Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing
Abstract
1. Introduction
2. Results
2.1. Synthesis of Dexketoprofen-Loaded Hydrogel DEXHY
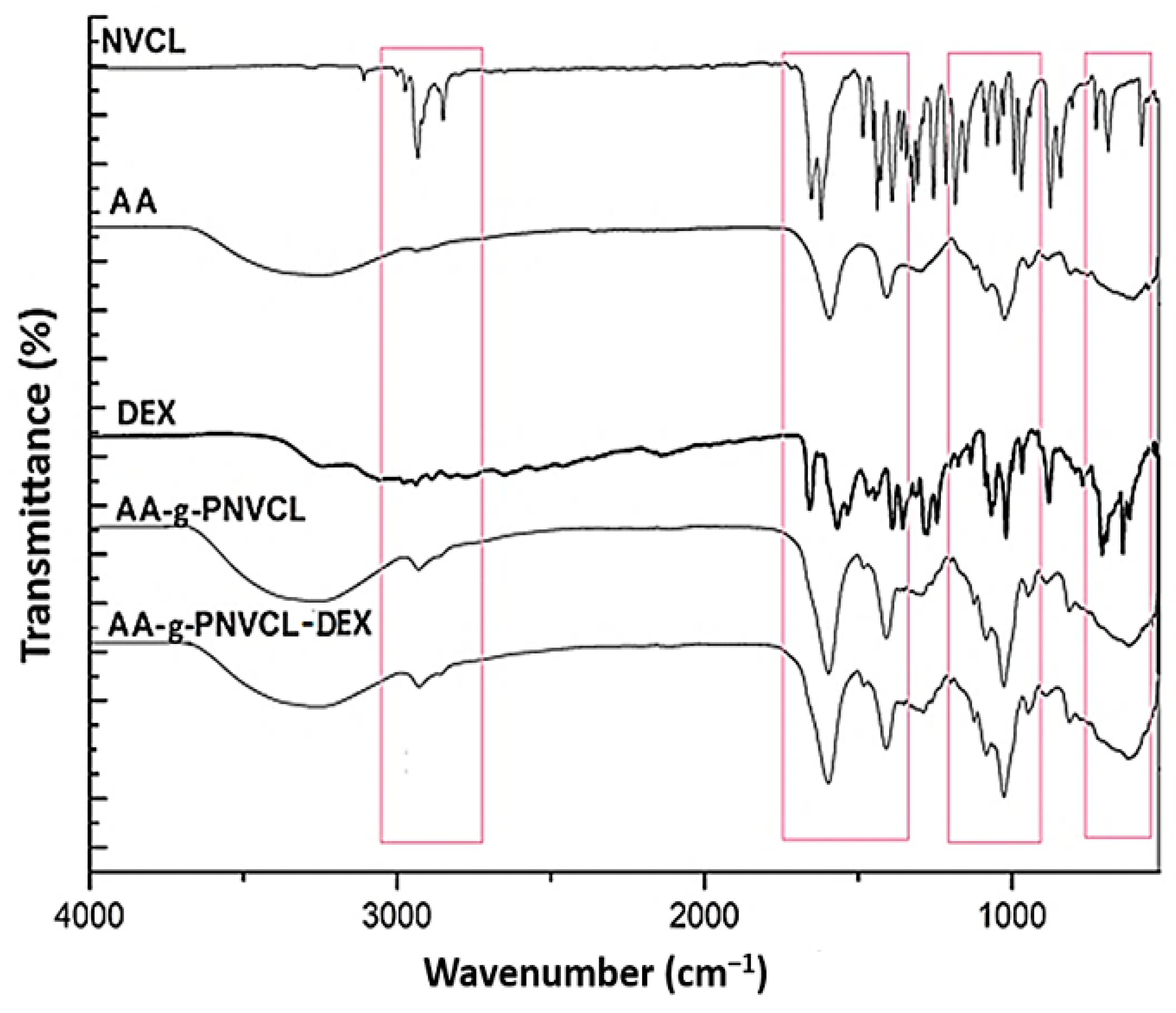
2.2. Infrared Spectroscopy (FTIR) Analysis
2.3. Differential Scanning Calorimetry (DSC) Analysis
2.4. Scanning Electron Microscopy (SEM) Analysis
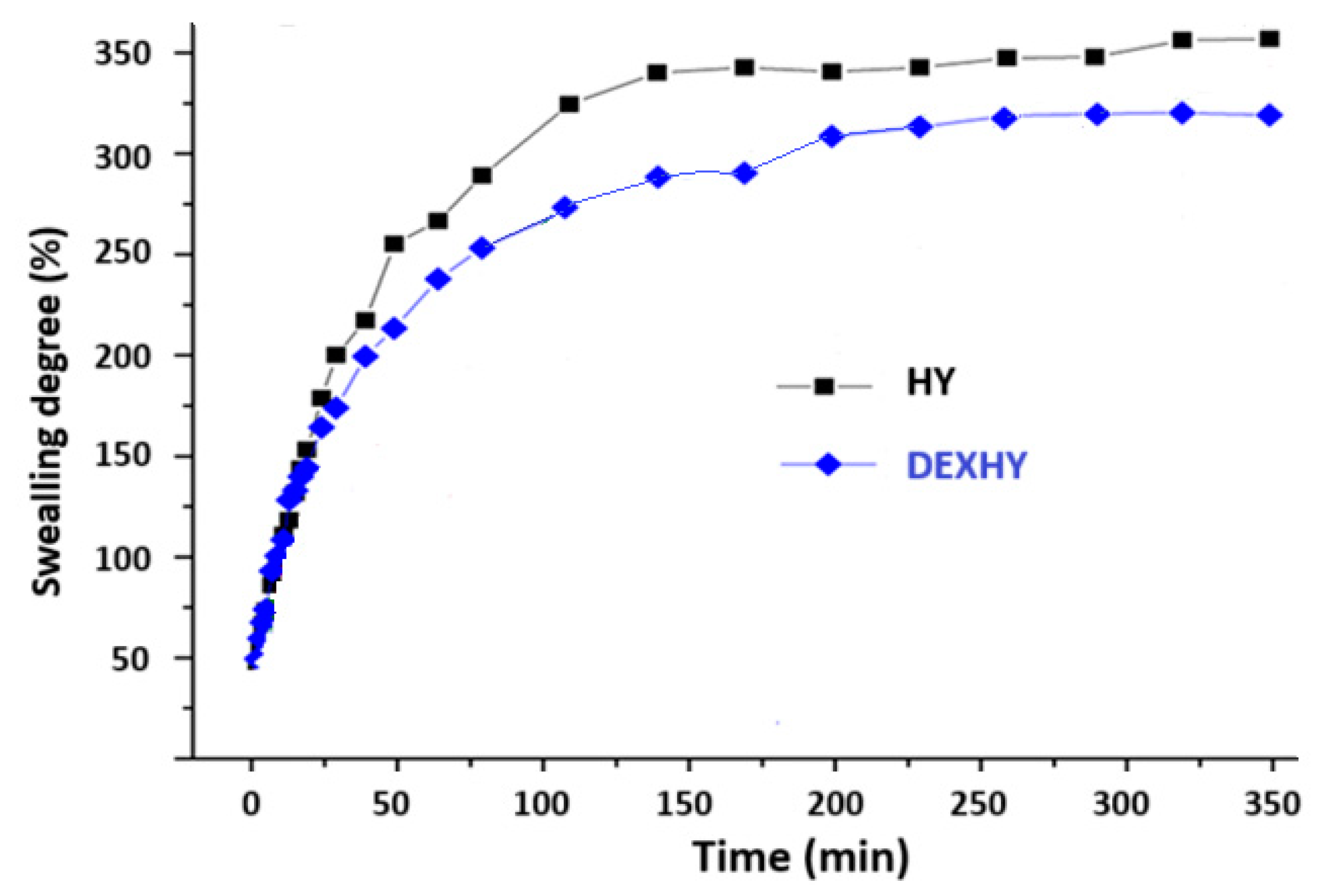
2.5. Swelling Behavior of Unloaded Hydrogel and Dexketoprofen-Loaded Hydrogel
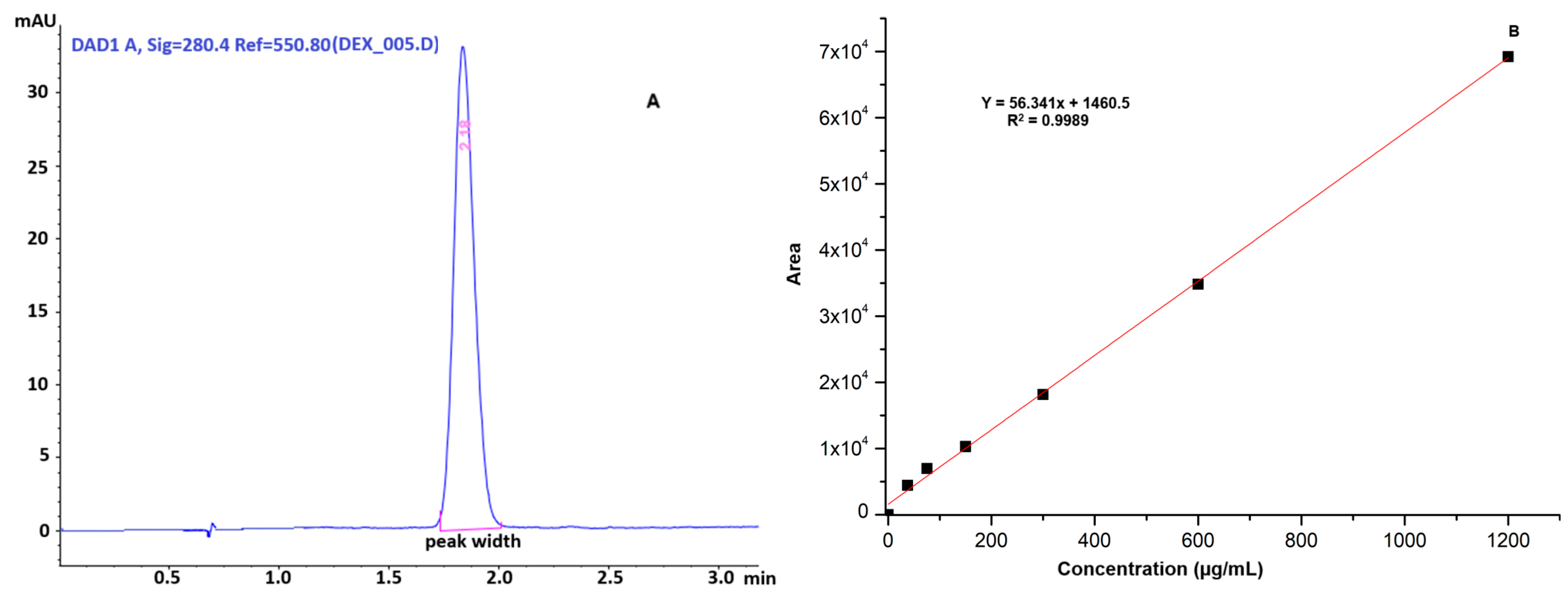
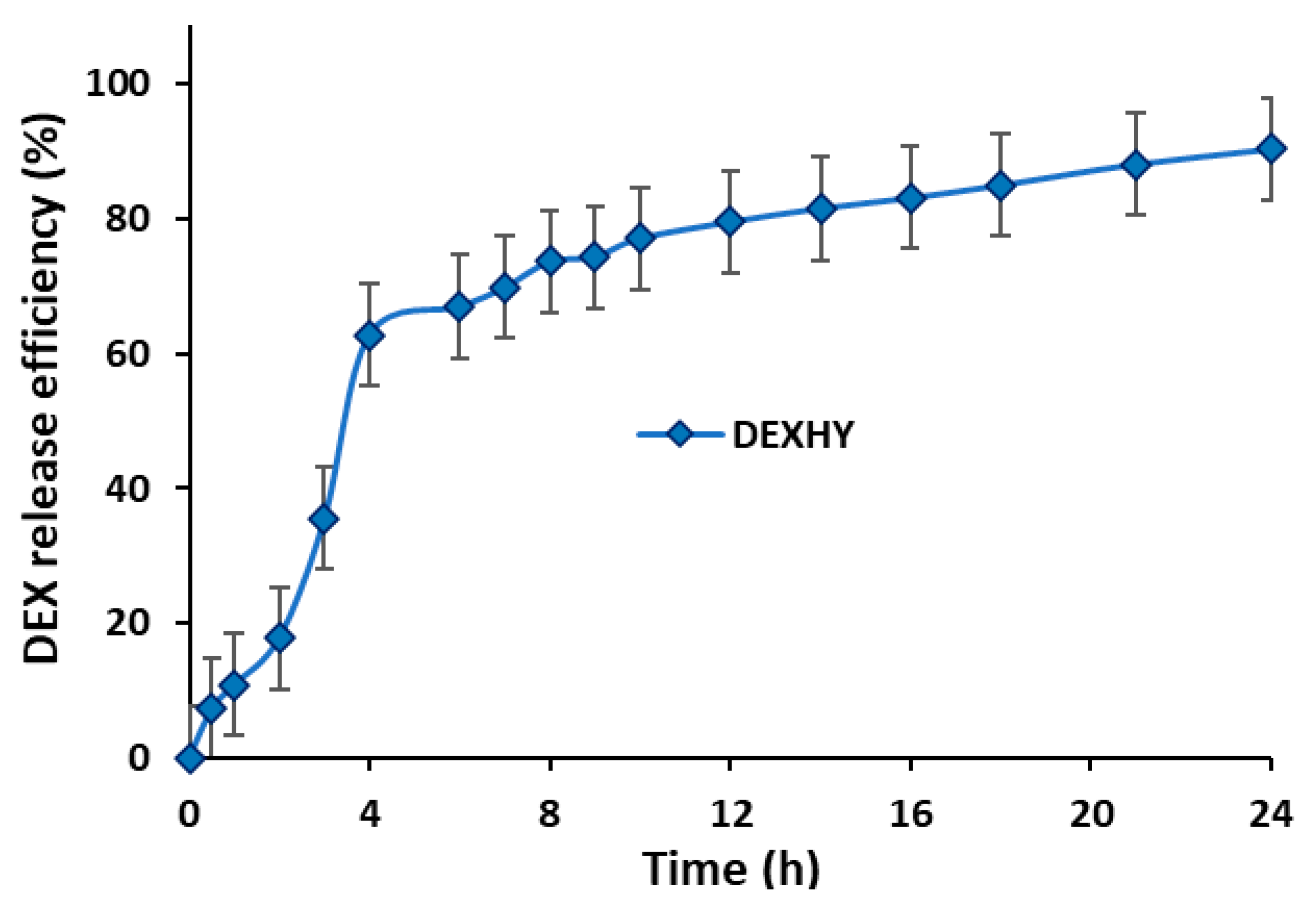
2.6. Drug-Loading Capacity and Release
2.7. Bioadhesion Properties
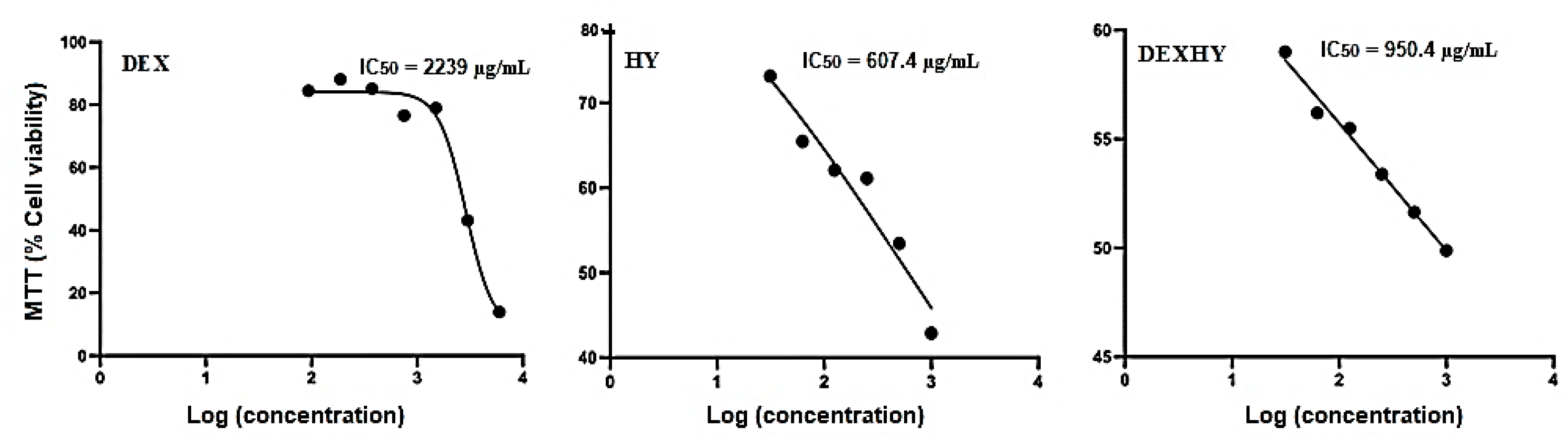
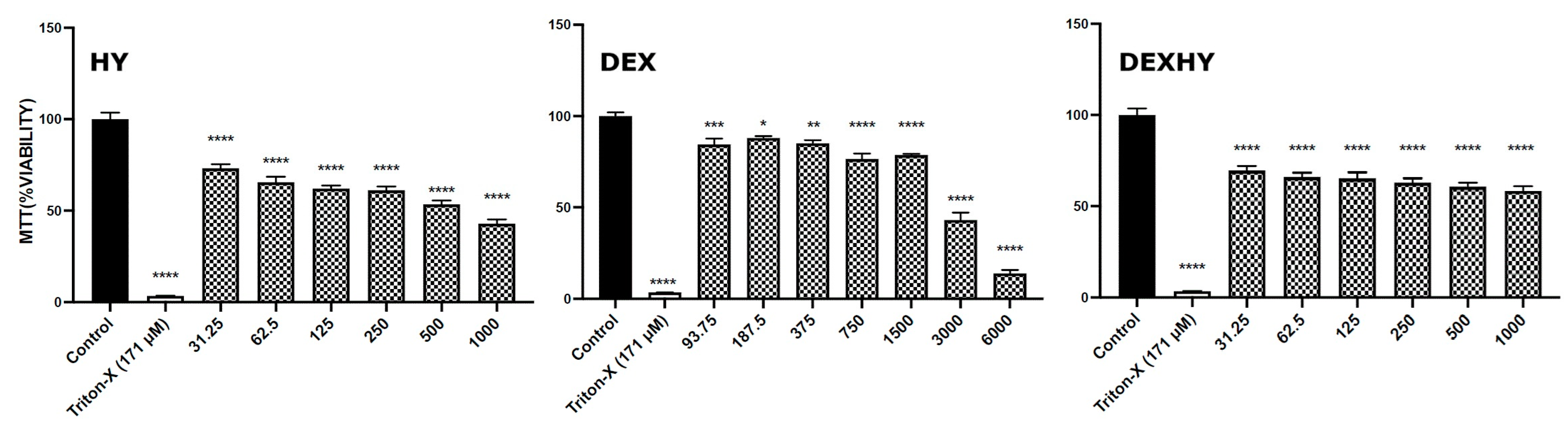
2.8. Hydrogel Cytocompatibility
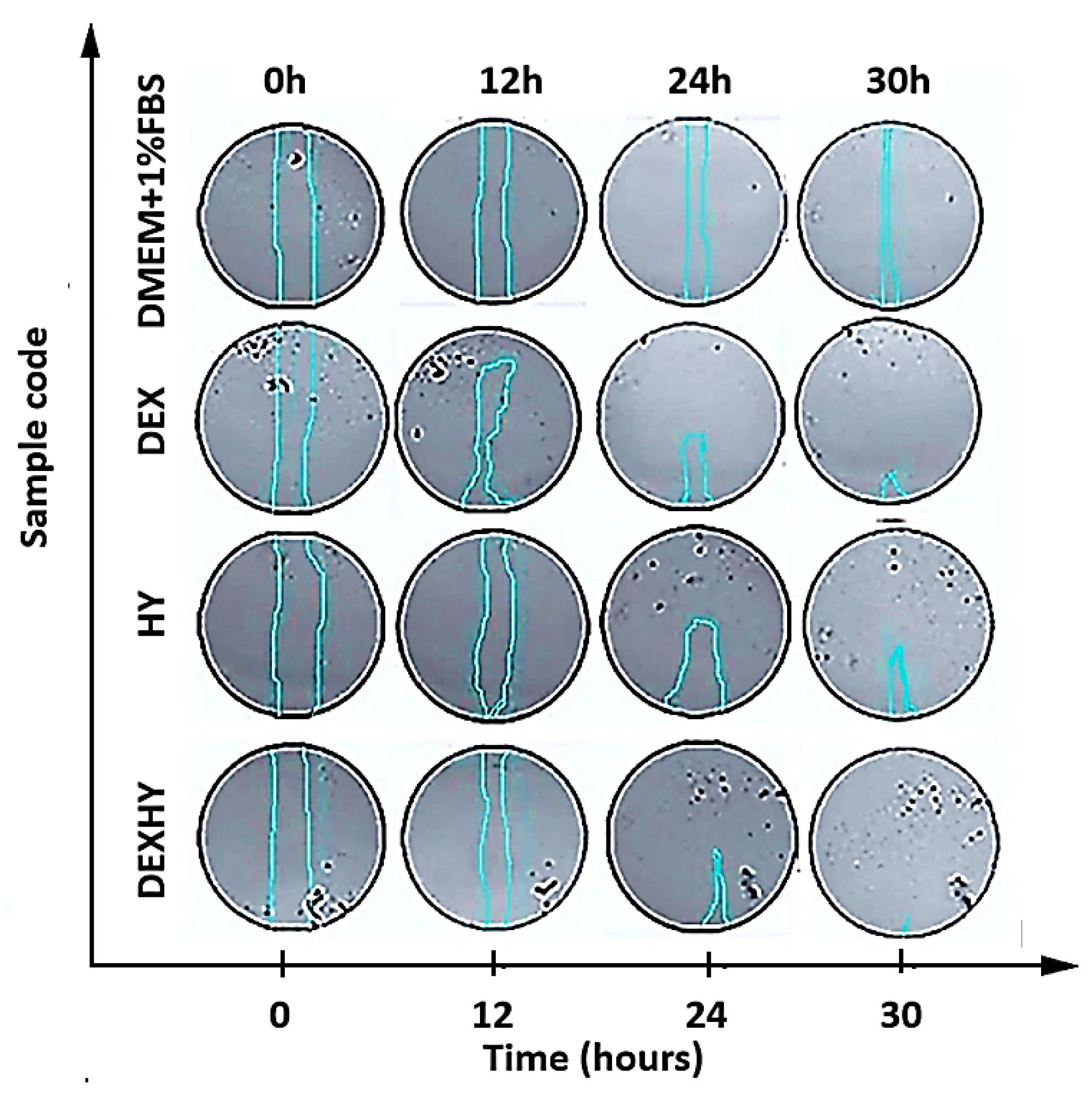
2.9. In Vitro Wound Healing Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Obtaining the Dexketoprofen-Loaded Hydrogel DEXHY
4.2.1. Synthesis of Alginate Copolymer Grafted with Poly(N-Vinylcaprolactam)
4.2.2. Preparation of Unloaded Hydrogel AA-g-PNVCL (HY)
4.2.3. Preparation of Dexketoprofen-Loaded Hydrogel AA-g-PNVCL-DEX (DEXHY)
4.3. Structural Analysis by FTIR
4.4. Analysis by DSC
4.5. Morphological Analysis by SEM
4.6. Swelling Study of Hydrogels
4.7. Drug-Loading Efficiency and Release Kinetics of Dexketoprofen
4.8. In Vitro Evaluation of Tissue Adhesive Interactions of Dexketoprofen-Loaded Hydrogel
4.9. In Vitro Cytotoxicity Studies
4.9.1. 3T3-L1 Cell Culture
4.9.2. Cell Viability Assay
4.10. Scratch Assay
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Pormohammad, A.; Monych, K.N.; Ghosh, S.; Turner, L.D.; Turner, J.R. Nanomaterials in Wound Healing and Infection Control. Antibiotics 2021, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Bowers, A.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician. 2020, 101, 159–166. [Google Scholar]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2023, 24, 641. [Google Scholar] [CrossRef]
- Li, M.; Hou, Q.; Zhong, L.; Zhao, Y.; Fu, X. Macrophage Related Chronic Inflammation in Non-Healing Wounds. Front. Immunol. 2021, 12, 681710. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in skin wound healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Tran, H.Q.; Shahriar, S.M.S.; Yan, Z.; Xie, J. Recent Advances in Functional Wound Dressings. Adv. Wound Care (New Rochelle) 2023, 12, 399–427. [Google Scholar] [CrossRef]
- Willenborg, S.; Injarabian, L.; Eming, S.A. Role of Macrophages in Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041216. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing applications. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef] [PubMed]
- Sindhi, K.; Pingili, R.B.; Beldar, V.; Bhattacharya, S.; Rahaman, J.; Mukherjee, D. The role of biomaterials-based scaffolds in advancing skin tissue construct. J. Tissue Viability 2025, 34, 100858. [Google Scholar] [CrossRef]
- Kumar, M.; Mahmood, S.; Chopra, S.; Bhatia, A. Biopolymer-based nanoparticles and their therapeutic potential in wound healing—A review. Int. J. Biol. Macromol. 2024, 267, 131335. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Sherje, A.P. Recent advances in biopolymer-based smart hydrogel for wound healing. J. Drug Deliv. Sci. Technol. 2024, 99, 105990. [Google Scholar] [CrossRef]
- Huang, C.; Dong, L.; Zhao, B.; Lu, Y.; Huang, S.; Yuan, Z.; Luo, G.; Xu, Y.; Qian, W. Anti-inflammatory hydrogel dressings and skin wound healing. Clin. Transl. Med. 2022, 12, e1094. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, F.; Hesari, R.; Jensen, T.; Obagi, S.; Rgeai, A.; Damiani, G.; Bunick, C.G.; Grada, A. Antimicrobial Wound Dressings: A Concise Review for Clinicians. Antibiotics 2023, 12, 1434. [Google Scholar] [CrossRef]
- Tiwari, R.; Pathak, K. Local Drug Delivery Strategies towards Wound Healing. Pharmaceutics 2023, 15, 634. [Google Scholar] [CrossRef]
- Chopra, H.; Mohanta, Y.K.; Mahanta, S.; Mohanta, T.K.; Singh, I.; Avula, S.K.; Mallick, S.P.; Rabaan, A.A.; AlSaihati, H.; Alsayyah, A.; et al. Recent updates in nanotechnological advances for wound healing: A narrative review. Nanotechnol. Rev. 2023, 12, 20230129. [Google Scholar] [CrossRef]
- Bibire, T.; Dănilă, R.; Yilmaz, C.N.; Verestiuc, L.; Nacu, I.; Ursu, R.G.; Ghiciuc, C.M. In vitro biological evaluation of an alginate-based hydrogel loaded with rifampicin for wound care. Pharmaceuticals 2024, 17, 943. [Google Scholar] [CrossRef]
- Bibire, T.; Timofte, D.V.; Dănilă, R.; Panainte, A.-D.; Yilmaz, C.N.; Bibire, N.; Agoroaei, L.; Ghiciuc, C.M. Rifampicin-loaded PLGA/Alginate-grafted pNVCL-based nanoparticles for wound healing. Appl. Sci. 2024, 14, 9799. [Google Scholar] [CrossRef]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for Surgical Applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Gao, M.; Guo, H.; Dong, X.; Wang, Z.; Yang, Z.; Shang, Q.; Wang, Q. Regulation of inflammation during wound healing: The function of mesenchymal stem cells and strategies for therapeutic enhancement. Front. Pharmacol. 2024, 15, 1345779. [Google Scholar] [CrossRef]
- Barbu, A.; Neamtu, B.; Zahan, M.; Iancu, G.M.; Bacila, C.; Miresan, V. Current Trends in Advanced Alginate-Based Wound Dressings for Chronic Wounds. J. Pers. Med. 2021, 11, 890. [Google Scholar] [CrossRef]
- Reczynska-Kolman, K.; Hartman, K.; Kwiecien, K.; Brzychczy-Włoch, M.; Pamuła, E. Composites Based on Gellan Gum, Alginate and Nisin-Enriched Lipid Nanoparticles for the Treatment of Infected Wounds. Int. J. Mol. Sci. 2021, 23, 321. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, L.; Zhang, P.; Zhang, J.; Mei, X.; Wang, D.; Zhang, Y.; Ren, X.; Chen, Z. Encapsulation of Green Tea Polyphenol Nanospheres in PVA/Alginate Hydrogel for Promoting Wound Healing of Diabetic Rats by Regulating PI3K/AKT Pathway. Mater. Sci. Eng. C 2020, 110, 110686. [Google Scholar] [CrossRef]
- Oveissi, F.; Tavakoli, N.; Minaiyan, M.; Mofid, M.R.; Taheri, A. Alginate Hydrogel Enriched with Ambystoma Mexicanum Epidermal Lipoxygenase-Loaded Pectin Nanoparticles for Enhanced Wound Healing. J. Biomater. Appl. 2020, 34, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.; Bhatt, A.; Mittal, R.K.; Abdellatif, M.H.; Farghaly, T.A. Polymer Grafting and its chemical reactions. Front. Bioeng. Biotechnol. 2023, 10, 104492. [Google Scholar] [CrossRef]
- Lu, W.; Luo, H.; Zhu, Z.; Wu, Y.; Luo, J.; Wang, H. Preparation and the biopharmaceutical evaluation for the metered dose transdermal spray of dexketoprofen. J. Drug Deliv. 2014, 2014, 697434. [Google Scholar] [CrossRef]
- Trivedi, D.; Goyal, A. Formulation and evaluation of transdermal patches containing dexketoprofen trometamol. Int. J. Pharm. Chem. Anal. 2020, 7, 87–97. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; Chavarría-Bolaños, D.; Jesús Pozos-Guillén, A.; Escobar-Barrios, V.A.; Cerda-Cristerna, B.I. Synthesis of a PVA drug delivery system for controlled release of a Tramadol–Dexketoprofen combination. J. Mater. Sci. Mater. Med. 2021, 32, 56. [Google Scholar] [CrossRef]
- Voycheva, C.; Slavkova, M.; Popova, T.; Tzankova, D.; Alexandra Tosheva, A.; Aluani, D.; Tzankova, V.; Ivanova, I.; Tzankov, S.; Spassova, I.; et al. Synthesis and characterization of PnVCL grafted agar with potential temperature-sensitive delivery of Doxorubicin. J. Drug Deliv. Sci. Technol. 2022, 76, 103725. [Google Scholar] [CrossRef]
- Marsili, L.; Dal Bo, M.; Eisele, G.; Donati, I.; Berti, F.; Toffoli, G. Characterization of Thermoresponsive Poly-N-Vinylcaprolactam Polymers for Biological Applications. Polymers 2021, 13, 2639. [Google Scholar] [CrossRef]
- Khan, S.; Minhas, M.U.; Akhtar, N.; Thakur, R.R.S. Sodium alginate/N-(Vinylcaprolactam) based supramolecular self-assembled subcutaneously administered in situ formed gels depot of 5-fluorouracil: Rheological analysis, in vitro cytotoxic potential, in vivo bioavailability and safety evaluation. Int. J. Biol. Macromol. 2022, 211, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.A.; Yenilmez, E.; Arslan, R.; Şenel, B.; Yazan, Y. Dexketoprofen trometamol loaded solid lipid nanoparticles (SLNs): Formulation, in vitro and in vivo evaluation. J. Res. Pharm. 2020, 24, 82–99. [Google Scholar] [CrossRef]
- Çulcu, Ö.; Tunçel, E.; Tamer, S.İ.; Tirnaksiz, F.F. Characterization of Thermosensitive Gels for the Sustained Delivery of Dexketoprofen Trometamol for Dermal Applications. J. Gazi Univ. Health Sci. Inst. 2020, 2, 1–12. [Google Scholar]
- Castillo-Henríquez, L.; Sanabria-Espinoza, P.; Murillo-Castillo, B.; Montes de Oca-Vásquez, G.; Batista-Menezes, D.; Calvo-Guzmán, B.; Ramírez-Arguedas, N.; Vega-Baudrit, J. Topical Chitosan-Based Thermo-Responsive Scaffold Provides Dexketoprofen Trometamol Controlled Release for 24 h Use. Pharmaceutics 2021, 13, 2100. [Google Scholar] [CrossRef]
- Iljazi, B.; Iljazi, N.; Jashari, A.; Mahmudi, N.; Ziberi, B. Alginate based hydrogels stability and their use for controlled release of ketoprofen. J. Math. Sci. 2021, 6, 153–164. [Google Scholar]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Abasalizadeh, F.; Moghaddam, S.V.; Alizadeh, E.; Akbari, E.; Kashani, E.; Bagher Fazljou, S.M.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The Usages and Potential Uses of Alginate for Healthcare Applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef]
- Halligan, E.; Zhuo, S.; Colbert, D.M.; Alsaadi, M.; Tie, B.S.H.; Bezerra, G.S.N.; Keane, G.; Geever, L.M. Modulation of the Lower Critical Solution Temperature of Thermoresponsive Poly(N-vinylcaprolactam) Utilizing Hydrophilic and Hydrophobic Monomers. Polymers 2023, 15, 1595. [Google Scholar] [CrossRef]
- Serap, D.; Murat, E.Y. Synthesis and Characterization of Thermosensitive Poly(N-Vinyl Caprolactam)-Grafted-Aminated Alginate Hydrogels. Macromol. Chem. Phys. 2019, 221, 1900412. [Google Scholar]
- Öztürk, A.; Tuba Kiyan, H. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.; Capsoni, D.; Milanese, C.; Cardini, A. Polymorphic quantification of dexketoprofen trometamol by differential scanning calorimetry. J. Therm. Anal. Calorim. 2023, 148, 1949–1958. [Google Scholar] [CrossRef]
- Lozinsky, V.; Simenel, I.; Kurskaya, E.; Kulakova, V.; Galaev, I.; Mattiasson, B.; Grinberg, V.; Grinberg, N.; Khokhlov, A. Synthesis of N-vinylcaprolactam polymers in water-containing media. Polymer 2000, 41, 6507–6518. [Google Scholar] [CrossRef]
- Nakamura, K.; Kinoshita, E.; Hatakeyama, T.; Hatakeyama, H. Thermal properties of alginic acid-polylysine molecular composites. In Hydrocolloids, 1st ed.; Nishinari, K., Ed.; Elsevier Science: Tokyo, Japan, 2000; pp. 189–195. [Google Scholar]
- Román, S.S.; Almeida, G.N.; del Arco, M.; Martín, C. Dex-ketoprofen and aceclofenac release from layered double hydroxide and SBA-15 ordered mesoporous material. Appl. Clay Sci. 2016, 121–122, 9–16. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous Hydrogels: Present Challenges and Future Opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Akbarzadeh Solbu, A.; Caballero, D.; Damigos, S.; Kundu, S.C.; Reis, R.L.; Halaas, O.; Chahal, A.S.; Strand, B.L. Assessing cell migration in hydrogels: An overview of relevant materials and methods. Mater. Today Bio 2022, 18, 100537. [Google Scholar]
- Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. [Google Scholar] [CrossRef]
- Huang, J.; Fan, C.; Ma, Y.; Huang, G. Exploring Thermal Dynamics in Wound Healing: The Impact of Temperature and Microenvironment. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1251–1258. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; Fischer, T.; Hayn, A.; Mierke, C.T.; Burgess, J.K.; Harmsen, M.C.A. Beginner’s Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications. Gels 2022, 8, 535. [Google Scholar] [CrossRef]
- Priya, A.S.; Premanand, R.; Ragupathi, I.; Bhaviripudi, V.R.; Aepuru, R.; Kannan, K.; Shanmugaraj, K. Comprehensive Review of Hydrogel Synthesis, Characterization, and Emerging Applications. J. Compos. Sci. 2024, 8, 457. [Google Scholar] [CrossRef]
- Farzan, M.; Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. The processes behind drug loading and release in porous drug delivery systems. Eur. J. Pharm. Biopharm. 2023, 189, 133–151. [Google Scholar] [CrossRef]
- Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, X.; Ma, X.; Wang, W.; Yan, F.; Zhao, X.; Chu, X.; Xu, W.; Sun, C. A review of the properties and applications of bioadhesive hydrogels. Polym. Chem. 2021, 12, 3721–3739. [Google Scholar] [CrossRef]
- Djekic, L.; Martinovic, M. In Vitro, Ex Vivo and In Vivo Methods for Characterization of Bioadhesiveness of Drug Delivery Systems. In Bioadhesives in Drug Delivery, 1st ed.; Mittal, K.L., Bakshi, I.S., Narang, J.K., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 3–98. [Google Scholar]
- Mishra, N.; Nisha, R.; Singh, N.; Maurya, P.; Singh, P.; Pal, R.R.; Singh, S.; Saraf, S.A. Bioadhesive and phase change polymers for drug delivery. In Smart Polymeric Nano-Constructs in Drug Delivery, 1st ed.; Vyas, S.P., Agrawal, U., Sharma, R., Eds.; Academic Press: West Bengal, India, 2022; pp. 151–186. [Google Scholar]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Gökçe, B.; Gençer, N.; Arslan, O.; Turkoğlu, S.A.; Alper, M.; Köçkar, F. Evaluation of in vitro effects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J. Enzyme Inhib. Med. Chem. 2011, 27, 37–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abate, M.; Citro, M.; Pisanti, S.; Caputo, M.; Martinelli, M. Keratinocytes Migration Promotion, Proliferation Induction, and Free Radical Injury Prevention by 3-Hydroxytirosol. Int. J. Mol. Sci. 2021, 22, 2438. [Google Scholar] [CrossRef]
- Bosch, D.J.; Nieuwenhuijs-Moeke, G.J.; van Meurs, M.; Abdulahad, W.H.; Struys, M.M. R F. Immune Modulatory Effects of Nonsteroidal Anti-inflammatory Drugs in the Perioperative Period and TheirConsequence on Postoperative Outcome. Anesthesiology 2022, 136, 843–860. [Google Scholar] [CrossRef]
- Shah, D.K.; Ghosh, S.; More, N.; Choppadandi, M.; Sinha, M.; Srivalliputtur, S.B.; Velayutham, R.; Kapusetti, G. ECM-mimetic, NSAIDs loaded thermo-responsive, immunomodulatory hydrogel for rheumatoid arthritis treatment. BMC Biotechnol. 2024, 24, 26. [Google Scholar] [CrossRef]
- Neeraj, G.; Nikunj, T.; Sanjay, B.; Rajeev, T. Nanostructured Lipid Carrier-Mediated Transdermal Delivery of Aceclofenac hydrogel present on effective therapeutic approach for inflammatory diseases. Front. Pharmacol. 2024, 12, 713616. [Google Scholar]
- Miranda, D.G.; Ramos, L.P.; Lopes, N.F.S.; Silva, N.V.H.F.; Soares, C.P.; Rodrigues, F.P.; Morais, V.P.; Sani-Taiariol, T.; Baldan, M.R.; Vasconcellos, L.M.R.; et al. Ketoprofen Associated with Hyaluronic Acid Hydrogel for Temporomandibular Disorder Treatment: An In Vitro Study. Gels 2024, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Ristić, I.; Cakić, S.; Petrović, S.D. Biomedical Applications of Thermosensitive Hydrogels for Controlled/Modulated Piroxicam Delivery. Gels 2023, 9, 70. [Google Scholar] [CrossRef]
- Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-Loaded Hydrogels Improve Motor and Cognitive Functions in a Rat Mild Traumatic Brain Injury Model. Int. J. Mol. Sci. 2022, 23, 11153. [Google Scholar] [CrossRef]
- Dildar, K.; Maimoona, Q.; Naveed, A.; Muhammad, A.; Kifayat, S.; Asim, R. Development of an intelligent, stimuli-responsive transdermal system for efficient delivery of ibuprofen against rheumatoid arthiritis. Int. J. Pharm. 2021, 610, 121242. [Google Scholar]
- Büyükfidan, D.; Bilimgut, B.; Tekeli, A.; Take, G.; Ceyhan, A.; Erdoğan, D. The effects of dexketoprofen trometamol on incisional wound healing in a rat model: 9AP7-7. Eur. J. Anaesthesiol. 2011, 28, 44–145. [Google Scholar] [CrossRef]
- Kaçaroğlu, D. Assessing the Effects of Lornoxicam and Dexketoprofen on Mouse Fibroblast Cells. Lokman Hekim Health Sci. 2024, 4, 181–187. [Google Scholar] [CrossRef]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.G.; Jiang, D.; et al. Fibroblasts—The cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef]
- Cialdai, F.; Risaliti, C.; Monici, M. Role of fibroblasts in wound healing and tissue remodeling on Earth and in space. Front. Bioeng. Biotechnol. 2022, 10, 958381. [Google Scholar] [CrossRef]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and Evaluation of a Thermoresponsive Degradable Chitosan-Grafted PNIPAAm Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Interpretation of Mucoadhesive Properties of Polymer Gel Preparations Using a Tensile Strength Method. J. Pharm. Pharmacol. 2001, 53, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Eslin, U.K.; Cagla, E.C.; Ayla, A. Colorimetric Cytotoxicity Assays. In Cytotoxicity—Understanding Cellular Damage and Response; Sukumaran, A., Mansour, M.A., Eds.; IntechOpen: London, UK, 2022; pp. 2–14. [Google Scholar]
- Martinotti, S.; Ranzato, E. Scratch Wound Healing Assay. Methods Mol Biol. 2020, 2109, 225–229. [Google Scholar] [PubMed]
- Orsu, P.; Haider, H.Y.; Koyyada, A. Bioengineering for curcumin loaded carboxymethyl guargum/reduced graphene oxide nanocomposites for chronic wound healing applications. Int. J. Pharm. 2021, 606, 120928. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; bin Abdul Rahim, A.A.; Mok, P.; Liu, D. An effective device to enable consistent scratches for in vitro scratch assays. BMC Biotechnol. 2023, 23, 32. [Google Scholar] [CrossRef]






| Hydrogel | Min. (µm) | Max. (µm) |
|---|---|---|
| HY | 12.14 ± 6.15 | 86.05 ± 6.12 |
| DEXHY | 10.21 ± 7.08 | 70.22 ± 7.74 |
| Sample | n | k (min−1) |
|---|---|---|
| HY | 0.39 | 0.21 |
| DEXHY | 0.35 | 0.19 |
| Sample | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | ||
|---|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | n | k | |
| DEXHY | 0.9678 | 0.9373 | 0.9455 | 0.9883 | 0.5336 | 0.21 |
| Hydrogel Code | Details |
|---|---|
| HY (AA-g-PNVCL) | Dexketoprofen-free hydrogel was obtained through ethanol precipitation followed by lyophilization of the polymeric matrix consisting of alginate grafted with poly(N-vinylcaprolactam) (PNVCL). That polymeric matrix resulted from the radical polymerization of NVCL and grafting of the formed PNVCL onto alginate through hydrogen bonding. |
| DEXHY (AA-g-PNVCL-DEX) | Dexketoprofen-loaded hydrogel obtained by the in situ loading of HY (AA-g-PNVCL) with dexketoprofen. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibire, T.; Panainte, A.-D.; Yilmaz, C.N.; Timofte, D.V.; Dănilă, R.; Bibire, N.; Păduraru, L.; Ghiciuc, C.M. Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing. Int. J. Mol. Sci. 2025, 26, 3051. https://doi.org/10.3390/ijms26073051
Bibire T, Panainte A-D, Yilmaz CN, Timofte DV, Dănilă R, Bibire N, Păduraru L, Ghiciuc CM. Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing. International Journal of Molecular Sciences. 2025; 26(7):3051. https://doi.org/10.3390/ijms26073051
Chicago/Turabian StyleBibire, Tudor, Alina-Diana Panainte, Catalina Natalia Yilmaz, Daniel Vasile Timofte, Radu Dănilă, Nela Bibire, Larisa Păduraru, and Cristina Mihaela Ghiciuc. 2025. "Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing" International Journal of Molecular Sciences 26, no. 7: 3051. https://doi.org/10.3390/ijms26073051
APA StyleBibire, T., Panainte, A.-D., Yilmaz, C. N., Timofte, D. V., Dănilă, R., Bibire, N., Păduraru, L., & Ghiciuc, C. M. (2025). Dexketoprofen-Loaded Alginate-Grafted Poly(N-vinylcaprolactam)-Based Hydrogel for Wound Healing. International Journal of Molecular Sciences, 26(7), 3051. https://doi.org/10.3390/ijms26073051







