Sublethal Concentrations of 2C-I and 25I-NBOMe Designer Drugs Impact Caenorhabditis elegans Development and Reproductive Behavior
Abstract
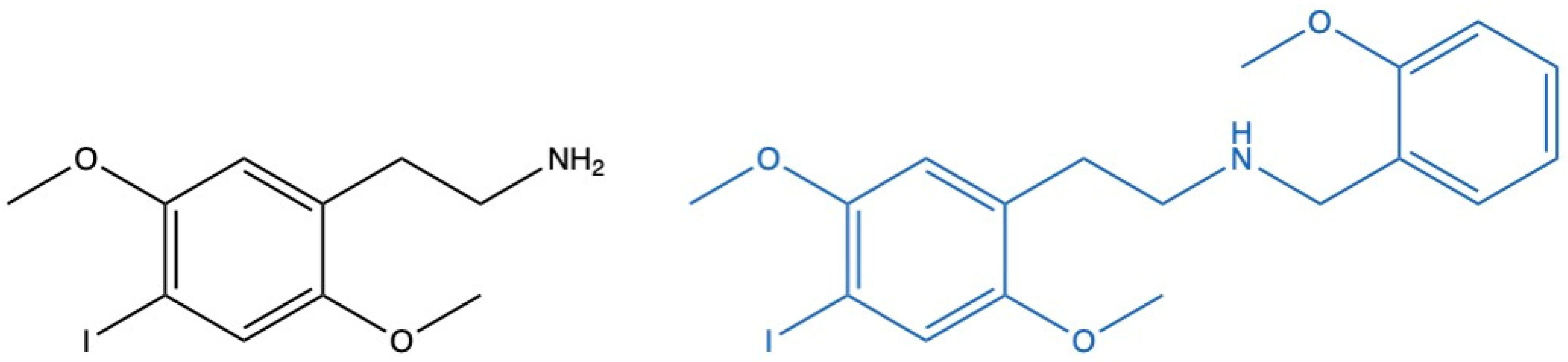
1. Introduction
2. Results
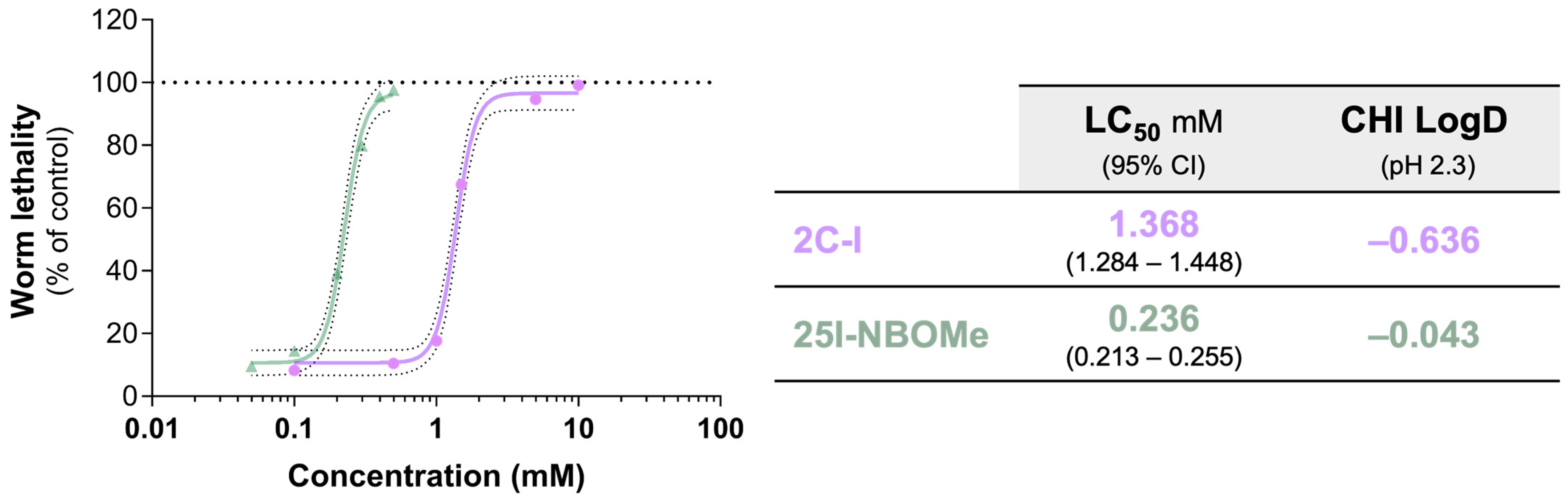
2.1. Impact of 2C-I and 25I-NBOMe on C. elegans Survival
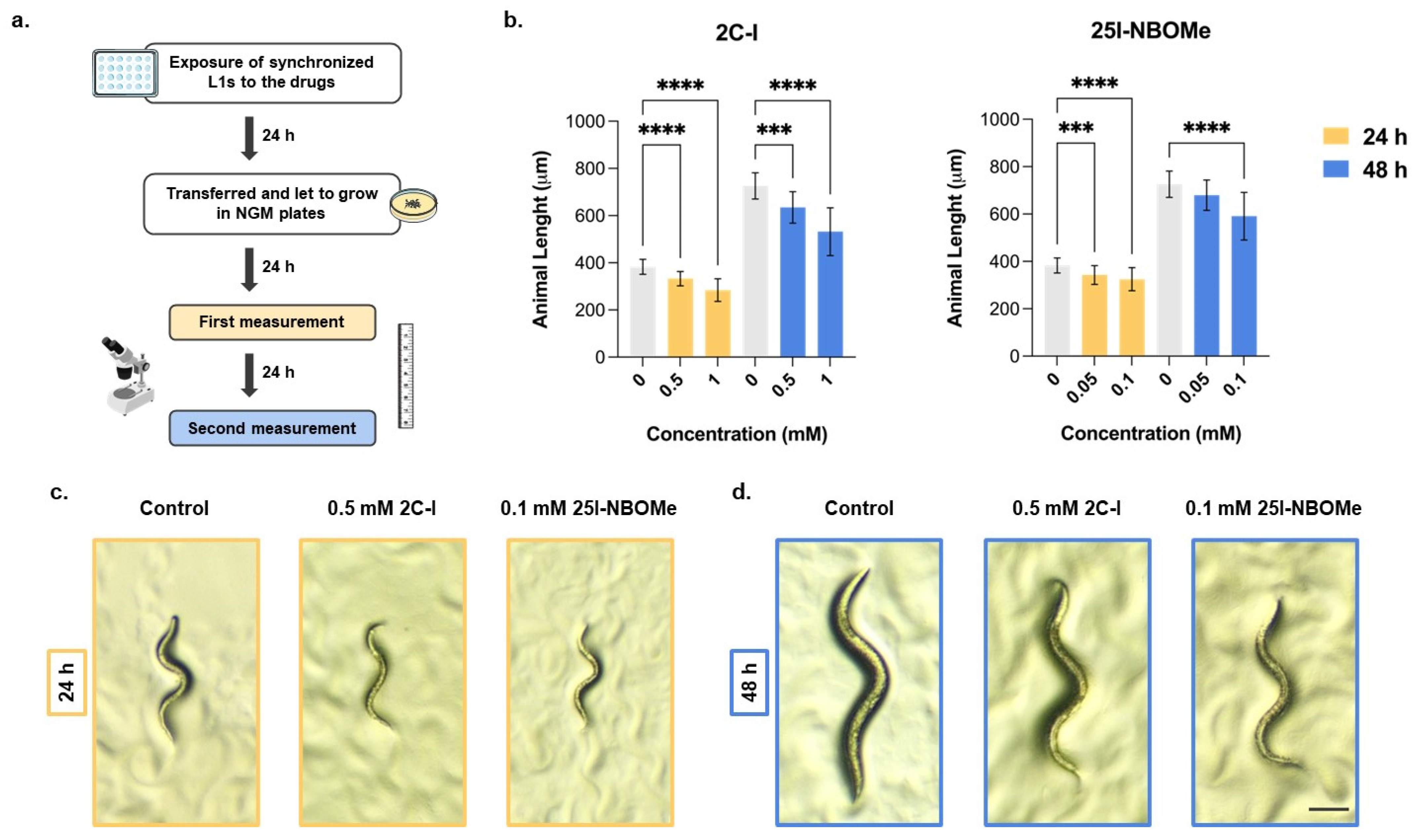
2.2. Impact of 2C-I and 25I-NBOMe on C. elegans Development over Time
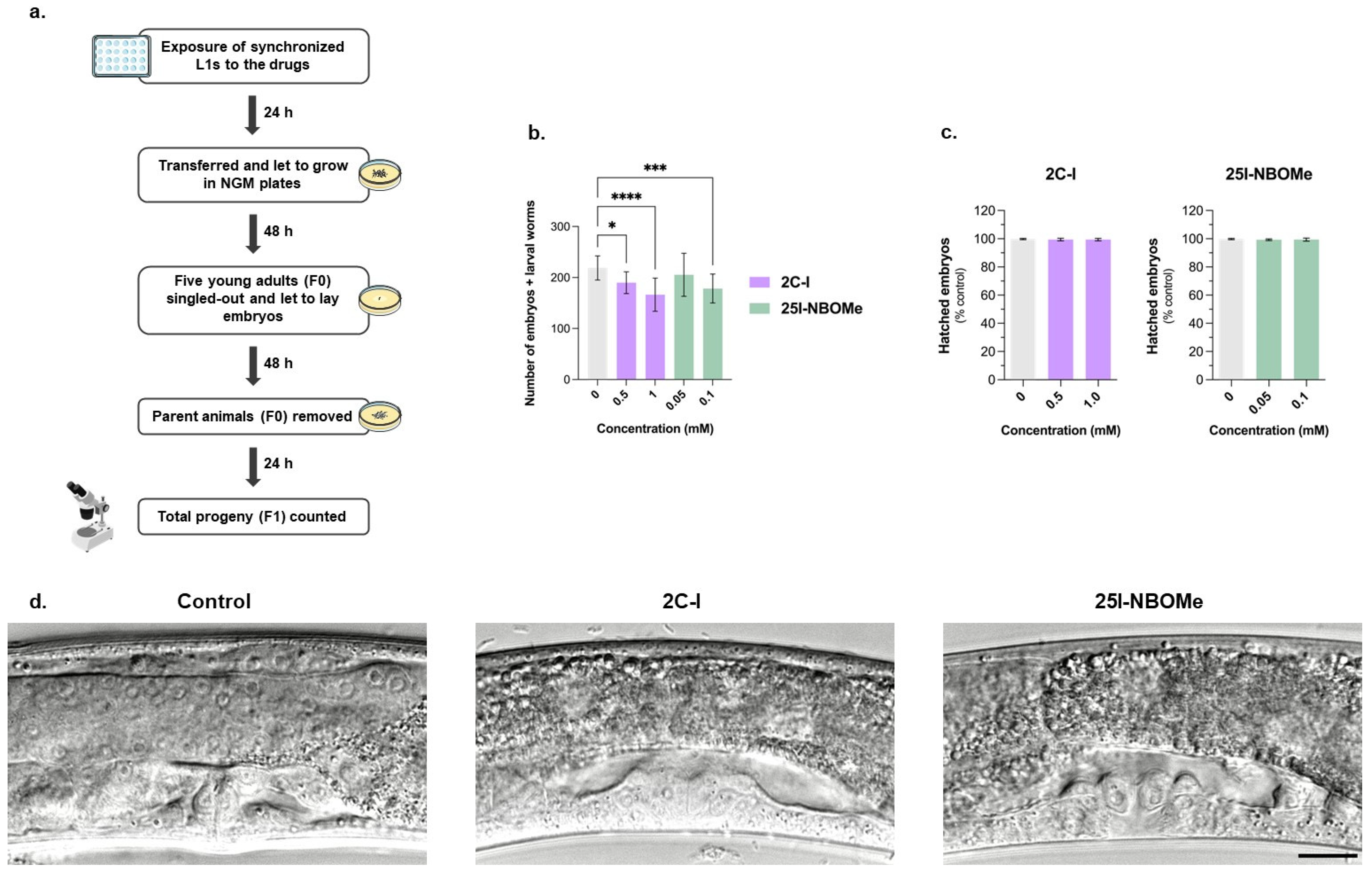
2.3. Impact of 2C-I and 25I-NBOMe on C. elegans Reproductive Behavior
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. C. elegans Maintenance
4.3. Synchronization at the L1 Stage
4.4. Drugs Exposure
4.5. Animal Length
4.6. Embryonic Viability and Brood Size
4.7. Differential Interference Contrast (DIC) Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simao, A.Y.; Antunes, M.; Cabral, E.; Oliveira, P.; Rosendo, L.M.; Brinca, A.T.; Alves, E.; Marques, H.; Rosado, T.; Passarinha, L.A.; et al. An Update on the Implications of New Psychoactive Substances in Public Health. Int. J. Environ. Res. Public Health 2022, 19, 4869. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. Annual Report 2009: The State of the Drugs Problem in Europe; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Shulgin, A.; Shulgin, A. PIHKAL: A Chemical Love Story; Transform Press: Berkeley, CA, USA, 1991. [Google Scholar]
- Heim, R. Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur: Entwicklung eines neuen Struktur-Wirkungskonzepts. Ph.D. Thesis, Freie Universität, Berlin, Germany, 2004. [Google Scholar]
- Elmore, J.S.; Decker, A.M.; Sulima, A.; Rice, K.C.; Partilla, J.S.; Blough, B.E.; Baumann, M.H. Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats. Neuropharmacology 2018, 142, 240–250. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 2014, 77, 200–207. [Google Scholar] [CrossRef]
- Herian, M.; Wojtas, A.; Kaminska, K.; Swit, P.; Wach, A.; Golembiowska, K. Hallucinogen-Like Action of the Novel Designer Drug 25I-NBOMe and Its Effect on Cortical Neurotransmitters in Rats. Neurotox. Res. 2019, 36, 91–100. [Google Scholar] [CrossRef]
- Herian, M.; Wojtas, A.; Sobocinska, M.K.; Skawski, M.; Gonzalez-Marin, A.; Golembiowska, K. Contribution of serotonin receptor subtypes to hallucinogenic activity of 25I-NBOMe and to its effect on neurotransmission. Pharmacol. Rep. 2020, 72, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Kim, Y.H.; Kim, S.J.; Suh, S.K.; Cha, H.J. Abuse potential of 2-(4-iodo-2, 5-dimethoxyphenyl)N-(2-methoxybenzyl)ethanamine (25INBOMe); in vivo and ex vivo approaches. Neurochem. Int. 2019, 125, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bosak, A.; LoVecchio, F.; Levine, M. Recurrent seizures and serotonin syndrome following “2C-I” ingestion. J. Med. Toxicol. 2013, 9, 196–198. [Google Scholar] [CrossRef]
- Drees, J.C.; Stone, J.A.; Wu, A.H. Morbidity involving the hallucinogenic designer amines MDA and 2C-I. J. Forensic. Sci. 2009, 54, 1485–1487. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Angerer, V.; Kithinji, J.; Grumann, C.; Auwarter, V. Bad trip due to 25I-NBOMe: A case report from the EU project SPICE II plus. Clin. Toxicol. 2017, 55, 922–924. [Google Scholar] [CrossRef]
- Kueppers, V.B.; Cooke, C.T. 25I-NBOMe related death in Australia: A case report. Forensic. Sci. Int. 2015, 249, e15–e18. [Google Scholar] [CrossRef]
- Lowe, L.M.; Peterson, B.L.; Couper, F.J. A Case Review of the First Analytically Confirmed 25I-NBOMe-Related Death in Washington State. J. Anal. Toxicol. 2015, 39, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Shanks, K.G.; Sozio, T.; Behonick, G.S. Fatal Intoxications with 25B-NBOMe and 25I-NBOMe in Indiana During 2014. J. Anal. Toxicol. 2015, 39, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Poklis, J.L.; Poklis, A. “My friend said it was good LSD”: A suicide attempt following analytically confirmed 25I-NBOMe ingestion. J. Psychoact. Drugs 2014, 46, 379–382. [Google Scholar] [CrossRef]
- Thornton, S.L.; Hoehn, S.; Gerona, R.R. Seizures, Systemic Inflammatory Response, and Rhabdomyolysis Associated With Laboratory-Confirmed 2C-I and 25-I Exposure. Pediatr. Emerg. Care 2018, 34, e181–e183. [Google Scholar] [CrossRef]
- Gil-Martins, E.; Cagide-Fagín, F.; Martins, D.; Borer, A.; Barbosa, D.J.; Fernandes, C.; Chavarria, D.; Remião, F.; Borges, F.; Silva, R. Mechanistic Insights into the Neurotoxicity of 2,5-Dimethoxyphenethylamines (2C) and Corresponding N-(2-methoxybenzyl)phenethylamine (NBOMe) Drugs. J. Xenobiot. 2024, 14, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Gil-Martins, E.; Cagide, F.; da Fonseca, C.; Benfeito, S.; Fernandes, C.; Chavarria, D.l.; Remião, F.; Silva, R.; Borges, F. Unraveling the In Vitro Toxicity Profile of Psychedelic 2C Phenethylamines and Their N-Benzylphenethylamine (NBOMe) Analogues. Pharmaceuticals 2023, 16, 1158. [Google Scholar] [CrossRef]
- Rickli, A.; Luethi, D.; Reinisch, J.; Buchy, D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs). Neuropharmacology 2015, 99, 546–553. [Google Scholar] [CrossRef]
- Gil-Martins, E.; Cagide, F.; Borer, A.; Barbosa, D.J.; Fernandes, C.; Chavarria, D.; Remião, F.; Borges, F.; Silva, R. The Role of Mitochondrial Dysfunction and Calcium Dysregulation in 2C-I and 25I-NBOMe-Induced Neurotoxicity. Chem. Biol. Interact. 2025, 411, 111425. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Anal. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef]
- Tejeda-Benitez, L.; Olivero-Verbel, J. Caenorhabditis elegans, a Biological Model for Research in Toxicology. Rev. Environ. Contam. Toxicol. 2016, 237, 1–35. [Google Scholar] [CrossRef]
- Nyaanga, J.; Goss, C.; Zhang, G.; Ahmed, H.N.; Andersen, E.J.; Miller, I.R.; Rozenich, J.K.; Swarthout, I.L.; Vaughn, J.A.; Mangan, N.M.; et al. Changes in body shape implicate cuticle stretch in C. elegans growth control. Cells. Dev. 2022, 170, 203780. [Google Scholar] [CrossRef] [PubMed]
- Engleman, E.A.; Katner, S.N.; Neal-Beliveau, B.S. Caenorhabditis elegans as a Model to Study the Molecular and Genetic Mechanisms of Drug Addiction. Prog. Mol. Biol. Transl. Sci. 2016, 137, 229–252. [Google Scholar] [CrossRef]
- Wittkowski, P.; Marx-Stoelting, P.; Violet, N.; Fetz, V.; Schwarz, F.; Oelgeschläger, M.; Schönfelder, G.; Vogl, S. Caenorhabditis elegans As a Promising Alternative Model for Environmental Chemical Mixture Effect Assessment—A Comparative Study. Environ. Sci. Technol. 2019, 53, 12725–12733. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Pears, C.; Woollard, A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017, 7, 9839. [Google Scholar] [CrossRef]
- Harlow, P.H.; Perry, S.J.; Widdison, S.; Daniels, S.; Bondo, E.; Lamberth, C.; Currie, R.A.; Flemming, A.J. The nematode Caenorhabditis elegans as a tool to predict chemical activity on mammalian development and identify mechanisms influencing toxicological outcome. Sci. Rep. 2016, 6, 22965. [Google Scholar] [CrossRef]
- Alvarez-Alarcon, N.; Osorio-Mendez, J.J.; Ayala-Fajardo, A.; Garzon-Mendez, W.F.; Garavito-Aguilar, Z.V. Zebrafish and Artemia salina in vivo evaluation of the recreational 25C-NBOMe drug demonstrates its high toxicity. Toxicol. Rep. 2021, 8, 315–323. [Google Scholar] [CrossRef]
- de Barros, W.A.; Nunes, C.D.S.; Souza, J.; Nascimento, I.; Figueiredo, I.M.; de Aquino, T.M.; Vieira, L.; Farias, D.; Santos, J.C.C.; de Fatima, A. The new psychoactive substances 25H-NBOMe and 25H-NBOH induce abnormal development in the zebrafish embryo and interact in the DNA major groove. Curr. Res. Toxicol. 2021, 2, 386–398. [Google Scholar] [CrossRef]
- Kawahara, G.; Maeda, H.; Kikura-Hanajiri, R.; Yoshida, K.I.; Hayashi, Y.K. The psychoactive drug 25B-NBOMe recapitulates rhabdomyolysis in zebrafish larvae. Forensic. Toxicol. 2017, 35, 369–375. [Google Scholar] [CrossRef]
- Sammi, S.R.; Jameson, L.E.; Conrow, K.D.; Leung, M.C.K.; Cannon, J.R. Caenorhabditis elegans Neurotoxicity Testing: Novel Applications in the Adverse Outcome Pathway Framework. Front. Toxicol. 2022, 4, 826488. [Google Scholar] [CrossRef]
- Neicun, J.; Yang, J.C.; Shih, H.; Nadella, P.; van Kessel, R.; Negri, A.; Czabanowska, K.; Brayne, C.; Roman-Urrestarazu, A. Lifetime prevalence of novel psychoactive substances use among adults in the USA: Sociodemographic, mental health and illicit drug use correlates. Evidence from a population-based survey 2007–2014. PLoS ONE 2020, 15, e0241056. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. ESPAD Report 2019: Results from the European School Survey Project on Alcohol. and Other Drugs; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hamidullah, S.; Thorpe, H.H.A.; Frie, J.A.; McCurdy, R.D.; Khokhar, J.Y. Adolescent Substance Use and the Brain: Behavioral, Cognitive and Neuroimaging Correlates. Front. Hum. Neurosci. 2020, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- ELNahas, G.; Thibaut, F. Perinatal Psychoactive Substances Use: A Rising Perinatal Mental Health Concern. J. Clin. Med. 2023, 12, 2175. [Google Scholar] [CrossRef]
- Hayer, S.; Garg, B.; Wallace, J.; Prewitt, K.C.; Lo, J.O.; Caughey, A.B. Prenatal methamphetamine use increases risk of adverse maternal and neonatal outcomes. Am. J. Obstet. Gynecol. 2024, 231, e351–e356. [Google Scholar] [CrossRef]
- Hall, D.H.; Altun, Z.F.C. elegans Atlas; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2007. [Google Scholar]
- Bora, S.; Vardhan, G.S.H.; Deka, N.; Khataniar, L.; Gogoi, D.; Baruah, A. Paraquat exposure over generation affects lifespan and reproduction through mitochondrial disruption in C. elegans. Toxicology 2021, 447, 152632. [Google Scholar] [CrossRef]
- Souto, C.; Goethel, G.; Peruzzi, C.P.; Cestonaro, L.V.; Garcia, I.; Avila, D.S.; Eifler-Lima, V.; Carmo, H.; Bastos, M.L.; Garcia, S.C.; et al. Piperazine designer drugs elicit toxicity in the alternative in vivo model Caenorhabditis elegans. J. Anal. Toxicol. 2020, 40, 363–372. [Google Scholar] [CrossRef]
- Wang, S.; Chu, Z.; Zhang, K.; Miao, G. Cadmium-induced serotonergic neuron and reproduction damages conferred lethality in the nematode Caenorhabditis elegans. Chemosphere 2018, 213, 11–18. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Zhang, J.; Cheng, J.; Lin, J.; Peng, Y.; Liu, Y.; Zhang, C.; Yan, J.; Niu, S. Toxic Effects of Methamphetamine on Reproductive Systems, Embryo Development, and Newborns: An Evidence-Based Review. Curr. Med. Chem. 2024, 29, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Borumandnia, N.; Majd, H.A.; Khadembashi, N.; Alaii, H. Assessing the Trend of Infertility Rate in 198 Countries and Territories in Last Decades. Iran. J. Public. Health. 2021, 50, 1735–1737. [Google Scholar] [CrossRef]
- Sternberg, P.W. Vulval development. WormBook 2005, 1–28. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2024: Trends and Developments; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Yücel, D. Ketamine induces apical extracellular matrix modifications in Caenorhabditis elegans. Sci. Rep. 2022, 12, 22122. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef] [PubMed]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Johnson, R.A.; Janowsky, A. Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT(2A) receptors. Biochem. Pharmacol. 2018, 158, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Oami, E.; Kutsuna, N.; Ishiura, S.; Suo, S. Dopamine regulates body size in Caenorhabditis elegans. Dev. Biol. 2016, 412, 128–138. [Google Scholar] [CrossRef]
- Schreiber, M.A.; McIntire, S.L. A Caenorhabditis elegans p38 MAP kinase pathway mutant protects from dopamine, methamphetamine, and MDMA toxicity. Neurosci. Lett. 2011, 498, 99–103. [Google Scholar] [CrossRef]
| Control | 2C-I | 25I-NBOMe | ||
| 0 mM | 0.5 mM | 1.0 mM | 0.05 mM | 0.10 mM |
| Mean survival rate (%) | ||||
| 95.83 | 89.51 | 82.53 ** | 90.44 | 85.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Martins, E.; Barbosa, D.J.; Cagide, F.; Remião, F.; Borges, F.; Silva, R. Sublethal Concentrations of 2C-I and 25I-NBOMe Designer Drugs Impact Caenorhabditis elegans Development and Reproductive Behavior. Int. J. Mol. Sci. 2025, 26, 3039. https://doi.org/10.3390/ijms26073039
Gil-Martins E, Barbosa DJ, Cagide F, Remião F, Borges F, Silva R. Sublethal Concentrations of 2C-I and 25I-NBOMe Designer Drugs Impact Caenorhabditis elegans Development and Reproductive Behavior. International Journal of Molecular Sciences. 2025; 26(7):3039. https://doi.org/10.3390/ijms26073039
Chicago/Turabian StyleGil-Martins, Eva, Daniel José Barbosa, Fernando Cagide, Fernando Remião, Fernanda Borges, and Renata Silva. 2025. "Sublethal Concentrations of 2C-I and 25I-NBOMe Designer Drugs Impact Caenorhabditis elegans Development and Reproductive Behavior" International Journal of Molecular Sciences 26, no. 7: 3039. https://doi.org/10.3390/ijms26073039
APA StyleGil-Martins, E., Barbosa, D. J., Cagide, F., Remião, F., Borges, F., & Silva, R. (2025). Sublethal Concentrations of 2C-I and 25I-NBOMe Designer Drugs Impact Caenorhabditis elegans Development and Reproductive Behavior. International Journal of Molecular Sciences, 26(7), 3039. https://doi.org/10.3390/ijms26073039











