Modulation of Structural and Physical-Chemical Properties of Fish Gelatin Hydrogel by Natural Polysaccharides
Abstract
1. Introduction
2. Results and Discussion
2.1. PXRD Overview of Hydrogel Phase State
2.2. SAXS Structural Characterization of Studied Systems
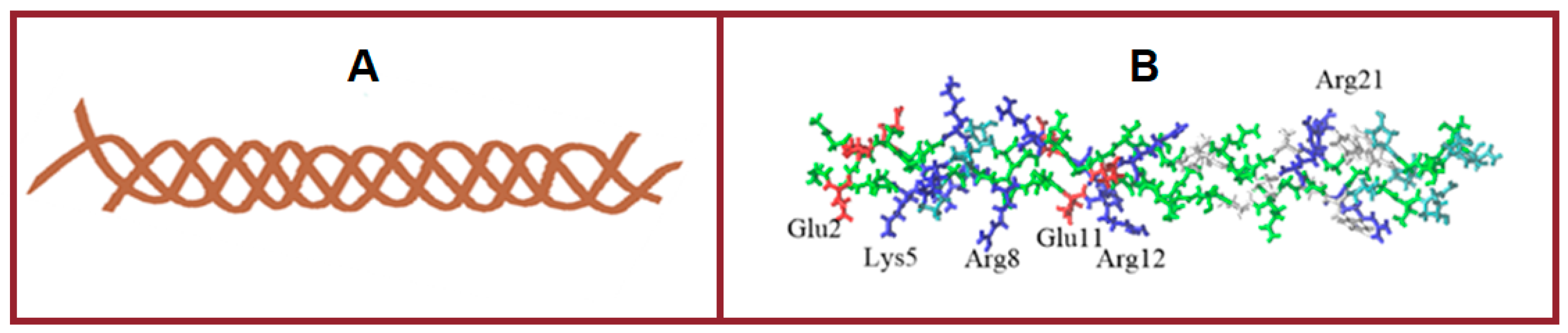
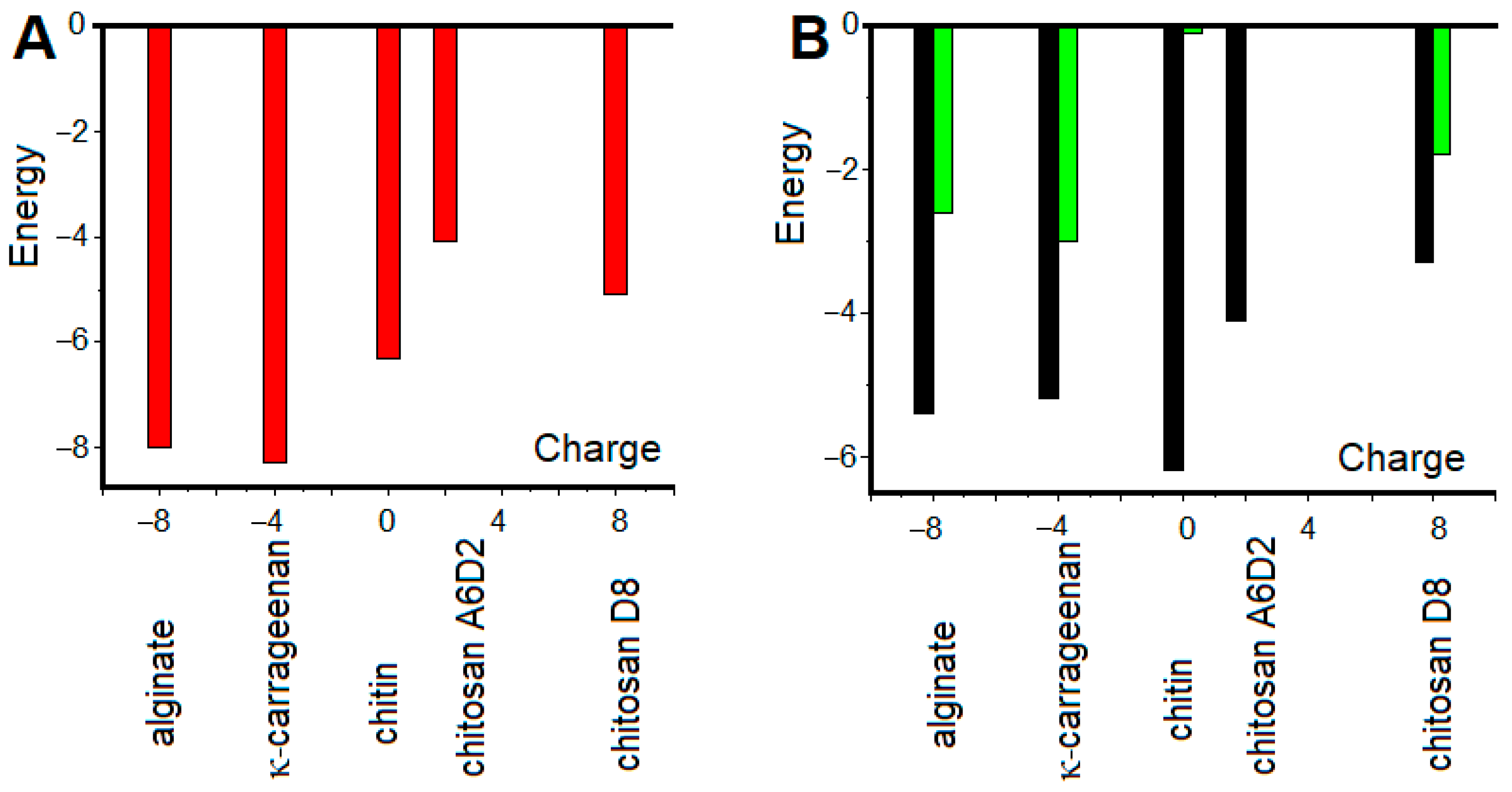
2.3. Molecular Modelling of Gelatin-Polysaccharide Interactions
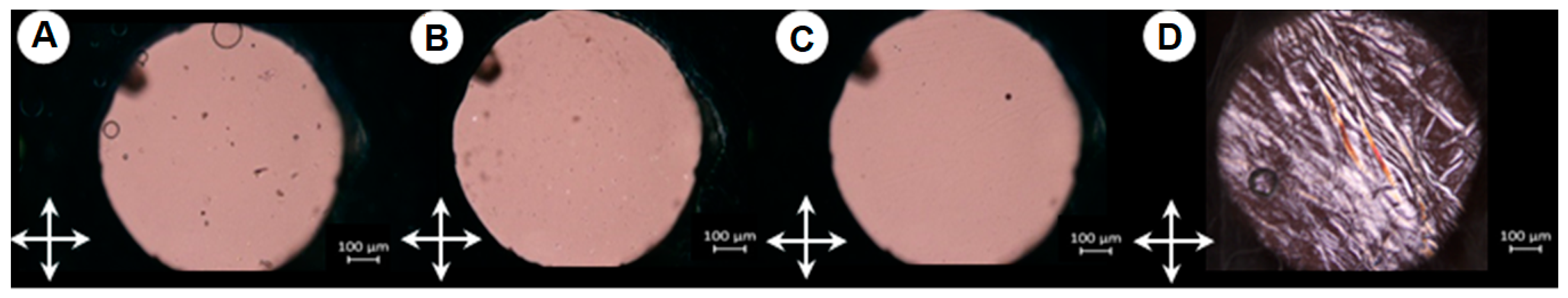
2.4. SEM Visualization of Hydrogel Morphology
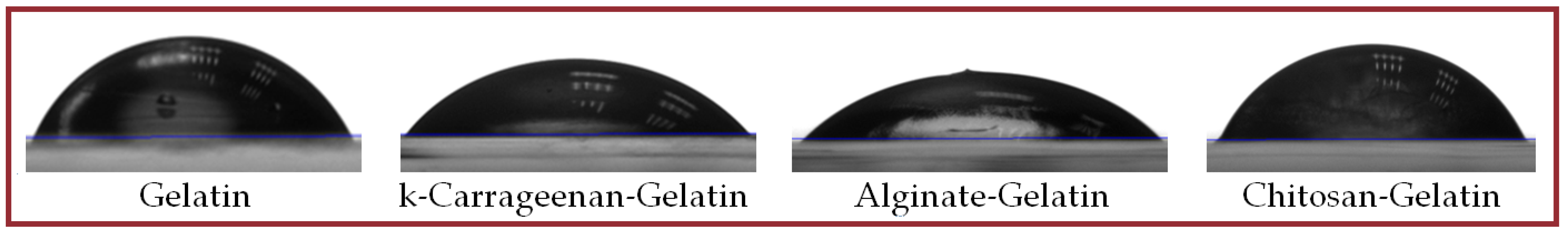
2.5. Some Physicochemical Properties of Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fish Gelatin-Polysaccharide Systems
3.3. Small-Angle and Wide-Angle X-Ray Scattering
3.4. Molecular Dynamics Study of Gelatin-Polysaccharide Interactions
3.4.1. Protein Modeling
3.4.2. Polysaccharides Modeling
3.4.3. Molecular Docking
3.4.4. Molecular Dynamics
3.4.5. Scanning Electron Microscopy
3.5. Physical-Chemical Studies of Gelatin-Polysaccharide Hydrogels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, X.T.; Rioux, L.-E.; Turgeon, S.L. Formation and functional properties of protein-polysaccharide electrostatic hydrogels in comparison to protein or polysaccharide hydrogels. Adv. Coll. Interface Sci. 2017, 239, 127–135. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Grokhovsky, V.A.; Nikiforova, A.A.; Sedov, I.A.; Faizullin, D.A.; Zuev, Y.F. Rheological Properties of Fish and Mammalian Gelatin Hydrogels as Bases for Potential Practical Formulations. Gels 2024, 10, 486. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Liu, X.; You, X.; Zhang, H.J. Degradable Gelatin-Based Supramolecular Coating for Green Paper Sizing. ACS Appl. Mater. Interfaces 2021, 13, 1367–1376. [Google Scholar]
- Ruan, H.; Bek, M.; Pandit, S.; Aulova, A.; Zhang, J.; Bjellheim, P.; Lovmar, M.; Mijakovic, I.; Kádár, R. Biomimetic Antibacterial Gelatin Hydrogels with Multifunctional Properties for Biomedical Applications. ACS Appl. Mater. Interfaces 2023, 15, 54249–54265. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Gelatin as It Is: History and Modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Effect of Pork Skin Gelatin on the Physical Properties of Pork Myofibrillar Protein Gel and Restructured Ham with Microbial Transglutaminase. Gels 2022, 8, 822. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Nitsuwat, S.; Zhang, P.; Ng, K.; Fang, Z. Fish gelatin as an alternative to mammalian gelatin for food industry: A meta-analysis. LWT-Food Sci. Technol. 2021, 141, 110899. [Google Scholar] [CrossRef]
- Athanasopoulou, E.; Michailidi, A.; Ladakis, D.; Kalliampakou, K.I.; Flemetakis, E.; Koutinas, A.; Tsironi, T. Extraction of Fish Protein Concentrates from Discards and Combined Application with Gelatin for the Development of Biodegradable Food Packaging. Sustainability 2023, 15, 12062. [Google Scholar] [CrossRef]
- Su, X.-N.; Khan, M.F.; Ai, X.; Liu, D.-L.; Liu, X.-F.; Zhao, Q.-L.; Cheong, K.-L.; Zhong, S.-Y.; Li, R. Fabrication, modification, interaction mechanisms, and applications of fish gelatin: A comprehensive review. Int. J. Biol. Macromol. 2025, 288, 138723. [Google Scholar] [CrossRef]
- Ramli, S.S.; Nizar, N.N.A.; Heng, J.Y.Y.; Karde, V.; Abidin, S.A.S.Z.; Taib, M.N. Chapter 9—Gelatin Substitute. In Innovation of Food Products in Halal Supply Chain Worldwide; Nizar, N.N.A., Abidin, S.A.S.Z., Bujang, A., Eds.; Academic Press: London, UK, 2023; pp. 87–109. [Google Scholar]
- Blanco, M.; Vazquez, J.A.; Perez-Martín, R.I.; Sotelo, C.G. Hydrolysates of fish skin collagen: An opportunity for valorizing fish industry byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Zuev, Y.F.; Derkach, S.R.; Bogdanova, L.R.; Voron’ko, N.G.; Kuchina, Y.A.; Gubaidullin, A.T.; Lunev, I.V.; Gnezdilov, O.I.; Sedov, I.A.; Larionov, R.A.; et al. Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel. Gels 2023, 9, 990. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Salahuddin, B.; Wang, S.; Sangian, D.; Aziz, S.; Gu, Q. Hybrid Gelatin Hydrogels in Nanomedicine Applications. ACS Appl. Bio Mater. 2021, 4, 2886–2906. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Ma, C.; Choi, J.-B.; Jang, Y.-S.; Kim, S.-Y.; Bae, T.-S.; Kim, Y.-K.; Park, J.-M.; Lee, M.-H. Mammalian and Fish Gelatin Methacryloyl−Alginate Interpenetrating Polymer Network Hydrogels for Tissue Engineering. ACS Omega 2021, 6, 17433–17441. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Majidiyan, N.; Jelyani, A.Z.; Moreno, A.; Hadian, Z.; Khanegah, A.M. Alginate/Fish Gelatin-Encapsulated Lactobacillus acidophilus: A Study on Viability and Technological Quality of Bread during Baking and Storage. Foods 2021, 10, 2215. [Google Scholar] [CrossRef]
- Kolotova, D.S.; Borovinskaya, E.V.; Bordiyan, V.V.; Zuev, Y.F.; Salnikov, V.V.; Zueva, O.S.; Derkach, S.R. Phase Behavior of Aqueous Mixtures of Sodium Alginate with Fish Gelatin: Effects of pH and Ionic Strength. Polymers 2023, 15, 2253. [Google Scholar] [CrossRef]
- Mathew, S.A.; Arumainathan, S. Crosslinked Chitosan−Gelatin Biocompatible Nanocomposite as a Neuro Drug Carrier. ACS Omega 2022, 7, 18732–18744. [Google Scholar] [CrossRef]
- Mottalib, A.; Islam, H.; Dhar, M.C.; Akhtar, K.; Goni, A. Preparation and Characterization of New Biodegradable Packaging Materials Based on Gelatin Extracted from Tenualosa ilisha Fish Scales with Cellulose Nanocrystals. ACS Omega 2024, 9, 51175–51190. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Prasad Shastri, V.; Forget, A. Hydrogel-Forming Algae Polysaccharides: From Seaweed to Biomedical Applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, S.; Das, S.; De, S.; Paul, P.; Dua, T.K.; Sahu, R.; Nandi, G. Current Status of Marine Animal Derived Polysaccharides in Sustainable Food Packaging. ACS Food Sci. Technol. 2023, 3, 1877–1889. [Google Scholar] [CrossRef]
- Rees, D.A. Structure, Conformation, and Mechanism in the Formation of Polysaccharide Gels and Networks. Adv. Carbohydr. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [CrossRef]
- Morris, E.R.; Rees, D.A.; Robinson, G. Cation-Specific Aggregation of Carrageenan Helices: Domain Model of Polymer Gel Structure. J. Mol. Biol. 1980, 138, 349–362. [Google Scholar] [CrossRef]
- Michel, A.S.; Mestdagh, M.M.; Axelos, M.A.V. Physico-Chemical Properties of Carrageenan Gels in Presence of Various Cations. Int. J. Biol. Macromol. 1997, 21, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Makshakova, O.N.; Faizullin, D.A.; Zuev, Y.F. Interplay between secondary structure and ion binding upon thermoreversible gelation of κ-carrageenan. Carbohydr. Polym. 2020, 227, 115342. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, O.A.; Tøndervik, A.; Sletta, H.; Skjåk-Bræk, G. Alginate Sequencing: An Analysis of Block Distribution in Alginates Using Specific Alginate Degrading Enzymes. Biomacromolecules 2012, 13, 106–116. [Google Scholar] [CrossRef]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-Induced Polysaccharide Gelation: Peculiarities of Alginate Egg-Box Association with Different Divalent Cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Zueva, O.S.; Khair, T.; Kazantseva, M.A.; Latypova, L.; Zuev, Y.F. Ions-Induced Alginate Gelation According to Elemental Analysis and a Combinatorial Approach. Int. J. Mol. Sci. 2023, 24, 16201. [Google Scholar] [CrossRef]
- Pérez-Madrigal, M.M.; Torras, J.; Casanovas, J.; Häring, M.; Alemán, C.; Díaz, D.D. Paradigm Shift for Preparing Versatile M2+-Free Gels from Unmodified Sodium Alginate. Biomacromolecules 2017, 18, 2967–2979. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Cross-Link. Hydrogels Rev. 2014, 4, 25–31. [Google Scholar]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; de Oliveira Franco, L.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Sereni, N.; Enache, A.A.; Sudre, G.; Montembault, A.; Rochas, C.; Durand, P.; Perrard-Durand, M.-H.; Bozga, G.; Puaux, J.-P.; Delair, T.; et al. Dynamic Structuration of Physical Chitosan Hydrogels. Langmuir 2017, 33, 12697–12707. [Google Scholar] [CrossRef] [PubMed]
- Ladet, S.; David, L.; Domard, A. Multi-membrane hydrogels. Nature 2008, 452, 76–79. [Google Scholar] [CrossRef]
- Latza, V.; Guerette, P.A.; Ding, D.; Amini, S.; Kumar, A.; Schmidt, I.; Keating, S.; Oxman, N.; Weaver, J.C.; Fratzl, P. Multi-scale thermal stability of a hard thermoplastic protein-based material. Nat. Commun. 2015, 6, 8313. [Google Scholar] [CrossRef]
- Montembault, A.; David, L.; Range, M. Morphology of Physical Chitosan Hydrogels. Langmuir 2010, 26, 17495–17504. [Google Scholar] [CrossRef]
- Popa-Nita, S.; Rochas, C.; David, L.; Domard, A. Structure of Natural Polyelectrolyte Solutions: Role of the Hydrophilic/Hydrophobic Interaction Balance. Langmuir 2009, 25, 6460–6468. [Google Scholar] [CrossRef]
- Boucard, N.; David, L.; Rochas, C.; Montembault, A.; Viton, C.; Domard, A. Polyelectrolyte Microstructure in Chitosan Aqueous and Alcohol Solutions. Biomacromolecules 2007, 8, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Zuev, Y.F.; Derkach, S.R.; Lunev, I.V.; Nikiforova, A.A.; Klimovitskaya, M.A.; Bogdanova, L.R.; Skvortsova, P.V.; Kurbanov, R.K.; Kazantseva, M.A.; Zueva, O.S. Water as a Structural Marker in Gelatin Hydrogels with Different Cross-Linking Nature. Int. J. Mol. Sci. 2024, 25, 11738. [Google Scholar] [CrossRef] [PubMed]
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 142–163. [Google Scholar]
- Zhang, T.; Sun, R.; Ding, M.; Li, L.; Tao, N.; Wang, X.; Zhong, J. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT Food Sci. Technol. 2020, 125, 109207. [Google Scholar] [CrossRef]
- Giraud-Guille, M.-M.; Besseau, L.; Chopin, C.; Durand, P.; Herbage, D. Structural aspects of fish skin collagen which forms ordered arrays via liquid crystalline states. Biomaterials 2000, 21, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Learner, T.J.S.; Smithen, P.; Krueger, J.W.; Schilling, M.R. (Eds.) Modern Paints Uncovered; Getty Publications: Los Angeles, CA, USA, 2008. [Google Scholar]
- Schmidt, V.; Giacomelli, C.; Soldi, V. Thermal stability of films formed by soy protein isolate-sodium dodecyl sulfate. Polym. Degrad. Stab. 2005, 87, 25–31. [Google Scholar] [CrossRef]
- Steyaert, I.; Rahier, H.; Van Vlierberghe, S.; Olijve, J.; De Clerck, K. Gelatin nanofibers: Analysis of triple helix dissociation temperature and cold-water-solubility. Food Hydrocoll. 2016, 57, 200–208. [Google Scholar] [CrossRef]
- Ghoshal, S.; Stapf, S.; Mattea, C. Protein renaturation in the gelatin film formation process. Appl. Magn. Reson. 2014, 45, 145–154. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-rays; Wiley: New York, NY, USA, 1955. [Google Scholar]
- Qazvini, N.T.; Bolisetty, S.; Adamcik, J.; Mezzenga, R. Self-Healing Fish Gelatin/Sodium Montmorillonite Biohybrid Coacervates: Structural and Rheological Characterization. Biomacromolecules 2012, 13, 2136–2147. [Google Scholar] [CrossRef]
- Schaefer, D.W. Fractal Models and the Structure of Materials. MRS Bull. 1988, 13, 22–27. [Google Scholar] [CrossRef]
- Yang, Z.; Hemar, Y.; Hilliou, L.; Gilbert, E.P.; McGillivray, D.J.; Williams, M.A.K.; Chaieb, S. Non-Linear Behaviour of Gelatin Networks Reveals a Hierarchical Structure. Biomacromolecules 2016, 17, 590–600. [Google Scholar] [CrossRef]
- Hung, K.-C.; Jeng, U.-S.; Hsu, S. Fractal Structure of Hydrogels Modulates Stem Cell Behavior. ACS Macro Lett. 2015, 4, 1056–1061. [Google Scholar] [CrossRef]
- Fontes-Candia, C.; Ström, A.; Lopez-Sanchez, P.; López-Rubio, A.; Martínez-Sanz, M. Rheological and structural characterization of carrageenan emulsion gels. Algal Res. 2020, 47, 101873. [Google Scholar] [CrossRef]
- Gubaidullin, A.T.; Makarova, A.O.; Derkach, S.R.; Voron’ko, N.G.; Kadyirov, A.I.; Ziganshina, S.A.; Salnikov, V.V.; Zueva, O.S.; Zuev, Y.F. Modulation of Molecular Structure and Mechanical Properties of k-Carrageenan-Gelatin Hydrogel with Multi-Walled Carbon Nanotubes. Polymers 2022, 14, 2346. [Google Scholar] [CrossRef]
- Zueva, O.S.; Gubaidullin, A.T.; Makarova, A.O.; Bogdanova, L.R.; Zakharova, L.Y.; Zuev, Y.F. Structural features of composite protein-polysaccharide hydrogel in the presence of a carbon nanomaterial. Russ. Chem. Bull. 2020, 69, 581–589. [Google Scholar] [CrossRef]
- Evmenenko, G.; Theunissen, E.; Mortensen, K.; Reynaers, H. SANS Study of Surfactant Ordering in k-Carrageenan/Cetylpyridinium Chloride Complexes. Polymer 2001, 42, 2907–2913. [Google Scholar] [CrossRef]
- Peesapati, S.; Jagannathareddy, D.K.; Roy, D. Mechanical Stretching of Nature Inspired Linear and Branched Polysaccharide Motifs through Steered Molecular. ACS Appl. Bio Mater. 2024, 7, 6114–6126. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Xu, X.; Men, X.; Yang, J.; Zhou, X.; Xue, Q. Facile fabrication of a superamphiphobic surface on the copper substrate. J. Colloid. Interface Sci. 2012, 367, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Galeeva, A.; Selivanova, N.; Galyametdinov, Y. Wetting and adhesive properties of biopolymer-based organized condensed phases as drug delivery systems. Liq. Cryst. Their Appl. 2023, 23, 38–48. [Google Scholar] [CrossRef]
- Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi, A.; Piergiovanni, L. Wetting of Biopolymer Coatings: Contact Angle Kinetics and Image Analysis Investigation. Langmuir 2011, 27, 7563–7574. [Google Scholar] [CrossRef]
- Selivanova, N.M.; Galeeva, A.I.; Galyametdinov, Y.G. Biocompatible delivery systems based on κ-carrageenan and nonionic surfactants. Liq. Cryst. Their App. 2020, 20, 28–39. [Google Scholar] [CrossRef]
- Selivanova, N.; Galeeva, A.; Galyametdinov, Y. Chitosan/lactic acid systems: Liquid crystalline behavior, rheological properties and riboflavin release in vitro. Int. J. Mol. Sci. 2022, 23, 13207. [Google Scholar] [CrossRef]
- Białopiotrowicz, T.; Jańczuk, B. Surface Properties of Gelatin Films. Langmuir 2002, 18, 9462–9468. [Google Scholar]
- DIFFRAC Plus Evaluation package EVA, Version 11. In User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005; 258p.
- TOPAS V3: General Profile and Structure Analysis Software for Powder Diffraction Data, Technical Reference; Bruker AXS: Karlsruhe, Germany, 2005.
- Glatter, O.; Kratky, O. (Eds.) Small Angle X-ray Scattering; Academic Press: London, UK; New York, NY, USA, 1982; ISBN 978-0-12-286280-9. [Google Scholar]
- Svergun, D.I.; Fêıgin, L.A.; Taylor, G.W. Structure Analysis by Small-Angle X-ray and Neutron Scattering; Plenum Press: New York, NY, USA, 1987; ISBN 978-0-306-42629-2. [Google Scholar]
- Da Vela, S.; Svergun, D.I. Methods, Development and Applications of Small-Angle X-ray Scattering to Characterize Biological Macromolecules in Solution. Curr. Res. Struct. Biol. 2020, 2, 164–170. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T.; Han, C.C. Small Angle Neutron Scattering Study on Poly(N-isopropyl Acrylamide) Gels near Their Volume-phase Transition Temperature. J. Chem. Phys. 1992, 97, 6829–6841. [Google Scholar] [CrossRef]
- McAulay, K.; Wang, H.; Fuentes-Caparrós, A.M.; Thomson, L.; Khunti, N.; Cowieson, N.; Cui, H.; Seddon, A.; Adams, D.J. Isotopic Control over Self-Assembly in Supramolecular Gels. Langmuir 2020, 36, 8626–8631. [Google Scholar] [CrossRef]
- Konarev, P.V.; Volkov, V.V.; Sokolova, A.V.; Koch, M.H.J.; Svergun, D.I. PRIMUS—A Windows-PC based system for small-angle scattering data analysis. J. Appl. Cryst. 2003, 36, 1277–1282. [Google Scholar] [CrossRef]
- SASView 3.0.0, University of Tennessee, 2009—2013. Available online: http://www.sasview.org (accessed on 21 March 2025).
- Rainey, J.K.; Goh, M.C. An interactive triple-helical collagen builder. Bioinformatics 2004, 20, 2458–2459. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods. 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Guvench, O.; Mallajosyula, S.S.; Raman, E.P.; Hatcher, E.; Vanommeslaeghe, K.; Foster, T.J.; Jamison, F.W., II; MacKerell, A.D., Jr. CHARMM additive all-atom force field for carbohydrate derivatives and its utility in polysaccharide and carbohydrate-protein modeling. J. Chem. Theory Comput. 2011, 7, 3162–3180. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.K.; Schulten, K. VMD—Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Ge, S.; Li, M.; Ji, N.; Liu, J.; Mul, H.; Xiong, L.; Sun, Q. Preparation of a Strong Gelatin–Short Linear Glucan Nanocomposite Hydrogel by an in Situ Self-Assembly Process. J. Agric. Food Chem. 2018, 66, 177–186. [Google Scholar] [CrossRef]
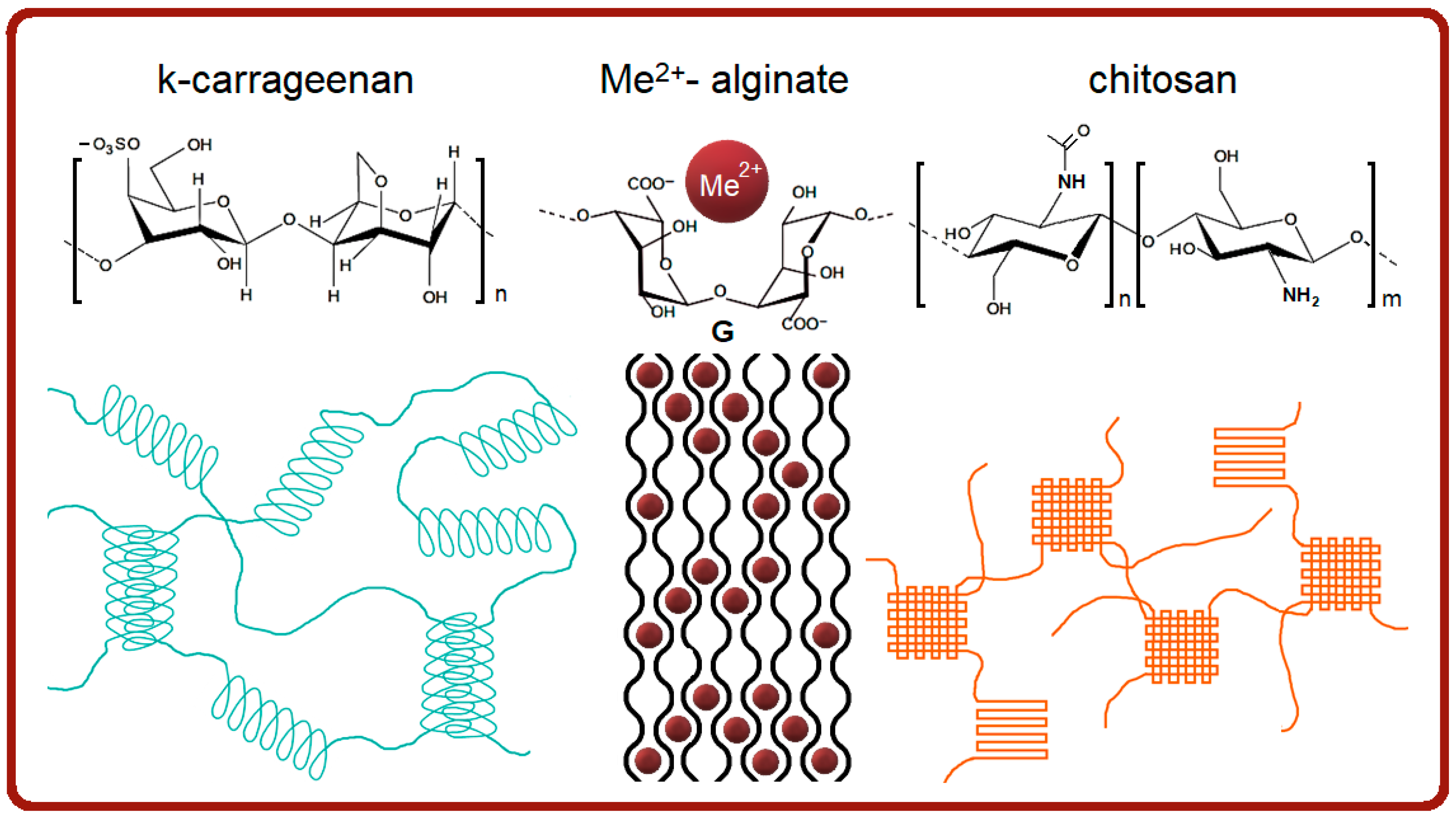
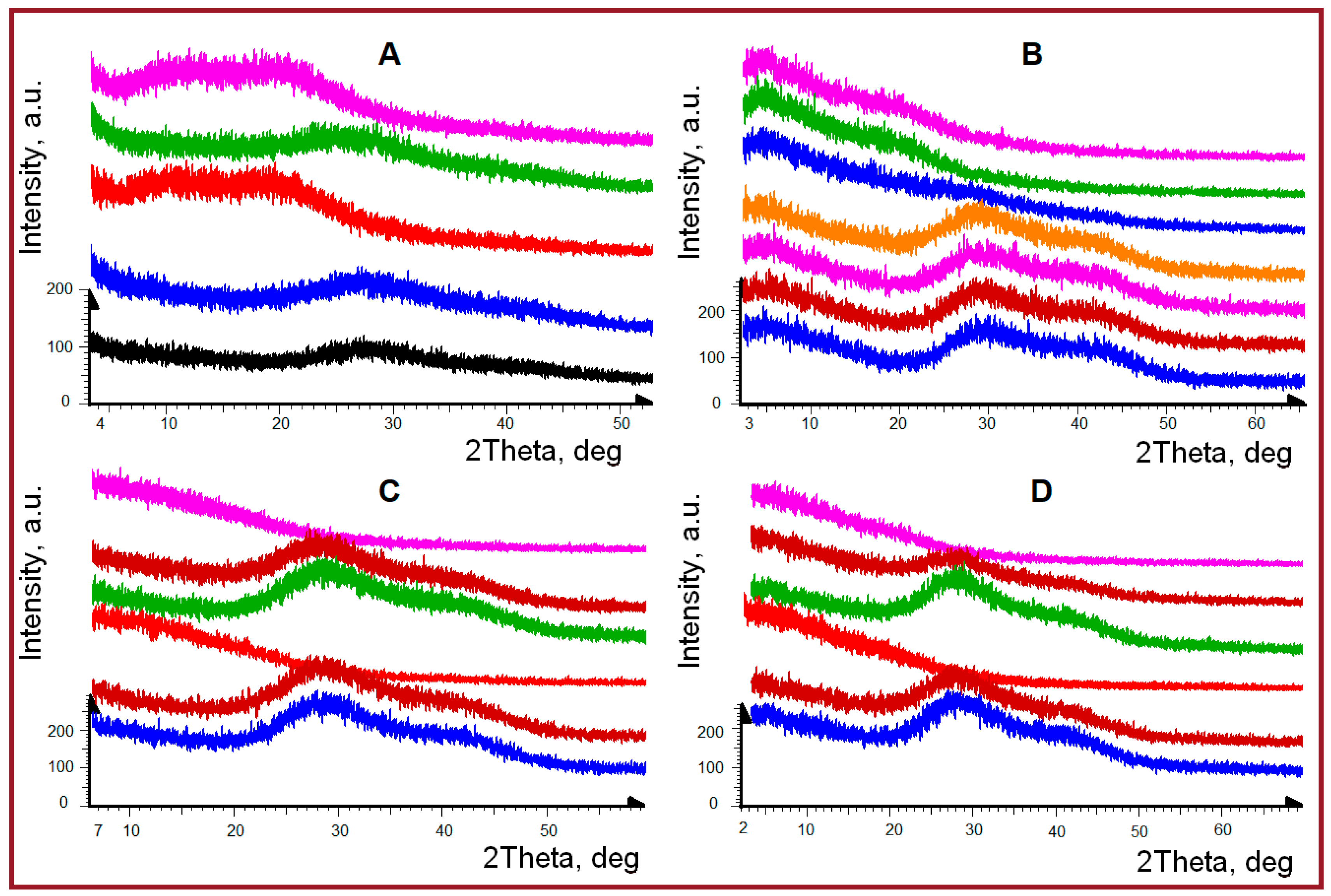
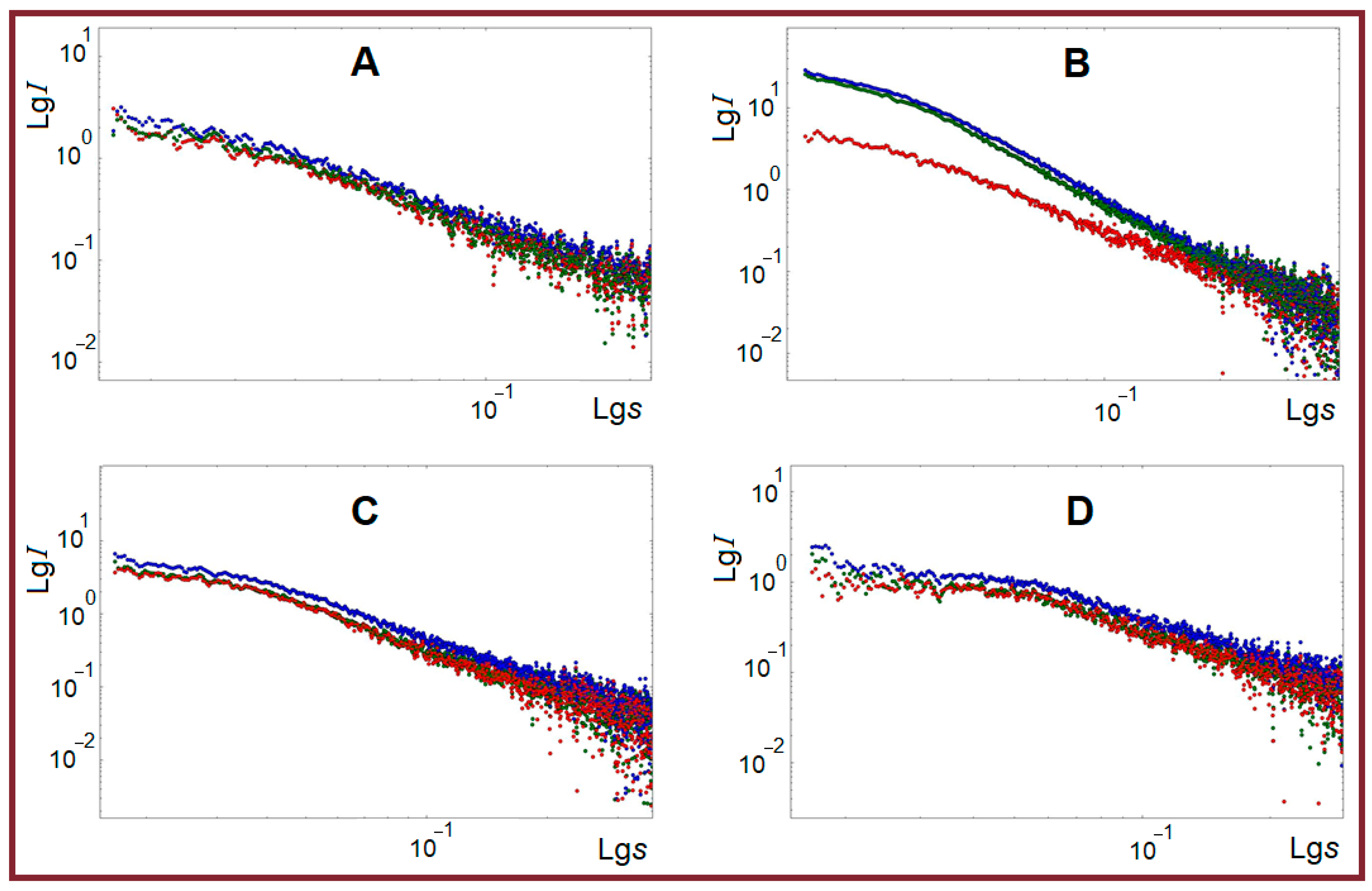
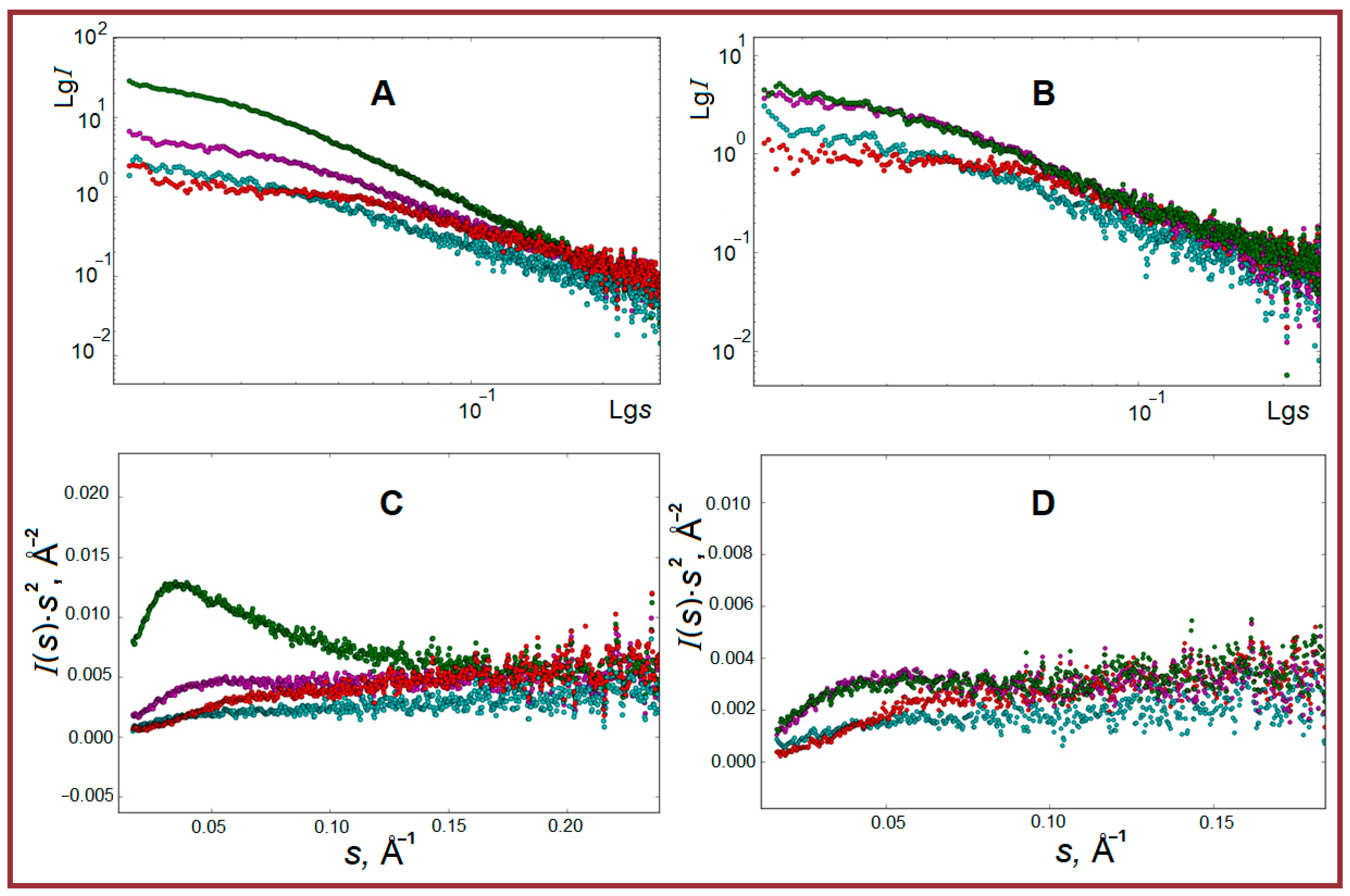



| Structural Parameter, Å | T, °C | Gelatin | κ-Carrageenan- Gelatin- | Alginate- Gelatin- | Chitosan- Gelatin- | |
|---|---|---|---|---|---|---|
| Sphere | Rg | 1 | 37.5 ± 2.0 | 50.6 ± 0.3 | 35.9 ± 0.7 | 22.0 ± 1.4 |
| 45 | 37.8 ± 2.3 | 41.8 ± 1.1 | 36.1 ± 1.0 | 20.2 ± 1.8 | ||
| Rsph | 1 | 48.4 | 65.3 | 46.4 | 28.4 | |
| 45 | 48.8 | 53.9 | 46.6 | 26.1 | ||
| Dmax | 1 | 119.05 | 192.18 | 107.56 | 71.9 | |
| 45 | 133.86 | 138.00 | 37.91 | 32.6 | ||
| G-L-gel- model | Ξ, static | 1 | 96.3 ± 5.7 | 41.3 ± 1.1 | 278.9 ± 8.4 | 174.1 ± 7.9 |
| 45 | 98.5 ± 6.8 | 50.3 ± 3.3 | 83.0 ± 4.2 | 358 ± 27 | ||
| ξ, dynamic | 1 | 21.2 ± 1.6 | 65.7 ± 1.1 | 35.3 ± 0.8 | 16.4 ± 1.2 | |
| 45 | 20.9 ± 2.1 | 24.3 ± 1.8 | 130.8 ± 4.6 | 15.8 ± 1.6 | ||
| Fractal dimension | df | 1 | 2.14 | 1.62 | 1.18 | 1.00 |
| 45 | 1.84 | 1.33 | 2.74 | 2.6 |
| Sample | D, nm | ζ, mV |
|---|---|---|
| 1% gelatin | 11.3 | −4.09 |
| 10% gelatin | 12.0 | |
| 1% gelatin/k-carrageenan | 4.5 | −2.00 |
| 1% gelatin/alginate | 14.6 | −6.63 |
| 1% gelatin/chitosan | 54.7 | 15.5 |
| Gelatin | k-Carrageenan-Gelatin | Alginate-Gelatin | Chitosan-Gelatin | |
|---|---|---|---|---|
| Contact angle, ° | 64.8 | 47.4 | 42.9 | 63.5 |
| Work of surface adhesion, mJ/m2 | 102.9 | 121.1 | 125.1 | 104.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubaidullin, A.T.; Galeeva, A.I.; Galyametdinov, Y.G.; Ageev, G.G.; Piryazev, A.A.; Ivanov, D.A.; Ermakova, E.A.; Nikiforova, A.A.; Derkach, S.R.; Zueva, O.S.; et al. Modulation of Structural and Physical-Chemical Properties of Fish Gelatin Hydrogel by Natural Polysaccharides. Int. J. Mol. Sci. 2025, 26, 2901. https://doi.org/10.3390/ijms26072901
Gubaidullin AT, Galeeva AI, Galyametdinov YG, Ageev GG, Piryazev AA, Ivanov DA, Ermakova EA, Nikiforova AA, Derkach SR, Zueva OS, et al. Modulation of Structural and Physical-Chemical Properties of Fish Gelatin Hydrogel by Natural Polysaccharides. International Journal of Molecular Sciences. 2025; 26(7):2901. https://doi.org/10.3390/ijms26072901
Chicago/Turabian StyleGubaidullin, Aidar T., Aliya I. Galeeva, Yuriy G. Galyametdinov, Georgiy G. Ageev, Alexey A. Piryazev, Dimitri A. Ivanov, Elena A. Ermakova, Alena A. Nikiforova, Svetlana R. Derkach, Olga S. Zueva, and et al. 2025. "Modulation of Structural and Physical-Chemical Properties of Fish Gelatin Hydrogel by Natural Polysaccharides" International Journal of Molecular Sciences 26, no. 7: 2901. https://doi.org/10.3390/ijms26072901
APA StyleGubaidullin, A. T., Galeeva, A. I., Galyametdinov, Y. G., Ageev, G. G., Piryazev, A. A., Ivanov, D. A., Ermakova, E. A., Nikiforova, A. A., Derkach, S. R., Zueva, O. S., & Zuev, Y. F. (2025). Modulation of Structural and Physical-Chemical Properties of Fish Gelatin Hydrogel by Natural Polysaccharides. International Journal of Molecular Sciences, 26(7), 2901. https://doi.org/10.3390/ijms26072901










