Circ_RUSC2 Sequesters miR-661 and Elevates TUSC2 Expression to Suppress Colorectal Cancer Progression
Abstract
1. Introduction
2. Results
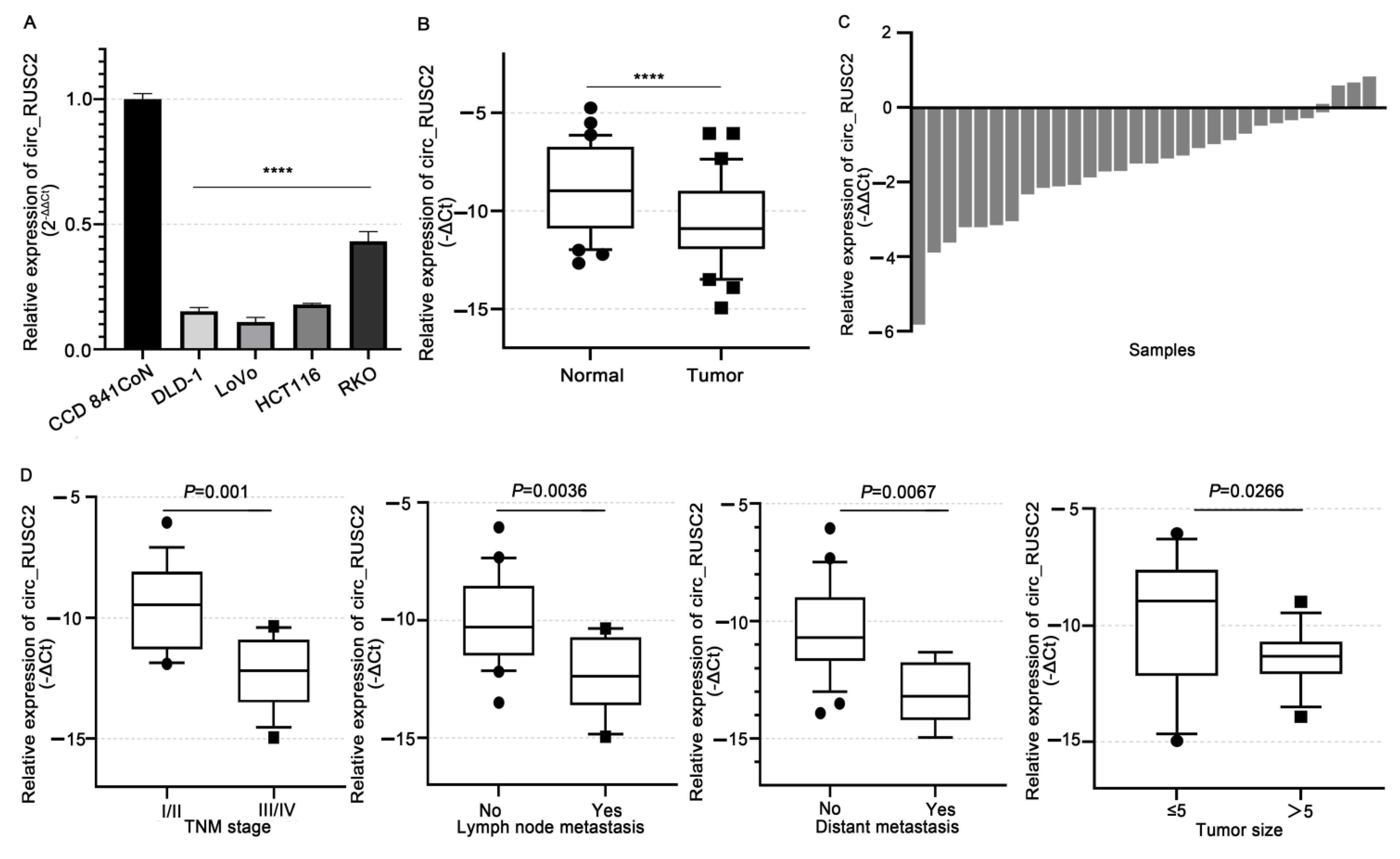
2.1. Circ_RUSC2 Is Notably Reduced in CRC Cells and Tissues, Correlating with Poor Clinicopathological Profiles
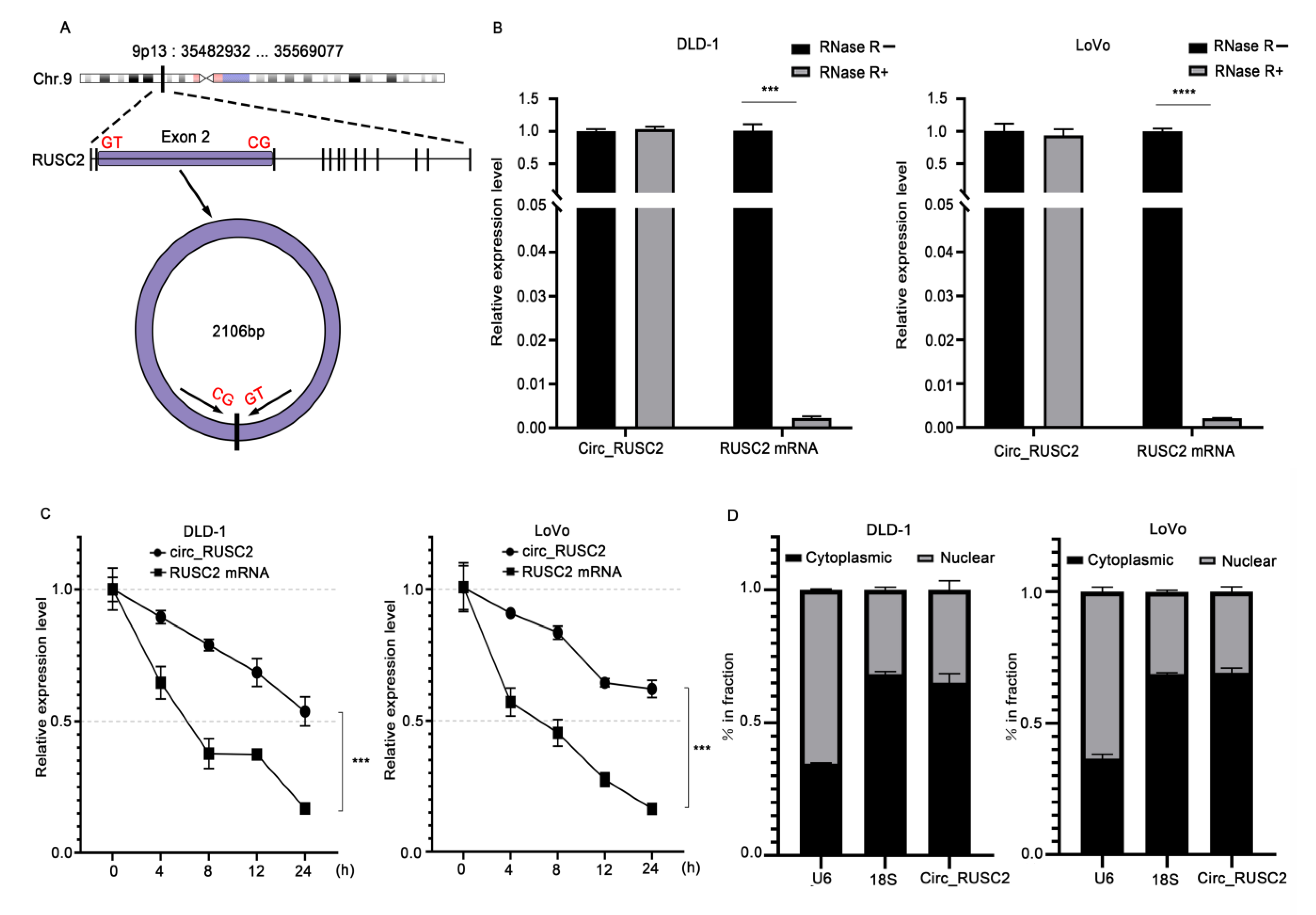
2.2. Circ_RUSC2 Is a Stable Cytoplasmic circRNA in CRC Cells
2.3. Circ_RUSC2 Sponges miR-661 as a ceRNA
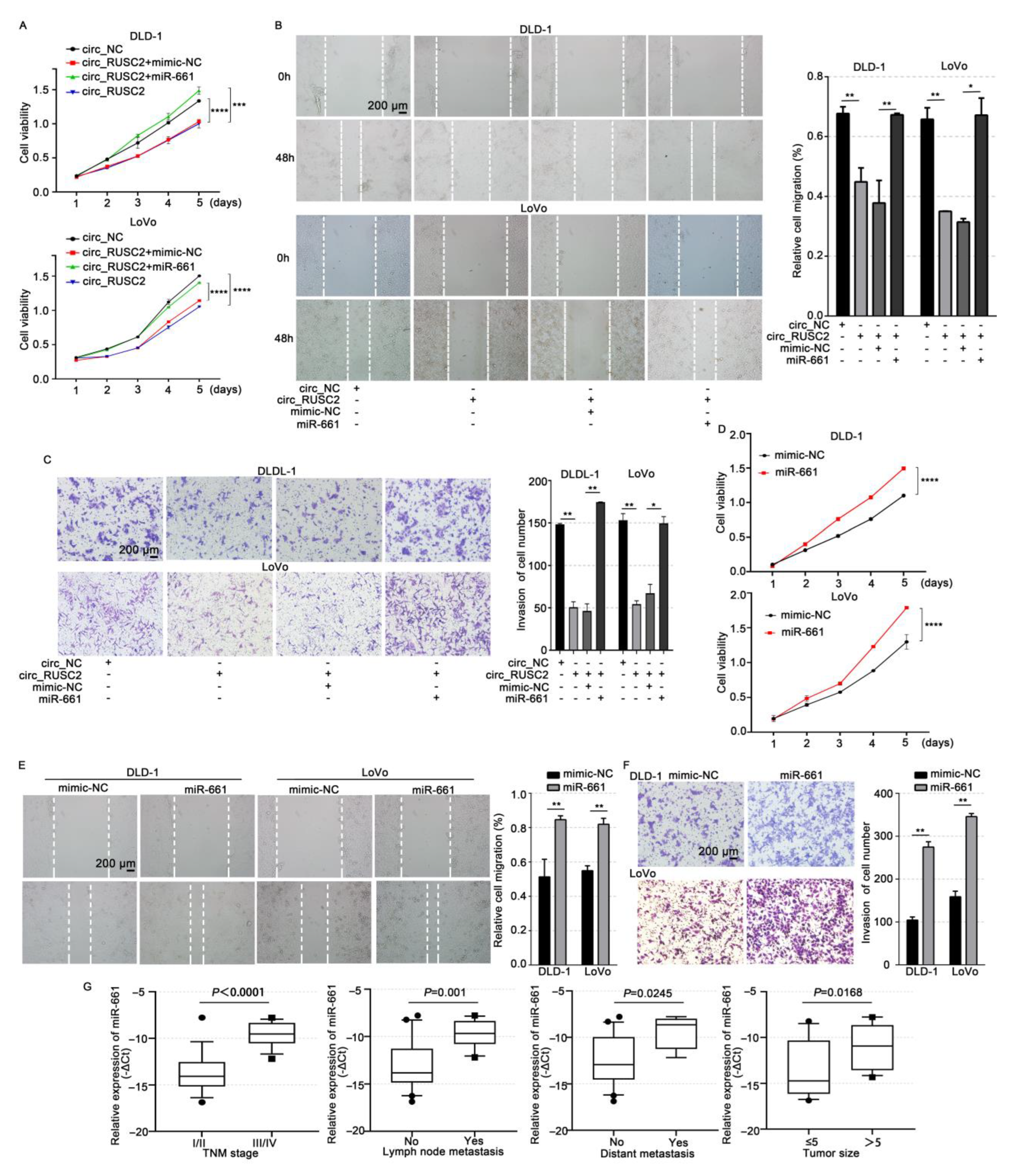
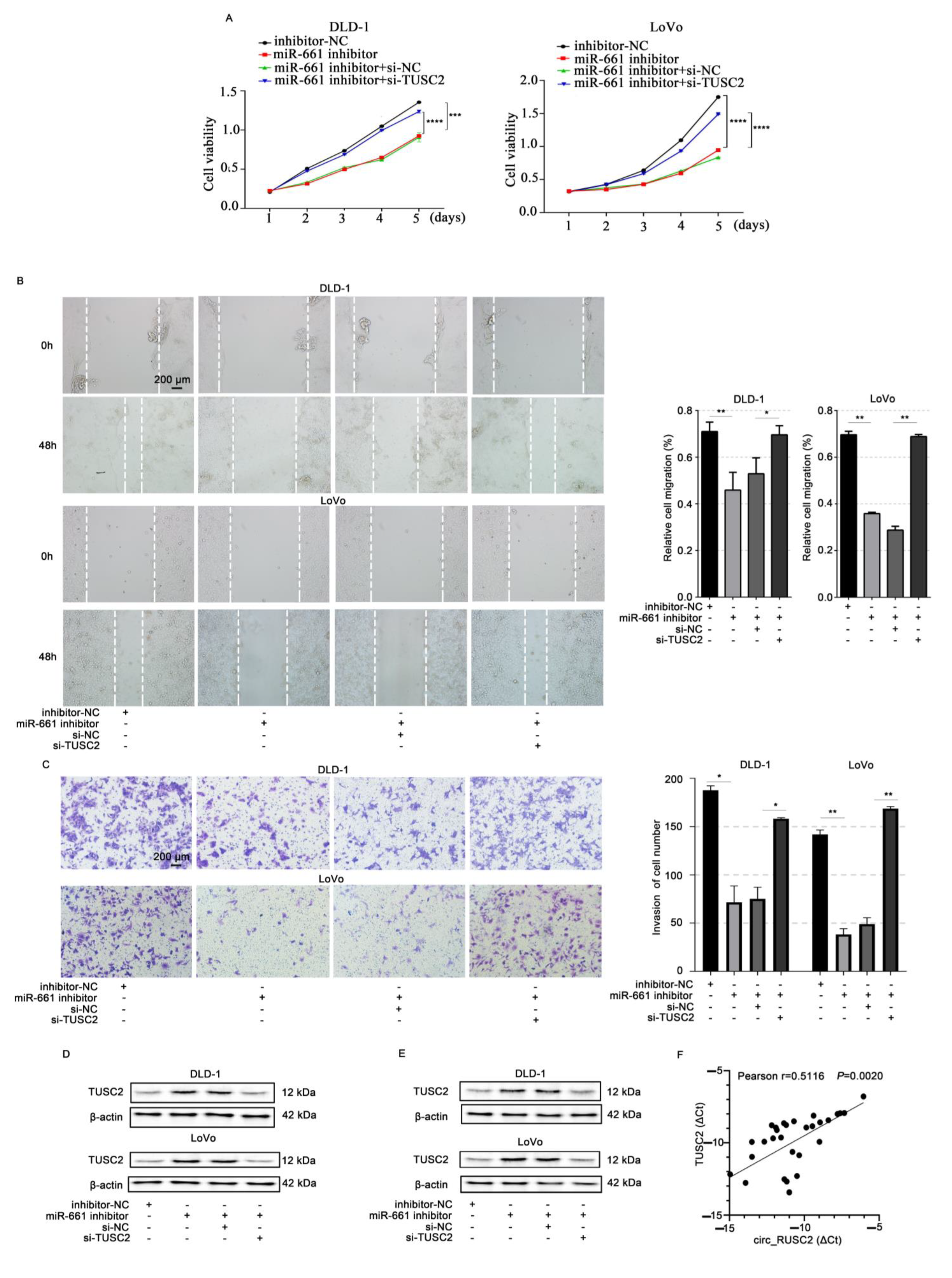
2.4. Circ_RUSC2 Inhibits the Proliferative, Migratory, and Invasive Behaviors of CRC Cells via Sponging miR-661
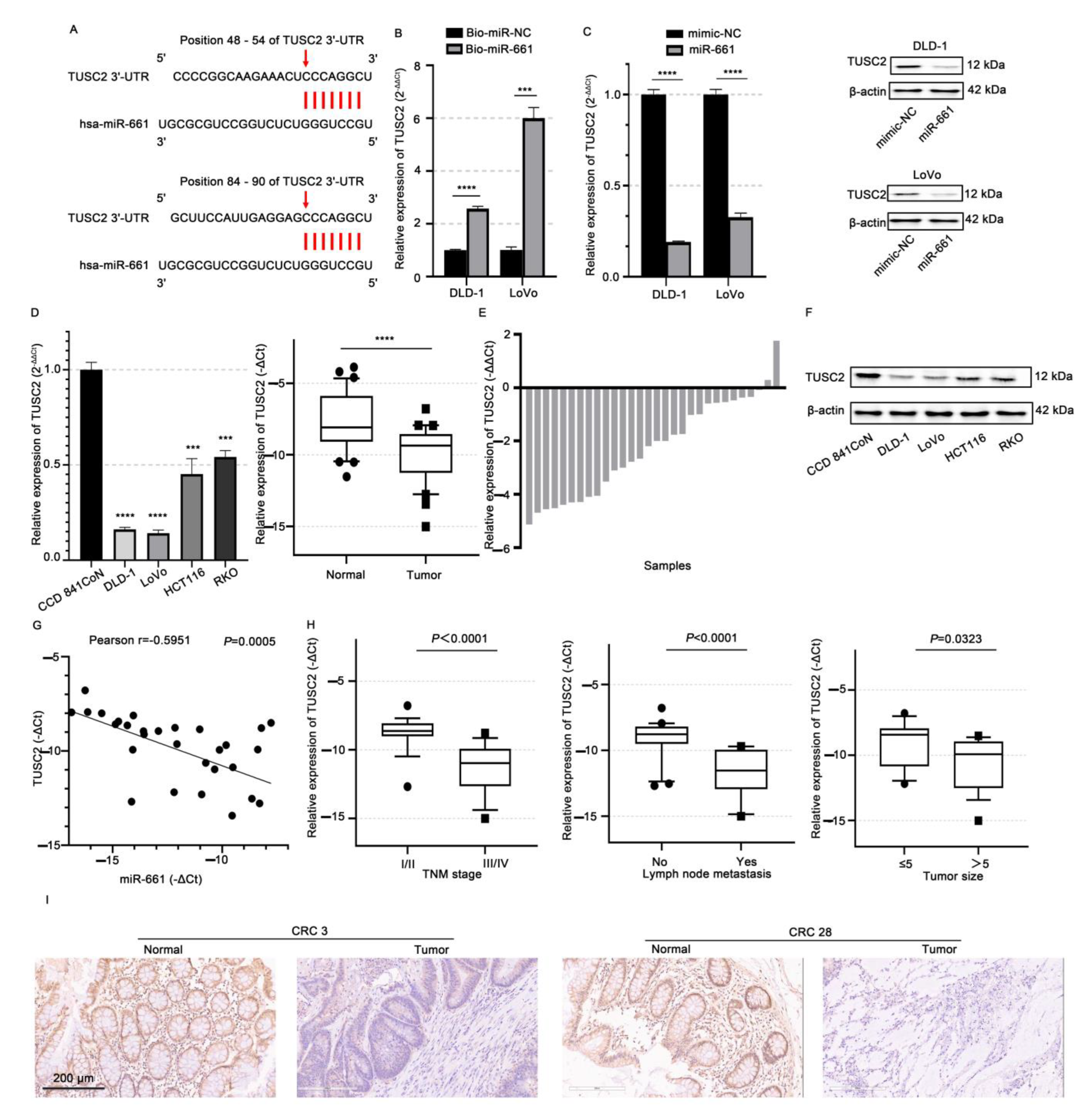
2.5. TUSC2 Is Targeted by miR-661 in CRC Cells
2.6. Circ_RUSC2 Upregulates TUSC2 Protein Expression of CRC Cells Through miR-661 Sponging
2.7. N6-Methyladenosine (m6A) Modification Stably Increases the circ_RUSC2 Expression and Is Possibly Mediated by METTL3
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Tissue Samples
4.3. Cell Transfection
4.4. RNA Isolation and Quantitative Reverse-Transcription PCR (qRT-PCR)
4.5. Actinomycin D and Ribonuclease (RNase) R Digestion Assays
4.6. Cytoplasmic and Nuclear RNA Fractionation
4.7. RNA Pull-Down Assay
4.8. Cell Counting Kit-8 (CCK-8) Assay
4.9. Scratch Wound Healing and Transwell Invasion Assays
4.10. Western Blot Analysis
4.11. Immunohistochemistry
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ou, Q.; Chang, Y.; Liu, J.; Yan, H.; Chen, L.; Guo, D.; Zhang, S.F. Mapping the intellectual structure and landscape of colorectal cancer immunotherapy: A bibliometric analysis. Hum. Vaccin. Immunother. 2024, 20, 2323861. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Chen, H.; Yu, Y.; Li, N.; Chen, W. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021, 134, 783–791. [Google Scholar] [CrossRef]
- He, M.; Pan, Y.; You, C.; Gao, H. CircRNAs in cancer therapy tolerance. Clin. Chim. Acta 2024, 558, 119684. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lu, Y. Roles of circRNAs in the progression of colorectal cancer: Novel strategies for detection and therapy. Cancer Gene Ther. 2024, 31, 831–841. [Google Scholar] [CrossRef]
- Kosik, K. Molecular biology: Circles reshape the RNA world. Nature 2013, 495, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhong, W.; Lyu, Z.; Peng, J.; Rong, Y.; Zeng, K.; Lai, J.; Wu, D.; Wang, J.; Li, Y.; et al. Circular RNA circTATDN3 promotes the Warburg effect and proliferation in colorectal cancer. Cancer Lett. 2024, 589, 216825. [Google Scholar] [CrossRef]
- Almalki, W.; Almujri, S. The dual roles of circRNAs in Wnt/β-Catenin signaling and cancer progression. Pathol. Res. Pract. 2024, 255, 155132. [Google Scholar] [CrossRef]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Li, P.; Wu, M. CircRNA: Functions and properties of a novel potential biomarker for cancer. Mol. Cancer 2017, 16, 94. [Google Scholar] [CrossRef]
- Hansen, T.; Jensen, T.; Clausen, B.; Bramsen, J.; Finsen, B.; Damgaard, C.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Jiang, X.; Tang, X. Comprehensive review for non-coding RNAs: From mechanisms to therapeutic applications. Biochem. Pharmacol. 2024, 224, 116218. [Google Scholar]
- Sun, J.; Wu, H.; Luo, J.; Qiu, Y.; Li, Y.; Xu, Y.; Liu, L.; Liu, X.; Zhang, Q. CircTBC1D22A inhibits the progression of colorectal cancer through autophagy regulated via miR-1825/ATG14 axis. iScience 2024, 27, 109168. [Google Scholar]
- Sun, J.; Zhang, Z.; Yang, S. Circ_RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem. Cell Biol. 2019, 97, 709–714. [Google Scholar]
- Farazi, T.; Hoell, J.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar]
- Liu, F.; Cai, Y.; Rong, X.; Chen, J.; Zheng, D.; Chen, L.; Zhang, J.; Luo, R.; Zhao, P.; Ruan, J. MiR-661 promotes tumor invasion and metastasis by directly inhibiting RB1 in non-small cell lung cancer. Mol. Cancer 2017, 16, 122. [Google Scholar] [PubMed]
- Zhou, G.; Yang, W.; Sun, B. Clinical impact of serum miR-661 in diagnosis and prognosis of non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5696–5701. [Google Scholar] [PubMed]
- Wang, Y.; Li, Y.; Wu, B.; Shi, C.; Li, C. MicroRNA-661 promotes non-small cell lung cancer progression by directly targeting RUNX3. Mol. Med. Rep. 2017, 16, 2113–2120. [Google Scholar]
- Zhu, T.; Yuan, J.; Wang, Y.; Gong, C.; Xie, Y.; Li, H. MiR-661 contributed to cell proliferation of human ovarian cancer cells by repressing INPP5J expression. Biomed. Pharmacother. 2015, 75, 123–128. [Google Scholar] [PubMed]
- Sui, C.; Qu, W.; Lian, Y.; Feng, C.; Zhan, Y. Hsa_circ_0069094 knockdown inhibits cell proliferation, migration, invasion and glycolysis, while induces cell apoptosis by miR-661/HMGA1 axis in breast cancer. Anticancer Drugs 2021, 32, 829–841. [Google Scholar]
- Yang, Z.; Zhang, Y.; Wang, X.; Huang, J.; Guo, W.; Wei, P.; Li, G.; Wang, Z.; Huang, Z.; Zhang, L. Putative biomarkers of malignant transformation of sinonasal inverted papilloma into squamous cell carcinoma. J. Int. Med. Res. 2019, 47, 2371–2380. [Google Scholar]
- Lv, F.; Zheng, K.; Yu, J.; Huang, Z. MicroRNA-661 expression is upregulated in pancreatic ductal adenocarcinoma and promotes cell proliferation. Oncol. Lett. 2018, 16, 6293–6298. [Google Scholar] [CrossRef]
- Lerman, M.; Minna, J. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: Identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000, 60, 6116–6133. [Google Scholar]
- Tekin, L.; Edgünlü, T.; Genç, D. Immunohistochemical and molecular evaluation of TUSC2 expression in breast cancer. Mol. Biol. Rep. 2024, 51, 394. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Regua, A.; Najjar, M.; Lo, H. Tumor Suppressor Candidate 2 (TUSC2): Discovery, functions, and cancer therapy. Cancers 2023, 15, 2455. [Google Scholar] [CrossRef]
- Shangguan, H.; Feng, H.; Lv, D.; Wang, J.; Tian, T.; Wang, X. Circular RNA circSLC25A16 contributes to the glycolysis of non-small-cell lung cancer through epigenetic modification. Cell Death Dis. 2020, 11, 437. [Google Scholar] [PubMed]
- Liu, F.; Gong, R.; He, B.; Chen, F.; Hu, Z. TUSC2P suppresses the tumor function of esophageal squamous cell carcinoma by regulating TUSC2 expression and correlates with disease prognosis. BMC Cancer 2018, 18, 894. [Google Scholar]
- Zhang, Z.; Wang, Q.; Zhang, M.; Zhang, W.; Zhao, L.; Yang, C.; Wang, B.; Jiang, K.; Ye, Y.; Shen, Z.; et al. Comprehensive analysis of the transcriptome-wide m6A methylome in colorectal cancer by MeRIP sequencing. Epigenetics 2021, 16, 425–435. [Google Scholar]
- Ouyang, P.; Li, K.; Xu, W.; Chen, C.; Shi, Y.; Tian, Y.; Gong, J.; Bao, Z. METTL3 recruiting M2-type immunosuppressed macrophages by targeting m6A-SNAIL-CXCL2 axis to promote colorectal cancer pulmonary metastasis. J. Exp. Clin. Cancer Res. 2024, 43, 111. [Google Scholar]
- Yang, R.; Yang, C.; Su, D.; Song, Y.; Min, J.; Qian, Z.; Shen, X.; Li, J.; Su, H. METTL3-mediated RanGAP1 promotes colorectal cancer progression through the MAPK pathway by recruiting YTHDF1. Cancer Gene Ther. 2024, 31, 562–573. [Google Scholar] [CrossRef]
- Bachmayr-Heyda, A.; Reiner, A.T.; Auer, K.; Sukhbaatar, N.; Aust, S.; Bachleitner-Hofmann, T.; Mesteri, I.; Grunt, T.W.; Zeillinger, R.; Pils, D. Correlation of circular RNA abundance with proliferation—Exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci. Rep. 2015, 5, 8057. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Huang, L.; Zhang, J.; Pan, F.; Li, B.; Yan, Y.; Jia, B.; Liu, H.; Li, S.; et al. Decreased expression of hsa_circ_001988 in colorectal cancer and its clinical significances. Int. J. Clin. Exp. Pathol. 2015, 8, 16020–16025. [Google Scholar]
- Zhuo, F.; Lin, H.; Chen, Z.; Huang, Z.; Hu, J. The expression profile and clinical significance of circRNA0003906 in colorectal cancer. OncoTargets Ther. 2017, 10, 5187–5193. [Google Scholar]
- Wang, J.; Li, X.; Lu, L.; He, L.; Hu, H.; Xu, Z. Circular RNA hsa_circ_0000567 can be used as a promising diagnostic biomarker for human colorectal cancer. J. Clin. Lab. Anal. 2018, 32, e22379. [Google Scholar] [PubMed]
- Song, W.; Fu, J.; Wu, J.; Ren, J.; Xiang, R.; Kong, C.; Fu, T. CircFBXW4 suppresses colorectal cancer progression by regulating the miR-338-5p/SLC5A7 axis. Adv. Sci. 2024, 11, e2300129. [Google Scholar] [CrossRef]
- Huang, M.; Gao, T.; Chen, X.; Yi, J.; Zhou, X. Circ_0087851 suppresses colorectal cancer malignant progression through triggering miR-593-3p/BAP1-mediated ferroptosis. J. Cancer Res. Clin. Oncol. 2024, 150, 204. [Google Scholar] [PubMed]
- Ali, M.A.; Matboli, M.; El-Khazragy, N.; Saber, O.; El-Nakeep, S.; Abdelzaher, H.M.; Shafei, A.E.; Mostafa, R. Investigating miRNA-661 and ATG4-B mRNA expression as potential biomarkers for hepatocellular carcinoma. Biomark. Med. 2018, 12, 245–256. [Google Scholar]
- Prudkin, L.; Behrens, C.; Liu, D.D.; Zhou, X.; Ozburn, N.C.; Bekele, B.N.; Minna, J.D.; Moran, C.; Roth, J.A.; Ji, L.; et al. Loss and reduction of FUS1 protein expression is a frequent phenomenon in the pathogenesis of lung cancer. Clin. Cancer Res. 2008, 14, 41–47. [Google Scholar] [PubMed]
- Ivanova, A.V.; Ivanov, S.V.; Prudkin, L.; Nonaka, D.; Liu, Z.; Tsao, A.; Wistuba, I.; Roth, J.; Pass, H.I. Mechanisms of FUS1/TUSC2 deficiency in mesothelioma and its tumorigenic transcriptional effects. Mol. Cancer 2009, 8, 91. [Google Scholar]
- Li, G.; Kawashima, H.; Ji, L.; Ogose, A.; Ariizumi, T.; Umezu, H.; Xu, Y.; Hotta, T.; Endo, N. Frequent absence of tumor suppressor FUS1 protein expression in human bone and soft tissue sarcomas. Anticancer Res. 2011, 31, 11–21. [Google Scholar]
- Xin, J.; Zhang, X.K.; Xin, D.Y.; Li, X.F.; Sun, D.K.; Ma, Y.Y.; Tian, L.Q. FUS1 acts as a tumor-suppressor gene by upregulating miR-197 in human glioblastoma. Oncol. Rep. 2015, 34, 868–876. [Google Scholar]
- Mariniello, R.M.; Maria Orlandella, F.; De Stefano, A.E.; Iervolino, P.L.; Smaldone, G.; Luciano, N.; Cervone, N.; Munciguerra, F.; Esposito, S.; Mirabelli, P.; et al. The TUSC2 tumour suppressor inhibits the malignant phenotype of human thyroid cancer cells via SMAC/DIABLO protein. Int. J. Mol. Sci. 2020, 21, 702. [Google Scholar] [CrossRef]
- Kondo, M.; Ji, L.; Kamibayashi, C.; Tomizawa, Y.; Randle, D.; Sekido, Y.; Yokota, J.; Kashuba, V.; Zabarovsky, E.; Kuzmin, I.; et al. Overexpression of candidate tumor suppressor gene FUS1 isolated from the 3p21.3 homozygous deletion region leads to G1 arrest and growth inhibition of lung cancer cells. Oncogene 2001, 20, 6258–6262. [Google Scholar]
- Ivanova, A.V.; Ivanov, S.V.; Pascal, V.; Lumsden, J.M.; Ward, J.M.; Morris, N.; Tessarolo, L.; Anderson, S.K.; Lerman, M.I. Autoimmunity, spontaneous tumourigenesis, and IL-15 insufficiency in mice with a targeted disruption of the tumour suppressor gene Fus1. J. Pathol. 2007, 211, 591–601. [Google Scholar] [PubMed]
- Guo, Y.; Guo, Y.; Chen, C.; Fan, D.; Wu, X.; Zhao, L.; Shao, B.; Sun, Z.; Ji, Z. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: Involvement of miR-30c-5p/TCF7 axis. Mol. Cancer 2021, 20, 93. [Google Scholar]
- Lai, J.; Zhou, Z.; Hu, K.; Yu, H.; Su, X.; Niu, X.; Li, H.; Mao, S. N6-methyladenosine methylation analysis of long noncoding RNAs and mRNAs in 5-FU-resistant colon cancer cells. Epigenetics 2024, 19, 2298058. [Google Scholar]
- Chen, X.Y.; Yang, Y.L.; Yu, Y.; Chen, Z.Y.; Fan, H.N.; Zhang, J.; Zhu, J.S. CircUGGT2 downregulation by METTL14-dependent m6A modification suppresses gastric cancer progression and cisplatin resistance through interaction with miR-186-3p/MAP3K9 axis. Pharmacol. Res. 2024, 204, 107206. [Google Scholar]
- Li, J.; Xu, X.; Xu, K.; Zhou, X.; Wu, K.; Yao, Y.; Liu, Z.; Chen, C.; Wang, L.; Sun, Z.; et al. N6-methyladenosine-modified circSLCO1B3 promotes intrahepatic cholangiocarcinoma progression via regulating HOXC8 and PD-L1. J. Exp. Clin. Cancer Res. 2024, 43, 119. [Google Scholar] [PubMed]
- Wu, P.; Fang, X.; Liu, Y.; Tang, Y.; Wang, W.; Li, X.; Fan, Y. N6-methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021, 12, 298. [Google Scholar] [PubMed]
- Chen, D.; Ma, W.; Ke, Z.; Xie, F. CircRNA hsa_circ_100395 regulates miR-1228/TCF21 pathway to inhibit lung cancer progression. Cell Cycle 2018, 17, 2080–2090. [Google Scholar]
- Chen, Q.; Liu, T.; Bao, Y.; Zhao, T.; Wang, J.; Wang, H.; Wang, A.; Gan, X.; Wu, Z.; Wang, L. CircRNA cRAPGEF5 inhibits the growth and metastasis of renal cell carcinoma via the miR-27a-3p/TXNIP pathway. Cancer Lett. 2020, 469, 68–77. [Google Scholar]
- Wang, K.; Long, B.O.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Li, D.; Xu, Y.; Guo, Y.; Mao, J.; Lu, Y. Circ_RUSC2 Sequesters miR-661 and Elevates TUSC2 Expression to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2025, 26, 2937. https://doi.org/10.3390/ijms26072937
Shi Y, Li D, Xu Y, Guo Y, Mao J, Lu Y. Circ_RUSC2 Sequesters miR-661 and Elevates TUSC2 Expression to Suppress Colorectal Cancer Progression. International Journal of Molecular Sciences. 2025; 26(7):2937. https://doi.org/10.3390/ijms26072937
Chicago/Turabian StyleShi, Yixin, Dingru Li, Yunchao Xu, Yijun Guo, Jun Mao, and Ying Lu. 2025. "Circ_RUSC2 Sequesters miR-661 and Elevates TUSC2 Expression to Suppress Colorectal Cancer Progression" International Journal of Molecular Sciences 26, no. 7: 2937. https://doi.org/10.3390/ijms26072937
APA StyleShi, Y., Li, D., Xu, Y., Guo, Y., Mao, J., & Lu, Y. (2025). Circ_RUSC2 Sequesters miR-661 and Elevates TUSC2 Expression to Suppress Colorectal Cancer Progression. International Journal of Molecular Sciences, 26(7), 2937. https://doi.org/10.3390/ijms26072937





