Gαi2 Induces Cell Migration in PC3 Prostate Cancer Cells in the Absence of Rac1 Activation
Abstract
1. Introduction
2. Results
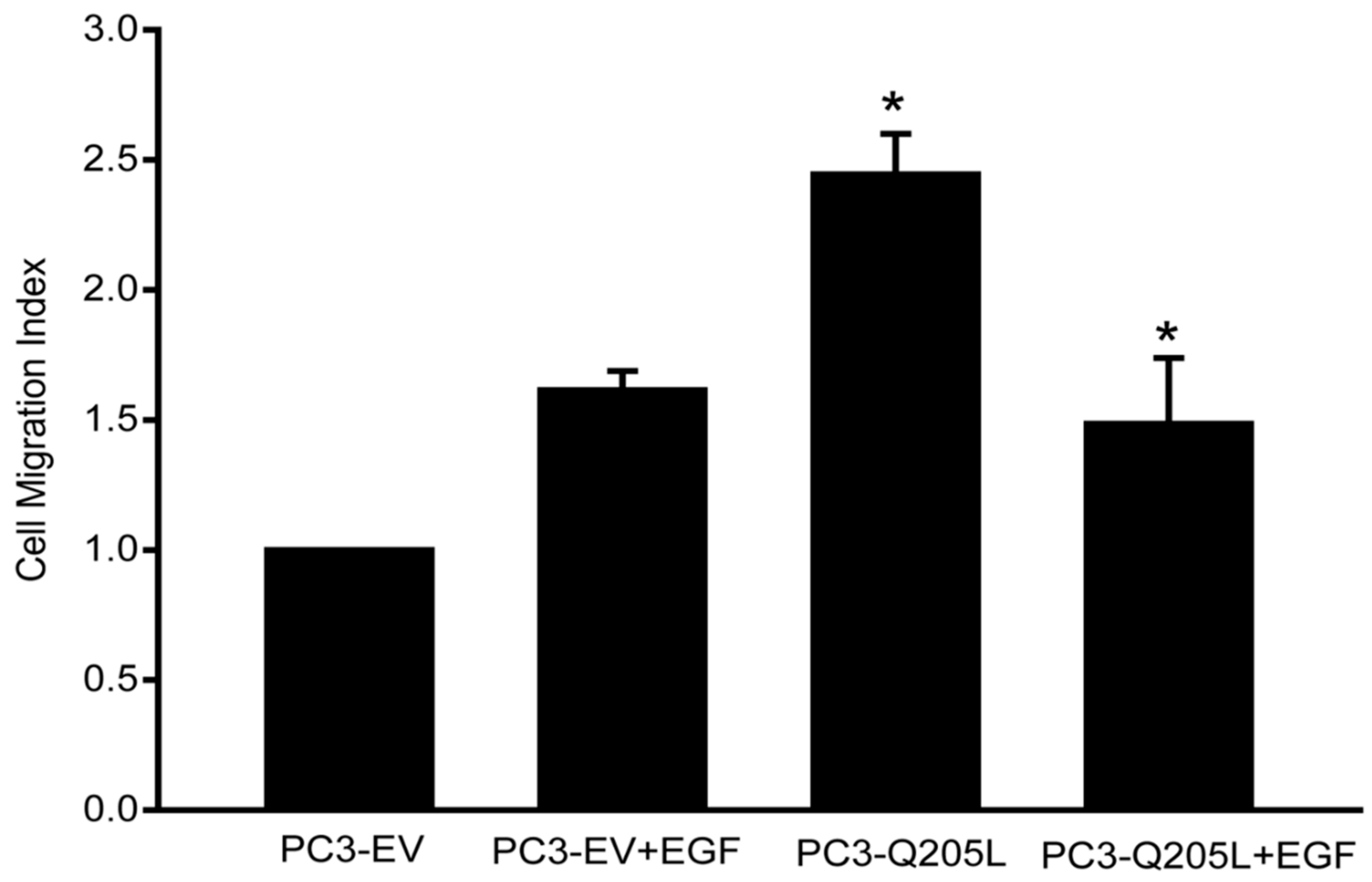
2.1. Overexpression of Constitutively Active Gαi2 in PC3 Cells Increases Cell Motility
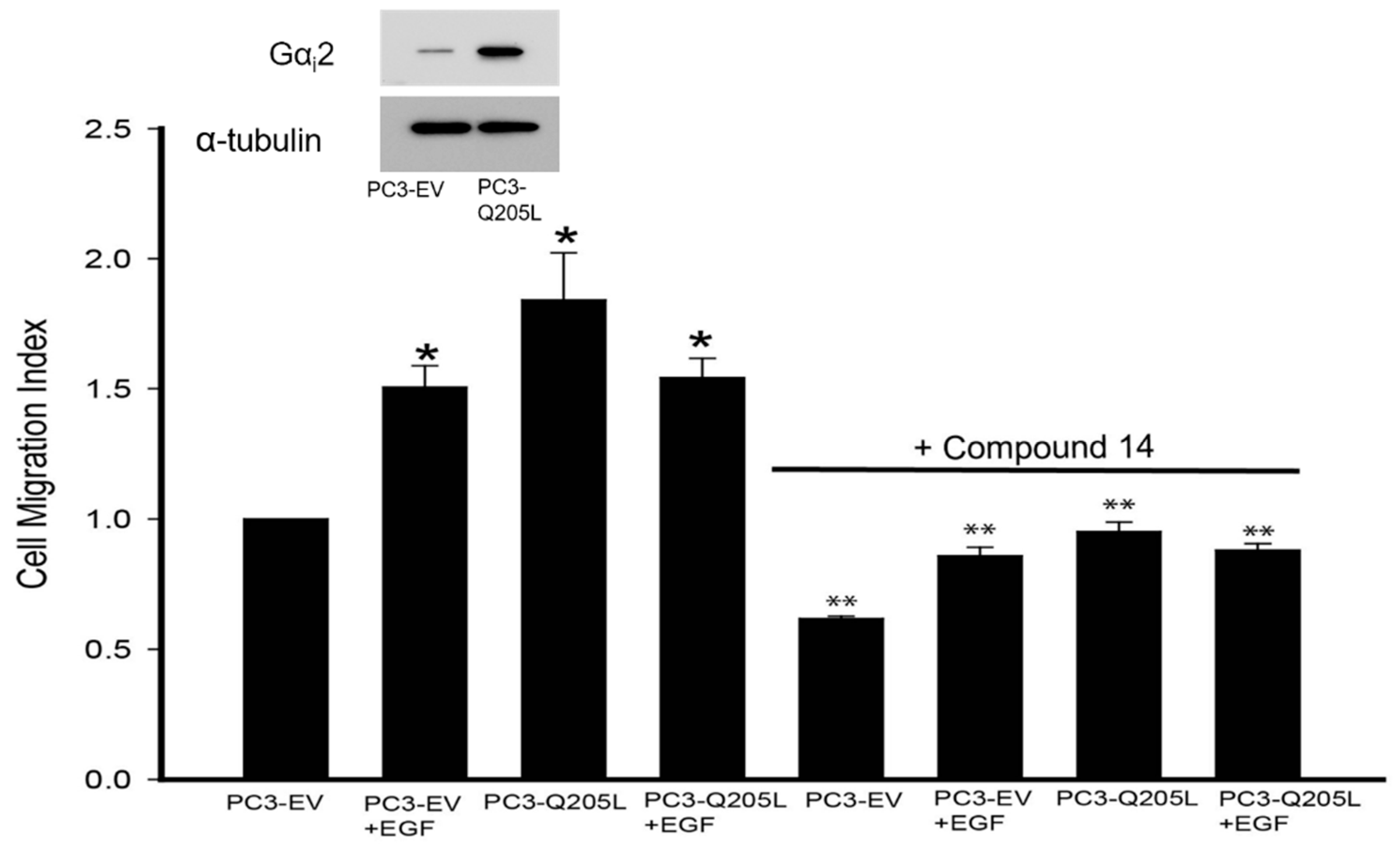
2.2. Gαi2 Inhibitor Blocked the Effects of Gαi2-Q205L on Cell Migration in PC3 Cells
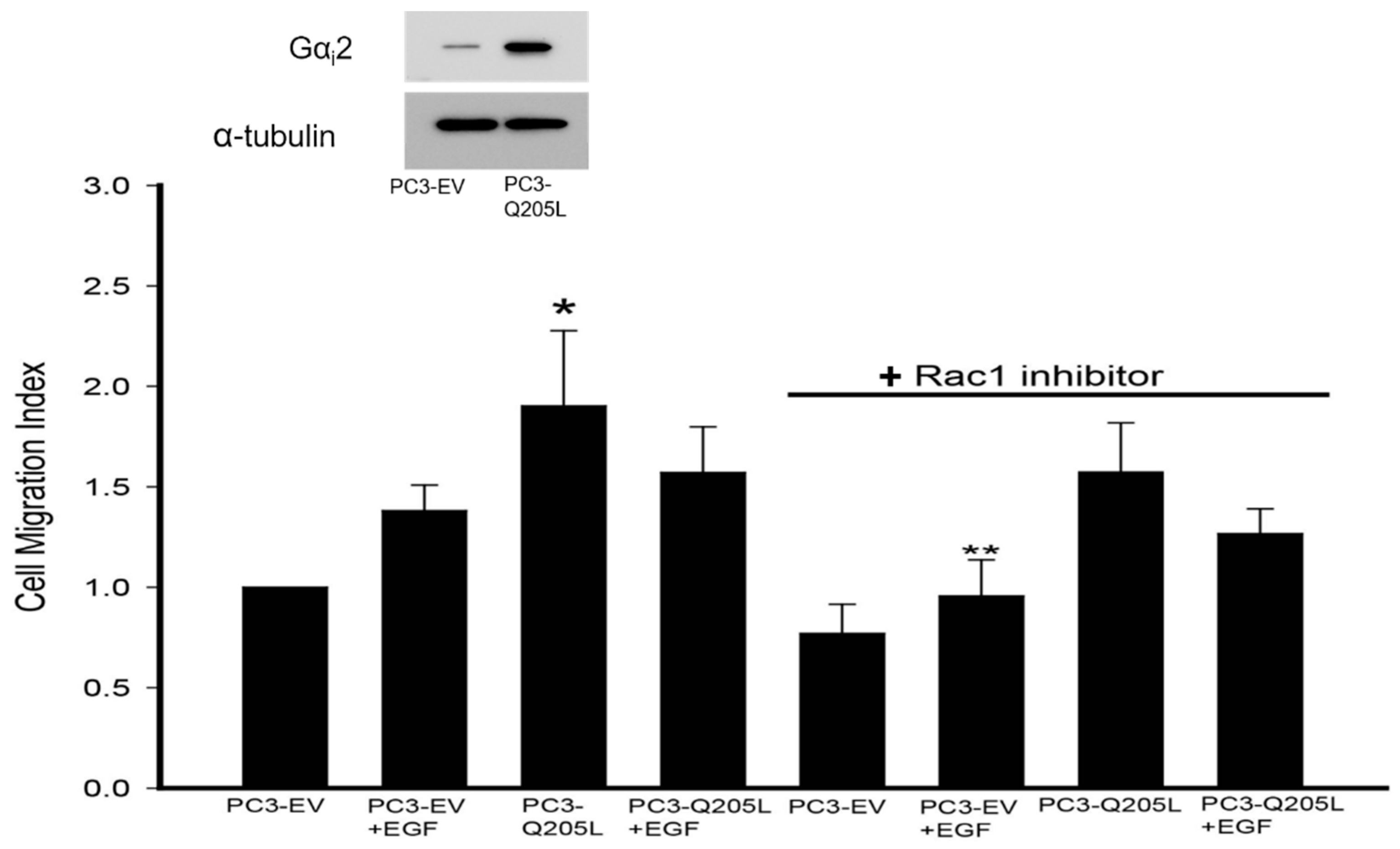
2.3. Rac1 Inhibitor Does Not Block Migration in PC3 Cells Overexpressing Constitutively Active Gαi2
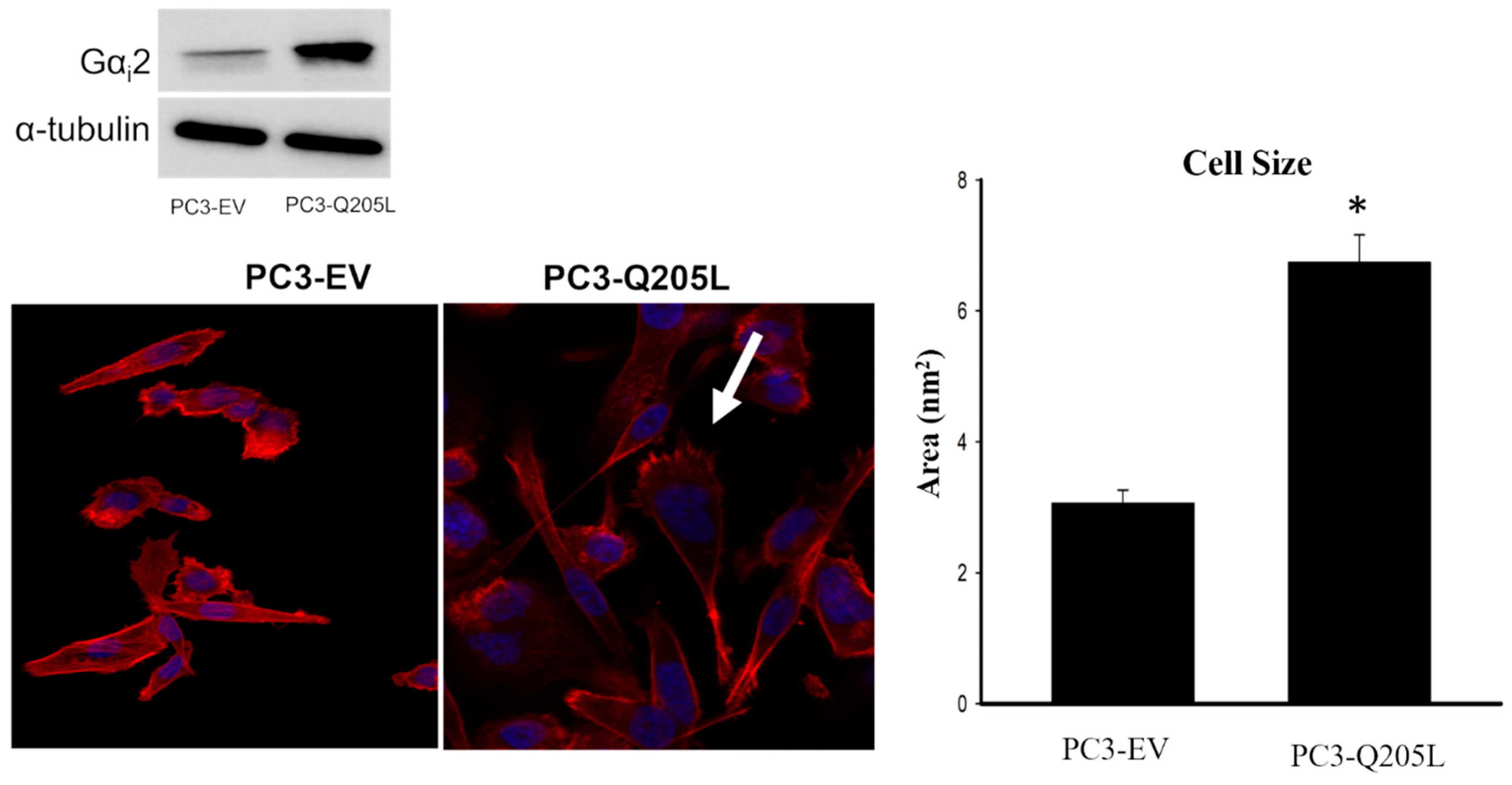
2.4. PC3 Cells Overexpressing Constitutively Active Gαi2 Increase in Cell Size and Lamellipodia Formation
2.5. Rac1-Dependent Activation of Wave2 Is Impaired in the Absence of Gαi2
3. Discussion
4. Materials and Methods
4.1. Cell Culture Chemicals and Reagents
4.2. Transient Transfection with Gαi2 siRNA
4.3. Overexpression of Constitutively Active Gαi2 (Gαi2-Q205L)
4.4. Western Blot Analysis
4.5. Cell Migration
4.6. Immunofluorescence and Actin Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeSantis, C.E.; Lin, C.C.; Mariotto, A.B.; Siegel, R.L.; Stein, K.D.; Kramer, J.L.; Alteri, R.; Robbins, A.S.; Jemal, A. Cancer treatment and survivorship statistics, 2014. CA A Cancer J. Clin. 2014, 64, 252–271. [Google Scholar] [CrossRef]
- Freedland, S.J.; Richhariya, A.; Wang, H.; Chung, K.; Shore, N.D. Treatment Patterns in Patients With Prostate Cancer and Bone Metastasis Among US Community-based Urology Group Practices. Urology 2012, 80, 293–298. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Scully, O.J.; Bay, B.H.; Yip, G.; Yu, Y. Breast cancer metastasis. Cancer Genom. Proteom. 2012, 9, 311–320. [Google Scholar]
- Rathinam, R.; Berrier, A.; Alahari, S.K. Role of Rho GTPases and their regulators in cancer progression. Front. Biosci. 2011, 16, 2561–2571. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef]
- Sun, L.; Ye, R.D. Role of G protein-coupled receptors in inflammation. Acta Pharmacol. Sin. 2012, 33, 342–350. [Google Scholar] [CrossRef]
- Ghosh, P.; Garcia-Marcos, M.; Bornheimer, S.J.; Farquhar, M.G. Activation of Galphai3 triggers cell migration via regulation of GIV. J. Cell Biol. 2008, 182, 381–393. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Fu, H.; Yan, J.; Wang, Y.; Guo, H.; Hao, X.; Xu, X.; Jin, T.; Zhang, N. Association between Galphai2 and ELMO1/Dock180 connects chemokine signalling with Rac activation and metastasis. Nat. Commun. 2013, 4, 1706. [Google Scholar] [CrossRef]
- Ward, J.D.; Dhanasekaran, D.N. LPA Stimulates the Phosphorylation of p130Cas via Galphai2 in Ovarian Cancer Cells. Genes Cancer 2012, 3, 578–591. [Google Scholar] [CrossRef]
- Zhong, M.; Clarke, S.; Vo, B.T.; Khan, S.A. The essential role of Gialpha2 in prostate cancer cell migration. Mol. Cancer Res. MCR 2012, 10, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Caggia, S.; Chunduri, H.; Millena, A.C.; Perkins, J.N.; Venugopal, S.V.; Vo, B.T.; Li, C.; Tu, Y.; Khan, S.A. Novel role of Gialpha2 in cell migration: Downstream of PI3-kinase-AKT and Rac1 in prostate cancer cells. J. Cell. Physiol. 2018, 234, 802–815. [Google Scholar] [CrossRef] [PubMed]
- SenGupta, S.; Parent, C.A.; Bear, J.E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 2021, 22, 529–547. [Google Scholar] [CrossRef]
- Krause, M.; Gautreau, A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15, 577–590. [Google Scholar] [CrossRef]
- Rana, P.S.; Alkrekshi, A.; Wang, W.; Markovic, V.; Sossey-Alaoui, K. The Role of WAVE2 Signaling in Cancer. Biomedicines 2021, 9, 1217. [Google Scholar] [CrossRef] [PubMed]
- Caggia, S.; Tapadar, S.; Wu, B.; Venugopal, S.V.; Garrett, A.S.; Kumar, A.; Stiffend, J.S.; Davis, J.S.; Oyelere, A.K.; Khan, S.A. Small Molecule Inhibitors Targeting Galphai2 Protein Attenuate Migration of Cancer Cells. Cancers 2020, 12, 1631. [Google Scholar] [CrossRef]
- Venugopal, S.V.; Caggia, S.; Gambrell-Sanders, D.; Khan, S.A. Differential roles and activation of mammalian target of rapamycin complexes 1 and 2 during cell migration in prostate cancer cells. Prostate 2020, 80, 412–423. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef]
- Roberts, P.J.; Mitin, N.; Keller, P.J.; Chenette, E.J.; Madigan, J.P.; Currin, R.O.; Cox, A.D.; Wilson, O.; Kirschmeier, P.; Der, C.J. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J. Biol. Chem. 2008, 283, 25150–25163. [Google Scholar] [CrossRef]
- Takenawa, T.; Miki, H. WASP and WAVE family proteins: Key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001, 114, 1801–1809. [Google Scholar] [CrossRef]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Haarer, E.L.; Theodore, C.J.; Guo, S.; Frier, R.B.; Campellone, K.G. Genomic instability caused by Arp2/3 complex inactivation results in micronucleus biogenesis and cellular senescence. PLoS Genet 2023, 19, e1010045. [Google Scholar] [CrossRef]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Wu, Y.; Wan, X.; Tang, X.; Xu, Y.; Chen, Q.; Liu, Y.; Liu, S. Rac1/PAK1 signaling contributes to bone cancer pain by Regulation dendritic spine remodeling in rats. Mol. Pain 2023, 19, 17448069231161031. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Xu, E.; Luo, Q.; Song, G. Rac1: A Regulator of Cell Migration and a Potential Target for Cancer Therapy. Molecules 2023, 28, 2976. [Google Scholar] [CrossRef]
- Porter, A.P.; Papaioannou, A.; Malliri, A. Deregulation of Rho GTPases in cancer. Small GTPases 2016, 7, 123–138. [Google Scholar] [CrossRef]
- Jin, T.; Xu, X.; Hereld, D. Chemotaxis, chemokine receptors and human disease. Cytokine 2008, 44, 1–8. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, Z.M.; Zhu, D.C.; Guo, Q.; Xu, J.J.; Lin, J.H.; Chen, Z.X.; Wu, Y.S. Inhibition of Rac1 activity by NSC23766 prevents cartilage endplate degeneration via Wnt/beta-catenin pathway. J. Cell. Mol. Med. 2020, 24, 3582–3592. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Chao, X.; Singh, S.P.; Thomason, P.; Yan, Y.; Dong, M.; Li, L.; Insall, R.H.; Cai, H. Leep1 interacts with PIP3 and the Scar/WAVE complex to regulate cell migration and macropinocytosis. J. Cell Biol. 2021, 220, e202010096. [Google Scholar] [CrossRef]
- Singh, S.P.; Thomason, P.A.; Insall, R.H. Extracellular Signalling Modulates Scar/WAVE Complex Activity through Abi Phosphorylation. Cells 2021, 10, 3485. [Google Scholar] [CrossRef]
- Patel, S.; McKeon, D.; Sao, K.; Yang, C.; Naranjo, N.M.; Svitkina, T.M.; Petrie, R.J. Myosin II and Arp2/3 cross-talk governs intracellular hydraulic pressure and lamellipodia formation. Mol. Biol. Cell 2021, 32, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Asokan, S.B.; Berginski, M.E.; Haynes, E.M.; Sharpless, N.E.; Griffith, J.D.; Gomez, S.M.; Bear, J.E. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 2012, 148, 973–987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Liu, H.; Cui, Y.; Deng, Y. Dragon’s Blood Regulates Rac1-WAVE2-Arp2/3 Signaling Pathway to Protect Rat Intestinal Epithelial Barrier Dysfunction Induced by Simulated Microgravity. Int. J. Mol. Sci. 2021, 22, 2722. [Google Scholar] [CrossRef] [PubMed]
- Yelland, T.; Le, A.H.; Nikolaou, S.; Insall, R.; Machesky, L.; Ismail, S. Structural Basis of CYRI-B Direct Competition with Scar/WAVE Complex for Rac1. Structure 2021, 29, 226–237.e224. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, R.; Caggia, S.; Khan, S.A. Gαi2 Induces Cell Migration in PC3 Prostate Cancer Cells in the Absence of Rac1 Activation. Int. J. Mol. Sci. 2025, 26, 2663. https://doi.org/10.3390/ijms26062663
Johnson R, Caggia S, Khan SA. Gαi2 Induces Cell Migration in PC3 Prostate Cancer Cells in the Absence of Rac1 Activation. International Journal of Molecular Sciences. 2025; 26(6):2663. https://doi.org/10.3390/ijms26062663
Chicago/Turabian StyleJohnson, Rarnice, Silvia Caggia, and Shafiq A. Khan. 2025. "Gαi2 Induces Cell Migration in PC3 Prostate Cancer Cells in the Absence of Rac1 Activation" International Journal of Molecular Sciences 26, no. 6: 2663. https://doi.org/10.3390/ijms26062663
APA StyleJohnson, R., Caggia, S., & Khan, S. A. (2025). Gαi2 Induces Cell Migration in PC3 Prostate Cancer Cells in the Absence of Rac1 Activation. International Journal of Molecular Sciences, 26(6), 2663. https://doi.org/10.3390/ijms26062663





