Prunin: An Emerging Anticancer Flavonoid
Abstract
:1. Introduction
1.1. Flavonoids and Their Significance in Biomedical Applications
1.2. Introduction to Prunin: Sources and Structure
1.3. Importance of Prunin in Cancer Research
2. Therapeutic Potential of Prunin and Mechanism of Action in Cancer
2.1. Effect of Prunin on Cell Cycle
2.2. Intrinsic and Extrinsic Pathways
2.3. The PI3K/Akt/mTOR Pathway
2.4. Antioxidant and Anti-Inflammatory Mechanisms
2.5. Activation of the P53 Pathway by Prunin
2.6. Activation of MAPK Pathway by Prunin
2.7. Modulation of Tumor Microenvironment (TME)
2.8. Suppression of Angiogenesis and Metastasis by Prunin
3. Potential for Combining Prunin with Conventional Therapies
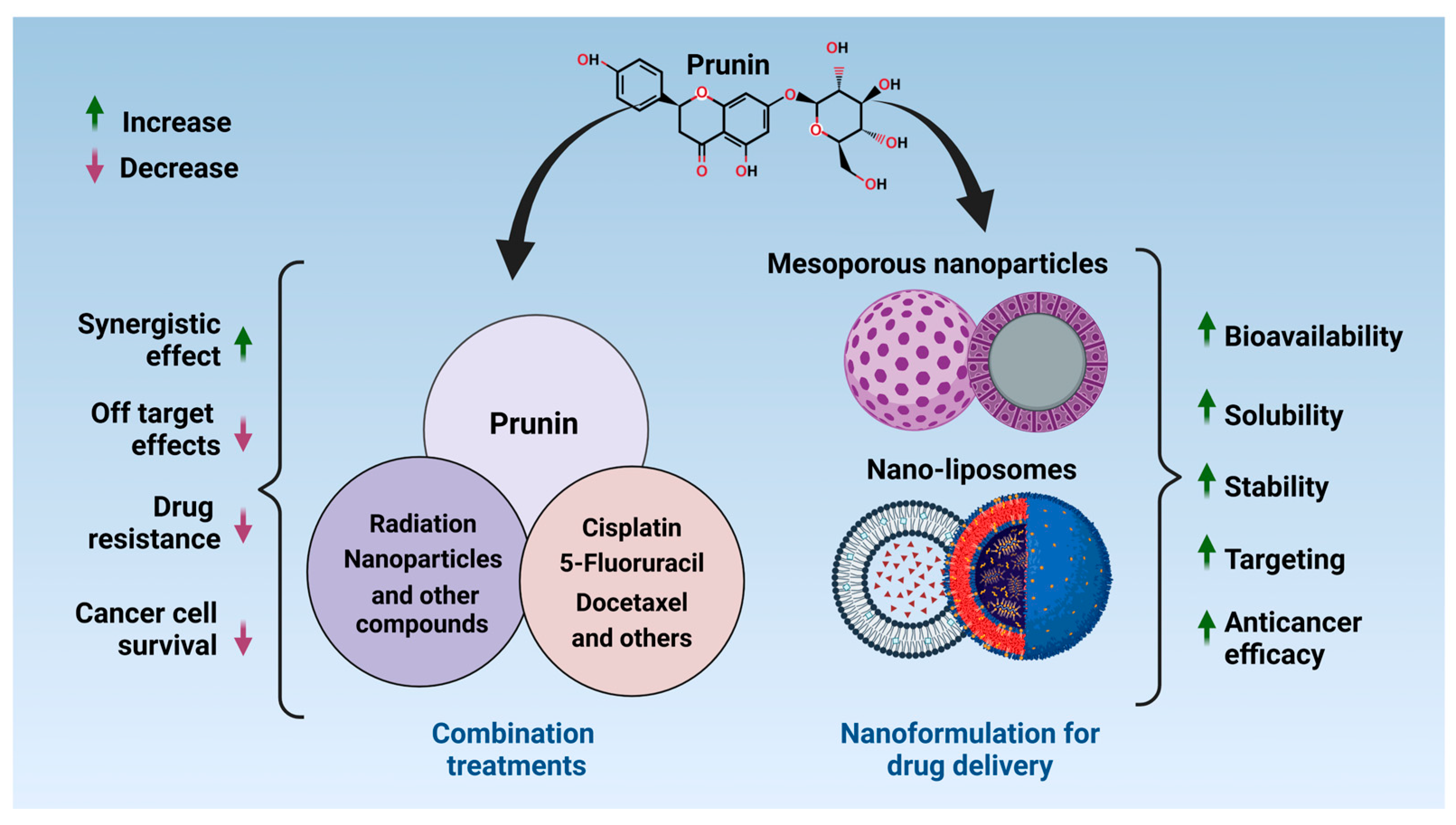
4. Nanotechnology and Drug Delivery Systems for Prunin
5. Conclusions and Future Perspectives
5.1. Summary of Key Findings
5.2. Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| ICIs | Immune checkpoint inhibitors |
| NK | Natural killer |
| VEGF | Vascular endothelial growth factor |
| CDK | Cyclin-dependent kinase |
| RNS | Reactive nitrogen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| MAPK | Mitogen-activated protein kinase |
| ERK | Extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| RTKs | Receptor tyrosine kinases |
| GPCRs | G-protein coupled receptors |
| EPR | Enhanced permeability and retention |
| AI | Artificial intelligence |
| TME | Tumor microenvironment |
References
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer Activity and Mechanism of Xanthohumol: A Prenylated Flavonoid From Hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Blanco, S.; Fernández, J.; López-Ibáñez, S.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Plant Phytochemicals in Food Preservation: Antifungal Bioactivity: A Review. J. Food Prot. 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Tzanova, M.; Atanasov, V.; Yaneva, Z.; Ivanova, D.; Dinev, T. Selectivity of Current Extraction Techniques for Flavonoids from Plant Materials. Processes 2020, 8, 1222. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of Cancer Metabolism: The Therapeutic Potential of Cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef] [PubMed]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsäläinen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A.K. Assessing the Response of Plant Flavonoids to UV Radiation: An Overview of Appropriate Techniques. Phytochem. Rev. 2015, 14, 273–297. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Rahimi, R.; Ghiasi, S.; Azimi, H.; Fakhari, S.; Abdollahi, M. A Review of the Herbal Phosphodiesterase Inhibitors; Future Perspective of New Drugs. Cytokine 2010, 49, 123–129. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Frenț, O.-D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin—Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef] [PubMed]
- Terahara, N. Flavonoids in Foods: A Review. Nat. Prod. Commun. 2015, 10, 1934578X1501000334. [Google Scholar] [CrossRef]
- Treutter, D.; Feucht, W.; Schmid, P.P.S. Ageing-Dependent Responses of Phloem Flavonoids of Prunus Avium Graftings: Flavanone-, Flavone- and Isoflavone-Glucosides. Sci. Hortic. 1987, 32, 183–193. [Google Scholar] [CrossRef]
- Castillo, J.; Benavente, O.; del Rio, J.A. Hesperetin 7-O-Glucoside and Prunin in Citrus Species (C. aurantium and C. paradisi). A Study of Their Quantitative Distribution in Immature Fruits and as Immediate Precursors of Neohesperidin and Naringin in Citrus Aurantium. J. Agric. Food Chem. 1993, 41, 1920–1924. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Jin, T.; Albillos, S.M.; Guo, F.; Howard, A.; Fu, T.-J.; Kothary, M.H.; Zhang, Y.-Z. Crystal Structure of Prunin-1, a Major Component of the Almond (Prunus dulcis) Allergen Amandin. J. Agric. Food Chem. 2009, 57, 8643–8651. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2019, 11, 28. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure–Function and Mechanisms of Action and Opportunities for Drug Development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Jung, H.A.; Ali, M.Y.; Bhakta, H.K.; Min, B.-S.; Choi, J.S. Prunin Is a Highly Potent Flavonoid from Prunus davidiana Stems That Inhibits Protein Tyrosine Phosphatase 1B and Stimulates Glucose Uptake in Insulin-Resistant HepG2 Cells. Arch. Pharm. Res. 2017, 40, 37–48. [Google Scholar] [CrossRef]
- Choi, J.S.; Yokozawa, T.; Oura, H. Antihyperlipidemic Effect of Flavonoids from Prunus davidiana. J. Nat. Prod. 1991, 54, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Céliz, G.; Audisio, M.C.; Daz, M. Antimicrobial Properties of Prunin, a Citric Flavanone Glucoside, and Its Prunin 6″-O-lauroyl Ester. J. Appl. Microbiol. 2010, 109, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Harikrishna, D.; Appa Rao, A.V.N.; Prabhakar, M.C. Pharmacological Investigation of Prunin-6″-O-p-Coumarate: A Flavonoid Glycoside. Indian J. Pharmacol. 2004, 36, 244–245. [Google Scholar]
- Ayub, H.; Nadeem, M.; Mohsin, M.; Ambreen, S.; Khan, F.A.; Oranab, S.; Rahim, M.A.; Zubair Khalid, M.; Zongo, E.; Zarlasht, M.; et al. A Comprehensive Review on the Availability of Bioactive Compounds, Phytochemicals, and Antioxidant Potential of Plum (Prunus domestica). Int. J. Food Prop. 2023, 26, 2388–2406. [Google Scholar] [CrossRef]
- Abraão, A.S.; Fernandes, N.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus lusitanica L. Fruits as a Novel Source of Bioactive Compounds with Antioxidant Potential: Exploring the Unknown. Antioxidants 2022, 11, 1738. [Google Scholar] [CrossRef]
- Berhow, M.A.; Vandercook, C.E. Biosynthesis of Naringin and Prunin in Detached Grapefruit. Phytochemistry 1989, 28, 1627–1630. [Google Scholar] [CrossRef]
- Dai, M.; Kang, X.; Wang, Y.; Huang, S.; Guo, Y.; Wang, R.; Chao, N.; Liu, L. Functional Characterization of Flavanone 3-Hydroxylase (F3H) and Its Role in Anthocyanin and Flavonoid Biosynthesis in Mulberry. Molecules 2022, 27, 3341. [Google Scholar] [CrossRef]
- Rehan, M. Biosynthesis of Diverse Class Flavonoids via Shikimate and Phenylpropanoid Pathway. In Bioactive Compounds-Biosynthesis, Characterization and Applications; Queiroz Zepka, L., Casagrande Do Nascimento, T., Jacob-Lopes, E., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83969-270-3. [Google Scholar]
- Liu, Y.; Qian, J.; Li, J.; Xing, M.; Grierson, D.; Sun, C.; Xu, C.; Li, X.; Chen, K. Hydroxylation Decoration Patterns of Flavonoids in Horticultural Crops: Chemistry, Bioactivity, and Biosynthesis. Hortic. Res. 2022, 9, uhab068. [Google Scholar] [CrossRef]
- Wu, R.; Qian, C.; Yang, Y.; Liu, Y.; Xu, L.; Zhang, W.; Ou, J. Integrative Transcriptomic and Metabolomic Analyses Reveal the Phenylpropanoid and Flavonoid Biosynthesis of Prunus mume. J. Plant Res. 2024, 137, 95–109. [Google Scholar] [CrossRef]
- Waris, M.H.; Muzaffar, N.; Mumtaz, M.A.; Afzal, A.M.; Iqbal, M.W.; Mumtaz, S.; Ali, M.; Alaraidh, I.A.; Okla, M.K.; Munnaf, S.A. High Performance Lanthanum-Doped Nickel Cobalt Ferrites on Titanium Carbide MXene Electrode Material for Superior Hybrid Device and Precision Creatinine Sensing. Appl. Phys. A 2025, 131, 220. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Yilmaz, B.; Pateiro, M.; Kumar, M.; Domínguez, R.; Shariati, M.A.; Hano, C.; Lorenzo, J.M. Valorization of By-Products from Prunus Genus Fruit Processing: Opportunities and Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 7795–7810. [Google Scholar] [CrossRef] [PubMed]
- Zan, S.; Wang, R.; Zhang, F.; Zhang, D.; Liu, B.; Meng, X. Composition Analysis of Rootstock Cherry (Prunus mahaleb L.), a Potential Source of Human Nutrition and Dietary Supplements. Eur. Food Res. Technol. 2022, 248, 1421–1435. [Google Scholar] [CrossRef]
- Nunes, A.R.; Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R. Prunus avium L. (Sweet Cherry) By-Products: A Source of Phenolic Compounds with Antioxidant and Anti-Hyperglycemic Properties—A Review. Appl. Sci. 2021, 11, 8516. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Martínez-Bailén, M.; Salido, S.; Altarejos, J. Antimicrobial Activity of Phenolics Isolated from the Pruning Wood Residue of European Plum (Prunus domestica L.). Ind. Crops Prod. 2022, 176, 114296. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, M.K.; Wani, T.F.; Sharma, A.; Nyorak, G. Varietal Wealth of Prunus Species. In Prunus-Recent Advances; Küden, A.B., Kuden, A., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83969-582-7. [Google Scholar]
- Vila-Real, H.; Alfaia, A.J.; Calado, A.R.; Ribeiro, M.H.L. Improvement of Activity and Stability of Soluble and Sol–Gel Immobilized Naringinase in Co-Solvent Systems. J. Mol. Catal. B Enzym. 2010, 65, 91–101. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated Flavonoids, Promising Nutraceuticals with Impressive Biological Activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharya, A.; Maulik, S.R. Chapter 3-Isolation and Characterization of Natural Dyes and Pigments. In Renewable Dyes and Pigments; Islam, U.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 37–48. ISBN 978-0-443-15213-9. [Google Scholar]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, Characterization, and Health Benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Arora, S.; Itankar, P. Extraction, Isolation and Identification of Flavonoid from Chenopodium Album Aerial Parts. J. Tradit. Complement. Med. 2018, 8, 476–482. [Google Scholar] [CrossRef]
- Youn, S.H.; Kim, H.J.; Kim, T.H.; Shin, C.S. Lipase-Catalyzed Acylation of Naringin with Palmitic Acid in Highly Concentrated Homogeneous Solutions. J. Mol. Catal. B Enzym. 2007, 46, 26–31. [Google Scholar] [CrossRef]
- Lyu, Y.; Zeng, W.; Du, G.; Chen, J.; Zhou, J. Efficient Bioconversion of Epimedin C to Icariin by a Glycosidase from Aspergillus nidulans. Bioresour. Technol. 2019, 289, 121612. [Google Scholar] [CrossRef]
- Zha, J.; Wu, X.; Gong, G.; Koffas, M.A.G. Pathway Enzyme Engineering for Flavonoid Production in Recombinant Microbes. Metab. Eng. Commun. 2019, 9, e00104. [Google Scholar] [CrossRef]
- Yadav, V.; Yadav, P.K.; Yadav, S.; Yadav, K.D.S. α-l-Rhamnosidase: A Review. Process Biochem. 2010, 45, 1226–1235. [Google Scholar] [CrossRef]
- Hădărugă, D.-I.; Hădărugă, N.-G. Flavanones in Plants and Humans: Properties and Applications. In Handbook of Food Bioactive Ingredients; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–53. ISBN 978-3-030-81404-5. [Google Scholar]
- Ribeiro, M.H.L. Glycosides. In Biotechnology of Bioactive Compounds; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 301–344. ISBN 9781118733103. [Google Scholar]
- Isidore, E.; Willig, G.; Brunissen, F.; Magro, C.; Monteux, C.; Ioannou, I. Selective Recovery of Glycosylated Phenolic Compounds from Nectarine Tree Branches (Prunus persica Var. nucipersica). Food Chem. Adv. 2024, 4, 100585. [Google Scholar] [CrossRef]
- Le Roy, J.; Huss, B.; Creach, A.; Hawkins, S.; Neutelings, G. Glycosylation Is a Major Regulator of Phenylpropanoid Availability and Biological Activity in Plants. Front. Plant Sci. 2016, 7, 735. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Li, R.; Huang, L.; Zhang, Z.; Chen, J.; Tang, H. Integrated Multispectroscopic Analysis and Molecular Docking Analyses of the Structure-Affinity Relationship and Mechanism of the Interaction of Flavonoids with Zein. Food Chem. 2022, 386, 132839. [Google Scholar] [CrossRef] [PubMed]
- Akher, F.B.; Ebrahimi, A.; Mostafavi, N. Characterization of π-Stacking Interactions between Aromatic Amino Acids and Quercetagetin. J. Mol. Struct. 2017, 1128, 13–20. [Google Scholar] [CrossRef]
- Deogratias, G.; Shadrack, D.M.; Munissi, J.J.E.; Kinunda, G.A.; Jacob, F.R.; Mtei, R.P.; Masalu, R.J.; Mwakyula, I.; Kiruri, L.W.; Nyandoro, S.S. Hydrophobic π-π Stacking Interactions and Hydrogen Bonds Drive Self-Aggregation of Luteolin in Water. J. Mol. Graph. Model. 2022, 116, 108243. [Google Scholar] [CrossRef]
- Jung, H.A.; Paudel, P.; Seong, S.H.; Min, B.-S.; Choi, J.S. Structure-Related Protein Tyrosine Phosphatase 1B Inhibition by Naringenin Derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2274–2280. [Google Scholar] [CrossRef]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Li, Y.; Paxton, J.W. The Effects of Flavonoids on the ABC Transporters: Consequences for the Pharmacokinetics of Substrate Drugs. Expert Opin. Drug Metab. Toxicol. 2013, 9, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Rebai, R.; Jasmin, L.; Boudah, A. Identification of Two Flavonoids as New and Safe Inhibitors of Kynurenine Aminotransferase II via Computational and In Vitro Study. Pharmaceuticals 2025, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.A.; Mitchell, A.E.; Shin, A.C.; Dehghani, F.; Shen, C.-L. Dietary Flavonoid Actions on Senescence, Aging, and Applications for Health. J. Nutr. Biochem. 2025, 139, 109862. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Yadav, S.; Yadava, S.; Yadav, K.D.S. α-l-Rhamnosidase from Aspergillus Flavus MTCC-9606 Isolated from Lemon Fruit Peel. Int. J. Food Sci. Technol. 2011, 46, 350–357. [Google Scholar] [CrossRef]
- Shakour, Z.T.A.; Fayek, N.M.; Farag, M.A. How Do Biocatalysis and Biotransformation Affect Citrus Dietary Flavonoids Chemistry and Bioactivity? A Review. Crit. Rev. Biotechnol. 2020, 40, 689–714. [Google Scholar] [CrossRef]
- Ali, M.Y.; Zamponi, G.W.; Abdul, Q.A.; Seong, S.H.; Min, B.-S.; Jung, H.A.; Choi, J.S. Prunin from Poncirus trifoliata (L.) Rafin Inhibits Aldose Reductase and Glucose-Fructose–Mediated Protein Glycation and Oxidation of Human Serum Albumin. J. Agric. Food Chem. 2024, 72, 7203–7218. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, Y.; Hu, J.; Zhang, L.; Benito, M.J.; Usmanov, D.; Nishanbaev, S.Z.; Song, X.; Zou, L.; Wu, Y. Quercetin and Kaempferol from Saffron Petals Alleviated Hydrogen Peroxide-Induced Oxidative Damage in B16 Cells. J. Sci. Food Agric. 2025, 105, 967–973. [Google Scholar] [CrossRef]
- Zhang, L.; Mohankumar, K.; Martin, G.; Mariyam, F.; Park, Y.; Han, S.J.; Safe, S. Flavonoids Quercetin and Kaempferol Are NR4A1 Antagonists and Suppress Endometriosis in Female Mice. Endocrinology 2023, 164, bqad133. [Google Scholar] [CrossRef]
- Speisky, H.; Arias-Santé, M.F.; Fuentes, J. Oxidation of Quercetin and Kaempferol Markedly Amplifies Their Antioxidant, Cytoprotective, and Anti-Inflammatory Properties. Antioxidants 2023, 12, 155. [Google Scholar] [CrossRef]
- Li, B.-C.; Wu, B.; Hou, X.; Ding, G.-B. Substrate Selectivities of GH78 α-L-Rhamnosidases from Human Gut Bacteria on Dietary Flavonoid Glycosides. Molecules 2025, 30, 980. [Google Scholar] [CrossRef]
- Chukwuma, C.I. Antioxidative, Metabolic and Vascular Medicinal Potentials of Natural Products in the Non-Edible Wastes of Fruits Belonging to the Citrus and Prunus Genera: A Review. Plants 2024, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, K.; Guo, M.; Zhang, G.; Li, X.; Shi, Y.; He, M.; Xu, D.; Chen, F.; Fan, J. Metabolomics Profiling Reveals P-Aminobenzoic Acid Enhances Resistance to Fusarium Head Blight in Wheat. Food Prod. Process. Nutr. 2025, 7, 14. [Google Scholar] [CrossRef]
- Yin, T.; Jiang, Y.; Shi, J. Effects of Alcalase Hydrolysis Combined with TGase-Type Glycosylation of Self-Assembled Zein for Curcumin Delivery: Stability, Bioavailability, and Antioxidant Properties. Int. J. Biol. Macromol. 2025, 303, 140735. [Google Scholar] [CrossRef] [PubMed]
- Kooptiwut, S.; Samon, K.; Semprasert, N.; Suksri, K.; Yenchitsomanus, P.-T. Prunetin Protects Against Dexamethasone-Induced Pancreatic Β-Cell Apoptosis via Modulation of P53 Signaling Pathway. Nat. Prod. Commun. 2020, 15, 1934578X20916328. [Google Scholar] [CrossRef]
- Veerana, M.; Mumtaz, S.; Rana, J.N.; Javed, R.; Panngom, K.; Ahmed, B.; Akter, K.; Choi, E.H. Recent Advances in Non-Thermal Plasma for Seed Germination, Plant Growth, and Secondary Metabolite Synthesis: A Promising Frontier for Sustainable Agriculture. Plasma Chem. Plasma Process. 2024, 44, 2263–2302. [Google Scholar] [CrossRef]
- Ullah, H.; De Filippis, A.; Khan, H.; Xiao, J.; Daglia, M. An Overview of the Health Benefits of Prunus Species with Special Reference to Metabolic Syndrome Risk Factors. Food Chem. Toxicol. 2020, 144, 111574. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Sugai, T.; Hanaya, K.; Higashibayashi, S. Semisynthesis of Prunetin, a Bioactive O-Methylated Isoflavone from Naringenin, by the Sequential Deacetylation of Chalcone Intermediates and Oxidative Rearrangement. Biosci. Biotechnol. Biochem. 2021, 85, 143–147. [Google Scholar] [CrossRef]
- Gordon, P.B.; Holen, I.; Seglen, P.O. Protection by Naringin and Some Other Flavonoids of Hepatocytic Autophagy and Endocytosis against Inhibition by Okadaic Acid. J. Biol. Chem. 1995, 270, 5830–5838. [Google Scholar] [CrossRef]
- Gonçalves, M.; Vale, N.; Silva, P. Neuroprotective Effects of Olive Oil: A Comprehensive Review of Antioxidant Properties. Antioxidants 2024, 13, 762. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Peng, H. Bioaccessibility and Bioavailability of Phenolic Compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kawabata, K.; Kakumoto, M.; Makita, H.; Hara, A.; Mori, H.; Satoh, K.; Hara, A.; Murakami, A.; Kuki, W.; et al. Citrus Auraptene Inhibits Chemically Induced Colonic Aberrant Crypt Foci in Male F344 Rats. Carcinogenesis 1997, 18, 2155–2161. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of Hyperglycemia and Hyperlipemia in Streptozotocin-Diabetic Rats by a Methanolic Extract of Prunus davidiana Stems and Its Main Component, Prunin. Planta Med. 1991, 57, 208–211. [Google Scholar] [CrossRef]
- Chen, J.; Chen, A.Y.; Huang, H.; Ye, X.; Rollyson, W.D.; Perry, H.E.; Brown, K.C.; Rojanasakul, Y.; Rankin, G.O.; Dasgupta, P.; et al. The Flavonoid Nobiletin Inhibits Tumor Growth and Angiogenesis of Ovarian Cancers via the Akt Pathway. Int. J. Oncol. 2015, 46, 2629–2638. [Google Scholar] [CrossRef]
- Ortuno, A.; Benavente-Garcia, O.; Castillo, J.; Alcaraz, M.; Vicente, V.; Del Rio, J.A. Beneficial Action of Citrus Flavonoids on Multiple Cancer-Related Biological Pathways. Curr. Cancer Drug Targets 2007, 7, 795–809. [Google Scholar] [CrossRef]
- Wang, I.-K.; Lin-Shiau, S.-Y.; Lin, J.-K. Induction of Apoptosis by Apigenin and Related Flavonoids through Cytochrome c Release and Activation of Caspase-9 and Caspase-3 in Leukaemia HL-60 Cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The Nutritional Flavanone Naringenin Triggers Antiestrogenic Effects by Regulating Estrogen Receptor α-Palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef]
- Sak, K. Site-Specific Anticancer Effects of Dietary Flavonoid Quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS Production in Response to High-Power Microwave Pulses Induces P53 Activation and DNA Damage in Brain Cells: Radiosensitivity and Biological Dosimetry Evaluation. Front. Cell Dev. Biol. 2023, 11, 1067861. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Jia, G.; Rizwan, M.; Imran, M.; Wei, D. Can WGX-50 Be a Potential Therapy to Treat Tumor by Inhibiting Mitochondrial Reactive Oxidative Species? Med. Hypotheses 2025, 196, 111583. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, C.; Wen, L.; Zhang, G.; Liu, X.; Wang, J.; Cui, L.; Li, R.; Nie, T.; Duan, J.; et al. Regulation of Reactive Oxygen Species and the Role of Mitochondrial Apoptotic-Related Genes in Rheumatoid Arthritis. Sci. Rep. 2025, 15, 2165. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Zhang, H.; Liu, Y.; Huo, L.; Jia, Z.; Xue, Y.; Sun, X.; Zhang, W. Inhibition of Transmembrane Member 16A Calcium-Activated Chloride Channels by Natural Flavonoids Contributes to Flavonoid Anticancer Effects. Br. J. Pharmacol. 2017, 174, 2334–2345. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Mumtaz, S.; Rana, J.N.; Lim, J.S.; Javed, R.; Choi, E.H.; Han, I. Effect of Plasma On-Time with a Fixed Duty Ratio on Reactive Species in Plasma-Treated Medium and Its Significance in Biological Applications. Int. J. Mol. Sci. 2023, 24, 5289. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Formation of Reactive Species via High Power Microwave Induced DNA Damage and Promoted Intrinsic Pathway-Mediated Apoptosis in Lung Cancer Cells: An in vitro Investigation. Fundam. Res. 2024, 4, 1542–1556. [Google Scholar] [CrossRef]
- Mumtaz, S.; Javed, R.; Rana, J.N.; Iqbal, M.; Choi, E.H. Pulsed High Power Microwave Seeds Priming Modulates Germination, Growth, Redox Homeostasis, and Hormonal Shifts in Barley for Improved Seedling Growth: Unleashing the Molecular Dynamics. Free Radic. Biol. Med. 2024, 222, 371–385. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Wong, K.Z.; Min, N.; Sun, J.; Ismail, N.K.B.M.; Tan, Y.J.; Lee, R.C.H.; Chu, J.J.H. Prunin Suppresses Viral IRES Activity and Is a Potential Candidate for Treating Enterovirus A71 Infection. Sci. Transl. Med. 2019, 11, eaar5759. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Nonaka, M.; Mochizuki, H.; Handa, K.; Hanada, H.; Hirota, K. Naringenin and Hesperetin Induce Growth Arrest, Apoptosis, and Cytoplasmic Fat Deposit in Human Preadipocytes. J. Agric. Food Chem. 2008, 56, 11030–11037. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising Anticancer Agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Siti, H.N.; Jalil, J.; Asmadi, A.Y.; Kamisah, Y. Rutin Modulates MAPK Pathway Differently from Quercetin in Angiotensin II-Induced H9c2 Cardiomyocyte Hypertrophy. Int. J. Mol. Sci. 2021, 22, 5063. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Chen, G.; Jing, C.; Liu, M.; Liang, B.; Gong, G.; Yu, M. Eriocitrin, a Dietary Flavonoid Suppressed Cell Proliferation, Induced Apoptosis through Modulation of JAK2/STAT3 and JNK/P38 MAPKs Signaling Pathway in MCF-7 Cells. J. Biochem. Mol. Toxicol. 2022, 36, e22943. [Google Scholar] [CrossRef]
- Cheng, M.; Li, T.; Hu, E.; Yan, Q.; Li, H.; Wang, Y.; Luo, J.; Tang, T. A Novel Strategy of Integrating Network Pharmacology and Transcriptome Reveals Antiapoptotic Mechanisms of Buyang Huanwu Decoction in Treating Intracerebral Hemorrhage. J. Ethnopharmacol. 2024, 319, 117123. [Google Scholar] [CrossRef]
- Zhao, C.; Song, W.; Wang, J.; Tang, X.; Jiang, Z. Immunoadjuvant-Functionalized Metal–Organic Frameworks: Synthesis and Applications in Tumor Immune Modulation. Chem. Commun. 2025, 61, 1962–1977. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Tang, Z.; Fu, Y.; Wang, L. Mirna-383-5p Functions as an Anti-Oncogene in Glioma through the Akt/MTOR Signaling Pathway by Targeting VEGFA. Curr. Cancer Drug Targets 2024, 24, 463–475. [Google Scholar] [CrossRef]
- Rao, U.S.M.; Dudekula, J.B.; Bhatt, S.; Kumar, M.S.; Shah, K.; Chauhan, N.S.; Shilpi, S. Chapter 18-Role of Phytopharmaceuticals in Inflammatory Disorders. In Phytopharmaceuticals and Herbal Drugs; Singh, M.R., Singh, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 433–451. ISBN 978-0-323-99125-4. [Google Scholar]
- Yang, Y.; Liu, Q.; Shi, X.; Zheng, Q.; Chen, L.; Sun, Y. Advances in Plant-Derived Natural Products for Antitumor Immunotherapy. Arch. Pharm. Res. 2021, 44, 987–1011. [Google Scholar] [CrossRef]
- Rattanapisit, K.; Suwanchaikasem, P.; Bulaon, C.J.I.; Guo, S.; Phoolcharoen, W. Plant-Derived Pembrolizumab in Conjugation with IL-15Rα-IL-15 Complex Shows Effective Anti-Tumor Activity. PLoS ONE 2025, 20, e0316790. [Google Scholar] [CrossRef]
- Pyo, Y.; Kwon, K.H.; Jung, Y.J. Anticancer Potential of Flavonoids: Their Role in Cancer Prevention and Health Benefits. Foods 2024, 13, 2253. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Hinterdorfer, P.; Lee, J.H.; Ko, K. Plant Production Systems for Recombinant Immunotherapeutic Proteins. Plant Biotechnol. Rep. 2025, 19, 1–14. [Google Scholar] [CrossRef]
- Trivedi, A.; Hasan, A.; Ahmad, R.; Siddiqui, S.; Srivastava, A.; Misra, A.; Mir, S.S. Flavonoid Myricetin as Potent Anticancer Agent: A Possibility towards Development of Potential Anticancer Nutraceuticals. Chin. J. Integr. Med. 2024, 30, 75–84. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-Derived Bioactive Compounds in Colon Cancer Treatment: An Updated Review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Elawad, M.A.; Ayaz, M.; Mosa, O.F.; Usman, A.; Hamdoon, A.A.E.; Almawash, S.; Salim, L.H.M.; Ahmed, A.; Elkhalifa, M.E.M. Polyphenols and Their Biogenic Nano-Formulations Targeting BACE1 as Anti-Amyloid Therapies; Meeting the Challenges of Bioavailability, Safety, and Specificity for the Treatment of Alzheimer’s Disease. Mol. Nutr. Food Res. 2024, 68, 2400525. [Google Scholar] [CrossRef]
- Marcucci, F.; Corti, A. How to Improve Exposure of Tumor Cells to Drugs—Promoter Drugs Increase Tumor Uptake and Penetration of Effector Drugs. Adv. Drug Deliv. Rev. 2012, 64, 53–68. [Google Scholar] [CrossRef]
- Pistollato, F.; Giampieri, F.; Battino, M. The Use of Plant-Derived Bioactive Compounds to Target Cancer Stem Cells and Modulate Tumor Microenvironment. Food Chem. Toxicol. 2015, 75, 58–70. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y. Targeting the Breast Tumor Microenvironment by Plant-Derived Products and Their Nanoformulations. J. Drug Deliv. Sci. Technol. 2024, 93, 105432. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Ahmad, I.; Mathew, S.P.; Bhattacharjee, C.K.; Jaganathan, B.G.; Poddar, S.; Patel, H.; Subramaniyan, V.; Chinni, S.V.; et al. Rhamnetin, a Nutraceutical Flavonoid Arrests Cell Cycle Progression of Human Ovarian Cancer (SKOV3) Cells by Inhibiting the Histone Deacetylase 2 Protein. J. Biomol. Struct. Dyn. 2024, 42, 13421–13436. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, J.; Wang, Z.; Peng, Y.; Zeng, L. Comprehensive Pan-Cancer Analysis Reveals ENC1 as a Promising Prognostic Biomarker for Tumor Microenvironment and Therapeutic Responses. Sci. Rep. 2024, 14, 25331. [Google Scholar] [CrossRef]
- Saadh, M.J.; Mustafa, M.A.; Malathi, H.; Ahluwalia, G.; Kaur, S.; Al-Dulaimi, M.A.A.H.; Alubiady, M.H.S.; Zain Al-Abdeen, S.H.; Shakier, H.G.; Ali, M.S.; et al. Targeting the Pancreatic Tumor Microenvironment by Plant-Derived Products and Their Nanoformulations. Med. Oncol. 2024, 41, 201. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.K.; Nguyen, N.P.N.; Nguyen, T.T.T.; Nguyen, K.N.; Do, B.D.; Nguyen, T.V.A.; Tran, A.K.; Nguyen, T.K.C.; Ho, Q.T.; Truong, D.-H.; et al. Extraction of Flavonoids from Durian (Durio zibethinus) Fruit Rinds and Evaluation of Their Antioxidant, Antidiabetic and Anticancer Properties. Int. J. Food Sci. Technol. 2024, 59, 1409–1420. [Google Scholar] [CrossRef]
- Lotfi, M.-S.; Rassouli, F.B. Natural Flavonoid Apigenin, an Effective Agent Against Nervous System Cancers. Mol. Neurobiol. 2024, 61, 5572–5583. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef]
- Mumtaz, S.; Lim, J.; Kaushik, N.K.; Choi, E.H. Biological Effects of Pulsed High-Power Microwaves. In Plasma Biosciences and Medicine; Choi, E.H., Ed.; Springer Nature: Singapore, 2023; pp. 281–307. ISBN 978-981-19-7935-4. [Google Scholar]
- Wu, T.-N.; Chen, H.-M.; Shyur, L.-F. Current Advancements of Plant-Derived Agents for Triple-Negative Breast Cancer Therapy through Deregulating Cancer Cell Functions and Reprogramming Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 13571. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, G.; Li, Y.; Shi, G.; Li, M. Potential Application of Plant-Based Functional Foods in the Development of Immune Boosters. Front. Pharmacol. 2021, 12, 637782. [Google Scholar] [CrossRef]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 4868417. [Google Scholar] [CrossRef]
- Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer Activity of the Plant Flavonoid Luteolin against Preclinical Models of Various Cancers and Insights on Different Signalling Mechanisms Modulated. Phyther. Res. 2021, 35, 3509–3532. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Qi, Y.-K.; Zheng, J.-S.; Liu, L. Mirror-Image Protein and Peptide Drug Discovery through Mirror-Image Phage Display. Chem 2024, 10, 2390–2407. [Google Scholar] [CrossRef]
- Saklani, A.; Kutty, S.K. Plant-Derived Compounds in Clinical Trials. Drug Discov. Today 2008, 13, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A Flavonoid with a Multifaceted Anticancer Potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A Mechanistic Review of the Anticancer Potential of Hesperidin, a Natural Flavonoid from Citrus Fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.R.; Sanati, M.; Ahmadi, S.S.; Kesharwani, P.; Sahebkar, A. Harnessing the Capacity of Phytochemicals to Enhance Immune Checkpoint Inhibitor Therapy of Cancers: A Focus on Brain Malignancies. Cancer Lett. 2024, 593, 216955. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.-M.; Kim, S.-J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in Cancer Immune Checkpoint Inhibitor Therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Mariappan, B.; Kaliyamurthi, V.; Binesh, A. Chapter 8-Medicinal Plants or Plant Derived Compounds Used in Aquaculture. In Recent Advances in Aquaculture Microbial Technology; Mathew, J., Jose, M.S., Radhakrishnan, E.K., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 153–207. ISBN 978-0-323-90261-8. [Google Scholar]
- Rahmani, A.H.; Almatroudi, A.; Allemailem, K.S.; Khan, A.A.; Almatroodi, S.A. The Potential Role of Fisetin, a Flavonoid in Cancer Prevention and Treatment. Molecules 2022, 27, 9009. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids Showed Anticancer Effects on the Ovarian Cancer Cells: Involvement of Reactive Oxygen Species, Apoptosis, Cell Cycle and Invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-Cancer Potential of Flavonoids: Recent Trends and Future Perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Budi, H.S.; Farhood, B. Tumor Microenvironment Remodeling in Oral Cancer: Application of Plant Derived-Natural Products and Nanomaterials. Environ. Res. 2023, 233, 116432. [Google Scholar] [CrossRef]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent Discoveries of Anticancer Flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Wan, H.; Zhou, S.; Li, C.; Zhou, H.; Wan, H.; Yang, J.; Yu, L. Ant Colony Algorithm-Enabled Back Propagation Neural Network and Response Surface Methodology Based Ultrasonic Optimization of Safflower Seed Alkaloid Extraction and Antioxidant. Ind. Crops Prod. 2024, 220, 119191. [Google Scholar] [CrossRef]
- Gao, T.-H.; Liao, W.; Lin, L.-T.; Zhu, Z.-P.; Lu, M.-G.; Fu, C.-M.; Xie, T. Curcumae rhizoma and Its Major Constituents against Hepatobiliary Disease: Pharmacotherapeutic Properties and Potential Clinical Applications. Phytomedicine 2022, 102, 154090. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Pan, Y.; Wang, S.; Ming, K.; Chi, Q.; Wang, C.; Xu, K. Rapid Identification of Astragalus Membranaceus Processing with Rice Water Based on Intelligent Color Recognition and Multi-Source Information Fusion Technology. Chin. Herb. Med. 2025. [Google Scholar] [CrossRef]
- Guo, Y.; Han, Z.; Zhang, J.; Lu, Y.; Li, C.; Liu, G. Development of a High-Speed and Ultrasensitive UV/Vis-CM for Detecting Total Triterpenes in Traditional Chinese Medicine and Its Application. Heliyon 2024, 10, e32239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, P.; Law, S.; Tian, H.; Leung, W.; Xu, C. Preventive Effect of Curcumin Against Chemotherapy-Induced Side-Effects. Front. Pharmacol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, X.; Luo, X.; Zhao, R.; Li, J.; Cai, H.; Ye, X.-Y.; Bai, R.; Xie, T. Combination of Chemotherapy and Gaseous Signaling Molecular Therapy: Novel β-Elemene Nitric Oxide Donor Derivatives against Leukemia. Drug Dev. Res. 2023, 84, 718–735. [Google Scholar] [CrossRef]
- Lodi, R.S.; Dong, X.; Wang, X.; Han, Y.; Liang, X.; Peng, C.; Peng, L. Current Research on the Medical Importance of Trametes Species. Fungal Biol. Rev. 2025, 51, 100413. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhuang, W.; Shu, X.; Wang, Z. Metabolic Variations of Flavonoids in Leaves of T. media and T. mairei Obtained by UPLC-ESI-MS/MS. Molecules 2019, 24, 3323. [Google Scholar] [CrossRef]
- De Marco, F.; Altieri, F.; Giuliani, S.; Falcone, I.; Falcucci, S.; Tedesco, M.; Becelli, R. A Combination of Flavonoids Suppresses Cell Proliferation and the E6 Oncogenic Pathway in Human Papillomavirus-Transformed Cells. Pathogens 2025, 14, 221. [Google Scholar] [CrossRef]
- Rajakumar, T.; Pugalendhi, P. Allyl Isothiocyanate Regulates Oxidative Stress, Inflammation, Cell Proliferation, Cell Cycle Arrest, Apoptosis, Angiogenesis, Invasion and Metastasis via Interaction with Multiple Cell Signaling Pathways. Histochem. Cell Biol. 2024, 161, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, M.; Liu, W.; Wu, J.; Tang, P. Role of Cell Division Cycle-Associated Proteins in Regulating Cell Cycle and Promoting Tumor Progression. Biochim. Biophys. Acta-Rev. Cancer 2024, 1879, 189147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mu, R.; Guo, X. Defensins Regulate Cell Cycle: Insights of Defensins on Cellular Proliferation and Division. Life Sci. 2024, 349, 122740. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Cha, H.-J.; Choi, E.O.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Kim, S.Y.; Hong, S.H.; Cheong, J.; et al. Isorhamnetin Induces Cell Cycle Arrest and Apoptosis Via Reactive Oxygen Species-Mediated AMP-Activated Protein Kinase Signaling Pathway Activation in Human Bladder Cancer Cells. Cancers 2019, 11, 1494. [Google Scholar] [CrossRef]
- Wu, J.; Song, Y.; Wang, J.; Wang, T.; Yang, L.; Shi, Y.; Song, B.; Yu, Z. Isorhamnetin inhibits hypertrophic scar formation through TGF-β1/Smad and TGF-β1/CREB3L1 signaling pathways. Heliyon 2024, 10, e33802. [Google Scholar] [CrossRef]
- Diehl, F.F.; Sapp, K.M.; Vander Heiden, M.G. The Bidirectional Relationship between Metabolism and Cell Cycle Control. Trends Cell Biol. 2024, 34, 136–149. [Google Scholar] [CrossRef]
- Stallaert, W.; Taylor, S.R.; Kedziora, K.M.; Taylor, C.D.; Sobon, H.K.; Young, C.L.; Limas, J.C.; Varblow Holloway, J.; Johnson, M.S.; Cook, J.G.; et al. The Molecular Architecture of Cell Cycle Arrest. Mol. Syst. Biol. 2022, 18, e11087. [Google Scholar] [CrossRef]
- Lu, R.; Liu, J.; Thakur, K.; Cao, H.; Mejuto, J.C.; Gandara, J.S.; Zhang, J.-G. Protopanaxadiol Triggers G0/G1 Cell Cycle Arrest and Apoptosis in Human Cervical Cancer HeLa Cells through the PPER Pathway. Food Biosci. 2024, 62, 105388. [Google Scholar] [CrossRef]
- Wang, J.-L.; Quan, Q.; Ji, R.; Guo, X.-Y.; Zhang, J.-M.; Li, X.; Liu, Y.-G. Isorhamnetin Suppresses PANC-1 Pancreatic Cancer Cell Proliferation through S Phase Arrest. Biomed. Pharmacother. 2018, 108, 925–933. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.-J.; Yu, L.-H.; Guan, Z.-R.; Jiang, Y.-P.; Hu, J.-H.; Yan, Y.-X.; Zhou, Z.-H.; Lou, J.-S. The Role of Ginkgo Folium on Antitumor: Bioactive Constituents and the Potential Mechanism. J. Ethnopharmacol. 2024, 321, 117202. [Google Scholar] [CrossRef]
- Deshpande, A.; Sicinski, P.; Hinds, P.W. Cyclins and Cdks in Development and Cancer: A Perspective. Oncogene 2005, 24, 2909–2915. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, X.; Fang, C.; Zhu, M.; Wang, Z.; Jian, L.; Tan, W.; Wang, Y.; Li, H.; Xu, X.; et al. Progesterone Enhances Niraparib Efficacy in Ovarian Cancer by Promoting Palmitoleic-Acid-Mediated Ferroptosis. Research 2025, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Koyu, H.; Kazan, A.; Nalbantsoy, A.; Yalcin, H.T.; Yesil-Celiktas, O. Cytotoxic, Antimicrobial and Nitric Oxide Inhibitory Activities of Supercritical Carbon Dioxide Extracted Prunus Persica Leaves. Mol. Biol. Rep. 2020, 47, 569–581. [Google Scholar] [CrossRef]
- Na, E.J.; Ryu, J.Y. Anti-Inflammatory Effects of Prunin on UVB-Irradiated Human Keratinocytes. Biomed. Dermatol. 2018, 2, 14. [Google Scholar] [CrossRef]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown Rot Strikes Prunus Fruit: An Ancient Fight Almost Always Lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef]
- Huang, M.-F.; Wang, Y.-X.; Chou, Y.-T.; Lee, D.-F. Therapeutic Strategies for RB1-Deficient Cancers: Intersecting Gene Regulation and Targeted Therapy. Cancers 2024, 16, 1558. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Liu, F.; Militi, S.; Hester, S.; Nibhani, R.; Deng, S.; Dunford, J.; Rendek, A.; Soonawalla, Z.; Fischer, R.; et al. The PRb/RBL2-E2F1/4-GCN5 Axis Regulates Cancer Stem Cell Formation and G0 Phase Entry/Exit by Paracrine Mechanisms. Nat. Commun. 2024, 15, 3580. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular Senescence: The Good, the Bad and the Unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef]
- Casagrande, F.; Darbon, J.-M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001, 61, 1205–1215. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chen, W.-J.; Lin-Shiau, S.-Y.; Ho, C.-T.; Lin, J.-K. Tangeretin Induces Cell-Cycle G1 Arrest through Inhibiting Cyclin-Dependent Kinases 2 and 4 Activities as Well as Elevating Cdk Inhibitors P21 and P27 in Human Colorectal Carcinoma Cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar] [CrossRef]
- AL-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Hogan, F.S.; Krishnegowda, N.K.; Mikhailova, M.; Kahlenberg, M.S. Flavonoid, Silibinin, Inhibits Proliferation and Promotes Cell-Cycle Arrest of Human Colon Cancer. J. Surg. Res. 2007, 143, 58–65. [Google Scholar] [CrossRef]
- Yang, S.; Chu, G.; Wu, J.; Zhang, G.; Du, L.; Lin, R. Enrichment and Evaluation of Antitumor Properties of Total Flavonoids from Juglans mandshurica Maxim. Molecules 2024, 29, 1976. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Chapter Four-Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. In Apoptosis in Health and Disease-Part A; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 125, pp. 73–120. ISBN 1876-1623. [Google Scholar]
- Liu, L.; Pang, Y.; Zhao, X.; Li, R.; Jin, C.; Xue, J.; Dong, R.; Liu, P. Curcumin Induces Apoptotic Cell Death and Protective Autophagy by Inhibiting AKT/MTOR/P70S6K Pathway in Human Ovarian Cancer Cells. Arch. Gynecol. Obstet. 2019, 299, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bai, Z.-F.; Zhang, Y.; Cui, H.; Zhou, H.-L. Flavonoids-Rich Extract from Bidens bipinnata L. Protects Pancreatic β-Cells against Oxidative Stress-Induced Apoptosis through Intrinsic and Extrinsic Pathways. J. Ethnopharmacol. 2021, 275, 114097. [Google Scholar] [CrossRef]
- Hongmei, Z. Extrinsic and Intrinsic Apoptosis Signal Pathway Review. In Apoptosis and Medicine; Ntuli, T.M., Ed.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Li, X.; Wang, T.; Zhou, Q.; Li, F.; Liu, T.; Zhang, K.; Wen, A.; Feng, L.; Shu, X.; Tian, S.; et al. Isorhamnetin Alleviates Mitochondrial Injury in Severe Acute Pancreatitis via Modulation of KDM5B/HtrA2 Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 3784. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.; Chen, Y.; Zhang, D.; Huang, W.; Shi, M.; Zhang, Y.; Wu, T.; Chen, Z.; Wu, L.; et al. Investigation of the Underlying Mechanism of Huangqi-Dangshen for Myasthenia Gravis Treatment via Molecular Docking and Network Pharmacology. Evid.-Based Complement. Altern. Med. 2023, 2023, 5301024. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative Cell Death in Cancer: Mechanisms and Therapeutic Opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Chen, L.; Wu, L.; Zhang, L.; Sun, B.; Wu, W.; Lei, Y.; Zhu, L.; Sun, T.; Liang, B.; Zhao, H.; et al. Effect of Metformin on Hepatocellular Carcinoma Patients with Type II Diabetes Receiving Transarterial Chemoembolization: A Multicenter Retrospective Cohort Study. Int. J. Surg. 2025, 111, 828–838. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Zhu, J.T.T.; Choi, R.C.Y.; Chu, G.K.Y.; Cheung, A.W.H.; Gao, Q.T.; Li, J.; Jiang, Z.Y.; Dong, T.T.X.; Tsim, K.W.K. Flavonoids Possess Neuroprotective Effects on Cultured Pheochromocytoma PC12 Cells: A Comparison of Different Flavonoids in Activating Estrogenic Effect and in Preventing β-Amyloid-Induced Cell Death. J. Agric. Food Chem. 2007, 55, 2438–2445. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Los, M.; Wesselborg, S.; Peter, M.E. Apoptosis Signaling by Death Receptors. Eur. J. Biochem. 1998, 254, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Hoque, M.; Alam, S.S.M.; Zughaibi, T.A.; Tabrez, S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/MTOR Pathway: A Prospective Role in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 6651. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.-Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/MTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chinnathambi, A.; Alharbi, S.A.; Shair, O.H.M.; Sethi, G.; Ahn, K.S. Farnesol Abrogates Epithelial to Mesenchymal Transition Process through Regulating Akt/MTOR Pathway. Pharmacol. Res. 2019, 150, 104504. [Google Scholar] [CrossRef]
- Tewari, D.; Patni, P.; Bishayee, A.; Sah, A.N.; Bishayee, A. Natural Products Targeting the PI3K-Akt-MTOR Signaling Pathway in Cancer: A Novel Therapeutic Strategy. Semin. Cancer Biol. 2022, 80, 1–17. [Google Scholar] [CrossRef]
- Chen, S.; Long, S.; Liu, Y.; Wang, S.; Hu, Q.; Fu, L. Evaluation of a Three-Gene Methylation Model for Correlating Lymph Node Metastasis in Postoperative Early Gastric Cancer Adjacent Samples. Front. Oncol. 2024, 14, 1432869. [Google Scholar] [CrossRef]
- Zhong, J.; Ding, S.; Zhang, X.; Di, W.; Wang, X.; Zhang, H.; Chen, Y.; Zhang, Y.; Hu, Y. To Investigate the Occurrence and Development of Colorectal Cancer Based on the PI3K/AKT/MTOR Signaling Pathway. Front. Biosci. 2023, 28, 37. [Google Scholar] [CrossRef]
- Yu, L.; Wei, J.; Liu, P. Attacking the PI3K/Akt/MTOR Signaling Pathway for Targeted Therapeutic Treatment in Human Cancer. Semin. Cancer Biol. 2022, 85, 69–94. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, C.; Um, J.-Y.; Sethi, G.; Ahn, K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/MTOR Signaling Cascade. Cancers 2019, 11, 254. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Zeng, F. Biological Functions and Health Benefits of Flavonoids in Fruits and Vegetables: A Contemporary Review. Foods 2025, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A.; Xu, P. Molecular Regulation of Autophagy Machinery by MTOR-Dependent and-Independent Pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin Induces Apoptosis and Autophagy in Primary Effusion Lymphoma Cells by Inhibiting PI3K/AKT/MTOR and STAT3 Signaling Pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Bai, D.; Zhao, Y.; Zhu, Q.; Zhou, Y.; Zhao, Y.; Zhang, T.; Guo, Q.; Lu, N. LZ205, a Newly Synthesized Flavonoid Compound, Exerts Anti-Inflammatory Effect by Inhibiting M1 Macrophage Polarization through Regulating PI3K/AKT/MTOR Signaling Pathway. Exp. Cell Res. 2018, 364, 84–94. [Google Scholar] [CrossRef]
- Saini, S.; Tuli, H.S.; Saini, R.V.; Saini, A.K.; Sak, K.; Kaur, D.; Shahwan, M.; Chauhan, R.; Chauhan, A. Flavonoid-Mediated Suppression of Tumor Angiogenesis: Roles of Ang-Tie/PI3K/AKT. Pathophysiology 2024, 31, 596–607. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Chen, H.; Qiao, D.; Guo, F.; Hu, X.; Qin, C.; Jin, X.; Zhang, K.; Wang, C.; et al. Role of FMRP in AKT/MTOR Pathway-Mediated Hippocampal Autophagy in Fragile X Syndrome. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2024, 134, 111036. [Google Scholar] [CrossRef]
- Araújo, C.B.; Alves Júnior, J.D.; Sato, M.R.; Costa, K.M.; Lima, J.R.; Damasceno, B.P.; Lima Junior, F.J.; Andréo, B.G.; Santos, V.L.; Oshiro-Junior, J.A. The Development and Pre-Clinical Anti-Inflammatory Efficacy of a New Transdermal Ureasil–Polyether Hybrid Matrix Loaded with Flavonoid-Rich Annona Muricata Leaf Extract. Pharmaceutics 2024, 16, 1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ye, B. Isolation of Prunin From Bauhinia Variegata and Its Antioxidant Activity in Rats Fed an Atherogenic Diet. Nat. Prod. Commun. 2020, 15, 1934578X20967875. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Cobo, A.; Ortega-Morente, E.; Gálvez, A.; Alejo-Armijo, A.; Salido, S.; Altarejos, J. Antimicrobial and Antioxidant Activities of Flavonoids Isolated from Wood of Sweet Cherry Tree (Prunus avium L.). J. Wood Chem. Technol. 2021, 41, 104–117. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, R.; Zhu, R.; Wu, X.; Liu, J.; Ma, Y.; Zhang, X.; Zhang, Y.; Yang, L.; Li, Y.; et al. Baicalin Ameliorates Neuroinflammation by Targeting TLR4/MD2 Complex on Microglia via PI3K/AKT/NF-ΚB Signaling Pathway. Neuropharmacology 2025, 267, 110296. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, J.; Yuan, T.; Yang, C.; Zhou, Q.; Shaukat, A.; Deng, G.; Wang, X. Luteolin Alleviates Inflammation Induced by Staphylococcus Aureus in Bovine Mammary Epithelial Cells by Attenuating NF-ΚB and MAPK Activation. Vet. Sci. 2025, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, L.; Wang, J.; Li, D.; Fei, H.; Chen, X.; Dong, J.; Sun, L. Fraxetin Inhibits IKKβ, Blocks NF-ΚB Pathway and NLRP3 Inflammasome Activation, and Alleviates Spleen Injury in Sepsis. Chem. Biol. Interact. 2025, 408, 111406. [Google Scholar] [CrossRef]
- Hou, X.L.; Tong, Q.; Wang, W.Q.; Shi, C.Y.; Xiong, W.; Chen, J.; Liu, X.; Fang, J.G. Suppression of Inflammatory Responses by Dihydromyricetin, a Flavonoid from Ampelopsis grossedentata, via Inhibiting the Activation of NF-ΚB and MAPK Signaling Pathways. J. Nat. Prod. 2015, 78, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid Fisetin Alleviates Kidney Inflammation and Apoptosis via Inhibiting Src-Mediated NF-ΚB P65 and MAPK Signaling Pathways in Septic AKI Mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, P.; Yang, J.; Zhang, Z.; Wang, H.; Guo, Y.; Liu, M. The Protective Effect of the Flavonoid Fraction of Abutilon Theophrasti Medic. Leaves on LPS-Induced Acute Lung Injury in Mice via the NF-ΚB and MAPK Signalling Pathways. Biomed. Pharmacother. 2019, 109, 1024–1031. [Google Scholar] [CrossRef]
- Yang, J.H.; Shin, B.Y.; Han, J.Y.; Kim, M.G.; Wi, J.E.; Kim, Y.W.; Cho, I.J.; Kim, S.C.; Shin, S.M.; Ki, S.H. Isorhamnetin Protects against Oxidative Stress by Activating Nrf2 and Inducing the Expression of Its Target Genes. Toxicol. Appl. Pharmacol. 2014, 274, 293–301. [Google Scholar] [CrossRef]
- Seo, K.; Yang, J.H.; Kim, S.C.; Ku, S.K.; Ki, S.H.; Shin, S.M. The Antioxidant Effects of Isorhamnetin Contribute to Inhibit COX-2 Expression in Response to Inflammation: A Potential Role of HO-1. Inflammation 2014, 37, 712–722. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhang, Z.; Tong, L.; Yu, S.; Liu, Y.; Yang, F. Flavonoid Compounds in Hippophae rhamnoides L. Protect Endothelial Cells from Oxidative Damage Through the PI3K/AKT-ENOS Pathway. Chem. Biodivers. 2024, 21, e202400300. [Google Scholar] [CrossRef]
- Pal, C. Small Molecules Targeting Mitochondria: A Mechanistic Approach to Combating Doxorubicin-Induced Cardiotoxicity. Cardiovasc. Toxicol. 2024, 25, 216–247. [Google Scholar] [CrossRef]
- Mihaylova, R.; Gevrenova, R.; Petrova, A.; Savov, Y.; Zheleva-Dimitrova, D.; Balabanova, V.; Momekov, G.; Simeonova, R. Mitigating Effects of Tanacetum balsamita L. on Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Plants 2024, 13, 2086. [Google Scholar] [CrossRef]
- Sattari, M.; Amri, J.; Shahaboddin, M.E.; Sattari, M.; Tabatabaei-Malazy, O.; Azmon, M.; Meshkani, R.; Panahi, G. The Protective Effects of Fisetin in Metabolic Disorders: A Focus on Oxidative Stress and Associated Events. J. Diabetes Metab. Disord. 2024, 23, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zha, Y.; Zang, Y.; Gao, Y.; Sun, J.; Liu, Y.; Wang, Z.; Wei, Z.; Wang, M.; Yang, Y. Isorhamnetin Improves Diabetes-Induced Erectile Dysfunction in Rats through Activation of the PI3K/AKT/ENOS Signaling Pathway. Biomed. Pharmacother. 2024, 177, 116987. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, Y.; Liu, S.; Luo, F.; Tang, D.; Yu, Y.; Xie, Y. Isorhamnetin Induces Cell Cycle Arrest and Apoptosis by Triggering DNA Damage and Regulating the AMPK/MTOR/P70S6K Signaling Pathway in Doxorubicin-Resistant Breast Cancer. Phytomedicine 2023, 114, 154780. [Google Scholar] [CrossRef]
- Song, B.; Yang, P.; Zhang, S. Cell Fate Regulation Governed by P53: Friends or Reversible Foes in Cancer Therapy. Cancer Commun. 2024, 44, 297–360. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Famusiwa, C.D.; Falode, J.A.; Ojelabi, A.O.; Mistura, A.N.; Ogunbiyi, D.O.; Jeje, T.O.; Akinlolu, O.S.; Ogedengbe, O.O.; Ojo, O.A. Ocimum gratissimum L. Leaf Flavonoid-Rich Extracts Reduced the Expression of P53 and VCAM in Streptozotocin-Induced Cardiomyopathy Rats. Phytomedicine Plus 2024, 4, 100548. [Google Scholar] [CrossRef]
- Efe, G.; Rustgi, A.K.; Prives, C. P53 at the Crossroads of Tumor Immunity. Nat. Cancer 2024, 5, 983–995. [Google Scholar] [CrossRef]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating P53-Based Therapies for Cancer into the Clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef]
- Fischer, M.; Sammons, M.A. Determinants of P53 DNA Binding, Gene Regulation, and Cell Fate Decisions. Cell Death Differ. 2024, 31, 836–843. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Harnessing the Synergy of Nanosecond High-Power Microwave Pulses and Cisplatin to Increase the Induction of Apoptosis in Cancer Cells through the Activation of ATR/ATM and Intrinsic Pathways. Free Radic. Biol. Med. 2024, 225, 221–235. [Google Scholar] [CrossRef]
- Rana, J.N.; Mumtaz, S.; Han, I.; Choi, E.H. Unveiling the Therapeutic Potential of Soft Plasma Jet and Nitric-Oxide Enriched Plasma-Activated Water (NO-PAW) on Oral Cancer YD-10B Cells: A Comprehensive Investigation of Direct and Indirect Treatments. Plasma Chem. Plasma Process. 2025, 1–28. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Yang, W.; Sheng, H.; Jia, B.; Cheng, P.; Xu, S.; Hong, X.; Jiang, C.; Yang, Y.; et al. Multiple Roles of P53 in Cancer Development: Regulation of Tumor Microenvironment, M6A Modification and Diverse Cell Death Mechanisms. J. Adv. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Long, Y.; Maimaitijiang, A.; Su, Z.; Li, W.; Li, J. Unraveling the Guardian: P53’s Multifaceted Role in the DNA Damage Response and Tumor Treatment Strategies. Int. J. Mol. Sci. 2024, 25, 12928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Z.; Tavana, O.; Gu, W. Understanding the Complexity of P53 in a New Era of Tumor Suppression. Cancer Cell 2024, 42, 946–967. [Google Scholar] [CrossRef]
- Indeglia, A.; Murphy, M.E. Elucidating the Chain of Command: Our Current Understanding of Critical Target Genes for P53-Mediated Tumor Suppression. Crit. Rev. Biochem. Mol. Biol. 2024, 59, 128–138. [Google Scholar] [CrossRef]
- Prakash, D.; Sudhandiran, G. Dietary Flavonoid Fisetin Regulates Aluminium Chloride-Induced Neuronal Apoptosis in Cortex and Hippocampus of Mice Brain. J. Nutr. Biochem. 2015, 26, 1527–1539. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on Uses and Properties of Citrus Flavonoids: New Findings in Anticancer, Cardiovascular, and Anti-Inflammatory Activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Signal Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Carceller, J.M.; Martínez Galán, J.P.; Monti, R.; Bassan, J.C.; Filice, M.; Iborra, S.; Yu, J.; Corma, A. Selective Synthesis of Citrus Flavonoids Prunin and Naringenin Using Heterogeneized Biocatalyst on Graphene Oxide. Green Chem. 2019, 21, 839–849. [Google Scholar] [CrossRef]
- Vila-Real, H.; Alfaia, A.J.; Bronze, M.R.; Calado, A.R.T.; Ribeiro, M.H.L. Enzymatic Synthesis of the Flavone Glucosides, Prunin and Isoquercetin, and the Aglycones, Naringenin and Quercetin, with Selective α-L-Rhamnosidase and β-D-Glucosidase Activities of Naringinase. Enzym. Res. 2011, 2011, 692618. [Google Scholar] [CrossRef]
- Uchida, Y.; Ferdousi, F.; Zheng, Y.-W.; Oda, T.; Isoda, H. Global Gene Expression Profiling Reveals Isorhamnetin Induces Hepatic-Lineage Specific Differentiation in Human Amniotic Epithelial Cells. Front. Cell Dev. Biol. 2020, 8, 578036. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Hetta, M.H.; Rateb, M.E.; Selim, M.A.; AboulMagd, A.M.; Badria, F.A.; Abdelmohsen, U.R.; Alhadrami, H.A.; Hassan, H.M. Bioassay-Guided Isolation, Metabolic Profiling, and Docking Studies of Hyaluronidase Inhibitors from Ravenala madagascariensis. Molecules 2020, 25, 1714. [Google Scholar] [CrossRef]
- Chau, T.P.; Devanesan, S.; Ayub, R.; Perumal, K. Identification and Characterization of Major Bioactive Compounds from Andrographis paniculata (Burm. f.) Extracts Showed Multi-Biomedical Applications. Environ. Res. 2024, 242, 117763. [Google Scholar] [CrossRef] [PubMed]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic Effects of Flavonoids and Chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta-Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S. Targeting MAPK Signaling: A Promising Approach for Treating Inflammatory Lung Disease. Pathol.-Res. Pract. 2024, 254, 155122. [Google Scholar] [CrossRef]
- Edvinsson, L.; Krause, D.N. Switching Off Vascular MAPK Signaling: A Novel Strategy to Prevent Delayed Cerebral Ischemia Following Subarachnoid Hemorrhage. Transl. Stroke Res. 2024. [Google Scholar] [CrossRef]
- Lin, H.-H. An Alternative Mode of GPCR Transactivation: Activation of GPCRs by Adhesion GPCRs. Int. J. Mol. Sci. 2025, 26, 552. [Google Scholar] [CrossRef]
- Roskoski, R. ERK1/2 MAP Kinases: Structure, Function, and Regulation. Pharmacol. Res. 2012, 66, 105–143. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, O.P.; Thompson-Bonilla, M.d.R.; Jaramillo-Flores, M.E. Association between Obesity and Breast Cancer: Molecular Bases and the Effect of Flavonoids in Signaling Pathways. Crit. Rev. Food Sci. Nutr. 2020, 60, 3770–3792. [Google Scholar] [CrossRef]
- Shi, X.; Yu, Q.; Wang, K.; Fu, Y.; Zhang, S.; Liao, Z.; Li, Y.; Cai, T. Active Ingredients Isorhamnetin of Croci srigma Inhibit Stomach Adenocarcinomas Progression by MAPK/MTOR Signaling Pathway. Sci. Rep. 2023, 13, 12607. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, G.; Zhang, Y.; Li, X.; Wang, R. Involvement of the NF-ΚB Signaling Pathway in the Renoprotective Effects of Isorhamnetin in a Type 2 Diabetic Rat Model. Biomed. Rep. 2016, 4, 628–634. [Google Scholar] [CrossRef]
- Gao, L.; Yao, R.; Liu, Y.; Wang, Z.; Huang, Z.; Du, B.; Zhang, D.; Wu, L.; Xiao, L.; Zhang, Y. Isorhamnetin Protects against Cardiac Hypertrophy through Blocking PI3K–AKT Pathway. Mol. Cell. Biochem. 2017, 429, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, T.; Chen, K.; Xia, Y.; Dai, W.; Xu, S.; Xu, L.; Wang, F.; Wu, L.; Li, J.; et al. Isorhamnetin: A Hepatoprotective Flavonoid Inhibits Apoptosis and Autophagy via P38/PPAR-α Pathway in Mice. Biomed. Pharmacother. 2018, 103, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-L.; Yen, G.-C. Modulation of Akt, JNK, and P38 Activation Is Involved in Citrus Flavonoid-Mediated Cytoprotection of PC12 Cells Challenged by Hydrogen Peroxide. J. Agric. Food Chem. 2009, 57, 2576–2582. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Shih, P.-H.; Yen, G.-C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef]
- Jayashankar, B.; Mishra, K.P.; Kumar, M.S.Y.; Udayasankar, K.; Misra, K.; Ganju, L.; Singh, S.B. A Supercritical CO2 Extract from Seabuckthorn Leaves Inhibits Pro-Inflammatory Mediators via Inhibition of Mitogen Activated Protein Kinase P38 and Transcription Factor Nuclear Factor-ΚB. Int. Immunopharmacol. 2012, 13, 461–467. [Google Scholar] [CrossRef]
- Lian, J.-J.; Cheng, B.-F.; Gao, Y.-X.; Xue, H.; Wang, L.; Wang, M.; Yang, H.-J.; Feng, Z.-W. Protective Effect of Kaempferol, a Flavonoid Widely Present in Varieties of Edible Plants, on IL-1β-Induced Inflammatory Response via Inhibiting MAPK, Akt, and NF-ΚB Signalling in SW982 Cells. J. Funct. Foods 2016, 27, 214–222. [Google Scholar] [CrossRef]
- Cai, J.; Wen, H.; Zhou, H.; Zhang, D.; Lan, D.; Liu, S.; Li, C.; Dai, X.; Song, T.; Wang, X.; et al. Naringenin: A Flavanone with Anti-Inflammatory and Anti-Infective Properties. Biomed. Pharmacother. 2023, 164, 114990. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Alonazi, R.; Elekhnawy, E.; Samra, R.M.; Alqarni, M.H.; Badreldin, H.; Magdy, G. HPLC-PDA and in vivo Anti-Inflammatory Potential of Isorhamnetin-3-O-β-D-Glucoside from Zygophyllum simplex L. J. Ethnopharmacol. 2025, 338, 119089. [Google Scholar] [CrossRef]
- Jnawali, H.N.; Jeon, D.; Jeong, M.-C.; Lee, E.; Jin, B.; Ryoo, S.; Yoo, J.; Jung, I.D.; Lee, S.J.; Park, Y.-M.; et al. Antituberculosis Activity of a Naturally Occurring Flavonoid, Isorhamnetin. J. Nat. Prod. 2016, 79, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Mandić, L.; Sadžak, A.; Šegota, S. Anti-Oxidative, Anti-Inflammatory and Anti-Apoptotic Effects of Flavonols: Targeting Nrf2, NF-ΚB and P53 Pathways in Neurodegeneration. Antioxidants 2021, 10, 1628. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential Mechanisms of Quercetin in Cancer Prevention: Focus on Cellular and Molecular Targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef]
- Kumari, N.; Radha; Kumar, M.; Puri, S.; Zhang, B.; Rais, N.; Pundir, A.; Chandran, D.; Raman, P.; Dhumal, S.; et al. Peach (Prunus persica (L.) Batsch) Seeds and Kernels as Potential Plant-Based Functional Food Ingredients: A Review of Bioactive Compounds and Health-Promoting Activities. Food Biosci. 2023, 54, 102914. [Google Scholar] [CrossRef]
- Chi, G.; Zhong, W.; Liu, Y.; Lu, G.; Lü, H.; Wang, D.; Sun, F. Isorhamnetin Protects Mice from Lipopolysaccharide-Induced Acute Lung Injury via the Inhibition of Inflammatory Responses. Inflamm. Res. 2016, 65, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, L.; Wei, Y.; Cui, Y.; Li, X.; Wei, Y.; Zhang, S.; Zhang, L.; Zhou, H.; Wang, G.; et al. Isorhamnetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting MTOR Signaling Pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 387–399. [Google Scholar] [CrossRef]
- Liu, G.; Jiang, C.; Li, D.; Yao, L.; Lin, Y.; Wang, B.; Qiu, J.; Wang, W.; Wang, W. Isorhamnetin Alleviates Esophageal Mucosal Injury in a Chronic Model of Reflux Esophagitis. Eur. J. Pharmacol. 2019, 864, 172720. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Liu, Q.; Zhou, X.; Wang, X.; Li, H.; Zhang, W.; Yuan, H.; Sun, C. Flavonoids Regulate Tumor-Associated Macrophages–From Structure-Activity Relationship to Clinical Potential (Review). Pharmacol. Res. 2022, 184, 106419. [Google Scholar] [CrossRef]
- Shahrezaei, A.; Sohani, M.; Sohouli, M.; Taherkhani, S.; Nasirinezhad, F. The Involvement and Significance of M2 Macrophages in Neuropathic Pain Following Spinal Cord Injury: A Systematic Review. J. Physiol. Sci. 2024, 74, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, K.; Wang, X.; Zhao, Y.; Shi, J.; Liu, Z. Roles of IL-4, IL-13, and Their Receptors in Lung Cancer. J. Interferon Cytokine Res. 2024, 44, 399–407. [Google Scholar] [CrossRef]
- Guo, J.; Yan, W.; Duan, H.; Wang, D.; Zhou, Y.; Feng, D.; Zheng, Y.; Zhou, S.; Liu, G.; Qin, X. Therapeutic Effects of Natural Products on Liver Cancer and Their Potential Mechanisms. Nutrients 2024, 16, 1642. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Coombs, M.R.P. Editorial: Immune Modulation by Flavonoids. Front. Immunol. 2022, 13, 899577. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Q.; Liang Cheng, M.; Ma, L.; Zhi Meng, Q.; Duan, L.; Chen, Y.; Wu Tan, J.; Chen, M.; Ting Liang, T.; et al. Effect of the Miaoyao Fanggan Sachet-Derived Isorhamnetin on TLR2/4 and NKp46 Expression in Mice. J. Ethnopharmacol. 2012, 144, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, P.; Lamenza, F.F.; Shrestha, S.; Roth, P.; Jagadeesha, S.; Pracha, H.; Horn, N.A.; Oghumu, S. Berry Extracts and Their Bioactive Compounds Mitigate LPS and DNFB-Mediated Dendritic Cell Activation and Induction of Antigen Specific T-Cell Effector Responses. Antioxidants 2023, 12, 1667. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of MTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef]
- Chen, Q.; Song, S.; Wang, Z.; Shen, Y.; Xie, L.; Li, J.; Jiang, L.; Zhao, H.; Feng, X.; Zhou, Y.; et al. Isorhamnetin Induces the Paraptotic Cell Death through ROS and the ERK/MAPK Pathway in OSCC Cells. Oral Dis. 2021, 27, 240–250. [Google Scholar] [CrossRef]
- Jaramillo, S.; Lopez, S.; Varela, L.M.; Rodriguez-Arcos, R.; Jimenez, A.; Abia, R.; Guillen, R.; Muriana, F.J.G. The Flavonol Isorhamnetin Exhibits Cytotoxic Effects on Human Colon Cancer Cells. J. Agric. Food Chem. 2010, 58, 10869–10875. [Google Scholar] [CrossRef]
- Yuan, J.; Ofengeim, D. A Guide to Cell Death Pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Meier, P.; Legrand, A.J.; Adam, D.; Silke, J. Immunogenic Cell Death in Cancer: Targeting Necroptosis to Induce Antitumour Immunity. Nat. Rev. Cancer 2024, 24, 299–315. [Google Scholar] [CrossRef]
- Pham, D.-C.; Shibu, M.A.; Mahalakshmi, B.; Velmurugan, B.K. Effects of Phytochemicals on Cellular Signaling: Reviewing Their Recent Usage Approaches. Crit. Rev. Food Sci. Nutr. 2020, 60, 3522–3546. [Google Scholar] [CrossRef] [PubMed]
- Jayashankar, B.; Singh, D.; Tanwar, H.; Mishra, K.P.; Murthy, S.; Chanda, S.; Mishra, J.; Tulswani, R.; Misra, K.; Singh, S.B.; et al. Augmentation of Humoral and Cellular Immunity in Response to Tetanus and Diphtheria Toxoids by Supercritical Carbon Dioxide Extracts of Hippophae rhamnoides L. Leaves. Int. Immunopharmacol. 2017, 44, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Poursoleiman, F.; Biregani, A.N.; Esmailzadeh, A. Flavonoids Target Different Molecules of Autophagic and Metastatic Pathways in Cancer Cells. Cancer Cell Int. 2023, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, Y. Flavonoids with Anti-Angiogenesis Function in Cancer. Molecules 2024, 29, 1570. [Google Scholar] [CrossRef]
- Weng, C.-J.; Yen, G.-C. Flavonoids, a Ubiquitous Dietary Phenolic Subclass, Exert Extensive in vitro Anti-Invasive and in vivo Anti-Metastatic Activities. Cancer Metastasis Rev. 2012, 31, 323–351. [Google Scholar] [CrossRef]
- Masarkar, N.; Pal, M.; Roy, M.; Yadav, A.K.; Pandya, B.; Lokhande, S.; Kanwar, J.R.; Ray, S.K.; Mukherjee, S. In-silico Screening of Bioactive Compounds of Moringa oleifera as Potential Inhibitors Targeting HIF-1α/VEGF/GLUT-1 Pathway against Breast Cancer. J. Complement. Integr. Med. 2024, 22, 149–164. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69, 4–10. [Google Scholar] [CrossRef]
- Shaw, P.; Dwivedi, S.K.D.; Bhattacharya, R.; Mukherjee, P.; Rao, G. VEGF Signaling: Role in Angiogenesis and Beyond. Biochim. Biophys. Acta-Rev. Cancer 2024, 1879, 189079. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Brown, J.S.; Gatenby, R.A.; Ibrahim-Hashim, A. A Gene for All Seasons: The Evolutionary Consequences of HIF-1 in Carcinogenesis, Tumor Growth and Metastasis. Semin. Cancer Biol. 2024, 102–103, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, S.; Abubakar, M.; Farid, M.A.; Zia, R.; Nazir, S.; Razzaque, H.; Ali, A.; Ali, Z.; Mahmood, A.; Al-Masry, W.; et al. Detection of Toxic Cypermethrin Pesticides in Drinking Water by Simple Graphitic Electrode Modified with Kraft Lignin@Ni@g-C3N4 Nano-Composite. J. Mater. Chem. B 2024, 12, 9364–9374. [Google Scholar] [CrossRef] [PubMed]
- Laack, E.; Scheffler, A.; Burkholder, I.; Boeters, I.; Andritzky, B.; Schuch, G.; Görn, M.; Vohwinkel, G.; Edler, L.; Fiedler, W.; et al. Pretreatment Vascular Endothelial Growth Factor (VEGF) and Matrix Metalloproteinase-9 (MMP-9) Serum Levels in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 2005, 50, 51–58. [Google Scholar] [CrossRef]
- Kpeli, G.W.; Conrad, K.M.; Bralower, W.; Byrne, C.E.; Boue, S.M.; Burow, M.E.; Mondrinos, M.J. Xenohormetic Phytochemicals Inhibit Neovascularization in Microphysiological Models of Vasculogenesis and Tumor Angiogenesis. Adv. Biol. 2024, 8, 2300480. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, L.; Zhang, H.; Li, Y.; Lai, S. Effects of Isorhamnetin on Protein Expression of VEGF, MMP-2 and Endostatin in Lewis Lung Cancer Mouse. Int. J. Clin. Exp. Med. 2017, 10, 11488–11495. [Google Scholar]
- Kang, L.; Gao, X.-H.; Liu, H.-R.; Men, X.; Wu, H.-N.; Cui, P.-W.; Oldfield, E.; Yan, J.-Y. Structure–Activity Relationship Investigation of Coumarin–Chalcone Hybrids with Diverse Side-Chains as Acetylcholinesterase and Butyrylcholinesterase Inhibitors. Mol. Divers. 2018, 22, 893–906. [Google Scholar] [CrossRef]
- Biswas, P.; Kaium, M.A.; Islam Tareq, M.M.; Tauhida, S.J.; Hossain, M.R.; Siam, L.S.; Parvez, A.; Bibi, S.; Hasan, M.H.; Rahman, M.M.; et al. The Experimental Significance of Isorhamnetin as an Effective Therapeutic Option for Cancer: A Comprehensive Analysis. Biomed. Pharmacother. 2024, 176, 116860. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Wu, Z.; Cao, W.; Wang, Y.; Deng, X.; Zhou, Y. Identification of Anti-Nociceptive Constituents from the Pollen of Typha angustifolia L. Using Effect-Directed Fractionation. Nat. Prod. Res. 2020, 34, 1041–1045. [Google Scholar] [CrossRef]
- Hui, Q.; Yang, N.; Xiong, C.; Zhou, S.; Zhou, X.; Jin, Q.; Xu, X. Isorhamnetin Suppresses the Epithelial-Mesenchymal Transition of the Retinal Pigment Epithelium Both in Vivo and in Vitro through Nrf2-Dependent AKT/GSK-3β Pathway. Exp. Eye Res. 2024, 240, 109823. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Shi, J.; Wang, Z.; Liang, Y.; Yu, J.; Wang, H.; Song, Z.; Tang, Z.; Zhang, D.; et al. Isorhamnetin Alleviates Renal Fibrosis by Inducing Endogenous Hydrogen Sulfide and Regulating Thiol-Based Redox State in Obstructed Kidneys. Biomolecules 2024, 14, 1233. [Google Scholar] [CrossRef]
- Martínez, G.; Mijares, M.R.; De Sanctis, J.B. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 84–104. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef]
- Kim, M.H. Flavonoids Inhibit VEGF/BFGF-Induced Angiogenesis in Vitro by Inhibiting the Matrix-Degrading Proteases. J. Cell. Biochem. 2003, 89, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Khater, M.; Greco, F.; Osborn, H.M.I. Antiangiogenic Activity of Flavonoids: A Systematic Review and Meta-Analysis. Molecules 2020, 25, 4712. [Google Scholar] [CrossRef]
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and Osteogenic Effects of Flavonoids in Bone Regeneration. Biotechnol. Bioeng. 2022, 119, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Bhattacharya, H.; Singh, S.K.; Dua, K.; Dey, A.; Jha, N.K. Recent Advances in Flavonoid-Based Nanocarriers as an Emerging Drug Delivery Approach for Cancer Chemotherapy. Drug Discov. Today 2023, 28, 103409. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Tan, X.; Hu, Q.; Pan, H.; Zhao, M.; Guo, C.; Zeng, J.; Ma, X.; Zhao, Y. Flavonoids and Gastric Cancer Therapy: From Signaling Pathway to Therapeutic Significance. Drug Des. Dev. Ther. 2024, 18, 3233–3253. [Google Scholar] [CrossRef]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Słonimska, P.; Sachadyn, P.; Zieliński, J.; Skrzypski, M.; Pikuła, M. Chemotherapy-Mediated Complications of Wound Healing: An Understudied Side Effect. Adv. Wound Care 2024, 13, 187–199. [Google Scholar] [CrossRef]
- Labe, S.; Jones, G.; Dailey, H.; Bhasker, J.; Kanwar, R.; Crago, M.; Fitzgerald, B.; Mikhail, D.; Hafiz, S.; Kramer, C.; et al. D-CRSE: Diminishing Chemotherapy-Related Side Effects through Patient Education, a Mixed-Methods Pilot Study. J. Psychosoc. Oncol. 2025, 43, 1–15. [Google Scholar] [CrossRef]
- Zhai, K.; Mazurakova, A.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Flavonoids Synergistically Enhance the Anti-Glioblastoma Effects of Chemotherapeutic Drugs. Biomolecules 2021, 11, 1841. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Sulaiman, A.A.; Balch, C.; Chauhan, H.; Alhadidi, Q.M.; Tiwari, A.K. Natural Polyphenols in Cancer Chemoresistance. Nutr. Cancer 2016, 68, 879–891. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Suhail, M.; Tarique, M.; Tabrez, S. Targeting PI3K/Akt/MTOR Pathway by Different Flavonoids: A Cancer Chemopreventive Approach. Int. J. Mol. Sci. 2021, 22, 12455. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, N.; Sun, T.; Ma, W.; Zhang, B.; Li, W. Xanthohumol Inhibits TGF-Β1-Induced Cardiac Fibroblasts Activation via Mediating PTEN/Akt/MTOR Signaling Pathway. Drug Des. Devel. Ther. 2020, 14, 5431–5439. [Google Scholar] [CrossRef]
- Talapko, J.; Talapko, D.; Katalinić, D.; Kotris, I.; Erić, I.; Belić, D.; Vasilj Mihaljević, M.; Vasilj, A.; Erić, S.; Flam, J.; et al. Health Effects of Ionizing Radiation on the Human Body. Medicina 2024, 60, 653. [Google Scholar] [CrossRef] [PubMed]
- Lohani, M.; Ahuja, M.; Buabeid, M.A.; Schwartz, D.; Shannon, D.; Suppiramaniam, V.; Kemppainen, B.; Dhanasekaran, M. Anti-Oxidative and DNA Protecting Effects of Flavonoids-Rich Scutellaria lateriflora. Nat. Prod. Commun. 2013, 8, 1934578X1300801019. [Google Scholar] [CrossRef]
- Arcas, M.C.; Botía, J.M.; Ortuño, A.M.; Del Río, J.A. UV Irradiation Alters the Levels of Flavonoids Involved in the Defence Mechanism of Citrus Aurantium Fruits against Penicillium Digitatum. Eur. J. Plant Pathol. 2000, 106, 617–622. [Google Scholar] [CrossRef]
- Yahyapour, R.; Shabeeb, D.; Cheki, M.; Musa, A.E.; Farhood, B.; Rezaeyan, A.; Amini, P.; Fallah, H.; Najafi, M. Radiation Protection and Mitigation by Natural Antioxidants and Flavonoids: Implications to Radiotherapy and Radiation Disasters. Curr. Mol. Pharmacol. 2018, 11, 285–304. [Google Scholar] [CrossRef]
- Tiwari, P.; Mishra, K.P. Flavonoids Sensitize Tumor Cells to Radiation: Molecular Mechanisms and Relevance to Cancer Radiotherapy. Int. J. Radiat. Biol. 2020, 96, 360–369. [Google Scholar] [CrossRef]
- Singh, S.; Ahuja, A.; Sharma, H.; Maheshwari, P. An Overview of Dietary Flavonoids as a Nutraceutical Nanoformulation Approach to Life-Threatening Diseases. Curr. Pharm. Biotechnol. 2023, 24, 1740–1773. [Google Scholar] [CrossRef]
- Fonseca, M.; Rehman, M.; Soares, R.; Fonte, P. The Impact of Flavonoid-Loaded Nanoparticles in the UV Protection and Safety Profile of Topical Sunscreens. Biomolecules 2023, 13, 493. [Google Scholar] [CrossRef] [PubMed]
- Jasim, A.J.; Sulaiman, G.M.; Ay, H.; Mohammed, S.A.A.; Mohammed, H.A.; Jabir, M.S.; Khan, R.A. Preliminary Trials of the Gold Nanoparticles Conjugated Chrysin: An Assessment of Anti-Oxidant, Anti-Microbial, and in Vitro Cytotoxic Activities of a Nanoformulated Flavonoid. Nanotechnol. Rev. 2022, 11, 2726–2741. [Google Scholar] [CrossRef]
- Teng, H.; Zheng, Y.; Cao, H.; Huang, Q.; Xiao, J.; Chen, L. Enhancement of Bioavailability and Bioactivity of Diet-Derived Flavonoids by Application of Nanotechnology: A Review. Crit. Rev. Food Sci. Nutr. 2023, 63, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement Strategies for the Oral Bioavailability of Poorly Water-Soluble Flavonoids: An Overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Zverev, Y.F.; Rykunova, A.Y. Modern Nanocarriers as a Factor in Increasing the Bioavailability and Pharmacological Activity of Flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Puri, A.; Bharati, D.; Singh, S. Polyphenol-Encapsulated Nanoparticles for the Treatment of Chronic Metabolic Diseases. In Role of Flavonoids in Chronic Metabolic Diseases; Scrivener Publishing LLC: Austin, TX, USA, 2024; pp. 375–416. ISBN 9781394238071. [Google Scholar]
- Chen, H.; Wang, G.; Li, X.; Wang, J.; Wang, X.; Wang, Y.; Liu, Z.; Liu, J.; Ding, Y.; Guo, J.; et al. Multi-Functional D-Alpha-Tocopheryl Polyethylene Glycol Succinate Surface Modified Nanocrystals Improve the Stability and Oral Bioavailability of Pueraria Flavonoids. J. Drug Deliv. Sci. Technol. 2024, 95, 105623. [Google Scholar] [CrossRef]
- Puspadewi, R.; Milanda, T.; Muhaimin, M.; Chaerunisaa, A.Y. Nanoparticle-Encapsulated Plant Polyphenols and Flavonoids as an Enhanced Delivery System for Anti-Acne Therapy. Pharmaceuticals 2025, 18, 209. [Google Scholar] [CrossRef]
- Mustafa, G.M.; Younas, B.; Waseem, M.; Ateeq-ur-Rehman; Noor, N.A.; Elhindi, K.M.; Mumtaz, S. Investigation of Optoelectronic and Thermoelectric Characteristics of Tl2Os(Cl/Br)6 Double Perovskites for Renewable Energy Applications. Mater. Sci. Semicond. Process. 2025, 192, 109420. [Google Scholar] [CrossRef]
- Ciceu, A.; Fenyvesi, F.; Hermenean, A.; Ardelean, S.; Dumitra, S.; Puticiu, M. Advancements in Plant-Based Therapeutics for Hepatic Fibrosis: Molecular Mechanisms and Nanoparticulate Drug Delivery Systems. Int. J. Mol. Sci. 2024, 25, 9346. [Google Scholar] [CrossRef]
- Li, N.; Wang, M.; Lyu, Z.; Shan, K.; Chen, Z.; Chen, B.; Chen, Y.; Hu, X.; Dou, B.; Zhang, J.; et al. Medicinal Plant-Based Drug Delivery System for Inflammatory Bowel Disease. Front. Pharmacol. 2023, 14, 1158945. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Bravo-Díaz, C.; Sova, M.; Kristl, J.; Saso, L. Nanotechnology-Based Drug Delivery to Improve the Therapeutic Benefits of NRF2 Modulators in Cancer Therapy. Antioxidants 2021, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Song, J.; Jing, Z.; Qin, H.; Wu, Y.; Zhou, J.; Zang, X. Stimulus Responsive Nanocarrier for Enhanced Antitumor Responses Against Hepatocellular Carcinoma. Int. J. Nanomed. 2024, 19, 13339–13355. [Google Scholar] [CrossRef]
- Sharma, A.; Wairkar, S. Flavonoids for Treating Pulmonary Fibrosis: Present Status and Future Prospects. Phyther. Res. 2024, 38, 4406–4423. [Google Scholar] [CrossRef] [PubMed]
- Majid, I.; Majid, D.; Makroo, H.A.; Dar, B.N. Enhancing the Bioavailability and Gut Health Benefits of Quercetin from Sprouted Onions: A Comprehensive Review in the Context of Food-Derived Bioactives. Food Chem. Adv. 2024, 4, 100725. [Google Scholar] [CrossRef]
- Attar, E.S.; Chaudhari, V.H.; Deokar, C.G.; Dyawanapelly, S.; Devarajan, P. V Nano Drug Delivery Strategies for an Oral Bioenhanced Quercetin Formulation. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 495–514. [Google Scholar] [CrossRef]
- Arif, M.; Rauf, A.; Akhter, T. A Comprehensive Review on Crosslinked Network Systems of Zinc Oxide-Organic Polymer Composites. Int. J. Biol. Macromol. 2024, 274, 133250. [Google Scholar] [CrossRef]
- Shree Harini, K.; Ezhilarasan, D. Flavonoids-Based Nanomedicines for the Treatment of Liver Fibrosis: A Recent Progress. J. Drug Deliv. Sci. Technol. 2024, 93, 105467. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological Applications and Health-Promoting Properties of Flavonols: An Updated View. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Sharma, H.; Anand, A.; Halagali, P.; Inamdar, A.; Pathak, R.; Taghizadeh-Hesary, F.; Ashique, S. Advancement of Nanoengineered Flavonoids for Chronic Metabolic Diseases. In Role of Flavonoids in Chronic Metabolic Diseases; Scrivener Publishing LLC: Austin, TX, USA, 2024; pp. 459–510. ISBN 9781394238071. [Google Scholar]
- Arif, M.; Rauf, A.; Raza, H.; Moussa, S.B.; Haroon, S.M.; Alzahrani, A.Y.A.; Akhter, T. Catalytic Reduction of Nitroarenes by Palladium Nanoparticles Decorated Silica@poly(Chitosan-N-Isopropylacrylamide-Methacrylic Acid) Hybrid Microgels. Int. J. Biol. Macromol. 2024, 275, 133633. [Google Scholar] [CrossRef]
- Patel, D.; Sethi, N.; Patel, P.; Shah, S.; Patel, K. Exploring the Potential of P-Glycoprotein Inhibitors in the Targeted Delivery of Anti-Cancer Drugs: A Comprehensive Review. Eur. J. Pharm. Biopharm. 2024, 198, 114267. [Google Scholar] [CrossRef]
- Alexander, A.; Ajazuddin; Patel, R.J.; Saraf, S.; Saraf, S. Recent Expansion of Pharmaceutical Nanotechnologies and Targeting Strategies in the Field of Phytopharmaceuticals for the Delivery of Herbal Extracts and Bioactives. J. Control. Release 2016, 241, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug Delivery Based Pharmacological Enhancement and Current Insights of Quercetin with Therapeutic Potential against Oral Diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.; Rauf, A.; Akhter, T. A Review on Ag Nanoparticles Fabricated in Microgels. RSC Adv. 2024, 14, 19381–19399. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Samborska, K.; Boostani, S.; Geranpour, M.; Hosseini, H.; Dima, C.; Khoshnoudi-Nia, S.; Rostamabadi, H.; Falsafi, S.R.; Shaddel, R.; Akbari-Alavijeh, S.; et al. Green Biopolymers from By-Products as Wall Materials for Spray Drying Microencapsulation of Phytochemicals. Trends Food Sci. Technol. 2021, 108, 297–325. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Kapoor, B.; Gulati, M.; Kumar, R.; Ramanunny, A.K.; Awasthi, A.; Dua, K. Treatment Strategies against Diabetes: Success so Far and Challenges Ahead. Eur. J. Pharmacol. 2019, 862, 172625. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, J.; An, C.; Li, X.; Xu, S.; He, Y.; Zhang, X.; Liu, L.; Hu, K.; Liang, M. Mechanism of LncRNA Gm2044 in Germ Cell Development. Front. Cell Dev. Biol. 2024, 12, 1410914. [Google Scholar] [CrossRef]
- Birnbaum, D.T.; Brannon-Peppas, L. Microparticle Drug Delivery Systems. In Drug Delivery Systems in Cancer Therapy; Brown, D.M., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 117–135. ISBN 978-1-59259-427-6. [Google Scholar]
- Kohane, D.S. Microparticles and Nanoparticles for Drug Delivery. Biotechnol. Bioeng. 2007, 96, 203–209. [Google Scholar] [CrossRef]
- Kállai-Szabó, N.; Farkas, D.; Lengyel, M.; Basa, B.; Fleck, C.; Antal, I. Microparticles and Multi-Unit Systems for Advanced Drug Delivery. Eur. J. Pharm. Sci. 2024, 194, 106704. [Google Scholar] [CrossRef]
- Dong, Q.; Jiang, Z. Platinum–Iron Nanoparticles for Oxygen-Enhanced Sonodynamic Tumor Cell Suppression. Inorganics 2024, 12, 331. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Mohammadian, E.; Rhim, J.-W.; Jafari, S.M. PH-Sensitive (Halochromic) Smart Packaging Films Based on Natural Food Colorants for the Monitoring of Food Quality and Safety. Trends Food Sci. Technol. 2020, 105, 93–144. [Google Scholar] [CrossRef]
- Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaproń, B.; Plech, T.; Szymanowska, D.; Karaźniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae Flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. [Google Scholar] [CrossRef] [PubMed]
- Kar, K. Functionalization of Food Polyphenols for Nanodeliveries. In Polyphenols: Food, Nutraceutical, and Nanotherapeutic Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 134–154. ISBN 9781394188864. [Google Scholar]
- Arif, M.; Raza, H.; Moussa, S.B.; Alzahrani, A.Y.A.; Akhter, T. Poly(Chitosan-N-Vinylcaprolactam-Methacrylic Acid) Microgels as Microreactor for Ag(I) Ions Extraction and in-Situ Silver Nanoparticles Formation to Reduce the Toxins. Int. J. Biol. Macromol. 2024, 282, 136906. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Zhang, Y.; Nie, G. Emerging Nanotechnological Approaches to Regulating Tumor Vasculature for Cancer Therapy. J. Control. Release 2023, 362, 647–666. [Google Scholar] [CrossRef]
- Jia, S.; Huang, S.; Jimo, R.; AXi, Y.; Lu, Y.; Kong, Z.; Ma, J.; Li, H.; Luo, X.; Qu, Y.; et al. In-Situ Forming Carboxymethyl Chitosan Hydrogel Containing Paeonia Suffruticosa Andr. Leaf Extract for Mixed Infectious Vaginitis Treatment by Reshaping the Micro-Biota. Carbohydr. Polym. 2024, 339, 122255. [Google Scholar] [CrossRef]
- Kaushal, N.; Singh, M.; Singh Sangwan, R. Flavonoids: Food Associations, Therapeutic Mechanisms, Metabolism and Nanoformulations. Food Res. Int. 2022, 157, 111442. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- Anusha Siddiqui, S.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel Insights on Extraction and Encapsulation Techniques of Elderberry Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 5937–5952. [Google Scholar] [CrossRef]
- Borah, M.S.; Tiwari, A.; Sridhar, K.; Narsaiah, K.; Nayak, P.K.; Stephen Inbaraj, B. Recent Trends in Valorization of Food Industry Waste and By-Products: Encapsulation and In Vitro Release of Bioactive Compounds. Foods 2023, 12, 3823. [Google Scholar] [CrossRef]
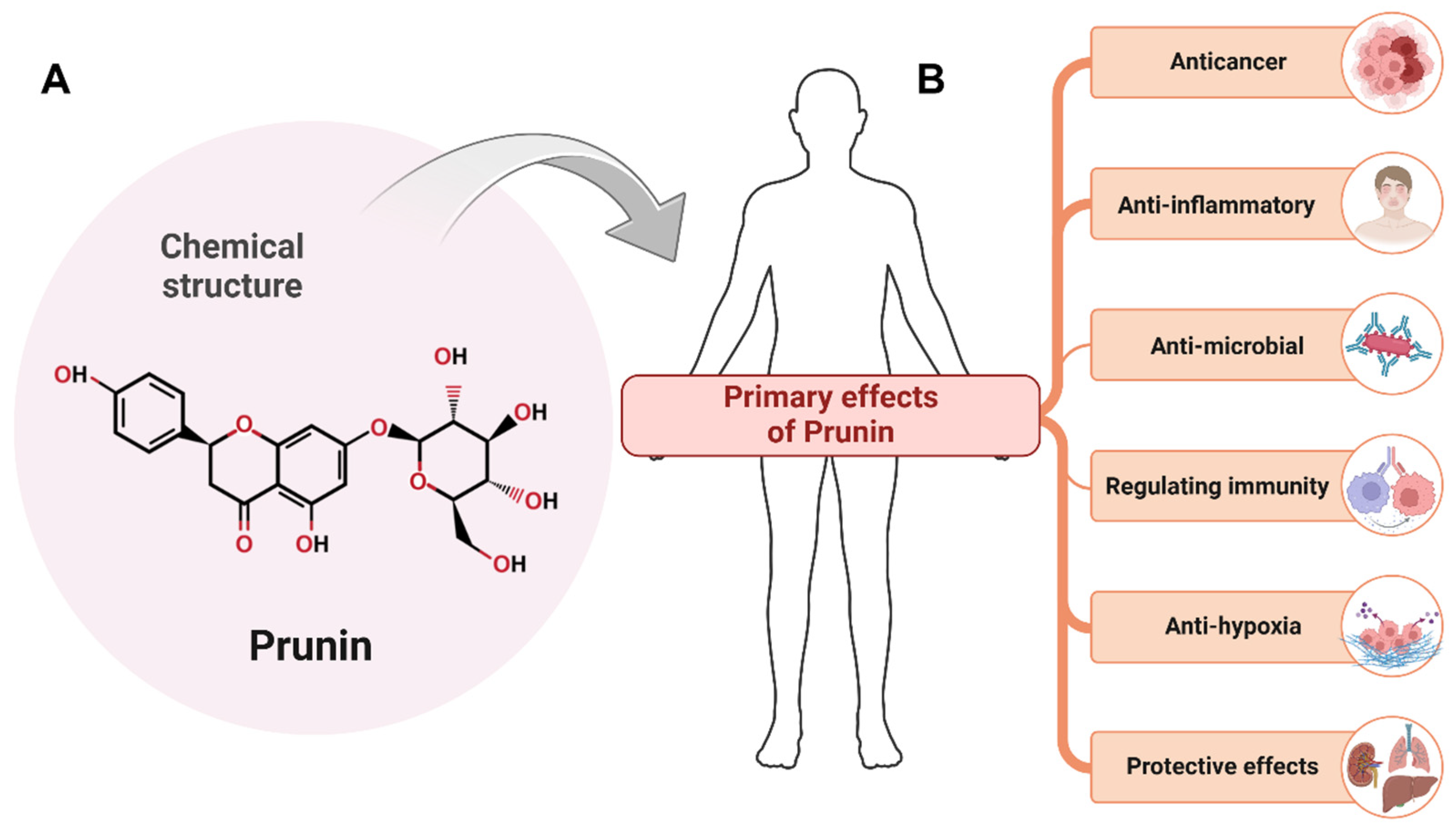
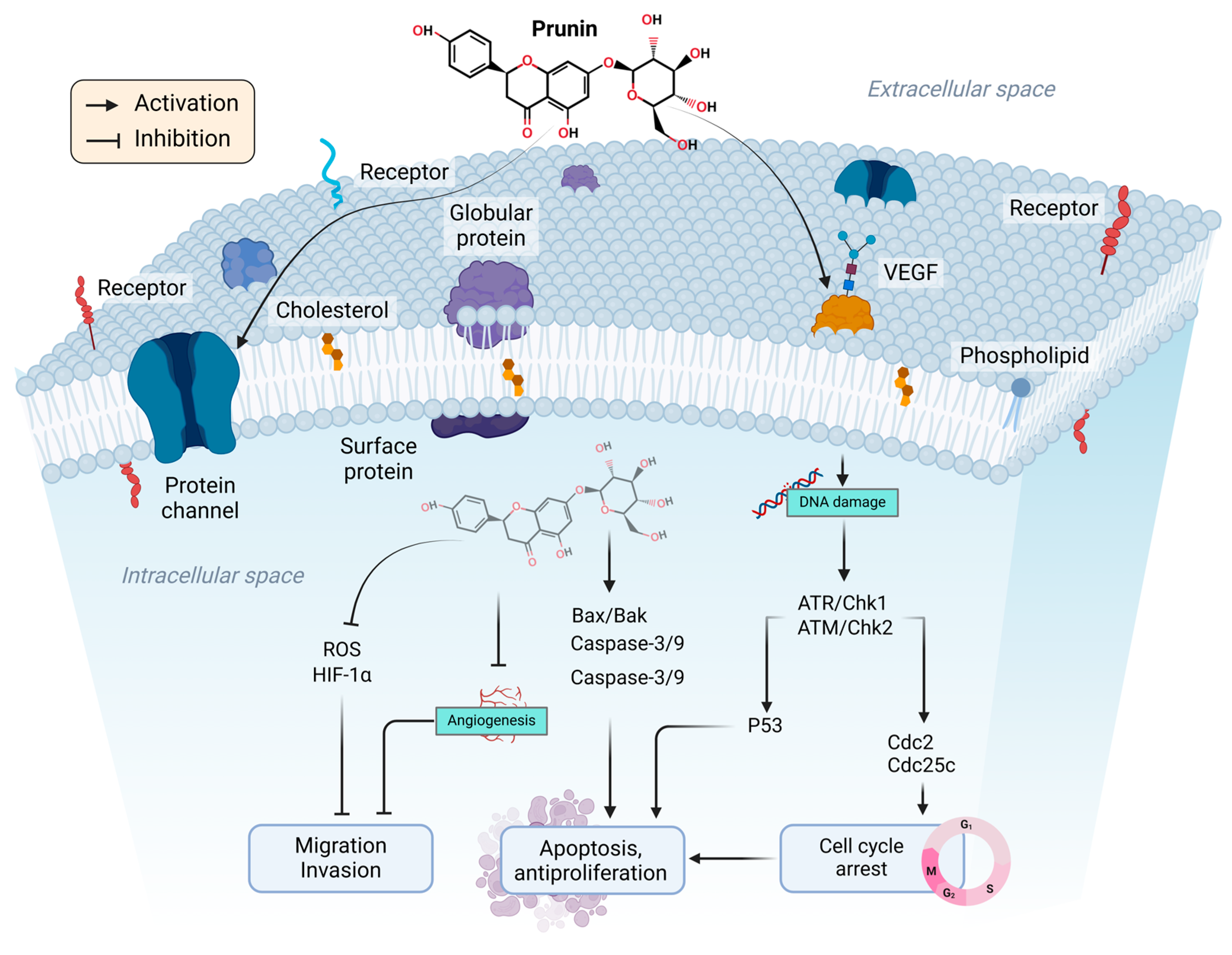


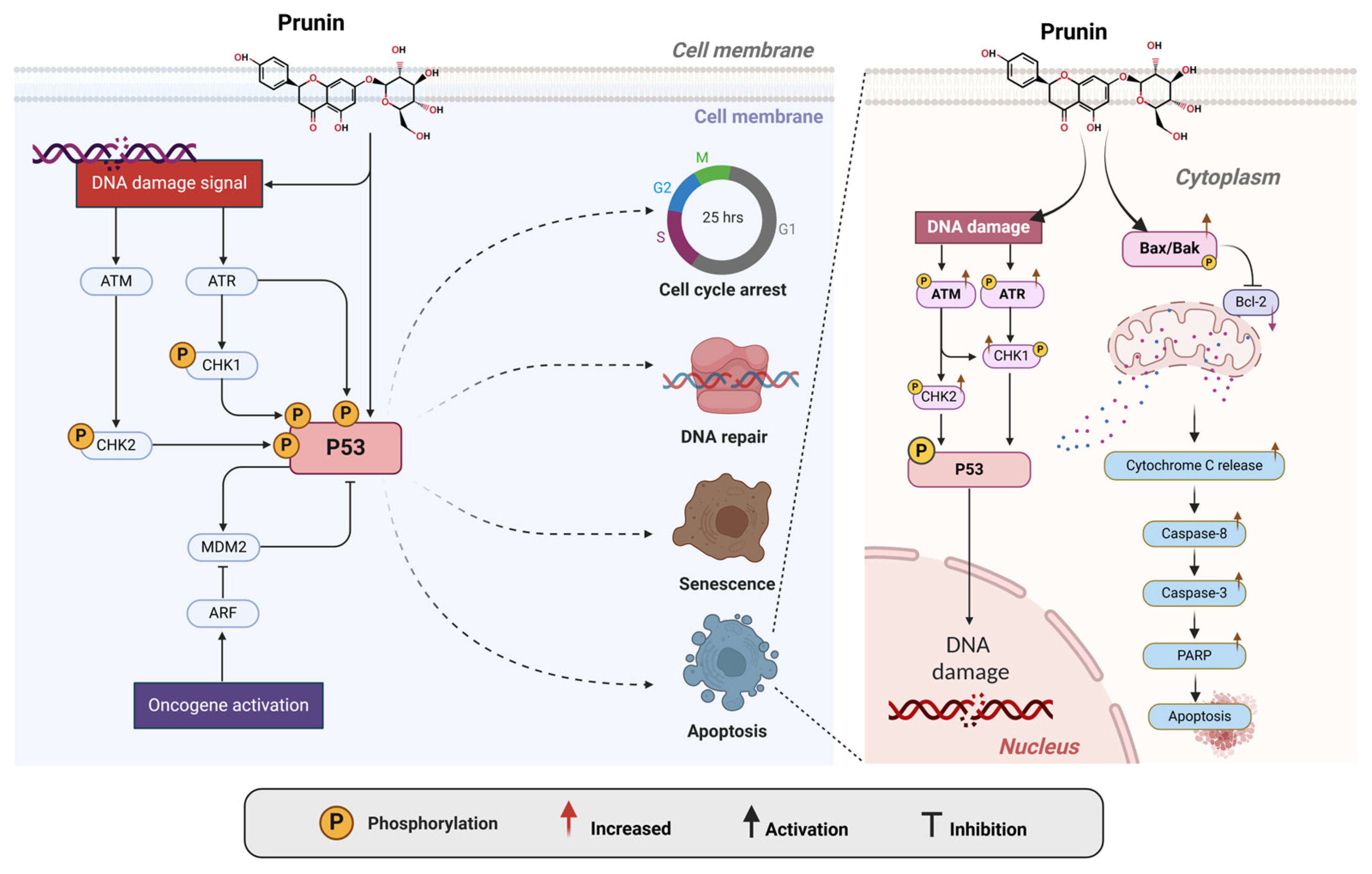
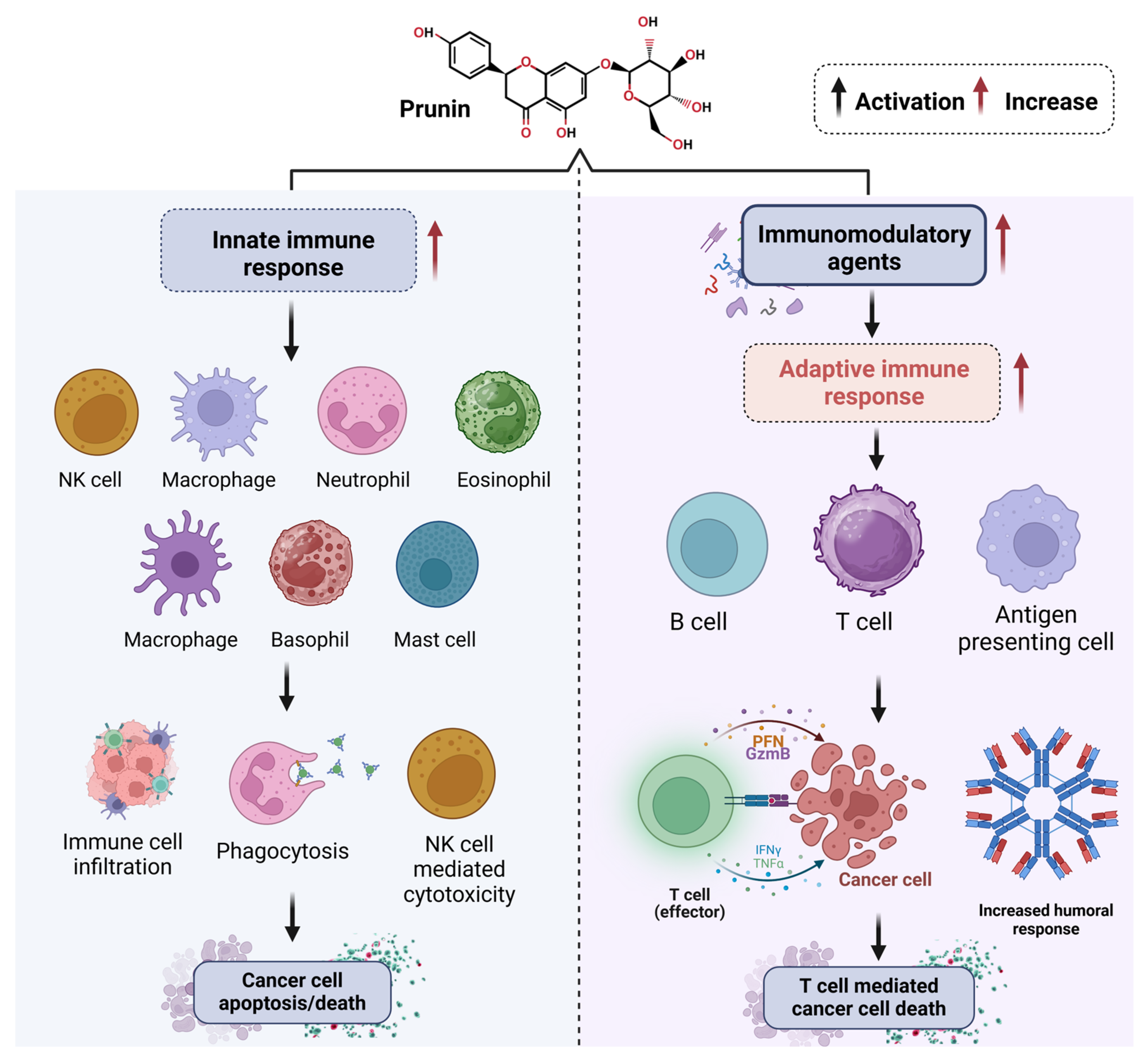
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. https://doi.org/10.3390/ijms26062678
Rana JN, Mumtaz S. Prunin: An Emerging Anticancer Flavonoid. International Journal of Molecular Sciences. 2025; 26(6):2678. https://doi.org/10.3390/ijms26062678
Chicago/Turabian StyleRana, Juie Nahushkumar, and Sohail Mumtaz. 2025. "Prunin: An Emerging Anticancer Flavonoid" International Journal of Molecular Sciences 26, no. 6: 2678. https://doi.org/10.3390/ijms26062678
APA StyleRana, J. N., & Mumtaz, S. (2025). Prunin: An Emerging Anticancer Flavonoid. International Journal of Molecular Sciences, 26(6), 2678. https://doi.org/10.3390/ijms26062678






