The Impact of the Exposome on Alzheimer’s Disease: The Influence of Nutrition
Abstract
:1. Alzheimer’s Disease and the Exposome
1.1. Introduction to AD
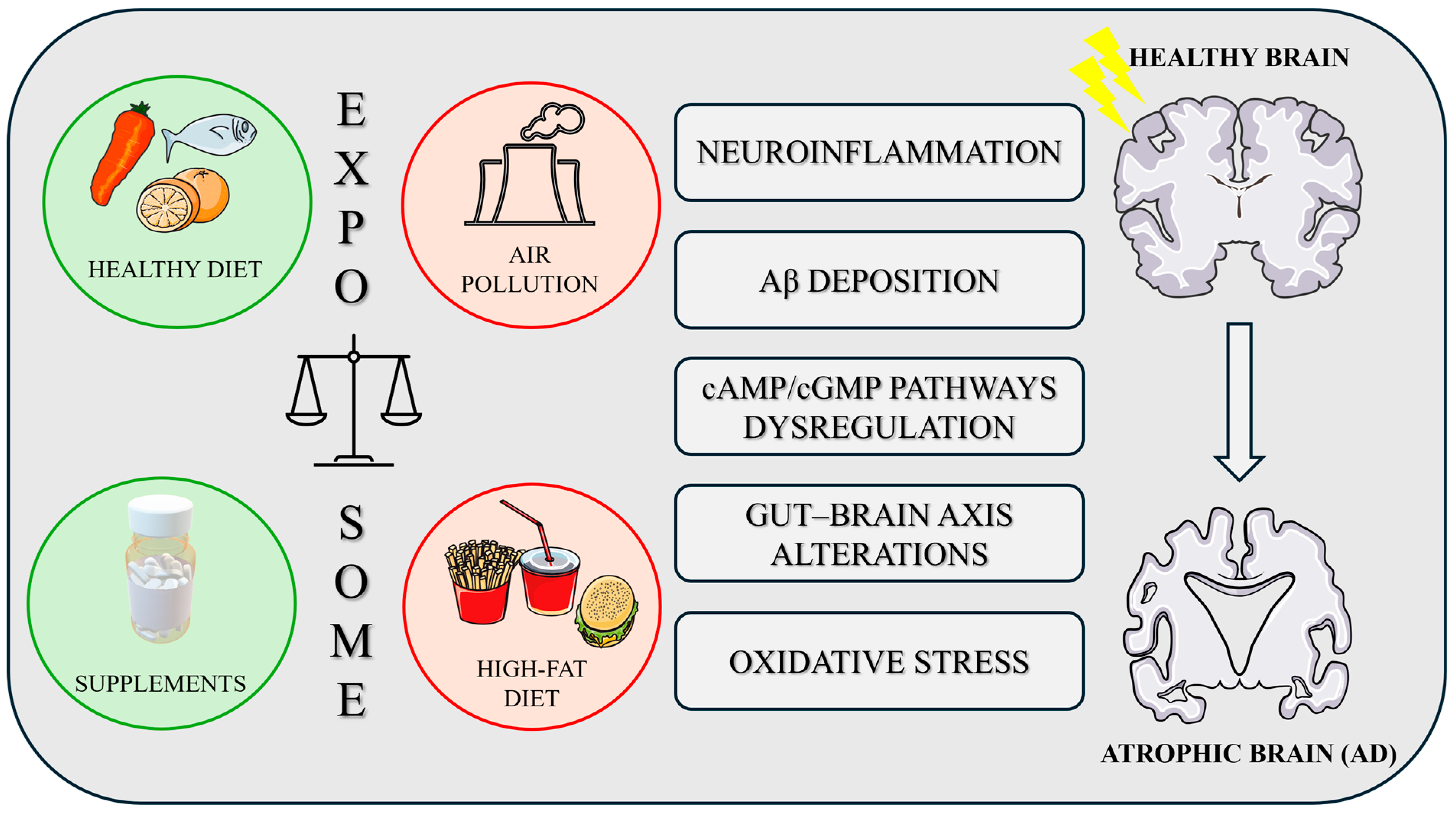
1.2. Exposome and AD
1.3. Exposome and Nutrition
1.4. Nutrition and AD
1.5. cAMP/cGMP Pathways and AD
1.6. Phosphodiesterase Enzymes (PDEs)
1.7. PDEs and AD
1.8. PDEs and Nutrition
2. Transgenic AD Mouse Models and Nutrition
2.1. Tg2576 Mouse Model
2.2. APP/PS1 Mouse Model
2.3. 5xFAD Mouse Model
2.4. 3xTg-AD Mouse Model
| Transgenic AD Mouse Model | Mechanisms | References |
|---|---|---|
| Tg2576 |
| [183,186,191,192] |
| [184,185] | |
| [187,188] | |
| [186,187,188] | |
| APP/PS1 |
| [194,199] |
| [196] | |
| [195] | |
| [196,201] | |
| 5xFAD |
| [203] |
| [205] | |
| [204] | |
| 3xTg-AD |
| [212,215,216] |
| [209,210] | |
| [215] | |
| [211] |
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Aguzzoli, C.S.; Anstey, K.J.; Atri, A.; Barbarino, P.; Benoist, C.; Brijnath, B.; Bruno, M.A.; Cose, L.; Darge, D.; Dean, W.; et al. World Alzheimer Report 2024 Global Changes in Attitudes to Dementia Contributors: Survey Translators. Alzheimer’s Disease International: London, UK, 2024. [Google Scholar]
- Zhong, S.; Lou, J.; Ma, K.; Shu, Z.; Chen, L.; Li, C.; Ye, Q.; Zhou, L.; Shen, Y.; Ye, X.; et al. Disentangling In-Vivo Microstructural Changes of White and Gray Matter in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Brain Imaging Behav. 2023, 17, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Żukowska, J.; Moss, S.J.; Subramanian, V.; Acharya, K.R. Molecular Basis of Selective Amyloid-β Degrading Enzymes in Alzheimer’s Disease. FEBS J. 2024, 291, 2999–3029. [Google Scholar] [PubMed]
- Zott, B.; Nästle, L.; Grienberger, C.; Unger, F.; Knauer, M.M.; Wolf, C.; Keskin-Dargin, A.; Feuerbach, A.; Busche, M.A.; Skerra, A.; et al. β-Amyloid Monomer Scavenging by an Anticalin Protein Prevents Neuronal Hyperactivity in Mouse Models of Alzheimer’s Disease. Nat. Commun. 2024, 15, 5819. [Google Scholar] [CrossRef]
- Hong, S.; Beja-Glasser, V.F.; Nfonoyim, B.M.; Frouin, A.; Li, S.; Ramakrishnan, S.; Merry, K.M.; Shi, Q.; Rosenthal, A.; Barres, B.A.; et al. Complement and Microglia Mediate Early Synapse Loss in Alzheimer Mouse Models. Science 2016, 352, 712–716. [Google Scholar] [CrossRef]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble Amyloid β-Protein Dimers Isolated from Alzheimer Cortex Directly Induce Tau Hyperphosphorylation and Neuritic Degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef]
- Kadowaki, H.; Nishitoh, H.; Urano, F.; Sadamitsu, C.; Matsuzawa, A.; Takeda, K.; Masutani, H.; Yodoi, J.; Urano, Y.; Nagano, T.; et al. Amyloid β Induces Neuronal Cell Death through ROS-Mediated ASK1 Activation. Cell Death Differ. 2005, 12, 19–24. [Google Scholar] [CrossRef]
- Zubčić, K.; Franić, D.; Pravica, M.; Hof, P.R.; Šimić, G.; Boban, M. Effects of Heterologous Human Tau Protein Expression in Yeast Models of Proteotoxic Stress Response. CNS Neurosci. Ther. 2024, 30, e14304. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Tau Protein Hyperphosphorylation and Aggregation in Alzheimer’s Disease and Other Tauopathies, and Possible Neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Wegmann, S.; Maury, E.A.; Kirk, M.J.; Saqran, L.; Roe, A.; DeVos, S.L.; Nicholls, S.; Fan, Z.; Takeda, S.; Cagsal-Getkin, O.; et al. Removing Endogenous Tau Does Not Prevent Tau Propagation yet Reduces Its Neurotoxicity. EMBO J. 2015, 34, 3028–3041. [Google Scholar] [CrossRef]
- Congdon, E.E.; Duff, K.E. Is Tau Aggregation Toxic or Protective? J. Alzheimer’s Dis. 2008, 14, 453–457. [Google Scholar] [CrossRef]
- Zuppe, H.; Reed, E. Common Cytokine Receptor Gamma Chain Family Cytokines Activate MAPK, PI3K, and JAK/STAT Pathways in Microglia to Influence Alzheimer’s Disease. Front Mol. Neurosci. 2024, 17, 1441691. [Google Scholar] [CrossRef] [PubMed]
- Swanson, A.; Wolf, T.; Sitzmann, A.; Willette, A.A. Neuroinflammation in Alzheimer’s Disease: Pleiotropic Roles for Cytokines and Neuronal Pentraxins. Behav. Brain Res. 2018, 347, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common Neurodegenerative Pathways in Obesity, Diabetes, and Alzheimer’s Disease. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The Link between Comorbidities, Genetics, and Alzheimer’s Disease. J. Neuroinflammation 2018, 15, 276. [Google Scholar] [CrossRef]
- Terao, I.; Kodama, W. Comparative Efficacy, Tolerability and Acceptability of Donanemab, Lecanemab, Aducanumab and Lithium on Cognitive Function in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and Network Meta-Analysis. Ageing Res. Rev. 2024, 94, 102203. [Google Scholar] [CrossRef]
- Fedele, E. Anti-Amyloid Therapies for Alzheimer’s Disease and the Amyloid Cascade Hypothesis. Int. J. Mol. Sci. 2023, 24, 14499. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Herrera, P.A.; Campos-Romero, S.; Szabo, W.; Martínez, P.; Guajardo, V.; Rojas, G. Understanding the Relationship between Depression and Chronic Diseases Such as Diabetes and Hypertension: A Grounded Theory Study. Int. J. Environ. Res. Public Health 2021, 18, 12130. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, J.; Winkel, I.; Mroczko, B. Metabolic and Immune System Dysregulation: Unraveling the Connections between Alzheimer’s Disease, Diabetes, Inflammatory Bowel Diseases, and Rheumatoid Arthritis. J. Clin. Med. 2024, 13, 5057. [Google Scholar] [CrossRef]
- Casserly, I.P.; Topol, E.J. Convergence of Atherosclerosis and Alzheimer’s Disease: Cholesterol, Inflammation, and Misfolded Proteins. Discov. Med. 2004, 4, 149–156. [Google Scholar] [PubMed]
- Chatterjee, S.; Mudher, A. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef]
- Ownby, R.L.; Crocco, E.; Acevedo, A.; John, V.; Loewenstein, D. Depression and Risk for Alzheimer Disease. Arch. Gen. Psychiatry 2006, 63, 530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Hetrick, S.E.; Cuijpers, P.; Qin, B.; Barth, J.; Whittington, C.J.; Cohen, D.; Del Giovane, C.; Liu, Y.; Michael, K.D.; et al. Comparative Efficacy and Acceptability of Psychotherapies for Depression in Children and Adolescents: A Systematic Review and Network Meta-Analysis. World Psychiatry 2015, 14, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; D’Souza, G.X.; Shi, J.; Ritter, A.; Suazo, J.; Sabbagh, M.N. The Cause of Alzheimer’s Disease: The Theory of Multipathology Convergence to Chronic Neuronal Stress. Aging Dis. 2022, 13, 37. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Lukens, J.R.; Zimring, J.C. The Role of PIMT in Alzheimer’s Disease Pathogenesis: A Novel Hypothesis. Alzheimer’s Dement. 2023, 19, 5296–5302. [Google Scholar] [CrossRef]
- Rudge, J.D. A New Hypothesis for Alzheimer’s Disease: The Lipid Invasion Model. J. Alzheimers Dis. Rep. 2022, 6, 129–161. [Google Scholar] [CrossRef]
- Ganz, T.; Fainstein, N.; Ben-Hur, T. When the Infectious Environment Meets the AD Brain. Mol. Neurodegener. 2022, 17, 53. [Google Scholar] [CrossRef]
- Abraham, M.J.; El Sherbini, A.; El-Diasty, M.; Askari, S.; Szewczuk, M.R. Restoring Epigenetic Reprogramming with Diet and Exercise to Improve Health-Related Metabolic Diseases. Biomolecules 2023, 13, 318. [Google Scholar] [CrossRef]
- Galkin, F.; Kovalchuk, O.; Koldasbayeva, D.; Zhavoronkov, A.; Bischof, E. Stress, Diet, Exercise: Common Environmental Factors and Their Impact on Epigenetic Age. Ageing Res. Rev. 2023, 88, 101956. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Z.; Peng, C. Effects of Aerobic Exercise on Cognitive Function and Quality of Life in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. BMJ Open 2025, 15, e090623. [Google Scholar] [CrossRef] [PubMed]
- de Sá Leitão, C.V.F.; de Faria Moraes, B.; Leite, G.A.P.D.; Duarte, A.G.; da Silva, M.V.G.; de Oliveira, G.M.; Andrade, F.A.B.; da Silva, J.A.B.; dos Santos, R.C.C.; Figueiredo, G.S.; et al. Twelve Weeks of Exercise Training Improves Cognitive Status, Physical Performance and Quality of Life in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2025, 104, 102655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Hao, Y.-N.; Ge, Y.-J.; Zhang, Y.; Huang, L.-Y.; Fu, Y.; Zhang, D.-D.; Ou, Y.-N.; Cao, X.-P.; Feng, J.-F.; et al. Variables Associated with Cognitive Function: An Exposome-Wide and Mendelian Randomization Analysis. Alzheimers Res. Ther. 2025, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P. Complementing the Genome with an “Exposome”: The Outstanding Challenge of Environmental Exposure Measurement in Molecular Epidemiology. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1847–1850. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near Major Roads and the Incidence of Dementia, Parkinson’s Disease, and Multiple Sclerosis: A Population-Based Cohort Study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Hussain, R.; Graham, U.; Elder, A.; Nedergaard, M. Air Pollution, Glymphatic Impairment, and Alzheimer’s Disease. Trends Neurosci. 2023, 46, 901–911. [Google Scholar] [CrossRef]
- Finch, C.E.; Kulminski, A.M. The Alzheimer’s Disease Exposome. Alzheimer’s Dement. 2019, 15, 1123–1132. [Google Scholar] [CrossRef]
- Granov, R.; Vedad, S.; Wang, S.-H.; Durham, A.; Shah, D.; Pasinetti, G.M. The Role of the Neural Exposome as a Novel Strategy to Identify and Mitigate Health Inequities in Alzheimer’s Disease and Related Dementias. Mol. Neurobiol. 2025, 62, 1205–1224. [Google Scholar] [CrossRef]
- Dagnino, S.; Macherone, A. Unraveling The Exposome: A Practical View; Springer International Publishing AG, part of Springer Nature 2019: Cham, Switzerland, 2019; ISBN 9783319893211. [Google Scholar]
- Vermeulen, R.; Schymanski, E.L.; Barabási, A.-L.; Miller, G.W. The Exposome and Health: Where Chemistry Meets Biology. Science 2020, 367, 392–396. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Azzarelli, B.; Acuna, H.; Garcia, R.; Gambling, T.M.; Osnaya, N.; Monroy, S.; Del Rosario Tizapantzi, M.; Carson, J.L.; Villarreal-Calderon, A.; et al. Air Pollution and Brain Damage. Toxicol. Pathol. 2002, 30, 373–389. [Google Scholar] [CrossRef]
- Calderon-Garciduenas, L.; Maronpot, R.R.; Torres-Jardon, R.; Henriquez-Roldan, C.; Schoonhoven, R.; Acuna-Ayala, H.; Villarreal-Calderon, A.; Nakamura, J.; Fernando, R.; Reed, W.; et al. DNA Damage in Nasal and Brain Tissues of Canines Exposed to Air Pollutants Is Associated with Evidence of Chronic Brain Inflammation and Neurodegeneration. Toxicol. Pathol. 2003, 31, 524–538. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. The Olfactory Vector Hypothesis of Neurodegenerative Disease: Is It Viable? Ann. Neurol. 2008, 63, 7–15. [Google Scholar] [CrossRef]
- Ehsanifar, M.; Montazeri, Z.; Taheri, M.A.; Rafati, M.; Behjati, M.; Karimian, M. Hippocampal Inflammation and Oxidative Stress Following Exposure to Diesel Exhaust Nanoparticles in Male and Female Mice. Neurochem. Int. 2021, 145, 104989. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.C.T.; Fagundes, L.S.; Barbosa, F.; Bernardi, R.; Rhoden, C.R.; Saldiva, P.H.N.; do Valle, A.C. Pre and Post-Natal Exposure to Ambient Level of Air Pollution Impairs Memory of Rats: The Role of Oxidative Stress. Inhal. Toxicol. 2010, 22, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chen, Y.-H.; Chien, C.-C.; Yan, Y.-H.; Chen, H.-C.; Chuang, H.-C.; Hsieh, H.-I.; Cho, K.-H.; Kuo, L.-W.; Chou, C.C.-K.; et al. Three Month Inhalation Exposure to Low-Level PM2.5 Induced Brain Toxicity in an Alzheimer’s Disease Mouse Model. PLoS ONE 2021, 16, e0254587. [Google Scholar] [CrossRef]
- You, R.; Ho, Y.-S.; Chang, R.C.-C. The Pathogenic Effects of Particulate Matter on Neurodegeneration: A Review. J. Biomed. Sci. 2022, 29, 15. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Kavanaugh, M.; Block, M.; D’Angiulli, A.; Delgado-Chávez, R.; Torres-Jardón, R.; González-Maciel, A.; Reynoso-Robles, R.; Osnaya, N.; Villarreal-Calderon, R.; et al. Neuroinflammation, Hyperphosphorylated Tau, Diffuse Amyloid Plaques, and Down-Regulation of the Cellular Prion Protein in Air Pollution Exposed Children and Young Adults. J. Alzheimer’s Dis. 2012, 28, 93–107. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Jáuregui, O.; Queipo-Ortuño, M.I.; Andrés-Lacueva, C. Characterization of the Human Exposome by a Comprehensive and Quantitative Large-Scale Multianalyte Metabolomics Platform. Anal. Chem. 2020, 92, 13767–13775. [Google Scholar] [CrossRef]
- Zhang, P.; Carlsten, C.; Chaleckis, R.; Hanhineva, K.; Huang, M.; Isobe, T.; Koistinen, V.M.; Meister, I.; Papazian, S.; Sdougkou, K.; et al. Defining the Scope of Exposome Studies and Research Needs from a Multidisciplinary Perspective. Environ. Sci. Technol. Lett. 2021, 8, 839–852. [Google Scholar]
- Sillé, F.C.M.; Karakitsios, S.; Kleensang, A.; Koehler, K.; Maertens, A.; Prasse, C.; Quiros-Alcala, L.; Ramachandran, G.; Rappaport, S.M.; Rule, A.M.; et al. The Exposome—A New Approach for Risk Assessment. ALTEX 2020, 37, 3–23. [Google Scholar] [CrossRef]
- Rushing, B.R.; Thessen, A.E.; Soliman, G.A.; Ramesh, A.; Sumner, S.C.J. The Exposome and Nutritional Pharmacology and Toxicology: A New Application for Metabolomics. Exposome 2023, 3, osad008. [Google Scholar] [CrossRef]
- Rattan, S.I.S.; Kaur, G. Nutrition, Food and Diet in Health and Longevity: We Eat What We Are. Nutrients 2022, 14, 5376. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass Spectrometry-based Metabolomics. Mass. Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Grand-Guillaume Perrenoud, A.; Guillarme, D.; Boccard, J.; Veuthey, J.-L.; Barron, D.; Moco, S. Ultra-High Performance Supercritical Fluid Chromatography Coupled with Quadrupole-Time-of-Flight Mass Spectrometry as a Performing Tool for Bioactive Analysis. J. Chromatogr. A 2016, 1450, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Liebich, H.M.; Gesele, E. Profiling of Organic Acids by Capillary Gas Chromatography–Mass Spectrometry after Direct Methylation in Urine Using Trimethyloxonium Tetrafluoroborate. J. Chromatogr. A 1999, 843, 237–245. [Google Scholar] [CrossRef]
- Sasaki, K.; Sagawa, H.; Suzuki, M.; Yamamoto, H.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomics Platform with Capillary Electrophoresis Coupled with High-Resolution Mass Spectrometry for Plasma Analysis. Anal. Chem. 2019, 91, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, C.R.; Kavitake, D.; Jaiswal, K.K.; Jaiswal, K.S.; Reddy, G.B.; Agarwal, V.; Shetty, P.H. NMR-Based Metabolomics as a Significant Tool for Human Nutritional Research and Health Applications. Food Biosci. 2023, 53, 102538. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Laraba, D.; Sanoner, P.; Drilleau, J.-F. Reversed-Phase HPLC Following Thiolysis for Quantitative Estimation and Characterization of the Four Main Classes of Phenolic Compounds in Different Tissue Zones of a French Cider Apple Variety (Malus Domestica Var. Kermerrien). J. Agric. Food Chem. 1998, 46, 1698–1705. [Google Scholar] [CrossRef]
- Mazanova, A.O. Development and Validation Of Immunoenzyme Test-System For Determination Of 25-Hydroxyvitamin D In Blood Serum. Biotechnol. Acta 2016, 9, 28–36. [Google Scholar] [CrossRef]
- Tabung, F.K.; Smith-Warner, S.A.; Chavarro, J.E.; Wu, K.; Fuchs, C.S.; Hu, F.B.; Chan, A.T.; Willett, W.C.; Giovannucci, E.L. Development and Validation of an Empirical Dietary Inflammatory Index. J. Nutr. 2016, 146, 1560–1570. [Google Scholar] [CrossRef]
- Neveu, V.; Moussy, A.; Rouaix, H.; Wedekind, R.; Pon, A.; Knox, C.; Wishart, D.S.; Scalbert, A. Exposome-Explorer: A Manually-Curated Database on Biomarkers of Exposure to Dietary and Environmental Factors. Nucleic Acids Res. 2017, 45, D979–D984. [Google Scholar] [CrossRef]
- Vieujean, S.; Caron, B.; Haghnejad, V.; Jouzeau, J.-Y.; Netter, P.; Heba, A.-C.; Ndiaye, N.C.; Moulin, D.; Barreto, G.; Danese, S.; et al. Impact of the Exposome on the Epigenome in Inflammatory Bowel Disease Patients and Animal Models. Int. J. Mol. Sci. 2022, 23, 7611. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Vojdani, E. The Role of Exposomes in the Pathophysiology of Autoimmune Diseases I: Toxic Chemicals and Food. Pathophysiology 2021, 28, 513–543. [Google Scholar] [CrossRef] [PubMed]
- Dietary Patterns to Prevent and Manage Diet-Related Disease Across the Lifespan; Snair, M., Ed.; National Academies Press: Washington, DC, USA, 2023; ISBN 978-0-309-71329-0. [Google Scholar]
- Santos, L. The Impact of Nutrition and Lifestyle Modification on Health. Eur. J. Intern. Med. 2022, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Fruits and Vegetables in the Prevention of Cellular Oxidative Damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary Intake of Natural Antioxidants: Vitamins and Polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef]
- Khubchandani, J.; Batra, K. Diet Fads and Supplements: Navigating the Allure, Risks, and Reality. J. Med. Surg. Public Health 2024, 4, 100168. [Google Scholar] [CrossRef]
- Nohesara, S.; Abdolmaleky, H.M.; Dickerson, F.; Pinto-Tomás, A.A.; Jeste, D.V.; Thiagalingam, S. Maternal Gut Microbiome-Mediated Epigenetic Modifications in Cognitive Development and Impairments: A New Frontier for Therapeutic Innovation. Nutrients 2024, 16, 4355. [Google Scholar] [CrossRef]
- Gold, E.; Perez de Bronner, S.; Goday, P.S. Nutrition Considerations in the Transgender and Gender-diverse Patient. Nutr. Clin. Pract. 2024, 39, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; Painer, J.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J.; Stenvinkel, P. Manipulating the Exposome to Enable Better Ageing. Biochem. J. 2021, 478, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Młynarska, E.; Jakubowska, P.; Frąk, W.; Gajewska, A.; Sornowska, J.; Skwira, S.; Wasiak, J.; Rysz, J.; Franczyk, B. Associations of Microbiota and Nutrition with Cognitive Impairment in Diseases. Nutrients 2024, 16, 3570. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Xia, B.; Jin, X.; Zou, Q.; Zeng, Z.; Zhao, W.; Yan, S.; Li, L.; Yuan, S.; et al. High-Fiber Diet Mitigates Maternal Obesity-Induced Cognitive and Social Dysfunction in the Offspring via Gut-Brain Axis. Cell Metab. 2021, 33, 923–938.e6. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Ederveen, T.H.A.; Rizzo, F.; Weisz, A.; Collado, M.C.; Muratori, F.; Gross, G.; Alkema, W.; Iozzo, P. Maternal Pre-Pregnancy Overweight and Neonatal Gut Bacterial Colonization Are Associated with Cognitive Development and Gut Microbiota Composition in Pre-School-Age Offspring. Brain Behav. Immun. 2022, 100, 311–320. [Google Scholar] [CrossRef]
- Contu, L.; Hawkes, C. A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int. J. Mol. Sci. 2017, 18, 1093. [Google Scholar] [CrossRef]
- Monthé-Drèze, C.; Rifas-Shiman, S.L.; Gold, D.R.; Oken, E.; Sen, S. Maternal Obesity and Offspring Cognition: The Role of Inflammation. Pediatr. Res. 2019, 85, 799–806. [Google Scholar] [CrossRef]
- Quan, W.; Xu, Y.; Luo, J.; Zeng, M.; He, Z.; Shen, Q.; Chen, J. Association of Dietary Meat Consumption Habits with Neurodegenerative Cognitive Impairment: An Updated Systematic Review and Dose–Response Meta-Analysis of 24 Prospective Cohort Studies. Food Funct. 2022, 13, 12590–12601. [Google Scholar] [CrossRef]
- Lockyer, S.; Spiro, A.; Berry, S.; He, J.; Loth, S.; Martinez-Inchausti, A.; Mellor, D.; Raats, M.; Sokolović, M.; Vijaykumar, S.; et al. How Do We Differentiate Not Demonise—Is There a Role for Healthier Processed Foods in an Age of Food Insecurity? Proceedings of a Roundtable Event. Nutr. Bull. 2023, 48, 278–295. [Google Scholar] [CrossRef]
- Bhardwaj, R.L.; Parashar, A.; Parewa, H.P.; Vyas, L. An Alarming Decline in the Nutritional Quality of Foods: The Biggest Challenge for Future Generations’ Health. Foods 2024, 13, 877. [Google Scholar] [CrossRef]
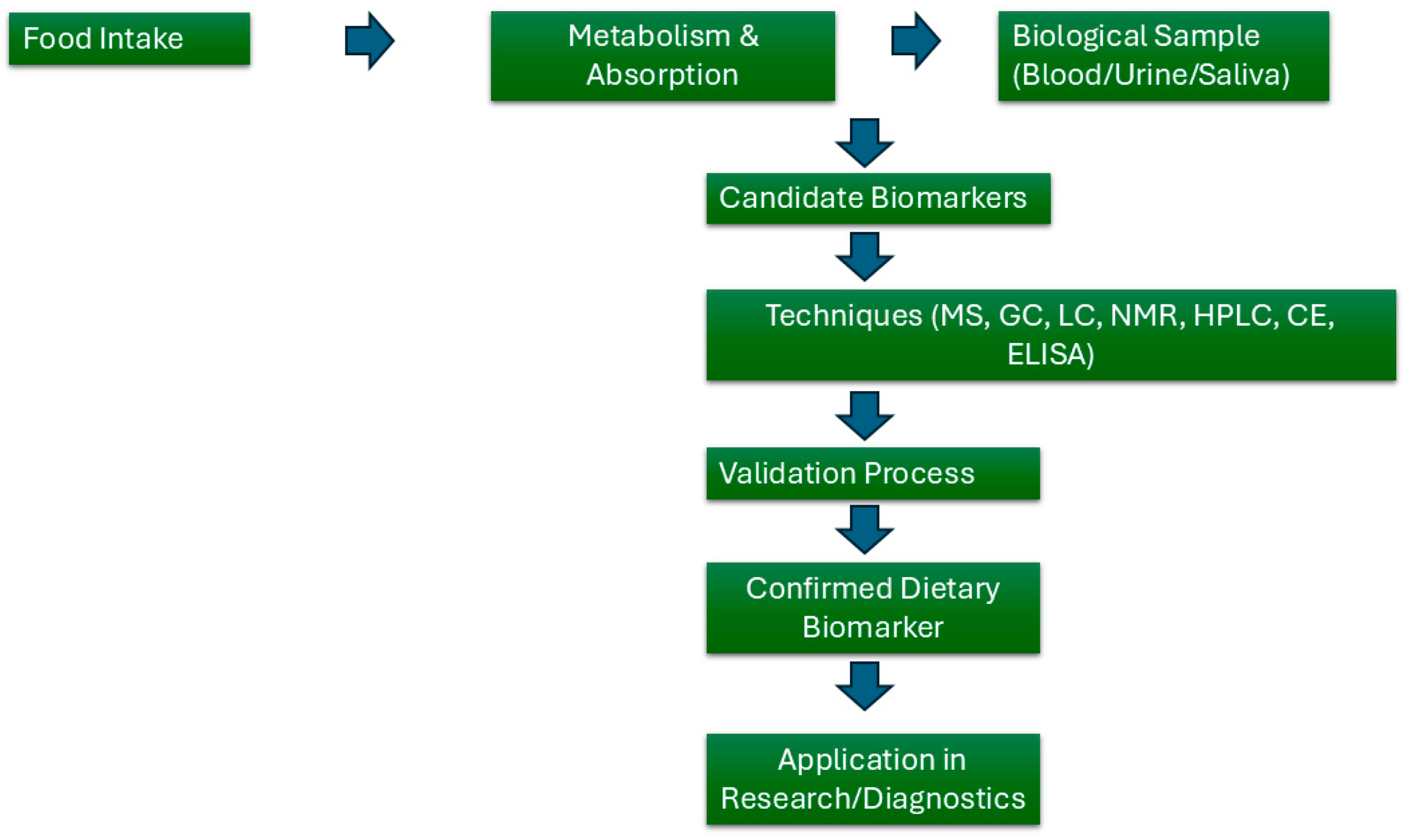
- McNamara, A.E.; Brennan, L. Potential of Food Intake Biomarkers in Nutrition Research. Proc. Nutr. Soc. 2020, 79, 487–497. [Google Scholar] [CrossRef]
- Vinci, G.; Prencipe, S.A.; Ruggieri, R.; Ruggeri, M. How Much Does Overnutrition Weigh? The Environmental and Social Impacts of Metabolic Food Waste in Italy. Sci. Total Environ. 2024, 947, 174420. [Google Scholar] [CrossRef] [PubMed]
- Franco, S.; Barbanera, M.; Moscetti, R.; Cicatiello, C.; Secondi, L.; Massantini, R. Overnutrition Is a Significant Component of Food Waste and Has a Large Environmental Impact. Sci. Rep. 2022, 12, 8166. [Google Scholar] [CrossRef]
- Serafini, M.; Toti, E. Unsustainability of Obesity: Metabolic Food Waste. Front. Nutr. 2016, 3, 40. [Google Scholar] [CrossRef]
- Unsal, P.; Guner, M.; Ozsurekci, C.; Balli, N.; Bas, A.O.; Ozturk, Y.; Dikmeer, A.; Burkuk, S.; Koca, M.; Balci, C.; et al. Prevalence of Nutrition Disorders and Nutrition-related Conditions in Older Patients with Alzheimer’s Disease. Nutr. Clin. Pract. 2023, 38, 1142–1153. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Gao, M.; Bai, X.; Chen, Z. Neuroprotective Effect of Several Phytochemicals and Its Potential Application in the Prevention of Neurodegenerative Diseases. Geriatrics 2016, 1, 29. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The Role of Ketogenic Diet in the Treatment of Neurological Diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Xie, G.; Tian, W.; Wei, T.; Liu, F. The Neuroprotective Effects of β-Hydroxybutyrate on Aβ-Injected Rat Hippocampus in Vivo and in Aβ-Treated PC-12 Cells in Vitro. Free Radic. Res. 2015, 49, 139–150. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Garcia-Rios, A.; Delgado-Lista, J.; Delgado-Casado, N.; Perez-Martinez, P.; Rodriguez-Cantalejo, F.; Fuentes, F.; Cruz-Teno, C.; Tunez, I.; Tasset-Cuevas, I.; et al. Postprandial Effects of the Mediterranean Diet on Oxidant and Antioxidant Status in Elderly Men and Women. J. Am. Geriatr. Soc. 2011, 59, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- van Lent, D.M.; Mesa, H.G.; Short, M.I.; Gonzales, M.M.; Aparicio, H.J.; Salinas, J.; Yuan, C.; Jacques, P.F.; Beiser, A.; Seshadri, S.; et al. Association between Dietary Inflammatory Index Score and Incident Dementia. Alzheimer’s Dement. 2024. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Manly, J.J.; Mayeux, R.P.; Brickman, A.M. An Inflammation-Related Nutrient Pattern Is Associated with Both Brain and Cognitive Measures in a Multiethnic Elderly Population. Curr. Alzheimer Res. 2018, 15, 493–501. [Google Scholar] [CrossRef]
- Duggan, M.R.; Butler, L.; Peng, Z.; Daya, G.N.; Moghekar, A.; An, Y.; Rapp, S.R.; Hayden, K.M.; Shadyab, A.H.; Natale, G.; et al. Plasma Proteins Related to Inflammatory Diet Predict Future Cognitive Impairment. Mol. Psychiatry 2023, 28, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Leurgans, S.E.; Agrawal, S.; Aggarwal, N.T.; Cherian, L.J.; James, B.D.; Dhana, K.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay and Mediterranean Diets with Alzheimer Disease Pathology. Neurology 2023, 100, e2259–e2268. [Google Scholar] [CrossRef]
- Fernández-Sanz, P.; Ruiz-Gabarre, D.; García-Escudero, V. Modulating Effect of Diet on Alzheimer’s Disease. Diseases 2019, 7, 12. [Google Scholar] [CrossRef]
- Couch, C.A.; Ament, Z.; Patki, A.; Kijpaisalratana, N.; Bhave, V.; Jones, A.C.; Armstrong, N.D.; Cheung, K.L.; Kimberly, W.T.; Tiwari, H.K.; et al. The Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet and Metabolites in Chronic Kidney Disease. Nutrients 2024, 16, 2458. [Google Scholar] [CrossRef]
- Ameen, A.O.; Freude, K.; Aldana, B.I. Fats, Friends or Foes: Investigating the Role of Short- and Medium-Chain Fatty Acids in Alzheimer’s Disease. Biomedicines 2022, 10, 2778. [Google Scholar] [CrossRef]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the Ketogenic Agent AC-1202 in Mild to Moderate Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a Medium-Chain Triglyceride-Based Ketogenic Formula on Cognitive Function in Patients with Mild-to-Moderate Alzheimer’s Disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Mazzei, G.; Ikegami, R.; Abolhassani, N.; Haruyama, N.; Sakumi, K.; Saito, T.; Saido, T.C.; Nakabeppu, Y. A High-Fat Diet Exacerbates the Alzheimer’s Disease Pathology in the Hippocampus of the AppNL−F/NL−F Knock-in Mouse Model. Aging Cell 2021, 20, e13429. [Google Scholar] [CrossRef] [PubMed]
- Soininen, H.; Solomon, A.; Visser, P.J.; Hendrix, S.B.; Blennow, K.; Kivipelto, M.; Hartmann, T. 36-Month LipiDiDiet Multinutrient Clinical Trial in Prodromal Alzheimer’s Disease. Alzheimer’s Dement. 2021, 17, 29–40. [Google Scholar] [CrossRef]
- Dilmore, A.H.; Martino, C.; Neth, B.J.; West, K.A.; Zemlin, J.; Rahman, G.; Panitchpakdi, M.; Meehan, M.J.; Weldon, K.C.; Blach, C.; et al. Effects of a Ketogenic and Low-Fat Diet on the Human Metabolome, Microbiome, and Foodome in Adults at Risk for Alzheimer’s Disease. Alzheimer’s Dement. 2023, 19, 4805–4816. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Ding, W.; Wu, J.; Liu, F.; Liu, J.; Zhang, J.; Wang, J. The Improvement Effects of Sika Deer Antler Protein in an Alzheimer’s Disease Mouse Model via the Microbe–Gut–Brain Axis. Food Sci. Nutr. 2025, 13, e4656. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Wang, S.; Mishra, S.P.; Jain, S.; Yadav, H. Protection of Alzheimer’s Disease Progression by a Human-Origin Probiotics Cocktail. Sci. Rep. 2025, 15, 1589. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Asl, Z.; Sepehri, G.; Salami, M. Probiotic Treatment Improves the Impaired Spatial Cognitive Performance and Restores Synaptic Plasticity in an Animal Model of Alzheimer’s Disease. Behav. Brain Res. 2019, 376, 112183. [Google Scholar] [CrossRef]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and Bifidobacteria Ameliorate Memory and Learning Deficits and Oxidative Stress in β-Amyloid (1–42) Injected Rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef]
- Li, S.-C.; Hsu, W.-F.; Chang, J.-S.; Shih, C.-K. Combination of Lactobacillus Acidophilus and Bifidobacterium Animalis Subsp. Lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. Phosphodiesterase 4D: An Enzyme to Remember. Br. J. Pharmacol. 2015, 172, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Fedele, E.; Ricciarelli, R. Memory Enhancers for Alzheimer’s Dementia: Focus on CGMP. Pharmaceuticals 2021, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate–Nitrite–Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug. Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary Flavonoids and Nitrate: Effects on Nitric Oxide and Vascular Function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef]
- López, D.; Orta, X.; Casós, K.; Sáiz, M.P.; Puig-Parellada, P.; Farriol, M.; Mitjavila, M.T. Upregulation of Endothelial Nitric Oxide Synthase in Rat Aorta after Ingestion of Fish Oil-Rich Diet. Am. J. Physiol. -Heart Circ. Physiol. 2004, 287, H567–H572. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Fedele, E. CAMP, CGMP and Amyloid β: Three Ideal Partners for Memory Formation. Trends Neurosci. 2018, 41, 255–266. [Google Scholar] [CrossRef]
- Sanders, O.; Rajagopal, L. Phosphodiesterase Inhibitors for Alzheimer’s Disease: A Systematic Review of Clinical Trials and Epidemiology with a Mechanistic Rationale. J. Alzheimers Dis. Rep. 2020, 4, 185–215. [Google Scholar] [CrossRef]
- Thal, L.J.; Salmon, D.P.; Lasker, B.; Bower, D.; Klauber, M.R. The Safety and Lack of Efficacy of Vinpocetine in Alzheimer’s Disease. J. Am. Geriatr. Soc. 1989, 37, 515–520. [Google Scholar] [CrossRef]
- Arlt, S.; Schwedhelm, E.; Kölsch, H.; Jahn, H.; Linnebank, M.; Smulders, Y.; Jessen, F.; Böger, R.H.; Popp, J. Dimethylarginines, Homocysteine Metabolism, and Cerebrospinal Fluid Markers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 31, 751–758. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Harrington, R.J.; Tsai, A.; Liao, P.; Harrington, M.G. Free Amino Acid and Dipeptide Changes in the Body Fluids from Alzheimer’s Disease Subjects. Amino Acids 2007, 32, 213–224. [Google Scholar] [CrossRef]
- Ibáñez, C.; Simó, C.; Martín-Álvarez, P.J.; Kivipelto, M.; Winblad, B.; Cedazo-Mínguez, A.; Cifuentes, A. Toward a Predictive Model of Alzheimer’s Disease Progression Using Capillary Electrophoresis–Mass Spectrometry Metabolomics. Anal. Chem. 2012, 84, 8532–8540. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, Y.; Nakaya, J. Effect of Oral Administration of L-Arginine on Senile Dementia. Am. J. Med. 2000, 108, 439. [Google Scholar] [CrossRef]
- Fonar, G.; Polis, B.; Meirson, T.; Maltsev, A.; Elliott, E.; Samson, A.O. Intracerebroventricular Administration of L-Arginine Improves Spatial Memory Acquisition in Triple Transgenic Mice via Reduction of Oxidative Stress and Apoptosis. Transl. Neurosci. 2018, 9, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Minamisawa, M.; Sato, Y.; Ishiguro, E.; Taniai, T.; Sakamoto, T.; Kawai, G.; Saito, T.; Saido, T.C. Amelioration of Alzheimer’s Disease by Gut-Pancreas-Liver-Brain Interaction in an App Knock-In Mouse Model. Life 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, K.; Serrano-Cuevas, L.; Almeida-Gutiérrez, E.; Flores-Chavez, S.; Mejía-Aranguré, J.M.; Garcia-delaTorre, P. Citrulline Supplementation Improves Spatial Memory in a Murine Model for Alzheimer’s Disease. Nutrition 2021, 90, 111248. [Google Scholar] [CrossRef]
- Rajendra, A.; Bondonno, N.P.; Rainey-Smith, S.R.; Gardener, S.L.; Hodgson, J.M.; Bondonno, C.P. Potential Role of Dietary Nitrate in Relation to Cardiovascular and Cerebrovascular Health, Cognition, Cognitive Decline and Dementia: A Review. Food Funct. 2022, 13, 12572–12589. [Google Scholar] [CrossRef]
- Morris, M.C.; Wang, Y.; Barnes, L.L.; Bennett, D.A.; Dawson-Hughes, B.; Booth, S.L. Nutrients and Bioactives in Green Leafy Vegetables and Cognitive Decline. Neurology 2018, 90, e214–e222. [Google Scholar] [CrossRef]
- Rajendra, A.; Bondonno, N.P.; Murray, K.; Zhong, L.; Rainey-Smith, S.R.; Gardener, S.L.; Blekkenhorst, L.C.; Ames, D.; Maruff, P.; Martins, R.N.; et al. Habitual Dietary Nitrate Intake and Cognition in the Australian Imaging, Biomarkers and Lifestyle Study of Ageing: A Prospective Cohort Study. Clin. Nutr. 2023, 42, 1251–1259. [Google Scholar] [CrossRef]
- Rajendra, A.; Bondonno, N.P.; Zhong, L.; Radavelli-Bagatini, S.; Murray, K.; Rainey-Smith, S.R.; Gardener, S.L.; Blekkenhorst, L.C.; Magliano, D.J.; Shaw, J.E.; et al. Plant but Not Animal Sourced Nitrate Intake Is Associated with Lower Dementia-Related Mortality in the Australian Diabetes, Obesity, and Lifestyle Study. Front. Nutr. 2024, 11, 1327042. [Google Scholar] [CrossRef]
- Hunt, T.; Pontifex, M.G.; Vauzour, D. (Poly)Phenols and Brain Health—beyond Their Antioxidant Capacity. FEBS Lett. 2024, 598, 2949–2962. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of Fish and Polyunsaturated Fatty Acids and Mild-to-Severe Cognitive Impairment Risks: A Dose-Response Meta-Analysis of 21 Cohort Studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Arshad, N.; Visweswariah, S.S. Cyclic Nucleotide Signaling in Intestinal Epithelia: Getting to the Gut of the Matter. WIREs Syst. Biol. Med. 2013, 5, 409–424. [Google Scholar] [CrossRef]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; de Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and Molecular Genetics of the Phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef]
- Ahmad, F.; Murata, T.; Shimizu, K.; Degerman, E.; Maurice, D.; Manganiello, V. Cyclic Nucleotide Phosphodiesterases: Important Signaling Modulators and Therapeutic Targets. Oral Dis. 2015, 21, e25–e50. [Google Scholar] [CrossRef]
- Lugnier, C. Cyclic Nucleotide Phosphodiesterase (PDE) Superfamily: A New Target for the Development of Specific Therapeutic Agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef] [PubMed]
- Newton, A.C.; Bootman, M.D.; Scott, J.D. Second Messengers. Cold Spring Harb. Perspect. Biol. 2016, 8, a005926. [Google Scholar] [CrossRef] [PubMed]
- Bondarev, A.D.; Attwood, M.M.; Jonsson, J.; Chubarev, V.N.; Tarasov, V.V.; Liu, W.; Schiöth, H.B. Recent Developments of Phosphodiesterase Inhibitors: Clinical Trials, Emerging Indications and Novel Molecules. Front. Pharmacol. 2022, 13, 1057083. [Google Scholar] [CrossRef]
- Heckman, P.R.A.; Blokland, A.; Bollen, E.P.P.; Prickaerts, J. Phosphodiesterase Inhibition and Modulation of Corticostriatal and Hippocampal Circuits: Clinical Overview and Translational Considerations. Neurosci. Biobehav. Rev. 2018, 87, 233–254. [Google Scholar] [CrossRef]
- Gulisano, W.; Tropea, M.R.; Arancio, O.; Palmeri, A.; Puzzo, D. Sub-Efficacious Doses of Phosphodiesterase 4 and 5 Inhibitors Improve Memory in a Mouse Model of Alzheimer’s Disease. Neuropharmacology 2018, 138, 151–159. [Google Scholar] [CrossRef]
- Hesse, R.; Lausser, L.; Gummert, P.; Schmid, F.; Wahler, A.; Schnack, C.; Kroker, K.S.; Otto, M.; Tumani, H.; Kestler, H.A.; et al. Reduced CGMP Levels in CSF of AD Patients Correlate with Severity of Dementia and Current Depression. Alzheimers Res. Ther. 2017, 9, 17. [Google Scholar] [CrossRef]
- Kelly, M.P. Cyclic Nucleotide Signaling Changes Associated with Normal Aging and Age-Related Diseases of the Brain. Cell Signal 2018, 42, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Götz, M.E.; Ozawa, H.; Luckhaus, C.; Saito, T.; Rösler, M.; Riederer, P. Hippocampal Level of Neural Specific Adenylyl Cyclase Type I Is Decreased in Alzheimer’s Disease. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2000, 1535, 60–68. [Google Scholar] [CrossRef]
- Xiang, Y.; Naik, S.; Zhao, L.; Shi, J.; Ke, H. Emerging Phosphodiesterase Inhibitors for Treatment of Neurodegenerative Diseases. Med. Res. Rev. 2024, 44, 1404–1445. [Google Scholar] [CrossRef] [PubMed]
- Brandt, R.; Lee, G.; Teplow, D.B.; Shalloway, D.; Abdel-Ghany, M. Differential Effect of Phosphorylation and Substrate Modulation on Tau’s Ability to Promote Microtubule Growth and Nucleation. J. Biol. Chem. 1994, 269, 11776–11782. [Google Scholar] [CrossRef]
- Schneider, A.; Biernat, J.; von Bergen, M.; Mandelkow, E.; Mandelkow, E.-M. Phosphorylation That Detaches Tau Protein from Microtubules (Ser262, Ser214) Also Protects It against Aggregation into Alzheimer Paired Helical Filaments. Biochemistry 1999, 38, 3549–3558. [Google Scholar] [CrossRef]
- Villa, V.; Montalto, G.; Caudano, F.; Fedele, E.; Ricciarelli, R. Selective Inhibition of Phosphodiesterase 4D Increases Tau Phosphorylation at Ser214 Residue. BioFactors 2022, 48, 1111–1117. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Wang, H.; Wang, C.; Wilson, S.P.; Xu, J.; Zhang, H.-T. RNA Interference-Mediated Knockdown of Long-Form Phosphodiesterase-4D (PDE4D) Enzyme Reverses Amyloid-Β42-Induced Memory Deficits in Mice. J. Alzheimer’s Dis. 2013, 38, 269–280. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.-M.; Zhuo, Y.-Y.; Zhou, H.; Lin, H.-B.; Cheng, Y.-F.; Xu, J.-P.; Zhang, H.-T. The Phosphodiesterase-4 Inhibitor Rolipram Reverses Aβ-Induced Cognitive Impairment and Neuroinflammatory and Apoptotic Responses in Rats. Int. J. Neuropsychopharmacol. 2012, 15, 749–766. [Google Scholar] [CrossRef]
- Kumari, S.; Bagri, K.; Deshmukh, R. Connecting Dots: Preclinical Foundations to Clinical Realities of PDE4 Inhibitors in Alzheimer’s Disease. Inflammopharmacology 2025, 33, 593–603. [Google Scholar] [CrossRef]
- Prickaerts, J.; Kerckhoffs, J.; Possemis, N.; van Overveld, W.; Verbeek, F.; Grooters, T.; Sambeth, A.; Blokland, A. Roflumilast and Cognition Enhancement: A Translational Perspective. Biomed. Pharmacother. 2024, 181, 117707. [Google Scholar] [CrossRef]
- Donders, Z.; Skorupska, I.J.; Willems, E.; Mussen, F.; Broeckhoven, J.V.; Carlier, A.; Schepers, M.; Vanmierlo, T. Beyond PDE4 Inhibition: A Comprehensive Review on Downstream CAMP Signaling in the Central Nervous System. Biomed. Pharmacother. 2024, 177, 117009. [Google Scholar] [CrossRef]
- Bonkale, W.L.; Winblad, B.; Ravid, R.; Cowburn, R.F. Reduced Nitric Oxide Responsive Soluble Guanylyl Cyclase Activity in the Superior Temporal Cortex of Patients with Alzheimer’s Disease. Neurosci. Lett. 1995, 187, 5–8. [Google Scholar] [CrossRef] [PubMed]
- BALTRONS, M.; PIFARRE, P.; FERRER, I.; CAROT, J.; GARCIA, A. Reduced Expression of NO-Sensitive Guanylyl Cyclase in Reactive Astrocytes of Alzheimer Disease, Creutzfeldt–Jakob Disease, and Multiple Sclerosis Brains. Neurobiol. Dis. 2004, 17, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, A.; Gil-Bea, F.; García-Barroso, C.; Cedazo-Minguez, Á.; Ramírez, M.J.; Franco, R.; García-Osta, A.; Oyarzabal, J.; Cuadrado-Tejedor, M. Decreased Levels of Guanosine 3′, 5′-monophosphate (c GMP) in Cerebrospinal Fluid (CSF) Are Associated with Cognitive Decline and Amyloid Pathology in A Lzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2015, 41, 471–482. [Google Scholar] [CrossRef]
- Yew, D.T.; Wong, H.W.; Li, W.P.; Lai, H.W.L.; Yu, W.A. Nitric Oxide Synthase Neurons in Different Areas of Normal Aged and Alzheimer’s Brains. Neuroscience 1999, 89, 675–686. [Google Scholar] [CrossRef]
- Gunn, A.P.; Wong, B.X.; McLean, C.; Fowler, C.; Barnard, P.J.; Duce, J.A.; Roberts, B.R. Increased Glutaminyl Cyclase Activity in Brains of Alzheimer’s Disease Individuals. J. Neurochem. 2021, 156, 979–987. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, D.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.; Bi, R.; Yao, Y. A Systematic Integrated Analysis of Brain Expression Profiles Reveals YAP1 and Other Prioritized Hub Genes as Important Upstream Regulators in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Sanders, O. Sildenafil for the Treatment of Alzheimer’s Disease: A Systematic Review. J. Alzheimers Dis. Rep. 2020, 4, 91–106. [Google Scholar] [CrossRef]
- Prieto, G.A.; Trieu, B.H.; Dang, C.T.; Bilousova, T.; Gylys, K.H.; Berchtold, N.C.; Lynch, G.; Cotman, C.W. Pharmacological Rescue of Long-Term Potentiation in Alzheimer Diseased Synapses. J. Neurosci. 2017, 37, 1197–1212. [Google Scholar] [CrossRef] [PubMed]
- Abouelmagd, M.E.; Abdelmeseh, M.; Elrosasy, A.; Saad, Y.H.; Alnajjar, A.Z.; Eid, M.; Hassan, A.; Abbas, A. Phosphodiesterase-5 Inhibitors Use and the Risk of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurol. Sci. 2024, 45, 5261–5270. [Google Scholar] [CrossRef]
- Crescioli, C.; Paronetto, M.P. The Emerging Role of Phosphodiesterase 5 Inhibition in Neurological Disorders: The State of the Art. Cells 2024, 13, 1720. [Google Scholar] [CrossRef]
- Justo, A.F.O.; Toscano, E.C. de B.; Farias-Itao, D.S.; Suemoto, C.K. The Action of Phosphodiesterase-5 Inhibitors on β-Amyloid Pathology and Cognition in Experimental Alzheimer’s Disease: A Systematic Review. Life Sci. 2023, 320, 121570. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, T.; Pacjuk, O.; Hernández-Huguet, S.; Körner, J.; Scherer, K.; Richling, E. Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents. Medicines 2017, 4, 80. [Google Scholar] [CrossRef]
- Göttel, C.; Niesen, S.; Daub, V.; Werle, T.; Bakuradze, T.; Winterhalter, P.; Richling, E. In Vitro Inhibition of Phosphodiesterase 3B (PDE 3B) by Anthocyanin-Rich Fruit Juice Extracts and Selected Anthocyanins. Int. J. Mol. Sci. 2020, 21, 6934. [Google Scholar] [CrossRef]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An Overview of New Possible Treatments of Alzheimer’s Disease, Based on Natural Products and Semi-Synthetic Compounds. Curr. Med. Chem. 2017, 24, 3749–3773. [Google Scholar] [CrossRef] [PubMed]
- HAYASHI, S.; OZAWA, H. Studies on 3, 7-Dimethyl-1-(5-Oxo-Hexyl)-Xanthine (BL 191). I. Cyclic 3’, 5’-Nucleotide Phosphodiesterase (PDE) and the Inhibitory Effect of BL 191 on PDE in Rat Brain and Heart. Chem. Pharm. Bull. 1974, 22, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.; Magnussen, H.; Dent, G. Theophylline and Selective PDE Inhibitors as Bronchodilators and Smooth Muscle Relaxants. Eur. Respir. J. 1995, 8, 637–642. [Google Scholar] [CrossRef]
- Orhan, I.E.; Rauf, A.; Saleem, M.; Khalil, A.A. Natural Molecules as Talented Inhibitors of Nucleotide Pyrophosphatases/ Phosphodiesterases (PDEs). Curr. Top Med. Chem. 2022, 22, 209–228. [Google Scholar] [CrossRef]
- Singh, A.; Kumar Singh, N. Pre-Clinical Evidence-Based Neuroprotective Potential of Naringin against Alzheimer’s Disease-like Pathology: A Comprehensive Review. Curr. Pharm. Biotechnol. 2024, 25, 1112–1123. [Google Scholar] [CrossRef]
- Ribaudo, G.; Memo, M.; Gianoncelli, A. A Perspective on Natural and Nature-Inspired Small Molecules Targeting Phosphodiesterase 9 (PDE9): Chances and Challenges against Neurodegeneration. Pharmaceuticals 2021, 14, 58. [Google Scholar] [CrossRef]
- Giacobbe, J.; Benoiton, B.; Zunszain, P.; Pariante, C.M.; Borsini, A. The Anti-Inflammatory Role of Omega-3 Polyunsaturated Fatty Acids Metabolites in Pre-Clinical Models of Psychiatric, Neurodegenerative, and Neurological Disorders. Front. Psychiatry 2020, 11, 122. [Google Scholar] [CrossRef]
- Thompson, A.S.; Jennings, A.; Bondonno, N.P.; Tresserra-Rimbau, A.; Parmenter, B.H.; Hill, C.; Perez-Cornago, A.; Kühn, T.; Cassidy, A. Higher Habitual Intakes of Flavonoids and Flavonoid-Rich Foods Are Associated with a Lower Incidence of Type 2 Diabetes in the UK Biobank Cohort. Nutr. Diabetes 2024, 14, 32. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of Platelet Aggregation by Olive Oil Phenols via CAMP-Phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. New Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Fekete, M.; Varga, P.; Ungvari, Z.; Fekete, J.T.; Buda, A.; Szappanos, Á.; Lehoczki, A.; Mózes, N.; Grosso, G.; Godos, J.; et al. The Role of the Mediterranean Diet in Reducing the Risk of Cognitive Impairement, Dementia, and Alzheimer’s Disease: A Meta-Analysis. Geroscience 2025, online ahead of print. [Google Scholar] [CrossRef]
- Duff, K. Transgenic Mouse Models of Alzheimer’s Disease: Phenotype and Mechanisms of Pathogenesis. Biochem. Soc. Symp. 2001, 67, 195–202. [Google Scholar] [CrossRef]
- Kosel, F.; Pelley, J.M.S.; Franklin, T.B. Behavioural and Psychological Symptoms of Dementia in Mouse Models of Alzheimer’s Disease-Related Pathology. Neurosci. Biobehav. Rev. 2020, 112, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Quan, M.; Wei, Y.; Wang, W.; Xu, L.; Wang, Q.; Jia, J. Critical Thinking of Alzheimer’s Transgenic Mouse Model: Current Research and Future Perspective. Sci. China Life Sci. 2023, 66, 2711–2754. [Google Scholar] [CrossRef]
- De Plano, L.M.; Saitta, A.; Oddo, S.; Caccamo, A. Navigating Alzheimer’s Disease Mouse Models: Age-Related Pathology and Cognitive Deficits. Biomolecules 2024, 14, 1405. [Google Scholar] [CrossRef]
- Sharman, M.J.; Gyengesi, E.; Liang, H.; Chatterjee, P.; Karl, T.; Li, Q.-X.; Wenk, M.R.; Halliwell, B.; Martins, R.N.; Münch, G. Assessment of Diets Containing Curcumin, Epigallocatechin-3-Gallate, Docosahexaenoic Acid and α-Lipoic Acid on Amyloid Load and Inflammation in a Male Transgenic Mouse Model of Alzheimer’s Disease: Are Combinations More Effective? Neurobiol. Dis. 2019, 124, 505–519. [Google Scholar] [CrossRef]
- Braidy, N.; Essa, M.M.; Poljak, A.; Selvaraju, S.; Al-Adawi, S.; Manivasagm, T.; Thenmozhi, A.J.; Ooi, L.; Sachdev, P.; Guillemin, G.J. Consumption of Pomegranates Improves Synaptic Function in a Transgenic Mice Model of Alzheimer’s Disease. Oncotarget 2016, 7, 64589–64604. [Google Scholar] [CrossRef] [PubMed]
- Essa, M.M.; Subash, S.; Akbar, M.; Al-Adawi, S.; Guillemin, G.J. Long-Term Dietary Supplementation of Pomegranates, Figs and Dates Alleviate Neuroinflammation in a Transgenic Mouse Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0120964. [Google Scholar] [CrossRef]
- Essa, M.; Braidy, N.; Awlad-Thani, K.; Vaishnav, R.; Al-Asmi, A.; Guillemin, G.; Al-Adawi, S.; Subash, S. Diet Rich in Date Palm Fruits Improves Memory, Learning and Reduces Beta Amyloid in Transgenic Mouse Model of Alzheimer′s Disease. J. Ayurveda Integr. Med. 2015, 6, 111. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Asmi, A.; Al-Adawi, S.; Vaishnav, R. Chronic Dietary Supplementation of 4% Figs on the Modification of Oxidative Stress in Alzheimer’s Disease Transgenic Mouse Model. Biomed. Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Braidy, N.; Al-Jabri, A.; Vaishnav, R.; Al-Adawi, S.; Al-Asmi, A.; Guillemin, G.J. Consumption of Fig Fruits Grown in Oman Can Improve Memory, Anxiety, and Learning Skills in a Transgenic Mice Model of Alzheimer’s Disease. Nutr. Neurosci. 2016, 19, 475–483. [Google Scholar] [CrossRef]
- Lim, G.P.; Calon, F.; Morihara, T.; Yang, F.; Teter, B.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. A Diet Enriched with the Omega-3 Fatty Acid Docosahexaenoic Acid Reduces Amyloid Burden in an Aged Alzheimer Mouse Model. J. Neurosci. 2005, 25, 3032–3040. [Google Scholar] [CrossRef]
- Zhuo, J.-M.; Praticò, D. Acceleration of Brain Amyloidosis in an Alzheimer’s Disease Mouse Model by a Folate, Vitamin B6 and B12-Deficient Diet. Exp. Gerontol. 2010, 45, 195–201. [Google Scholar] [CrossRef]
- Bernardo, A.; McCord, M.; Troen, A.M.; Allison, J.D.; McDonald, M.P. Impaired Spatial Memory in APP-Overexpressing Mice on a Homocysteinemia-Inducing Diet. Neurobiol. Aging 2007, 28, 1195–1205. [Google Scholar] [CrossRef]
- Zhuo, J.-M.; Portugal, G.; Kruger, W.; Wang, H.; Gould, T.; Pratico, D. Diet-Induced Hyperhomocysteinemia Increases Amyloid-beta Formation and Deposition in a Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2010, 7, 140–149. [Google Scholar] [CrossRef]
- Huang, H.; Nie, S.; Cao, M.; Marshall, C.; Gao, J.; Xiao, N.; Hu, G.; Xiao, M. Characterization of AD-like Phenotype in Aged APPSwe/PS1dE9 Mice. Age 2016, 38, 303–322. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate Polyphenols and Extract Inhibit Nuclear Factor of Activated T-Cell Activity and Microglial Activation In Vitro and in a Transgenic Mouse Model of Alzheimer Disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Lee, J.-H.; Heo, H.J.; Cho, H.Y.; Kim, H.K.; Kim, C.-J.; Kim, M.O.; Suh, S.H.; Shin, D.-H. Punica Granatum Protects Against Oxidative Stress in PC12 Cells and Oxidative Stress-Induced Alzheimer’s Symptoms in Mice. J. Med. Food 2011, 14, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A Attenuates Memory Impairment and Neuroinflammation in APP/PS1 Mice. J. Neuroinflammation. 2019, 16, 62. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Vitorica, J.; Gómez-Ariza, J.L. Deciphering Metabolic Abnormalities Associated with Alzheimer’s Disease in the APP/PS1 Mouse Model Using Integrated Metabolomic Approaches. Biochimie 2015, 110, 119–128. [Google Scholar] [CrossRef]
- Smach, M.A.; Jacob, N.; Golmard, J.-L.; Charfeddine, B.; Lammouchi, T.; Ben Othman, L.; Dridi, H.; Bennamou, S.; Limem, K. Folate and Homocysteine in the Cerebrospinal Fluid of Patients with Alzheimer’s Disease or Dementia: A Case Control Study. Eur. Neurol. 2011, 65, 270–278. [Google Scholar] [CrossRef]
- Liu, H.; Tian, T.; Qin, S.; Li, W.; Zhang, X.; Wang, X.; Gao, Y.; Huang, G. Folic Acid Deficiency Enhances Abeta Accumulation in APP/PS1 Mice Brain and Decreases Amyloid-Associated MiRNAs Expression. J. Nutr. Biochem. 2015, 26, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quinto, J.; Rodriguez de Turco, E.B.; DeRosa, S.; Howard, A.; Cruz-Sanchez, F.; Sambamurti, K.; Refolo, L.; Petanceska, S.; Pappolla, M.A. Hyperhomocysteinemic Alzheimer’s Mouse Model of Amyloidosis Shows Increased Brain Amyloid β Peptide Levels. Neurobiol. Dis. 2006, 22, 651–656. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Chen, R.; Lin, Y.; Xu, J.; Fang, Z.; Ru, Y.; Fan, C.; Wu, G. Fecal Microbiota Transplantation from Methionine-Restricted Diet Mouse Donors Improves Alzheimer’s Learning and Memory Abilities Through Short-Chain Fatty Acids. Foods 2025, 14, 101. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Sarroca, S.; Ivanova, A.; Puigoriol-Illamola, D.; Aguado, F.; Camins, A.; Sanfeliu, C.; Pallàs, M. Epigenetic Mechanisms Underlying Cognitive Impairment and Alzheimer Disease Hallmarks in 5XFAD Mice. Aging 2016, 8, 664–684. [Google Scholar] [CrossRef]
- Reilly, A.M.; Tsai, A.P.; Lin, P.B.; Ericsson, A.C.; Oblak, A.L.; Ren, H. Metabolic Defects Caused by High-Fat Diet Modify Disease Risk through Inflammatory and Amyloidogenic Pathways in a Mouse Model of Alzheimer’s Disease. Nutrients 2020, 12, 2977. [Google Scholar] [CrossRef]
- Li, L.; Yang, C.; Jia, M.; Wang, Y.; Zhao, Y.; Li, Q.; Gong, J.; He, Y.; Xu, K.; Liu, X.; et al. Synbiotic Therapy with Clostridium Sporogenes and Xylan Promotes Gut-Derived Indole-3-Propionic Acid and Improves Cognitive Impairments in an Alzheimer’s Disease Mouse Model. Food Funct. 2024, 15, 7865–7882. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, D.; Dedemadi, A.-G.; Voulgari, M.-L.; Georgiou, E.; Dafnis, I.; Mountaki, C.; Panagopoulou, E.A.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; et al. Corinthian Currants Promote the Expression of Paraoxonase-1 and Enhance the Antioxidant Status in Serum and Brain of 5xFAD Mouse Model of Alzheimer’s Disease. Biomolecules 2024, 14, 426. [Google Scholar] [CrossRef]
- Galeano, P.; de Ceglia, M.; Mastrogiovanni, M.; Campanelli, L.; Medina-Vera, D.; Campolo, N.; Novack, G.V.; Rosell-Valle, C.; Suárez, J.; Aicardo, A.; et al. The Effect of Fat Intake with Increased Omega-6-to-Omega-3 Polyunsaturated Fatty Acid Ratio in Animal Models of Early and Late Alzheimer’s Disease-like Pathogenesis. Int. J. Mol. Sci. 2023, 24, 17009. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Wang, J.; Woodie, L.N.; Greene, M.W.; Kaddoumi, A. Oleocanthal Ameliorates Metabolic and Behavioral Phenotypes in a Mouse Model of Alzheimer’s Disease. Molecules 2023, 28, 5592. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Liu, J.; Lee, S.-J.; Lee, D.-Y.; Zhang, G.; Kim, Y. Curcumin Mitigates the High-Fat High-Sugar Diet-Induced Impairment of Spatial Memory, Hepatic Metabolism, and the Alteration of the Gut Microbiome in Alzheimer’s Disease-Induced (3xTg-AD) Mice. Nutrients 2024, 16, 240. [Google Scholar] [CrossRef]
- Broderick, T.L.; Rasool, S.; Li, R.; Zhang, Y.; Anderson, M.; Al-Nakkash, L.; Plochocki, J.H.; Geetha, T.; Babu, J.R. Neuroprotective Effects of Chronic Resveratrol Treatment and Exercise Training in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 7337. [Google Scholar] [CrossRef]
- Paula, P.-C.; Angelica Maria, S.-G.; Luis, C.-H.; Gloria Patricia, C.-G. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef]
- Dal-Pan, A.; Dudonné, S.; Bourassa, P.; Bourdoulous, M.; Tremblay, C.; Desjardins, Y.; Calon, F. Cognitive-Enhancing Effects of a Polyphenols-Rich Extract from Fruits without Changes in Neuropathology in an Animal Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 55, 115–135. [Google Scholar] [CrossRef]
- Julien, C.; Tremblay, C.; Phivilay, A.; Berthiaume, L.; Émond, V.; Julien, P.; Calon, F. High-Fat Diet Aggravates Amyloid-Beta and Tau Pathologies in the 3xTg-AD Mouse Model. Neurobiol. Aging 2010, 31, 1516–1531. [Google Scholar] [CrossRef]
- Liang, Z.; Gong, X.; Zhao, Y.; Zhao, Y.; Yu, J.; Huang, T.; Yang, C.; Wu, L.; Huang, M.; Wang, X.; et al. Long-Term High-Fat Diet Consumption Aggravates Β-Amyloid Deposition and Tau Pathology Accompanied by Microglial Activation in an Alzheimer’s Disease Model. Mol. Nutr. Food. Res. 2024, 68, e2300669. [Google Scholar] [CrossRef]
- Tournissac, M.; Vandal, M.; Tremblay, C.; Bourassa, P.; Vancassel, S.; Emond, V.; Gangloff, A.; Calon, F. Dietary Intake of Branched-chain Amino Acids in a Mouse Model of Alzheimer’s Disease: Effects on Survival, Behavior, and Neuropathology. Alzheimer’s Dement.: Transl. Res. Clin. Interv. 2018, 4, 677–687. [Google Scholar] [CrossRef]
- Marwarha, G.; Claycombe-Larson, K.; Lund, J.; Schommer, J.; Ghribi, O. A Diet Enriched in Palmitate and Deficient in Linoleate Exacerbates Oxidative Stress and Amyloid-β Burden in the Hippocampus of 3xTg-AD Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 219–237. [Google Scholar] [CrossRef]
- Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. High Tryptophan Diet Reduces CA1 Intraneuronal Β-amyloid in the Triple Transgenic Mouse Model of Alzheimer’s Disease. Aging Cell 2012, 11, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Bubier, J.A.; Chesler, E.J.; Weinstock, G.M. Host Genetic Control of Gut Microbiome Composition. Mamm. Genome 2021, 32, 263–281. [Google Scholar] [CrossRef]
- Onisiforou, A.; Charalambous, E.G.; Zanos, P. Shattering the Amyloid Illusion: The Microbial Enigma of Alzheimer’s Disease Pathogenesis—From Gut Microbiota and Viruses to Brain Biofilms. Microorganisms 2025, 13, 90. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaco, M.; Torazza, C.; Fedele, E.; Grilli, M. The Impact of the Exposome on Alzheimer’s Disease: The Influence of Nutrition. Int. J. Mol. Sci. 2025, 26, 3015. https://doi.org/10.3390/ijms26073015
Monaco M, Torazza C, Fedele E, Grilli M. The Impact of the Exposome on Alzheimer’s Disease: The Influence of Nutrition. International Journal of Molecular Sciences. 2025; 26(7):3015. https://doi.org/10.3390/ijms26073015
Chicago/Turabian StyleMonaco, Martina, Carola Torazza, Ernesto Fedele, and Massimo Grilli. 2025. "The Impact of the Exposome on Alzheimer’s Disease: The Influence of Nutrition" International Journal of Molecular Sciences 26, no. 7: 3015. https://doi.org/10.3390/ijms26073015
APA StyleMonaco, M., Torazza, C., Fedele, E., & Grilli, M. (2025). The Impact of the Exposome on Alzheimer’s Disease: The Influence of Nutrition. International Journal of Molecular Sciences, 26(7), 3015. https://doi.org/10.3390/ijms26073015








