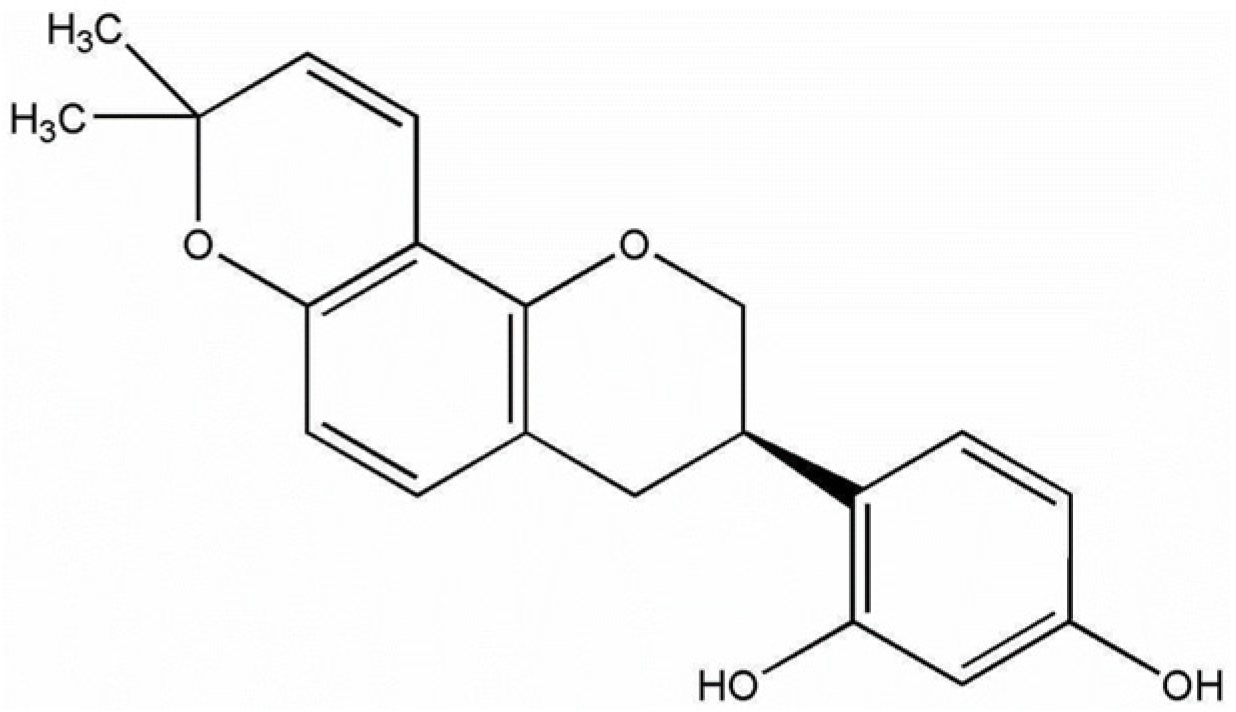
Glabridin Alleviates Oxidative Stress-Induced Osteoporosis by Targeting the Akt/NF-ĸB and Akt/GSK-3β Pathways
Abstract
1. Introduction
2. Results
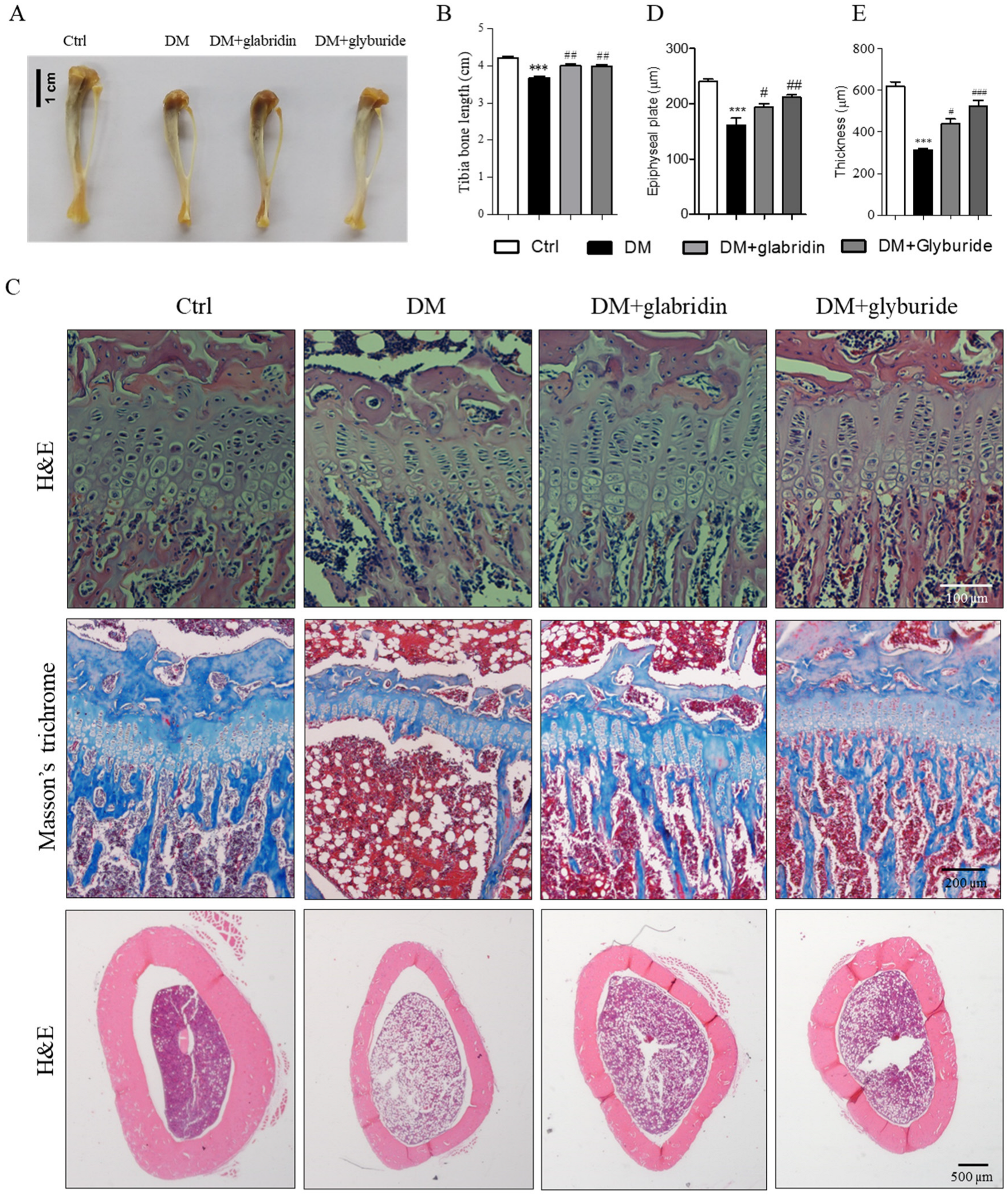
2.1. Glabridin Protected Against High Glucose-Induced Bone Loss in the Tibia of Rats
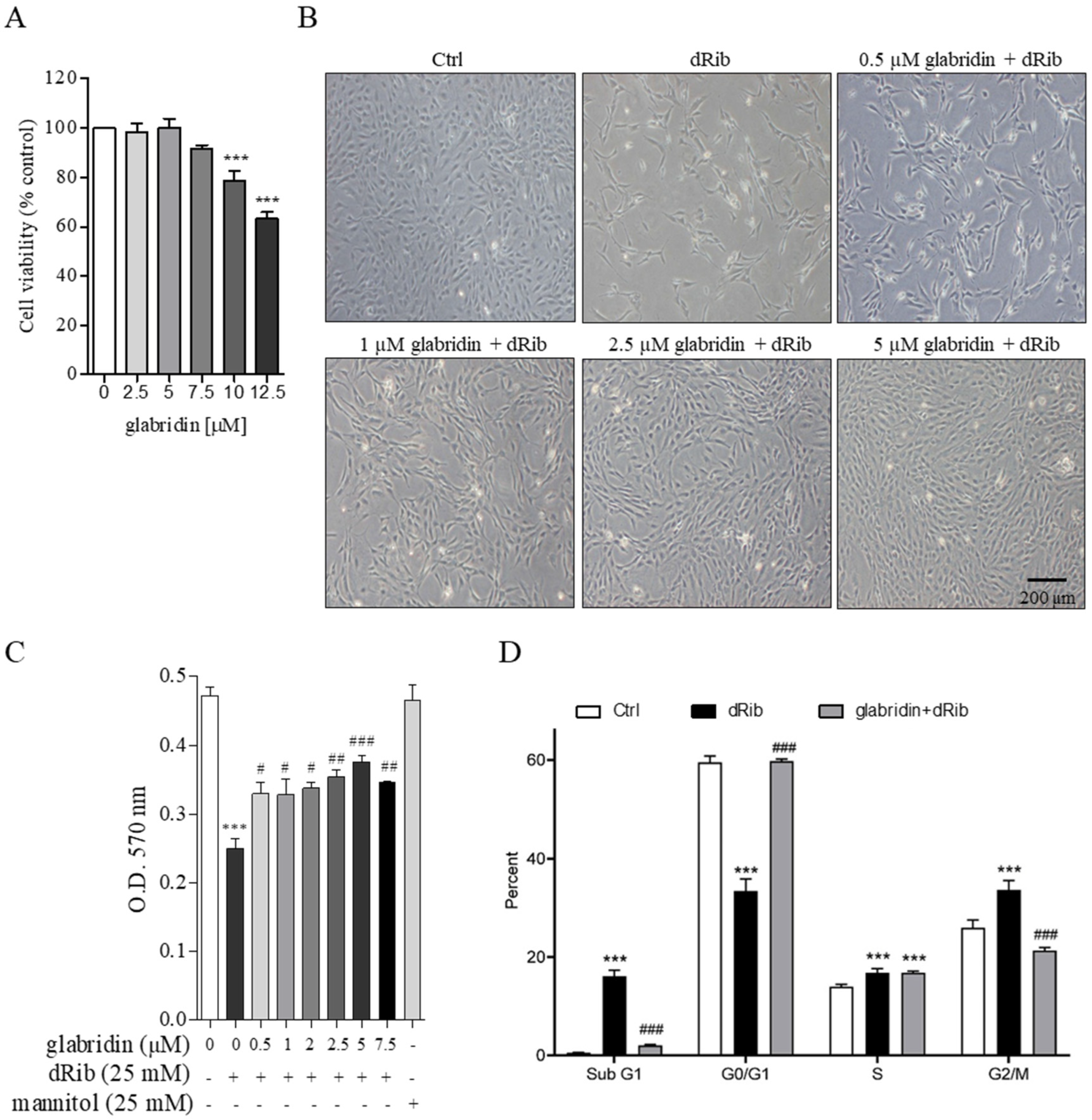
2.2. Glabridin Attenuated Oxidative Stress-Induced Cell Cycle Arrest in MC3T3-E1 Preosteoblasts
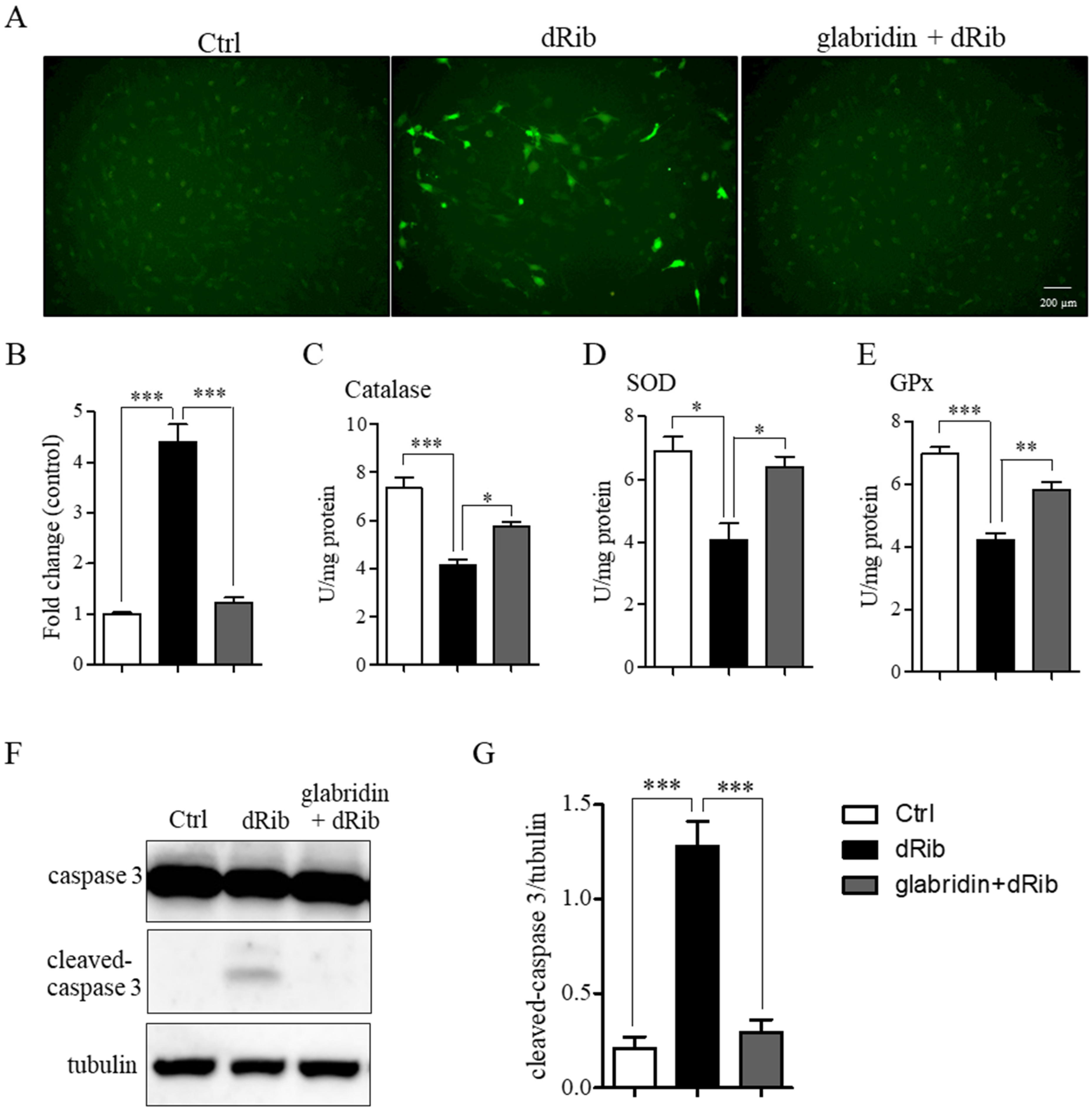
2.3. Glabridin Prevented 2-Deoxy-D-ribose-Induced ROS Production and Apoptosis
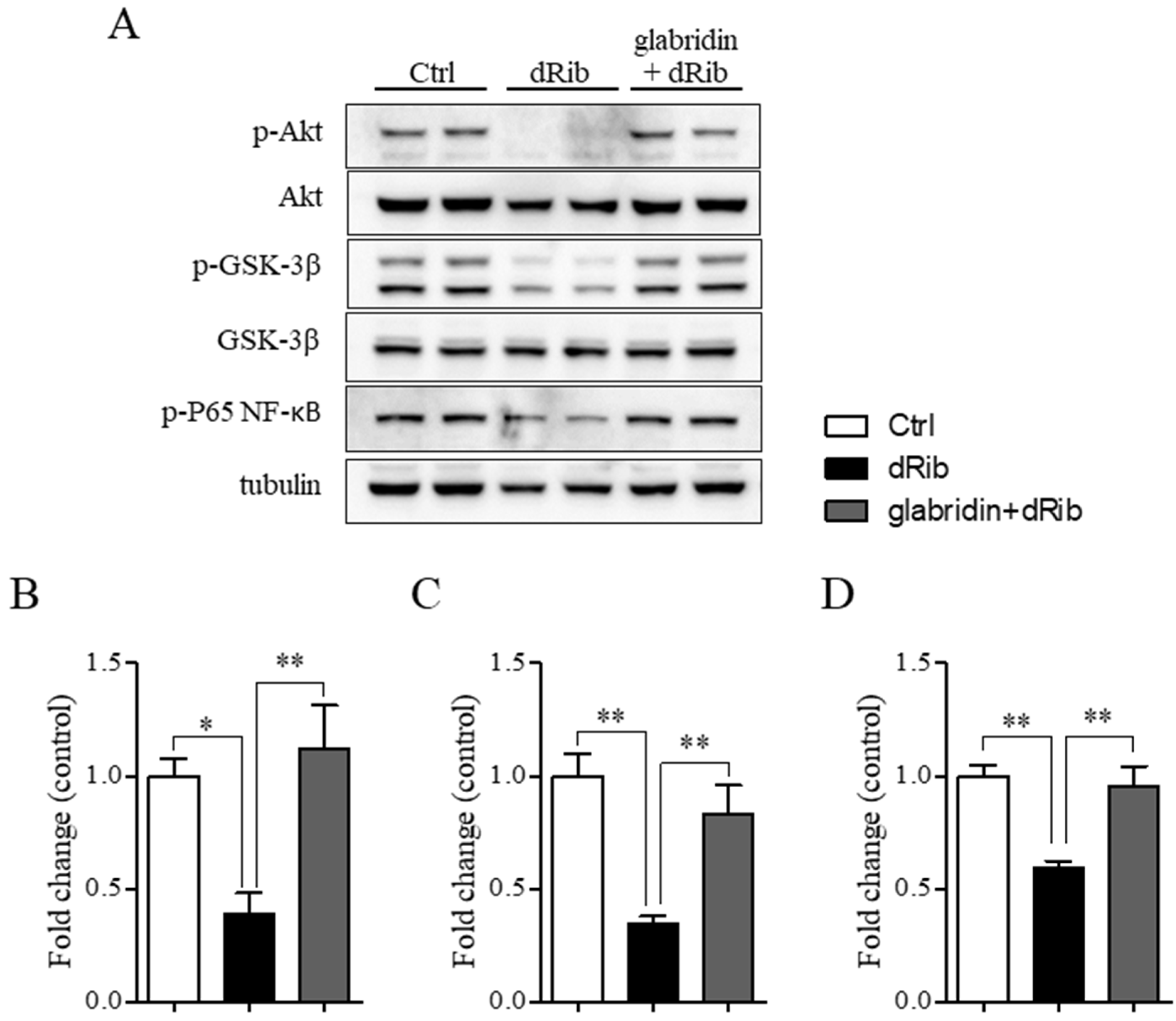
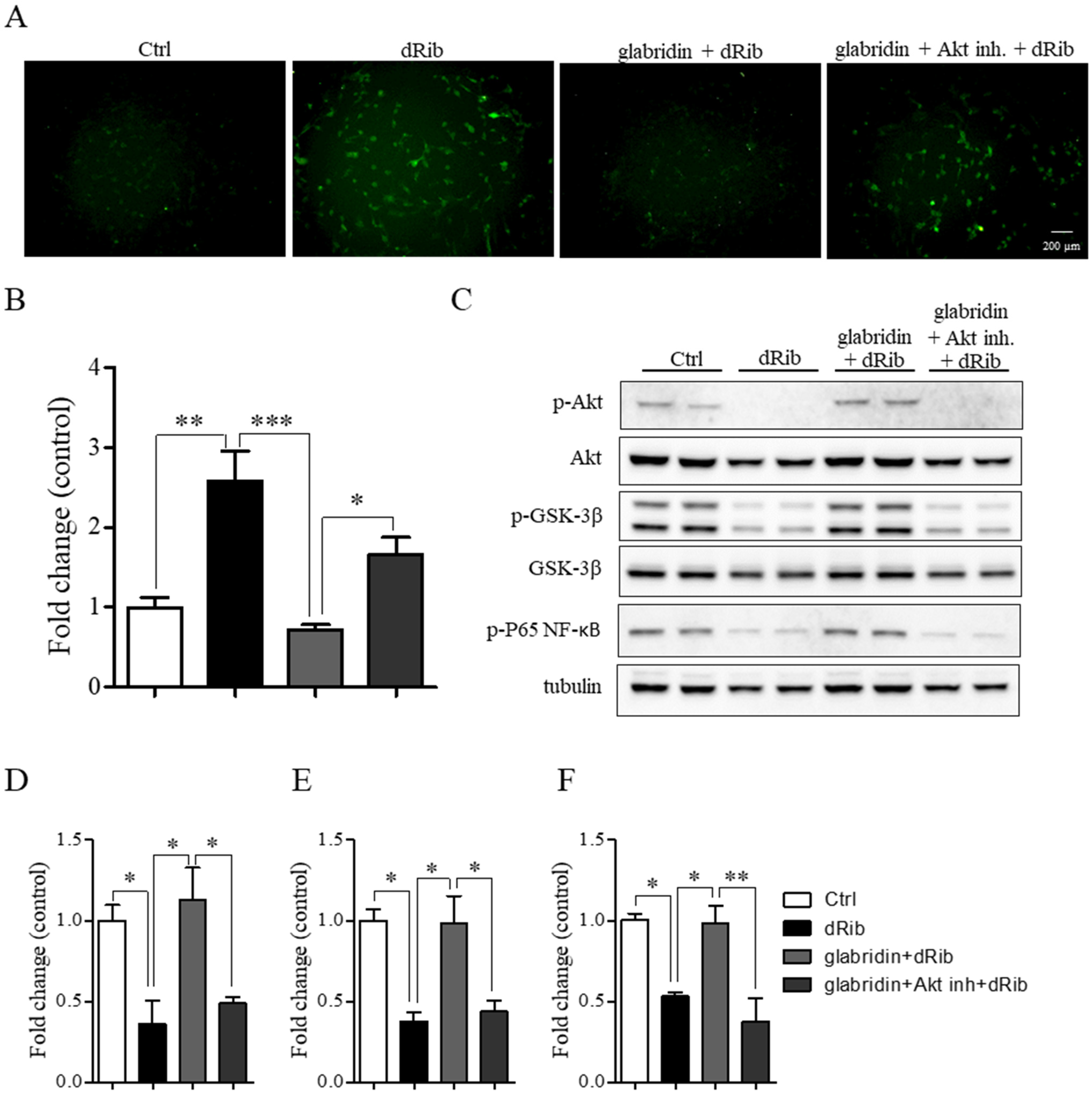
2.4. Glabridin Targets the Akt/NF-ĸB and Akt/GSK-3β Pathways in MC3T3-E1 Preosteoblasts in Oxidative Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Approval
4.2. Animals and Experimental Procedures
4.3. Histological Study
4.4. Cell Culture and Treatment
4.5. Cell Viability Assay
4.6. Flow Cytometry
4.7. H2DCFDA Assay
4.8. Antioxidant Enzyme Activity Assay
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NF-κB | nuclear factor kappa B |
| GSK-3β | Glycogen synthase kinase-3 beta |
| Akt | protein kinase B |
| SOD | superoxide dismutase |
| GPx | glutathione peroxidase |
| CAT | catalase |
| ROS | reactive oxygen species |
| DM | diabetes mellitus |
| RANKL | receptor activator of nuclear factor kappa beta ligand |
| ALP | Alkaline phosphatase |
| BMPs | bone morphogenetic proteins |
| OPN | Osteopontin |
| OPG | osteoprotegerin |
| OC | osteocalcin |
References
- Ishtaya, G.A.; Anabtawi, Y.M.; Zyoud, S.E.H.; Sweileh, W.M. Osteoporosis knowledge and beliefs in diabetic patients: A cross sectional study from Palestine. BMC Musculoskelet. Disord. 2018, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Tay, D.; Huang, X.; Hao, Y. The burden of inpatient care for diabetic and non-diabetic patients with osteoporotic hip fractures—Does it differ? An analysis of patients recruited into a fracture liaison service in Southeast Asia. Arch. Osteoporos. 2018, 13, 27. [Google Scholar] [PubMed]
- Yang, L.; Liu, J.; Shan, Q.; Geng, G.; Shao, P. High glucose inhibits proliferation and differentiation of osteoblast in alveolar bone by inducing pyroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liao, J.; Wang, X.; Feng, Z. High glucose promotes apoptosis and autophagy of MC3T3-E1 osteoblasts. Arch. Med. Sci. 2023, 19, 138–150. [Google Scholar] [CrossRef]
- Sahasrabudhe, R.A.; Limaye, T.Y.; Gokhale, V.S. Insulin injection site adverse effect in a type 1 diabetes patient: An unusual presentation. J. Clin. Diagn. Res. 2017, 11, OD10. [Google Scholar]
- Both, T.; Zillikens, M.C.; Schreuders-Koedam, M.; Vis, M.; Lam, W.K.; Weel, A.E.; van Leeuwen, J.P.; van Hagen, P.M.; van der Eerden, B.C.; van Daele, P.L. Hydroxychloroquine affects bone resorption both in vitro and in vivo. Cell Physiol. Biochem. 2018, 233, 1424–1433. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Anti-oxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar]
- Bhuyan, U.; Handique, J.G. Plant polyphenols as potent antioxidants: Highlighting the mechanism of antioxidant activity and synthesis/development of some polyphenol conjugates. Stud. Nat. Prod. Chem. 2022, 75, 243–266. [Google Scholar]
- Lee, S.E.; Park, Y.S. The emerging roles of antioxidant enzymes by dietary phytochemicals in vascular diseases. Life 2021, 11, 199. [Google Scholar] [CrossRef]
- Singh, V.; Pal, A.; Darokar, M.P. A polyphenolic flavonoid glabridin: Oxidative stress response in multidrug-resistant Staphylococcus aureus. Free Radic. Biol. Med. 2015, 87, 48–57. [Google Scholar] [CrossRef]
- Weng, J.; Wang, Y.; Tan, Z.; Yuan, Y.; Huang, S.; Li, Z.; Li, Y.; Zhang, L.; Du, Z. Glabridin reduces neuroinflammation by modulating inflammatory signals in LPS-induced in vitro and in vivo models. Inflammopharmacology 2024, 32, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, I.; Madar, Z.; Szuchman-Sapir, A.; Tamir, S. Glabridin, a phytoestrogen from licorice root, up-regulates manganese superoxide dismutase, catalase and paraoxonase 2 under glucose stress. Phytother. Res. 2011, 25, 659–667. [Google Scholar] [CrossRef]
- Kim, H.S.; Suh, K.S.; Sul, D.; Kim, B.J.; Lee, S.K.; Jung, W.W. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int. J. Mol. Med. 2012, 29, 169–177. [Google Scholar] [PubMed]
- Kim, H.S.; Suh, K.S.; Ko, A.; Sul, D.; Choi, D.; Lee, S.K.; Jung, W.W. The flavonoid glabridin attenuates 2-deoxy-D-ribose-induced oxidative damage and cellular dysfunction in MC3T3-E1 osteoblastic cells. Int. J. Mol. Med. 2013, 31, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the diverse biological effects of glabridin. Drug Des. Devel. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Komolkriengkrai, M.; Nopparat, J.; Vongvatcharanon, U.; Anupunpisit, V.; Khimmaktong, W. Effect of glabridin on collagen deposition in liver and amelioration of hepatocyte destruction in diabetes rats. Exp. Ther. Med. 2019, 18, 1164–1174. [Google Scholar] [CrossRef]
- Nopparat, J.; Khimmaktong, W.; Pholpramool, C.; Tipbunjong, C. Hypoglycemic effect of flavonoid glabridin prevents homeostatic disruption of native achilles tendon in streptozotocin-induced type 1 diabetic rats. Sains. Malays. 2023, 52, 579–588. [Google Scholar] [CrossRef]
- Tan, H.; Chen, J.; Li, Y.; Li, Y.; Zhong, Y.; Li, G.; Liu, L.; Li, Y. Glabridin, a bioactive component of licorice, ameliorates diabetic nephropathy by regulating ferroptosis and the VEGF/Akt/ERK pathways. Mol. Med. 2022, 28, 58. [Google Scholar] [CrossRef]
- Hua, Y.; Bi, R.; Zhang, Y.; Xu, L.; Guo, J.; Li, Y. Different bone sites-specific response to diabetes rat models: Bone density, histology and microarchitecture. PLoS ONE 2018, 13, e0205503. [Google Scholar] [CrossRef]
- Minami, M.; Ikoma, K.; Onishi, O.; Horii, M.; Itoh, K.; Takahashi, K. Histological assessment of cortical bone changes in diabetic rats. J. Orthop. Res. 2022, 17, 568. [Google Scholar] [CrossRef]
- Wu, F.; Jin, Z.; Jin, J. Hypoglycemic effects of glabridin, a polyphenolic flavonoid from licorice, in an animal model of diabetes mellitus. Mol. Med. Rep. 2013, 7, 1278–1282. [Google Scholar] [PubMed]
- Rivoira, M.; Rodríguez, V.; Picotto, G.; Battaglino, R.; de Talamoni, N.T. Naringin prevents bone loss in a rat model of type 1 Diabetes mellitus. Arch. Biochem. Biophys. 2018, 637, 56–63. [Google Scholar]
- Zhou, X.; Zhang, Z.; Jiang, W.; Hu, M.; Meng, Y.; Li, W.; Zhou, X.; Wang, C. Naringenin is a potential anabolic treatment for bone loss by modulating osteogenesis, osteoclastogenesis, and macrophage polarization. Front. Pharmacol. 2022, 13, 872188. [Google Scholar]
- Lu, R.; Zheng, Z.; Yin, Y.; Jiang, Z. Genistein prevents bone loss in type 2 diabetic rats induced by streptozotocin. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef]
- Su Wei Poh, M.; Voon Chen Yong, P.; Viseswaran, N.; Chia, Y.Y. Estrogenicity of glabridin in Ishikawa cells. PLoS ONE 2015, 10, e0121382. [Google Scholar]
- Heo, J.S.; Lee, S.G.; Kim, H.O. The flavonoid glabridin induces OCT4 to enhance osteogenetic potential in mesenchymal stem cells. Stem. Cells Int. 2017, 2017, 6921703. [Google Scholar]
- Wongdee, K.; Charoenphandhu, N. Osteoporosis in diabetes mellitus: Possible cellular and molecular mechanisms. World J. Diabetes 2011, 2, 41. [Google Scholar]
- Ham, J.R.; Lee, H.I.; Choi, R.Y.; Ryu, H.S.; Yee, S.T.; Kang, K.Y.; Lee, M.K. Heshouwu (Polygonum multiflorum Thunb.) extract attenuates bone loss in diabetic mice. Prev. Nutr. Food. Sci. 2019, 24, 121–127. [Google Scholar]
- Huang, P.H.; Cheng, Y.T.; Chan, Y.J.; Chen, S.J.; Ciou, J.Y.; Lu, W.C.; Hsu, W.J.; Wang, C.C.R.; Li, P.H. Anti-hyperlipidemic and antioxidant ability of HeShouWu (roots of Polygonum multiflorum Thunb.) and its complex formula. Arab. J. Chem. 2023, 16, 105280. [Google Scholar]
- Surinlert, P.; Thitiphatphuvanon, T.; Khimmaktong, W.; Pholpramoo, C.; Tipbunjong, C. Hyperglycemia induced C2C12 myoblast cell cycle arrest and skeletal muscle atrophy by modulating sirtuins gene expression in rats. Pol. J. Vet. Sci. 2021, 24, 563–572. [Google Scholar]
- Nagy, T.; Fisi, V.; Frank, D.; Kátai, E.; Nagy, Z.; Miseta, A. Hyperglycemia-induced aberrant cell proliferation; a metabolic challenge mediated by protein O-GlcNAc modification. Cells 2019, 8, 999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, S.; Wan, Z.; Chen, C.; Zhang, X.; Li, B.; Huang, G.; Chen, L.; He, Z.; Huang, Z. Glabridin arrests cell cycle and inhibits proliferation of hepatocellular carcinoma by suppressing braf/MEK signaling pathway. Tumour Biol. 2016, 37, 5837–5846. [Google Scholar]
- Chen, C.T.; Chen, Y.T.; Hsieh, Y.H.; Weng, C.J.; Yeh, J.C.; Yang, S.F.; Lin, C.W.; Yang, J.S. Glabridin induces apoptosis and cell cycle arrest in oral cancer cells through the JNK1/2 signaling pathway. Environ. Toxicol. 2018, 33, 679–685. [Google Scholar]
- Lin, X.; Li, L.; Wu, S.; Tian, J.; Zheng, W. Activation of GPR30 promotes osteogenic differentiation of MC3T3-E1 cells: An implication in osteoporosis. IUBMB Life 2019, 71, 1751–1759. [Google Scholar] [PubMed]
- Prall, O.J.; Sarcevic, B.; Musgrove, E.A.; Watts, C.W.; Sutherland, R.L. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J. Biol. Chem. 1997, 272, 10882–10894. [Google Scholar]
- Doisneau-Sixou, S.; Sergio, C.; Carroll, J.; Hui, R.; Musgrove, E.; Sutherland, R. Estrogen and antiestrogen regulation of cell cycle progression in breast cancer cells. Endocr. Relat. Cancer. 2003, 10, 179–186. [Google Scholar] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar]
- Medeiros, C.; Wallace, J.M. High glucose-induced inhibition of osteoblast like MC3T3-E1 differentiation promotes mitochondrial perturbations. PLoS ONE 2022, 17, e0270001. [Google Scholar]
- Botolin, S.; McCabe, L.R. Chronic hyperglycemia modulates osteoblast gene expression through osmotic and non-osmotic pathways. J. Cell. Biochem. 2006, 99, 411–424. [Google Scholar]
- Liu, Q.; Yang, Z.; Xie, C.; Ling, L.; Hu, H.; Cao, Y.; Huang, Y.; Zhu, Q.; Hua, Y. The hyperglycemia and hyperketonemia impaired bone microstructures: A pilot study in rats. Front. Endocrinol. 2020, 11, 590575. [Google Scholar]
- Lu, H.; Hu, H.; Yang, Y.; Li, S. The inhibition of reactive oxygen species (ROS) by antioxidants inhibits the release of an autophagy marker in ectopic endometrial cells. Taiwan. J. Obstet. Gynecol. 2020, 59, 256–261. [Google Scholar]
- Reis, J.; Gorgulla, C.; Massari, M.; Marchese, S.; Valente, S.; Noce, B.; Basile, L.; Törner, R.; Cox III, H.; Viennet, T. Targeting ROS production through inhibition of NADPH oxidases. Nat. Chem. Biol. 2023, 19, 1540–1550. [Google Scholar]
- Awang Daud, D.M.; Ahmedy, F.; Baharuddin, D.M.P.; Zakaria, Z.A. Oxidative stress and antioxidant enzymes activity after cycling at different intensity and duration. Appl. Sci. 2022, 12, 9161. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [PubMed]
- Allen, D.A.; Yaqoob, M.M.; Harwood, S.M. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J. Nutr. Biochem. 2005, 16, 705–713. [Google Scholar] [PubMed]
- Tipbunjong, C.; Kitiyanant, Y.; Chaturapanich, G.; Sornkaew, N.; Suksamrarn, A.; Kitiyanant, N.; Esser, K.A.; Pholpramool, C. Natural diarylheptanoid compounds from Curcuma comosa Roxb. promote differentiation of mouse myoblasts C2C12 cells selectively via ER alpha receptors. Med. Chem. Res. 2017, 26, 274–286. [Google Scholar]
- Veratti, E.; Rossi, T.; Giudice, S.; Benassi, L.; Bertazzoni, G.; Morini, D.; Azzoni, P.; Bruni, E.; Giannetti, A.; Magnoni, C. 18β-glycyrrhetinic acid and glabridin prevent oxidative DNA fragmentation in UVB-irradiated human keratinocyte cultures. Anticancer. Res. 2011, 31, 2209–2215. [Google Scholar]
- Tamir, S.; Eizenberg, M.; Somjen, D.; Stern, N.; Shelach, R.; Kaye, A.; Vaya, J. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000, 60, 5704–5709. [Google Scholar]
- Xi, J.C.; Zang, H.Y.; Guo, L.X.; Xue, H.B.; Liu, X.D.; Bai, Y.B.; Ma, Y.Z. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J. Recept. Signal. Transduct. 2015, 35, 640–645. [Google Scholar]
- Ko, K.I.; Syverson, A.L.; Kralik, R.M.; Choi, J.; DerGarabedian, B.P.; Chen, C.; Graves, D.T. Diabetes-induced NF-κB dysregulation in skeletal stem cells prevents resolution of inflammation. Diabetes 2019, 68, 2095–2106. [Google Scholar]
- Dedert, C.; Salih, L.; Xu, F. Progranulin protects against hyperglycemia-induced neuronal dysfunction through GSK3β signaling. Cells 2023, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.P.; Carmody, R.J. NF-κB and the transcriptional control of inflammation. Int. J. Biochem. Cell Biol. 2018, 335, 41–84. [Google Scholar]
- Zuo, Y.; Xiao, T.; Qiu, X.; Liu, Z.; Zhang, S.; Zhou, N. Adiponectin reduces apoptosis of diabetic cardiomyocytes by regulating miR-711/TLR4 axis. Diabetol. Metab. Syndr. 2022, 14, 131. [Google Scholar]
- Lee, K.H.; Yoo, C.G. Simultaneous inactivation of GSK-3β suppresses quercetin-induced apoptosis by inhibiting the JNK pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L782–L789. [Google Scholar] [PubMed]
- Shen, W.; Taylor, B.; Jin, Q.; Nguyen-Tran, V.; Meeusen, S.; Zhang, Y.Q.; Kamireddy, A.; Swafford, A.; Powers, A.F.; Walker, J. Inhibition of DYRK1A and GSK3B induces human β-cell proliferation. Nat. Commun. 2015, 6, 8372. [Google Scholar] [PubMed]
- Eiraku, N.; Chiba, N.; Nakamura, T.; Amir, M.S.; Seong, C.H.; Ohnishi, T.; Kusuyama, J.; Noguchi, K.; Matsuguchi, T. BMP9 directly induces rapid GSK3-β phosphorylation in a Wnt-independent manner through class I PI3K-Akt axis in osteoblasts. Faseb J. 2019, 33, 12124–12134. [Google Scholar]
- Li, C.X.; Li, T.H.; Zhu, M.; Lai, J.; Wu, Z.P. Pharmacological properties of glabridin (a flavonoid extracted from licorice): A comprehensive review. J. Funct. Foods. 2021, 85, 104638. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tipbunjong, C.; Khimmaktong, W.; Hengpratom, T.; Thitiphatphuvanon, T.; Pholpramool, C.; Surinlert, P. Glabridin Alleviates Oxidative Stress-Induced Osteoporosis by Targeting the Akt/NF-ĸB and Akt/GSK-3β Pathways. Int. J. Mol. Sci. 2025, 26, 2949. https://doi.org/10.3390/ijms26072949
Tipbunjong C, Khimmaktong W, Hengpratom T, Thitiphatphuvanon T, Pholpramool C, Surinlert P. Glabridin Alleviates Oxidative Stress-Induced Osteoporosis by Targeting the Akt/NF-ĸB and Akt/GSK-3β Pathways. International Journal of Molecular Sciences. 2025; 26(7):2949. https://doi.org/10.3390/ijms26072949
Chicago/Turabian StyleTipbunjong, Chittipong, Wipapan Khimmaktong, Tanaporn Hengpratom, Thanvarin Thitiphatphuvanon, Chumpol Pholpramool, and Piyaporn Surinlert. 2025. "Glabridin Alleviates Oxidative Stress-Induced Osteoporosis by Targeting the Akt/NF-ĸB and Akt/GSK-3β Pathways" International Journal of Molecular Sciences 26, no. 7: 2949. https://doi.org/10.3390/ijms26072949
APA StyleTipbunjong, C., Khimmaktong, W., Hengpratom, T., Thitiphatphuvanon, T., Pholpramool, C., & Surinlert, P. (2025). Glabridin Alleviates Oxidative Stress-Induced Osteoporosis by Targeting the Akt/NF-ĸB and Akt/GSK-3β Pathways. International Journal of Molecular Sciences, 26(7), 2949. https://doi.org/10.3390/ijms26072949




