Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm
Abstract
:1. BPDCN
1.1. Introduction
1.2. The Role of Plasmacytoid Dendritic Cells (pDCs) in Immunity
1.3. Comprehensive Diagnostic, Immunophenotypic, and Molecular Perspectives in BPDCN
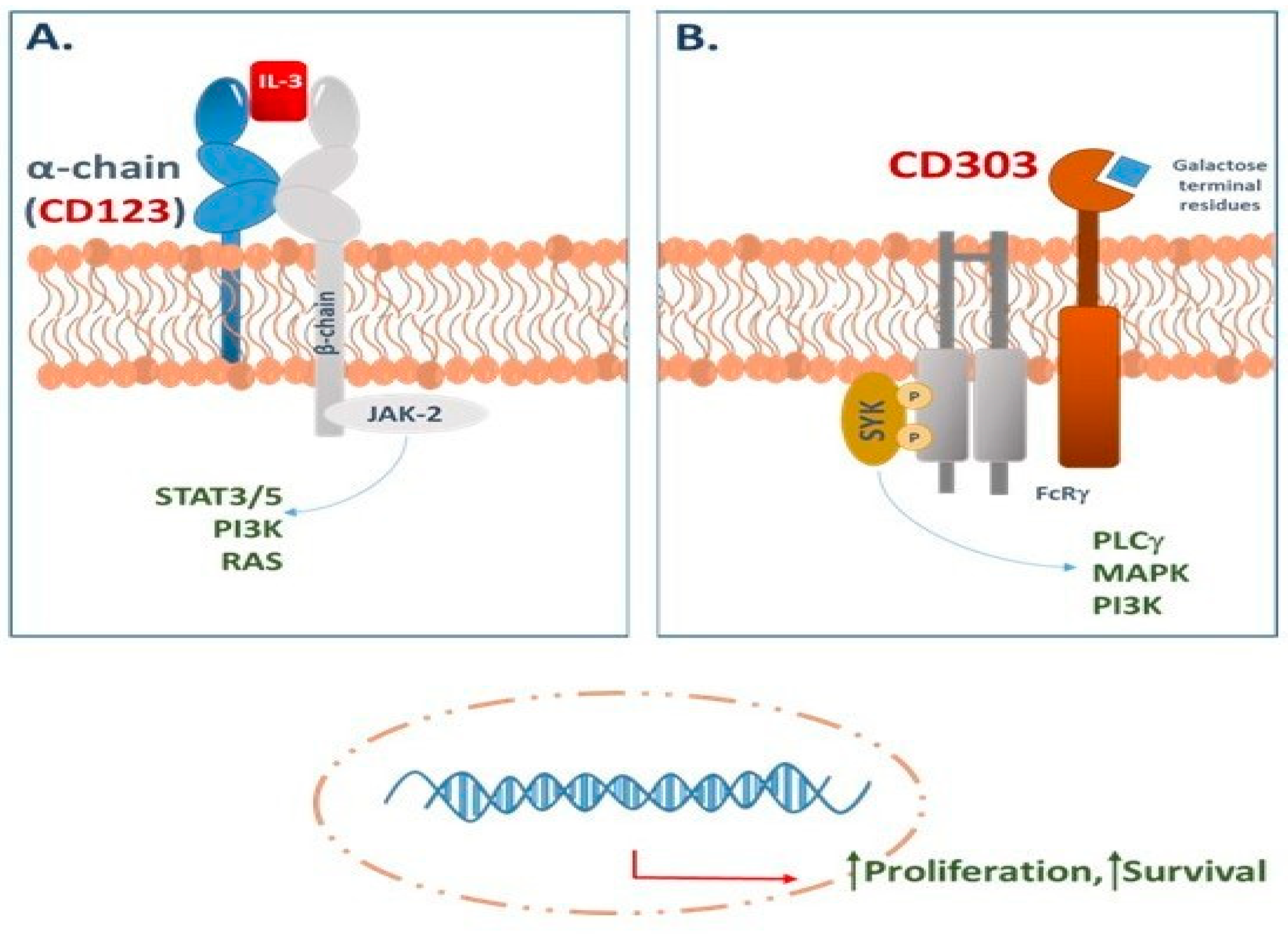
1.4. CD123 in BPDCN: An Emerging Therapeutic Target
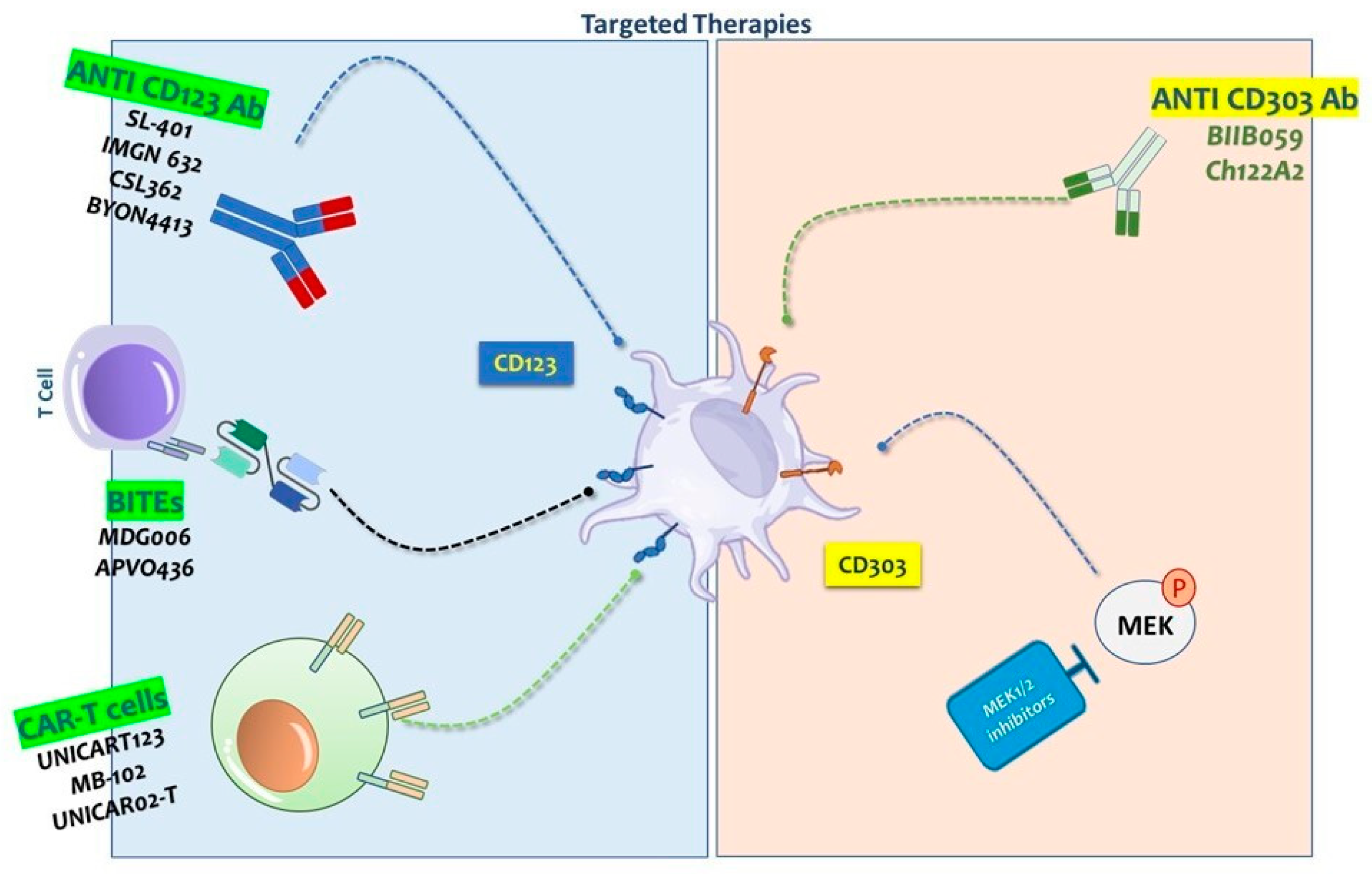
2. CD123-Targeted Therapies
2.1. Monoclonal Antibody-Based Therapies Targeting CD123 in Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN)
2.2. Tagraxofusp (SL-401)
2.3. Pivekimab Sunirine (IMGN632): A Next-Generation CD123-ADC with Emerging Clinical Efficacy in BPDCN
2.4. Other CD123-Targeted Monoclonal Antibodies Therapies
2.5. Bispecific T Cell Engagers
2.6. Advances in CD123-Targeted Chimeric Antigen Receptor (CAR) T-Cell Therapy for BPDCN and Hematologic Malignancies
3. CD303-Targeted Therapies
3.1. CD303 Is a Useful Diagnostic Marker in BPDCN
3.2. The Role of CD303 in Targeted Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: An Italian multicenter study. Haematologica 2013, 98, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; Corazzelli, G.; De Filippi, R.; Pinto, A. Dendritic cells in hematological malignancies. Crit. Rev. Oncol. Hematol. 2016, 108, 86–96. [Google Scholar] [CrossRef]
- Liu, K.; Nussenzweig, M.C. Development and homeostasis of dendritic cells. Eur. J. Immunol. 2010, 40, 2099–2102. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.; Park, S.H.; Jo, J.C. Plasmacytoid dendritic cell neoplasms. Blood Res. 2023, 58, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Garnache-Ottou, F.; Feuillard, J.; Saas, P. Plasmacytoid dendritic cell leukaemia/lymphoma: Towards a well defined entity? Br. J. Haematol. 2007, 136, 539–548. [Google Scholar] [CrossRef]
- Martin-Martin, L.; Lopez, A.; Vidriales, B.; Caballero, M.D.; Rodrigues, A.S.; Ferreira, S.I.; Lima, M.; Almeida, S.; Valverde, B.; Martinez, P.; et al. Classification and clinical behavior of blastic plasmacytoid dendritic cell neoplasms according to their maturation-associated immunophenotypic profile. Oncotarget 2015, 6, 19204–19216. [Google Scholar] [CrossRef] [PubMed]
- Reimer, P.; Rudiger, T.; Kraemer, D.; Kunzmann, V.; Weissinger, F.; Zettl, A.; Konrad Muller-Hermelink, H.; Wilhelm, M. What is CD4+CD56+ malignancy and how should it be treated? Bone Marrow Transpl. 2003, 32, 637–646. [Google Scholar] [CrossRef]
- Garnache-Ottou, F.; Feuillard, J.; Ferrand, C.; Biichle, S.; Trimoreau, F.; Seilles, E.; Salaun, V.; Garand, R.; Lepelley, P.; Maynadie, M.; et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br. J. Haematol. 2009, 145, 624–636. [Google Scholar] [CrossRef]
- Xiao, W.; Chan, A.; Waarts, M.R.; Mishra, T.; Liu, Y.; Cai, S.F.; Yao, J.; Gao, Q.; Bowman, R.L.; Koche, R.P.; et al. Plasmacytoid dendritic cell expansion defines a distinct subset of RUNX1-mutated acute myeloid leukemia. Blood 2021, 137, 1377–1391. [Google Scholar] [CrossRef]
- Pagano, L.; Valentini, C.G.; Grammatico, S.; Pulsoni, A. Blastic plasmacytoid dendritic cell neoplasm: Diagnostic criteria and therapeutical approaches. Br. J. Haematol. 2016, 174, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Mezzanzanica, D.; Canevari, S.; Mazzoni, A.; Figini, M.; Colnaghi, M.I.; Waks, T.; Schindler, D.G.; Eshhar, Z. Transfer of chimeric receptor gene made of variable regions of tumor-specific antibody confers anticarbohydrate specificity on T cells. Cancer Gene Ther. 1998, 5, 401–407. [Google Scholar] [PubMed]
- Testa, U.; Pelosi, E.; Castelli, G. CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies. Cancers 2019, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Qiu, P.; Zeng, Z.; Jorgensen, J.L.; Mak, D.H.; Burks, J.K.; Schober, W.; McQueen, T.J.; Cortes, J.; Tanner, S.D.; et al. Single-cell mass cytometry reveals intracellular survival/proliferative signaling in FLT3-ITD-mutated AML stem/progenitor cells. Cytom. A 2015, 87, 346–356. [Google Scholar] [CrossRef]
- Jordan, C.T.; Upchurch, D.; Szilvassy, S.J.; Guzman, M.L.; Howard, D.S.; Pettigrew, A.L.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.A.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef]
- Moseman, E.A.; Liang, X.; Dawson, A.J.; Panoskaltsis-Mortari, A.; Krieg, A.M.; Liu, Y.J.; Blazar, B.R.; Chen, W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004, 173, 4433–4442. [Google Scholar] [CrossRef]
- Haniffa, M.; Collin, M.; Ginhoux, F. Ontogeny and functional specialization of dendritic cells in human and mouse. Adv. Immunol. 2013, 120, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Thomson, A.W.; Rogers, N.M. Dendritic Cells as Sensors, Mediators, and Regulators of Ischemic Injury. Front. Immunol. 2019, 10, 2418. [Google Scholar] [CrossRef]
- Wang, W.; Khoury, J.D.; Miranda, R.N.; Jorgensen, J.L.; Xu, J.; Loghavi, S.; Li, S.; Pemmaraju, N.; Nguyen, T.; Medeiros, L.J.; et al. Immunophenotypic characterization of reactive and neoplastic plasmacytoid dendritic cells permits establishment of a 10-color flow cytometric panel for initial workup and residual disease evaluation of blastic plasmacytoid dendritic cell neoplasm. Haematologica 2021, 106, 1047–1055. [Google Scholar] [CrossRef]
- Deotare, U.; Yee, K.W.; Le, L.W.; Porwit, A.; Tierens, A.; Musani, R.; Barth, D.; Torlakovic, E.; Schimmer, A.; Schuh, A.C.; et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: 10-Color flow cytometry diagnosis and HyperCVAD therapy. Am. J. Hematol. 2016, 91, 283–286. [Google Scholar] [CrossRef]
- Garnache-Ottou, F.; Vidal, C.; Biichle, S.; Renosi, F.; Poret, E.; Pagadoy, M.; Desmarets, M.; Roggy, A.; Seilles, E.; Soret, L.; et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019, 3, 4238–4251. [Google Scholar] [CrossRef]
- Riaz, W.; Zhang, L.; Horna, P.; Sokol, L. Blastic plasmacytoid dendritic cell neoplasm: Update on molecular biology, diagnosis, and therapy. Cancer Control 2014, 21, 279–289. [Google Scholar] [CrossRef]
- Sapienza, M.R.; Pileri, A.; Derenzini, E.; Melle, F.; Motta, G.; Fiori, S.; Calleri, A.; Pimpinelli, N.; Tabanelli, V.; Pileri, S. Blastic Plasmacytoid Dendritic Cell Neoplasm: State of the Art and Prospects. Cancers 2019, 11, 595. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Yang, M.; Wang, L.; Jin, J. New perspectives in genetics and targeted therapy for blastic plasmacytoid dendritic cell neoplasm. Crit. Rev. Oncol. Hematol. 2020, 149, 102928. [Google Scholar] [CrossRef]
- Sugita, M.; Guzman, M.L. CD123 as a Therapeutic Target Against Malignant Stem Cells. Hematol. Oncol. Clin. North Am. 2020, 34, 553–564. [Google Scholar] [CrossRef]
- Mardiros, A.; Dos Santos, C.; McDonald, T.; Brown, C.E.; Wang, X.; Budde, L.E.; Hoffman, L.; Aguilar, B.; Chang, W.C.; Bretzlaff, W.; et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 2013, 122, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Broughton, S.E.; Dhagat, U.; Hercus, T.R.; Nero, T.L.; Grimbaldeston, M.A.; Bonder, C.S.; Lopez, A.F.; Parker, M.W. The GM-CSF/IL-3/IL-5 cytokine receptor family: From ligand recognition to initiation of signaling. Immunol. Rev. 2012, 250, 277–302. [Google Scholar] [CrossRef]
- Cruz, N.M.; Sugita, M.; Ewing-Crystal, N.; Lam, L.; Galetto, R.; Gouble, A.; Smith, J.; Hassane, D.C.; Roboz, G.J.; Guzman, M.L. Selection and characterization of antibody clones are critical for accurate flow cytometry-based monitoring of CD123 in acute myeloid leukemia. Leuk. Lymphoma 2018, 59, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Goverman, J.; Gomez, S.M.; Segesman, K.D.; Hunkapiller, T.; Laug, W.E.; Hood, L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: Implications for T cell receptor complex formation and activation. Cell 1990, 60, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Pemmaraju, N.; Medeiros, B.C.; Carraway, H.E.; Frankfurt, O.; Forman, S.J.; Yang, X.A.; Konopleva, M.; et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014, 124, 385–392. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Lane, A.A.; Sweet, K.L.; Stein, A.S.; Vasu, S.; Blum, W.; Rizzieri, D.A.; Wang, E.S.; Duvic, M.; Sloan, J.M.; et al. Tagraxofusp in Blastic Plasmacytoid Dendritic-Cell Neoplasm. N. Engl. J. Med. 2019, 380, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Diaz Acedo, R.; Dominguez Munoz, M.A.; Navajas Laguna, C.; Morales Camacho, R.; Simon Pilo, I.; Calama Ruiz-Mateos, V.P.; Yebenes Ramirez, M.; Vahi Sanchez de Medina, M.; Artacho Criado, S.; Rodriguez Perez, A.; et al. Tagraxofusp as first-line treatment for blastic plasmacytoid dendritic cell neoplasm. Leuk Lymphoma 2022, 63, 1762–1764. [Google Scholar] [CrossRef]
- Stephansky, J.; Togami, K.; Ghandi, M.; Montero, J.; vonEgypt, N.; Lindsay, R.; Brooks, C.; Aster, J.C.; Johannessen, C.; Lane, A.A. Resistance to SL-401 in AML and BPDCN Is Associated with Loss of the Diphthamide Synthesis Pathway Enzyme DPH1 and Is Reversible By Azacitidine. Blood 2017, 130, 797. [Google Scholar] [CrossRef]
- Montero, J.; Stephansky, J.; Cai, T.Y.; Griffin, G.K.; Cabal-Hierro, L.; Togami, K.; Hogdal, L.J.; Galinsky, I.; Morgan, E.A.; Aster, J.C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Is Dependent on BCL2 and Sensitive to Venetoclax. Cancer Discov. 2017, 7, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Togami, K.; Pastika, T.; Stephansky, J.; Ghandi, M.; Christie, A.L.; Jones, K.L.; Johnson, C.A.; Lindsay, R.W.; Brooks, C.L.; Letai, A.; et al. DNA methyltransferase inhibition overcomes diphthamide pathway deficiencies underlying CD123-targeted treatment resistance. J. Clin. Investig. 2019, 129, 5005–5019. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.A.; Stein, A.S.; Garcia, J.S.; Garzon, J.L.; Galinsky, I.; Luskin, M.R.; Stone, R.M.; Winer, E.S.; Leonard, R.; Mughal, T.I.; et al. Safety and Efficacy of Combining Tagraxofusp (SL-401) with Azacitidine or Azacitidine and Venetoclax in a Phase 1b Study for CD123 Positive AML, MDS, or BPDCN. Blood 2021, 138, 2346. [Google Scholar] [CrossRef]
- Lane, A.A.; Garcia, J.S.; Raulston, E.G.; Garzon, J.L.; Galinsky, I.; Baxter, E.W.; Leonard, R.; DeAngelo, D.J.; Luskin, M.R.; Reilly, C.R.; et al. Phase 1b trial of tagraxofusp in combination with azacitidine with or without venetoclax in acute myeloid leukemia. Blood Adv. 2024, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gokbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.M.; Wei, A.; Dombret, H.; Foa, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Angelova, E.; Audette, C.; Kovtun, Y.; Daver, N.; Wang, S.A.; Pierce, S.; Konoplev, S.N.; Khogeer, H.; Jorgensen, J.L.; Konopleva, M.; et al. CD123 expression patterns and selective targeting with a CD123-targeted antibody-drug conjugate (IMGN632) in acute lymphoblastic leukemia. Haematologica 2019, 104, 749–755. [Google Scholar] [CrossRef]
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018, 2, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.G.; Montesinos, P.; DeAngelo, D.J.; Wang, E.S.; Papadantonakis, N.; Todisco, E.; Sweet, K.L.; Pemmaraju, N.; Lane, A.A.; Torres-Minana, L.; et al. Pivekimab sunirine (IMGN632), a novel CD123-targeting antibody-drug conjugate, in relapsed or refractory acute myeloid leukaemia: A phase 1/2 study. Lancet Oncol. 2024, 25, 388–399. [Google Scholar] [CrossRef]
- van der Lee, M.M.C.; van Achterberg, T.; Hersmus, K.; Brouwers-Vos, A.; van der Vleuten, M.; Pruis, M.; Kappers, W.; Verheijden, G.; Huls, G.; van Wigcheren, G.; et al. BYON4413, an in vivo active CD123-targeting antibody-drug conjugate, combines effectively with azacitidine and venetoclax in acute myeloid leukemia cell lines. Cancer Res. 2024, 84, 3129. [Google Scholar] [CrossRef]
- van der Lee, M.; van Achtenberg, T.; Brouwers-Vos, A.; van der Vleuten, M.; Kappers, W.; Verheijden, G.; Huls, G.A.; van Wigcheren, G.; Ubink, R.; Sesink, A.; et al. Potent in Vitro and In Vivo Efficacy of BYON4413, a Duba-Based Antibody-Drug Conjugate Targeting CD123 in Acute Myeloid Leukemia. Blood 2023, 142, 2795. [Google Scholar] [CrossRef]
- Kubasch, A.S.; Schulze, F.; Giagounidis, A.; Gotze, K.S.; Kronke, J.; Sockel, K.; Middeke, J.M.; Chermat, F.; Gloaguen, S.; Puttrich, M.; et al. Single agent talacotuzumab demonstrates limited efficacy but considerable toxicity in elderly high-risk MDS or AML patients failing hypomethylating agents. Leukemia 2020, 34, 1182–1186. [Google Scholar] [CrossRef]
- Montesinos, P.; Roboz, G.J.; Bulabois, C.E.; Subklewe, M.; Platzbecker, U.; Ofran, Y.; Papayannidis, C.; Wierzbowska, A.; Shin, H.J.; Doronin, V.; et al. Safety and efficacy of talacotuzumab plus decitabine or decitabine alone in patients with acute myeloid leukemia not eligible for chemotherapy: Results from a multicenter, randomized, phase 2/3 study. Leukemia 2021, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Bole-Richard, E.; Pemmaraju, N.; Cael, B.; Daguindau, E.; Lane, A.A. CD123 and More: How to Target the Cell Surface of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers 2022, 14, 2287. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, E.; Castelli, G.; Testa, U. CD123 a Therapeutic Target for Acute Myeloid Leukemia and Blastic Plasmocytoid Dendritic Neoplasm. Int. J. Mol. Sci. 2023, 24, 2718. [Google Scholar] [CrossRef] [PubMed]
- Zanotta, S.; Galati, D.; De Filippi, R.; Pinto, A. Breakthrough in Blastic Plasmacytoid Dendritic Cell Neoplasm Cancer Therapy Owing to Precision Targeting of CD123. Int. J. Mol. Sci. 2024, 25, 1454. [Google Scholar] [CrossRef]
- Comeau, M.R.; Gottschalk, R.; Daugherty, M.; Sewell, T.; Misher, L.; Jeannette, B.; Johnson, S.; Parr, L.; Kumer, J.; Jablonski, D.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity with limited cytokine release, is well tolerated in repeat dose toxicology studies in cynomolgus macaques. Cancer Res. 2019, 79, 1786. [Google Scholar] [CrossRef]
- Comeau, M.R.; Miller, R.E.; Bannink, J.; Johnson, S.; Bader, R.; Gottschalk, R.; Misher, L.; Mitchell, D.; Parr, L.; DeFrancesco, M.; et al. Characterization of APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity, in preclinical models of AML and nonhuman primates. Mol. Cancer Ther. 2018, 17, 1786. [Google Scholar] [CrossRef]
- Comeau, M.R.; Miller, R.E.; Bader, R.; Gottschalk, R.; Daugherty, M.; Sewell, T.; Misher, L.; Parr, L.; DeFrancesco, M.; Bienvenue, D.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR® molecule for redirected T-cell cytotoxicity, induces potent T-cell activation, proliferation and cytotoxicity with limited cytokine release. Cancer Res. 2018, 78, 1786. [Google Scholar] [CrossRef]
- Bole-Richard, E.; Fredon, M.; Biichle, S.; Anna, F.; Certoux, J.M.; Renosi, F.; Tse, F.; Molimard, C.; Valmary-Degano, S.; Jenvrin, A.; et al. CD28/4-1BB CD123 CAR T cells in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2020, 34, 3228–3241. [Google Scholar] [CrossRef]
- Cai, T.; Gouble, A.; Black, K.L.; Skwarska, A.; Naqvi, A.S.; Taylor, D.; Zhao, M.; Yuan, Q.; Sugita, M.; Zhang, Q.; et al. Targeting CD123 in blastic plasmacytoid dendritic cell neoplasm using allogeneic anti-CD123 CAR T cells. Nat. Commun. 2022, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Loff, S.; Dietrich, J.; Meyer, J.E.; Riewaldt, J.; Spehr, J.; von Bonin, M.; Grunder, C.; Swayampakula, M.; Franke, K.; Feldmann, A.; et al. Rapidly Switchable Universal CAR-T Cells for Treatment of CD123-Positive Leukemia. Mol. Ther. Oncolytics 2020, 17, 408–420. [Google Scholar] [CrossRef]
- Xue, T.; Budde, L.E. Immunotherapies Targeting CD123 for Blastic Plasmacytoid Dendritic Cell Neoplasm. Hematol. Oncol. Clin. North Am. 2020, 34, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.H.; Muhammad, N.; Tarique, M.; Usmani, D.; Naz, H.; Sarode, A. The Role of Cancer-Specific Target Antigens in CAR T Cell Therapy in Hematological Malignancies. Curr. Tissue Microenviron. Rep. 2024, 5, 61–67. [Google Scholar] [CrossRef]
- Das, S.; Khan, T.H.; Sarkar, D. Comprehensive Review on the Effect of Stem Cells in Cancer Progression. Curr. Tissue Microenviron. Rep. 2024, 5, 39–59. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.; Liu, Y.J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Mildner, A.; Jung, S. Development and function of dendritic cell subsets. Immunity 2014, 40, 642–656. [Google Scholar] [CrossRef]
- Diebold, S.S. Activation of dendritic cells by toll-like receptors and C-type lectins. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–30. [Google Scholar] [CrossRef]
- Wilson, N.R.; Bover, L.; Konopleva, M.; Han, L.; Neelapu, S.; Pemmaraju, N. CD303 (BDCA-2)—A potential novel target for therapy in hematologic malignancies. Leuk Lymphoma 2022, 63, 19–30. [Google Scholar] [CrossRef]
- El Hussein, S.; Wang, W. Flow Cytometry Profiling of Plasmacytoid Dendritic Cell Neoplasms. Cancers 2024, 16, 2118. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.; Yin, L.; Zhou, L.; Deng, Y.; Deng, H. Diagnosis, treatment, and genetic characteristics of blastic plasmacytoid dendritic cell neoplasm: A review. Medicine 2023, 102, e32904. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J.; Khoury, J.D.; Pemmaraju, N.; Fang, H.; Miranda, R.N.; Yin, C.C.; Hussein, S.E.; Jia, F.; Tang, Z.; et al. Immunophenotypic and Molecular Features of Acute Myeloid Leukemia with Plasmacytoid Dendritic Cell Differentiation Are Distinct from Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers 2022, 14, 3375. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, A.; Otero, K.; Czerkowicz, J.M.; Kerns, H.M.; Shapiro, R.I.; Ranger, A.M.; Otipoby, K.L.; Taylor, F.R.; Cameron, T.O.; Viney, J.L.; et al. Anti-BDCA2 monoclonal antibody inhibits plasmacytoid dendritic cell activation through Fc-dependent and Fc-independent mechanisms. EMBO Mol. Med. 2015, 7, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Werth, V.P.; Merola, J.F.; Stevenson, L.; Reynolds, T.L.; Naik, H.; Wang, W.; Christmann, R.; Gardet, A.; Pellerin, A.; et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Investig. 2019, 129, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Fournier, N.; Jacque, E.; Fontayne, A.; Derache, D.; Dupont, G.; Verhaeghe, L.; Baptista, L.; Dehenne, A.; Dezetter, A.-S.; Terrier, A.; et al. Improved in vitro and in vivo activity against CD303-expressing targets of the chimeric 122A2 antibody selected for specific glycosylation pattern. mAbs 2018, 10, 651–663. [Google Scholar] [CrossRef]
- Janovec, V.; Aouar, B.; Font-Haro, A.; Hofman, T.; Trejbalova, K.; Weber, J.; Chaperot, L.; Plumas, J.; Olive, D.; Dubreuil, P.; et al. The MEK1/2-ERK Pathway Inhibits Type I IFN Production in Plasmacytoid Dendritic Cells. Front. Immunol. 2018, 9, 364. [Google Scholar] [CrossRef]
- Zhang, Q.; Hossain, D.M.; Nechaev, S.; Kozlowska, A.; Zhang, W.; Liu, Y.; Kowolik, C.M.; Swiderski, P.; Rossi, J.J.; Forman, S.; et al. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood 2013, 121, 1304–1315. [Google Scholar] [CrossRef]

| Study ID | Therapeutic Strategy | Condition/Disease | Phase | Status |
|---|---|---|---|---|
| NCT03113643 | Tagraxofusp + azacitidine ± venetoclax | AML, MDS, and BPDCN | I | Recruiting |
| NCT03386513 | IMGN632 | AML, ALL, BPDCN, MPN | I/II | Active, not recruiting |
| NCT04086264 | IMGN632 alone or + azacitidine ± venetoclax | CD123-Positive AML | I/II | Active, not recruiting |
| NCT06359002 | BYON4413 | R/R AML, and MDS | I | Recruiting |
| NCT04681105 | Bispecific antibodies Flotetuzumab | AML, BPDCN | I | Active, not recruiting |
| NCT03203369 | UCART123 | BPDCN | I | Terminated |
| NCT04109482 | Chimeric Antigen Receptor T cells MB-102 | BPDCN | I/II | Terminated |
| NCT02159495 | Chimeric Antigen Receptor T cells CD123+ CAR T cells | AML, BPDCN | I | Active, not recruiting |
| NCT04230265 | Chimeric Antigen Receptor T cells UniCAR02-T + TM123 | AML, BPDCN | I | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galati, D.; Zanotta, S.; Florio, F.; Mele, S.; De Filippi, R.; Pinto, A. Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm. Int. J. Mol. Sci. 2025, 26, 2732. https://doi.org/10.3390/ijms26062732
Galati D, Zanotta S, Florio F, Mele S, De Filippi R, Pinto A. Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm. International Journal of Molecular Sciences. 2025; 26(6):2732. https://doi.org/10.3390/ijms26062732
Chicago/Turabian StyleGalati, Domenico, Serena Zanotta, Fabrizia Florio, Sara Mele, Rosaria De Filippi, and Antonio Pinto. 2025. "Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm" International Journal of Molecular Sciences 26, no. 6: 2732. https://doi.org/10.3390/ijms26062732
APA StyleGalati, D., Zanotta, S., Florio, F., Mele, S., De Filippi, R., & Pinto, A. (2025). Immunotherapies Targeting CD123 and CD303: A New Frontier in Treating Blastic Plasmacytoid Dendritic Cell Neoplasm. International Journal of Molecular Sciences, 26(6), 2732. https://doi.org/10.3390/ijms26062732







