Downregulated Expression of miR-200c-3p in Plasma Exosome as a Potential Biomarker in Takayasu’s Arteritis
Abstract
1. Introduction
2. Results
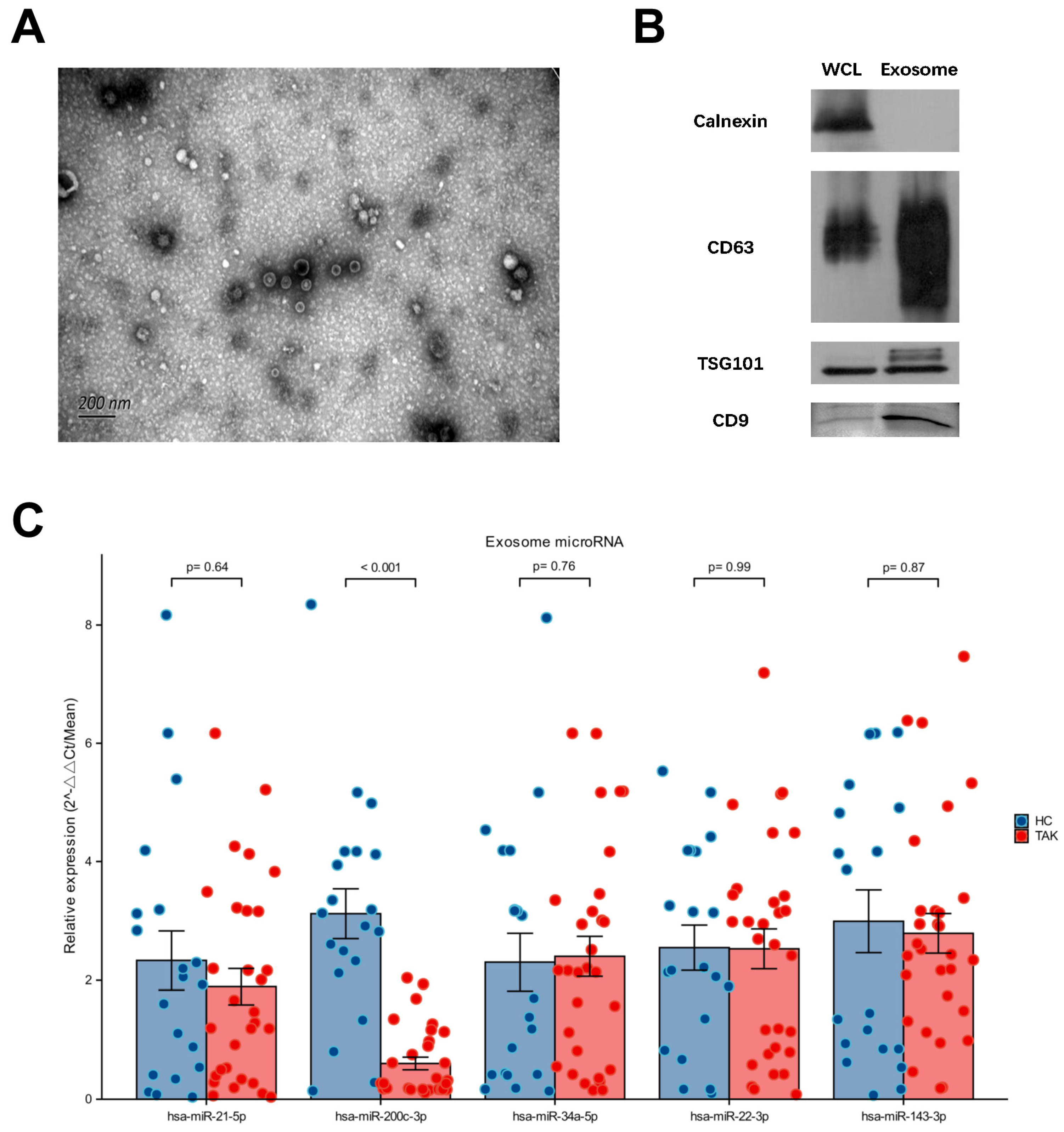
2.1. Downregulated Expression of miR-200c-3p in Plasma Exosome Was Validated in Untreated TAK Patients
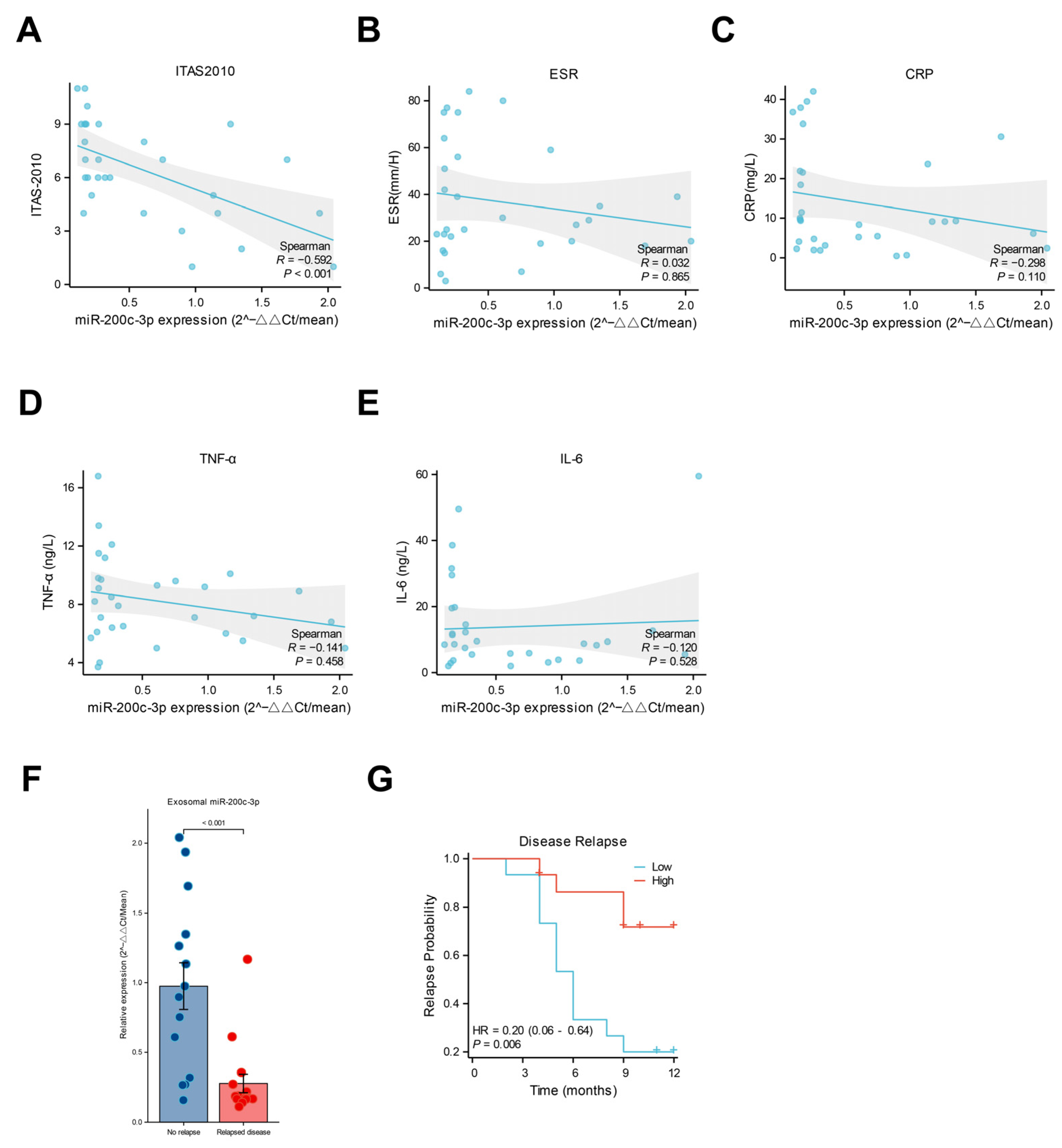
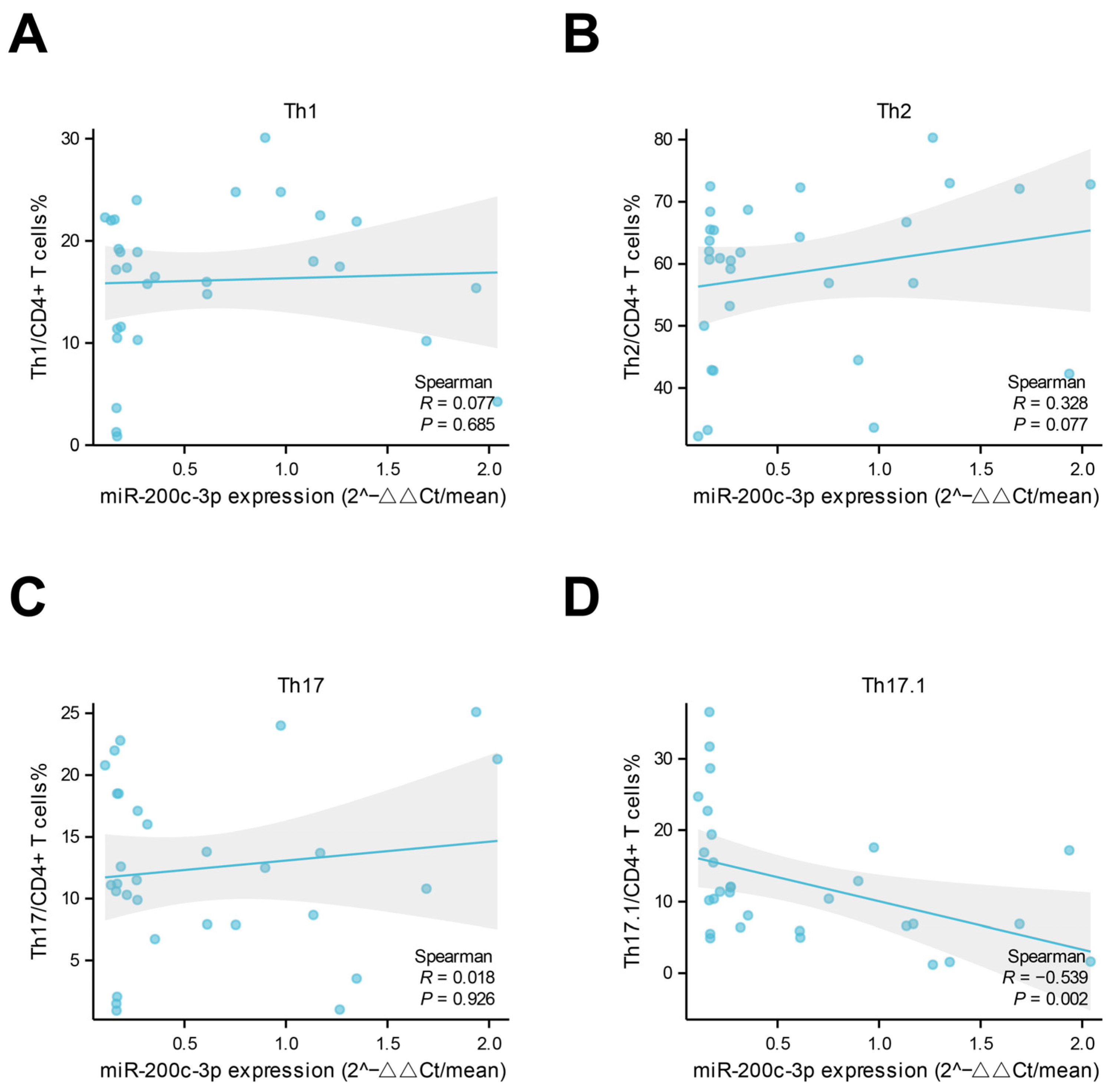
2.2. Lower Levels of miR-200c-3p in Plasma-Derived Exosomes Were Associated with Vascular Inflammation and Th1 Response
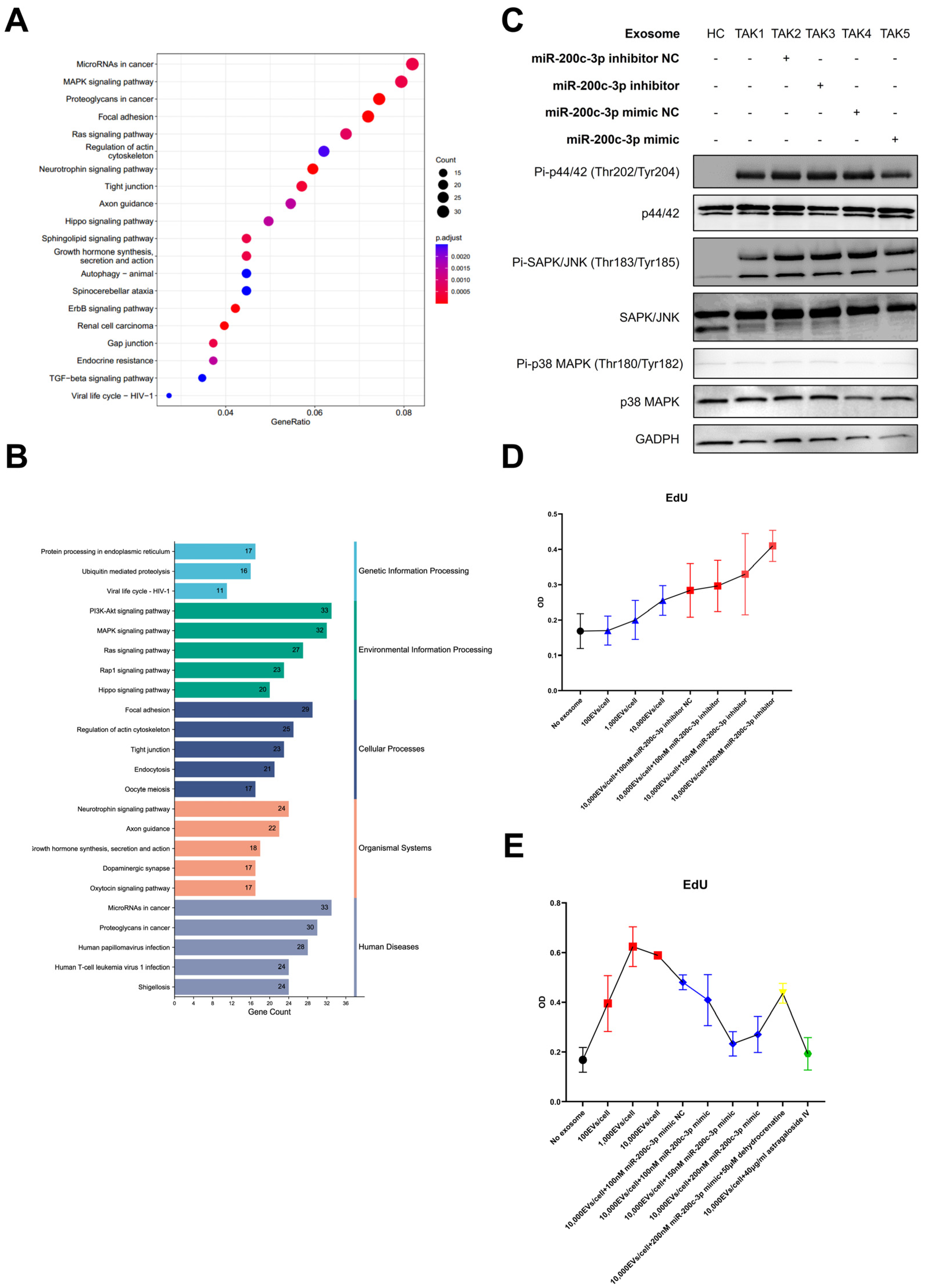
2.3. Enrichment Analysis of Targeted Genes of miR-200c-3p and In Vitro Experiments Suggested the Possible Pathogenic Role of the MAPK Pathway in TAK
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Plasma Preparation and Total Exosome Isolation
4.3. MicroRNA Validation by Quantitative Polymerase Chain Reaction (qPCR)
4.4. Peripheral Blood Mononuclear Cells (PBMCs) Collection and Analysis by Flow Cytometry
4.5. Cell Culture
4.6. Western Blot
4.7. EdU Assay
4.8. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saadoun, D.; Vautier, M.; Cacoub, P. Medium- and Large-Vessel Vasculitis. Circulation 2021, 143, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Karabayas, M.; Basu, N.; Cid, M.C.; Goel, R.; Goodyear, C.S.; Grayson, P.C.; McAdoo, S.P.; Mason, J.C.; Owen, C.; et al. Large-vessel vasculitis. Nat. Rev. Dis. Primers 2022, 7, 93. [Google Scholar] [CrossRef]
- Jae, N.; Dimmeler, S. Noncoding RNAs in Vascular Diseases. Circ. Res. 2020, 126, 1127–1145. [Google Scholar] [CrossRef]
- Gu, F.; Huang, X.; Huang, W.; Zhao, M.; Zheng, H.; Wang, Y.; Chen, R. The role of miRNAs in Behcet’s disease. Front. Immunol. 2023, 14, 1249826. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, Z.; Wang, D.; Ni, H.; Zhang, L.; Wang, Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics 2019, 9, 6901–6919. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Shi, B.; Liu, W.; Gong, J.; Gao, J.; Sun, Y.; Yang, P. Circulating exosomal microRNAs as biomarkers of lupus nephritis. Front. Immunol. 2023, 14, 1326836. [Google Scholar] [CrossRef]
- Huang, B.; Li, J.; Tian, X.; Zeng, X. Analysis of Differentially Expressed MicroRNA and Protein Expression Profiles Carried by Exosomes in the Plasma of Patients with Takayasu’s Arteritis. Chin. J. Intern. Med. 2023, 62, 57–65. [Google Scholar] [CrossRef]
- Guo, S.; Li, J.; Pang, S.; Li, J.; Tian, X. Exosome miR-199a-5p modulated vascular remodeling and inflammatory infiltration of Takayasu’s arteritis. Arthritis Res. Ther. 2025, 27, 11. [Google Scholar] [CrossRef]
- Tombetti, E.; Di Chio, M.C.; Sartorelli, S.; Papa, M.; Salerno, A.; Bottazzi, B.; Bozzolo, E.P.; Greco, M.; Rovere-Querini, P.; Baldissera, E.; et al. Systemic pentraxin-3 levels reflect vascular enhancement and progression in Takayasu arteritis. Arthritis Res. Ther. 2014, 16, 479. [Google Scholar] [CrossRef]
- Kong, F.; Huang, X.; Su, L.; Liao, Q.; Wang, C.; Zhao, Y. Risk factors for cerebral infarction in Takayasu arteritis: A single-centre case-control study. Rheumatology 2021, 61, 281–290. [Google Scholar] [CrossRef]
- Misra, R.; Danda, D.; Rajappa, S.M.; Ghosh, A.; Gupta, R.; Mahendranath, K.M.; Jeyaseelan, L.; Lawrence, A.; Bacon, P.A.; Indian Rheumatology Vasculitis, g. Development and initial validation of the Indian Takayasu Clinical Activity Score (ITAS2010). Rheumatology 2013, 52, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Jiang, L. MiR-200c-3p promotes ox-LDL-induced endothelial to mesenchymal transition in human umbilical vein endothelial cells through SMAD7/YAP pathway. J. Physiol. Sci. 2021, 71, 30. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Chen, J.; Yang, M.; Afzal, T.A.; An, W.; Maguire, E.M.; He, S.; Luo, J.; Wang, X.; et al. miRNA-200c-3p promotes endothelial to mesenchymal transition and neointimal hyperplasia in artery bypass grafts. J. Pathol. 2021, 253, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Bakhashab, S.; Yuen Yeoh, M.L.; Coulson, D.J.; Steel, S.C.; Ray, S.L.; Weaver, J.U. Deciphering the Role of miR-200c-3p in Type 1 Diabetes (Subclinical Cardiovascular Disease) and Its Correlation with Inflammation and Vascular Health. Int. J. Mol. Sci. 2022, 23, 15659. [Google Scholar] [CrossRef]
- Abdolahi, S.; Hosseini, M.; Rezaei, R.; Mohebbi, S.R.; Rostami-Nejad, M.; Mojarad, E.N.; Mirjalali, H.; Yadegar, A.; Asadzadeh Aghdaei, H.; Zali, M.R.; et al. Evaluation of miR-200c-3p and miR-421-5p levels during immune responses in the admitted and recovered COVID-19 subjects. Infect. Genet. Evol. 2022, 98, 105207. [Google Scholar] [CrossRef]
- Song, Y.; Tran, M.; Wang, L.; Shin, D.J.; Wu, J. MiR-200c-3p targets SESN1 and represses the IL-6/AKT loop to prevent cholangiocyte activation and cholestatic liver fibrosis. Lab. Investig. 2022, 102, 485–493. [Google Scholar] [CrossRef]
- Singh, K.; Rathore, U.; Rai, M.K.; Behera, M.R.; Jain, N.; Ora, M.; Bhadauria, D.; Sharma, S.; Pande, G.; Gambhir, S.; et al. Novel Th17 Lymphocyte Populations, Th17.1 and PD1+Th17, are Increased in Takayasu Arteritis, and Both Th17 and Th17.1 Sub-Populations Associate with Active Disease. J. Inflamm. Res. 2022, 15, 1521–1541. [Google Scholar] [CrossRef] [PubMed]
- Keser, G.; Aksu, K.; Direskeneli, H. Discrepancies between vascular and systemic inflammation in large vessel vasculitis: An important problem revisited. Rheumatology 2018, 57, 784–790. [Google Scholar] [CrossRef]
- Hopkins, P.N. Molecular biology of atherosclerosis. Physiol. Rev. 2013, 93, 1317–1542. [Google Scholar] [CrossRef]
- Zheng, Y.; Han, Z.; Zhao, H.; Luo, Y. MAPK: A Key Player in the Development and Progression of Stroke. CNS Neurol. Disord. Drug Targets 2020, 19, 248–256. [Google Scholar] [CrossRef]
- Hadjadj, J.; Canaud, G.; Mirault, T.; Samson, M.; Bruneval, P.; Regent, A.; Goulvestre, C.; Witko-Sarsat, V.; Costedoat-Chalumeau, N.; Guillevin, L.; et al. mTOR pathway is activated in endothelial cells from patients with Takayasu arteritis and is modulated by serum immunoglobulin G. Rheumatology 2018, 57, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, W.; Yang, Y.; Zhao, J.; Li, M.; Wang, Y.; Tian, X.; Zeng, X. Clinical Characteristics of Adult Patients with Systemic Vasculitis: Data of 1348 Patients from a Single Center. Rheumatol. Immunol. Res. 2021, 2, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P.; Michel, B.A.; Bloch, D.A.; Hunder, G.G.; Calabrese, L.H.; Edworthy, S.M.; Fauci, A.S.; Leavitt, R.Y.; Lie, J.T.; Lightfoot, R.W., Jr.; et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990, 33, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Kerr, G. Takayasu’s arteritis. Curr. Opin. Rheumatol. 1994, 6, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021, 73, 1349–1365. [Google Scholar] [CrossRef]

| Age (years) | Sex | Disease Duration (months) | Asthenia | Abdominal pain | Abortion | Hypertension | Stroke | ESR (mm/h) | hs-CRP (mg/L) | IL-6 (ng/L) | TNF-α (ng/L) | Numano Classification | ITAS-2010 Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | F | 3 | Y | N | N | Y | Y | 7 | 5.44 | 5.9 | 9.6 | II | 7 |
| 2 | 46 | F | 34 | Y | N | N | Y | N | 80 | 8.35 | 2 | 9.3 | V | 8 |
| 3 | 15 | M | 1 | Y | N | N | Y | Y | 6 | 2.26 | 2 | 8.2 | V | 9 |
| 4 | 38 | M | 1 | Y | N | N | Y | N | 18 | 30.59 | 12.7 | 8.9 | V | 7 |
| 5 | 40 | F | 49 | N | N | N | N | N | 39 | 6.11 | 5.5 | 6.8 | II | 4 |
| 6 | 35 | F | 2 | Y | N | N | Y | N | 3 | 11.45 | 3.7 | 4 | V | 9 |
| 7 | 35 | F | 11 | N | N | N | N | N | 16 | 4.07 | 2.9 | 6.1 | V | 4 |
| 8 | 39 | F | 2 | N | N | N | N | N | 30 | 5.22 | 5.8 | 5 | III | 4 |
| 9 | 34 | F | 67 | Y | N | N | Y | N | 25 | 1.87 | 5.5 | 7.9 | V | 6 |
| 10 | 22 | F | 39 | Y | N | N | Y | Y | 23 | 36.79 | 8.5 | 5.7 | V | 11 |
| 11 | 42 | F | 114 | N | N | N | N | N | 20 | 2.42 | 59.5 | 5 | V | 1 |
| 12 | 33 | F | 44 | Y | N | N | Y | N | 25 | 21.56 | 8.6 | 7.1 | V | 10 |
| 13 | 37 | F | 14 | Y | N | N | Y | N | 75 | 10.01 | 31.56 | 3.7 | V | 9 |
| 14 | 22 | F | 9 | Y | N | N | Y | N | 23 | 21.92 | 19.53 | 16.8 | V | 8 |
| 15 | 20 | F | 3 | N | N | N | Y | N | 64 | 9.61 | 29.53 | 9.8 | V | 11 |
| 16 | 29 | F | 7 | N | N | N | N | N | 42 | 37.91 | 38.56 | 9.1 | V | 9 |
| 17 | 31 | F | 19 | N | N | N | Y | N | 51 | 9.35 | 11.76 | 11.5 | V | 6 |
| 18 | 45 | F | 59 | N | N | N | N | N | 15 | 18.44 | 11.34 | 13.4 | V | 7 |
| 19 | 32 | M | 22 | N | N | N | Y | N | 77 | 33.82 | 19.8 | 9.7 | V | 6 |
| 20 | 32 | F | 40 | N | N | N | N | N | 22 | 39.48 | 49.53 | 11.2 | III | 5 |
| 21 | 19 | F | 10 | Y | N | N | N | N | 39 | 42.02 | 7.5 | 8.5 | V | 6 |
| 22 | 37 | F | 57 | Y | N | N | N | N | 56 | 1.95 | 12.22 | 12.1 | V | 7 |
| 23 | 25 | F | 6 | Y | N | N | N | N | 75 | 4.75 | 14.55 | 6.4 | V | 9 |
| 24 | 29 | M | 13 | N | N | N | N | N | 84 | 3.1 | 9.5 | 6.5 | II | 6 |
| 25 | 23 | F | 17 | N | N | N | Y | N | 19 | 0.47 | 3.1 | 7.1 | V | 3 |
| 26 | 24 | F | 35 | N | N | N | Y | N | 59 | 0.66 | 3.9 | 9.2 | V | 1 |
| 27 | 22 | F | 48 | N | N | N | Y | N | 20 | 23.67 | 3.66 | 6 | II | 5 |
| 28 | 27 | F | 56 | Y | N | N | N | N | 27 | 9.13 | 8.77 | 10.1 | V | 4 |
| 29 | 23 | F | 13 | Y | N | N | N | N | 29 | 9.13 | 8.31 | 5.5 | V | 9 |
| 30 | 18 | F | 9 | N | Y | N | Y | N | 35 | 9.29 | 9.35 | 7.2 | V | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Fang, C.; Huang, B.; Zeng, X.; Li, J.; Tian, X. Downregulated Expression of miR-200c-3p in Plasma Exosome as a Potential Biomarker in Takayasu’s Arteritis. Int. J. Mol. Sci. 2025, 26, 2881. https://doi.org/10.3390/ijms26072881
Du L, Fang C, Huang B, Zeng X, Li J, Tian X. Downregulated Expression of miR-200c-3p in Plasma Exosome as a Potential Biomarker in Takayasu’s Arteritis. International Journal of Molecular Sciences. 2025; 26(7):2881. https://doi.org/10.3390/ijms26072881
Chicago/Turabian StyleDu, Lihong, Chenglong Fang, Biqing Huang, Xiaofeng Zeng, Jing Li, and Xinping Tian. 2025. "Downregulated Expression of miR-200c-3p in Plasma Exosome as a Potential Biomarker in Takayasu’s Arteritis" International Journal of Molecular Sciences 26, no. 7: 2881. https://doi.org/10.3390/ijms26072881
APA StyleDu, L., Fang, C., Huang, B., Zeng, X., Li, J., & Tian, X. (2025). Downregulated Expression of miR-200c-3p in Plasma Exosome as a Potential Biomarker in Takayasu’s Arteritis. International Journal of Molecular Sciences, 26(7), 2881. https://doi.org/10.3390/ijms26072881







