Contributions of Dietary Patterns and Factors to Regulation of Rheumatoid Disease
Abstract
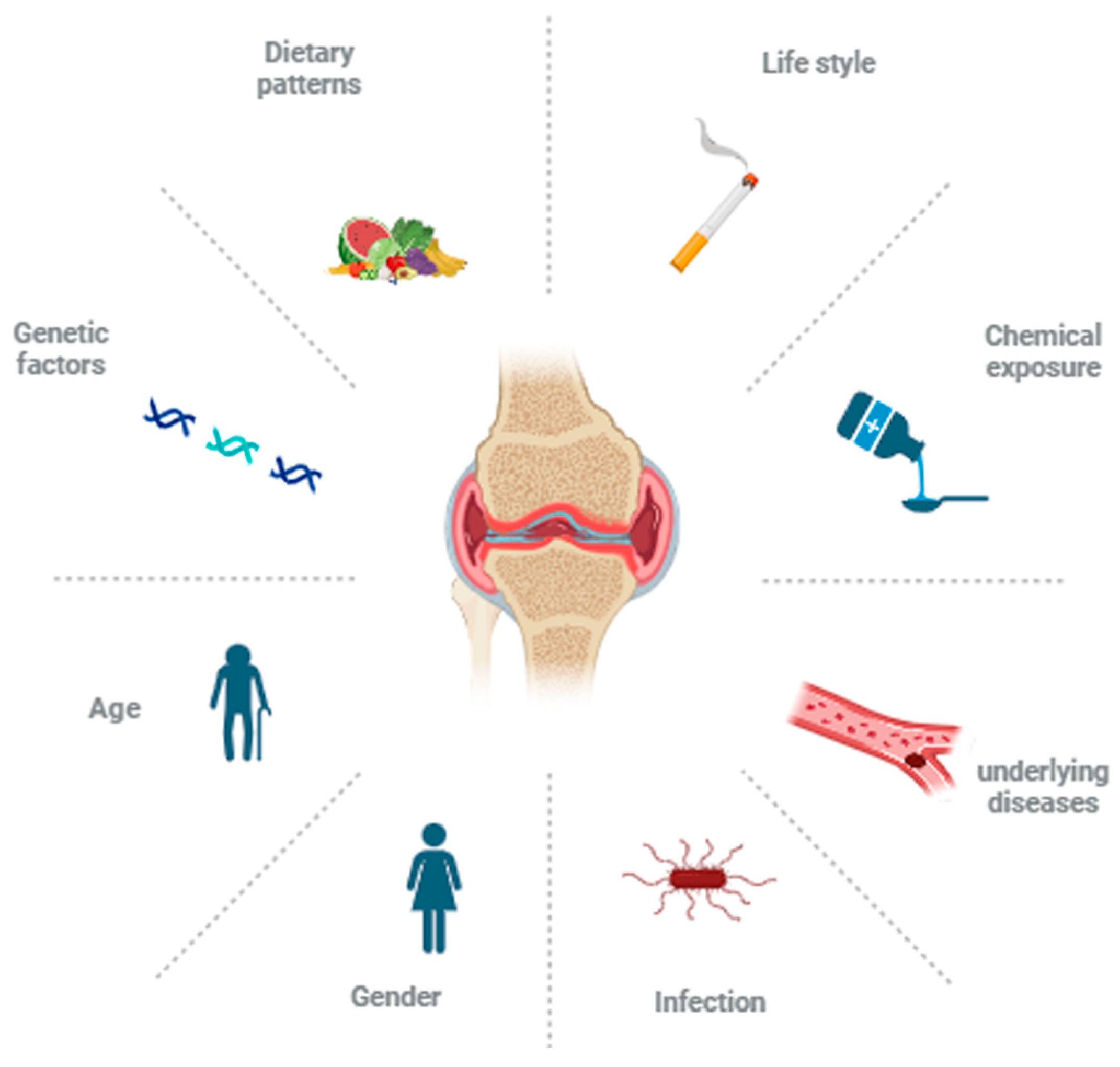
1. Introduction
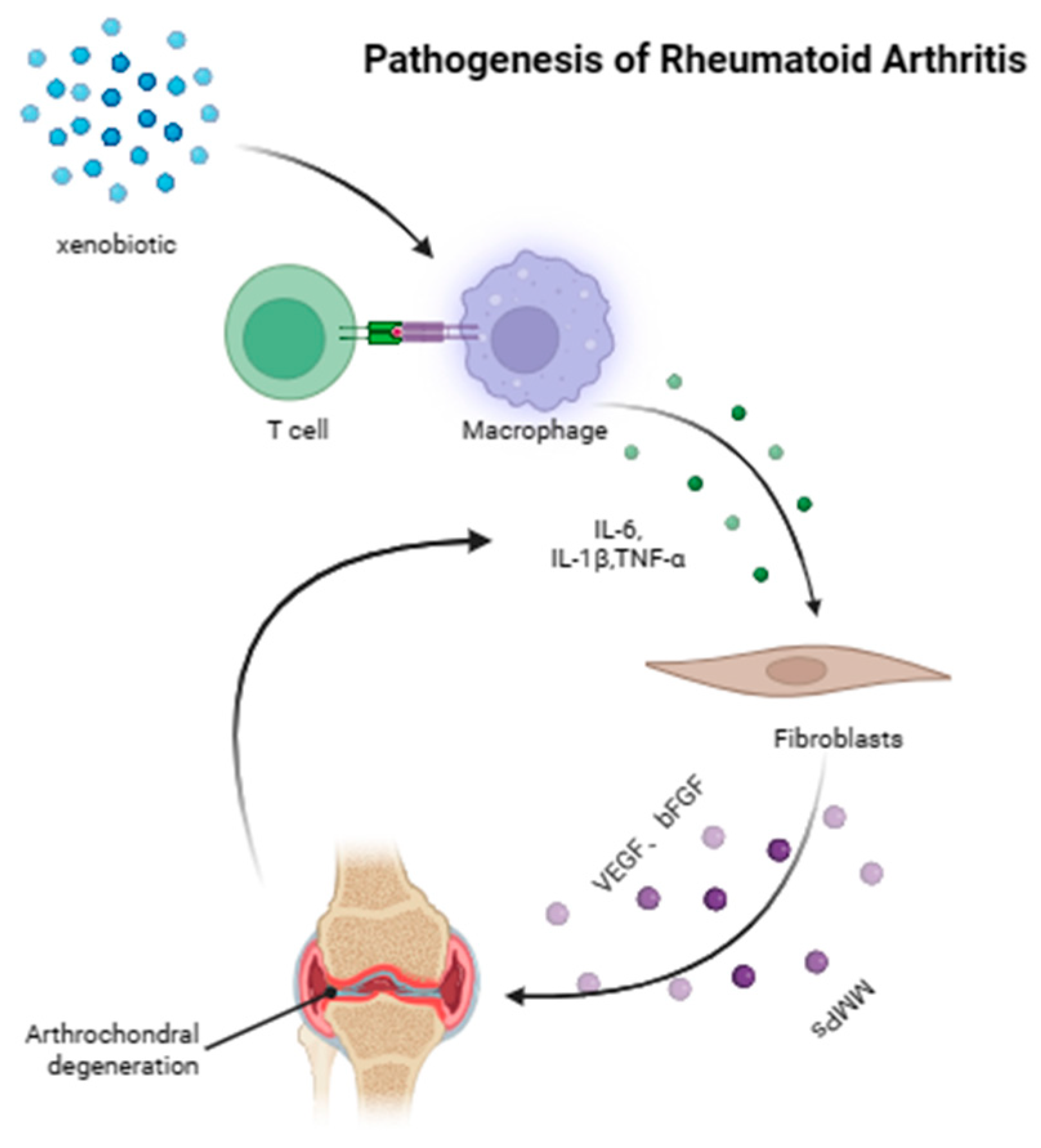
2. The Pathogenesis of RA
3. Effects of Different Dietary Patterns on RA Progress
3.1. Unbalanced Diet Patterns
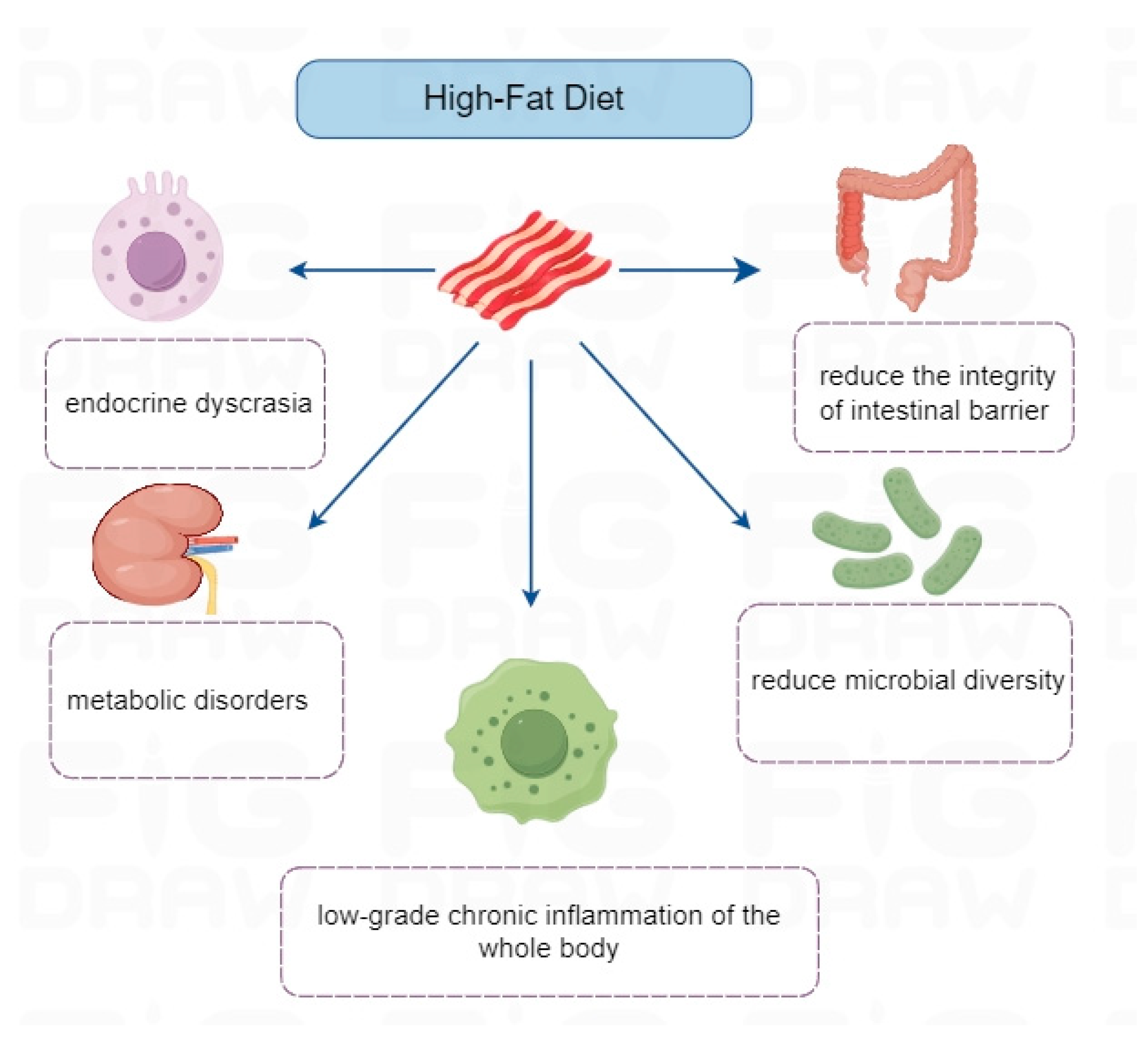
3.1.1. High-Fat Diet
3.1.2. High-Sugar Diet
3.1.3. High-Salt Diet
3.2. Balanced Diet Patterns
- (1)
- Anti-inflammatory effect: The MD is rich in antioxidants and anti-inflammatory components, such as polyphenols and omega-3 fatty acids, which can reduce the level of systemic inflammation [38,40]. In addition, the MD improved the symptoms of RA and lowered the level of inflammation. Studies have shown that the levels of inflammatory markers (such as CRP and IL-6) in the blood of RA patients with MD are reduced [111].
- (2)
- Lipid profile improvement: Healthy fats in the MD (such as olive oil and fish oil) can improve the lipid profile by reducing the level of low-density lipoprotein (LDL) and increasing the level of high-density lipoprotein (HDL), thus lowering the risk of cardiovascular diseases and indirectly improving the health status of patients with RA [102].
- (3)
- Immunoregulation: Some ingredients in the MD (such as prebiotics and probiotics) help to maintain gut microbiota balance and subsequently regulate immune system function, which may relieve the symptoms of RA [112]. The literature has shown that an imbalance in the gut microbiota is closely related to the occurrence of RA [113]. This imbalance may lead to abnormal reactions of the immune system, thus promoting inflammation. The prospect of probiotics as a potential intervention method is emphasized, and it is believed that by restoring the balance of the intestinal microflora, intestinal permeability could be improved, thus alleviating the symptoms of RA and regulating the immune response [113].
- (4)
- Weight management: The MD is usually low in calories and high in fiber, which helps maintain a healthy weight and relieves the symptoms of RA [114].
- (5)
- Intestinal health promotion: The MD is usually associated with an increase in intestinal microbial diversity. The Mediterranean diet is rich in fiber, which helps promote intestinal health and improve the diversity of the intestinal microflora, which may be related to the regulation of inflammatory reactions [115].
3.3. Mitigating Factors
3.3.1. Dietary Fiber
3.3.2. Probiotics
3.3.3. Vitamins
3.3.4. Minerals
3.3.5. Other Factor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| RA | Rheumatoid arthritis |
| MD | Mediterranean diet |
| HLA | Human leucocyte antigen |
| PTPN22 | Protein tyrosine phosphatase, non-receptor type 22 |
| CTLA-4 | Cytotoxic T lymphocyte-associated antigen-4 |
| CRP | C-reaction protein |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| MMP | Metalloproteinase |
| AGEs | Advanced glycation end products |
| SGK1 | Serum glucocorticoid kinase |
| DASH | Dietary Approaches to Stop Hypertension |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| IDF | Insoluble dietary fiber |
| SDF | Soluble dietary fiber |
| SCFAs | Short-chain fatty acids |
| MTX | Methotrexate |
| MK-4 | Menadione-4 |
| CIA | Arthritis induced by collagen |
| AMPK | Adenosine 5‘-monophosphate (AMP)-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Mcinnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. Mass. Med. Soc. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef]
- Rosa-Gonçalves, D.; Bernardes, M.; Costa, L. Quality of life and functional capacity in patients with rheumatoid arthritis-Cross-sectional study. Reum. Clin. (Engl. Ed.) 2018, 14, 360–366. [Google Scholar]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: A systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e594–e610. [CrossRef] [PubMed]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- van Heemst, J.; van der Woude, D.; Huizinga, T.W.; Toes, R.E. HLA and rheumatoid arthritis: How do they connect? Ann. Med. 2014, 46, 304–310. [Google Scholar] [CrossRef]
- Charpin, C.; Balandraud, N.; Guis, S.; Roudier, C.; Toussirot, E.; Rak, J.; Lambert, N.; Martin, M.; Reviron, D.; Roudier, J.; et al. HLA-DRB1*0404 is strongly associated with high titers of anti-cyclic citrullinated peptide antibodies in rheumatoid arthritis. Clin. Exp. Rheumatol. 2008, 26, 627–631. [Google Scholar]
- Rodríguez-Fernández, J.L. Antigen presentation by dendritic cells in rheumatoid arthritis. Curr. Top. Med. Chem. 2013, 13, 712–719. [Google Scholar] [CrossRef]
- Berthelot, J.M.; Darrieutort-Laffite, C.; Le Goff, B. Contribution of HLA DRB1, PTPN22, and CTLA4, to RA dysbiosis. Jt. Bone Spine 2022, 89, 105446. [Google Scholar] [CrossRef] [PubMed]
- Venetsanopoulou, A.I.; Alamanos, Y.; Voulgari, P.V.; Drosos, A.A. Epidemiology of rheumatoid arthritis: Genetic and environmental influences. Expert. Rev. Clin. Immunol. 2022, 18, 923–931. [Google Scholar] [CrossRef]
- Arleevskaya, M.I.; Manukyan, G.; Inoue, R.; Aminov, R. Editorial: Microbial and Environmental Factors in Autoimmune and Inflammatory Diseases. Front. Immunol. 2017, 8, 243. [Google Scholar] [CrossRef] [PubMed]
- Christman, L.M.; Gu, L. Efficacy and mechanisms of dietary polyphenols in mitigating rheumatoid arthritis. J. Funct. Foods 2020, 71, 104003. [Google Scholar] [CrossRef]
- Giannini, D.; Antonucci, M.; Petrelli, F.; Bilia, S.; Alunno, A.; Puxeddu, I. One year in review 2020: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2020, 38, 387–397. [Google Scholar] [CrossRef]
- Hitchon, C.A.; Chandad, F.; Ferucci, E.D.; Willemze, A.; Ioan-Facsinay, A.; van der Woude, D.; Markland, J.; Robinson, D.; Elias, B.; Newkirk, M.; et al. Antibodies to porphyromonas gingivalis are associated with anticitrullinated protein antibodies in patients with rheumatoid arthritis and their relatives. J. Rheumatol. 2010, 37, 1105–1112. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Rashid, T.; Dybwad, A.; Sioud, M.; Haugen, M.; Førre, O.; Ebringer, A. Decrease in anti-Proteus mirabilis but not anti-Escherichia coli antibody levels in rheumatoid arthritis patients treated with fasting and a one year vegetarian diet. Ann. Rheum. Dis. 1995, 54, 221–224. [Google Scholar] [CrossRef]
- Blaschke, S.; Schwarz, G.; Moneke, D.; Binder, L.; Müller, G.; Reuss-Borst, M. Epstein-Barr virus infection in peripheral blood mononuclear cells, synovial fluid cells, and synovial membranes of patients with rheumatoid arthritis. J. Rheumatol. 2000, 27, 866–873. [Google Scholar]
- da Rocha Sobrinho, H.M.; Jarach, R.; da Silva, N.A.; Shio, M.T.; Jancar, S.; Timenetsky, J.; Oliveira, M.A.; Dorta, M.L.; Ribeiro-Dias, F. Mycoplasmal lipid-associated membrane proteins and Mycoplasma arthritidis mitogen recognition by serum antibodies from patients with rheumatoid arthritis. Rheumatol. Int. 2011, 31, 951–957. [Google Scholar] [CrossRef]
- Parks, C.G.; Hoppin, J.A.; De Roos, A.J.; Costenbader, K.H.; Alavanja, M.C.; Sandler, D.P. Rheumatoid Arthritis in Agricultural Health Study Spouses: Associations with Pesticides and Other Farm Exposures. Environ. Health Perspect. 2016, 124, 1728–1734. [Google Scholar] [CrossRef]
- Solomon, D.H.; Reed, G.W.; Kremer, J.M.; Curtis, J.R.; Farkouh, M.E.; Harrold, L.R.; Hochberg, M.C.; Tsao, P.; Greenberg, J.D. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036. [Google Scholar] [CrossRef]
- Liu, Y.; Hazlewood, G.S.; Kaplan, G.G.; Eksteen, B.; Barnabe, C. Impact of Obesity on Remission and Disease Activity in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2017, 69, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; Farina, N.; Dagna, L. Obesity and its role in the management of rheumatoid and psoriatic arthritis. Clin. Rheumatol. 2020, 39, 1039–1047. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alarcón-de-la-Lastra, C.; Sánchez-Hidalgo, M. An update on dietary phenolic compounds in the prevention and management of rheumatoid arthritis. Food Funct. 2016, 7, 2943–2969. [Google Scholar] [CrossRef]
- Dessein, P.H.; Stanwix, A.E.; Solomon, A. How Could Physical Activity Reduce Inflammation and Inflammatory Gene Expression in Rheumatoid Arthritis? J. Rheumatol. 2022, 49, 1299–1302. [Google Scholar] [CrossRef]
- Bungau, S.G.; Behl, T.; Singh, A.; Sehgal, A.; Singh, S.; Chigurupati, S.; Vijayabalan, S.; Das, S.; Palanimuthu, V.R. Targeting Probiotics in Rheumatoid Arthritis. Nutrients 2021, 13, 3376. [Google Scholar] [CrossRef]
- Metsios, G.S.; Kitas, G.D. Physical activity, exercise and rheumatoid arthritis: Effectiveness, mechanisms and implementation. Best. Pract. Res. Clin. Rheumatol. 2018, 32, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, X.; Yang, Y.; Zhi, K.; Chen, Y.; Zhao, J.; Cui, W.; Zhao, X.; Zhang, Z.; An, Y.; et al. Association between passive smoking and the risk of rheumatoid arthritis: A systematic review and meta-analysis. Clin. Rheumatol. 2023, 42, 663–672. [Google Scholar] [CrossRef]
- Hu, H.; Xu, A.; Gao, C.; Wang, Z.; Wu, X. The effect of physical exercise on rheumatoid arthritis: An overview of systematic reviews and meta-analysis. J. Adv. Nurs. 2021, 77, 506–522. [Google Scholar] [CrossRef]
- Swardh, E.; Brodin, N. Effects of aerobic and muscle strengthening exercise in adults with rheumatoid arthritis: A narrative review summarising a chapter in Physical activity in the prevention and treatment of disease (FYSS 2016). Br. J. Sports Med. 2016, 50, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Redwan, E.M. Interplay of Microbiota and Citrullination in the Immunopathogenesis of Rheumatoid Arthritis. Probiotics Antimicrob. Proteins 2022, 14, 99–113. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Johansson, K.; Askling, J.; Alfredsson, L.; Di Giuseppe, D. Mediterranean diet and risk of rheumatoid arthritis: A population-based case-control study. Arthritis Res. Ther. 2018, 20, 175. [Google Scholar]
- Skoczyńska, M.; Świerkot, J. The role of diet in rheumatoid arthritis. Reumatologia 2018, 56, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Manzel, A.; Muller, D.N.; Hafler, D.A.; Erdman, S.E.; Linker, R.A.; Kleinewietfeld, M. Role of “Western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 2014, 14, 404. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou-Athanassiou, I.; Athanassiou, L.; Athanassiou, P. The Effect of Omega-3 Fatty Acids on Rheumatoid Arthritis. Mediterr. J. Rheumatol. 2020, 31, 190–194. [Google Scholar] [CrossRef]
- Zorgetto-Pinheiro, V.A.; Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Hiane, P.A.; Pott, A.; Guimaraes, R.C.A.; Bogo, D. Omega-3 Fatty Acids and Balanced Gut Microbiota on Chronic Inflammatory Diseases: A Close Look at Ulcerative Colitis and Rheumatoid Arthritis Pathogenesis. J. Med. Food 2022, 25, 341–354. [Google Scholar] [CrossRef]
- Jalili, M.; Hekmatdoost, A. Dietary omega-3 fatty acids and their influence on inflammation via Toll-like receptor pathways. Nutrition 2021, 85, 111070. [Google Scholar] [CrossRef] [PubMed]
- Bellan, M.; Sainaghi, P.P.; Pirisi, M. Role of Vitamin D in Rheumatoid Arthritis. Adv. Exp. Med. Biol. 2017, 996, 155–168. [Google Scholar] [PubMed]
- Moradi, A.; Nezamoleslami, S.; Nezamoleslami, S.; Clark, C.C.T.; Sohouli, M.H.; Ghiasvand, R. The association between dietary total antioxidant capacity with risk of rheumatoid arthritis in adults: A case-control study. Clin. Nutr. ESPEN 2022, 51, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, S. Dietary fiber intake associated with risk of rheumatoid arthritis among U.S. adults: NHANES 2010–2020. Medicine 2023, 102, e33357. [Google Scholar] [CrossRef]
- Rasouli-Saravani, A.; Jahankhani, K.; Moradi, S.; Gorgani, M.; Shafaghat, Z.; Mirsanei, Z.; Mehmandar, A.; Mirzaei, R. Role of microbiota short-chain fatty acids in the pathogenesis of autoimmune diseases. Biomed. Pharmacother. 2023, 162, 114620. [Google Scholar] [CrossRef]
- Coutant, F.; Miossec, P. Evolving concepts of the pathogenesis of rheumatoid arthritis with focus on the early and late stages. Curr. Opin. Rheumatol. 2020, 32, 57–63. [Google Scholar] [CrossRef]
- Scherer, H.U.; Haupl, T.; Burmester, G.R. The etiology of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102400. [Google Scholar] [CrossRef]
- de Hair, M.J.; van de Sande, M.G.; Ramwadhdoebe, T.H.; Hansson, M.; Landewé, R.; van der Leij, C.; Maas, M.; Serre, G.; van Schaardenburg, D.; Klareskog, L.; et al. Features of the synovium of individuals at risk of developing rheumatoid arthritis: Implications for understanding preclinical rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 513–522. [Google Scholar] [CrossRef]
- Elshabrawy, H.A.; Chen, Z.; Volin, M.V.; Ravella, S.; Virupannavar, S.; Shahrara, S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015, 18, 433–448. [Google Scholar] [CrossRef]
- Mehana, E.E.; Khafaga, A.F.; El-Blehi, S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Pulik, L.; Legosz, P.; Motyl, G. Matrix metalloproteinases in rheumatoid arthritis and osteoarthritis: A state of the art review. Reumatologia 2023, 61, 191–201. [Google Scholar] [CrossRef]
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Praditsorn, P.; Purnamasari, S.D.; Sranacharoenpong, K.; Arai, Y.; Sundermeir, S.M.; Gittelsohn, J.; Hadi, H.; Nishi, N. Measures of Perceived Neighborhood Food Environments and Dietary Habits: A Systematic Review of Methods and Associations. Nutrients 2022, 14, 1788. [Google Scholar] [CrossRef] [PubMed]
- Bouchard-Mercier, A.; Paradis, A.M.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Associations between dietary patterns and gene expression profiles of healthy men and women: A cross-sectional study. Nutr. J. 2013, 12, 24. [Google Scholar] [CrossRef]
- Xia, Y.; Gu, Y.; Yu, F.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Du, H.; Shi, H.; Guo, X.; et al. Association between dietary patterns and metabolic syndrome in Chinese adults: A propensity score-matched case-control study. Sci. Rep. 2016, 6, 34748. [Google Scholar] [CrossRef]
- Deahl-Greenlaw, A.; Marks, S. Meeting the New 2015–2020 Dietary Guidelines for Americans. Dela. J. Public Health 2016, 2, 22–23. [Google Scholar] [CrossRef]
- Bovolini, A.; Garcia, J.; Andrade, M.A.; Duarte, J.A. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021, 42, 199–214. [Google Scholar] [CrossRef]
- Bäcklund, R.; Drake, I.; Bergström, U.; Compagno, M.; Sonestedt, E.; Turesson, C. Diet and the risk of rheumatoid arthritis-A systematic literature review. Semin. Arthritis Rheum. 2023, 58, 152118. [Google Scholar] [CrossRef]
- Rohr, M.W.; Narasimhulu, C.A.; Rudeski-Rohr, T.A.; Parthasarathy, S. Negative Effects of a High-Fat Diet on Intestinal Permeability: A Review. Adv. Nutr. 2020, 11, 77–91. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhang, J.; Ren, S.; Cao, Q.; Kong, H.; Xu, Q.; Liu, R. High-fat diet stimulated butyric acid metabolism dysbiosis, altered microbiota, and aggravated inflammatory response in collagen-induced arthritis rats. Nutr. Metab. 2024, 21, 95. [Google Scholar] [CrossRef]
- Min, Y.; Heo, Y.; Feng, F.; Kim, D.; Kim, M.; Yang, J.; Kim, H.J.; Jee, Y.; Ghosh, M.; Kang, I.; et al. High-Sucrose Diet Accelerates Arthritis Progression in a Collagen-Induced Rheumatoid Arthritis Model. Mol. Nutr. Food Res. 2023, 67, e2300244. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 2014, 66, 121–129. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, J.W.; Netea, M.G. A salty taste to autoimmunity. N. Engl. J. Med. 2013, 368, 2520–2521. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Amital, H.; Shoenfeld, Y. The role of dietary sodium in autoimmune diseases: The salty truth. Autoimmun. Rev. 2018, 17, 1069–1073. [Google Scholar] [CrossRef]
- Hager, J.; Bang, H.; Hagen, M.; Frech, M.; Trager, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef]
- Bai, Y.; Li, Y.; Marion, T.; Tong, Y.; Zaiss, M.M.; Tang, Z.; Zhang, Q.; Liu, Y.; Luo, Y. Resistant starch intake alleviates collagen-induced arthritis in mice by modulating gut microbiota and promoting concomitant propionate production. J. Autoimmun. 2021, 116, 102564. [Google Scholar] [CrossRef]
- Vaghef-Mehrabany, E.; Alipour, B.; Homayouni-Rad, A.; Sharif, S.K.; Asghari-Jafarabadi, M.; Zavvari, S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition 2014, 30, 430–435. [Google Scholar] [CrossRef]
- Mohammed, A.T.; Khattab, M.; Ahmed, A.M.; Turk, T.; Sakr, N.; Adham, M.K.; Abdelhalim, M.; Sawaf, B.; Hirayama, K.; Huy, N.T. The therapeutic effect of probiotics on rheumatoid arthritis: A systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017, 36, 2697–2707. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhuo, F.; Wang, H.; Geng, X.; Xu, B.; Yin, L.; Sun, H.; Yan, X. Additive Effects of VDBP and 1,25(OH)2D3 on the Viability and Apoptosis of Rheumatoid Arthritis Synovial Fibroblasts. Front. Endocrinol. 2020, 11, 583229. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Hsu, F.C.; Nevitt, M.; Booth, S.L.; Kwoh, C.K.; McAlindon, T.E.; Vermeer, C.; Drummen, N.; Harris, T.B.; et al. The association between vitamin K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthr. Cartil. 2015, 23, 370–378. [Google Scholar] [CrossRef]
- Krisanits, B.; Randise, J.F.; Burton, C.E.; Findlay, V.J.; Turner, D.P. Pubertal mammary development as a “susceptibility window” for breast cancer disparity. Adv. Cancer Res. 2020, 146, 57–82. [Google Scholar]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef] [PubMed]
- Rhea, E.M.; Salameh, T.S.; Logsdon, A.F.; Hanson, A.J.; Erickson, M.A.; Banks, W.A. Blood-Brain Barriers in Obesity. AAPS J. 2017, 19, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, K.; Shen, Y.; Peng, X.; Tan, H.; Liu, L.; Xie, Q.; Wang, Y. Incidence of collagen-induced arthritis is elevated by a high-fat diet without influencing body weight in mice. RMD Open 2024, 10, e003869. [Google Scholar] [CrossRef]
- Iannone, F.; Fanizzi, R.; Notarnicola, A.; Scioscia, C.; Anelli, M.G.; Lapadula, G. Obesity reduces the drug survival of second line biological drugs following a first TNF-α inhibitor in rheumatoid arthritis patients. Jt. Bone Spine 2015, 82, 187–191. [Google Scholar] [CrossRef]
- Jhun, J.Y.; Yoon, B.Y.; Park, M.K.; Oh, H.J.; Byun, J.K.; Lee, S.Y.; Min, J.K.; Park, S.H.; Kim, H.Y.; Cho, M.L. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp. Mol. Med. 2012, 44, 424–431. [Google Scholar] [CrossRef]
- Kim, S.J.; Chen, Z.; Essani, A.B.; Elshabrawy, H.A.; Volin, M.V.; Fantuzzi, G.; McInnes, I.B.; Baker, J.F.; Finn, P.; Kondos, G.; et al. Differential impact of obesity on the pathogenesis of RA or preclinical models is contingent on the disease status. Ann. Rheum. Dis. 2017, 76, 731–739. [Google Scholar] [CrossRef]
- Turesson, C.; Bergstrom, U.; Pikwer, M.; Nilsson, J.A.; Jacobsson, L.T. High serum cholesterol predicts rheumatoid arthritis in women, but not in men: A prospective study. Arthritis Res. Ther. 2015, 17, 284. [Google Scholar] [CrossRef]
- Turesson, C.; Bergström, U.; Pikwer, M.; Nilsson, J.; Jacobsson, L.T. A high body mass index is associated with reduced risk of rheumatoid arthritis in men, but not in women. Rheumatology 2016, 55, 307–314. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, X.; Cheng, H.; Zhang, D.F. [Research Progress in High-Sugar Diet and Inflammatory Diseases]. Sichuan Da Xue Xue Bao Yi Xue Ban 2022, 53, 538–542. [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015.
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31 (Suppl. S1), S61–S78. [Google Scholar] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Almiron-Roig, E. Frontiers in Neuroscience Human Perceptions and Preferences for Fat-Rich Foods. In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; Taylor & Francis Group, LLC.: Boca Raton, FL, USA, 2010. [Google Scholar]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef]
- Stanhope, K.L. Sugar consumption, metabolic disease and obesity: The state of the controversy. Crit. Rev. Clin. Lab. Sci. 2016, 53, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Hu, Y.; Costenbader, K.H.; Gao, X.; Al-Daabil, M.; Sparks, J.A.; Solomon, D.H.; Hu, F.B.; Karlson, E.W.; Lu, B. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am. J. Clin. Nutr. 2014, 100, 959–967. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Laiguillon, M.C.; Courties, A.; Houard, X.; Auclair, M.; Sautet, A.; Capeau, J.; Fève, B.; Berenbaum, F.; Sellam, J. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: Toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Espino-Gonzalez, E.; Dalbram, E.; Mounier, R.; Gondin, J.; Farup, J.; Jessen, N.; Treebak, J.T. Impaired skeletal muscle regeneration in diabetes: From cellular and molecular mechanisms to novel treatments. Cell Metab. 2024, 36, 1204–1236. [Google Scholar] [PubMed]
- Dieppe, P. Epidemiology of the Rheumatic Diseases Second Edition. Silman, A.J., Hochberg, M.C., Eds.; Oxford University Press: Oxford, UK, 2001; p. 377, ISBN: 0192631497. Int. J. Epidemiol. 2002, 31, 1079–1080. [Google Scholar] [CrossRef]
- Zhang, Y.; Pool, A.H.; Wang, T.; Liu, L.; Kang, E.; Zhang, B.; Ding, L.; Frieda, K.; Palmiter, R.; Oka, Y. Parallel neural pathways control sodium consumption and taste valence. Cell 2023, 186, 5751–5765.e16. [Google Scholar] [CrossRef]
- Kaldor, J.C.; Thow, A.M.; Schönfeldt, H. Using regulation to limit salt intake and prevent non-communicable diseases: Lessons from South Africa’s experience. Public Health Nutr. 2019, 22, 1316–1325. [Google Scholar] [CrossRef]
- WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012.
- Salgado, E.; Bes-Rastrollo, M.; de Irala, J.; Carmona, L.; Gómez-Reino, J.J. High Sodium Intake Is Associated With Self-Reported Rheumatoid Arthritis: A Cross Sectional and Case Control Analysis Within the SUN Cohort. Medicine 2015, 94, e0924. [Google Scholar] [CrossRef] [PubMed]
- Sundström, B.; Johansson, I.; Rantapää-Dahlqvist, S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: Results from a nested case-control study. Rheumatology 2015, 54, 487–493. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Meyers Mathieu, K.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet. 2023, 123, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Vitiello, V.; Germani, A.; Capuzzo Dolcetta, E.; Donini, L.M.; Del Balzo, V. The New Modern Mediterranean Diet Italian Pyramid. Ann. Ig. Med. Prev. Comunita 2016, 28, 179–186. [Google Scholar]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean diet and health status: An updated meta-analysis and a proposal for a literature-based adherence score. Public. Health Nutr. 2014, 17, 2769–2782. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; et al. Modern vision of the Mediterranean diet. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E36–E43. [Google Scholar]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Philippou, E. Nutrition and its role in prevention and management of rheumatoid arthritis. Autoimmun. Rev. 2023, 22, 103333. [Google Scholar] [CrossRef]
- Blenkinsopp, H.C.; Seidler, K.; Barrow, M. Microbial Imbalance and Intestinal Permeability in the Pathogenesis of Rheumatoid Arthritis: A Mechanism Review with a Focus on Bacterial Translocation, Citrullination, and Probiotic Intervention. J. Am. Nutr. Assoc. 2024, 43, 59–76. [Google Scholar] [CrossRef]
- Pitaraki, E.E. The role of Mediterranean diet and its components on the progress of osteoarthritis. J. Frailty Sarcopenia Falls 2017, 2, 45–52. [Google Scholar] [CrossRef]
- Kimble, R.; Gouinguenet, P.; Ashor, A.; Stewart, C.; Deighton, K.; Matu, J.; Griffiths, A.; Malcomson, F.C.; Joel, A.; Houghton, D.; et al. Effects of a mediterranean diet on the gut microbiota and microbial metabolites: A systematic review of randomized controlled trials and observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 8698–8719. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Danneskiold-Samsøe, N.B.; Dias de Freitas Queiroz Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Maróstica Júnior, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Sun, Y.; Zhang, X. Combined Physical Exercise and Diet: Regulation of Gut Microbiota to Prevent and Treat of Metabolic Disease: A Review. Nutrients 2022, 14, 4774. [Google Scholar] [CrossRef]
- Gill, P.A.; van Zelm, M.C.; Muir, J.G.; Gibson, P.R. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment. Pharmacol. Ther. 2018, 48, 15–34. [Google Scholar] [CrossRef]
- Dürholz, K.; Hofmann, J.; Iljazovic, A.; Häger, J.; Lucas, S.; Sarter, K.; Strowig, T.; Bang, H.; Rech, J.; Schett, G.; et al. Dietary Short-Term Fiber Interventions in Arthritis Patients Increase Systemic SCFA Levels and Regulate Inflammation. Nutrients 2020, 12, 3207. [Google Scholar] [CrossRef]
- Rosser, E.C.; Piper, C.J.M.; Matei, D.E.; Blair, P.A.; Rendeiro, A.F.; Orford, M.; Alber, D.G.; Krausgruber, T.; Catalan, D.; Klein, N.; et al. Microbiota-Derived Metabolites Suppress Arthritis by Amplifying Aryl-Hydrocarbon Receptor Activation in Regulatory B Cells. Cell Metab. 2020, 31, 837–851.e10. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Britton, R.A.; Hoffmann, D.E.; Khoruts, A. Probiotics and the Microbiome-How Can We Help Patients Make Sense of Probiotics? Gastroenterology 2021, 160, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Furneri, P.M. Applications of Probiotics and Their Potential Health Benefits. Int. J. Mol. Sci. 2023, 24, 15915. [Google Scholar] [CrossRef]
- Szajewska, H.; Canani, R.B.; Guarino, A.; Hojsak, I.; Indrio, F.; Kolacek, S.; Orel, R.; Shamir, R.; Vandenplas, Y.; van Goudoever, J.B.; et al. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 495–506. [Google Scholar] [CrossRef]
- Abdallah, A.; Elemba, E.; Zhong, Q.; Sun, Z. Gastrointestinal Interaction between Dietary Amino Acids and Gut Microbiota: With Special Emphasis on Host Nutrition. Curr. Protein Pept. Sci. 2020, 21, 785–798. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; Jenmalm, M.C.; Prescott, S.L. The gut microbiota and its role in the development of allergic disease: A wider perspective. Clin. Exp. Allergy 2015, 45, 43–53. [Google Scholar] [CrossRef]
- Girardin, M.; Frossard, J.L. The role of probiotics in the treatment of inflammatory bowel diseases. Rev. Med. Suisse 2012, 8, 1674–1676, 1678. [Google Scholar]
- Zamani, B.; Golkar, H.R.; Farshbaf, S.; Emadi-Baygi, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Akhavan, R.; Taghizadeh, M.; Memarzadeh, M.R.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: A randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016, 19, 869–879. [Google Scholar] [CrossRef]
- Yamashita, M.; Matsumoto, K.; Endo, T.; Ukibe, K.; Hosoya, T.; Matsubara, Y.; Nakagawa, H.; Sakai, F.; Miyazaki, T. Preventive Effect of Lactobacillus helveticus SBT2171 on Collagen-Induced Arthritis in Mice. Front. Microbiol. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Kumar, A.; Sharma, P.; Singh, R. Lactobacillus casei and Lactobacillus acidophilus regulate inflammatory pathway and improve antioxidant status in collagen-induced arthritic rats. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2013, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Whitfield, G.K.; Hsieh, J.C.; Thompson, P.D.; Barthel, T.K.; Bartik, L.; Egan, J.B.; Wu, Y.; Kubicek, J.L.; et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010, 121, 88–97. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W. The Roles of Vitamin D and Its Analogs in Inflammatory Diseases. Curr. Top. Med. Chem. 2016, 16, 1242–1261. [Google Scholar] [CrossRef] [PubMed]
- Azzeh, F.S.; Kensara, O.A. Vitamin D Is a Good Marker for Disease Activity of Rheumatoid Arthritis Disease. Dis. Markers 2015, 2015, 260725. [Google Scholar] [CrossRef]
- Tsugawa, N.; Shiraki, M. Vitamin K Nutrition and Bone Health. Nutrients 2020, 12, 1909. [Google Scholar] [CrossRef]
- Okamoto, H. Vitamin K and rheumatoid arthritis. IUBMB Life 2008, 60, 355–361. [Google Scholar] [CrossRef]
- Meki, A.R.; Hamed, E.A.; Ezam, K.A. Effect of green tea extract and vitamin C on oxidant or antioxidant status of rheumatoid arthritis rat model. Indian. J. Clin. Biochem. 2009, 24, 280–287. [Google Scholar] [CrossRef]
- Kou, H.; Qing, Z.; Guo, H.; Zhang, R.; Ma, J. Effect of vitamin E supplementation in rheumatoid arthritis: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 166–172. [Google Scholar] [CrossRef]
- Turrubiates-Hernandez, F.J.; Marquez-Sandoval, Y.F.; Gonzalez-Estevez, G.; Reyes-Castillo, Z.; Munoz-Valle, J.F. The Relevance of Selenium Status in Rheumatoid Arthritis. Nutrients 2020, 12, 3007. [Google Scholar] [CrossRef] [PubMed]
- Walwadkar, S.D.; Suryakar, A.N.; Katkam, R.V.; Kumbar, K.M.; Ankush, R.D. Oxidative stress and calcium-phosphorus levels in Rheumatoid arthritis. Indian J. Clin. Biochem. IJCB 2006, 21, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Malhotra, S.; Welling, M.N.; Mantri, S.B.; Desai, K. In vitro and in vivo antioxidant, cytotoxic, and anti-chronic inflammatory arthritic effect of selenium nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 993–1003. [Google Scholar] [CrossRef]
- Cejka, D.; Hayer, S.; Niederreiter, B.; Sieghart, W.; Fuereder, T.; Zwerina, J.; Schett, G. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum. 2010, 62, 2294–2302. [Google Scholar] [CrossRef]
- Schonenberger, K.A.; Schupfer, A.C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef] [PubMed]
- Raad, T.; Griffin, A.; George, E.S.; Larkin, L.; Fraser, A.; Kennedy, N.; Tierney, A.C. Dietary Interventions with or without Omega-3 Supplementation for the Management of Rheumatoid Arthritis: A Systematic Review. Nutrients 2021, 13, 3506. [Google Scholar] [CrossRef]
- Mathieu, S.; Pereira, B.; Daien, C.; Tournadre, A.; Soubrier, M. Omega 3 Fatty Acids Intake Does Not Decrease the Risk of Rheumatoid Arthritis Occurrence: A Meta-Analysis. Comment on Tanski et al. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients 2022, 14, 1030. Nutrients 2023, 15, 539. [Google Scholar]
| Diet Patterns | Dietary Types | Influencing Mechanism | References |
|---|---|---|---|
| Unbalanced diet patterns | High-fat diet | Long-term high-fat diet may lead to endocrine disorders, metabolic disorders, systemic low-grade chronic inflammation, reduce microbial diversity, and the integrity of the intestinal barrier, thus leading to the induction or aggravation of RA. | [61,62] |
| High-sugar diet | High glucose intake aggravates RA symptoms by increasing blood glucose and insulin levels, increasing inflammatory factors, accelerating joint degeneration, reducing bone mineral density, and leading to weight gain. | [63,64] | |
| High-salt diet | Increasing the intake of sodium chloride may decrease the number of T regulatory cells by activating pro-inflammatory macrophages and Th 17 cells and affecting the sodium sensitivity of Th 17 cells, thus increasing the risk of RA. | [65,66] | |
| Balanced diet patterns | Dietary fiber-rich diet | Dietary fiber may help alleviate the symptoms of rheumatoid arthritis by regulating inflammation, improving intestinal microbial community, controlling body weight, enhancing immune function, and maintaining intestinal barrier. | [67,68] |
| Probiotics-rich diet | Probiotics can significantly alleviate the occurrence and development of rheumatoid arthritis through immunomodulation, inhibiting inflammation, maintaining intestinal microbial balance, enhancing intestinal barrier function, and improving nutrient absorption. | [69,70] | |
| Vitamins-rich diet | Vitamins may play an active role in the management and treatment of rheumatoid arthritis through anti-inflammatory reaction, immunomodulation, antioxidation, and promoting bone health. | [71,72] |
| Study Size and Duration | Probiotic Strain | Results | References |
|---|---|---|---|
| Forty-six patients with RA (8 weeks) | Lactobacillus casei | Disease activity score was significantly decreased by the intervention, a statistically significant improvement in all pro-inflammatory biomarkers except for IL-1β. | [69] |
| Sixty patients with RA (8 weeks) | Lactobacillus acidophilus Lactobacillus casei Bifidobacterium bifidum | Taking probiotic supplements for 8 weeks among patients with RA had beneficial effects on DAS-28, insulin levels, HOMA-B, and hs-CRP levels. | [133] |
| Male DBA/1J mouse model of RA (7 weeks) | L. helveticus SBT2171 | The ability of L. helveticus SBT2171 to downregulate the abundance of immune cells and the subsequent production of CII-specific antibodies and IL-6, thereby suppressing the CIA symptoms. | [134] |
| Male Wistar rat model of RA (4 weeks) | Lactobacillus casei and Lactobacillus acidophilus | Treatment with Lactobacillus casei and Lactobacillus acidophilus significantly downregulated pro-inflammatory cytokines and upregulated anti-inflammatory cytokines and significantly reduced the oxidative stress and arthritis score of synovial tissues. | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, X.; Fang, J.; Li, Y.; Yu, Y.; Wang, J.; Sun, B. Contributions of Dietary Patterns and Factors to Regulation of Rheumatoid Disease. Int. J. Mol. Sci. 2025, 26, 2674. https://doi.org/10.3390/ijms26062674
Zhang J, Wang X, Fang J, Li Y, Yu Y, Wang J, Sun B. Contributions of Dietary Patterns and Factors to Regulation of Rheumatoid Disease. International Journal of Molecular Sciences. 2025; 26(6):2674. https://doi.org/10.3390/ijms26062674
Chicago/Turabian StyleZhang, Jingjie, Xueli Wang, Juan Fang, Yingying Li, Yonghui Yu, Jing Wang, and Baoguo Sun. 2025. "Contributions of Dietary Patterns and Factors to Regulation of Rheumatoid Disease" International Journal of Molecular Sciences 26, no. 6: 2674. https://doi.org/10.3390/ijms26062674
APA StyleZhang, J., Wang, X., Fang, J., Li, Y., Yu, Y., Wang, J., & Sun, B. (2025). Contributions of Dietary Patterns and Factors to Regulation of Rheumatoid Disease. International Journal of Molecular Sciences, 26(6), 2674. https://doi.org/10.3390/ijms26062674








