Vasoactive Intestinal Peptide (VIP) in COVID-19 Therapy—Shedding of ACE2 and TMPRSS2 via ADAM10
Abstract
:1. Introduction
2. Results
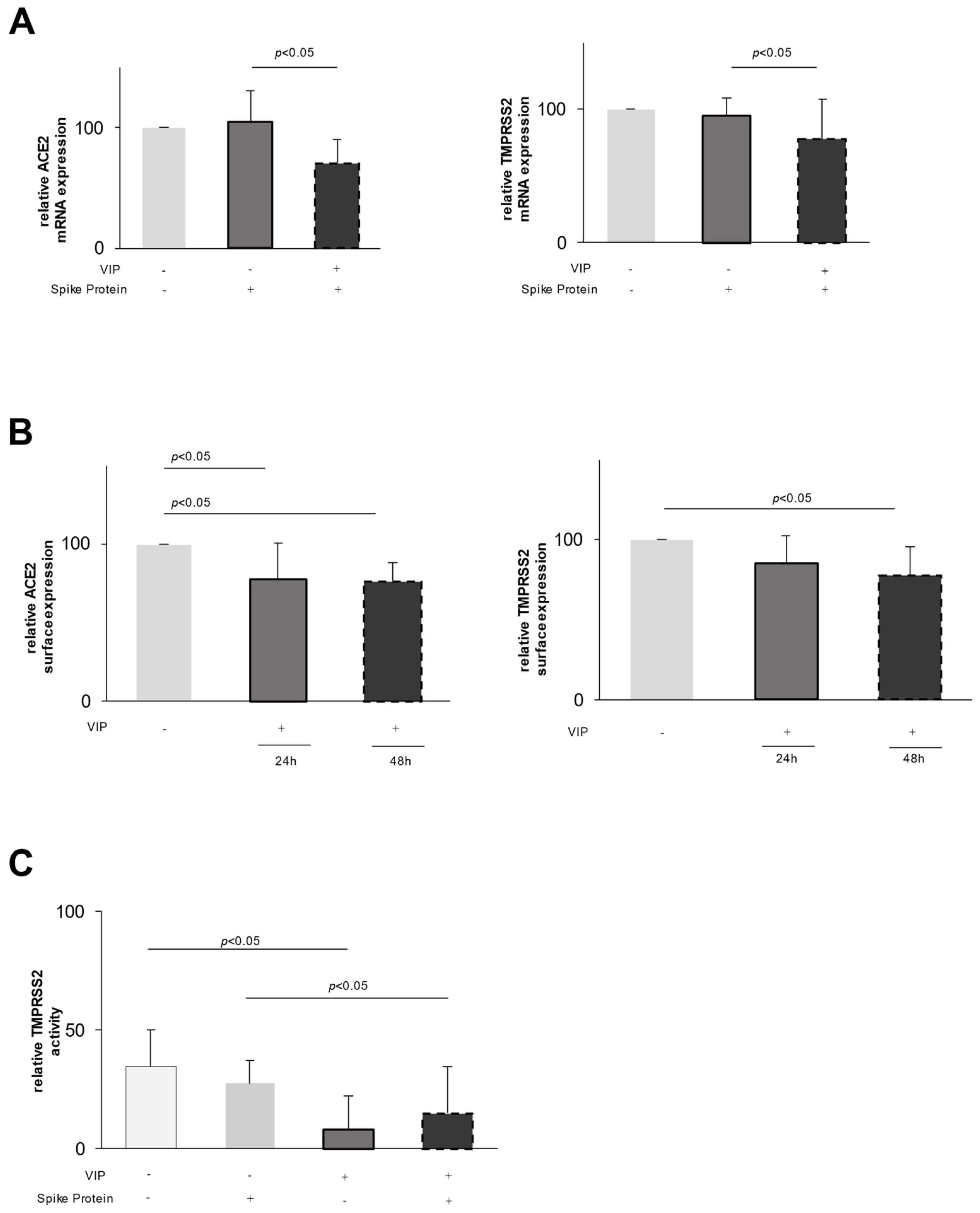
2.1. Effect of VIP on Epithelial Cells
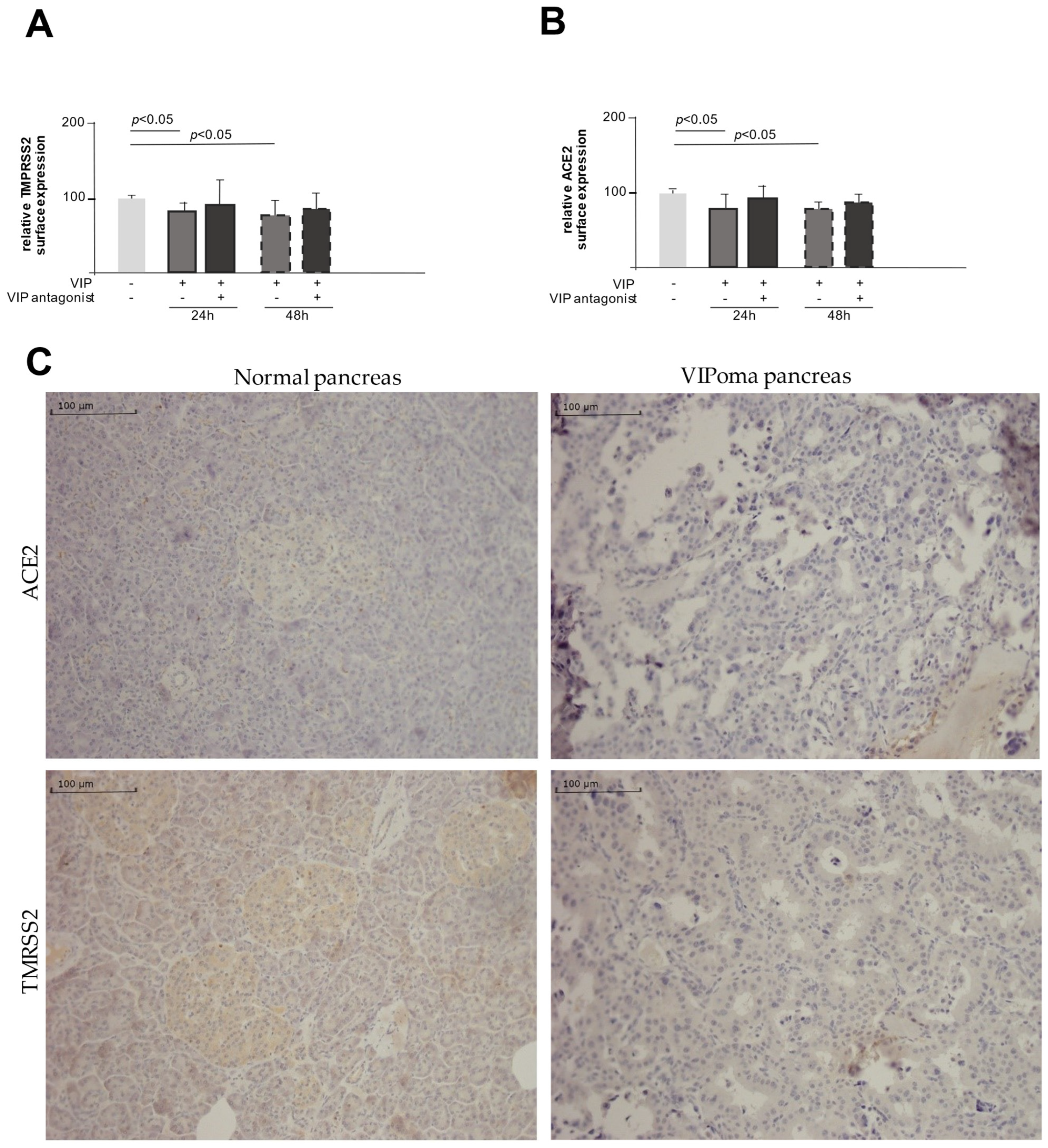
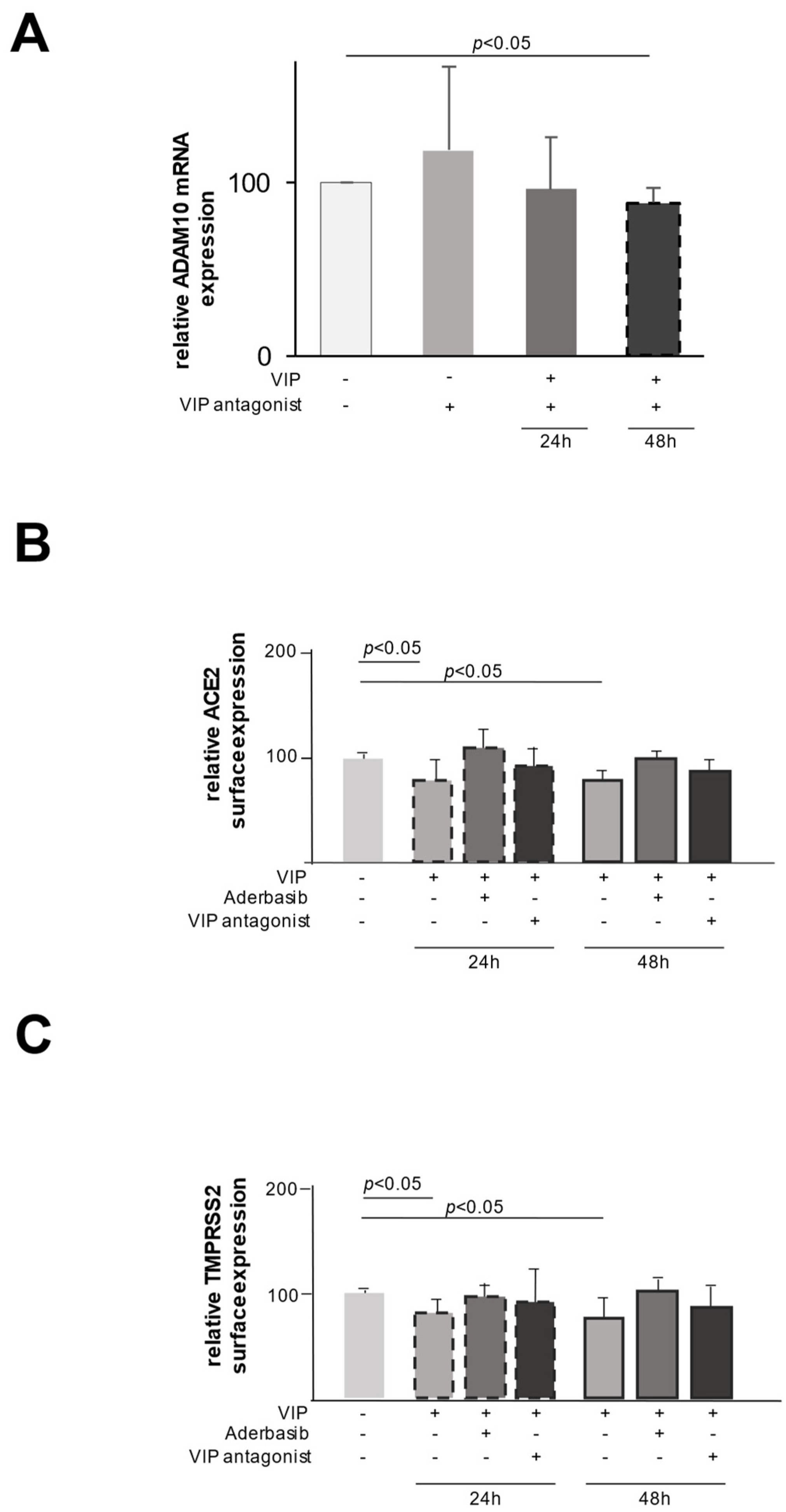
2.2. VIP Mediates TMPRSS2 and ACE2 Shedding via ADAM10
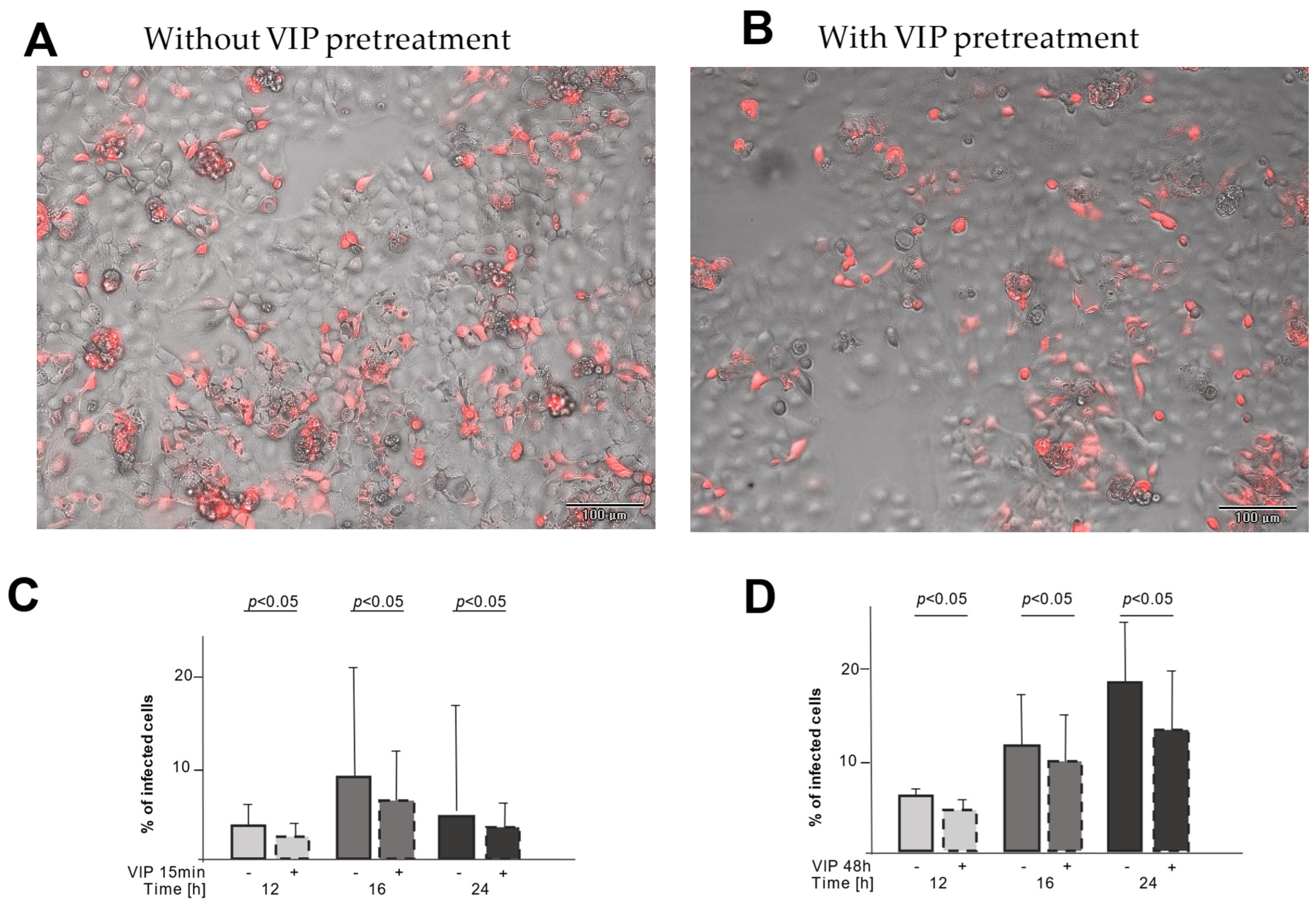
2.3. VIP Treatment Reduces the Number of Infected CaCo-2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Stimulation Assays
4.2. Harvesting Cells with RNA Isolation Kit from ExtractMe®
4.3. Real-Time PCR for mRNA Expression
4.4. Flow Cytometry
4.5. Fluorescence-Based Assay for Measuring TMPRSS2 Activity
4.6. Immunohistochemistry
4.7. Measuring SARS-CoV-2 Pseudovirus Infection Rate in Cell Cultures
4.8. Data Presentation and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus Dashboard. 2023. Available online: www.who.int (accessed on 11 November 2022).
- Paules, C.I.; Fauci, A.S. COVID-19: The therapeutic landscape. Med 2021, 2, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T.; et al. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef]
- Gulick, R.M.; Pau, A.K.; Daar, E.; Evans, L.; Gandhi, R.T.; Tebas, P.; Ridzon, R.; Masur, H.; Lane, H.C.; NIH COVID-19 Treatment Guidelines Panel; et al. National Institutes of Health COVID-19 Treatment Guidelines Panel: Perspectives and Lessons Learned. Ann. Intern. Med. 2024, 177, 1547–1557. [Google Scholar] [CrossRef]
- Roche, N.; Crichton, M.L.; Goeminne, P.C.; Cao, B.; Humbert, M.; Shteinberg, M.; Antoniou, K.M.; Ulrik, C.S.; Parks, H.; Wang, C.; et al. Update June 2022: Management of hospitalised adults with coronavirus disease 2019 (COVID-19): A European Respiratory Society living guideline. Eur. Respir. J. 2022, 60, 2200803. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Iwata, M.; Greenberg, B.H. Ectodomain shedding of ACE and ACE2 as regulators of their protein functions. Curr. Enzym. Inhib. 2011, 7, 42–55. [Google Scholar] [CrossRef]
- Niehues, R.V.; Wozniak, J.; Wiersch, F.; Lilienthal, E.; Tacken, N.; Schumertl, T.; Garbers, C.; Ludwig, A.; Düsterhöft, S. The collectrin-like part of theSARS-CoV-1 and -2receptorACE2is shed by the metalloproteinasesADAM10andADAM17. FASEB J. 2022, 36, e22234. [Google Scholar] [CrossRef]
- Saftig, P.; Lichtenthaler, S.F. The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog. Neurobiol. 2015, 135, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.; Koo, C.Z.; Tomlinson, M.G. Regulation of ADAM10 by the TspanC8 Family of Tetraspanins and Their Therapeutic Potential. Int. J. Mol. Sci. 2021, 22, 6707. [Google Scholar] [CrossRef]
- Arduise, C.; Abache, T.; Li, L.; Billard, M.; Chabanon, A.; Ludwig, A.; Mauduit, P.; Boucheix, C.; Rubinstein, E.; Le Naour, F. Tetraspanins regulate ADAM10-mediated cleavage of TNF-α and epidermal growth factor. J. Immunol. 2008, 181, 7002–7013. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.P.; Look, D.C.; Tan, P.; Shi, L.; Hickey, M.; Gakhar, L.; Chappell, M.C.; Wohlford-Lenane, C.; McCray, P.B., Jr. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L84–L96. [Google Scholar] [CrossRef] [PubMed]
- Said, S.I.; Mutt, V. Isolation from porcine-intestinal wall of a vasoactive octacosapeptide related to secretin and to glucagon. Eur. J. Biochem. 1972, 28, 199–204. [Google Scholar] [CrossRef]
- Gardner, J.D.; Cerda, J.J. In vitro inhibition of intestinal fluid and electrolyte transfer by a non-beta islet cell tumor. Proc. Soc. Exp. Biol. Med. 1966, 123, 361–364. [Google Scholar] [CrossRef]
- Delgado, M.; Pozo, D.; Ganea, D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Varela, N.; Chorny, A.; Delgado, M. Therapeutical approaches of vasoactive intestinal peptide as a pleiotropic immunomodulator. Curr. Pharm. Des. 2007, 13, 1113–1139. [Google Scholar] [CrossRef]
- Said, S.I.; Hamidi, S.A.; Dickman, K.G.; Szema, A.M.; Lyubsky, S.; Lin, R.Z.; Jiang, Y.-P.; Chen, J.J.; Waschek, J.A.; Kort, S. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation 2007, 115, 1260–1268. [Google Scholar] [CrossRef]
- Prasse, A.; Zissel, G.; Lützen, N.; Schupp, J.; Schmiedlin, R.; Gonzalez-Rey, E.; Rensing-Ehl, A.; Bacher, G.; Cavalli, V.; Bevec, D.; et al. Inhaled vasoactive intestinal peptide exerts immunoregulatory effects in sarcoidosis. Am. J. Respir. Crit. Care Med. 2010, 182, 540–548. [Google Scholar] [CrossRef]
- Temerozo, J.R.; Sacramento, C.Q.; Fintelman-Rodrigues, N.; Pão, C.R.R.; de Freitas, C.S.; Dias, S.S.G.; Ferreira, A.C.; Mattos, M.; Soares, V.C.; Teixeira, L.; et al. VIP plasma levels associate with survival in severe COVID-19 patients, correlating with protective effects in SARS-CoV-2-infected cells. J. Leukoc. Biol. 2022, 111, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.G.; Lavin, P.; Schoenfeld, D.A.; Lee, R.A.; Lenhardt, R.; Park, D.J.; Fernandez, J.P.; Morganroth, M.L.; Javitt, J.C.; Jayaweera, D. The Use of IV Vasoactive Intestinal Peptide (Aviptadil) in Patients With Critical COVID-19 Respiratory Failure: Results of a 60-Day Randomized Controlled Trial. Crit. Care Med. 2022, 50, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Chelbi-Alix, M.K.; Boissard, C.; Sripati, C.E.; Rosselin, G.; Thang, M.N. VIP induces in HT-29 cells 2′5′oligoadenylate synthetase and antiviral state via interferon β/α synthesis. Peptides 1991, 12, 1085–1093. [Google Scholar] [CrossRef]
- Vota, D.; Torti, M.; Paparini, D.; Giovannoni, F.; Merech, F.; Hauk, V.; Calo, G.; Ramhorst, R.; Garcia, C.; Leirós, C.P. Zika virus infection of first trimester trophoblast cells affects cell migration, metabolism and immune homeostasis control. J. Cell. Physiol. 2021, 236, 4913–4925. [Google Scholar] [CrossRef]
- Sacchi, T.B.; Bani, D.; Biliotti, G. Immunocytochemical and ultrastructural abnormalities of islet tissue in patients with VIP-producing tumors of the pancreas. Pancreas 1992, 7, 601–610. [Google Scholar] [CrossRef]
- Dreymueller, D.; Uhlig, S.; Ludwig, A. ADAM-family metalloproteinases in lung inflammation: Potential therapeutic targets. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L325–L343. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Böttcher-Friebertshäuser, E.; Lohoff, M. TMPRSS2, a novel host-directed drug target against SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 251. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, P.; Jiang, J.; Liu, Y.; Yan, R.; Shu, S.; Hu, B.; Xiao, H.; Cai, K.; Yuan, S.; et al. Advances in developing ACE2 derivatives against SARS-CoV-2. Lancet Microbe 2023, 4, e369–e378. [Google Scholar] [CrossRef]
- Zipeto, D.; Palmeira, J.d.F.; Argañaraz, G.A.; Argañaraz, E.R. ACE2/ADAM17/TMPRSS2 Interplay May Be the Main Risk Factor for COVID-19. Front. Immunol. 2020, 11, 576745. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Stamatelopoulos, K.; Terpos, E.; Tsitsilonis, O.E.; Aivalioti, E.; Paraskevis, D.; Kastritis, E.; Pavlakis, G.N.; Dimopoulos, M.A. Insights to SARS-CoV-2 life cycle, pathophysiology, and rationalized treatments that target COVID-19 clinical complications. J. Biomed. Sci. 2021, 28, 9. [Google Scholar] [CrossRef]
- Sharif-Askari, N.S.; Sharif-Askari, F.S.; Alabed, M.; Temsah, M.-H.; Al Heialy, S.; Hamid, Q.; Halwani, R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther.-Methods Clin. Dev. 2020, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Adedeji, A.O.; Severson, W.; Jonsson, C.; Singh, K.; Weiss, S.R.; Sarafianos, S.G. Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 2013, 87, 8017–8028. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.-P.; Zhou, R.; Chan, K.-H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e12. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Yang, C.X.; Tam, A.; Shaipanich, T.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R.; Sin, D.D. ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur. Respir. J. 2020, 55, 2000688. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Son, K.J.; Han, C.H.; Park, S.C.; Jung, J.Y. Impact of COPD on COVID-19 prognosis: A nationwide population-based study in South Korea. Sci. Rep. 2021, 11, 3735. [Google Scholar] [CrossRef]
- Saccon, E.; Chen, X.; Mikaeloff, F.; Rodriguez, J.E.; Szekely, L.; Vinhas, B.S.; Krishnan, S.; Byrareddy, S.N.; Frisan, T.; Végvári, Á.; et al. Cell-type-resolved quantitative proteomics map of interferon response against SARS-CoV-2. iScience 2021, 24, 102420. [Google Scholar] [CrossRef]
- Shuai, H.; Chu, H.; Hou, Y.; Yang, D.; Wang, Y.; Hu, B.; Huang, X.; Zhang, X.; Chai, Y.; Cai, J.-P.; et al. Differential immune activation profile of SARS-CoV-2 and SARS-CoV infection in human lung and intestinal cells: Implications for treatment with IFN-β and IFN inducer. J. Infect. 2020, 81, e1–e10. [Google Scholar] [CrossRef]
- Zupin, L.; Fontana, F.; Clemente, L.; Ruscio, M.; Ricci, G.; Crovella, S. Effect of Short Time of SARS-CoV-2 Infection in CaCo-2 Cells. Viruses 2022, 14, 704. [Google Scholar] [CrossRef]
- Bojkova, D.; Klann, K.; Koch, B.; Widera, M.; Krause, D.; Ciesek, S.; Cinatl, J.; Münch, C. Proteomics of SARS-CoV-2-infected host cells reveals therapy targets. Nature 2020, 583, 469–472. [Google Scholar] [CrossRef]
- Gutzler, C.; Höhne, K.; Müller-Quernheim, J.; Zissel, G.; Frye, B.C. Vasoactive intestinal peptide (VIP) suppresses ACE2- and TMPRSS2 expression in stimulated epithelial cells. Eur. Respir. J. 2021, 58 (Suppl. S65), PA2317. [Google Scholar] [CrossRef]
| Primer | Accession Number | Sequence | Tm (°C) |
|---|---|---|---|
| GAPDH_LISP | NM_002046.7 | 5′-CAC CAG GGC TGC TTT TAA CT-3′ | 55 |
| GAPDH_RISP | 5′-GAT CTC GCT CCT GGA AGA TG-3′ | 54 | |
| hsACE2 | NM_001371415 | 5′-GCC CTC TGC ACA AAT GTG ACA TCT-3′ | 59 |
| hsACE2 | 5′-TTT CCA ATG CTA GGG TCC AGG GTT-3′ | 61 | |
| hsTMPRSS2 | NM_001135099.1 | 5′-CTG CAG GGA CAT GGG CTA TAA GAA-3′ | 58 |
| hsTMPRSS2 | 5′-GAT ATC GAC ATT GCC GGC ACT T-3′ | 58 | |
| hsADAM10 | NM_001110.4 | 5′-TGG TGC TCA TGT ACC TCC CAA A-3′ | 56 |
| hsADAM10 | 5′-GGT GTG CAC TCT GTT CCA GAA TCA-3′ | 58 |
| Antibody | Supplier | Dilution |
|---|---|---|
| mouse anti-hACE2 AlexaFluor647 | R&D Systems, Inc., Minneapolis, MN, USA | 1:30 |
| mouse anti-TMPRSS2-FITC | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:30 |
| mouse IgG1 FITC | ImmunoTools, Friesoythe, Germany | 1:30 |
| mouse IgG2a AlexaFluor647 | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | 1:30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutzler, C.; Höhne, K.; Bani, D.; Kayser, G.; Fähndrich, S.; Ambros, M.; Hug, M.J.; Rieg, S.; Falcone, V.; Müller-Quernheim, J.; et al. Vasoactive Intestinal Peptide (VIP) in COVID-19 Therapy—Shedding of ACE2 and TMPRSS2 via ADAM10. Int. J. Mol. Sci. 2025, 26, 2666. https://doi.org/10.3390/ijms26062666
Gutzler C, Höhne K, Bani D, Kayser G, Fähndrich S, Ambros M, Hug MJ, Rieg S, Falcone V, Müller-Quernheim J, et al. Vasoactive Intestinal Peptide (VIP) in COVID-19 Therapy—Shedding of ACE2 and TMPRSS2 via ADAM10. International Journal of Molecular Sciences. 2025; 26(6):2666. https://doi.org/10.3390/ijms26062666
Chicago/Turabian StyleGutzler, Charlotte, Kerstin Höhne, Daniele Bani, Gian Kayser, Sebastian Fähndrich, Michael Ambros, Martin J. Hug, Siegbert Rieg, Valeria Falcone, Joachim Müller-Quernheim, and et al. 2025. "Vasoactive Intestinal Peptide (VIP) in COVID-19 Therapy—Shedding of ACE2 and TMPRSS2 via ADAM10" International Journal of Molecular Sciences 26, no. 6: 2666. https://doi.org/10.3390/ijms26062666
APA StyleGutzler, C., Höhne, K., Bani, D., Kayser, G., Fähndrich, S., Ambros, M., Hug, M. J., Rieg, S., Falcone, V., Müller-Quernheim, J., Zissel, G., & Frye, B. C. (2025). Vasoactive Intestinal Peptide (VIP) in COVID-19 Therapy—Shedding of ACE2 and TMPRSS2 via ADAM10. International Journal of Molecular Sciences, 26(6), 2666. https://doi.org/10.3390/ijms26062666






