Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review
Abstract
:1. Introduction
2. Periodontitis as a Risk Factor for DM
2.1. Overview
2.2. Mechanistic Details
2.3. Further Evidence Linking DM to Modified Periodontal DNA Methylation and Vice Versa
2.4. ROS as a Key Mechanistic Controller of Periodontitis-Associated DM
2.5. The Role of Proinflammatory Cytokines in Periodontitis as Epigenetic Modulators of DM
3. Could Epigenetic Modulators Protect Against Cyclical Pathological Amplification Between PD and DM?
3.1. Natural Potential Epigenetic Modulators in Chronic Disease
3.2. CBD as a Novel Epigenetic Modulator of the Periodontitis–DM Axis
3.3. Metformin Plus CBD as a Novel Synergistic Therapeutic
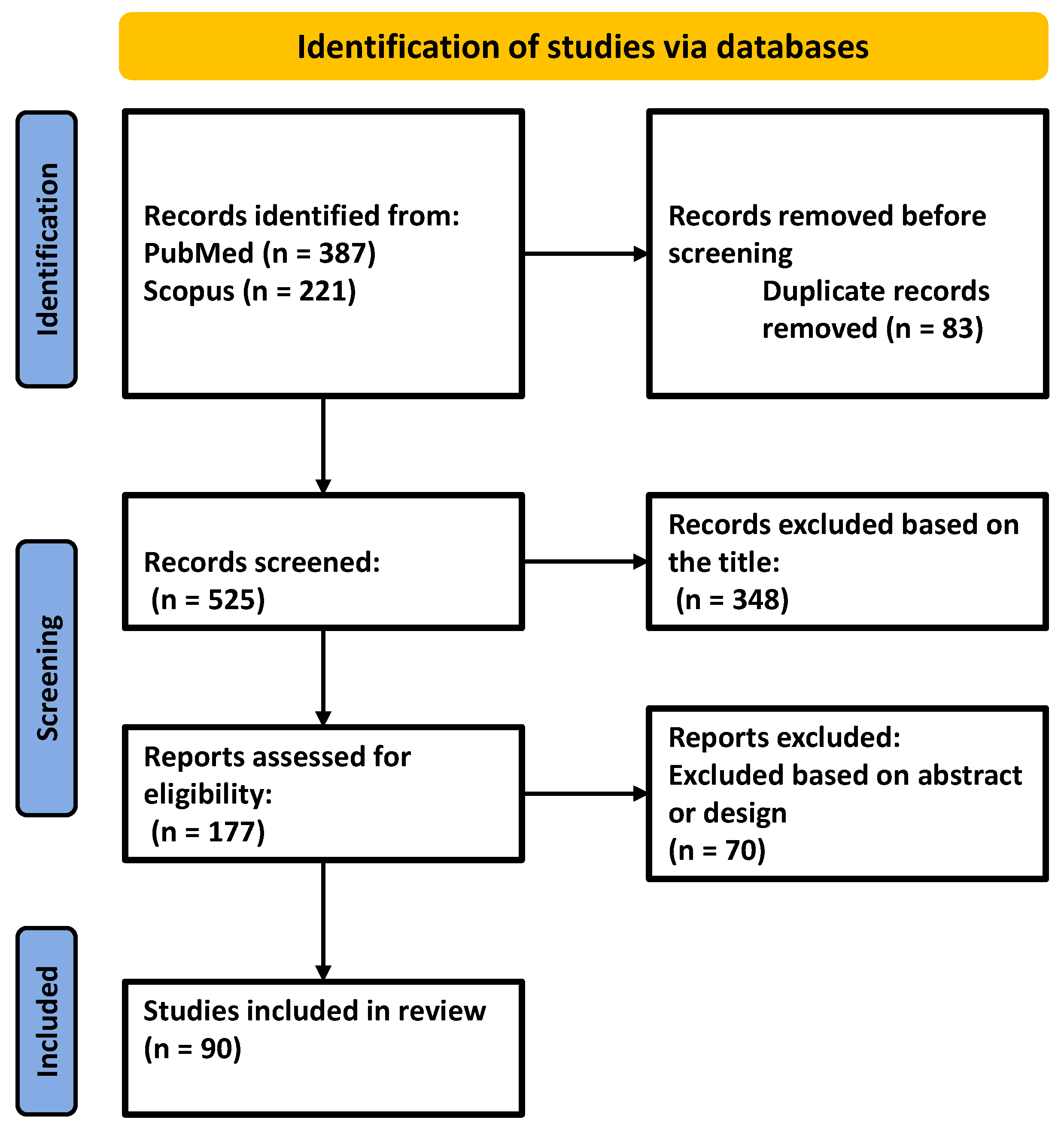
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BETs | bromodomain-containing proteins |
| CAT | catalase |
| CBD | cannabidiol |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DM | diabetes mellitus |
| DNMT-1 | DNA methyltransferase-1 |
| DNMT3b | DNA methyltransferase-3 beta |
| DNMTs | DNA methyltransferases |
| ECM | extracellular matrix |
| HATs | histone acetyltransferases |
| HDACs | histone deacetylases |
| HFD | high-fat diet |
| hPDLSCs | human periodontal ligament stem cells |
| HSC | hematopoietic stem cell |
| IHC | immunohistochemistry |
| ILs | interleukins |
| IRS-1 | insulin receptor substrate-1 |
| LPSs | lipopolysaccharides |
| MDA | malondialdehyde |
| MMPs | matrix metalloproteinases |
| NAD+ | nicotinamide adenine dinucleotide |
| NLRP3 | NOD-like receptor family pyrin domain-containing 3 |
| Nox2 | NADPH oxidase 2 |
| OPG | osteoprotegerin |
| PDLFs | periodontal ligament fibroblasts |
| PGs | prostaglandins |
| PPAR-α | peroxisome proliferator-activated receptors alpha |
| RANKL | receptor activator of NF-κB ligand |
| SIRT6 | sirtuin-6 |
| SOD | superoxide dismutase |
| TAOC | total antioxidant capacity |
| TET1 | ten-eleven translocation methyl cytosine dioxygenase 1 |
| THC | Δ9-tetrahydrocannabinol |
| TIMPs | tissue inhibitors of metalloproteinases |
| TNF-α | tumor necrosis factor alpha |
| TRAF6 | tumor necrosis factor receptor-associated factor 6 |
| TXNIP | thioredoxin |
References
- Dong, Z.; Wu, L.; Hong, H. Mitochondrial Dysfunction in the Pathogenesis and Treatment of Oral Inflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 15483. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Genco, R.J.; Sanz, M. Clinical and Public Health Implications of Periodontal and Systemic Diseases: An Overview. Periodontol. 2000 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Shapira, L. An Update on the Evidence for Pathogenic Mechanisms That May Link Periodontitis and Diabetes. J. Clin. Periodontol. 2018, 45, 150–166. [Google Scholar] [CrossRef]
- Su, Y.; Ye, L.; Hu, C.; Zhang, Y.; Liu, J.; Shao, L. Periodontitis as a Promoting Factor of T2D: Current Evidence and Mechanisms. Int. J. Oral Sci. 2023, 15, 25. [Google Scholar] [CrossRef]
- Deng, J.; Golub, L.M.; Lee, H.-M.; Raja, V.; Johnson, F.; Kucine, A.; Lee, W.; Xu, T.-M.; Gu, Y. A Novel Modified-Curcumin Promotes Resolvin-Like Activity and Reduces Bone Loss in Diabetes-Induced Experimental Periodontitis. J. Inflamm. Res. 2021, 14, 5337–5347. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Kim, W.-J.; Ryoo, H.-M.; Kim, H.-G.; Kim, K.-H.; Ku, Y.; Seol, Y.-J. Current Advances of Epigenetics in Periodontology from ENCODE Project: A Review and Future Perspectives. Clin. Epigenetics 2021, 13, 92. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of Epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Ferioli, M.; Zauli, G.; Maiorano, P.; Milani, D.; Mirandola, P.; Neri, L.M. Role of Physical Exercise in the Regulation of Epigenetic Mechanisms in Inflammation, Cancer, Neurodegenerative Diseases, and Aging Process. J. Cell. Physiol. 2019, 234, 14852–14864. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Strahl, B.D. Interpreting the Language of Histone and DNA Modifications. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2014, 1839, 627–643. [Google Scholar] [CrossRef]
- Jurdziński, K.T.; Potempa, J.; Grabiec, A.M. Epigenetic Regulation of Inflammation in Periodontitis: Cellular Mechanisms and Therapeutic Potential. Clin. Epigenetics 2020, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Tao, F. Mechanisms for Orofacial Pain: Roles of Immunomodulation, Metabolic Reprogramming, Oxidative Stress and Epigenetic Regulation. Biomedicines 2025, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Domingos, L.B.; Silva, N.R.; Chaves Filho, A.J.M.; Sales, A.J.; Starnawska, A.; Joca, S. Regulation of DNA Methylation by Cannabidiol and Its Implications for Psychiatry: New Insights from In Vivo and In Silico Models. Genes 2022, 13, 2165. [Google Scholar] [CrossRef]
- Melas, P.A.; Scherma, M.; Fratta, W.; Cifani, C.; Fadda, P. Cannabidiol as a Potential Treatment for Anxiety and Mood Disorders: Molecular Targets and Epigenetic Insights from Preclinical Research. Int. J. Mol. Sci. 2021, 22, 1863. [Google Scholar] [CrossRef] [PubMed]
- Kicman, A.; Toczek, M. The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease. Int. J. Mol. Sci. 2020, 21, 6740. [Google Scholar] [CrossRef]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and Therapeutic Targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef]
- Jirasek, P.; Jusku, A.; Simanek, V.; Frankova, J.; Storch, J.; Vacek, J. Cannabidiol and Periodontal Inflammatory Disease: A Critical Assessment. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2022, 166, 155–160. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Wu, H.; Huang, S.; Chen, L.; Gui, Q.; Zhou, W.; Yang, Y.; Wu, Y.; Zhang, H.; et al. Periodontitis in Elderly Patients with Type 2 Diabetes Mellitus: Impact on Gut Microbiota and Systemic Inflammation. Aging 2020, 12, 25956–25980. [Google Scholar] [CrossRef]
- Menendez, J.A.; Alarcón, T. Senescence-Inflammatory Regulation of Reparative Cellular Reprogramming in Aging and Cancer. Front. Cell Dev. Biol. 2017, 5, 49. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Q.; Chen, Q. The Cytokine Network Involved in the Host Immune Response to Periodontitis. Int. J. Oral Sci. 2019, 11, 30. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G. Epigenetic Modifications in Diabetes. Metabolism 2022, 126, 154920. [Google Scholar] [CrossRef] [PubMed]
- Palioto, D.B.; Finoti, L.S.; Kinane, D.F.; Benakanakere, M. Epigenetic and Inflammatory Events in Experimental Periodontitis Following Systemic Microbial Challenge. J. Clin. Periodontol. 2019, 46, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Feng, Q.; Chen, W.; Bai, X. Catalpol Antagonizes LPS-Mediated Inflammation and Promotes Osteoblast Differentiation through the miR-124-3p/DNMT3b/TRAF6 Axis. Acta Histochem. 2024, 126, 152118. [Google Scholar] [CrossRef]
- Ilievski, V.; Bhat, U.G.; Suleiman-Ata, S.; Bauer, B.A.; Toth, P.T.; Olson, S.T.; Unterman, T.G.; Watanabe, K. Oral Application of a Periodontal Pathogen Impacts SerpinE1 Expression and Pancreatic Islet Architecture in Prediabetes. J. Periodontal Res. 2017, 52, 1032–1041. [Google Scholar] [CrossRef]
- Diomede, F.; Thangavelu, S.R.; Merciaro, I.; D’Orazio, M.; Bramanti, P.; Mazzon, E.; Trubiani, O. Porphyromonas Gingivalis Lipopolysaccharide Stimulation in Human Periodontal Ligament Stem Cells: Role of Epigenetic Modifications to the Inflammation. Eur. J. Histochem. 2017, 61, 2826. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, M.; Hernández-Lemus, E. Periodontal Inflammation and Systemic Diseases: An Overview. Front. Physiol. 2021, 12, 709438. [Google Scholar] [CrossRef]
- Li, B.; Xin, Z.; Gao, S.; Li, Y.; Guo, S.; Fu, Y.; Xu, R.; Wang, D.; Cheng, J.; Liu, L.; et al. SIRT6-Regulated Macrophage Efferocytosis Epigenetically Controls Inflammation Resolution of Diabetic Periodontitis. Theranostics 2023, 13, 231–249. [Google Scholar] [CrossRef]
- Li, Y.; Du, Z.; Xie, X.; Zhang, Y.; Liu, H.; Zhou, Z.; Zhao, J.; Lee, R.S.; Xiao, Y.; Ivanoviski, S.; et al. Epigenetic Changes Caused by Diabetes and Their Potential Role in the Development of Periodontitis. J. Diabetes Investig. 2021, 12, 1326–1335. [Google Scholar] [CrossRef]
- Bala, S.V.; Appukuttan, D.; Subramaniam, S.; Prakash, P.S.G.; Cholan, P.K.; Victor, D.J. Association of Receptor for Advanced Glycation End Products G82S Polymorphism with Chronic Periodontitis in Type II Diabetic and Non-Diabetic South Indians. Gene 2019, 708, 30–37. [Google Scholar] [CrossRef]
- Zhao, M.; Xie, Y.; Gao, W.; Li, C.; Ye, Q.; Li, Y. Diabetes Mellitus Promotes Susceptibility to Periodontitis—Novel Insight into the Molecular Mechanisms. Front. Endocrinol. 2023, 14, 1192625. [Google Scholar] [CrossRef]
- Christensen, A.A.; Gannon, M. The Beta Cell in Type 2 Diabetes. Curr. Diabetes Rep. 2019, 19, 81. [Google Scholar] [CrossRef]
- Poser, M.; Sing, K.E.A.; Ebert, T.; Ziebolz, D.; Schmalz, G. The Rosetta Stone of Successful Ageing: Does Oral Health Have a Role? Biogerontology 2023, 24, 867–888. [Google Scholar] [CrossRef]
- Gutiérrez, G.D.; Bender, A.S.; Cirulli, V.; Mastracci, T.L.; Kelly, S.M.; Tsirigos, A.; Kaestner, K.H.; Sussel, L. Pancreatic β Cell Identity Requires Continual Repression of Non–β Cell Programs. J. Clin. Investig. 2016, 127, 244–259. [Google Scholar] [CrossRef] [PubMed]
- Bi, R.; Yang, Y.; Liao, H.; Ji, G.; Ma, Y.; Cai, L.; Li, J.; Yang, J.; Sun, M.; Liang, J.; et al. Porphyromonas Gingivalis Induces an Inflammatory Response via the cGAS-STING Signaling Pathway in a Periodontitis Mouse Model. Front. Microbiol. 2023, 14, 1183415. [Google Scholar] [CrossRef]
- Misawa, M.Y.O.; Silvério Ruiz, K.G.; Nociti, F.H.; Albiero, M.L.; Saito, M.T.; Nóbrega Stipp, R.; Condino-Neto, A.; Holzhausen, M.; Palombo, H.; Villar, C.C. Periodontal Ligament-derived Mesenchymal Stem Cells Modulate Neutrophil Responses via Paracrine Mechanisms. J. Periodontol. 2019, 90, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Wang, J.; Xiao, Z.; Yang, Y.; Yang, Z.; Ai, K. ROS-Scavenging Nanomaterials to Treat Periodontitis. Front. Chem. 2020, 8, 595530. [Google Scholar] [CrossRef]
- Lian, D.; Dai, L.; Xie, Z.; Zhou, X.; Liu, X.; Zhang, Y.; Huang, Y.; Chen, Y. Periodontal Ligament Fibroblasts Migration Injury via ROS/TXNIP/Nlrp3 Inflammasome Pathway with Porphyromonas Gingivalis Lipopolysaccharide. Mol. Immunol. 2018, 103, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Almerich-Silla, J.M.; Montiel-Company, J.M.; Pastor, S.; Serrano, F.; Puig-Silla, M.; Dasí, F. Oxidative Stress Parameters in Saliva and Its Association with Periodontal Disease and Types of Bacteria. Dis. Markers 2015, 2015, 653537. [Google Scholar] [CrossRef]
- Tóthová, L.; Celec, P. Oxidative Stress and Antioxidants in the Diagnosis and Therapy of Periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: An Organ-Based Analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef]
- Cordani, M.; Resines-Urien, E.; Gamonal, A.; Milán-Rois, P.; Salmon, L.; Bousseksou, A.; Costa, J.S.; Somoza, Á. Water Soluble Iron-Based Coordination Trimers as Synergistic Adjuvants for Pancreatic Cancer. Antioxidants 2021, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Yuan, Q.; Guryanova, O.A. Epigenetic Mechanisms in Hematologic Aging and Premalignant Conditions. Epigenomes 2023, 7, 32. [Google Scholar] [CrossRef]
- Dionigi, C.; Larsson, L.; Difloe-Geisert, J.C.; Zitzmann, N.U.; Berglundh, T. Cellular Expression of Epigenetic Markers and Oxidative Stress in Periodontitis Lesions of Smokers and Non-smokers. J. Periodontal Res. 2022, 57, 952–959. [Google Scholar] [CrossRef]
- Ostuni, R.; Natoli, G.; Cassatella, M.A.; Tamassia, N. Epigenetic Regulation of Neutrophil Development and Function. Semin. Immunol. 2016, 28, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Lumb, K.J. Protein-Protein Interactions: A Structural View of Inhibition Strategies and the IL-23/IL-17 Axis. In Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 121, pp. 253–303. ISBN 978-0-12-816846-2. [Google Scholar]
- Montero, J.; Lopez-Valverde, N.; Ferrera, M.; Lopez-Valverde, A. Changes in Crevicular Cytokines after Application of Melatonin in Patients with Periodontal Disease. J. Clin. Exp. Dent. 2017, 9, e1081–e1087. [Google Scholar] [CrossRef]
- Rastgoo Haghi, A.; Khorami, N.; Fotoohi, M.; Moradi, A. MIF and MMP-9 Serum Changes in Type II Diabetes and Non-Diabetic Subjects: A Short Communication. Iran. J. Pathol. 2021, 16, 444–447. [Google Scholar] [CrossRef]
- Acharya, A.; Thakur, S.; Muddapur, M. Evaluation of Serum Interleukin-10 Levels as a Predictor of Glycemic Alteration in Chronic Periodontitis and Type 2 Diabetes Mellitus. J. Indian. Soc. Periodontol. 2015, 19, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Fang, F.; Qiu, W. The Role of Adiponectin in Periodontitis: Current State and Future Prospects. Biomed. Pharmacother. 2021, 137, 111358. [Google Scholar] [CrossRef]
- Purnamasari, D.; Khumaedi, A.I.; Soeroso, Y.; Marhamah, S. The Influence of Diabetes and or Periodontitis on Inflammation and Adiponectin Level. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2176–2182. [Google Scholar] [CrossRef]
- Deschner, J.; Eick, S.; Damanaki, A.; Nokhbehsaim, M. The Role of Adipokines in Periodontal Infection and Healing. Mol. Oral Microbiol. 2014, 29, 258–269. [Google Scholar] [CrossRef]
- Priyanka, A.; Sindhu, G.; Shyni, G.; Preetha Rani, M.; Nisha, V.; Raghu, K. Bilobalide Abates Inflammation, Insulin Resistance and Secretion of Angiogenic Factors Induced by Hypoxia in 3T3-L1 Adipocytes by Controlling NF-κB and JNK Activation. Int. Immunopharmacol. 2017, 42, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Wanichkittikul, N.; Laohapand, P.; Mansa-nguan, C.; Thanakun, S. Periodontal Treatment Improves Serum Levels of Leptin, Adiponectin, and C-Reactive Protein in Thai Patients with Overweight or Obesity. Int. J. Dent. 2021, 2021, 6660097. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Huang, Y.; Gao, P.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Leptin Aggravates Periodontitis by Promoting M1 Polarization via NLRP3. J. Dent. Res. 2022, 101, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, C.; Wu, X.; Zhang, W.; Sun, Y.; Shrestha, A. Leptin Regulates OPG and RANKL Expression in Gingival Fibroblasts and Tissues of Chronic Periodontitis Patients. Int. J. Med. Sci. 2021, 18, 2431–2437. [Google Scholar] [CrossRef]
- Luchian, I.; Goriuc, A.; Sandu, D.; Covasa, M. The Role of Matrix Metalloproteinases (MMP-8, MMP-9, MMP-13) in Periodontal and Peri-Implant Pathological Processes. Int. J. Mol. Sci. 2022, 23, 1806. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.S.; Alblowi, J.A. Effect of Subantimicrobial Dose Doxycycline Treatment on Gingival Crevicular Fluid Levels of MMP-9 and MMP-13 in Periodontitis Stage 2, Grade B in Subjects with Type 2 Diabetes Mellitus. J. Immunol. Res. 2020, 2020, 2807259. [Google Scholar] [CrossRef]
- Waligóra, J.; Kuśnierz-Cabala, B.; Pawlica-Gosiewska, D.; Gawlik, K.; Chomyszyn-Gajewska, M.; Pytko-Polończyk, J. Salivary Matrix Metalloproteinase-9 (MMP-9) as a Biomarker of Periodontitis in Pregnant Patients with Diabetes. Dent. Med. Probl. 2023, 60, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Alam, M.R.; Kamal, M.A.; Seo, K.J.; Singh, L.R. AGE-RAGE Axis Culminates into Multiple Pathogenic Processes: A Central Road to Neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1155175. [Google Scholar] [CrossRef]
- Arshad, R.; Ismail, W.A.; Zara, B.; Naseer, R.; Minhas, S.; Ansari, M.; Akhter, F.; Khokhar, S.A.; Alqahtani, A.A.; Abutayyem, H.; et al. Salivary MMP-9 Levels in Chronic Periodontitis Patients with Type-II Diabetes Mellitus. Molecules 2022, 27, 2174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Q.; Tang, W.; Hu, S.-Q.; Fu, X.-L.; Wu, H.; Shen, W.-Q.; Chen, H.-L. Effect of Matrix Metalloproteinases on the Healing of Diabetic Foot Ulcer: A Systematic Review. J. Tissue Viability 2023, 32, 51–58. [Google Scholar] [CrossRef]
- Seutter, S.; Winfield, J.; Esbitt, A.; Snyder, S.; Magner, A.; Kim, K.; Carcuffe, C.; Schmoyer, J.; Kamrani, P.; Mercando, J.; et al. Interleukin 1β and Prostaglandin E2 Affect Expression of DNA Methylating and Demethylating Enzymes in Human Gingival Fibroblasts. Int. Immunopharmacol. 2020, 78, 105920. [Google Scholar] [CrossRef]
- He, J.; Qin, M.; Chen, Y.; Hu, Z.; Xie, F.; Ye, L.; Hui, T. Epigenetic Regulation of Matrix Metalloproteinases in Inflammatory Diseases: A Narrative Review. Cell Biosci. 2020, 10, 86. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Teng, W.; Zhao, C.; Ye, X. Quantitative Evaluation of MMP-9 and TIMP-1 Promoter Methylation in Chronic Periodontitis. DNA Cell Biol. 2018, 37, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Favale, N.; Farina, R.; Carrieri, A.; Simonelli, A.; Severi, M.; Sabbioni, S.; Trombelli, L.; Scapoli, C. Functional Profile of Oral Plaque Microbiome: Further Insight into the Bidirectional Relationship between Type 2 Diabetes and Periodontitis. Mol. Oral Microbiol. 2024, 39, 62–79. [Google Scholar] [CrossRef]
- Leira, Y.; Vivancos, J.; Diz, P.; Martín, Á.; Carasol, M.; Frank, A. The Association between Periodontitis and Cerebrovascular Disease, and Dementia. Scientific Report of the Working Group of the Spanish Society of Periodontology and the Spanish Society of Neurology. Neurol. (Engl. Ed.) 2024, 39, 302–311. [Google Scholar] [CrossRef]
- Men, B.; Li, Y.; Jiang, S. Updates on the Role of Periodontitis-Related Epigenetics, Inflammation, Oral Microbiome, and Treatment in Cardiovascular Risk. J. Inflamm. Res. 2024, 17, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.M.; Loren, P.; Paez, I.; Martínez, C.; Chaparro, A.; Salazar, L.A. MicroRNAs: The Missing Link between Hypertension and Periodontitis? Int. J. Mol. Sci. 2024, 25, 1992. [Google Scholar] [CrossRef]
- Bascones-Martinez, A.; González-Febles, J. Epigenetics and Periodontitis: A Source of Connection to Systemic Diseases. In Translational Oral Health Research; Meurman, J.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 19–31. ISBN 978-3-319-78204-1. [Google Scholar]
- Cao, X.; Huo, P.; Li, W.; Li, P.; He, L.; Meng, H. Interactions among Moderate/Severe Periodontitis, ADIPOQ-Rs1501299, and LEPR-Rs1137100 Polymorphisms on the Risk of Type 2 Diabetes in a Chinese Population. Arch. Oral Biol. 2019, 103, 26–32. [Google Scholar] [CrossRef]
- Lee, H.; Joo, J.; Song, J.; Kim, H.; Kim, Y.H.; Park, H.R. Immunological Link between Periodontitis and Type 2 Diabetes Deciphered by Single-cell RNA Analysis. Clin. Transl. Med. 2023, 13, e1503. [Google Scholar] [CrossRef]
- Kang, J.; Lee, H.; Joo, J.; Song, J.; Kim, H.; Kim, Y.H.; Park, H.R. Comparison of Genetic and Epigenetic Profiles of Periodontitis According to the Presence of Type 2 Diabetes. MedComm 2024, 5, e620. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Koldobskiy, M.A.; Göndör, A. Epigenetic Modulators, Modifiers and Mediators in Cancer Aetiology and Progression. Nat. Rev. Genet. 2016, 17, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Hull, E.E.; Montgomery, M.R.; Leyva, K.J. HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. BioMed Res. Int. 2016, 2016, 8797206. [Google Scholar] [CrossRef]
- Akone, S.H.; Ntie-Kang, F.; Stuhldreier, F.; Ewonkem, M.B.; Noah, A.M.; Mouelle, S.E.M.; Müller, R. Natural Products Impacting DNA Methyltransferases and Histone Deacetylases. Front. Pharmacol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Naidoo, V.; Carrera, I.; Corzo, L.; Cacabelos, R. Natural Bioactive Products as Epigenetic Modulators for Treating Neurodegenerative Disorders. Pharmaceuticals 2023, 16, 216. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids Infused Mouthwash Products Are as Effective as Chlorhexidine on Inhibition of Total-Culturable Bacterial Content in Dental Plaque Samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W.; Nedamat, K. The Current and Potential Application of Medicinal Cannabis Products in Dentistry. Dent. J. 2021, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- David, C.; Elizalde-Hernández, A.; Barboza, A.; Cardoso, G.; Santos, M.; Moraes, R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193. [Google Scholar] [CrossRef]
- Monteiro Viana, J.C.; Da Silva Gomes, G.E.; Duarte Oliveira, F.J.; Marques De Araújo, L.N.; Teles, G.; Mourão, C.F.; De Vasconcelos Gurgel, B.C. The Role of Different Types of Cannabinoids in Periodontal Disease: An Integrative Review. Pharmaceutics 2024, 16, 893. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Yu, S.; Li, C.; Gao, B.; Zhou, X. Cannabidiol Attenuates Periodontal Inflammation through Inhibiting TLR4/NF-κB Pathway. J. Periodontal Res. 2023, 58, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Rapino, C.; Di Francesco, A.; Dainese, E.; D’Addario, C.; Maccarrone, M. Epigenetic Control of Skin Differentiation Genes by Phytocannabinoids. Br. J. Pharmacol. 2013, 170, 581–591. [Google Scholar] [CrossRef]
- Massimini, M.; Dalle Vedove, E.; Bachetti, B.; Di Pierro, F.; Ribecco, C.; D’Addario, C.; Pucci, M. Polyphenols and Cannabidiol Modulate Transcriptional Regulation of Th1/Th2 Inflammatory Genes Related to Canine Atopic Dermatitis. Front. Vet. Sci. 2021, 8, 606197. [Google Scholar] [CrossRef]
- Jîtcă, G.; Ősz, B.E.; Vari, C.E.; Rusz, C.-M.; Tero-Vescan, A.; Pușcaș, A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants 2023, 12, 485. [Google Scholar] [CrossRef]
- Pantell, M.S.; Silveira, P.P.; De Mendonça Filho, E.J.; Wing, H.; Brown, E.M.; Keeton, V.F.; Pokhvisneva, I.; O’Donnell, K.J.; Neuhaus, J.; Hessler, D.; et al. Associations between Social Adversity and Biomarkers of Inflammation, Stress, and Aging in Children. Pediatr. Res. 2024, 95, 1553–1563. [Google Scholar] [CrossRef]
- Zanelati, T.; Biojone, C.; Moreira, F.; Guimarães, F.; Joca, S. Antidepressant-like Effects of Cannabidiol in Mice: Possible Involvement of 5-HT 1A Receptors. Br. J. Pharmacol. 2010, 159, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Aikins, J.; Warshal, D.; Ostrovsky, O. Can Cannabidiol Affect the Efficacy of Chemotherapy and Epigenetic Treatments in Cancer? Biomolecules 2021, 11, 766. [Google Scholar] [CrossRef]
- Gerecke, C.; Egea Rodrigues, C.; Homann, T.; Kleuser, B. The Role of Ten-Eleven Translocation Proteins in Inflammation. Front. Immunol. 2022, 13, 861351. [Google Scholar] [CrossRef] [PubMed]
- Antonyová, V.; Kejík, Z.; Brogyanyi, T.; Kaplánek, R.; Veselá, K.; Abramenko, N.; Ocelka, T.; Masařík, M.; Matkowski, A.; Gburek, J.; et al. Non-Psychotropic Cannabinoids as Inhibitors of TET1 Protein. Bioorganic Chem. 2022, 124, 105793. [Google Scholar] [CrossRef]
- Reece, A.S.; Hulse, G.K. Impacts of Cannabinoid Epigenetics on Human Development: Reflections on Murphy et. al. ‘Cannabinoid Exposure and Altered DNA Methylation in Rat and Human Sperm’ Epigenetics 2018; 13: 1208-1221. Epigenetics 2019, 14, 1041–1056. [Google Scholar] [CrossRef]
- Wanner, N.M.; Colwell, M.; Drown, C.; Faulk, C. Developmental Cannabidiol Exposure Increases Anxiety and Modifies Genome-Wide Brain DNA Methylation in Adult Female Mice. Clin. Epigenetics 2021, 13, 4. [Google Scholar] [CrossRef]
- Lanza Cariccio, V.; Scionti, D.; Raffa, A.; Iori, R.; Pollastro, F.; Diomede, F.; Bramanti, P.; Trubiani, O.; Mazzon, E. Treatment of Periodontal Ligament Stem Cells with MOR and CBD Promotes Cell Survival and Neuronal Differentiation via the PI3K/Akt/mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2341. [Google Scholar] [CrossRef]
- Rajan, T.S.; Giacoppo, S.; Iori, R.; De Nicola, G.R.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E. Anti-Inflammatory and Antioxidant Effects of a Combination of Cannabidiol and Moringin in LPS-Stimulated Macrophages. Fitoterapia 2016, 112, 104–115. [Google Scholar] [CrossRef]
- Ala, M.; Ala, M. Metformin for Cardiovascular Protection, Inflammatory Bowel Disease, Osteoporosis, Periodontitis, Polycystic Ovarian Syndrome, Neurodegeneration, Cancer, Inflammation and Senescence: What Is Next? ACS Pharmacol. Transl. Sci. 2021, 4, 1747–1770. [Google Scholar] [CrossRef]
- Sun, T.; Liu, J.; Xie, C.; Yang, J.; Zhao, L.; Yang, J. Metformin Attenuates Diabetic Renal Injury via the AMPK-Autophagy Axis. Exp. Ther. Med. 2021, 21, 578. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFκB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef]
- Agius, L.; Ford, B.E.; Chachra, S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020, 21, 3240. [Google Scholar] [CrossRef] [PubMed]
- Koethe, D.; Rohleder, C.; Kracht, L.; Leweke, F.M. Cannabidiol Enhances Cerebral Glucose Utilization and Ameliorates Psychopathology and Cognition: A Case Report in a Clinically High-Risk Mental State. Front. Psychiatry 2023, 14, 1088459. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, I.; Pozo-Morales, M.; Romero-Zerbo, S.Y.; Espinosa-Jimenez, V.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Cobo-Vuilleumier, N.; Gauthier, B.R.; Bermúdez-Silva, F.J. Abnormal Cannabidiol Ameliorates Inflammation Preserving Pancreatic Beta Cells in Mouse Models of Experimental Type 1 Diabetes and Beta Cell Damage. Biomed. Pharmacother. 2022, 145, 112361. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.D.S.; Jantsch, J.; Fraga, G.D.F.; Dias, V.S.; Eller, S.; De Oliveira, T.F.; Giovenardi, M.; Guedes, R.P. Cannabidiol Treatment Improves Metabolic Profile and Decreases Hypothalamic Inflammation Caused by Maternal Obesity. Front. Nutr. 2023, 10, 1150189. [Google Scholar] [CrossRef]
- Jiang, N.; Zhong, B.; Huang, J.; Li, W.; Zhang, S.; Zhu, X.; Ni, C.; Shen, J. Transarterial Chemoembolization Combined with Molecularly Targeted Agents plus Immune Checkpoint Inhibitors for Unresectable Hepatocellular Carcinoma: A Retrospective Cohort Study. Front. Immunol. 2023, 14, 1205636. [Google Scholar] [CrossRef]
- He, K.-Y.; Lei, X.-Y.; Wu, D.-H.; Zhang, L.; Li, J.-Q.; Li, Q.-T.; Yin, W.-T.; Zhao, Z.-L.; Liu, H.; Xiang, X.-Y.; et al. Akkermansia Muciniphila Protects the Intestine from Irradiation-Induced Injury by Secretion of Propionic Acid. Gut Microbes 2023, 15, 2293312. [Google Scholar] [CrossRef]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-Induced Hyperpermeability of the Human Gut In Vitro and In Vivo—A Randomized, Placebo-Controlled, Double-Blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Okamoto, Y.; Yamamoto, I.; Watanabe, K. Cannabidiol Is a Potent Inhibitor of the Catalytic Activity of Cytochrome P450 2C19. Drug Metab. Pharmacokinet. 2013, 28, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-J.; Wang, J.-S.; Markowitz, J.S.; Donovan, J.L.; Gibson, B.B.; Gefroh, H.A.; DeVane, C.L. Characterization of P-Glycoprotein Inhibition by Major Cannabinoids from Marijuana. J. Pharmacol. Exp. Ther. 2006, 317, 850–857. [Google Scholar] [CrossRef] [PubMed]
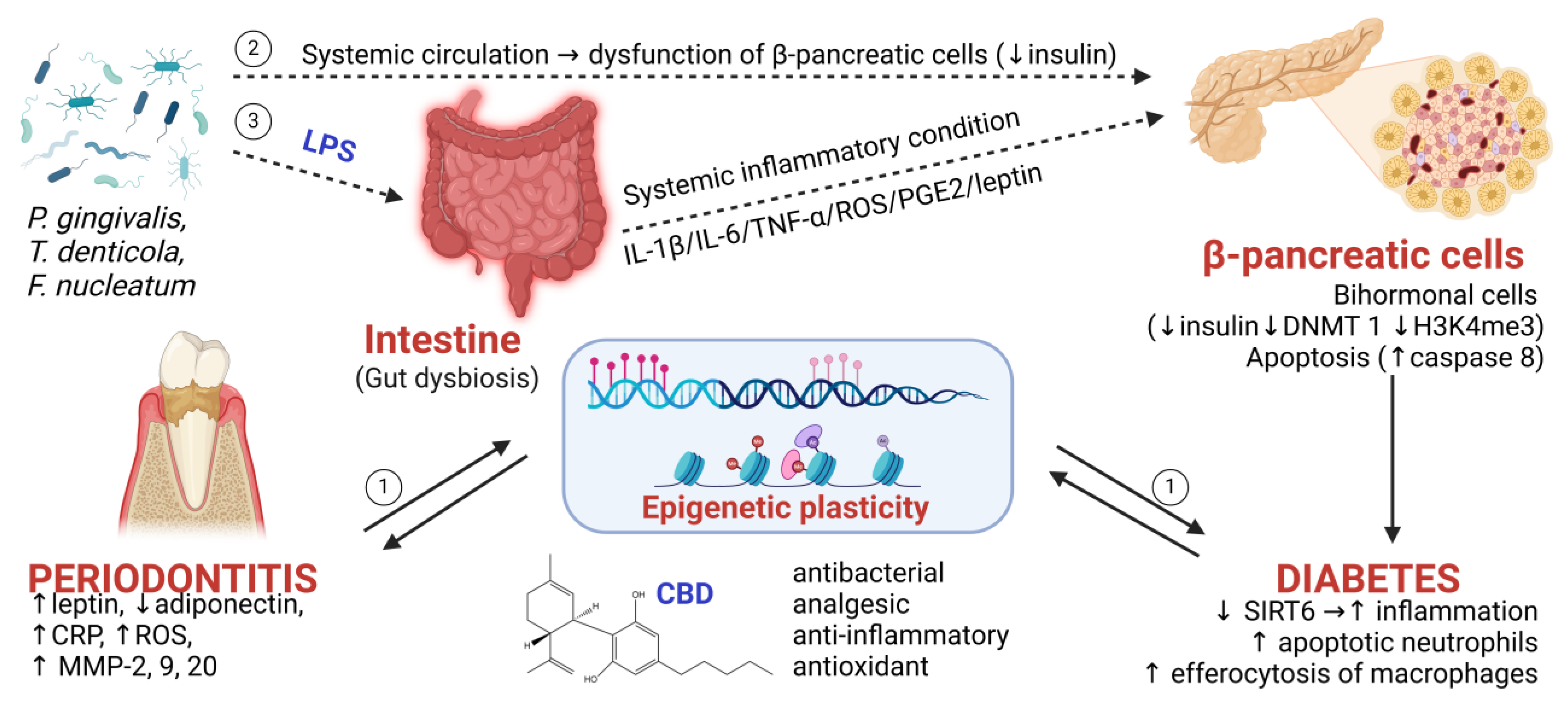
| General Mechanism | Study Type | Administration Pathway | Mechanistic Details | Reference |
|---|---|---|---|---|
| Gut dysbiosis induced by oral bacteria | In vivo, C57BL/6 mice | Ligature-induced periodontitis + the administration of P. gingivalis by gavage |
| [22] |
| In vitro, human osteoblast hFOB1.19 cells | 0, 2, 5, or 10 µg/mL LPSs |
| [23] | |
| β-cell apoptosis and β-pancreatic bi(poli)-hormonal cells | In vivo, C57BL/6 mice | 22 weeks oral application of P. gingivalis |
| [24] |
| In vivo, NKX2.2ΔBeta mice | - |
| [31,33] | |
| Epigenetic plasticity responsible for the inducement and dissemination of inflammation | In vitro/in vivo (murine model) | Ligature ligation and oral infection with P. gingivalis |
| [3,26,34] |
| In vitro, CD105-enriched periodontal ligament-derived mesenchymal stem cells | P. gingivalis total protein extract (PgPE) (0 or 2 ug/mL) for 3 h |
| [35,36] | |
| In vitro, Mouse periodontal ligament fibroblasts (mPDLFs) | LPSs (0.5 mM, 1 mM, and 2 mM) |
| [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tero-Vescan, A.; Slevin, M.; Pușcaș, A.; Sita, D.; Ștefănescu, R. Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review. Int. J. Mol. Sci. 2025, 26, 2853. https://doi.org/10.3390/ijms26072853
Tero-Vescan A, Slevin M, Pușcaș A, Sita D, Ștefănescu R. Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review. International Journal of Molecular Sciences. 2025; 26(7):2853. https://doi.org/10.3390/ijms26072853
Chicago/Turabian StyleTero-Vescan, Amelia, Mark Slevin, Amalia Pușcaș, Dragoș Sita, and Ruxandra Ștefănescu. 2025. "Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review" International Journal of Molecular Sciences 26, no. 7: 2853. https://doi.org/10.3390/ijms26072853
APA StyleTero-Vescan, A., Slevin, M., Pușcaș, A., Sita, D., & Ștefănescu, R. (2025). Targeting Epigenetic Plasticity to Reduce Periodontitis-Related Inflammation in Diabetes: CBD, Metformin, and Other Natural Products as Potential Synergistic Candidates for Regulation? A Narrative Review. International Journal of Molecular Sciences, 26(7), 2853. https://doi.org/10.3390/ijms26072853






