The Multifaceted Impact of Karrikin Signaling in Plants
Abstract
:1. Introduction
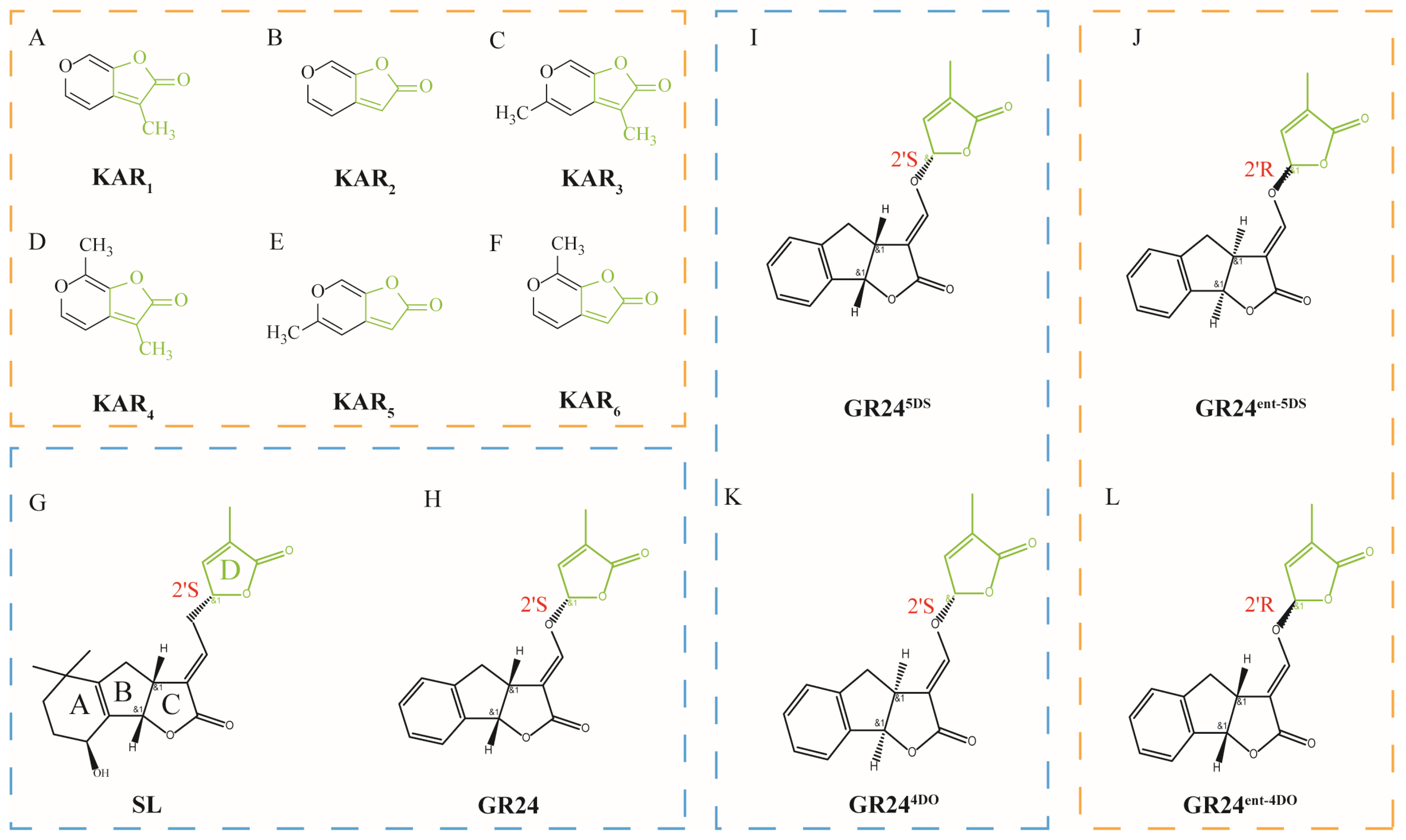
2. Structure of KARs
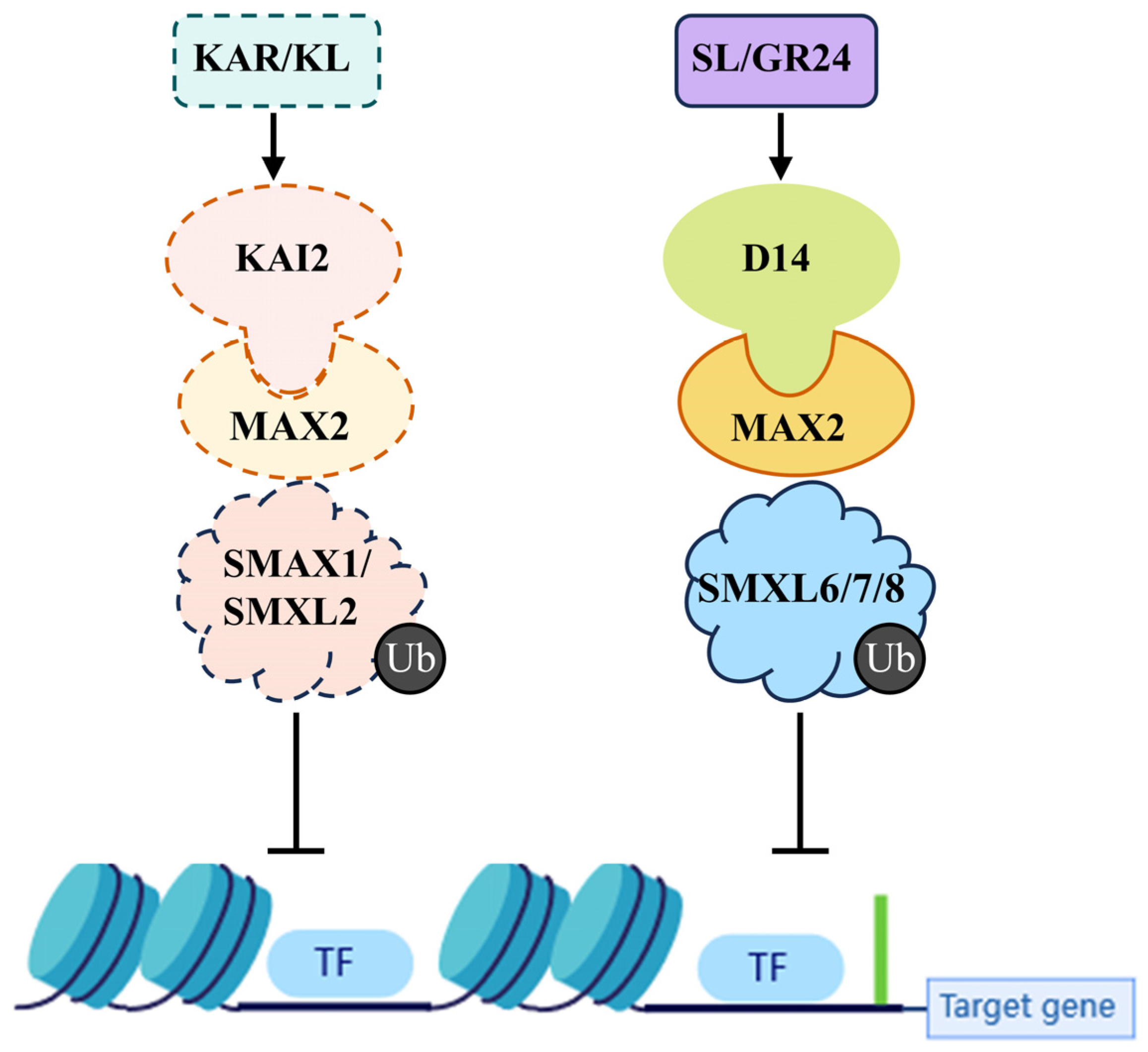
3. Signal Transduction of KARs
3.1. The KAR Receptor KAI2 Protein
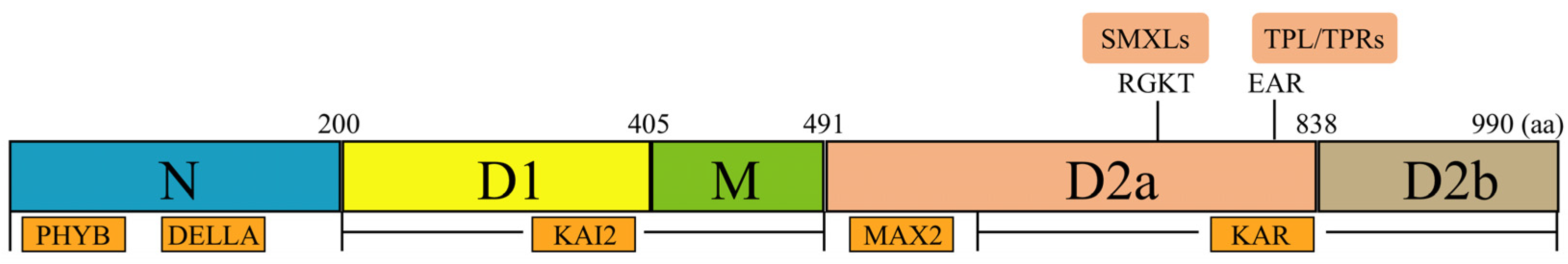
3.1.1. The Structure of KAI2 Protein
3.1.2. Functional Significance of KAI2 Hydrolysis
3.1.3. KL, the Endogenous Ligand for KAI2
3.2. The Coreceptor MAX2
3.3. The Repressor SMXL Proteins
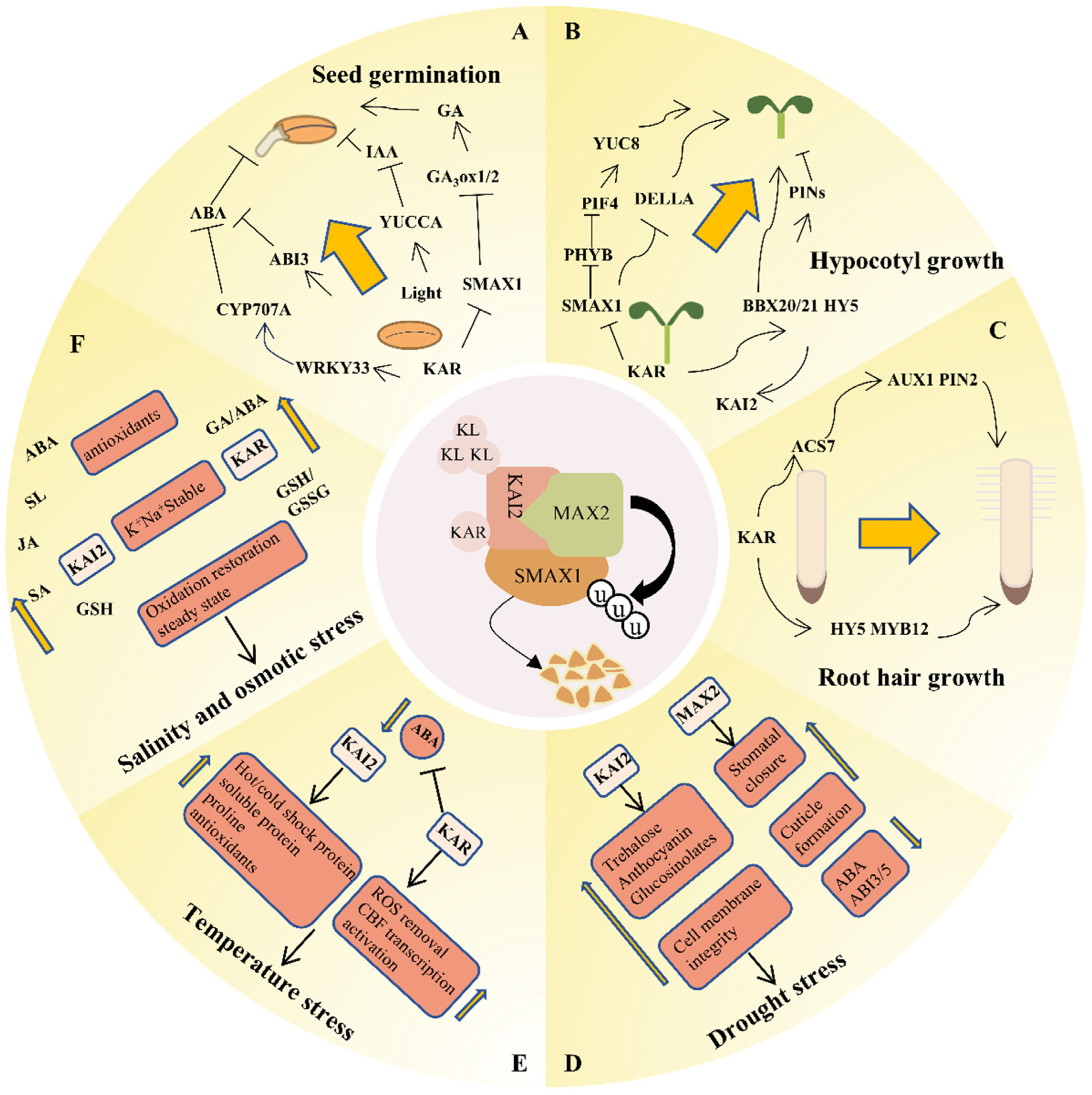
4. Functions of KARs
4.1. Seed Germination
4.2. Hypocotyl Growth and Seedling Photomorphogenesis
4.3. Regulation of Root Development
4.4. Response to Stress
4.4.1. Drought Stress
4.4.2. Temperature Stress
4.4.3. Salinity and Osmotic Stress
4.5. Other Functions
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Baldwin, I.T.; Staszak-Kozinski, L.; Davidson, R. Up in smoke: I. smoke-derived germination cues for postfire annual, Nicotiana attenuata torr. Ex. Watson. J. Chem. Ecol. 1994, 20, 2345–2371. [Google Scholar] [CrossRef]
- Lamont, B.B.; He, T.; Yan, Z. Evolutionary history of fire-stimulated resprouting, flowering, seed release and germination. Biol. Rev. Camb. Philos. Soc. 2019, 94, 903–928. [Google Scholar] [CrossRef]
- Chiwocha, S.D.S.; Dixon, K.W.; Flematti, G.R.; Ghisalberti, E.L.; Merritt, D.J.; Nelson, D.C.; Riseborough, J.A.M.; Smith, S.M.; Stevens, J.C. Karrikins: A new family of plant growth regulators in smoke. Plant Sci. Int. J. Exp. Plant Biol. 2009, 177, 252–256. [Google Scholar] [CrossRef]
- Dixon, K.W.; Merritt, D.J.; Flematti, G.R.; Ghisalberti, E.L. Karrikinolide—A phytoreactive compound derived from smoke with applications in horticulture, ecological restoration and agriculture. Acta Hortic. 2009, 813, 155–170. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. A compound from smoke that promotes seed germination. Science 2004, 305, 977. [Google Scholar] [CrossRef]
- Flematti, G.R.; Goddard-Borger, E.D.; Merritt, D.J.; Ghisalberti, E.L.; Trengove, R.D. Preparation of 2H-furo[2,3-c]pyran-2-one derivatives and evaluation of their germination-promoting activity. J. Agric. Food Chem. 2007, 55, 2189–2194. [Google Scholar] [CrossRef]
- Flematti, G.R.; Ghisalberti, E.L.; Dixon, K.W.; Trengove, R.D. Identification of alkyl substituted 2H-furo[2,3-c] pyran-2-ones as germination stimulants present in smoke. J. Agric. Food Chem. 2009, 57, 9475–9480. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Riseborough, J.-A.; Flematti, G.R.; Stevens, J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol. 2009, 149, 863–873. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Dev. Camb. Engl. 2012, 139, 1285–1295. [Google Scholar] [CrossRef]
- Kagiyama, M.; Hirano, Y.; Mori, T.; Kim, S.; Kyozuka, J.; Seto, Y.; Yamaguchi, S.; Hakoshima, T. Structures of D 14 and D 14 L in the strigolactone and karrikin signaling pathways. Genes Cells 2013, 18, 147–160. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Z.; La Clair, J.J.; Chory, J.; Noel, J.P. Smoke-derived karrikin perception by the α/β-hydrolase KAI2 from Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 8284–8289. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Q.; Yu, H.; Ma, H.; Li, X.; Yang, J.; Chu, J.; Xie, Q.; Wang, Y.; Smith, S.M.; et al. Strigolactone and karrikin signaling pathways elicit ubiquitination and proteolysis of SMXL2 to regulate hypocotyl elongation in Arabidopsis. Plant Cell 2020, 32, 2251–2270. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C. Karrikin perception and signalling. New Phytol. 2022, 237, 1525–1541. [Google Scholar] [CrossRef]
- Varshney, K.; Gutjahr, C. KAI2 can do: Karrikin receptor function in plant development and response to abiotic and biotic factors. Plant Cell Physiol. 2023, 64, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Melville, K.T.; Waters, M.T. Karrikin signalling: Impacts on plant development and abiotic stress tolerance. J. Exp. Bot. 2024, 75, 1174–1186. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Scaffidi, A.; Sun, Y.K.; Flematti, G.R.; Smith, S.M. The karrikin response system of Arabidopsis. Plant J. Cell Mol. Biol. 2014, 79, 623–631. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Zwanenburg, B. Tentative molecular mechanism for germination stimulation of Striga and Orobanche seeds by strigol and its synthetic analogs. J. Agric. Food Chem. 1992, 40, 1066–1070. [Google Scholar] [CrossRef]
- Scaffidi, A.; Waters, M.T.; Sun, Y.K.; Skelton, B.W.; Dixon, K.W.; Ghisalberti, E.L.; Flematti, G.R.; Smith, S.M. Strigolactone hormones and their stereoisomers signal through two related receptor proteins to induce different physiological responses in Arabidopsis. Plant Physiol. 2014, 165, 1221–1232. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Pospíšil, T.; Ćavar Zeljković, S. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef]
- Flematti, G.R.; Scaffidi, A.; Waters, M.T.; Smith, S.M. Stereospecificity in strigolactone biosynthesis and perception. Planta 2016, 243, 1361–1373. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S.P. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Machin, D.C.; Hamon-Josse, M.; Bennett, T. Fellowship of the rings: A saga of strigolactones and other small signals. New Phytol. 2020, 225, 621–636. [Google Scholar] [CrossRef]
- Yoneyama, K. Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 2020, 45, 45–53. [Google Scholar] [CrossRef]
- Nomura, T.; Seto, Y.; Kyozuka, J. Unveiling the complexity of strigolactones: Exploring structural diversity, biosynthesis pathways, and signaling mechanisms. J. Exp. Bot. 2024, 75, 1134–1147. [Google Scholar] [CrossRef]
- Guercio, A.M.; Torabi, S.; Cornu, D.; Dalmais, M.; Bendahmane, A.; Le Signor, C.; Pillot, J.-P.; Le Bris, P.; Boyer, F.-D.; Rameau, C.; et al. Structural and functional analyses explain pea KAI2 receptor diversity and reveal stereoselective catalysis during signal perception. Commun. Biol. 2022, 5, 126. [Google Scholar] [CrossRef]
- Martinez, S.E.; Conn, C.E.; Guercio, A.M.; Sepulveda, C.; Fiscus, C.J.; Koenig, D.; Shabek, N.; Nelson, D.C. A karrikin insensitive2 paralog in lettuce mediates highly sensitive germination responses to karrikinolide. Plant Physiol. 2022, 190, 1440–1456. [Google Scholar] [CrossRef]
- Arellano-Saab, A.; Skarina, T.; Xu, Z.; McErlean, C.S.P.; Savchenko, A.; Lumba, S.; Stogios, P.J.; McCourt, P. Structural analysis of a hormone-bound Striga strigolactone receptor. Nat. Plants 2023, 9, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Kodama, K.; Xie, X.; Kyozuka, J. The D14 and KAI2 orthologs of gymnosperms sense strigolactones and KL mimics, respectively, and the signals are transduced to control downstream genes. Plant Cell Physiol. 2023, 64, 1057–1065. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281. [Google Scholar] [CrossRef]
- Sun, X.-D.; Ni, M. Hyposensitive to light, an alpha/beta fold protein, acts downstream of elongated hypocotyl 5 to regulate seedling de-etiolation. Mol. Plant 2011, 4, 116–126. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Zhou, X.E.; Wu, Z.-S.; Yi, W.; Xu, Y.; Li, S.; Xu, T.-H.; Liu, Y.; Chen, R.-Z.; Kovach, A.; et al. Crystal structures of two phytohormone signal-transducing α/β hydrolases: Karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 2013, 23, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.K.; Yao, J.; Scaffidi, A.; Melville, K.T.; Davies, S.F.; Bond, C.S.; Smith, S.M.; Flematti, G.R.; Waters, M.T. Divergent receptor proteins confer responses to different karrikins in two ephemeral weeds. Nat. Commun. 2020, 11, 1264. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miyakawa, T.; Nosaki, S.; Nakamura, A.; Lyu, Y.; Nakamura, H.; Ohto, U.; Ishida, H.; Shimizu, T.; Asami, T.; et al. Structural analysis of HTL and D14 proteins reveals the basis for ligand selectivity in Striga. Nat. Commun. 2018, 9, 3947. [Google Scholar] [CrossRef]
- Bürger, M.; Honda, K.; Kondoh, Y.; Hong, S.; Watanabe, N.; Osada, H.; Chory, J. Crystal structure of Arabidopsis DWARF14-like2 (DLK2) reveals a distinct substrate binding pocket architecture. Plant Direct 2022, 6, e446. [Google Scholar] [CrossRef]
- Takei, S.; Uchiyama, Y.; Bürger, M.; Suzuki, T.; Okabe, S.; Chory, J.; Seto, Y. A divergent clade KAI2 protein in the root parasitic plant Orobanche minor is a highly sensitive strigolactone receptor and is involved in the perception of sesquiterpene lactones. Plant Cell Physiol. 2023, 64, 996–1007. [Google Scholar] [CrossRef]
- Stirling, S.A.; Guercio, A.M.; Patrick, R.M.; Huang, X.-Q.; Bergman, M.E.; Dwivedi, V.; Kortbeek, R.W.J.; Liu, Y.-K.; Sun, F.; Tao, W.A.; et al. Volatile communication in plants relies on a KAI2-mediated signaling pathway. Science 2024, 383, 1318–1325. [Google Scholar] [CrossRef]
- Waters, M.T.; Scaffidi, A.; Moulin, S.L.Y.; Sun, Y.K.; Flematti, G.R.; Smith, S.M. A Selaginella moellendorffii Ortholog of karrikin insensitive2 functions in Arabidopsis development but cannot mediate responses to karrikins or strigolactones. Plant Cell 2015, 27, 1925–1944. [Google Scholar] [CrossRef]
- de Saint Germain, A.; Clavé, G.; Badet-Denisot, M.-A.; Pillot, J.-P.; Cornu, D.; Le Caer, J.-P.; Burger, M.; Pelissier, F.; Retailleau, P.; Turnbull, C.; et al. A histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016, 12, 787–794. [Google Scholar] [CrossRef]
- Seto, Y.; Yasui, R.; Kameoka, H.; Tamiru, M.; Cao, M.; Terauchi, R.; Sakurada, A.; Hirano, R.; Kisugi, T.; Hanada, A.; et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Shabek, N.; Ticchiarelli, F.; Mao, H.; Hinds, T.R.; Leyser, O.; Zheng, N. Structural plasticity of D3-D14 ubiquitin ligase in strigolactone signalling. Nature 2018, 563, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Bürger, M.; Mashiguchi, K.; Lee, H.J.; Nakano, M.; Takemoto, K.; Seto, Y.; Yamaguchi, S.; Chory, J. Structural basis of karrikin and non-natural strigolactone perception in Physcomitrella patens. Cell Rep. 2019, 26, 855–865.e5. [Google Scholar] [CrossRef]
- Yao, J.; Waters, M.T. Perception of karrikins by plants: A continuing enigma. J. Exp. Bot. 2020, 71, 1774–1781. [Google Scholar] [CrossRef]
- Chen, J.; Shukla, D. Multiple modes of substrate hydrolysis-induced covalent modification of strigolactones receptors. bioRxiv. 2022. [Google Scholar] [CrossRef]
- Guercio, A.M.; Gilio, A.K.; Pawlak, J.; Shabek, N. Structural insights into rice KAI2 receptor provide functional implications for perception and signal transduction. J. Biol. Chem. 2024, 300, 107593. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miyakawa, T.; Nakamura, H.; Nakamura, A.; Imamura, Y.; Asami, T.; Tanokura, M. Structural basis of unique ligand specificity of KAI2-like protein from parasitic weed Striga hermonthica. Sci. Rep. 2016, 6, 31386. [Google Scholar] [CrossRef]
- Lee, I.; Kim, K.; Lee, S.; Lee, S.; Hwang, E.; Shin, K.; Kim, D.; Choi, J.; Choi, H.; Cha, J.S.; et al. A missense allele of karrikin-insensitive2 impairs ligand-binding and downstream signaling in Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 3609–3623. [Google Scholar] [CrossRef]
- Conn, C.E.; Nelson, D.C. Evidence that karrikin-insensitive2 (KAI2) receptors may perceive an unknown signal that is not karrikin or strigolactone. Front. Plant Sci. 2015, 6, 1219. [Google Scholar] [CrossRef]
- Flematti, G.R.; Waters, M.T.; Scaffidi, A.; Merritt, D.J.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikin and cyanohydrin smoke signals provide clues to new endogenous plant signaling compounds. Mol. Plant 2013, 6, 29–37. [Google Scholar] [CrossRef]
- Sun, Y.K.; Flematti, G.R.; Smith, S.M.; Waters, M.T. Reporter gene-facilitated detection of compounds in Arabidopsis leaf extracts that activate the karrikin signaling pathway. Front. Plant Sci. 2016, 7, 1799. [Google Scholar] [CrossRef] [PubMed]
- Khosla, A.; Morffy, N.; Li, Q.; Faure, L.; Chang, S.H.; Yao, J.; Zheng, J.; Cai, M.L.; Stanga, J.; Flematti, G.R.; et al. Structure-function analysis of SMAX1 reveals domains that mediate its karrikin-induced proteolysis and interaction with the receptor KAI2. Plant Cell 2020, 32, 2639–2659. [Google Scholar] [CrossRef]
- Yao, J.; Mashiguchi, K.; Scaffidi, A.; Akatsu, T.; Melville, K.T.; Morita, R.; Morimoto, Y.; Smith, S.M.; Seto, Y.; Flematti, G.R.; et al. An allelic series at the karrikin insensitive2 locus of Arabidopsis thaliana decouples ligand hydrolysis and receptor degradation from downstream signalling. Plant J. Cell Mol. Biol. 2018, 96, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Scaffidi, A.; Meng, Y.; Melville, K.T.; Komatsu, A.; Khosla, A.; Nelson, D.C.; Kyozuka, J.; Flematti, G.R.; Waters, M.T. Desmethyl butenolides are optimal ligands for karrikin receptor proteins. New Phytol. 2021, 230, 1003–1016. [Google Scholar] [CrossRef]
- Sepulveda, C.; Guzmán, M.A.; Li, Q.; Villaécija-Aguilar, J.A.; Martinez, S.E.; Kamran, M.; Khosla, A.; Liu, W.; Gendron, J.M.; Gutjahr, C.; et al. Karrikin up-regulated F-box 1 (KUF1) imposes negative feedback regulation of karrikin and KAI2 ligand metabolism in Arabidopsis thaliana. Proc. Natl. Acad. Sci USA 2022, 119, e2112820119. [Google Scholar] [CrossRef]
- Nelson, D.C.; Scaffidi, A.; Dun, E.A.; Waters, M.T.; Flematti, G.R.; Dixon, K.W.; Beveridge, C.A.; Ghisalberti, E.L.; Smith, S.M. F-box protein MAX2 has dual roles in karrikin and strigolactone signaling in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 8897–8902. [Google Scholar] [CrossRef] [PubMed]
- Stirnberg, P.; Furner, I.J.; Ottoline Leyser, H.M. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. Cell Mol. Biol. 2007, 50, 80–94. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of plant hormone response pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef]
- Xu, G.; Ma, H.; Nei, M.; Kong, H. Evolution of F-box genes in plants: Different modes of sequence divergence and their relationships with functional diversification. Proc. Natl. Acad. Sci. USA 2009, 106, 835–840. [Google Scholar] [CrossRef]
- Temmerman, A.; Marquez-Garcia, B.; Depuydt, S.; Bruznican, S.; De Cuyper, C.; De Keyser, A.; Boyer, F.-D.; Vereecke, D.; Struk, S.; Goormachtig, S. MAX2-dependent competence for callus formation and shoot regeneration from Arabidopsis thaliana root explants. J. Exp. Bot. 2022, 73, 6272–6291. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, F.; Yang, X.; Li, W.; Chen, S.; Yue, X.; Jia, Q.; Sun, X. The MAX2-KAI2 module promotes salicylic acid-mediated immune responses in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 1566–1584. [Google Scholar] [CrossRef] [PubMed]
- Tal, L.; Palayam, M.; Ron, M.; Young, A.; Britt, A.; Shabek, N. A conformational switch in the SCF-D3/MAX2 ubiquitin ligase facilitates strigolactone signalling. Nat. Plants 2022, 8, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Tal, L.; Guercio, A.M.; Varshney, K.; Young, A.; Gutjahr, C.; Shabek, N. C-terminal conformational changes in SCF-D3/MAX2 ubiquitin ligase are required for KAI2-mediated signaling. New Phytol. 2023, 239, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Stanga, J.P.; Smith, S.M.; Briggs, W.R.; Nelson, D.C. Suppressor of more axillary growth2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 2013, 163, 318–330. [Google Scholar] [CrossRef]
- Moturu, T.R.; Thula, S.; Singh, R.K.; Nodzynski, T.; Vareková, R.S.; Friml, J.; Simon, S. Molecular evolution and diversification of the SMXL gene family. J. Exp. Bot. 2018, 69, 2367–2378. [Google Scholar] [CrossRef]
- Walker, C.H.; Siu-Ting, K.; Taylor, A.; O’Connell, M.J.; Bennett, T. Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 2019, 17, 70. [Google Scholar] [CrossRef]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, X.; Liu, S.; Liu, Y.; Li, H.; Wang, Z.; Kan, J.; Yang, Q.; Li, X. Comprehensive evolutionary analysis of the SMXL gene family in rosaceae: Further insights into its origin, expansion, diversification, and role in regulating pear branching. Int. J. Mol. Sci. 2024, 25, 2971. [Google Scholar] [CrossRef]
- Soundappan, I.; Bennett, T.; Morffy, N.; Liang, Y.; Stanga, J.P.; Abbas, A.; Leyser, O.; Nelson, D.C. SMAX1-like/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 2015, 27, 3143–3159. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, H.G.; Choi, H.-Y.; Lee, S.; Park, C.-M. Complexity of SMAX1 signaling during seedling establishment. Trends Plant Sci. 2023, 28, 902–912. [Google Scholar] [CrossRef]
- Xu, P.; Hu, J.; Chen, H.; Cai, W. SMAX1 interacts with DELLA protein to inhibit seed germination under weak light conditions via gibberellin biosynthesis in Arabidopsis. Cell Rep. 2023, 42, 112740. [Google Scholar] [CrossRef]
- Chang, W.; Qiao, Q.; Li, Q.; Li, X.; Li, Y.; Huang, X.; Wang, Y.; Li, J.; Wang, B.; Wang, L. Non-transcriptional regulatory activity of SMAX1 and SMXL2 mediates karrikin-regulated seedling response to red light in Arabidopsis. Mol. Plant 2024, 17, 1054–1072. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Komatsu, A.; Shimazaki, S.; Naramoto, S.; Inoue, K.; Xie, X.; Ishizaki, K.; Kohchi, T.; Kyozuka, J. Major components of the karrikin insensitive2-dependent signaling pathway are conserved in the liverwort Marchantia polymorpha. Plant Cell 2021, 33, 2395–2411. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.F.; Contaldi, F.; Lo Celso, F.; Baratto, C.M.; Grossi-de-Sa, M.F.; Barone, G.; Ferrante, A.; Martinelli, F. Identification and expression profile of the SMAX/SMXL family genes in chickpea and lentil provide important players of biotechnological interest involved in plant branching. Planta 2023, 259, 1. [Google Scholar] [CrossRef]
- Guillory, A.; Lopez-Obando, M.; Bouchenine, K.; Le Bris, P.; Lécureuil, A.; Pillot, J.-P.; Steinmetz, V.; Boyer, F.-D.; Rameau, C.; de Saint Germain, A.; et al. Suppressor of MAX2 1-like (SMXL) homologs are MAX2-dependent repressors of Physcomitrium patens growth. Plant Cell 2024, 36, 1655–1672. [Google Scholar] [CrossRef]
- Li, Q.; Martín-Fontecha, E.S.; Khosla, A.; White, A.R.F.; Chang, S.; Cubas, P.; Nelson, D.C. The strigolactone receptor D14 targets SMAX1 for degradation in response to GR24 treatment and osmotic stress. Plant Commun. 2022, 3, 100303. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, A.; Guillory, A.; Bonhomme, S.; Goormachtig, S.; Struk, S. Masks start to drop: Suppressor of MAX2 1-like proteins reveal their many faces. Front. Plant Sci. 2022, 13, 887232. [Google Scholar] [CrossRef]
- Villaécija-Aguilar, J.A.; Hamon-Josse, M.; Carbonnel, S.; Kretschmar, A.; Schmidt, C.; Dawid, C.; Bennett, T.; Gutjahr, C. SMAX1/SMXL2 regulate root and root hair development downstream of KAI2-mediated signalling in Arabidopsis. PLoS Genet. 2019, 15, e1008327. [Google Scholar] [CrossRef]
- Bursch, K.; Niemann, E.T.; Nelson, D.C.; Johansson, H. Karrikins control seedling photomorphogenesis and anthocyanin biosynthesis through a HY5-BBX transcriptional module. Plant J. Cell Mol. Biol. 2021, 107, 1346–1362. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Guerringue, Y.; Matthus, E.; Jamieson, F.J.C.; Davies, J.M. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2019, 98, 607–621. [Google Scholar] [CrossRef]
- Carbonnel, S.; Das, D.; Varshney, K.; Kolodziej, M.C.; Villaécija-Aguilar, J.A.; Gutjahr, C. The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 21757–21765. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Hong, K.; Zeng, L.; Wang, L.; Kang, S.; Qu, M.; Dai, J.; Zou, L.; Zhu, L.; Tang, Z.; et al. Karrikin signaling acts parallel to and additively with strigolactone signaling to regulate rice mesocotyl elongation in darkness. Plant Cell 2020, 32, 2780–2805. [Google Scholar] [CrossRef]
- Ho-Plágaro, T.; Morcillo, R.J.L.; Tamayo-Navarrete, M.I.; Huertas, R.; Molinero-Rosales, N.; López-Ráez, J.A.; Macho, A.P.; García-Garrido, J.M. DLK2 regulates arbuscule hyphal branching during arbuscular mycorrhizal symbiosis. New Phytol. 2021, 229, 548–562. [Google Scholar] [CrossRef]
- Hamon-Josse, M.; Villaécija-Aguilar, J.A.; Ljung, K.; Leyser, O.; Gutjahr, C.; Bennett, T. KAI2 regulates seedling development by mediating light-induced remodelling of auxin transport. New Phytol. 2022, 235, 126–140. [Google Scholar] [CrossRef]
- Villaécija-Aguilar, J.A.; Körösy, C.; Maisch, L.; Hamon-Josse, M.; Petrich, A.; Magosch, S.; Chapman, P.; Bennett, T.; Gutjahr, C. KAI2 promotes Arabidopsis root hair elongation at low external phosphate by controlling local accumulation of AUX1 and PIN2. Curr. Biol. CB 2022, 32, 228–236.e3. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Liang, X.; Tian, H.; Watanabe, Y.; Nguyen, K.H.; Tran, C.D.; Abdelrahman, M.; Xu, K.; Mostofa, M.G.; Ha, C.V.; et al. Suppressor of MAX2 1 (SMAX1) and SMAX1-like2 (SMXL2) negatively regulate drought resistance in Arabidopsis thaliana. Plant Cell Physiol. 2023, 63, 1900–1913. [Google Scholar] [CrossRef]
- Banerjee, A.; Tripathi, D.K.; Roychoudhury, A. The karrikin “calisthenics”: Can compounds derived from smoke help in stress tolerance? Physiol. Plant. 2019, 165, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lian, Y.; Wang, C. Comparing and contrasting the multiple roles of butenolide plant growth regulators: Strigolactones and karrikins in plant development and adaptation to abiotic stresses. Int. J. Mol. Sci. 2019, 20, 6270. [Google Scholar] [CrossRef]
- Ullah, G.; Ibrahim, M.; Nawaz, G.; Khatoon, A.; Jamil, M.; Rehman, S.U.; Ali, E.A.; Tariq, A. Plant-derived smoke mitigates the inhibitory effects of the auxin inhibitor 2,3,5-Triiodo Benzoic Acid (TIBA) by enhancing root architecture and biochemical parameters in maize. Plants 2023, 12, 2604. [Google Scholar] [CrossRef]
- Kępczyński, J.; Kępczyńska, E. Plant-derived smoke and karrikin 1 in seed priming and seed biotechnology. Plants 2023, 12, 2378. [Google Scholar] [CrossRef]
- Bunsick, M.; Toh, S.; Wong, C.; Xu, Z.; Ly, G.; McErlean, C.S.P.; Pescetto, G.; Nemrish, K.E.; Sung, P.; Li, J.D.; et al. SMAX1-dependent seed germination bypasses GA signalling in Arabidopsis and Striga. Nat. Plants 2020, 6, 646–652. [Google Scholar] [CrossRef]
- Brun, G.; Thoiron, S.; Braem, L.; Pouvreau, J.-B.; Montiel, G.; Lechat, M.-M.; Simier, P.; Gevaert, K.; Goormachtig, S.; Delavault, P. CYP707As are effectors of karrikin and strigolactone signalling pathways in Arabidopsis thaliana and parasitic plants. Plant Cell Environ. 2019, 42, 2612–2626. [Google Scholar] [CrossRef]
- Bunsick, M.; Lumba, S. ShHTL7 requires functional brassinosteroid signaling to initiate GA-independent germination. Plant Signal. Behav. 2021, 16, 1855845. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Kitaoka, K.; Suzuki, T.; Kuruma, M.; Hagihara, S.; Yamaguchi, S.; Fukui, K.; Seto, Y. Desmethyl type germinone, a specific agonist for the HTL/KAI2 receptor, induces the Arabidopsis seed germination in a gibberellin-independent manner. Biochem. Biophys. Res. Commun. 2023, 649, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.C.; Flematti, G.R.; Riseborough, J.-A.; Ghisalberti, E.L.; Dixon, K.W.; Smith, S.M. Karrikins enhance light responses during germination and seedling development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 7095–7100. [Google Scholar] [CrossRef]
- Gupta, S.; Plačková, L.; Kulkarni, M.G.; Doležal, K.; Van Staden, J. Role of smoke stimulatory and inhibitory biomolecules in phytochrome-regulated seed germination of Lactuca sativa. Plant Physiol. 2019, 181, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Nam, B.E.; Park, C.-M. Environmentally adaptive reshaping of plant photomorphogenesis by karrikin and strigolactone signaling. J. Integr. Plant Biol. 2023, 66, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Bączek-Kwinta, R. An interplay of light and smoke compounds in photoblastic seeds. Plants 2022, 11, 1773. [Google Scholar] [CrossRef]
- Struk, S.; De Cuyper, C.; Jacobs, A.; Braem, L.; Walton, A.; De Keyser, A.; Depuydt, S.; Vu, L.D.; De Smet, I.; Boyer, F.-D.; et al. Unraveling the MAX2 protein network in Arabidopsis thaliana: Identification of the protein phosphatase PAPP5 as a novel MAX2 interactor. Mol. Cell. Proteomics MCP 2021, 20, 100040. [Google Scholar] [CrossRef]
- Ruduś, I.; Cembrowska-Lech, D.; Jaworska, A.; Kępczyński, J. Involvement of ethylene biosynthesis and perception during germination of dormant Avena fatua L. caryopses induced by KAR1 or GA3. Planta 2019, 249, 719–738. [Google Scholar] [CrossRef]
- Sami, A.; Riaz, M.W.; Zhou, X.; Zhu, Z.; Zhou, K. Alleviating dormancy in Brassica oleracea seeds using NO and KAR1 with ethylene biosynthetic pathway, ROS and antioxidant enzymes modifications. BMC Plant Biol. 2019, 19, 577. [Google Scholar] [CrossRef]
- Stanga, J.P.; Morffy, N.; Nelson, D.C. Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta 2016, 243, 1397–1406. [Google Scholar] [CrossRef]
- Lee, I.; Choi, S.; Lee, S.; Soh, M.-S. KAI2-KL signaling intersects with light-signaling for photomorphogenesis. Plant Signal. Behav. 2019, 14, e1588660. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Kim, J.Y.; Park, C.-M. SMAX1 potentiates phytochrome b-mediated hypocotyl thermomorphogenesis. Plant Cell 2022, 34, 2671–2687. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Jinbo, H.; Cai, W. Karrikin signaling regulates hypocotyl shade avoidance response by modulating auxin homeostasis in Arabidopsis. New Phytol. 2022, 236, 1748–1761. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, Y.-J.; Lee, J.-H.; Park, C.-M. SMAX1 integrates karrikin and light signals into GA-mediated hypocotyl growth during seedling establishment. Plant Cell Physiol. 2022, 63, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Swarbreck, S.M.; Mohammad-Sidik, A.; Davies, J.M. Common components of the strigolactone and karrikin signaling pathways suppress root branching in Arabidopsis. Plant Physiol. 2020, 184, 18–22. [Google Scholar] [CrossRef]
- Struk, S.; Braem, L.; Matthys, C.; Walton, A.; Vangheluwe, N.; Van Praet, S.; Jiang, L.; Baster, P.; De Cuyper, C.; Boyer, F.-D.; et al. Transcriptional analysis in the Arabidopsis roots reveals new regulators that link rac-GR24 treatment with changes in flavonol accumulation, root hair elongation and lateral root density. Plant Cell Physiol. 2022, 63, 104–119. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rehman, N.U.; Yu, S.; Zhou, Y.; Haq, B.U.; Wang, J.; Li, P.; Zeng, Z.; Zhao, J. GmMAX2-D14 and -KAI interaction-mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. Cell Mol. Biol. 2020, 101, 334–351. [Google Scholar] [CrossRef]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H.; et al. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Ha, C.V.; Watanabe, Y.; Osakabe, Y.; Leyva-González, M.A.; Sato, M.; Toyooka, K.; Voges, L.; et al. The karrikin receptor KAI2 promotes drought resistance in Arabidopsis thaliana. PLoS Genet. 2017, 13, e1007076. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nguyen, K.H.; Chu, H.D.; Watanabe, Y.; Osakabe, Y.; Sato, M.; Toyooka, K.; Seo, M.; Tian, L.; Tian, C.; et al. Comparative functional analyses of DWARF14 and karrikin insensitive2 in drought adaptation of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2020, 103, 111–127. [Google Scholar] [CrossRef]
- Li, W.; Gupta, A.; Tian, H.; Nguyen, K.H.; Tran, C.D.; Watanabe, Y.; Tian, C.; Li, K.; Yang, Y.; Guo, J.; et al. Different strategies of strigolactone and karrikin signals in regulating the resistance of Arabidopsis thaliana to water-deficit stress. Plant Signal. Behav. 2020, 15, 1789321. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shan, Q.; Ding, E.; Gu, T.; Gong, B. Karrikin increases tomato cold tolerance via strigolactone and the abscisic acid signaling network. Plant Sci. 2023, 332, 111720. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Mostofa, M.G.; Tran, C.D.; El-Sayed, M.; Li, W.; Sulieman, S.; Tanaka, M.; Seki, M.; Tran, L.-S.P. The karrikin receptor karrikin insensitive2 positively regulates heat stress tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2023, 63, 1914–1926. [Google Scholar] [CrossRef]
- Shah, F.A.; Ni, J.; Yao, Y.; Hu, H.; Wei, R.; Wu, L. Overexpression of karrikins receptor gene Sapium sebiferum KAI2 promotes the cold stress tolerance via regulating the redox homeostasis in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 657960. [Google Scholar] [CrossRef]
- Wang, L.; Waters, M.T.; Smith, S.M. Karrikin-KAI2 signalling provides Arabidopsis seeds with tolerance to abiotic stress and inhibits germination under conditions unfavourable to seedling establishment. New Phytol. 2018, 219, 605–618. [Google Scholar] [CrossRef]
- Shah, F.A.; Ni, J.; Tang, C.; Chen, X.; Kan, W.; Wu, L. Karrikinolide alleviates salt stress in wheat by regulating the redox and K+/Na+ homeostasis. Plant Physiol. Biochem. PPB 2021, 167, 921–933. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Abdelrahman, M.; Rahman, M.M.; Tran, C.D.; Nguyen, K.H.; Watanabe, Y.; Itouga, M.; Li, W.; Wang, Z.; Mochida, K.; et al. Karrikin receptor KAI2 coordinates salt tolerance mechanisms in Arabidopsis thaliana. Plant Cell Physiol. 2023, 63, 1927–1942. [Google Scholar] [CrossRef]
- Shah, F.A.; Wei, X.; Wang, Q.; Liu, W.; Wang, D.; Yao, Y.; Hu, H.; Chen, X.; Huang, S.; Hou, J.; et al. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Ha, C.V.; Watanabe, Y.; Tran, L.-S.P. Crosstalk between the cytokinin and MAX2 signaling pathways in growth and callus formation of Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2019, 511, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Baldwin, G.; Yang, C.; Lu, R.; Meng, S.; Huang, J.; Wang, M.; Baldwin, I.T. Field-work reveals a novel function for MAX2 in a native tobacco’s high-light adaptions. Plant Cell Environ. 2023, 47, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, A.; Kodama, K.; Mizuno, Y.; Fujibayashi, M.; Naramoto, S.; Kyozuka, J. Control of vegetative reproduction in Marchantia polymorpha by the KAI2-ligand signaling pathway. Curr. Biol. CB 2023, 33, 1196–1210.e4. [Google Scholar] [CrossRef]
- Oláh, D.; Molnár, Á.; Soós, V.; Kolbert, Z. Nitric oxide is associated with strigolactone and karrikin signal transduction in Arabidopsis roots. Plant Signal. Behav. 2021, 16, 1868148. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, Z.-X.; Sun, H.; Guo, L.-P. Smoke-isolated karrikins stimulated tanshinones biosynthesis in Salvia miltiorrhiza through endogenous nitric oxide and jasmonic acid. Molecules 2019, 24, 1229. [Google Scholar] [CrossRef]
- Shah, A.A.; Khan, W.U.; Yasin, N.A.; Akram, W.; Ahmad, A.; Abbas, M.; Ali, A.; Safdar, M.N. Butanolide alleviated cadmium stress by improving plant growth, photosynthetic parameters and antioxidant defense system of Brassica oleracea. Chemosphere 2020, 261, 127728. [Google Scholar] [CrossRef]
- Ma, B.; Zhu, J.; Huang, X. Diversification of plant suppressor of MAX2 1 (SMAX1)-like genes and genome-wide identification and characterization of cotton SMXL gene family. BMC Plant Biol. 2023, 23, 419. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, H.; He, Y.; Hao, Y.; Yan, J.; Liu, S.; Huang, X.; Yan, Z.; Zhang, D.; Ban, X.; et al. Regulatory mechanisms of strigolactone perception in rice. Cell 2024, 187, 7551–7567. [Google Scholar] [CrossRef] [PubMed]

| Species | Abiotic Stress | Mechanism of Tolerance | Reference |
|---|---|---|---|
| Arabidopsis thaliana | Drought | Reduced leaf water loss due to the increased sensitivity to ABA and enhanced detoxification ability for active oxygen | [86] |
| Arabidopsis thaliana | Drought | Cuticle thickness and ABA gene expression | [110] |
| Arabidopsis thaliana | Drought | Stomatal closure, cuticle protection, anthocyanin biosynthesis, ABA insensitivity | [111] |
| Arabidopsis thaliana | Drought | KAI2 is more efficacious in attenuating electrolyte leakage, promoting cuticle formation, and diminishing permeability compared to D14 | [112] |
| Arabidopsis thaliana | Drought | Promotes the biosynthesis of glucosinolates and trehalose | [113] |
| Arabidopsis thaliana | Temperature | SMAX1 interacts with PHYB to relieve the inhibition of PIF4 | [104] |
| Solanum lycopersicum | Temperature | Influence of ABA content | [114] |
| Arabidopsis thaliana | Temperature | Heat shock protein regulation and heat shock-dependent transcription | [115] |
| Sapium sebiferum | Temperature | Redox homeostasis, cold shock proteins | [116] |
| Arabidopsis thaliana | Osmotic | Enhanced expression of DREB2A, WRKY33, and ERF5 genes | [117] |
| Triticum aestivum | Osmotic | Maintaining the redox homeostasis and the K+/Na+ homeostasis | [118] |
| Arabidopsis thaliana | Osmotic | Redox homeostasis, antioxidant enzyme activity | [119] |
| Sapium sebiferum | Osmotic | Regulates redox homeostasis and alters ABA signaling gene expression | [120] |
| Arabidopsis thaliana | Osmotic | D14 mediates osmotic-stress-induced SMAX1 degradation | [76] |
| Species | Function | Mechanism of Tolerance | Reference |
|---|---|---|---|
| Arabidopsis thaliana | Enhance the development of lateral roots while concurrently postponing the formation of root calli | MAX2 serves a regulatory function in mediating the interaction among cytokinin, auxin, and light signals during the onset of callus formation | [60] |
| Arabidopsis thaliana | Enhance immune defense response | Dependent on salicylic acid (SA) signaling | [62] |
| Arabidopsis thaliana | Affects wounded tissue and seed development | CK and MAX2 regulate development independently and interactively | [121] |
| Nicotiana attenuata | Adaptation to high-light response | NaMAX2 regulates the antioxidant system | [122] |
| Marchantia polymorpha | Promotes gemma cup formation and gemma initiation | Controlled by the ON/OFF switch of KAI2-related signals | [123] |
| Arabidopsis thaliana | Adjusting NO signal | rac-GR24 and SLs enhance GSNOR protein levels | [124] |
| Salvia miltiorrhiza | KAR1 regulates the secondary metabolism of medicinal plants | KAR1 induces the generation of nitric oxide (NO), jasmonic acid (JA), and T-I in the hair-like root | [125] |
| Brassica oleracea | Relieve the toxicity of cadmium | The reduction of the level of MDA, EL, and H2O2 and the improvement of the antioxidant mechanism | [126] |
| Gossypium sp. | Promote stem elongation and axillary bud development | Degradation of GhSMAX1-1 and GhSMAX1-2 | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Q.; Wang, H.; Qiu, Y.; Wang, D.; Xia, Y.; Zhang, Y.; Pei, M.; Zhao, Y.; Xu, X.; Zhang, H. The Multifaceted Impact of Karrikin Signaling in Plants. Int. J. Mol. Sci. 2025, 26, 2775. https://doi.org/10.3390/ijms26062775
Deng Q, Wang H, Qiu Y, Wang D, Xia Y, Zhang Y, Pei M, Zhao Y, Xu X, Zhang H. The Multifaceted Impact of Karrikin Signaling in Plants. International Journal of Molecular Sciences. 2025; 26(6):2775. https://doi.org/10.3390/ijms26062775
Chicago/Turabian StyleDeng, Qilin, Hongyang Wang, Yanhong Qiu, Dexin Wang, Yang Xia, Yumeng Zhang, Manying Pei, Yinling Zhao, Xiulan Xu, and Haijun Zhang. 2025. "The Multifaceted Impact of Karrikin Signaling in Plants" International Journal of Molecular Sciences 26, no. 6: 2775. https://doi.org/10.3390/ijms26062775
APA StyleDeng, Q., Wang, H., Qiu, Y., Wang, D., Xia, Y., Zhang, Y., Pei, M., Zhao, Y., Xu, X., & Zhang, H. (2025). The Multifaceted Impact of Karrikin Signaling in Plants. International Journal of Molecular Sciences, 26(6), 2775. https://doi.org/10.3390/ijms26062775






