Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes
Abstract
1. Introduction
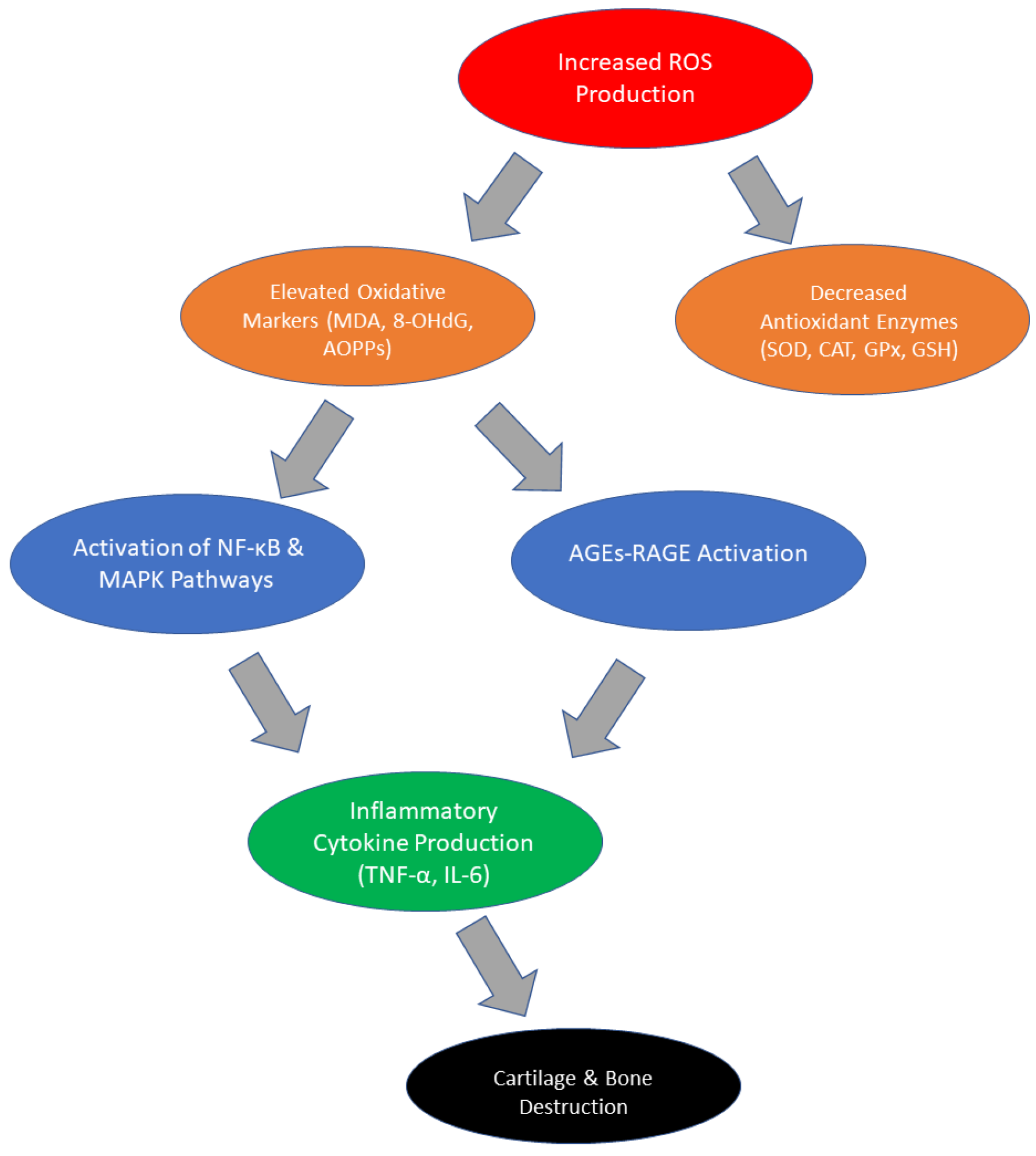
2. Changes in Oxidative Stress Parameters in RA
3. Current Therapeutic Options and Their Limitations
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) and Glucocorticosteroids
4. Potential Antioxidant Therapies for RA
4.1. Curcumin
4.2. Resveratrol and Other Polyphenols
4.3. N-Acetylcysteine (NAC)
4.4. Sulforaphane (SFN)
4.5. Propolis
4.6. Molecular Hydrogen Therapies
4.7. The Role of Vitamins in Antioxidant Therapy
5. The Importance of Antioxidant Therapies
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crowley, T.; O’Neil, J.D.; Adams, H.; Thomas, A.M.; Filer, A.; Buckley, C.D.; Clark, A.R. Priming in Response to Pro-Inflammatory Cytokines Is a Feature of Adult Synovial but Not Dermal Fibroblasts. Arthritis Res. Ther. 2017, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Islander, U.; Jochems, C.; Lagerquist, M.K.; Forsblad-d’Elia, H.; Carlsten, H. Estrogens in Rheumatoid Arthritis; the Immune System and Bone. Mol. Cell. Endocrinol. 2010, 335, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Sur, L.M.; Gaga, R.; Duca, E.; Sur, G.; Lupan, I.; Sur, D.; Samasca, G.; Lazea, C.; Lazar, C. Different Chronic Disorders That Fall within the Term Juvenile Idiopathic Arthritis. Life 2021, 11, 398. [Google Scholar] [CrossRef]
- Dar, W.R.; Mir, I.A.; Siddiq, S.; Nadeem, M.; Singh, G. The Assessment of Fatigue in Rheumatoid Arthritis Patients and Its Impact on Their Quality of Life. Clin. Pract. 2022, 12, 591–598. [Google Scholar] [CrossRef]
- Katz, P. Causes and Consequences of Fatigue in Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2017, 29, 269–276. [Google Scholar] [CrossRef]
- Kapitány, A.; Zilahi, E.; Szántó, S.; Szücs, G.; Szabó, Z.; Végvári, A.; Rass, P.; Sipka, S.; Szegedi, G.; Szekanecz, Z. Association of Rheumatoid Arthritis with HLA-DR1 and HLA-DR4 in Hungary. Ann. N. Y. Acad. Sci 2005, 1051, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Layh-Schmitt, G.; Lu, S.; Navid, F.; Brooks, S.R.; Lazowick, E.; Davis, K.M.; Montagna, C.; Gadina, M.; Colbert, R.A. Generation and Differentiation of Induced Pluripotent Stem Cells Reveal Ankylosing Spondylitis Risk Gene Expression in Bone Progenitors. Clin. Rheumatol. 2016, 36, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Terry, F.E.; Gutierrez, A.H.; Hirano, T.; Hoshi, M.; Mizuno, Y.; Martin, W.; Yasunaga, S.; Niiro, H.; Fujio, K.; et al. Individual and Population-Level Variability in HLA-DR Associated Immunogenicity Risk of Biologics Used for the Treatment of Rheumatoid Arthritis. Front. Immunol. 2024, 15, 1377911. [Google Scholar] [CrossRef]
- Chang, K.; Yang, S.; Kim, S.; Han, K.; Park, S.; Shin, J. Smoking and Rheumatoid Arthritis. Int. J. Mol. Sci. 2014, 15, 22279–22295. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Terao, C. The Impact of Cigarette Smoking on Risk of Rheumatoid Arthritis: A Narrative Review. Cells 2020, 9, 475. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Y.; Wang, R.; He, Y.; Ge, H.; Yang, Z.; Jin, Z.; Jin, H.; Lv, S.; Zhan, H. The Global Magnitude and Temporal Trend of Rheumatoid Arthritis Burden Attributable to Smoking from 1990 to 2019. Lara D. Rheumatol. 2023, 63, 689–697. [Google Scholar] [CrossRef]
- Scherer, H.U.; Häupl, T.; Burmester, G.R. The Etiology of Rheumatoid Arthritis. J. Autoimmunol. 2020, 110, 102400. [Google Scholar] [CrossRef] [PubMed]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef]
- Yao, X.; Yang, Y.; Jiang, Z.; Ma, W.; Yao, X. The Causal Impact of Saturated Fatty Acids on Rheumatoid Arthritis: A Bidirectional Mendelian Randomisation Study. Front. Nutri. 2024, 11, 1337256. [Google Scholar] [CrossRef]
- Jang, S.; Kwon, E.-J.; Lee, J.J. Rheumatoid Arthritis: Pathogenic Roles of Diverse Immune Cells. Int. J. Mol. Sci. 2022, 23, 905. [Google Scholar] [CrossRef]
- Pulik, Ł.; Łęgosz, P.; Motyl, G. Matrix Metalloproteinases in Rheumatoid Arthritis and Osteoarthritis: A State of the Art Review. Rheumatology 2023, 61, 191–201. [Google Scholar] [CrossRef]
- Tutan, D.; Doğan, A.G. Pan-Immune-Inflammation Index as a Biomarker for Rheumatoid Arthritis Progression and Diagnosis. Cureus 2023, 15, e46609. [Google Scholar] [CrossRef]
- Veglia, M.; D’Ippolito, S.; Marana, R.; Di Nicuolo, F.; Castellani, R.; Bruno, V.; Fiorelli, A.; Ria, F.; Maulucci, G.; De Spirito, M.; et al. Human IGG Antinuclear Antibodies Induce Pregnancy Loss in Mice by Increasing Immune Complex Deposition in Placental Tissue:In VivoStudy. Am. J. Reprod. Immunol. 2015, 74, 542–552. [Google Scholar] [CrossRef]
- Di Simone, N.; Castellani, R.; Raschi, E.; Borghi, M.O.; Meroni, P.L.; Caruso, A. Anti-Beta-2 Glycoprotein I Antibodies Affect Bcl-2 and Bax Trophoblast Expression without Evidence of Apoptosis. Ann. N. Y. Acad. Sci. 2006, 1069, 364–376. [Google Scholar] [CrossRef]
- Di Simone, N.; Castellani, R.; Caliandro, D.; Caruso, A. Monoclonal Anti-Annexin V Antibody Inhibits Trophoblast Gonadotropin Secretion and Induces Syncytiotrophoblast Apoptosis. Biol. Reprod. 2001, 65, 1766–1770. [Google Scholar] [CrossRef]
- Bilski, R.; Kamiński, P.; Kupczyk, D.; Jeka, S.; Baszyński, J.; Tkaczenko, H.; Kurhaluk, N. Environmental and Genetic Determinants of Ankylosing Spondylitis. Int. J. Mol. Sci. 2024, 25, 7814. [Google Scholar] [CrossRef] [PubMed]
- Pertsinidou, E.; Saevarsdottir, S.; Manivel, V.A.; Klareskog, L.; Alfredsson, L.; Mathsson-Alm, L.; Hansson, M.; Cornillet, M.; Serre, G.; Holmdahl, R.; et al. In Early Rheumatoid Arthritis, Anticitrullinated Peptide Antibodies Associate with Low Number of Affected Joints and Rheumatoid Factor Associates with Systemic Inflammation. Ann. Rheum. Dis. 2023, 83, 277–287. [Google Scholar] [CrossRef]
- Iyengar, K.P.; Vaish, A.; Nune, A. Anti-Cyclic Citrullinated Peptide Antibody (ACPA) and Rheumatoid Arthritis: Clinical Relevance. J. Orthop. Trauma 2021, 24, 101729. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2021, 63, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Zamudio-Cuevas, Y.; Martínez-Flores, K.; Martínez-Nava, G.A.; Clavijo-Cornejo, D.; Fernández-Torres, J.; Sánchez-Sánchez, R. Rheumatoid Arthritis and Oxidative Stress, a Review of a Decade. Mol. Cell Biol. 2022, 68, 174–184. [Google Scholar] [CrossRef]
- Kaur, G.; Sharma, A.; Bhatnagar, A. Role of Oxidative Stress in Pathophysiology of Rheumatoid Arthritis: Insights into NRF2-KEAP1 Signalling. Autoimmunity 2021, 54, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fan, D.; Cao, X.; Ye, Q.; Wang, Q.; Zhang, M.; Xiao, C. The Role of Reactive Oxygen Species in the Rheumatoid Arthritis-Associated Synovial Microenvironment. Antioxidants 2022, 11, 1153. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Didion, S. Cellular and Oxidative Mechanisms Associated with Interleukin-6 Signaling in the Vasculature. Int. J. Mol. Sci. 2017, 18, 2563. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Jing, W.; Liu, C.; Su, C.; Liu, L.; Chen, P.; Li, X.; Zhang, X.; Yuan, B.; Wang, H.; Du, X. Role of Reactive Oxygen Species and Mitochondrial Damage in Rheumatoid Arthritis and Targeted Drugs. Front. Immunol. 2023, 14, 1107670. [Google Scholar] [CrossRef]
- Tudorachi, N.B.; Totu, E.E.; Fifere, A.; Ardeleanu, V.; Mocanu, V.; Mircea, C.; Isildak, I.; Smilkov, K.; Cărăuşu, E.M. The Implication of Reactive Oxygen Species and Antioxidants in Knee Osteoarthritis. Antioxidants 2021, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhang, G.; Zhang, X.; Zhao, Q. Nrf2-Mediated Ferroptosis Inhibition: A Novel Approach for Managing Inflammatory Diseases. Inflammopharmacology 2024, 32, 2961–2986. [Google Scholar] [CrossRef] [PubMed]
- Khadim, R.M.; Al-Fartusie, F.S. Evaluation of Some Trace Elements and Antioxidants in Sera of Patients with Rheumatoid Arthritis: A Case–Control Study. Clin. Rheumatol. 2022, 42, 55–65. [Google Scholar] [CrossRef]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Jazayeri, S.H.; Chamari, M.; Nazari, S.A. Beta-Carotene, Vitamin E, MDA, Glutathione Reductase and Arylesterase Activity Levels in Patients with Active Rheumatoid Arthritis. Iran. J. Public Health 2011, 40, 102. [Google Scholar]
- Nabih, G.A.; Sheshtawy, N.E.; Mikkawy, D.M.E.E.; Kamel, M.A. Serum Malondialdehyde as a Marker of Oxidative Stress in Rheumatoid Arthritis. Egypt. Rheumatol. Rehabil. 2024, 51, 43. [Google Scholar] [CrossRef]
- Hassan, W.M. Oxidative DNA Damage and Zinc Status in Patients with Rheumatoid Arthritis in Duhok, Iraq. Cureus 2024, 16, e52860. [Google Scholar] [CrossRef] [PubMed]
- Rall, L.C.; Roubenoff, R.; Meydani, S.N.; Han, S.N.; Meydani, M. Urinary 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Marker of Oxidative Stress in Rheumatoid Arthritis and Aging: Effect of Progressive Resistance Training. J. Nutr. Biochem. 2000, 11, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kanai, T.; Okada, M. Rheumatoid Arthritis and Reactive Oxygen Species: A Review. Curr. Issues Mol. Biol. 2023, 45, 3000–3015. [Google Scholar] [CrossRef]
- Quiñonez-Flores, C.M.; González-Chávez, S.A.; Del Río Nájera, D.; Pacheco-Tena, C. Oxidative Stress Relevance in the Pathogenesis of the Rheumatoid Arthritis: A Systematic Review. BioMed Res. Int. 2016, 2016, 6097417. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N. Increased Reactive Oxygen Species Formation and Oxidative Stress in Rheumatoid Arthritis. PLoS ONE 2016, 11, e0152925. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Hadi, R.A.; Al-Rawi, Z.S.; Padron, V.A.; Stohs, S.J. The Glutathione Defense System in the Pathogenesis of Rheumatoid Arthritis. J. Appl. Toxicol. 2001, 21, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Kadry, M.O. Liposomal Glutathione as a Promising Candidate for Immunological Rheumatoid Arthritis Therapy. Heliyon 2019, 5, e02162. [Google Scholar] [CrossRef] [PubMed]
- Kryl’skii, E.D.; Popova, T.N.; Kirilova, E.M. Activity of Glutathione Antioxidant System and NADPH-Generating Enzymes in Rats with Experimental Rheumatoid Arthritis. Exp. Biol. Med. 2015, 160, 24–27. [Google Scholar] [CrossRef]
- Lou, A.; Wang, L.; Lai, W.; Zhu, D.; Wu, W.; Wang, Z.; Cai, Z.; Yang, M. Advanced Oxidation Protein Products Induce Inflammatory Responses and Invasive Behaviour in Fibroblast-like Synoviocytes via the RAGE-NF-κB Pathway. Bone Jt. Res. 2021, 10, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Najafizadeh, S.R.; Amiri, K.; Moghaddassi, M.; Khanmohammadi, S.; Mirmiranpour, H.; Nakhjavani, M. Advanced Glycation End Products, Advanced Oxidation Protein Products, and Ferric Reducing Ability of Plasma in Patients with Rheumatoid Arthritis: A Focus on Activity Scores. Clin. Rheumatol. 2021, 40, 4019–4026. [Google Scholar] [CrossRef]
- De Groot, L.; Hinkema, H.; Westra, J.; Smit, A.J.; Kallenberg, C.G.; Bijl, M.; Posthumus, M.D. Advanced Glycation Endproducts Are Increased in Rheumatoid Arthritis Patients with Controlled Disease. Arthritis Res. Ther. 2011, 13, R205. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, R.; Delanghe, J.R.; Speeckaert, M.M. The Potential Influence of Advanced Glycation End Products and (s)RAGE in Rheumatic Diseases. Int. J. Mol. Sci. 2023, 24, 2894. [Google Scholar] [CrossRef]
- Tarannum, A. Nitroxidized-Albumin Advanced Glycation End Product and Rheumatoid Arthritis. Arch. Rheumatol. 2019, 34, 461–475. [Google Scholar] [CrossRef]
- Monu, N.; Agnihotri, P.; Saquib, M.; Sarkar, A.; Chakraborty, D.; Kumar, U.; Biswas, S. Transthyretin and Receptor for Advanced Glycation End Product’s Differential Levels Associated with the Pathogenesis of Rheumatoid Arthritis. J. Inflamm. Res. 2021, 14, 5581–5596. [Google Scholar] [CrossRef]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for Advanced Glycation End Products and Its Involvement in Inflammatory Diseases. Int. J. Inflamm. 2013, 2013, 403460. [Google Scholar] [CrossRef]
- Rojas, A.; Lindner, C.; Schneider, I.; Gonzalez, I.; Uribarri, J. The RAGE Axis: A Relevant Inflammatory Hub in Human Diseases. Biomolecules 2024, 14, 412. [Google Scholar] [CrossRef]
- Rojas, A.; Morales, M.; Gonzalez, I.; Araya, P. Inhibition of RAGE Axis Signaling: A Pharmacological Challenge. Curr. Drug Targets 2018, 20, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Feng, J.-T.; Zhang, L.; Kuang, Y. Clinical Significance of Serum Total Oxidant/Antioxidant Status for the Disease Activity in Active Rheumatoid Arthritis. Int. J. Clin. Exp. Pathol. 2017, 10, 8895–8900. [Google Scholar]
- Bekar, C.; Armagan, B.; Sari, A.; Ayaz, A. Evaluation of Serum Total Antioxidant Level, Nutritional Status and Mediterranean Diet Adherence of Adult Women with Rheumatoid Arthritis: A Case-Control Study. Br. J. Nutr. 2025, 133, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Altindag, O.; Karakoc, M.; Kocyigit, A.; Celik, H.; Soran, N. Increased DNA Damage and Oxidative Stress in Patients with Rheumatoid Arthritis. Clin. Biochem. 2006, 40, 167–171. [Google Scholar] [CrossRef]
- García-González, A.; Gaxiola-Robles, R.; Zenteno-Savín, T. Oxidative Stress in Patients with Rheumatoid Arthritis. Rev. Investig. Clin. 2015, 67, 46–53. [Google Scholar]
- Veselinovic, M.; Barudzic, N.; Vuletic, M.; Zivkovic, V.; Tomic-Lucic, A.; Djuric, D.; Jakovljevic, V. Oxidative Stress in Rheumatoid Arthritis Patients: Relationship to Diseases Activity. Mol. Cell. Biochem. 2014, 391, 225–232. [Google Scholar] [CrossRef]
- Alzaidi, A.F.; Yağlioğlu, A.Ş.; Aziz, M.A.; Jabbar, N.M. The Relevance of Antioxidants, Oxidative Stress and Inflammatory Parameters in Rheumatoid Arthritis Patients. AIP Conf. Proc. 2023, 2820, 050016. [Google Scholar] [CrossRef]
- Vasanthi, P.; Nalini, G.; Rajasekhar, G. Status of Oxidative Stress in Rheumatoid Arthritis. Int. J. Rheum. Dis. 2009, 12, 29–33. [Google Scholar] [CrossRef]
- Surapneni, K.M.; Gopan, V.S.C. Lipid Peroxidation and Antioxidant Status in Patients with Rheumatoid Arthritis. Indian J. Clin. Biochem. 2008, 23, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.Z.; Gheita, T.A.; Kenawy, S.A.; Fahim, A.T.; El-Sorougy, I.M.; Abdou, M.S. Oxidative Stress in Systemic Lupus Erythematosus and Rheumatoid Arthritis Patients: Relationship to Disease Manifestations and Activity. Int. J. Rheum. Dis. 2011, 14, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Alver, A.; Şentürk, A.; Çakirbay, H.; Menteşe, A.; Gökmen, F.; Keha, E.E.; Uçar, F. Carbonic Anhydrase II Autoantibody and Oxidative Stress in Rheumatoid Arthritis. Clin. Biochem. 2011, 44, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Wanchu, A.; Bhatnagar, A. Interaction between Oxidative Stress and Chemokines: Possible Pathogenic Role in Systemic Lupus Erythematosus and Rheumatoid Arthritis. Immunobiology 2011, 216, 1010–1017. [Google Scholar] [CrossRef]
- Sarban, S.; Kocyigit, A.; Yazar, M.; Isikan, U.E. Plasma Total Antioxidant Capacity, Lipid Peroxidation, and Erythrocyte Antioxidant Enzyme Activities in Patients with Rheumatoid Arthritis and Osteoarthritis. Clin. Biochem. 2005, 38, 981–986. [Google Scholar] [CrossRef]
- Jacobson, G.A.; Ives, S.J.; Narkowicz, C.; Jones, G. Plasma Glutathione Peroxidase (GSH-Px) Concentration Is Elevated in Rheumatoid Arthritis: A Case–Control Study. Clin. Rheumatol. 2012, 31, 1543–1547. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, J.; Chang, S.; Kuo, C.; See, L. Trends of Adverse Events and Mortality after DMARDs in Patients with Rheumatoid Arthritis: Interrupted Time-series Analysis. Immun. Inflamm. Dis. 2022, 10, e630. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G.; Negru, P.A.; Marcu, M.F.; Andronie-Cioara, F.L. In-Depth Bibliometric Analysis and Current Scientific Mapping Research in the Context of Rheumatoid Arthritis Pharmacotherapy. Biomed. Pharmacother. 2022, 154, 113614. [Google Scholar] [CrossRef]
- Aletaha, D. Toxicity Profiles of Traditional Disease Modifying Antirheumatic Drugs for Rheumatoid Arthritis. Ann. Rheum. Dis. 2003, 62, 482–486. [Google Scholar] [CrossRef]
- Kedia, A.K.; Mohansundaram, K.; Goyal, M.; Ravindran, V. Safety of Long-Term Use of Four Common Conventional Disease Modifying Anti-Rheumatic Drugs in Rheumatoid Arthritis. J. R. Coll. Physicians Edinb. 2021, 51, 237–245. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, L. Side Effects of Methotrexate Therapy for Rheumatoid Arthritis: A Systematic Review. Eur. J. Med. Chem. 2018, 158, 502–516. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, Y.; Luo, W.-W.; Cheng, X.; Xiang, H.-R.; Zhang, Q.-Z.; He, J.; Peng, W.-X. The Risk of Adverse Effects of TNF-A Inhibitors in Patients with Rheumatoid Arthritis: A Network Meta-Analysis. Front. Immunol. 2022, 13, 814429. [Google Scholar] [CrossRef]
- Watanabe, A.; Matsumoto, T.; Igari, H.; Sawa, J.; Yamaguchi, Y.; Sakatani, M. Risk of Developing Active Tuberculosis in Rheumatoid Arthritis Patients on Adalimumab in Japan. Int. J. Tuberc. Lung Dis. 2015, 20, 101–108. [Google Scholar] [CrossRef]
- Scheinfeld, N. Adalimumab: A Review of Side Effects. Expert Opin. Drug Saf. 2005, 4, 637–641. [Google Scholar] [CrossRef]
- Minozzi, S.; Bonovas, S.; Lytras, T.; Pecoraro, V.; González-Lorenzo, M.; Bastiampillai, A.J.; Gabrielli, E.M.; Lonati, A.C.; Moja, L.; Cinquini, M.; et al. Risk of Infections Using Anti-TNF Agents in Rheumatoid Arthritis, Psoriatic Arthritis, and Ankylosing Spondylitis: A Systematic Review and Meta-Analysis. Expert Opin. Drug Saf. 2016, 15, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Charles-Schoeman, C.; Buch, M.H.; Dougados, M.; Bhatt, D.L.; Giles, J.T.; Ytterberg, S.R.; Koch, G.G.; Vranic, I.; Wu, J.; Wang, C.; et al. Risk of Major Adverse Cardiovascular Events with Tofacitinib versus Tumour Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis with or without a History of Atherosclerotic Cardiovascular Disease: A Post Hoc Analysis from ORAL Surveillance. Ann. Rheum. Dis. 2022, 82, 119–129. [Google Scholar] [CrossRef]
- Goldman, A.; Galper, B.-E.L.; Druyan, A.; Grossman, C.; Sharif, K.; Shechtman, L.; Moshkovits, Y.; Lahat, A.; Ben-Zvi, I. Adverse Cardiovascular Events in Rheumatoid Arthritis Patients Treated with JAK Inhibitors: An Analysis of Postmarketing Spontaneous Safety Reports. Semin. Arthritis Rheum. 2024, 67, 152461. [Google Scholar] [CrossRef]
- Szekanecz, Z.; Szűcs, G.; Kerekes, G. Antirheumatic Drugs and Cardiovascular Disease in Rheumatoid Arthritis. Rheumatol. Forum 2023, 9, 49–62. [Google Scholar] [CrossRef]
- Vonkeman, H.E.; Van De Laar, M.A.F.J. Nonsteroidal Anti-Inflammatory Drugs: Adverse Effects and Their Prevention. Semin. Arthritis Rheum. 2008, 39, 294–312. [Google Scholar] [CrossRef]
- Bobek, D. Use of Non-Steroidal Anti-Inflammatory Drugs in Patients with Advanced Active Rheumatoid Arthritis. Acta Clin. Croat. 2022, 61, 588. [Google Scholar] [CrossRef]
- Costello, R.; David, T.; Jani, M. Impact of Adverse Events Associated with Medications in the Treatment and Prevention of Rheumatoid Arthritis. Clin. Ther. 2019, 41, 1376–1396. [Google Scholar] [CrossRef] [PubMed]
- Petta, I.; Peene, I.; Elewaut, D.; Vereecke, L.; De Bosscher, K. Risks and Benefits of Corticosteroids in Arthritic Diseases in the Clinic. Biochem. Pharmacol. 2019, 165, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Malanga, G.A.; Capella, T. Corticosteroids: Review of the History, the Effectiveness, and Adverse Effects in the Treatment of Joint Pain. Pain Physician 2021, 24 (Suppl. 1), S233–S246. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Nanomedical Approaches in the Realm of Rheumatoid Arthritis. Ageing Res. Rev. 2023, 87, 101927. [Google Scholar] [CrossRef]
- Sharma, D.; Chaubey, P.; Suvarna, V. Role of Natural Products in Alleviation of Rheumatoid Arthritis—A Review. J. Food Biochem. 2021, 45, e13673. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, K.; Samanovic, A.M.; Veselinovic, M.; Zivkovic, V.; Mikhaylovsky, V.; Mikerova, M.; Reshetnikov, V.; Jakovljevic, V.; Turnic, T.N. Oxidative Stress Mediated Therapy in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1938. [Google Scholar] [CrossRef]
- Jin, W.; Botchway, B.O.A.; Liu, X. Curcumin Can Activate the NRF2/HO-1 Signaling Pathway and Scavenge Free Radicals in Spinal Cord Injury Treatment. Neurorehabilit. Neural Repair 2021, 35, 576–584. [Google Scholar] [CrossRef]
- Schiavoni, V.; Emanuelli, M.; Milanese, G.; Galosi, A.B.; Pompei, V.; Salvolini, E.; Campagna, R. NRF2 Signaling in Renal Cell Carcinoma: A Potential Candidate for the Development of Novel Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 13239. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of NRF2/KEAP1 Signaling by Phytotherapeutics in Periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Radzka, J.; Łapińska, Z.; Szwedowicz, U.; Gajewska-Naryniecka, A.; Gizak, A.; Kulbacka, J. Alternations of NF-ΚB Signaling by Natural Compounds in Muscle-Derived Cancers. Int. J. Mol. Sci. 2023, 24, 11900. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.-L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by SOX9/NF-KB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the NRF2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2019, 20, 116–133. [Google Scholar] [CrossRef]
- Ghoushi, E.; Poudineh, M.; Parsamanesh, N.; Jamialahmadi, T.; Sahebkar, A. Curcumin as a Regulator of Th17 Cells: Unveiling the Mechanisms. Food Chem. Mol. Sci. 2024, 8, 100198. [Google Scholar] [CrossRef]
- Kou, H.; Huang, L.; Jin, M.; He, Q.; Zhang, R.; Ma, J. Effect of Curcumin on Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1121655. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Jiang, M.; Huang, Y.; Liu, X.; Liu, L.; Gu, H. Resveratrol Exerts Anti-Osteoarthritic Effect by Inhibiting TLR4/NF-κB Signaling Pathway via the TLR4/Akt/FoxO1 Axis in IL-1β-Stimulated SW1353 Cells. Drug Des. Dev. Ther. 2020, 14, 2079–2090. [Google Scholar] [CrossRef]
- Yang, C.-M.; Chen, Y.-W.; Chi, P.-L.; Lin, C.-C.; Hsiao, L.-D. Resveratrol Inhibits BK-Induced COX-2 Transcription by Suppressing Acetylation of AP-1 and NF-κB in Human Rheumatoid Arthritis Synovial Fibroblasts. Biochem. Pharmacol. 2017, 132, 77–91. [Google Scholar] [CrossRef]
- Sheng, S.; Wang, X.; Liu, X.; Hu, X.; Shao, Y.; Wang, G.; Mao, D.; Li, C.; Chen, B.; Chen, X. The Role of Resveratrol on Rheumatoid Arthritis: From Bench to Bedside. Front. Pharmacol. 2022, 13, 829677. [Google Scholar] [CrossRef]
- De Carvalho, J.F.; Lerner, A. Resveratrol in Rheumatological Diseases: A Systematic Review. Eur. J. Rheumatol. 2023, 10, 163–168. [Google Scholar] [CrossRef]
- Long, Z.; Xiang, W.; He, Q.; Xiao, W.; Wei, H.; Li, H.; Guo, H.; Chen, Y.; Yuan, M.; Yuan, X.; et al. Efficacy and Safety of Dietary Polyphenols in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of 47 Randomized Controlled Trials. Front. Immunol. 2023, 14, 1024120. [Google Scholar] [CrossRef]
- Behl, T.; Mehta, K.; Sehgal, A.; Singh, S.; Sharma, N.; Ahmadi, A.; Arora, S.; Bungau, S. Exploring the Role of Polyphenols in Rheumatoid Arthritis. Crit. Rev. Food Sci. Nutr. 2021, 62, 5372–5393. [Google Scholar] [CrossRef]
- Santus, P.; Signorello, J.C.; Danzo, F.; Lazzaroni, G.; Saad, M.; Radovanovic, D. Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective. J. Clin. Med. 2024, 13, 4127. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; De Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Eghtedari, Y.; Oh, L.J.; Di Girolamo, N.; Watson, S.L. The Role of Topical N-Acetylcysteine in Ocular Therapeutics. Surv. Ophthalmol. 2021, 67, 608–622. [Google Scholar] [CrossRef]
- Panahi, Y.; Ghanei, M.; Rahimi, M.; Samim, A.; Vahedian-Azimi, A.; Atkin, S.L.; Sahebkar, A. Evaluation the Efficacy and Safety of N-acetylcysteine Inhalation Spray in Controlling the Symptoms of Patients with COVID-19: An Open-label Randomized Controlled Clinical Trial. J. Med. Virol. 2022, 95, e28393. [Google Scholar] [CrossRef]
- Wrotek, A.; Badyda, A.; Jackowska, T. Molecular Mechanisms of N-Acetylcysteine in RSV Infections and Air Pollution-Induced Alterations: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6051. [Google Scholar] [CrossRef]
- Riegger, J.; Leucht, F.; Palm, H.-G.; Ignatius, A.; Brenner, R.E. Initial Harm Reduction by N-Acetylcysteine Alleviates Cartilage Degeneration after Blunt Single-Impact Cartilage Trauma in Vivo. Int. J. Mol. Sci. 2019, 20, 2916. [Google Scholar] [CrossRef]
- Uehara, H.; Itoigawa, Y.; Morikawa, D.; Koga, A.; Tsurukami, H.; Maruyama, Y.; Ishijima, M. The Effect of Vitamin C and N-Acetylcysteine on Tendon-to-Bone Healing in a Rodent Model of Rotator Cuff Repair. Am. J. Sports Med. 2023, 51, 1596–1607. [Google Scholar] [CrossRef]
- He, T.; Ren, K.; Xiang, L.; Yao, H.; Huang, Y.; Gao, Y. Efficacy of N-Acetylcysteine as an Adjuvant Therapy for Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Br. J. Hosp. Med. 2024, 85, 1–16. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Sethi, S.; Kong, X.; Yarmus, L.; Brown, R.H.; Feller-Kopman, D.; Wise, R.; Biswal, S. Targeting Nrf2 Signaling Improves Bacterial Clearance by Alveolar Macrophages in Patients with COPD and in a Mouse Model. Sci. Transl. Med. 2011, 3, 78ra32. [Google Scholar] [CrossRef]
- Myzak, M.C.; Dashwood, W.M.; Orner, G.A.; Ho, E.; Dashwood, R.H. Sulforaphane Inhibits Histone Deacetylase in Vivo and Suppresses Tumorigenesis inApcminmice. FASEB J. 2006, 20, 506–508. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 Targeting by Sulforaphane: A Potential Therapy for Cancer Treatment. Crit. Rev. Food Sci. Nutr. 2016, 58, 1391–1405. [Google Scholar] [CrossRef]
- Liebman, S.E.; Le, T.H. Eat Your Broccoli: Oxidative Stress, NRF2, and Sulforaphane in Chronic Kidney Disease. Nutrients 2021, 13, 266. [Google Scholar] [CrossRef]
- Moon, S.-J.; Jhun, J.; Ryu, J.; Kwon, J.Y.; Kim, S.-Y.; Jung, K.; Cho, M.-L.; Min, J.-K. The Anti-Arthritis Effect of Sulforaphane, an Activator of Nrf2, Is Associated with Inhibition of Both B Cell Differentiation and the Production of Inflammatory Cytokines. PLoS ONE 2021, 16, e0245986. [Google Scholar] [CrossRef]
- Wu, F.; Gao, J.; Kang, J.; Wang, X.; Niu, Q.; Liu, J.; Zhang, L. B Cells in Rheumatoid Arthritis:Pathogenic Mechanisms and Treatment Prospects. Front. Immunol. 2021, 12, 750753. [Google Scholar] [CrossRef]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxidative Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Liang, J.; Jahraus, B.; Balta, E.; Ziegler, J.D.; Hübner, K.; Blank, N.; Niesler, B.; Wabnitz, G.H.; Samstag, Y. Sulforaphane Inhibits Inflammatory Responses of Primary Human T-Cells by Increasing ROS and Depleting Glutathione. Front. Immunol 2018, 9, 2584. [Google Scholar] [CrossRef]
- Xu, W.; Lu, H.; Yuan, Y.; Deng, Z.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of Flavonoids from Propolis via Nrf2 and NF-κB Pathways. Foods 2022, 11, 2439. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Pereira, P.M.; De Almeida-Junior, S.; De Melo Taveira, N.N.; De Melo, E.M.; Santos, M.F.C.; Nascimento, L.C.G.D.; Rodrigues, M.A.; Aldana-Mejía, J.A.; Silva, M.L.A.E.; Ambrósio, S.R.; et al. Therapeutic Efficacy of Brown Propolis from Araucaria Sp. in Modulating Rheumatoid Arthritis. Inflammopharmacology 2024, 33, 799–807. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Chavda, V.P.; Chaudhari, A.Z.; Teli, D.; Balar, P.; Vora, L. Propolis and Their Active Constituents for Chronic Diseases. Biomedicines 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okamoto, Y.; Fukui, T.; Masuzawa, T. Suppression of Interleukin 17 Production by Brazilian Propolis in Mice with Collagen-Induced Arthritis. Inflammopharmacology 2011, 20, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nattagh-Eshtivani, E.; Pahlavani, N.; Ranjbar, G.; Navashenaq, J.G.; Salehi-Sahlabadi, A.; Mahmudiono, T.; Shalaby, M.N.; Jokar, M.; Nematy, M.; Barghchi, H.; et al. Does Propolis Have Any Effect on Rheumatoid Arthritis? A Review Study. Food Sci. Nutr. 2022, 10, 1003–1020. [Google Scholar] [CrossRef]
- Parisi, V.; Vassallo, A.; Pisano, C.; Signorino, G.; Cardile, F.; Sorrentino, M.; Colelli, F.; Fucci, A.; D’Andrea, E.L.; De Tommasi, N.; et al. A Herbal Mixture from Propolis, Pomegranate, and Grape Pomace Endowed with Anti-Inflammatory Activity in an In Vivo Rheumatoid Arthritis Model. Molecules 2020, 25, 2255. [Google Scholar] [CrossRef]
- Zhong-Yong, L.; ZHi-Qing, D.; Li-Qiong, X.; Poorasadollah, E.; Shirvani, S. The Impact of Propolis Supplementation on Inflammatory Biomarkers: A Meta-Analysis and Systematic Review of Randomized Controlled Clinical Trials. Prostaglandins Other Lipid Mediat. 2024, 175, 106915. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Takahashi, K.; Sugioka, Y.; Inui, K.; Okano, T.; Mandai, K.; Yamada, Y.; Shintani, A.; Koike, T. Double-Blinded Randomized Controlled Trial to Reveal the Effects of Brazilian Propolis Intake on Rheumatoid Arthritis Disease Activity Index; BeeDAI. PLoS ONE 2021, 16, e0252357. [Google Scholar] [CrossRef]
- Ohta, S. Molecular Hydrogen as a Novel Antioxidant. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; He, Q.; Zhu, P.; Zhuang, Q.; Shen, J.; Zhang, X.; Zhao, M. Hydrogen: A Novel Option in Human Disease Treatment. Oxidative Med. Cell. Longev. 2020, 2020, 8384742. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Hiorth, M.; Klaveness, J. Molecular Hydrogen Therapy—A Review on Clinical Studies and Outcomes. Molecules 2023, 28, 7785. [Google Scholar] [CrossRef]
- Nogueira, J.E.; Branco, L.G.S. Recent Advances in Molecular Hydrogen Research Reducing Exercise-Induced Oxidative Stress and Inflammation. Curr. Pharm. Des. 2020, 27, 731–736. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Rikitake, M.; Seo, T.; Kurokawa, R.; Hara, Y.; Naritomi, Y.; Hara, H.; Nagao, T. Consumption of Water Containing a High Concentration of Molecular Hydrogen Reduces Oxidative Stress and Disease Activity in Patients with Rheumatoid Arthritis: An Open-Label Pilot Study. Med. Gas Res. 2012, 2, 27. [Google Scholar] [CrossRef]
- Ishibashi, T.; Sato, B.; Shibata, S.; Sakai, T.; Hara, Y.; Naritomi, Y.; Koyanagi, S.; Hara, H.; Nagao, T. Therapeutic Efficacy of Infused Molecular Hydrogen in Saline on Rheumatoid Arthritis: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Int. Immunopharmacol. 2014, 21, 468–473. [Google Scholar] [CrossRef]
- Meng, J.; Yu, P.; Jiang, H.; Yuan, T.; Liu, N.; Tong, J.; Chen, H.; Bao, N.; Zhao, J. Molecular Hydrogen Decelerates Rheumatoid Arthritis Progression through Inhibition of Oxidative Stress. Am. J. Transl. Res. 2016, 8, 4472–4477. [Google Scholar]
- Ishibashi, T. Therapeutic Efficacy of Molecular Hydrogen: A New Mechanistic Insight. Curr. Pharm. Des. 2019, 25, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Hijjawi, N.; Tout, F.S.; Azaizeh, B.; Aljaafreh, B. The Role of Vitamins D, B12, C, and K in Modulating Inflammation and Disease Management in Rheumatoid Arthritis: A Comprehensive Review. Clin. Rheumatol. 2024, 44, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and Autoimmune Rheumatic Diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef]
- Heidari, B.; Hajian-Tilaki, K.; Babaei, M. Vitamin D Deficiency and Rheumatoid Arthritis: Epidemiological, Immunological, Clinical and Therapeutic Aspects. Mediterr. J. Rheumatol. 2019, 30, 94–102. [Google Scholar] [PubMed]
- Su, Y.-W.; Lee, A.M.C.; Xu, X.; Hua, B.; Tapp, H.; Wen, X.-S.; Xian, C.J. Methotrexate Chemotherapy Causes Growth Impairments, Vitamin D Deficiency, Bone Loss, and Altered Intestinal Metabolism—Effects of Calcitriol Supplementation. Cancers 2023, 15, 4367. [Google Scholar] [CrossRef]
- Lourdudoss, C.; Wolk, A.; Nise, L.; Alfredsson, L.; Van Vollenhoven, R. Are Dietary Vitamin D, Omega-3 Fatty Acids and Folate Associated with Treatment Results in Patients with Early Rheumatoid Arthritis? Data from a Swedish Population-Based Prospective Study. BMJ Open 2017, 7, e016154. [Google Scholar] [CrossRef]
- El-Banna, H.S.; Gado, S.E. Vitamin D: Does It Help Tregs in Active Rheumatoid Arthritis Patients. Expert Rev. Clin. Immunol. 2020, 16, 847–853. [Google Scholar] [CrossRef]
- Patel, A.V.; Morgan, S.L.; Green, R.; Danila, M.I.; Merriman, T.R.; Wanzeck, K.; Ahmed, H.; Gaffo, A.L. Vitamin B12 Status and Hyperhomocysteinemia in Patients with Rheumatoid Arthritis Treated with Methotrexate and Folic Acid. Am. J. Med. Sci. 2024, 368, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, G.; Wognum, A.W.; Van Eijk, H.G.; Swaak, A.J. Anaemia in Rheumatoid Arthritis: The Role of Iron, Vitamin B12, and Folic Acid Deficiency, and Erythropoietin Responsiveness. Ann. Rheum. Dis. 1990, 49, 93–98. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.F.; Coles, S.J.; Bueno, A.A. Vitamin B12 (Cobalamin) Supplementation for the Management of Autoimmune Rheumatic Diseases: Potential Indications and Opportunity for Future Research. Rheumatol. Int. 2023, 44, 743–744. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R.; Imai, T.; Iwasaki, Y.; Okamoto, K.; Nakagawa, M.; Ohashi, Y.; Takase, T.; Hanada, T.; Shimizu, H.; Tashiro, K.; et al. Ultra-High-Dose Methylcobalamin in Amyotrophic Lateral Sclerosis: A Long-Term Phase II/III Randomised Controlled Study. J. Neurol. Neurosurg. Psychiatry 2019, 90, 451–457. [Google Scholar] [CrossRef]
- Gunes-Bayir, A.; Mendes, B.; Dadak, A. The Integral Role of Diets Including Natural Products to Manage Rheumatoid Arthritis: A Narrative Review. Curr. Issues Mol. Biol. 2023, 45, 5373–5388. [Google Scholar] [CrossRef]
- Isola, S.; Gammeri, L.; Furci, F.; Gangemi, S.; Pioggia, G.; Allegra, A. Vitamin C Supplementation in the Treatment of Autoimmune and Onco-Hematological Diseases: From Prophylaxis to Adjuvant Therapy. Int. J. Mol. Sci. 2024, 25, 7284. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Huang, S.; Chen, Q.; Wang, X.; Liu, H. Association between Rheumatoid Arthritis and Serum Vitamin C Levels in Adults: Based on the National Health and Nutrition Examination Survey Database. Prev. Med. Rep. 2024, 44, 102793. [Google Scholar] [CrossRef]
- Mikirova, N.; Casciari, J.; Rogers, A.; Taylor, P. Effect of High-Dose Intravenous Vitamin C on Inflammation in Cancer Patients. J. Transl. Med. 2012, 10, 189. [Google Scholar] [CrossRef]
- Rodríguez, C.R.-O.; Curiel, M.D. Vitamin K and Bone Health: A Review on the Effects of Vitamin K Deficiency and Supplementation and the Effect of Non-Vitamin K Antagonist Oral Anticoagulants on Different Bone Parameters. J. Osteoporos. 2019, 2019, 2069176. [Google Scholar] [CrossRef]
- Salma, N.; Ahmad, S.S.; Karim, S.; Ibrahim, I.M.; Alkreathy, H.M.; Alsieni, M.; Khan, M.A. Effect of Vitamin K on Bone Mineral Density and Fracture Risk in Adults: Systematic Review and Meta-Analysis. Biomedicines 2022, 10, 1048. [Google Scholar] [CrossRef]
- Stock, M.; Schett, G. Vitamin K-Dependent Proteins in Skeletal Development and Disease. Int. J. Mol. Sci. 2021, 22, 9328. [Google Scholar] [CrossRef] [PubMed]
- Jaminon, A.M.G.; Dai, L.; Qureshi, A.R.; Evenepoel, P.; Ripsweden, J.; Söderberg, M.; Witasp, A.; Olauson, H.; Schurgers, L.J.; Stenvinkel, P. Matrix Gla Protein Is an Independent Predictor of Both Intimal and Medial Vascular Calcification in Chronic Kidney Disease. Sci. Rep. 2020, 10, 6586. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Levels in RA Patients | References |
|---|---|---|
| Total Oxidant Status (TOS) | Elevated | [54,55,56] |
| Total Antioxidant Status (TAS) | Decreased | [54,55,56] |
| Oxidative Stress Index (OSI) | Elevated | [54,55,56] |
| MDA | Elevated | [57,58,59,60,61,62,63] |
| 8-OHdG | Elevated | [37,38,39] |
| SOD | Decreased | [37,41,59,63] |
| Elevated | [57,58,61] | |
| GSH | Decreased | [42,43,44,61,62,64] |
| Elevated | [57,63] | |
| CAT | Decreased | [59,65] |
| GPx | Decreased | [62,63,65] |
| Elevated | [57,61,66] | |
| AOPPs | Elevated | [45,46] |
| AGEs | Elevated | [46,47,48,49,50] |
| RAGE | Elevated | [48,51,52,53] |
| Substance | Mechanism of Action | Potential Benefits in RA | References |
|---|---|---|---|
| Curcumin | Inhibition of NF-κB, activation of Nrf2/HO-1, regulation of proinflammatory cytokines (TNF-α, IL-6, IL-1β) | Reduction in inflammation, alleviation of clinical symptoms, enhancement of DMARD efficacy | [88,91,92] |
| Resveratrol | Inhibition of osteoclast activation, suppression of NF-κB and MAPK, activation of PI3K/Akt, inhibition of TLR4 expression | Protection against bone resorption, reduction in extracellular matrix degradation, attenuation of inflammatory response | [96,97,98,99] |
| NAC | Precursor of glutathione, ROS neutralization, NF-κB inhibition, regulation of matrix metalloproteinases (MMPs) | Improvement in redox balance, reduction in oxidative stress, slowdown of joint destruction | [102,103,104,105] |
| Sulforaphane | Activation of Nrf2, inhibition of NF-κB, regulation of B and T lymphocytes, reduction in IL-17 | Decrease in autoantibody production, synovial membrane protection, reduction in IL-17 | [110,111,112,113] |
| Propolis | Inhibition of NF-κB, enhancement of antioxidant enzyme activity (SOD, CAT, GPx), modulation of Th17 and Treg lymphocytes | Reduction in oxidative damage, lowering of inflammatory markers, improvement of joint function | [117,118,119] |
| Molecular hydrogen | Neutralization of hydroxyl radicals (•OH) and peroxynitrite (ONOO−), activation of Nrf2, inhibition of NF-κB | Protection against DNA and mitochondrial damage, reduction in inflammatory markers, enhancement of cellular immunity | [127,128,129,131] |
| Vitamin D | Regulation of proinflammatory cytokines (TNF-α, IL-6, IL-17), bone protection, support for methotrexate therapy | Reduced osteoporosis risk, enhanced immune response, decreased methotrexate side effects | [136,137,138,139] |
| Vitamin B12 | Reduction in homocysteine levels, nerve regeneration, fatigue reduction | Decreased inflammation, improved neurological function, methotrexate therapy support | [141,142,143] |
| Vitamin C | ROS neutralization, collagen synthesis support, reduction in inflammatory markers (CRP, ESR) | Decreased cartilage degradation, reduction in CRP, improvement in connective tissue function | [146,147,148] |
| Vitamin K | Activation of osteocalcin and Matrix Gla-Protein (MGP), inhibition of osteoclast activity, reduction in inflammatory mediators | Improved bone mineralization, decreased inflammation, osteoporosis prevention | [149,150,151,152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilski, R.; Nuszkiewicz, J. Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes. Int. J. Mol. Sci. 2025, 26, 2873. https://doi.org/10.3390/ijms26072873
Bilski R, Nuszkiewicz J. Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes. International Journal of Molecular Sciences. 2025; 26(7):2873. https://doi.org/10.3390/ijms26072873
Chicago/Turabian StyleBilski, Rafał, and Jarosław Nuszkiewicz. 2025. "Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes" International Journal of Molecular Sciences 26, no. 7: 2873. https://doi.org/10.3390/ijms26072873
APA StyleBilski, R., & Nuszkiewicz, J. (2025). Antioxidant Therapies as Emerging Adjuncts in Rheumatoid Arthritis: Targeting Oxidative Stress to Enhance Treatment Outcomes. International Journal of Molecular Sciences, 26(7), 2873. https://doi.org/10.3390/ijms26072873







