | 0.01 |
| RMSE | 1.15 | 3.22 | 0.04 | 0.37 |
| Freundlich Equation | ||||
| R2 | 0.93 | 0.99 | 0.63 | 0.63 |
| 1/n | 0.14 | 0.10 | 0.05 | 0.13 |
| ΚF (mg·g−1) | 45.69 | 41.54 | 14.97 | 14.26 |
| χ2 | 60.55 | 24.51 | 0.28 | 0.01 |
| MPSD | 28.47 | 19.43 | 2.68 | 0.07 |
| RMSE | 1500.21 | 986.15 | 4.01 | 0.03 |
| qmax,exp (mg·g−1) | 98.29 | 73.45 | 12.90 | 13.87 |
| Heavy Metals | C0, mg·L−1 | T, K | ΔH0, KJ·mol−1 | ΔS0, JK−1 mol−1 | ΔG0, KJ·mol−1 |
|---|---|---|---|---|---|
| Pb(II) | 50 | 298 | 1.08 | 36.52 | 9.85 |
| 308 | 10.11 | ||||
| 318 | 10.48 | ||||
| 328 | 10.95 | ||||
| Cu(II) | 298 | 19.01 | 80.76 | 4.83 | |
| 308 | 6.16 | ||||
| 318 | 6.84 | ||||
| 328 | 7.26 | ||||
| Cd(II) | 298 | 18.02 | 66.91 | 1.81 | |
| 308 | 2.62 | ||||
| 318 | 3.59 | ||||
| 328 | 3.68 | ||||
| Zn(II) | 298 | 22.95 | 78.66 | 0.45 | |
| 308 | 1.17 | ||||
| 318 | 2.41 | ||||
| 328 | 2.64 |
| Maximum Adsorption Capacity, qmax (mg·g−1) | |||||
|---|---|---|---|---|---|
| Adsorbents | Pb(II) | Cu(II) | Cd(II) | Zn(II) | References |
| Chemically modified dragon fruit peel (DFP) | 97.08 | - | 86.20 | - | [39] |
| Incomplete incinerated Urtica dioica leaves (IIN) | 46.47 | - | [35] | ||
| Chemically modified orange peel | 73.53 | 15.27 | 13.7 | - | [61] |
| Kraft pulp-based carboxymethylated cellulose | 20.3 | 7.8 | - | 8.4 | [62] |
| Amino-modified wheat straw biochar | 46.84 | 19.79 | 10.37 | - | [51] |
| Commercial activated carbon (CGAC) | 20.3 | - | 27.3 | - | [16] |
| Unmodified Urtica dioica leaves (UDLs) | 1.493 | 1.49 | - | 1.039 | [36] |
| H2SO4-modified UDLs | 98.3 | 73.4 | 12.9 | 13.9 | In this study |
| TUDLs | 46.6 | 37.1 | 11.9 | 10.6 | In this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendsaikhan, E.; Bat-Amgalan, M.; Yunden, G.; Miyamoto, N.; Kano, N.; Kim, H.J. Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution. Int. J. Mol. Sci. 2025, 26, 2639. https://doi.org/10.3390/ijms26062639
Mendsaikhan E, Bat-Amgalan M, Yunden G, Miyamoto N, Kano N, Kim HJ. Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution. International Journal of Molecular Sciences. 2025; 26(6):2639. https://doi.org/10.3390/ijms26062639
Chicago/Turabian StyleMendsaikhan, Enkhtuul, Munkhpurev Bat-Amgalan, Ganchimeg Yunden, Naoto Miyamoto, Naoki Kano, and Hee Joon Kim. 2025. "Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution" International Journal of Molecular Sciences 26, no. 6: 2639. https://doi.org/10.3390/ijms26062639
APA StyleMendsaikhan, E., Bat-Amgalan, M., Yunden, G., Miyamoto, N., Kano, N., & Kim, H. J. (2025). Modified Urtica dioica Leaves as a Low-Cost and Effective Adsorbent for the Simultaneous Removal of Pb(II), Cu(II), Cd(II), and Zn(II) from Aqueous Solution. International Journal of Molecular Sciences, 26(6), 2639. https://doi.org/10.3390/ijms26062639






