Sex-Specific Anti-Inflammatory Effects of a Ketogenic Diet in a Mouse Model of Allergic Airway Inflammation
Abstract
:1. Introduction
2. Results
2.1. Impact of Ketogenic Diet on Allergen-Induced Mice
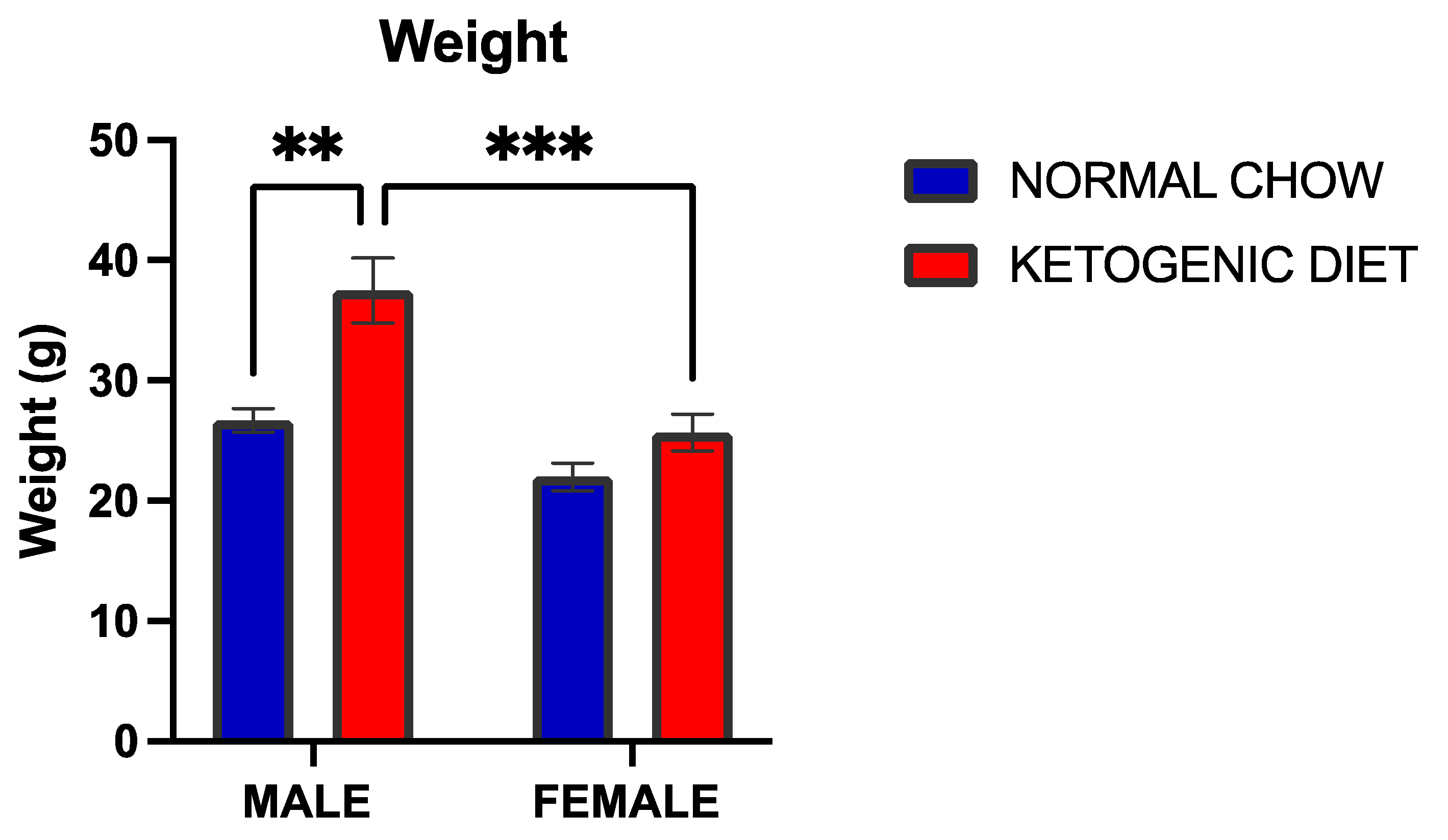
2.1.1. A Ketogenic Diet Induced a Significant Weight Gain in Allergen-Induced Male but Not Female Mice
2.1.2. Alveolar Macrophages Were Elevated in Allergen-Exposed Mice Fed a Ketogenic Diet
2.1.3. Eosinophils Were Reduced in Allergen-Induced Male Mice Fed a Ketogenic Diet
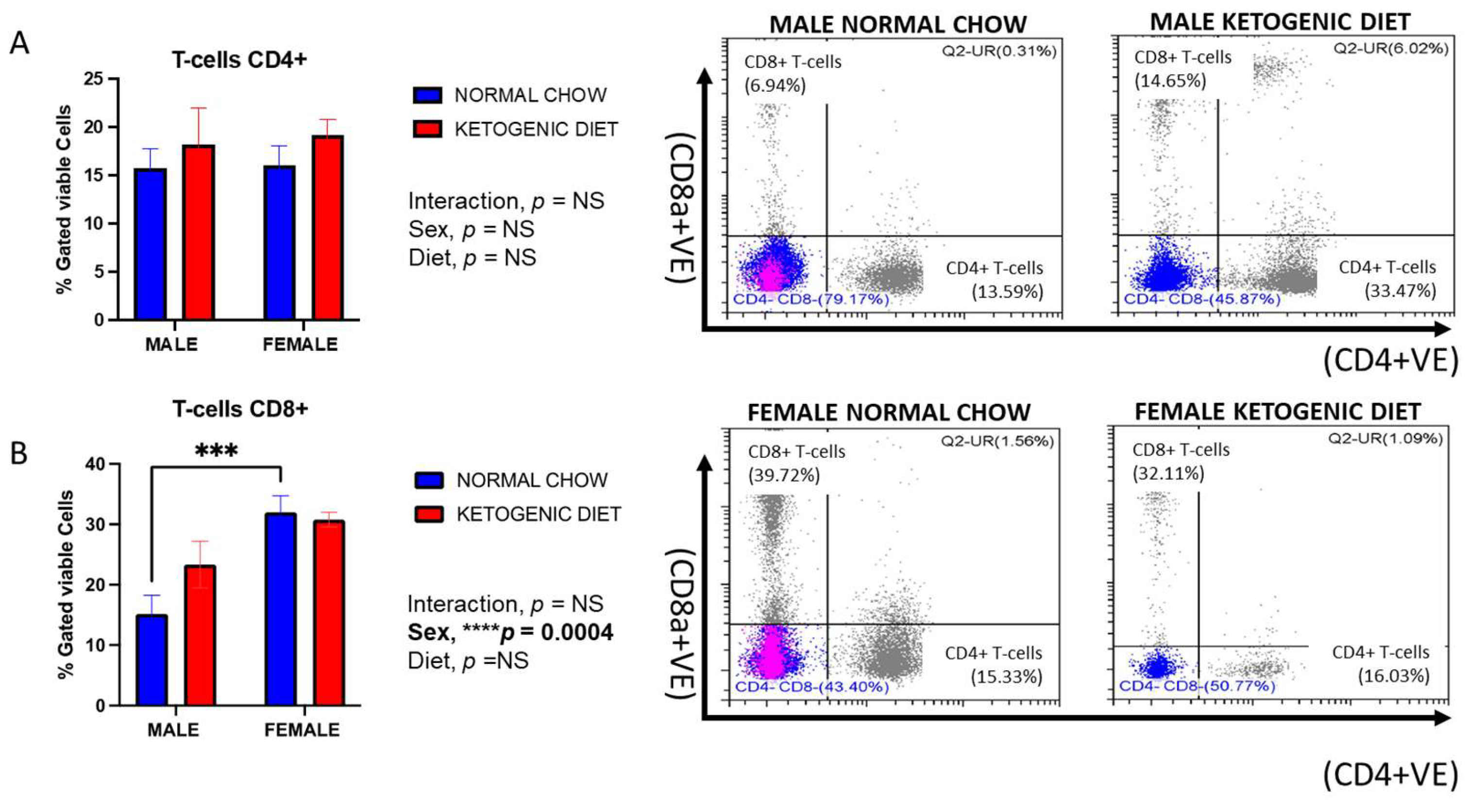
2.1.4. Sex Differences in CD8+ but Not CD4+ Lung T-Cells in Allergen-Induced Mice
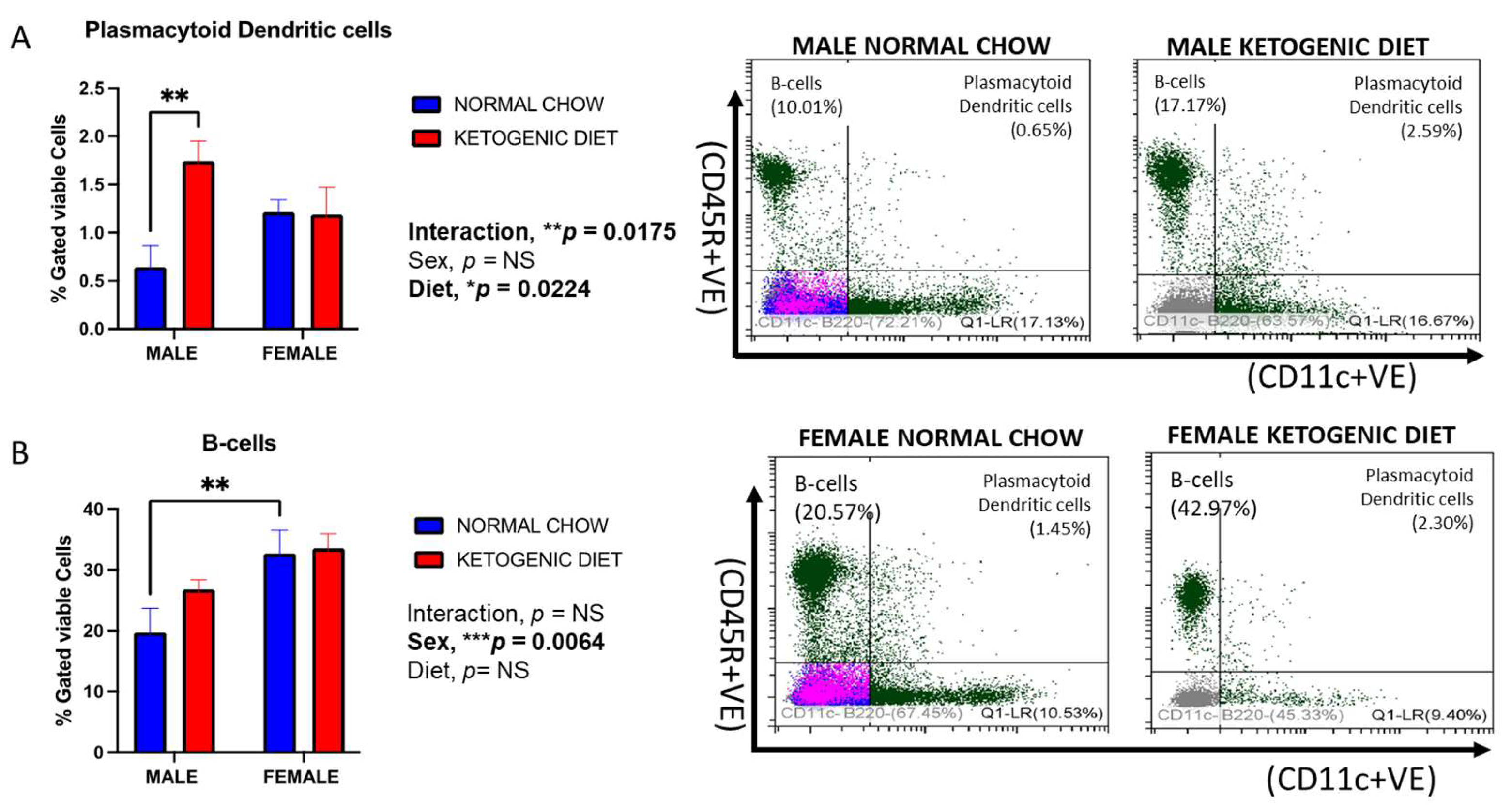
2.1.5. Sex Differences in Plasmacytoid Dendritic Cells in Response to a Ketogenic Diet and After Allergen Exposure
2.1.6. Sex Differences in B-Cells in Response to Ketogenic Diet After Allergen Exposure
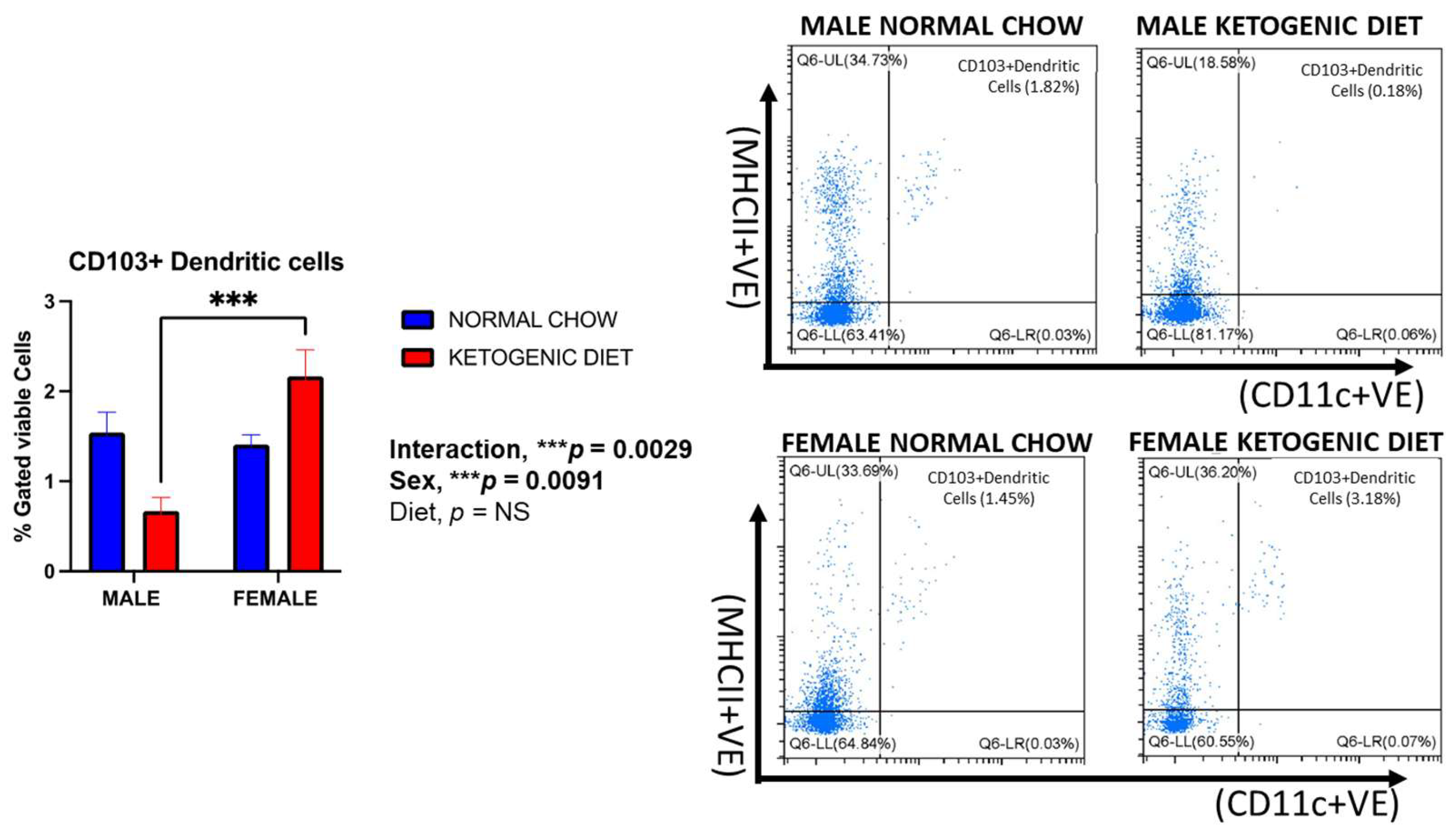
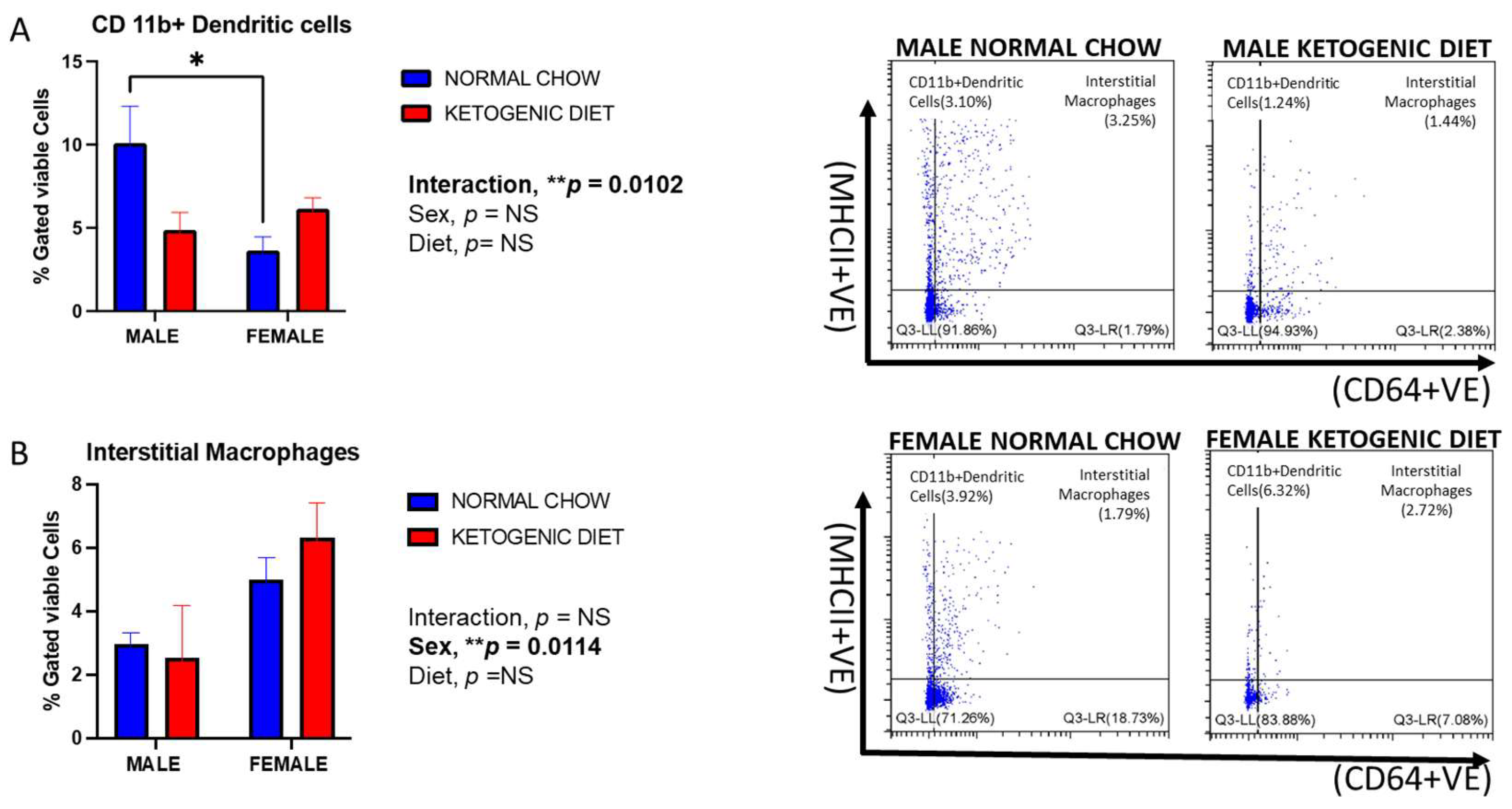
2.1.7. Sex Differences in CD103+ and CD11b+ Dendritic Cells in Allergen-Induced Mice Fed a Ketogenic Diet
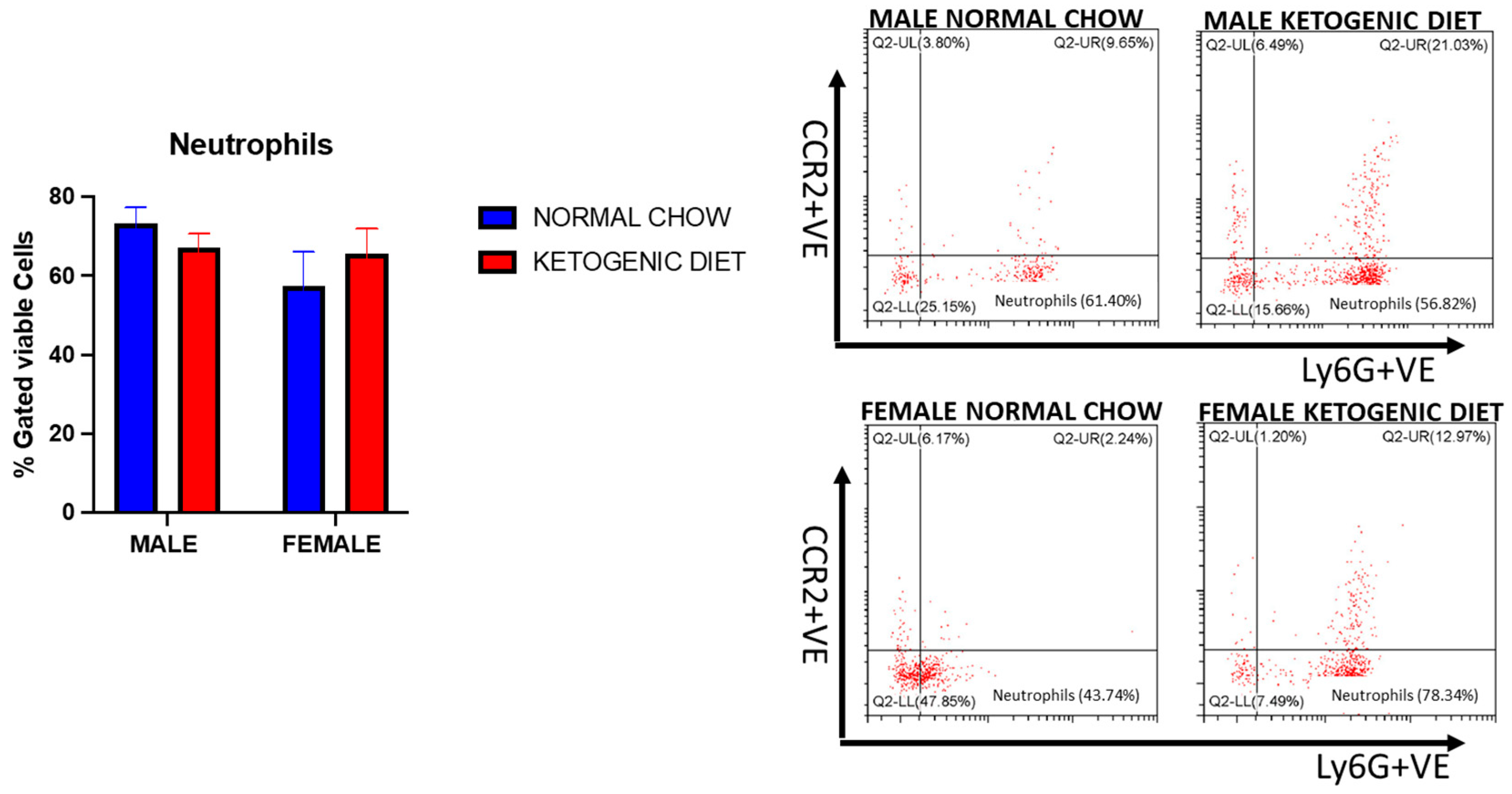
2.1.8. Sex Differences and Ketogenic Diet Effects on Lung Interstitial Alveolar Macrophages, Undifferentiated Monocytes, and Neutrophils in Allergen-Induced Mice
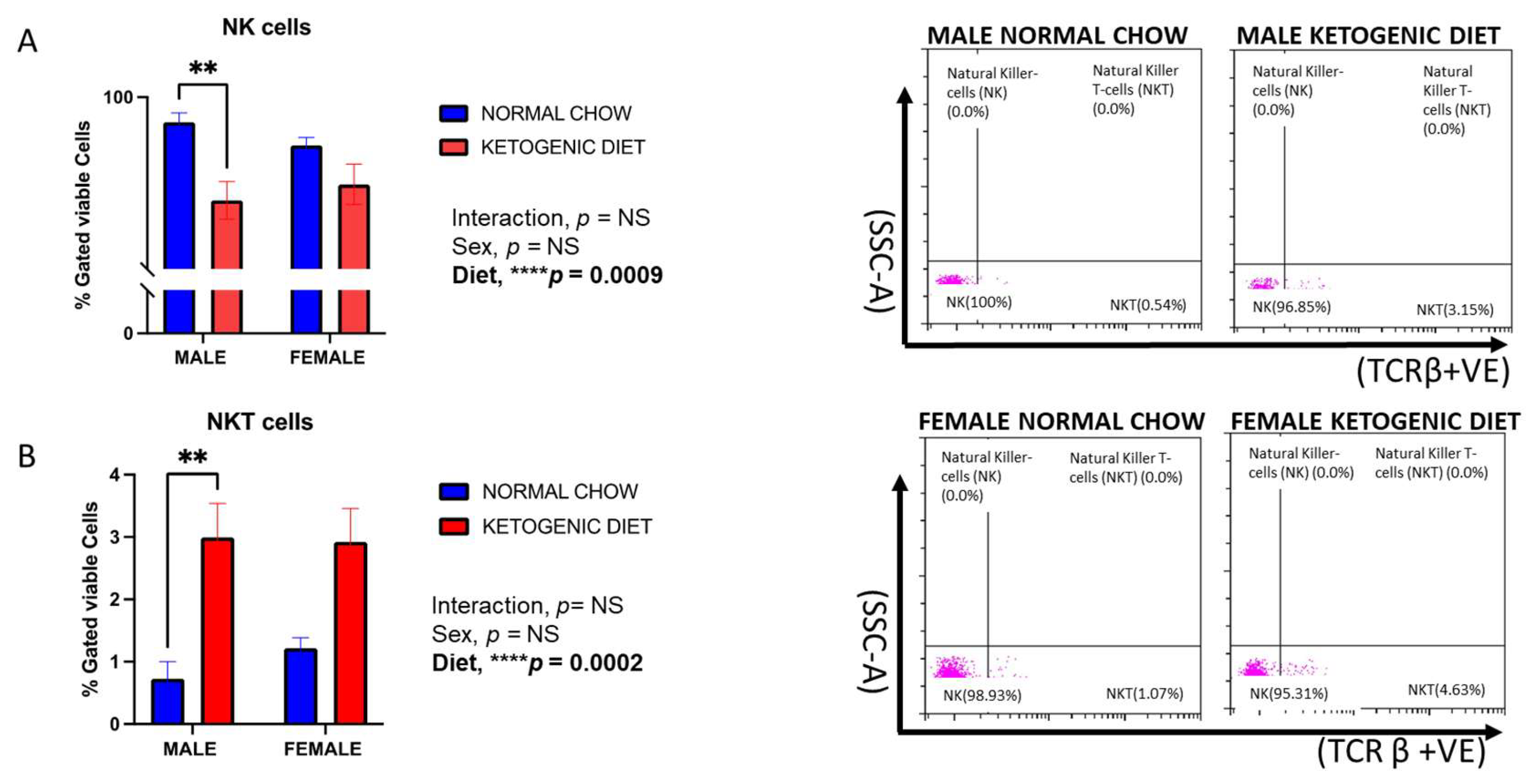
2.1.9. Natural Killer (NK) Cells and Natural Killer T-Cells (NKT) Response in Allergen-Induced Mice Fed a Ketogenic Diet
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Model
4.3. Diet Protocol
4.4. HDM Challenge
4.5. Isolation of Immune Cells and Flow Cytometry
4.6. Gating Strategy
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekpruke, C.D.; Silveyra, P. Sex Differences in Airway Remodeling and Inflammation: Clinical and Biological Factors. Front. Allergy 2022, 3, 875295. [Google Scholar]
- Naeem, A.; Silveyra, P. Sex Differences in Paediatric and Adult Asthma. Eur. Med. J. 2019, 4, 27–35. [Google Scholar]
- McKeever, T.M.; Britton, J. Diet and asthma. Am. J. Respir. Crit. Care Med. 2004, 170, 725–729. [Google Scholar]
- Romieu, I. Nutrition and lung health [State of the Art]. Int. J. Tuberc. Lung Dis. 2005, 9, 362–374. [Google Scholar]
- Lee, S.-C.; Yang, Y.-H.; Chuang, S.-Y.; Liu, S.-C.; Yang, H.-C.; Pan, W.-H. Risk of asthma associated with energy-dense but nutrient-poor dietary pattern in Taiwanese children. Asia Pac. J. Clin. Nutr. 2012, 21, 73–81. [Google Scholar] [PubMed]
- Dixon, A.E.; Holguin, F. Diet and metabolism in the evolution of asthma and obesity. Clin. Chest Med. 2019, 40, 97–106. [Google Scholar]
- Percopo, C.M.; McCullough, M.; Limkar, A.R.; Druey, K.M.; Rosenberg, H.F. Impact of controlled high-sucrose and high-fat diets on eosinophil recruitment and cytokine content in allergen-challenged mice. PLoS ONE 2021, 16, e0255997. [Google Scholar] [CrossRef]
- Li, Q.; Baines, K.J.; Gibson, P.G.; Wood, L.G. Changes in expression of genes regulating airway inflammation following a high-fat mixed meal in asthmatics. Nutrients 2016, 8, 30. [Google Scholar] [CrossRef]
- Wood, L.G.; Garg, M.L.; Gibson, P.G. A high-fat challenge increases airway inflammation and impairs bronchodilator recovery in asthma. J. Allergy Clin. Immunol. 2011, 127, 1133–1140. [Google Scholar]
- Spector, S.L.; Surette, M.E. Diet and asthma: Has the role of dietary lipids been overlooked in the management of asthma? Ann. Allergy Asthma Immunol. 2003, 90, 371–377. [Google Scholar]
- Waldman, H.S.; Heatherly, A.J.; Killen, L.G.; Hollingsworth, A.; Koh, Y.; O’Neal, E.K. A 3-Week, Low-Carbohydrate, High-Fat Diet Improves Multiple Serum Inflammatory Markers in Endurance-Trained Males. J. Strength Cond. Res. 2022, 36, 2502–2508. [Google Scholar] [CrossRef] [PubMed]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Florence, T.M. The role of free radicals in disease. Aust. N. Z. J. Ophthalmol. 1995, 23, 3–7. [Google Scholar] [CrossRef]
- Cheng, B.; Lu, H.; Bai, B.; Chen, J. d-β-Hydroxybutyrate inhibited the apoptosis of PC12 cells induced by H2O2 via inhibiting oxidative stress. Neurochem. Int. 2013, 62, 620–625. [Google Scholar] [CrossRef]
- Haces, M.L.; Hernández-Fonseca, K.; Medina-Campos, O.N.; Montiel, T.; Pedraza-Chaverri, J.; Massieu, L. Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp. Neurol. 2008, 211, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef]
- Alessandro, R.; Gerardo, B.; Alessandra, L.; Lorenzo, C.; Andrea, P.; Keith, G.; Yang, Z.; Antonio, P. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung 2015, 193, 939–945. [Google Scholar] [CrossRef]
- Feldman, D.; Huggins, S.; Norwitz, N.G. Short-term hyper-caloric high-fat feeding on a ketogenic diet can lower low-density lipoprotein cholesterol: The cholesterol drop experiment. Curr. Opin. Endocrinol. Diabetes 2022, 29, 434–439. [Google Scholar] [CrossRef]
- Ludwig, D.S. The Ketogenic Diet: Evidence for Optimism but High-Quality Research Needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar] [CrossRef]
- Walton, C.M.; Perry, K.; Hart, R.H.; Berry, S.L.; Bikman, B.T. Improvement in Glycemic and Lipid Profiles in Type 2 Diabetics with a 90-Day Ketogenic Diet. J. Diabetes Res. 2019, 2019, 8681959. [Google Scholar]
- Eneli, I.U.; Skybo, T.; A Camargo, C. Weight loss and asthma: A systematic review. Thorax 2008, 63, 671–676. [Google Scholar] [PubMed]
- Beuther, D.A.; Sutherland, E.R. Overweight, obesity, and incident asthma: A meta-analysis of prospective epidemiologic studies. Am. J. Respir. Crit. Care Med. 2007, 175, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, I.; Williams, L.; Thompson, C.; Scott, H.; Wood, L. Evidence for lifestyle interventions in asthma. Breathe 2019, 15, e50–e61. [Google Scholar]
- Abbasi, J. Interest in the ketogenic diet grows for weight loss and type 2 diabetes. JAMA 2018, 319, 215–217. [Google Scholar] [PubMed]
- Mank, M.M.; Reed, L.F.; Walton, C.J.; Barup, M.L.T.; Ather, J.L.; Poynter, M.E. Therapeutic ketosis decreases methacholine hyperresponsiveness in mouse models of inherent obese asthma. Am. J. Physiol. Cell. Mol. Physiol. 2022, 322, L243–L257. [Google Scholar]
- Commodore, S.; Ekpruke, C.D.; Rousselle, D.; Alford, R.; Babayev, M.; Sharma, S.; Buechlein, A.; Rusch, D.B.; Silveyra, P. Lung proinflammatory microRNA and cytokine expression in a mouse model of allergic inflammation: Role of sex chromosome complement and gonadal hormones. Physiol. Genom. 2024, 56, 179–193. [Google Scholar]
- Ekpruke, C.D.; Alford, R.; Parker, E.; Silveyra, P. Gonadal sex and chromosome complement influence the gut microbiome in a mouse model of allergic airway inflammation. Physiol. Genom. 2024, 56, 417–425. [Google Scholar] [CrossRef]
- Ekpruke, C.D.; Alford, R.; Rousselle, D.; Babayev, M.; Sharma, S.; Commodore, S.; Buechlein, A.; Rusch, D.B.; Silveyra, P. Transcriptomics analysis of allergen-induced inflammatory gene expression in the Four-Core Genotype mouse model. Physiol. Genom. 2024, 56, 235–245. [Google Scholar]
- Hsu, J.; Chen, J.; Mirabelli, M.C. Asthma Morbidity, Comorbidities, and Modifiable Factors Among Older Adults. J. Allergy Clin. Immunol. Pract. 2017, 6, 236–243.e7. [Google Scholar]
- Sood, A. Sex differences: Implications for the obesity-asthma association. Exerc. Sport Sci. Rev. 2011, 39, 48–56. [Google Scholar] [PubMed]
- Miles, K.N.; Skelton, M.R. Male mice placed on a ketogenic diet from postnatal day (P) 21 through adulthood have reduced growth, are hypoactive, show increased freezing in a conditioned fear paradigm, and have spatial learning deficits. Brain Res. 2020, 1734, 146697. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Pissios, P.; Otu, H.; Xue, B.; Asakura, K.; Furukawa, N.; Marino, F.E.; Liu, F.-F.; Kahn, B.B.; Libermann, T.A.; et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Metab. 2007, 292, E1724–E1739. [Google Scholar]
- Byrne, F.L.; Hargett, S.R.; Lahiri, S.; Roy, R.J.; Berr, S.S.; Caldwell, S.H.; Hoehn, K.L. Serial MRI Imaging Reveals Minimal Impact of Ketogenic Diet on Established Liver Tumor Growth. Cancers 2018, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ding, J.; Wu, X.; Huang, Z.; Kong, G.; Liu, Q.; Yang, Z.; Huang, Z.; Zhu, Q. Bone microstructure and metabolism changes under the combined intervention of ketogenic diet with intermittent fasting: An in vivo study of rats. Exp. Anim. 2019, 68, 371–380. [Google Scholar]
- A LaFountain, R.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; A Short, J.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Varley, B.J.; Hartwig, T.B.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar]
- Zengin, A.; Kropp, B.; Chevalier, Y.; Junnila, R.; Sustarsic, E.; Herbach, N.; Fanelli, F.; Mezzullo, M.; Milz, S.; Bidlingmaier, M.; et al. Low-carbohydrate, high-fat diets have sex-specific effects on bone health in rats. Eur. J. Nutr. 2016, 55, 2307–2320. [Google Scholar]
- Guidance Development Review Committee; Working Group for Clinical Studies of Cancer Immunotherapy; Working Group for Effector Cell Therapy; Working Group for CMC/Non-clinical Studies; Working Group for Cancer Vaccines and Adjuvants; Working Group for Anti-immune Checkpoint Therapy and Comprehensive Cancer Immunotherapy; Subcommittee, B. 2015 Guidance on cancer immunotherapy development in early-phase clinical studies. Cancer Sci. 2015, 106, 1761–1771. [Google Scholar]
- Chen, Z.; Huang, A.; Sun, J.; Jiang, T.; Qin, F.X.-F.; Wu, A. Inference of immune cell composition on the expression profiles of mouse tissue. Sci. Rep. 2017, 7, 40508. [Google Scholar]
- Li, Q.-X.; Feuer, G.; Ouyang, X.; An, X. Experimental animal modeling for immuno-oncology. Pharmacol. Ther. 2017, 173, 34–46. [Google Scholar] [PubMed]
- Hahne, F.; Khodabakhshi, A.H.; Bashashati, A.; Wong, C.; Gascoyne, R.D.; Weng, A.P.; Seyfert-Margolis, V.; Bourcier, K.; Asare, A.; Lumley, T.; et al. Per-channel basis normalization methods for flow cytometry data. Cytom. Part A J. Int. Soc. Adv. Cytom. 2009, 77, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; van Nimwegen, M.; Willart, M.A.M.; Muskens, F.; Boon, L.; Smit, J.J.; Coyle, A.; Clausen, B.E.; Hoogsteden, H.C.; Lambrecht, B.N.; et al. An Anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 2009, 183, 1074–1082. [Google Scholar] [PubMed]
- Liu, Y.-J. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005, 23, 275–306. [Google Scholar]
- Chen, J.; Flurkey, K.; E Harrison, D. A reduced peripheral blood CD4+ lymphocyte proportion is a consistent ageing phenotype. Mech. Ageing Dev. 2002, 123, 145–153. [Google Scholar] [CrossRef]
- Chen, J.; Harrison, D.E. Quantitative trait loci regulating relative lymphocyte proportions in mouse peripheral blood. Blood The J. Am. Soc. Hematol. 2002, 99, 561–566. [Google Scholar]
- Peters, L.L.; Cheever, E.M.; Ellis, H.R.; Magnani, P.A.; Svenson, K.L.; Von Smith, R.; Bogue, M.A. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol. Genom. 2002, 11, 185–193. [Google Scholar]
- Petkova, S.B.; Yuan, R.; Tsaih, S.-W.; Schott, W.; Roopenian, D.C.; Paigen, B. Genetic influence on immune phenotype revealed strain-specific variations in peripheral blood lineages. Physiol. Genom. 2008, 34, 304–314. [Google Scholar]
- Hensel, J.A.; Khattar, V.; Ashton, R.; Ponnazhagan, S. Characterization of immune cell subtypes in three commonly used mouse strains reveals gender and strain-specific variations. Lab. Investig. 2019, 99, 93–106. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Molony, R.D.; Kudo, E.; Sidorov, S.; Kong, Y.; Dixit, V.D.; Iwasaki, A. Ketogenic diet activates protective γδ T cell responses against influenza virus infection. Sci. Immunol. 2019, 4, eaav2026. [Google Scholar]
- Goldberg, E.L.; Shchukina, I.; Asher, J.L.; Sidorov, S.; Artyomov, M.N.; Dixit, V.D. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2020, 2, 50–61. [Google Scholar] [PubMed]
- McMurray, R.W.; Bradsher, R.W.; Steele, R.W.; Pilkington, N.S. Effect of prolonged modified fasting in obese persons on in vitro markers of immunity: Lymphocyte function and serum effects on normal neutrophils. Am. J. Med. Sci. 1990, 299, 379–385. [Google Scholar] [PubMed]
- Saeed, F.A.; Castle, G.E. Neutrophil chemiluminescence during phagocytosis is inhibited by abnormally elevated levels of acetoacetate: Implications for diabetic susceptibility to infections. Clin. Diagn. Lab. Immunol. 1998, 5, 740–743. [Google Scholar] [CrossRef]
- Hirschberger, S.; Strauß, G.; Effinger, D.; Marstaller, X.; Ferstl, A.; Müller, M.B.; Wu, T.; Hübner, M.; Rahmel, T.; Mascolo, H.; et al. Very-low-carbohydrate diet enhances human T-cell immunity through immunometabolic reprogramming. EMBO Mol. Med. 2021, 13, e14323. [Google Scholar]
- Link, V.M.; Subramanian, P.; Cheung, F.; Han, K.L.; Stacy, A.; Chi, L.; Sellers, B.A.; Koroleva, G.; Courville, A.B.; Mistry, S.; et al. Differential peripheral immune signatures elicited by vegan versus ketogenic diets in humans. Nat. Med. 2024, 30, 560–572. [Google Scholar] [PubMed]
- Möller, G.M.; E Overbeek, S.; Van Helden-Meeuwsen, C.G.; Van Haarst, J.M.; Prens, E.P.; Mulder, P.G.; Postma, D.S.; Hoogsteden, H.C. Increased numbers of dendritic cells in the bronchial mucosa of atopic asthmatic patients: Downregulation by inhaled corticosteroids. Clin. Exp. Allergy 1996, 26, 517–524. [Google Scholar]
- Yi, S.; Zhai, J.; Niu, R.; Zhu, G.; Wang, M.; Liu, J.; Huang, H.; Wang, Y.; Jing, X.; Kang, L.; et al. Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge. Nat. Commun. 2018, 9, 3879. [Google Scholar]
- Vroman, H.; Hendriks, R.W.; Kool, M. Dendritic Cell Subsets in Asthma: Impaired Tolerance or Exaggerated Inflammation? Front. Immunol. 2017, 8, 941. [Google Scholar]
- Morianos, I.; Semitekolou, M. Dendritic Cells: Critical Regulators of Allergic Asthma. Int. J. Mol. Sci. 2020, 21, 7930. [Google Scholar] [CrossRef]
- Conejero, L.; Khouili, S.C.; Martínez-Cano, S.; Izquierdo, H.M.; Brandi, P.; Sancho, D. Lung CD103+ dendritic cells restrain allergic airway inflammation through IL-12 production. J. Clin. Investig. 2017, 2, e90420. [Google Scholar]
- Semper, A.E.; Hartley, J.A. Dendritic cells in the lung: What is their relevance to asthma? Clin. Exp. Allergy 1996, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Onai, N.; Obata-Onai, A.; A Schmid, M.; Ohteki, T.; Jarrossay, D.; Manz, M.G. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007, 8, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.-S.J.; Fu, S.M.; Rose, C.E.; Gaskin, F.; Ju, S.-T.; Beaty, S.R. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing langerin and tight junction proteins. J. Immunol. 2006, 176, 2161–2172. [Google Scholar] [CrossRef]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell–associated antigen. J. Exp. Med. 2011, 208, 1789–1797. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Willart, M.A.; van Rijt, L.S.; Muskens, F.; Kool, M.; Baas, C.; Thielemans, K.; Bennett, C.; Clausen, B.E.; Hoogsteden, H.C.; et al. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 2008, 205, 1621–1634. [Google Scholar] [CrossRef]
- Beaty, S.R.; Rose, C.E.; Sung, S.-S.J. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 2007, 178, 1882–1895. [Google Scholar] [CrossRef]
- Shaw, D.M.; Keaney, L.; Maunder, E.; Dulson, D.K. Natural killer cell subset count and antigen-stimulated activation in response to exhaustive running following adaptation to a ketogenic diet. Exp. Physiol. 2023, 108, 706–714. [Google Scholar] [CrossRef]
- Kesarwani, P.; Kant, S.; Zhao, Y.; Miller, C.R.; Chinnaiyan, P. The Influence of the Ketogenic Diet on the Immune Tolerant Microenvironment in Glioblastoma. Cancers 2022, 14, 5550. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J. Role of Natural Killer Cells in Airway Inflammation. Allergy Asthma Immunol. Res. 2018, 10, 448–456. [Google Scholar] [CrossRef]
- Gorska, M.M. Natural killer cells in asthma. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 50–54. [Google Scholar]
- Lepretre, F.; Gras, D.; Chanez, P.; Duez, C. Natural killer cells in the lung: Potential role in asthma and virus-induced exacerbation? Eur. Respir. Rev. 2023, 32, 230036. [Google Scholar] [PubMed]
- Gutiérrez-Vera, C.; García-Betancourt, R.; Palacios, P.A.; Müller, M.; Montero, D.A.; Verdugo, C.; Ortiz, F.; Simon, F.; Kalergis, A.M.; González, P.A.; et al. Natural killer T cells in allergic asthma: Implications for the development of novel immunotherapeutical strategies. Front. Immunol. 2024, 15, 1364774. [Google Scholar]
- Hall, S.; Agrawal, D.K. Key mediators in the immunopathogenesis of allergic asthma. Int. Immunopharmacol. 2014, 23, 316–329. [Google Scholar] [PubMed]
- Batch, J.T.; Lamsal, S.P.; Adkins, M.; Sultan, S.; Ramirez, M.N. Advantages and Disadvantages of the Ketogenic Diet: A Review Article. Cureus 2020, 12, e9639. [Google Scholar] [CrossRef] [PubMed]
- Nasser, S.; Solé, T.; Vega, N.; Thomas, T.; Balcerczyk, A.; Strigini, M.; Pirola, L. Ketogenic diet administration to mice after a high-fat-diet regimen promotes weight loss, glycemic normalization and induces adaptations of ketogenic pathways in liver and kidney. Mol. Metab. 2022, 65, 101578. [Google Scholar]
- Di Lucente, J.; Persico, G.; Zhou, Z.; Jin, L.-W.; Ramsey, J.J.; Rutkowsky, J.M.; Montgomery, C.M.; Tomilov, A.; Kim, K.; Giorgio, M.; et al. Ketogenic diet and BHB rescue the fall of long-term potentiation in an Alzheimer’s mouse model and stimulates synaptic plasticity pathway enzymes. Commun. Biol. 2024, 7, 195. [Google Scholar]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar]
- Batacan, R.B.; Duncan, M.J.; Dalbo, V.J.; Buitrago, G.L.; Fenning, A.S. Effect of different intensities of physical activity on cardiometabolic markers and vascular and cardiac function in adult rats fed with a high-fat high-carbohydrate diet. J. Sport Health Sci. 2018, 7, 109–119. [Google Scholar] [CrossRef]
- Dias, M.d.M.e.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.d.O.; Pereira, S.S.; de Oliveira, L.L.; Peluzio, M.D.C.G.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar]
- Monaghan, K.L.; Farris, B.Y.; Zheng, W.; Wan, E.C.K. Characterization of Immune Cells and Proinflammatory Mediators in the Pulmonary Environment. J. Vis. Exp. 2020, 160, e61359. [Google Scholar]



| a. Set 1 | |||
| Immune Cell Type | Antibody | Surface Marker Expression | Clone |
| Alveolar macrophages | CD45-FITC | CD45+ Siglec F+ CD11b− | 30-F11 |
| Eosinophils | Siglec F-PE | CD45+ Siglec F+ CD11b+ | S17007L |
| CD103+ DCs | CD11c-Percp/Cy5.5 | CD45+ Siglec F− CD11b− CD103+ CD11c+ MHC II+ | N418 |
| CD11b+ DCs | CD11b-PE/Cy7 | CD45+ Siglec F− CD11b hi CD103− CD64− MHC II+ | M1/70 |
| Interstitial macrophages | CD64-APC | CD45+ Siglec F− CD11b hi CD103− CD64+ MHC II+ | X54-5/7.1 |
| CD103-BV421 | 2E7 | ||
| MHC II-BV510 | M5/114.15.2 | ||
| b. Set 2 | |||
| Immune Cell Type | Antibody | Surface Marker Expression | Clone |
| Monocytes/moDCs | CD45-FITC | CD45+ CD11b hi Ly6C hi/int CCR2+/− Ly6G− | 30-F11 |
| Neutrophils | Ly6C-PE | CD45+ CD11b hi Ly6C int CCR2− Ly6G+ | HK1.4 |
| CD11b-PE/Cy7 | M1/70 | ||
| CCR2-BV421 | SA203G11 | ||
| Ly6G-BV510 | 1A8 | ||
| c. Set 3 | |||
| Immune Cell Type | Antibody | Surface Marker Expression | Clone |
| Plasmacytoid DCs | CD45-FITC | CD45+ B220+ CD11c+ | 30-F11 |
| B-cells | CD8-PE | CD45+ B220+ CD11c− | 53-6.7 |
| CD4+ T-cells | NK1.1-Percp/Cy5.5 | CD45+ B220− CD11c− CD4+ CD8− | PK136 |
| CD8+ T-cells | CD11c-PE/Cy7 | CD45+ B220− CD11c− CD4− CD8+ | N418 |
| NK cells | APC-B220 | CD45+ B220− CD11c− CD4− CD8− NK1.1+ TCRb− | RA3-6B2 |
| NKT cells | CD4-BV421 | CD45+ B220− CD11c− CD4− CD8− NK1.1+ TCRb+ | GK1.5 |
| TCRb-BV510 | H57-597 | ||
| Target | Fluorochrome | CytoFLEX LX Laser/Detector | Quantity |
|---|---|---|---|
| B220/CD45R | APC | 640 nm/R660 | 0.25 µg per 106 cells in 100 µL volume |
| CCR2 | BV421 | 405 nm/V450 | 0.5 µg per million cells in 100 µL volume |
| CD103 | BV421 | 405 nm/V450 | 5 µL per million cells in 100 µL staining volume |
| CD11b | PE/Cy7 | 561 nm/Y763 | 0.25 µg per 106 cells in 100 µL volume |
| CD11c | PE/Cy7 | 561 nm/Y763 | 0.25 µg per 106 cells in 100 µL volume |
| CD11c | PerCP/Cyanine5.5 | 488 nm/B690 | 1.0 µg per million cells in 100 µL volume |
| CD4 | BV421 | 405 nm/V450 | 5 µL per million cells in 100 µl |
| CD45 | FITC | 488 nm/B525 | 0.25 µg per 106 cells in 100 µL volume |
| CD64 | APC | 640 nm/R660 | 1.0 µg per million cells in 100 µL volume |
| CD8 | PE | 561 nm/Y585 | 0.25 µg per 106 cells in 100 µL volume |
| Ly6c | PE | 561 nm/Y585 | 0.25 µg per 106 cells in 100 µL |
| Ly6g | BV510 | 405 nm/V525 | 0.5 µg per million cells in 100 µL volume |
| MHCII | BV510 | 405 nm/V525 | 5 µL per million cells in 100uL staining volume |
| Nk1.1 | PerCP/Cyanine5.5 | 488 nm/B690 | 1.0 µg per million cells in 100 µL volume |
| Siglec F | PE | 561 nm/Y585 | 0.25 µg per million cells in 100 µL volume |
| TCRb | BV510 | 405 nm/V525 | 5 µL per million cells in 100 µL staining volume |
| Dead cell stain | Zombie NIR | 640 nm/R763 | 1:100 for 1–10 million cells |
| TruStain FcX™ (anti-mouse CD16/32) Antibody | N/A | N/A | 1.0 µg per 106 cells in 100 µL volume |
| Product | Control Diet (D11112201) | Ketogenic Diet (D15010301) | ||
|---|---|---|---|---|
| gm% | kcal% | gm% | kcal% | |
| Protein | 19 | 20 | 17 | 10 |
| Carbohydrate | 63 | 65 | 2 | 1 |
| Fat | 7 | 15 | 66 | 89 |
| Total | 100 | 100 | ||
| kcal/gm | 3.81 | 6.6 | ||
| Ingredient | gm | kcal | gm | kcal |
| Casein | 200 | 800 | 100 | 400 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn Starch | 381 | 1524 | 0 | 0 |
| Maltodextrin 10 | 110 | 440 | 0 | 0 |
| Sucrose | 150 | 600 | 0 | 0 |
| Cellulose, BW200 | 75 | 0 | 50 | 0 |
| Inulin | 25 | 37.5 | 0 | 0 |
| Soybean Oil | 70 | 630 | 25 | 225 |
| Lard | 0 | 0 | 175.5 | 1580 |
| Cocoa Butter | 0 | 0 | 200 | 1800 |
| Mineral Mix, S10026 | 10 | 0 | 10 | 0 |
| Dicalcium Phosphate | 13 | 0 | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 | 5.5 | 0 |
| Potassium Citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 |
| Vitamin Mix, V10001 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 0 | 2 | 0 |
| FD&C Yellow Dye #5 | 0.025 | 0 | 0.025 | 0 |
| FD&C Red Dye #40 | 0 | 0 | 0.025 | 0 |
| FD&C Blue Dye #1 | 0.025 | 0 | 0 | 0 |
| Total | 1071.05 | 4084 | 610.55 | 4057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekpruke, C.D.; Borges-Sosa, O.; Hassel, C.A.; Rousselle, D.; Dinwiddie, L.; Babayev, M.; Bakare, A.; Silveyra, P. Sex-Specific Anti-Inflammatory Effects of a Ketogenic Diet in a Mouse Model of Allergic Airway Inflammation. Int. J. Mol. Sci. 2025, 26, 3046. https://doi.org/10.3390/ijms26073046
Ekpruke CD, Borges-Sosa O, Hassel CA, Rousselle D, Dinwiddie L, Babayev M, Bakare A, Silveyra P. Sex-Specific Anti-Inflammatory Effects of a Ketogenic Diet in a Mouse Model of Allergic Airway Inflammation. International Journal of Molecular Sciences. 2025; 26(7):3046. https://doi.org/10.3390/ijms26073046
Chicago/Turabian StyleEkpruke, Carolyn D., Omar Borges-Sosa, Christiane A. Hassel, Dustin Rousselle, Lyidia Dinwiddie, Maksat Babayev, Ahmed Bakare, and Patricia Silveyra. 2025. "Sex-Specific Anti-Inflammatory Effects of a Ketogenic Diet in a Mouse Model of Allergic Airway Inflammation" International Journal of Molecular Sciences 26, no. 7: 3046. https://doi.org/10.3390/ijms26073046
APA StyleEkpruke, C. D., Borges-Sosa, O., Hassel, C. A., Rousselle, D., Dinwiddie, L., Babayev, M., Bakare, A., & Silveyra, P. (2025). Sex-Specific Anti-Inflammatory Effects of a Ketogenic Diet in a Mouse Model of Allergic Airway Inflammation. International Journal of Molecular Sciences, 26(7), 3046. https://doi.org/10.3390/ijms26073046







