Study on the Quality Change and Regulation Mechanism of ‘Shannongsu’ Pear Under Low-Temperature Storage
Abstract
:1. Introduction
2. Results
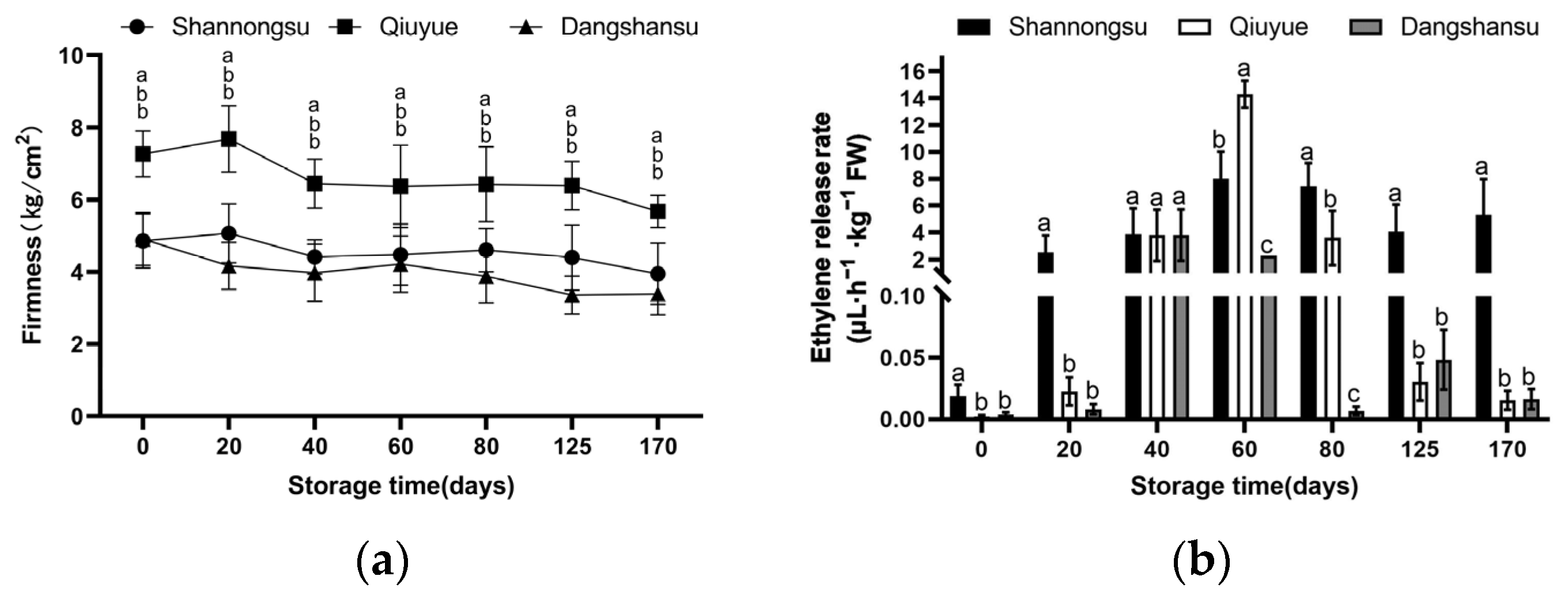
2.1. Changes in Firmness and Ethylene During Low-Temperature Storage
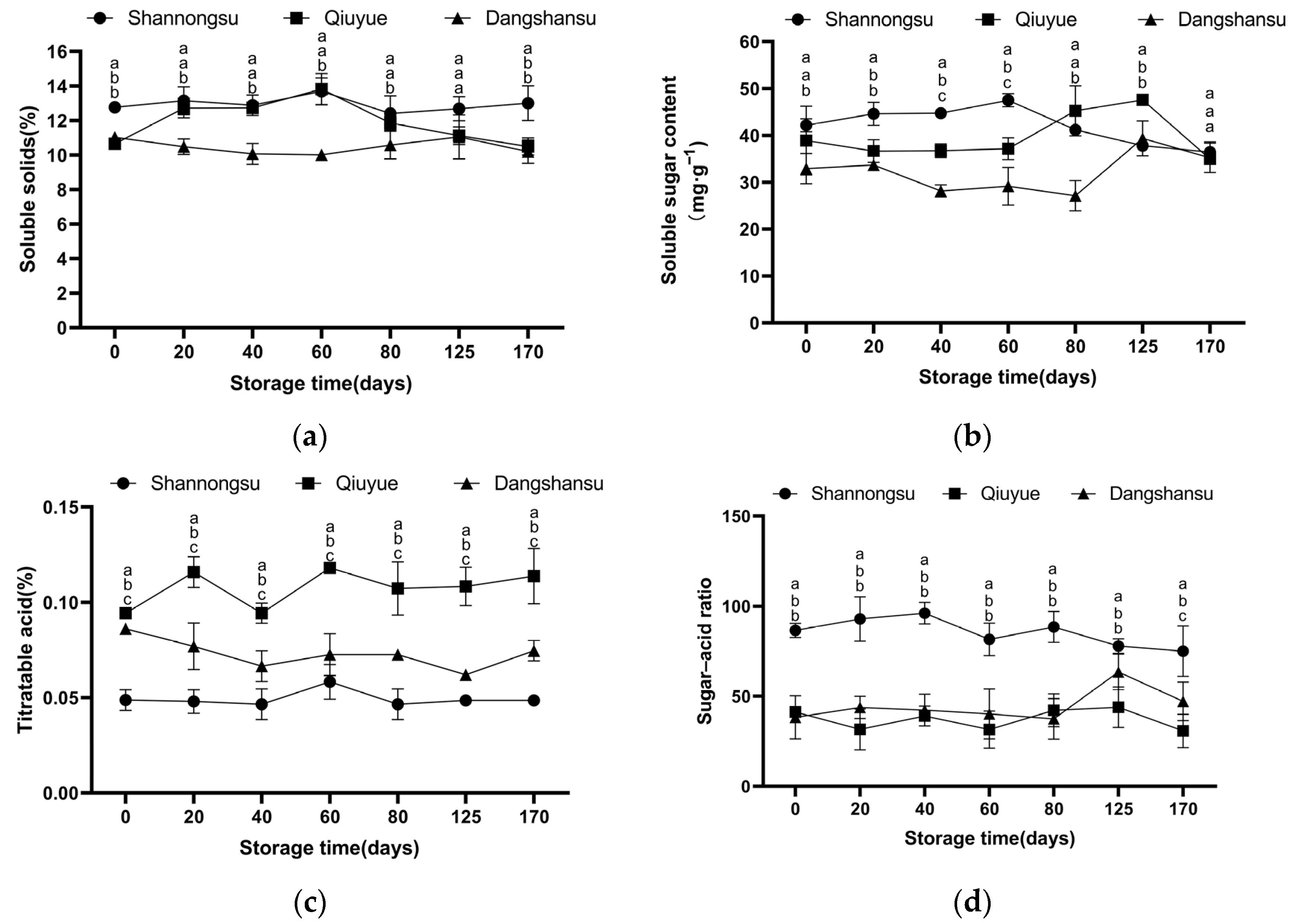
2.2. Changes in Sugar and Acid Contents During Low-Temperature Storage
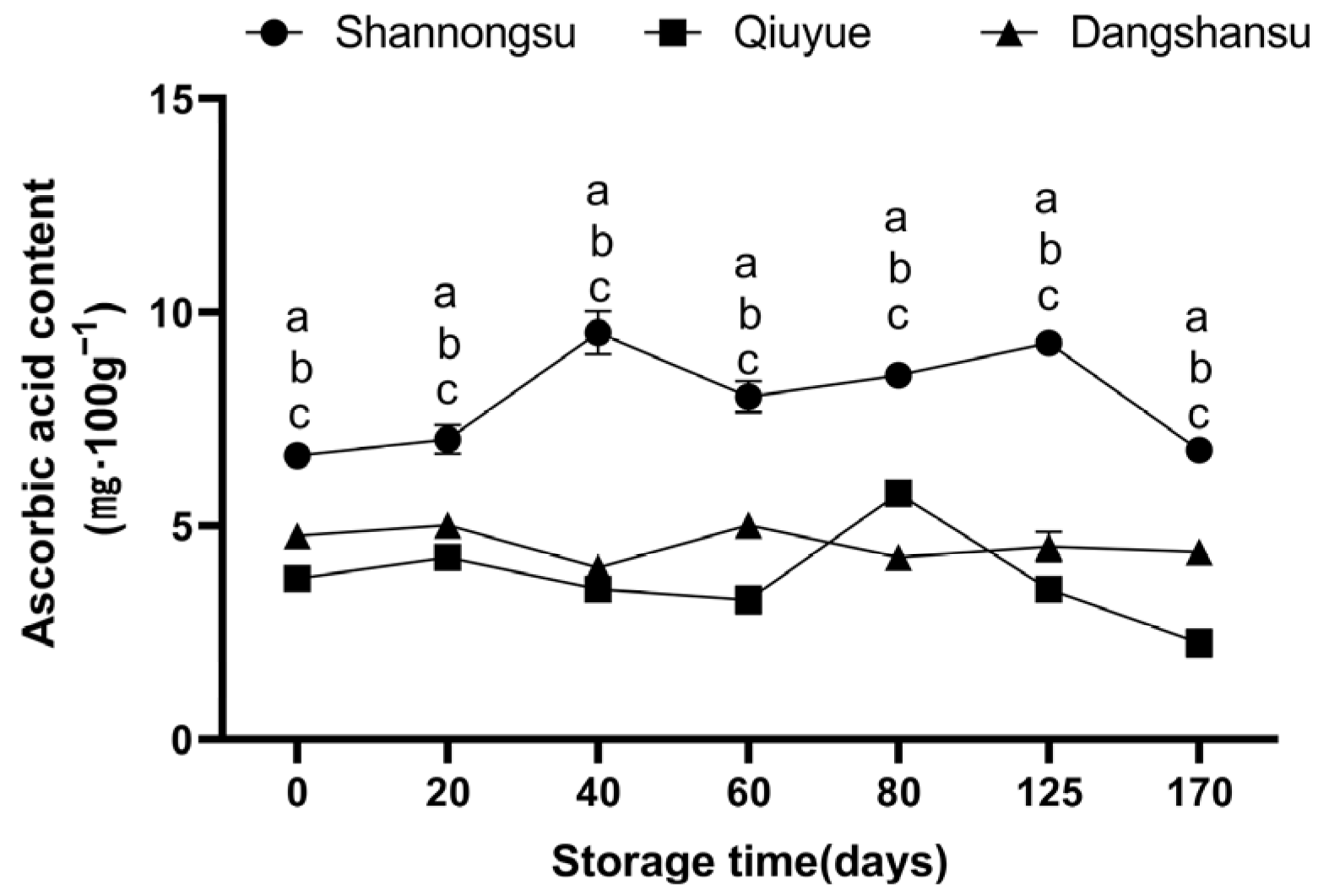
2.3. Changes in Ascorbic Acid Content During Low-Temperature Storage
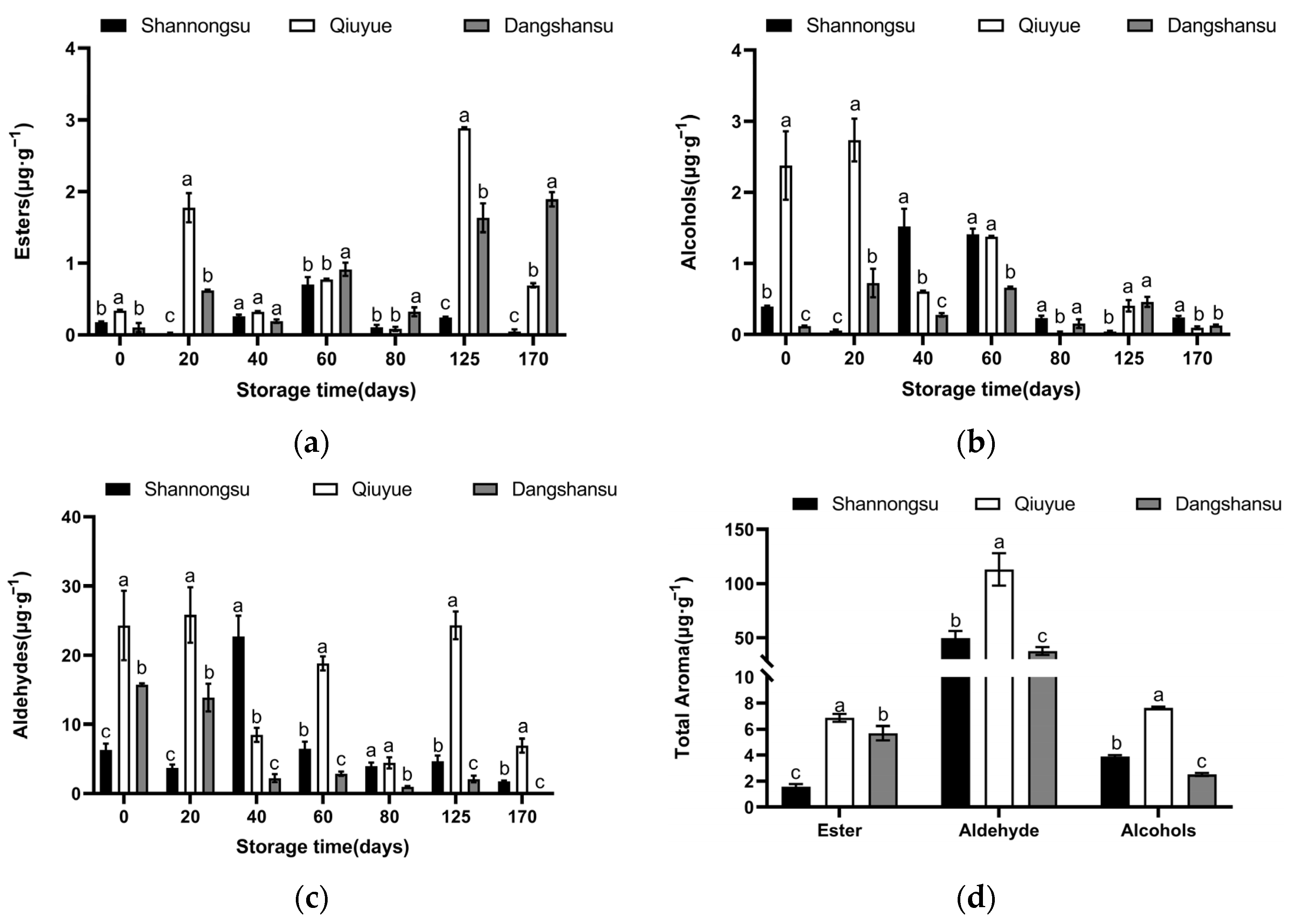
2.4. Changes in the Content of Aromatic Substances During Low-Temperature Storage
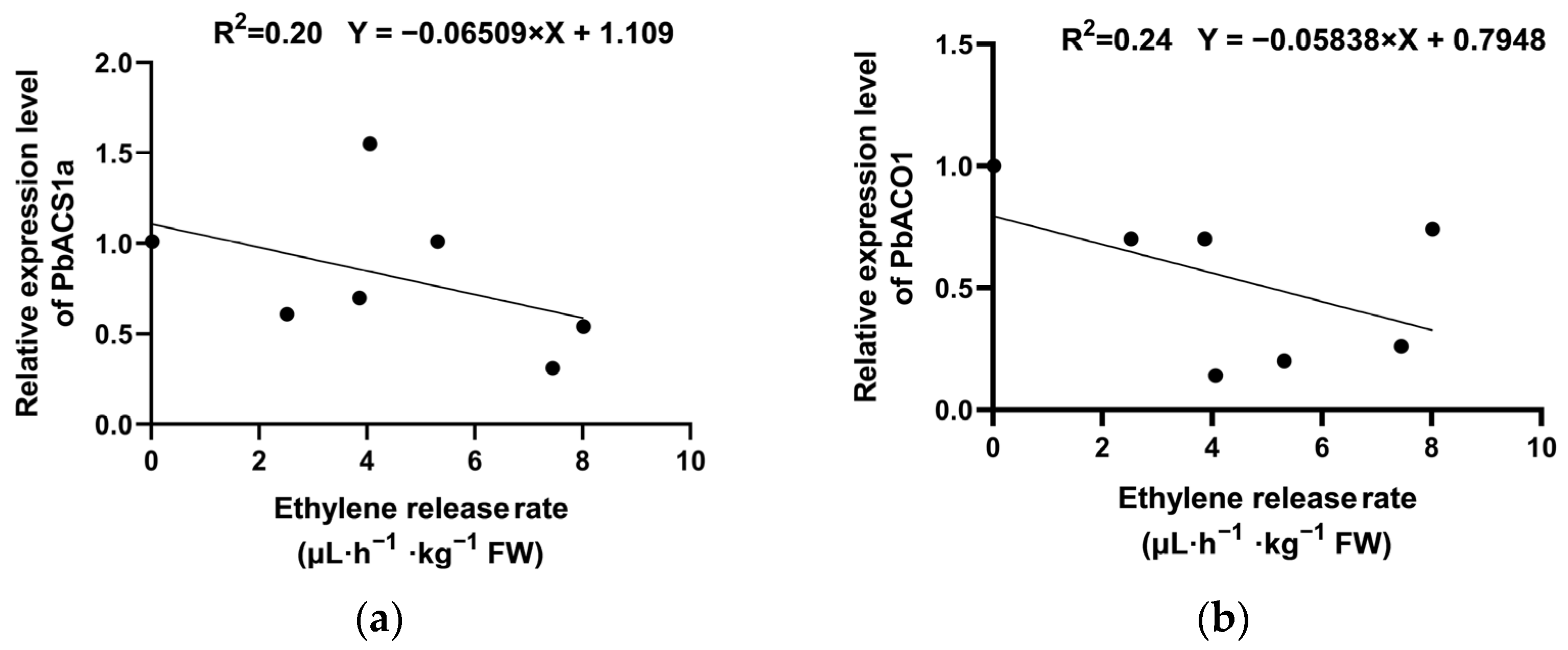
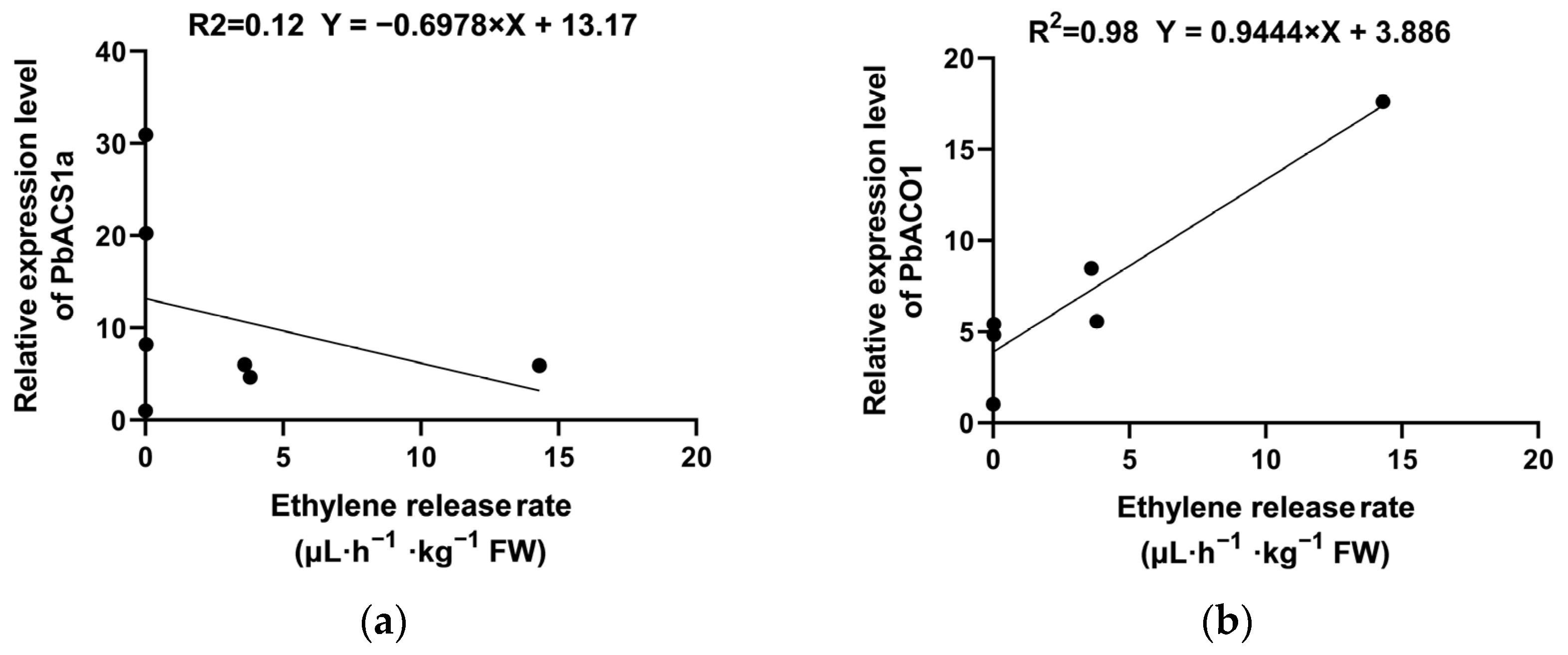
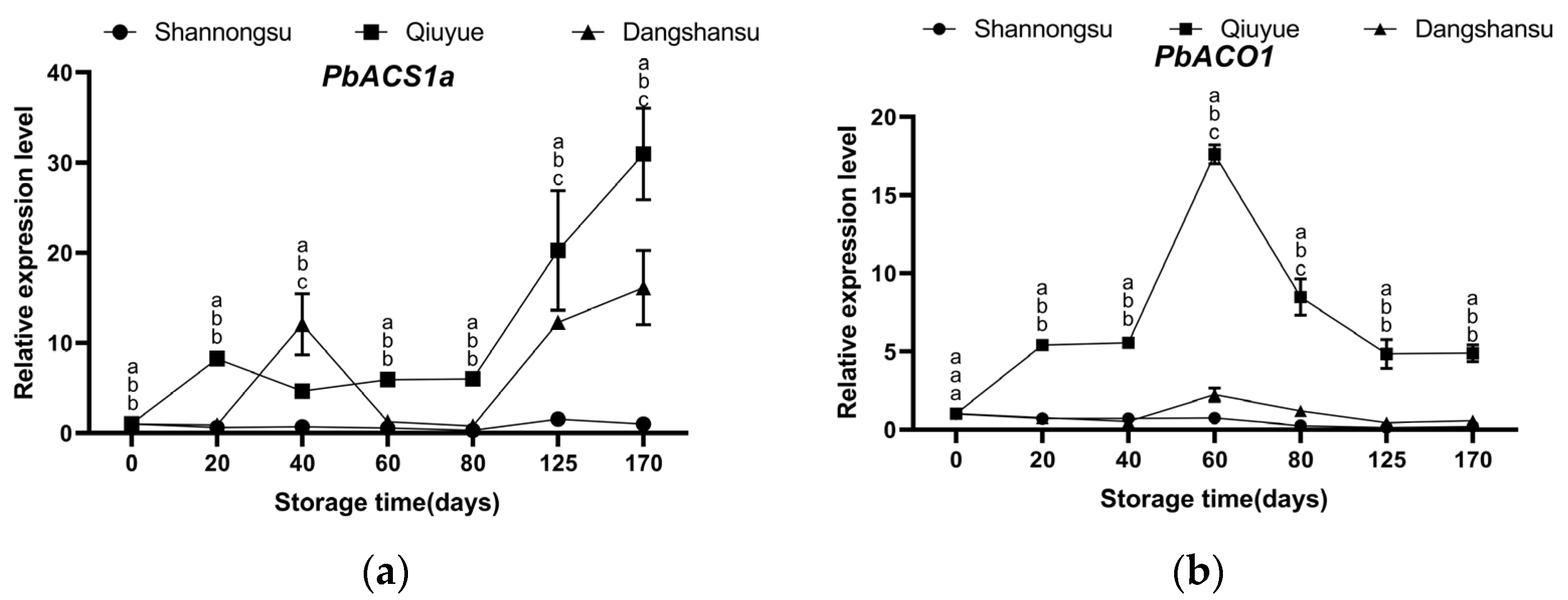
2.5. Changes in the Expression of Key Genes Regulating Ethylene Synthesis During Low-Temperature Storage
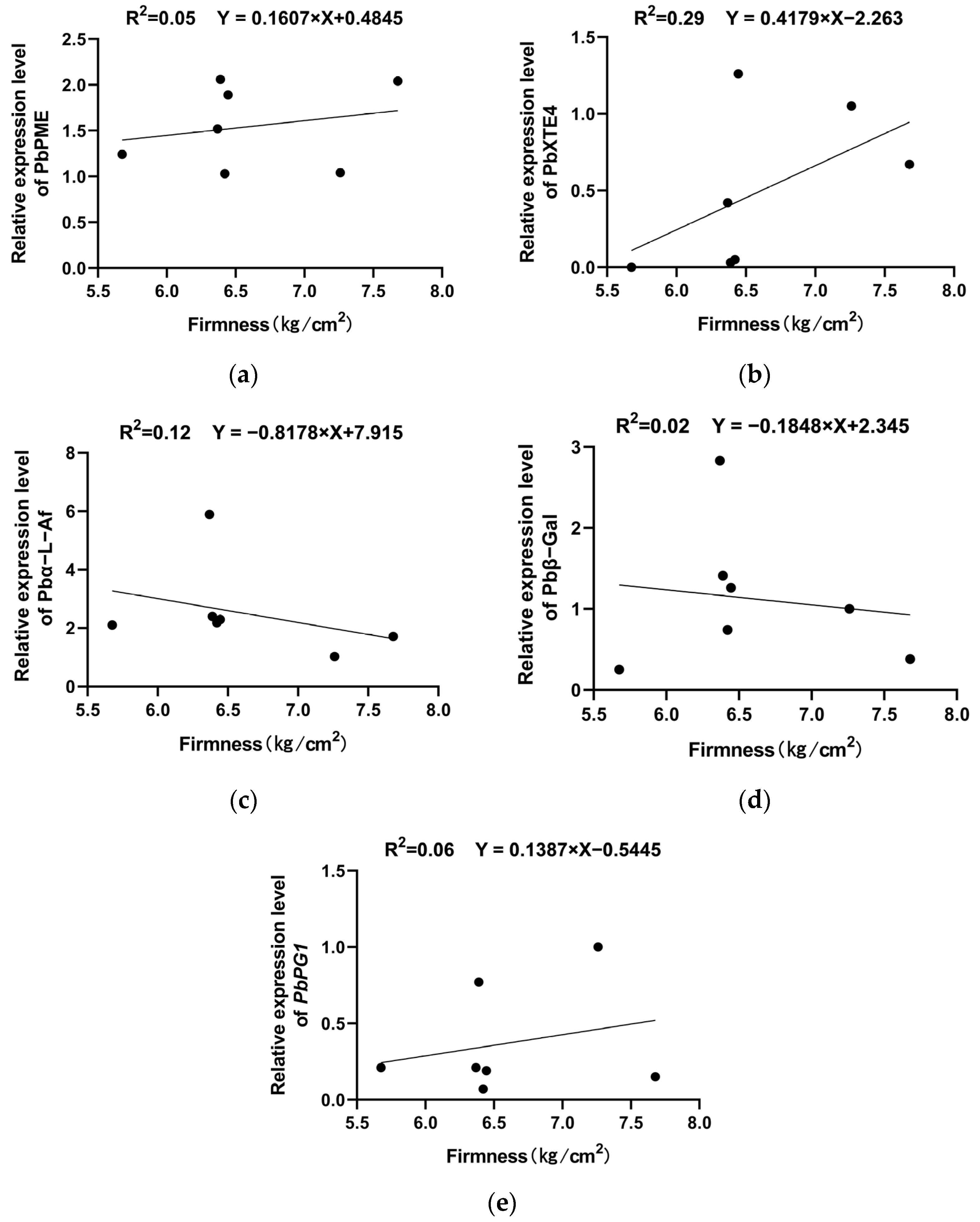
2.6. Changes in the Expression of Texture-Related Genes During Storage
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Determination of Firmness
4.3. Determination of Ethylene Production
4.4. Determination of Soluble Sugar Content
4.5. Determination of Titratable Acid Content
4.6. Determination of Ascorbic Acid Content
4.7. Extraction and Detection of Sugar Components
4.8. Extraction and Detection of Aromatic Substances
4.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Varieties | Sucrose (mg·mL−1) | Glucose (mg·mL−1) | Fructose (mg·mL−1) | Sorbitol (mg·mL−1) | Total Sugar (mg·mL−1) | Sweetness (mg·mL−1) |
|---|---|---|---|---|---|---|
| Shannongsu | 1.68 ± 0.04 b | 2.26 ± 0.04 b | 4.22 ± 0.08 a | 2.46 ± 0.05 a | 10.61 ± 0.21 a | 11.62 ± 0.23 a |
| Dangshansu | 0.15 ± 0.01 c | 2.68 ± 0.03 a | 4.26 ± 0.05 a | 2.09 ± 0.01 b | 9.18 ± 0.09 b | 10.31 ± 0.12 b |
| Qiuyue | 3.51 ± 0.04 a | 1.14 ± 0.01 c | 3.12 ± 0.02 b | 1.26 ± 0.07 c | 9.04 ± 0.03 b | 10.28 ± 0.08 b |
| Gene Name | Primer Sequence (5′–3′) | |
|---|---|---|
| PbACS1a | CAAGAACAAGCACAAGTGA | ATTGGTGAATGAGGAGACA |
| PbACO1 | ACCACTCCATTGTCATAAAC | GGTTGTAGAACGAGGCTAT |
| PbPG1 | GTTGTGGTCATTTGTTGTTG | CGGACACCCTATCATCAA |
| PbPME | GATGGCTTGAGTGGAATG | GCAACAGTGAACTTACCG |
| PbXET4 | TCCATTCCTACTCCATCTTC | AATCTGTCTTGACCCTACC |
| Pbβ-Gal | TGGTCCATCAGCATTCTT | TGTAGTTGTATCGGTCTCAT |
| Pbα-L-Af | GCCCTTTGACTTGAGATATG | ATCCAACTGACGAGAAGAA |



References
- Zhang, S.L.; Xie, Z.H. Current status, trends, main problems and the suggestions on development of pear industry in China. J. Fruit Sci. 2019, 36, 1067–1072. [Google Scholar]
- Li, B.; Qi, S.M.; Zhao, H.J.; Zhang, J.; Zhang, Z.Y. The Performance and Key Technique for High Quality and Yield of Shannong Crisp Pear in Mountainous Area of Central Shandong Province. J. Fruit Resour. 2023, 4, 87–89. [Google Scholar]
- Hou, Y.R.; Zhang, X.Y.; Gao, Z.F.; Chen, T.; Zhang, L.X. Relationships between fungal diversity and fruit quality of Yuluxiang pear during low temperature storage. Front. Microbiol. 2023, 14, 1132271. [Google Scholar]
- Yu, W.T.; Zhang, X.N.; Yan, W.W.; Sun, X.N.; Wang, Y.; Jia, X.H. Effects of 1-methylcyclopropene on skin greasiness and quality of ‘Yuluxiang’ pear during storage at 20 °C. J. Integr. Agric. 2024, 24, 76–2490. [Google Scholar] [CrossRef]
- Bapat, V.A.; Trivedi, P.K.; Ghosh, A.; Sane, V.A.; Ganapathi, T.R.; Nath, P. Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol. Adv. 2010, 28, 94–107. [Google Scholar]
- Lu, L.; Zuo, W.F.; Wang, C.C.; Li, C.X.; Feng, T.; Li, X.; Wang, C.; Yao, Y.X.; Zhang, Z.X.; Chen, X.S. Analysis of the Postharvest Storage Characteristics of the New Red-fleshed Apple Cultivar ‘Meihong’. Food Chem. 2021, 354, 129470. [Google Scholar]
- Tacken, E.; Ireland, H.; Gunaseelan, K.; Karunairetnam, S.; Wang, D.; Schultz, K.; Bowen, J.; Atkinson, R.G.; Johnston, J.W.; Putterill, J.; et al. The role of ethylene and cold temperature in the regulation of the apple polygalacturonase1 gene and fruit softening. Plant Physiol. 2010, 153, 294–305. [Google Scholar]
- Mworia, E.G.; Yoshikawa, T.; Salikon, N.; Oda, C.; Asiche, W.O.; Yokotani, N.; Abe, D.; Ushijima, K.; Nakano, R.; Kubo, Y. Low-temperature-modulated fruit ripening is independent of ethylene in ‘Sanuki Gold’ kiwifruit. J. Exp. Bot. 2012, 63, 963–971. [Google Scholar]
- Liu, J.P. Advances in research on key enzyme genes of ethylene biosynthesis. Chin. J. Trop. Agric. 2013, 33, 51–57. [Google Scholar]
- Choi, C.; Kang, I.K. Changes in cell wall components, and solubilization and depolymerization of pectin and neutral sugar polymers during softening of ‘Tsugaru’ Apples. J. Life Sci. 2006, 16, 834–839. [Google Scholar]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2009, 56, 147–154. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef]
- Wang, W.H.; Feng, H.; Xu, N. Comparisons on changes of several indexes related to the quality between common and truss tomatoes during storage period. Plant Physiol. Commun. 2008, 44, 93–96. [Google Scholar]
- Zhang, S.; Qi, S.; Li, B.; Zhang, J.; Cui, C.; Zhang, R.; Mao, Z.; Wang, N.; Chen, X.; Zhang, Z. Analysis of the Postharvest Storage Characteristics of Two New Pear Cultivars ‘Shannongsu’ and ‘Xincixiang’. Horticulturae 2023, 9, 281. [Google Scholar] [CrossRef]
- Liu, M.Y.; Wang, W.H.; Du, Y.M.; Jia, X.S. Effects of 1-MCP Treatment on Postharvest Quality and Physiology of Qiuyue Pear. Chin. Fruit Trees 2023, 15, 16–20. [Google Scholar]
- Huang, W.; Hu, N.; Xiao, Z.N.; Qiu, Y.P.; Yang, Y.; Yang, J.; Mao, X.; Wang, Y.C.; Li, Z.G.; Guo, H.W. A molecular framework of ethylene-mediated fruit growth and ripening processes in tomato. Plant Cell 2022, 34, 3280–3300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Liu, Z.; Lv, T.X.; Wei, Y.; Liu, W.T.; Wei, Y.J.; Yang, G.G.; Liu, L.; Li, T.; Wang, A. Exogenous Ca2+ promotes transcription factor phosphorylation to suppress ethylene biosynthesis in apple. Plant Physiol. 2023, 191, 2475–2488. [Google Scholar] [CrossRef]
- Cheng, S.; Jin, M.M.; He, X.Y.; Liu, J.; Zhang, M. Effects of exogenous ethylene on the cracking and cell wall metabolism of sweet potato. Food Ferment. Ind. 2020, 46, 161–166. [Google Scholar]
- Costa, F.; Sara, S.; Weg, W.E.V.D.; Guerra, W.; Cecchinel, M.; Dallivina, J.; Koller, B.; Sansivini, S. Role of the genes Md-ACO1 and Md-ACS1 in ethylene production and shelf life of apple (Malus domestica Borkh). Euphytica 2005, 141, 181–190. [Google Scholar] [CrossRef]
- Oraguzie, N.C.; Volz, R.K.; Whitworth, C.J.; Bassett, H.C.M.; Hall, A.J.; Gardiner, S.E. Influence of Md-ACS1 allelotype and harvest season within an apple germplasm collection on fruit softening during cold air storage. Postharvest Biol. Technol. 2007, 44, 212–219. [Google Scholar] [CrossRef]
- Dandekari, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Goulao, L.F.; Cosgrove, D.J.; Oliveira, C.M. Cloning, characterisation and expression analyses of cDNA clones encoding cell wall-modifying enzymes isolated from ripe apples. Postharvest Biol. Technol. 2008, 48, 37–51. [Google Scholar]
- Qi, X.D.; Li, S.H.; Wei, J.M.; Jia, Y.R. The Characteristics of Cell Wall Metabolism and Key Enzyme Genes Expression in Postharvest Gala Apple Fruit. Acta Agric. Boreali-Sin. 2012, 27, 55–60. [Google Scholar]
- Goulao, L.F.; Santos, J.; Sousa, I.; Oliveira, C.M. Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biol. Technol. 2006, 43, 307–318. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–340. [Google Scholar]
- Candelas, P.; Sara, P.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit softening and pectin disassembly: An overview of nanostructural pectin modifications assessed by atomic force microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar]
- Wang, D.D.; Yeats, T.H.; Uluisik, S.; Rose, J.K.C.; Seymour, G.B. Fruit softening: Revisiting the role of pectin. Trends Plant Sci. 2018, 23, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Z.; Cao, S. Promoter Variation of the Key Apple Fruit Texture Related Gene MdPG1 and the Upstream Regulation Analysis. Plants 2023, 12, 1452. [Google Scholar] [CrossRef]
- Oshita, S.; Shima, K.; Haruta, T.; Seo, Y.; Kawagoe, Y.; Nakayama, S.; Takahara, H. Discrimination of odors emanating from ‘La France’ pear by semi-conducting polymer sensors. Comput. Electron. Agric. 2000, 26, 209–216. [Google Scholar]
- Tian, C.P.; Wang, Y.L.; Liu, Z.C.; Wang, C.Z.; Sun, J.Z.; Wang, N.; Chen, X.S. Effects of 1-MCP and NO Treatments on Major Storage Quality Indices and Fatty Acid Metabolism Enzyme Activities in Golden Pear. Sci. Agric. Sin. 2010, 43, 2962–2972. [Google Scholar]
- Dou, S.J.; Chen, K.S.; Lv, J.L.; Zheng, J.T. Effects of different postharvest treatments on storage of huanghua pear fruit (Pyrus pyrifolia Nakai) and its physiological basis. Sci. Agric. Sin. 2003, 36, 82–88. [Google Scholar]
- Wang, R.C.; Tan, X.C.; Lv, C.P.; Xiong, X.Y. Fruit storeability and physio-biochemical changes during postharvest ripening in different clones of actinidia. J. Hunan Agric. Univ. 2000, 26, 46–49. [Google Scholar]
- Chen, X.S.; Guo, W.W.; Xu, J.; Cong, P.H.; Wang, L.R.; Liu, C.H.; Li, X.G.; Wu, S.J.; Yao, Y.X.; Chen, X.L. Genetic improvement and promotion of fruit quality of main fruit trees. Sci. Agric. Sin. 2015, 48, 3524–3540. [Google Scholar]
- Zhang, Z.Y.; Wang, N.; Jiang, S.H.; Xu, H.H.; Wang, Y.C.; Wang, C.Z.; Li, M.; Liu, J.X.; Qu, C.Z.; Liu, W.; et al. Analysis of thexyloglucan endotransglucosylase/hydrolase gene family during apple fruit ripening and softening. J. Agric. Food Chem. 2017, 65, 429–434. [Google Scholar]
- Li, H.S. Principles and Techniques of Plant Physiological and Biochemical Experiments; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Qian, J.; Zhou, J.; Di, B.; Liu, Y.; Zhang, G.; Yang, X. Using electrical impedance tomography for rapid determination of starch and soluble sugar contents in Rosa hybrida. Sci. Rep. 2021, 11, 2871. [Google Scholar]
- Jia, H.J.; Hirano, K.; Okamoto, G. Effect of fertilizer levels on tree growth and fruit quality of Hakuho’peaches (Prunus persica). J. Jpn. Soc. Hortic. Sci. 1999, 68, 487–493. [Google Scholar] [CrossRef]
- Filip, M.; Vlassa, M.; Coman, V.; Halmagyi, A. Simultaneous determination of glucose, fructose, sucrose and sorbitol in the leaf and fruit peel of different apple cultivars by the HPLC-RI optimized method. Food Chem. 2016, 199, 653–659. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Sun, Y.H. Study on Soluble Sugars and Organic Acids in Shandong Apples. J. Shandong Agric. Univ. 1995, 26, 355–360. [Google Scholar]
- Wang, C.Z.; Zhang, Y.M.; Xu, Y.T.; Dong, F.; Song, Y.; Liu, M.Y.; Liu, J.; Chen, X.S. Analysis of aroma components and Fatty acid metabolism related enzyme activities in red bud of Apple. Acta Hortic. Sin. 2012, 39, 2447–2456. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Qi, S.; Zhang, S.; Hu, R.; Li, L.; Zhou, X.; Wang, N.; Chen, X.; Zhang, Z. Study on the Quality Change and Regulation Mechanism of ‘Shannongsu’ Pear Under Low-Temperature Storage. Int. J. Mol. Sci. 2025, 26, 2900. https://doi.org/10.3390/ijms26072900
Chen C, Qi S, Zhang S, Hu R, Li L, Zhou X, Wang N, Chen X, Zhang Z. Study on the Quality Change and Regulation Mechanism of ‘Shannongsu’ Pear Under Low-Temperature Storage. International Journal of Molecular Sciences. 2025; 26(7):2900. https://doi.org/10.3390/ijms26072900
Chicago/Turabian StyleChen, Cong, Sumin Qi, Susu Zhang, Ruize Hu, Lu Li, Xinyue Zhou, Nan Wang, Xuesen Chen, and Zongying Zhang. 2025. "Study on the Quality Change and Regulation Mechanism of ‘Shannongsu’ Pear Under Low-Temperature Storage" International Journal of Molecular Sciences 26, no. 7: 2900. https://doi.org/10.3390/ijms26072900
APA StyleChen, C., Qi, S., Zhang, S., Hu, R., Li, L., Zhou, X., Wang, N., Chen, X., & Zhang, Z. (2025). Study on the Quality Change and Regulation Mechanism of ‘Shannongsu’ Pear Under Low-Temperature Storage. International Journal of Molecular Sciences, 26(7), 2900. https://doi.org/10.3390/ijms26072900







