Melatonin’s Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective
Abstract
:1. Introduction
2. Melatonin Secretion and Synthesis
2.1. Pineal Gland Interacts with SCN to Produce Melatonin
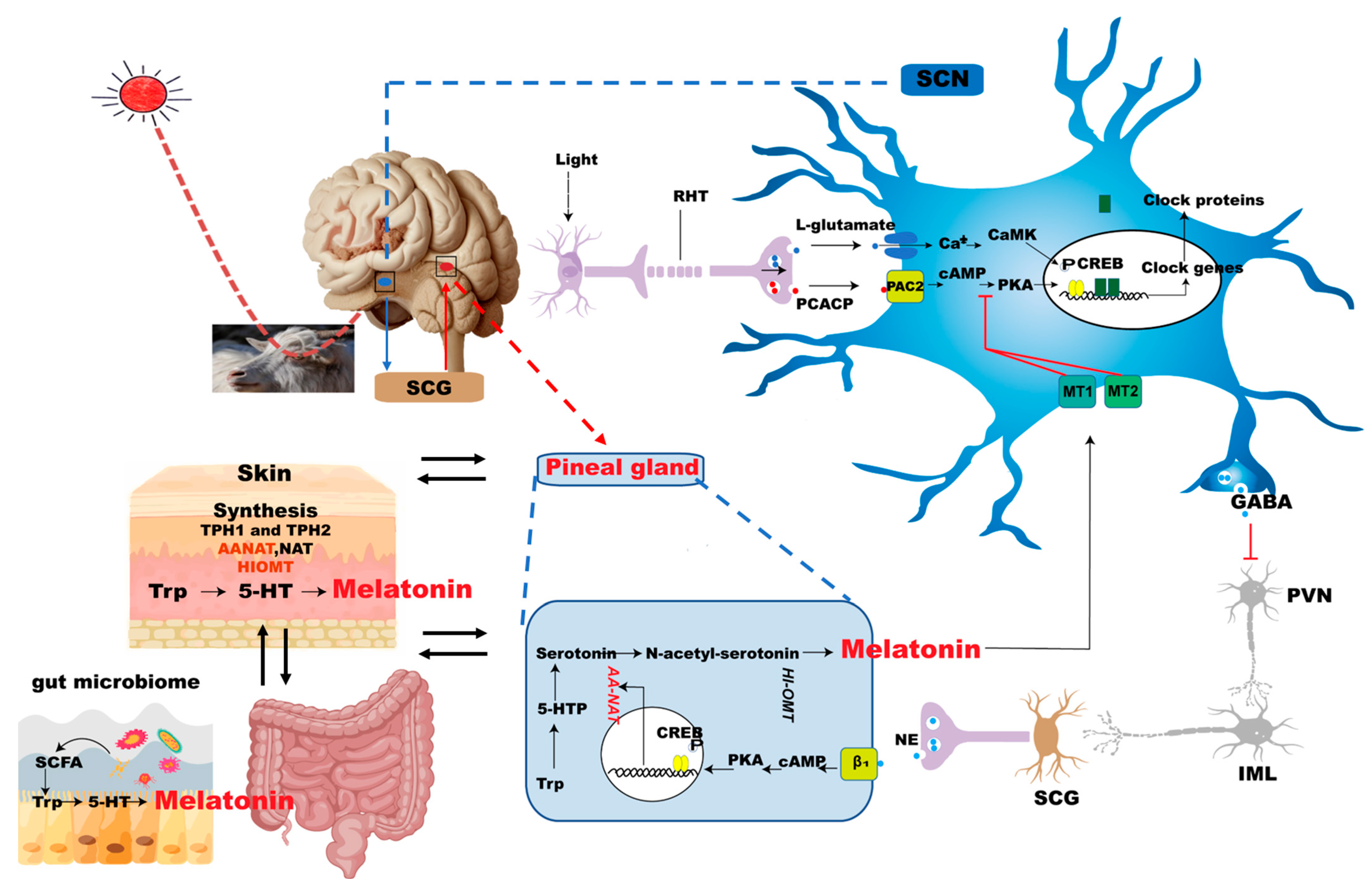
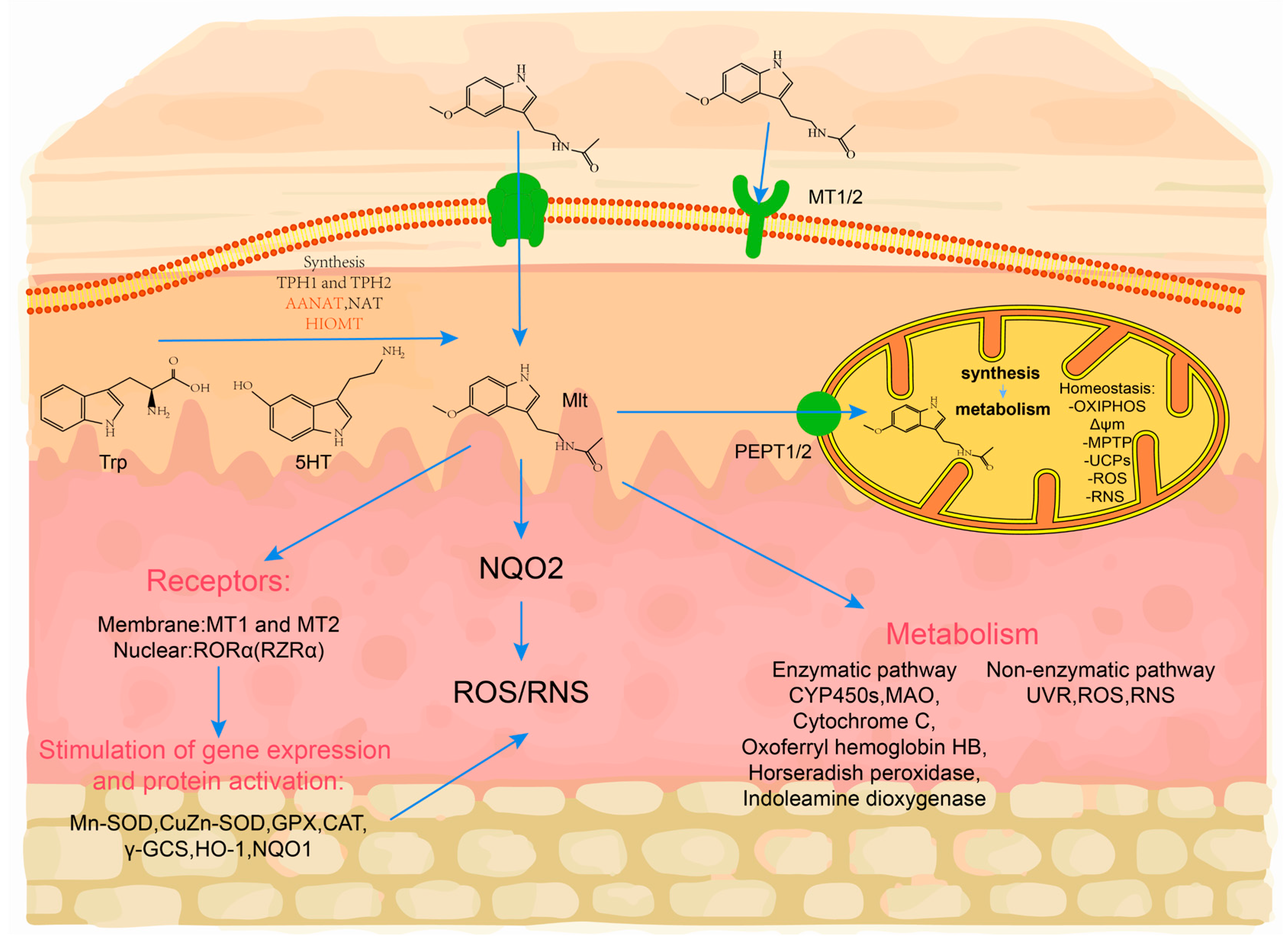
2.2. Melatonin Synthesis from the Skin
2.3. Melatonin Synthesis from Gut Microbiome
3. Laws and Regulation Mechanisms of Cashmere Growth
4. Hair Follicles Growth and Development
4.1. Mechanics of Hair Follicle Formation and Periodic Growth
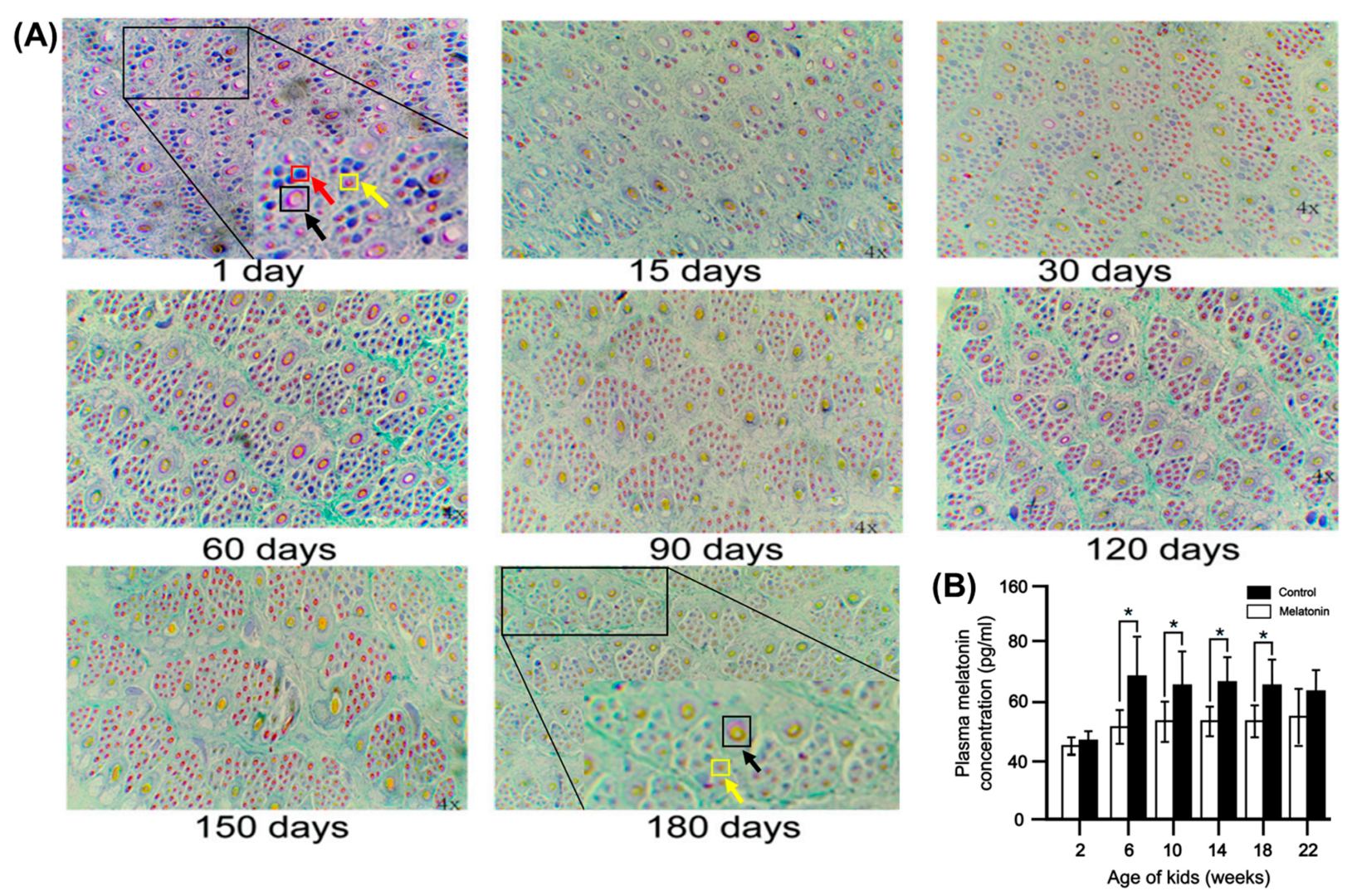
4.2. Regularity of Hair Follicle Development in Young Cashmere Goats
4.3. Law of Hair Follicle Development in Adult Cashmere Goats
4.4. Advances in the Melatonin-Regulated Growth and Development of Hair Follicles
4.5. Melatonin Induced the Combination of the Cashmere-Growth and Non-Growth-Cashmere Periods in Adult Goats
| Authors | Animals | Methods | Results |
|---|---|---|---|
| Betteridge [113] | Feral goats | Implantation of melatonin (18 mg) before and after the vernal equinox. | Cashmere grows early, falls off in autumn; second growth falls off in March. |
| Litherland [108] | Cashmere goats | Implantation of melatonin (18 mg) in the non-cashmere growth phase. | Promoted early growth of cashmere, but most melatonin-treated groups cashmere began to shed at week 19 of melatonin implantation. |
| Welch [109] | Feral goats | Continuous melatonin (1.86 mg/kg body weight) implantation or subcutaneous injection for two years. | In the melatonin treatment group, there were two instances of cashmere growth and the length of cashmere increased significantly, while in the control group, cashmere only grew in autumn and winter. |
| O‘Neill [110] | Lactating and non-lactating goats | Implantation of melatonin (18 mg) on 16 October, 30 October, and 13 November or 25 November | Cashmere goats grew wool twice a year; the length of the cashmere increased significantly. |
| Nixon [114] | New Zealand cashmere bearing goats | Implantation of melatonin (18 mg) in the spring (September in the southern hemisphere). | The PHFs began to grow wool after 14 days of implantation and the secondary follicles began to grow cashmere after 28 days. |
| Kloren [111] | Australian cashmere goats | Melatonin (26 mg) was implanted from July to October and from January to April. | In the melatonin-implantation group, cashmere began to grow at the end of July until it was shed on 1 December, and in January of the following year, cashmere began a new round of growth until it was shed in June of the same year. |
| Wuliji [115] | Spanish goats | During the non-cashmere growth period, 3 mg was taken orally daily for 5 weeks or 18 mg was implanted every 6 weeks. | Implanting melatonin induced the early growth of cashmere and increased cashmere yield. |
5. Mechanism of Melatonin Promoting the Hair Follicle Growth and Development
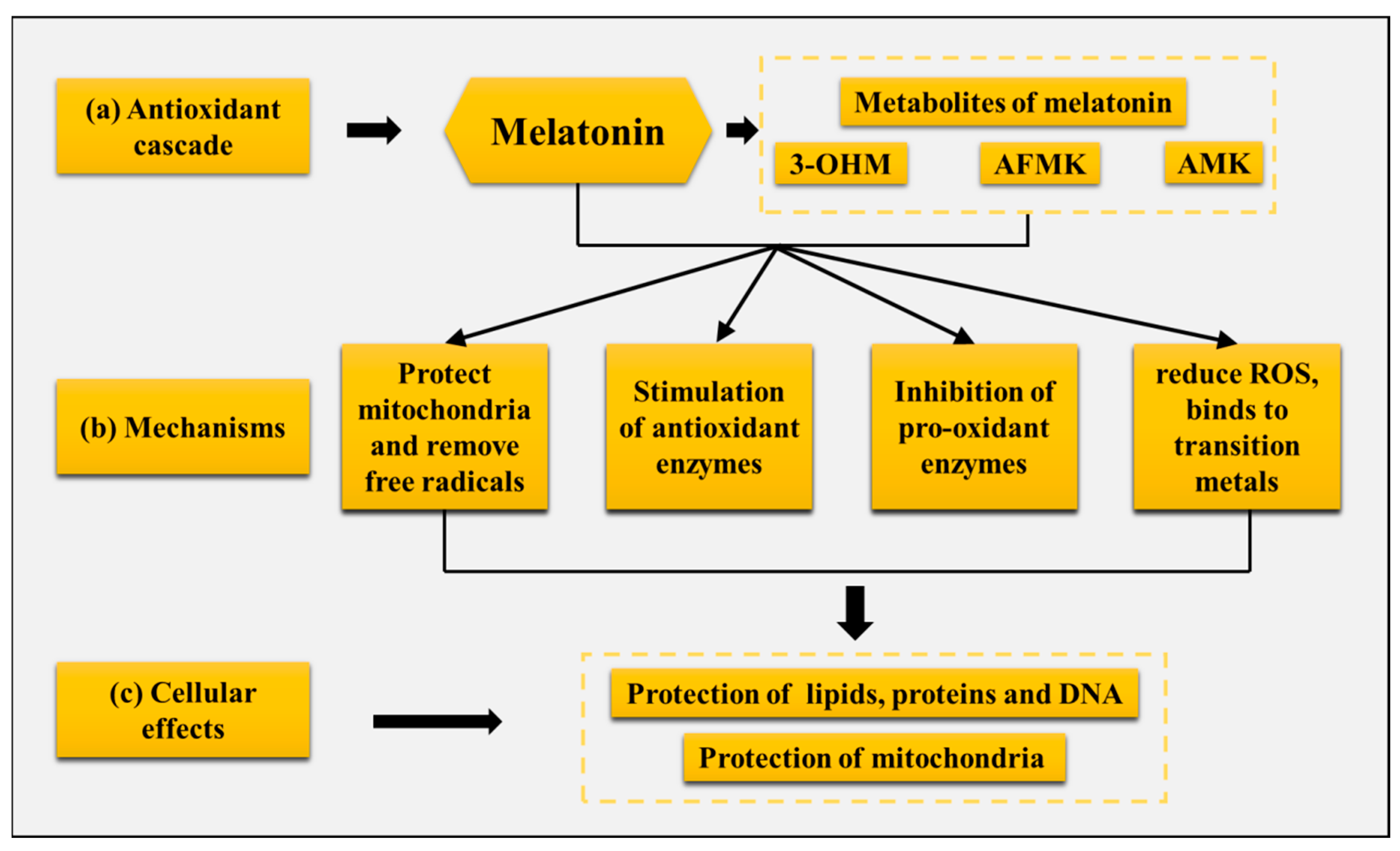
5.1. Melatonin Enhances the Development of Hair Follicles in Cashmere Goats by Improving the Body’s Antioxidant Capacity
5.2. Melatonin Receptor Expression in the Hair Follicle
5.3. Melatonin–Gut–Skin Axis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional hair follicle regeneration: An updated review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar]
- Aragona, F.; Giannetto, C.; Piccione, G.; Licata, P.; Deniz, Ö.; Fazio, F. Hair and Blood Trace Elements (Cadmium, Zinc, Chrome, Lead, Iron and Copper) Biomonitoring in the Athletic Horse: The Potential Role of Haematological Parameters as Biomarkers. Animals 2024, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [PubMed]
- Paterson, A.M.; Foldes, A. Melatonin and farm animals: Endogenous rhythms and exogenous applications. J. Pineal Res. 1994, 16, 167–177. [Google Scholar] [PubMed]
- Li, C.; Feng, C.; Ma, G.; Fu, S.; Chen, M.; Zhang, W.; Li, J. Time-course RNA-seq analysis reveals stage-specific and melatonin-triggered gene expression patterns during the hair follicle growth cycle in Capra hircus. BMC Genom. 2022, 23, 140. [Google Scholar]
- Yang, C.H.; Duan, C.H.; Wu, Z.Y.; Li, Y.; Luan, Y.Y.; Fu, X.J.; Zhang, C.X.; Zhang, W. Effects of melatonin administration to cashmere goats on cashmere production and hair follicle characteristics in two consecutive cashmere growth cycles. Domest. Anim. Endocrinol. 2021, 74, 106534. [Google Scholar]
- Yang, C.H.; Xu, J.H.; Ren, Q.C.; Duan, T.; Mo, F.; Zhang, W. Melatonin promotes secondary hair follicle development of early postnatal cashmere goat and improves cashmere quantity and quality by enhancing antioxidant capacity and suppressing apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar]
- Diao, X.; Yao, L.; Duan, T.; Qin, J.; He, L.; Zhang, W. Melatonin promotes the development of the secondary hair follicles by regulating circMPP5. J. Anim. Sci. Biotechnol. 2023, 14, 51. [Google Scholar]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes 1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar]
- Giannetto, C.; Carcangiu, V.; Luridiana, S.; Parmeggiani, A.; Piccione, G. Twenty-four-hour rhythm patterns of plasma melatonin in short-day and long-day breeders maintained under natural environmental conditions. Chronobiol. Int. 2020, 37, 974–979. [Google Scholar]
- Erren, T.C.; Reiter, R.J.; Meyer-Rochow, V.B. Frog transparency led to discovery of melatonin. Nature 2008, 451, 127. [Google Scholar] [PubMed]
- Li, Y.; Ma, J.; Yao, K.; Su, W.; Tan, B.; Wu, X.; Huang, X.; Li, T.; Yin, Y.; Tosini, G.; et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J. Pineal Res. 2020, 69, e12682. [Google Scholar] [PubMed]
- Leng, Y.; Musiek, E.S.; Hu, K.; Cappuccio, F.P.; Yaffe, K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol. 2019, 18, 307–318. [Google Scholar] [PubMed]
- Lee, J.S.; Cua, D.J. Melatonin Lulling Th17 Cells to Sleep. Cell 2015, 162, 1212–1214. [Google Scholar]
- López, A.; García, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; López, L.C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46, 188–198. [Google Scholar]
- Majidinia, M.; Reiter, R.J.; Shakouri, S.K.; Yousefi, B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res. Rev. 2018, 47, 198–213. [Google Scholar]
- Luchetti, F.; Canonico, B.; Bartolini, D.; Arcangeletti, M.; Ciffolilli, S.; Murdolo, G.; Piroddi, M.; Papa, S.; Reiter, R.J.; Galli, F. Melatonin regulates mesenchymal stem cell differentiation: A review. J. Pineal Res. 2014, 56, 382–397. [Google Scholar]
- Szewczyk-Golec, K.; Woźniak, A.; Reiter, R.J. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: Implications for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar]
- Kent, B.A.; Rahman, S.A.; St Hilaire, M.A.; Grant, L.K.; Rüger, M.; Czeisler, C.A.; Lockley, S.W. Publisher Correction: Circadian lipid and hepatic protein rhythms shift with a phase response curve different than melatonin. Nat. Commun. 2022, 13, 2241. [Google Scholar]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar]
- Slominski, A.T.; Kim, T.K.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [PubMed]
- Reiter, R.J.; Sharma, R.; Tan, D.X.; Chuffa, L.G.A.; da Silva, D.G.H.; Slominski, A.T.; Steinbrink, K.; Kleszczynski, K. Dual sources of melatonin and evidence for different primary functions. Front. Endocrinol. 2024, 15, 1414463. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the hair follicle. J. Pineal Res. 2008, 44, 1–15. [Google Scholar] [PubMed]
- Eşrefoğlu, M.; Seyhan, M.; Gül, M.; Parlakpinar, H.; Batçioğlu, K.; Uyumlu, B. Potent therapeutic effect of melatonin on aging skin in pinealectomized rats. J. Pineal Res. 2005, 39, 231–237. [Google Scholar]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar]
- Glover, J.D.; Sudderick, Z.R.; Shih, B.B.; Batho-Samblas, C.; Charlton, L.; Krause, A.L.; Anderson, C.; Riddell, J.; Balic, A.; Li, J.; et al. The developmental basis of fingerprint pattern formation and variation. Cell 2023, 186, 940–956.e920. [Google Scholar]
- Sevilla, A.; Chéret, J.; Slominski, R.M.; Slominski, A.T.; Paus, R. Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective. J. Pineal Res. 2022, 72, e12790. [Google Scholar] [CrossRef]
- Wang, J.; Fu, Y.; Huang, W.; Biswas, R.; Banerjee, A.; Broussard, J.A.; Zhao, Z.; Wang, D.; Bjerke, G.; Raghavan, S.; et al. MicroRNA-205 promotes hair regeneration by modulating mechanical properties of hair follicle stem cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2220635120. [Google Scholar]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; et al. Insights into male androgenetic alopecia using comparative transcriptome profiling: Hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187, 936–947. [Google Scholar]
- Akdis, C.A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 2021, 21, 739–751. [Google Scholar]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Sassone-Corsi, P. Communicating clocks shape circadian homeostasis. Science 2021, 371, eabd0951. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Aragona, F.; Fazio, F.; Piccione, G.; Giannetto, C. Chronophysiology of domestic animals. Chronobiol. Int. 2024, 41, 888–903. [Google Scholar] [CrossRef]
- Ricketts, E.J.; Joyce, D.S.; Rissman, A.J.; Burgess, H.J.; Colwell, C.S.; Lack, L.C.; Gradisar, M. Electric lighting, adolescent sleep and circadian outcomes, and recommendations for improving light health. Sleep Med. Rev. 2022, 64, 101667. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015, 58, 1–11. [Google Scholar] [CrossRef]
- Stein, R.M.; Kang, H.J.; McCorvy, J.D.; Glatfelter, G.C.; Jones, A.J.; Che, T.; Slocum, S.; Huang, X.P.; Savych, O.; Moroz, Y.S.; et al. Virtual discovery of melatonin receptor ligands to modulate circadian rhythms. Nature 2020, 579, 609–614. [Google Scholar] [CrossRef]
- Nassan, M.; Videnovic, A. Circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 2022, 18, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Zee, P.C.; Manthena, P. The brain’s master circadian clock: Implications and opportunities for therapy of sleep disorders. Sleep Med. Rev. 2007, 11, 59–70. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [PubMed]
- Saper, C.B.; Lu, J.; Chou, T.C.; Gooley, J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005, 28, 152–157. [Google Scholar] [PubMed]
- Benarroch, E.E. Suprachiasmatic nucleus and melatonin: Reciprocal interactions and clinical correlations. Neurology 2008, 71, 594–598. [Google Scholar] [PubMed]
- Klosen, P.; Lapmanee, S.; Schuster, C.; Guardiola, B.; Hicks, D.; Pevet, P.; Felder-Schmittbuhl, M.P. MT1 and MT2 melatonin receptors are expressed in nonoverlapping neuronal populations. J. Pineal Res. 2019, 67, e12575. [Google Scholar]
- Back, K.; Tan, D.X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar]
- Tan, D.X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar]
- Song, L.; He, M.; Sun, Q.; Wang, Y.; Zhang, J.; Fang, Y.; Liu, S.; Duan, L. Roseburia hominis Increases Intestinal Melatonin Level by Activating p-CREB-AANAT Pathway. Nutrients 2021, 14, 117. [Google Scholar] [CrossRef]
- Mul Fedele, M.L.; Galiana, M.D.; Golombek, D.A.; Muñoz, E.M.; Plano, S.A. Alterations in Metabolism and Diurnal Rhythms following Bilateral Surgical Removal of the Superior Cervical Ganglia in Rats. Front. Endocrinol. 2017, 8, 370. [Google Scholar]
- Maronde, E.; Stehle, J.H. The mammalian pineal gland: Known facts, unknown facets. Trends Endocrinol. Metab. 2007, 18, 142–149. [Google Scholar]
- Foster, R.; Bellingham, J. Opsins and melanopsins. Curr. Biol. 2002, 12, R543–R544. [Google Scholar] [CrossRef] [PubMed]
- Hannibal, J.; Fahrenkrug, J. Neuronal input pathways to the brain's biological clock and their functional significance. Adv. Anat. Embryol. Cell Biol. 2006, 182, 1–71. [Google Scholar]
- Perreau-Lenz, S.; Kalsbeek, A.; Garidou, M.L.; Wortel, J.; van der Vliet, J.; van Heijningen, C.; Simonneaux, V.; Pévet, P.; Buijs, R.M. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Giraudet, F.; Becquet, D.; Sage, D.; Laforge-Anglade, G.; Bosler, O.; François-Bellan, A.M. Vitamin A is a necessary factor for sympathetic-independent rhythmic activation of mitogen-activated protein kinase in the rat pineal gland. Eur. J. Neurosci. 2005, 21, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Ursinus, J.; Zhou, J.N.; Scheer, F.A.; Ai-Min, B.; Jockers, R.; van Heerikhuize, J.; Swaab, D.F. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J. Affect. Disord. 2013, 148, 357–367. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Szczesniewski, A.; Wortsman, J. Serotoninergic system in hamster skin. J. Investig. Dermatol. 2002, 119, 934–942. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J.; Szczesniewski, A.; Slugocki, G.; McNulty, J.; Kauser, S.; Tobin, D.J.; et al. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002, 16, 896–898. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Zmijewski, M.A.; Wortsman, J.; Paus, R. Melatonin in the skin: Synthesis, metabolism and functions. Trends Endocrinol. Metab. TEM 2008, 19, 17–24. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M.; Pittelkow, M.R.; Gütlich, M.; Lemke, K.R.; Rödl, W.; Swanson, N.N.; Hitzemann, K.; Ziegler, I. Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 1994, 263, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kromminga, A.; Dunlop, T.W.; Tychsen, B.; Conrad, F.; Suzuki, N.; Memezawa, A.; Bettermann, A.; Aiba, S.; Carlberg, C.; et al. A role of melatonin in neuroectodermal-mesodermal interactions: The hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 2005, 19, 1710–1712. [Google Scholar] [PubMed]
- Reiter, R.J.; Sharma, R.; Rosales-Corral, S.; de Campos Zuccari, D.A.P.; de Almeida Chuffa, L.G. Melatonin: A mitochondrial resident with a diverse skill set. Life Sci. 2022, 301, 120612. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.N.; Chuffa, L.G.A.; da Silva, D.G.H.; Rosales-Corral, S. Intrinsically synthesized melatonin in mitochondria and factors controlling its production. Histol. Histopathol. 2025, 40, 271–282. [Google Scholar]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62, 12390. [Google Scholar]
- Bubenik, G.A.; Pang, S.F.; Hacker, R.R.; Smith, P.S. Melatonin concentrations in serum and tissues of porcine gastrointestinal tract and their relationship to the intake and passage of food. J. Pineal Res. 1996, 21, 251–256. [Google Scholar]
- Bubenik, G.A.; Hacker, R.R.; Brown, G.M.; Bartos, L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J. Pineal Res. 1999, 26, 56–63. [Google Scholar] [CrossRef]
- Liu, B.; Fan, L.; Wang, Y.; Wang, H.; Yan, Y.; Chen, S.; Hung, I.; Liu, C.; Wei, H.; Ge, L.; et al. Gut microbiota regulates host melatonin production through epithelial cell MyD88. Gut Microbes 2024, 16, 2313769. [Google Scholar] [CrossRef]
- Lian, Z.; Xu, Y.; Wang, C.; Chen, Y.; Yuan, L.; Liu, Z.; Liu, Y.; He, P.; Cai, Z.; Zhao, J. Gut microbiota-derived melatonin from Puerariae Lobatae Radix-resistant starch supplementation attenuates ischemic stroke injury via a positive microbial co-occurrence pattern. Pharmacol. Res. 2023, 190, 106714. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar]
- Zhuo, Y.; Cao, M.; Gong, Y.; Tang, L.; Jiang, X.; Li, Y.; Yang, M.; Xu, S.; Li, J.; Che, L.; et al. Gut microbial metabolism of dietary fibre protects against high energy feeding induced ovarian follicular atresia in a pig model. Br. J. Nutr. 2021, 125, 38–49. [Google Scholar] [PubMed]
- Ervin, S.M.; Simpson, J.B.; Gibbs, M.E.; Creekmore, B.C.; Lim, L.; Walton, W.G.; Gharaibeh, R.Z.; Redinbo, M.R. Structural Insights into Endobiotic Reactivation by Human Gut Microbiome-Encoded Sulfatases. Biochemistry 2020, 59, 3939–3950. [Google Scholar] [PubMed]
- Lutfi, E.; Basili, D.; Falcinelli, S.; Morillas, L.; Carnevali, O.; Capilla, E.; Navarro, I. The probiotic Lactobacillus rhamnosus mimics the dark-driven regulation of appetite markers and melatonin receptors’ expression in zebrafish (Danio rerio) larvae: Understanding the role of the gut microbiome. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 256, 110634. [Google Scholar]
- Nocelli, C.; Cappelli, K.; Capomaccio, S.; Pascucci, L.; Mercati, F.; Pazzaglia, I.; Mecocci, S.; Antonini, M.; Renieri, C. Shedding light on cashmere goat hair follicle biology: From morphology analyses to transcriptomic landascape. BMC Genom. 2020, 21, 458. [Google Scholar]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [PubMed]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar]
- O’Brien, C.; Darcy-Dunne, M.R.; Murphy, B.A. The effects of extended photoperiod and warmth on hair growth in ponies and horses at different times of year. PLoS ONE 2020, 15, e0227115. [Google Scholar]
- Wu, J.-H.; Zhang, Y.-J.; Zhang, J.-X.; Chang, Z.-L.; Li, J.-Q.; Yan, Z.-W.; Husile; Zhang, W.-G. Hoxc13/β-catenin Correlation with Hair Follicle Activity in Cashmere Goat. J. Integr. Agric. 2012, 11, 1159–1166. [Google Scholar]
- Smale, L.; Nelson, R.J.; Zucker, I. Daylength influences pelage and plasma prolactin concentrations but not reproduction in the prairie vole, Microtus ochrogaster. J. Reprod. Fertil. 1988, 83, 99–106. [Google Scholar]
- Gong, W.; Liu, J.; Mu, Q.; Chahaer, T.; Liu, J.; Ding, W.; Bou, T.; Wu, Z.; Zhao, Y. Melatonin promotes proliferation of Inner Mongolia cashmere goat hair follicle papilla cells through Wnt10b. Genomics 2024, 116, 110844. [Google Scholar]
- Amini, H.; Rezabakhsh, A.; Heidarzadeh, M.; Hassanpour, M.; Hashemzadeh, S.; Ghaderi, S.; Sokullu, E.; Rahbarghazi, R.; Reiter, R.J. An Examination of the Putative Role of Melatonin in Exosome Biogenesis. Front. Cell Dev. Biol. 2021, 9, 686551. [Google Scholar]
- Yang, C.H.; Wu, Z.Y.; Li, Y.; Zhang, W. Effect of melatonin administration to lactating cashmere goats on milk production of dams and on hair follicle development in their offspring. Animal 2020, 14, 1241–1248. [Google Scholar] [PubMed]
- Duan, C.; Xu, J.; Sun, C.; Jia, Z.; Zhang, W. Effects of melatonin implantation on cashmere yield, fibre characteristics, duration of cashmere growth as well as growth and reproductive performance of Inner Mongolian cashmere goats. J. Anim. Sci. Biotechnol. 2015, 6, 22. [Google Scholar]
- Gáspár, E.; Hardenbicker, C.; Bodó, E.; Wenzel, B.; Ramot, Y.; Funk, W.; Kromminga, A.; Paus, R. Thyrotropin releasing hormone (TRH): A new player in human hair-growth control. FASEB J. 2010, 24, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.A.; Ramot, Y.; Hanning, A.; Poeggeler, B.; Bíró, T.; Gaspar, E.; Funk, W.; Griffiths, C.E.; Paus, R. Thyrotropin-releasing hormone and oestrogen differentially regulate prolactin and prolactin receptor expression in female human skin and hair follicles in vitro. Br. J. Dermatol. 2010, 162, 1127–1131. [Google Scholar]
- Oláh, A.; Gherardini, J.; Bertolini, M.; Chéret, J.; Ponce, L.; Kloepper, J.; Bíró, T.; Soeberdt, M.; Abels, C.; Paus, R. The Thyroid Hormone Analogue KB2115 (Eprotirome) Prolongs Human Hair Growth (Anagen) Ex Vivo. J. Investig. Dermatol. 2016, 136, 1711–1714. [Google Scholar]
- O’Sullivan, J.D.B.; Nicu, C.; Picard, M.; Chéret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The biology of human hair greying. Biol. Rev. Camb. Philos. Soc. 2021, 96, 107–128. [Google Scholar]
- Badarinath, K.; Dutta, A.; Hegde, A.; Pincha, N.; Gund, R.; Jamora, C. Interactions Between Epidermal Keratinocytes, Dendritic Epidermal T-Cells, and Hair Follicle Stem Cells. Methods Mol. Biol. 2019, 1879, 285–297. [Google Scholar]
- Kumamoto, T.; Shalhevet, D.; Matsue, H.; Mummert, M.E.; Ward, B.R.; Jester, J.V.; Takashima, A. Hair follicles serve as local reservoirs of skin mast cell precursors. Blood 2003, 102, 1654–1660. [Google Scholar]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar]
- Zhang, Y.; Tomann, P.; Andl, T.; Gallant, N.M.; Huelsken, J.; Jerchow, B.; Birchmeier, W.; Paus, R.; Piccolo, S.; Mikkola, M.L.; et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 2009, 17, 49–61. [Google Scholar] [PubMed]
- Kurek, D.; Garinis, G.A.; van Doorninck, J.H.; van der Wees, J.; Grosveld, F.G. Transcriptome and phenotypic analysis reveals Gata3-dependent signalling pathways in murine hair follicles. Development 2007, 134, 261–272. [Google Scholar] [PubMed]
- Nascimento, M.A.; Nonaka, C.F.; Barboza, C.A.; Freitas, R.A.; Pereira Pinto, L.; Souza, L.B. Immunoexpression of BMP-2 and BMP-4 and their receptors, BMPR-IA and BMPR-II, in ameloblastomas and adenomatoid odontogenic tumors. Arch. Oral Biol. 2017, 73, 223–229. [Google Scholar] [PubMed]
- Ahn, K.; Mishina, Y.; Hanks, M.C.; Behringer, R.R.; Crenshaw, E.B., 3rd. BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development 2001, 128, 4449–4461. [Google Scholar]
- Wang, Z.; Lin, L.; Zhang, J.S.; Zhong, X.; Bellusci, S.; Li, X. Editorial: The Fibroblast Growth Factor Signaling Pathway in Metabolic Regulation, Development, Disease, and Repair After Injury. Front. Pharmacol. 2020, 11, 586654. [Google Scholar]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar]
- Diao, X.; Yao, L.; Wang, X.; Li, S.; Qin, J.; Yang, L.; He, L.; Zhang, W. Hair Follicle Development and Cashmere Traits in Albas Goat Kids. Animals 2023, 13, 617. [Google Scholar] [CrossRef]
- Gong, G.; Fan, Y.; Yan, X.; Li, W.; Yan, X.; Liu, H.; Zhang, L.; Su, Y.; Zhang, J.; Jiang, W.; et al. Identification of Genes Related to Hair Follicle Cycle Development in Inner Mongolia Cashmere Goat by WGCNA. Front. Vet. Sci. 2022, 9, 894380. [Google Scholar]
- Su, R.; Zhang, W.G.; Sharma, R.; Chang, Z.L.; Yin, J.; Li, J.Q. Characterization of BMP2 gene expression in embryonic and adult Inner Mongolia Cashmere goat (Capra hircus) hair follicles. Can. J. Anim. Sci. 2009, 89, 457–462. [Google Scholar]
- Ge, W.; Wang, S.H.; Sun, B.; Zhang, Y.L.; Shen, W.; Khatib, H.; Wang, X. Melatonin promotes Cashmere goat (Capra hircus) secondary hair follicle growth: A view from integrated analysis of long non-coding and coding RNAs. Cell Cycle 2018, 17, 1255–1267. [Google Scholar] [PubMed]
- Zhang, W.; Wang, N.; Zhang, T.; Wang, M.; Ge, W.; Wang, X. Roles of Melatonin in Goat Hair Follicle Stem Cell Proliferation and Pluripotency Through Regulating the Wnt Signaling Pathway. Front. Cell Dev. Biol. 2021, 9, 686805. [Google Scholar]
- Diao, X.; Duan, C.; Yao, L.; Qin, J.; He, L.; Zhang, W. Melatonin Promotes the Development of Secondary Hair Follicles in Adult Cashmere Goats by Activating the Keap1-Nrf2 Signaling Pathway and Inhibiting the Inflammatory Transcription Factors NFκB and AP-1. Int. J. Mol. Sci. 2023, 24, 3403. [Google Scholar] [CrossRef]
- Mitchell, R.; Betteridge, K.; Gurnsey, M.; Welch, R. Fibre growth of cashmere-bearing goats given melatonin in late autumn and winter. N. Z. J. Agric. Res. 1991, 34, 419–425. [Google Scholar]
- Dicks, P.; Russel, A.; Lincoln, G. The effect of melatonin implants administered from December until April, on plasma prolactin, triiodothyronine and thyroxine concentrations and on the timing of the spring moult in cashmere goats. Anim. Sci. 1995, 60, 239–247. [Google Scholar]
- Cong, Y.; Deng, H.; Feng, Y.; Chen, Q.; Sun, Y. Melatonin implantation from winter solstice could extend the cashmere growth phase effectively. Small Rumin. Res. 2011, 99, 48–53. [Google Scholar]
- Litherland, A.J.; Paterson, D.J.; Parry, A.L. Melatonin for cashmere production. Proc. N. Z. Soc. Anim. Prod. 1990, 50, 339–343. [Google Scholar]
- Welch, R.; Gurnsey, M.; Betteridge, K.; Mitchell, R. Goat fibre response to melatonin given in spring in two consecutive years. Proc. N. Z. Soc. Anim. Prod. 1990, 22, 335–358. [Google Scholar]
- O’Neill, K.; Litherland, A.J.; Paterson, D.J. Melatonin for cashmere production in breeding does. Proc. N. Z. Soc. Anim. Prod. 1992, 52, 161–164. [Google Scholar]
- Klören, W.; Norton, B. Melatonin and fleece growth in Australian cashmere goats. Small Rumin. Res. 1995, 17, 179–185. [Google Scholar]
- Duan, C.; Xu, J.; Zhang, Y.; Zhang, W.; Sun, Y.; Jia, Z. Effects of melatonin implantation on cashmere growth, hormone concentrations and cashmere yield in cashmere-perennial-type Liaoning cashmere goats. Anim. Prod. Ence 2017, 7, 151–183. [Google Scholar] [CrossRef]
- Betteridge, K.; Welch, R.; Pomroy, W.; Lapwood, K.; Devantier, B. Out of season cashmere growth in feral goats. In Proceedings of the Second International Cashmere Conference; Lincoln College: Lincoln, New Zealand, 1987; pp. 137–142. [Google Scholar]
- Nixon, A.; Gurnseyb, M.; Betteridgec, K.; Mitchellc, R.; Welchc, R. Seasonal hair follicle activity and fibre growth in some New Zealand cashmere-bearing goats (Caprus hircus). J. Zool. 1991, 224, 589–598. [Google Scholar]
- Wuliji, T.; Litherland, A.; Goetsch, A.; Sahlu, T.; Puchala, R.; Dawson, L.; Gipson, T. Evaluation of melatonin and bromocryptine administration in Spanish goats: II. Effect on seasonal cashmere growth, yield and fiber characteristics of does. Small Rumin. Res. 2003, 49, 41–49. [Google Scholar]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar]
- Byeon, Y.; Lee, K.; Park, Y.I.; Park, S.; Back, K. Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 2013, 55, 371–376. [Google Scholar]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar]
- Hosseinzadeh, A.; Kamrava, S.K.; Joghataei, M.T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R.J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal Res. 2016, 61, 411–425. [Google Scholar]
- García, J.J.; López-Pingarrón, L.; Almeida-Souza, P.; Tres, A.; Escudero, P.; García-Gil, F.A.; Tan, D.X.; Reiter, R.J.; Ramírez, J.M.; Bernal-Pérez, M. Protective effects of melatonin in reducing oxidative stress and in preserving the fluidity of biological membranes: A review. J. Pineal Res. 2014, 56, 225–237. [Google Scholar]
- Mollaoglu, H.; Topal, T.; Ozler, M.; Uysal, B.; Reiter, R.J.; Korkmaz, A.; Oter, S. Antioxidant effects of melatonin in rats during chronic exposure to hyperbaric oxygen. J. Pineal Res. 2007, 42, 50–54. [Google Scholar] [PubMed]
- Chua, S.; Lee, F.Y.; Chiang, H.J.; Chen, K.H.; Lu, H.I.; Chen, Y.T.; Yang, C.C.; Lin, K.C.; Chen, Y.L.; Kao, G.S.; et al. The cardioprotective effect of melatonin and exendin-4 treatment in a rat model of cardiorenal syndrome. J. Pineal Res. 2016, 61, 438–456. [Google Scholar] [PubMed]
- Lowes, D.A.; Webster, N.R.; Murphy, M.P.; Galley, H.F. Antioxidants that protect mitochondria reduce interleukin-6 and oxidative stress, improve mitochondrial function, and reduce biochemical markers of organ dysfunction in a rat model of acute sepsis. Br. J. Anaesth. 2013, 110, 472–480. [Google Scholar]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria Synthesize Melatonin to Ameliorate Its Function and Improve Mice Oocyte’s Quality under in Vitro Conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar]
- Galano, A.; Medina, M.E.; Tan, D.X.; Reiter, R.J. Melatonin and its metabolites as copper chelating agents and their role in inhibiting oxidative stress: A physicochemical analysis. J. Pineal Res. 2015, 58, 107–116. [Google Scholar]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar]
- Lei, X.; Xu, Z.; Huang, L.; Huang, Y.; Tu, S.; Xu, L.; Liu, D. The potential influence of melatonin on mitochondrial quality control: A review. Front. Pharmacol. 2023, 14, 1332567. [Google Scholar]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. CMLS 2017, 74, 3913–3925. [Google Scholar]
- Lan, M.; Zhang, Y.; Wan, X.; Pan, M.H.; Xu, Y.; Sun, S.C. Melatonin ameliorates ochratoxin A-induced oxidative stress and apoptosis in porcine oocytes. Environ. Pollut. 2020, 256, 113374. [Google Scholar] [PubMed]
- Regodón, S.; Ramos, A.; Míguez, M.P.; Carrillo-Vico, A.; Rosado, J.A.; Jardín, I. Vaccination prepartum enhances the beneficial effects of melatonin on the immune response and reduces platelet responsiveness in sheep. BMC Vet. Res. 2012, 8, 84. [Google Scholar]
- Canto, F.; González, E.; Abecia, J.A. Effects of Implanting Exogenous Melatonin 40 Days before Lambing on Milk and Colostrum Quality. Animals 2022, 12, 1257. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Chassalevris, N.; Mazurkiewicz, J.; Maurer, M.; Paus, R. Murine skin as a target for melatonin bioregulation. Exp. Dermatol. 1994, 3, 45–50. [Google Scholar]
- Dicks, P.; Morgan, C.J.; Morgan, P.J.; Kelly, D.; Williams, L.M. The localisation and characterisation of insulin-like growth factor-I receptors and the investigation of melatonin receptors on the hair follicles of seasonal and non-seasonal fibre-producing goats. J. Endocrinol. 1996, 151, 55–63. [Google Scholar]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–148. [Google Scholar]
- Pégurier, C.; Curtet, S.; Nicolas, J.P.; Boutin, J.A.; Delagrange, P.; Renard, P.; Langlois, M. Synthesis of a small library of phenylalkylamide derivatives as melatoninergic ligands for human mt1 and MT2 receptors. Bioorganic Med. Chem. 2000, 8, 163–171. [Google Scholar]
- Paul, P.; Lahaye, C.; Delagrange, P.; Nicolas, J.P.; Canet, E.; Boutin, J.A. Characterization of 2-[125I]iodomelatonin binding sites in Syrian hamster peripheral organs. J. Pharmacol. Exp. Ther. 1999, 290, 334–340. [Google Scholar]
- Zhao, Y.H.; Liu, Z.H.; Wang, L.; Xiao, H.M.; Du, C.G.; Zhang, Y.J.; Su, R.; Li, J.Q. Expression of the RORα gene in Inner Mongolian cashmere goat hair follicles. Genet. Mol. Res. GMR 2015, 14, 380–388. [Google Scholar]
- Lu, Z.; Wu, J.; Wu, J.; Zhang, T.; Liu, J.; Mu, Q.; Terigele; Wu, Z.; Zhang, Y.; Su, R.; et al. Melatonin regulates the periodic growth of secondary hair follicles through the nuclear receptor RORα. Front. Vet. Sci. 2023, 10, 1203302. [Google Scholar]
- Ma, L.; Tao, S.; Song, T.; Lyu, W.; Li, Y.; Wang, W.; Shen, Q.; Ni, Y.; Zhu, J.; Zhao, J.; et al. Clostridium butyricum and carbohydrate active enzymes contribute to the reduced fat deposition in pigs. iMeta 2024, 3, e160. [Google Scholar] [PubMed]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium butyricum-Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol. Nutr. Food Res. 2023, 67, e2200884. [Google Scholar] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar]

| Authors | Animals | Methods | Results |
|---|---|---|---|
| Mitchell [105] | New Zealand cashmere goat ewes | Implantations of melatonin in May, June, July and August, respectively, at the dose of 125 mg/ goat. | Cashmere grows early, growth rate significantly higher; second growth falls off in March, early shedding occurs. |
| Dicks [106] | Angora goats | Melatonin (18 mg) was implanted in cashmere goats in the late period of cashmere growth. | As a result, the cashmere fell off in advance and did not prolong the cashmere growth period. |
| Cong [107] | Liaoning cashmere goats | Three consecutive implantations of melatonin (2 mg/kg BW) at the end of December, February, and April. | The control group stopped growing in March, while the implant group continued to grow until June. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Su, Z.; Zhang, W. Melatonin’s Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective. Int. J. Mol. Sci. 2025, 26, 2844. https://doi.org/10.3390/ijms26072844
Zheng Z, Su Z, Zhang W. Melatonin’s Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective. International Journal of Molecular Sciences. 2025; 26(7):2844. https://doi.org/10.3390/ijms26072844
Chicago/Turabian StyleZheng, Zibin, Zhenyu Su, and Wei Zhang. 2025. "Melatonin’s Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective" International Journal of Molecular Sciences 26, no. 7: 2844. https://doi.org/10.3390/ijms26072844
APA StyleZheng, Z., Su, Z., & Zhang, W. (2025). Melatonin’s Role in Hair Follicle Growth and Development: A Cashmere Goat Perspective. International Journal of Molecular Sciences, 26(7), 2844. https://doi.org/10.3390/ijms26072844





