Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization
Abstract
1. Introduction
2. Results
2.1. Establish a Set of Antibodies Suitable for Cross-Species Analysis of Proliferation and “Stemness”
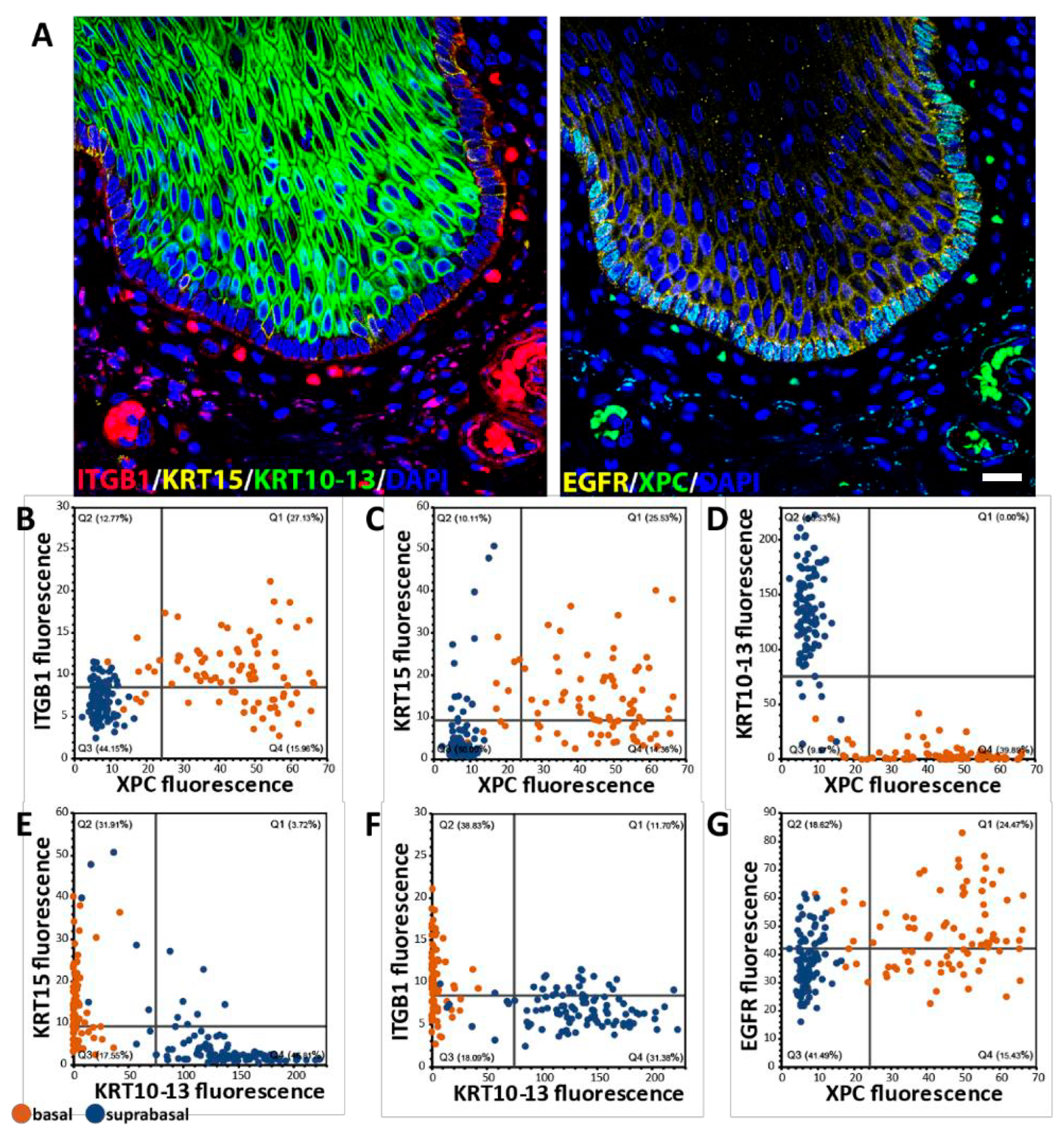
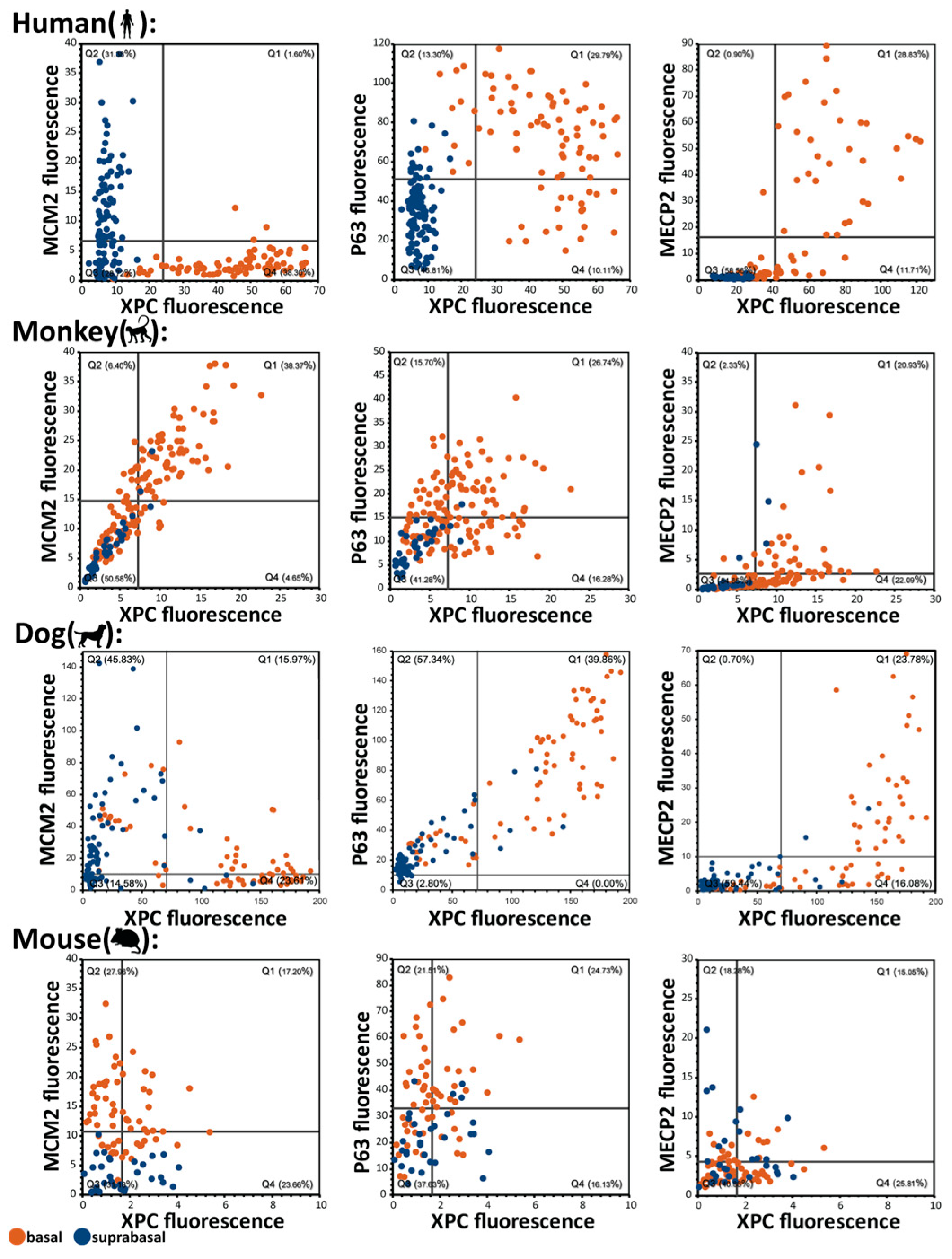
2.2. Basal-Suprabasal Cell Dichotomy in Humans and Absent in Other Analyzed Species: Basal Cell Quiescence and Accompanying MECP2 and XPC Expression Are Human Features
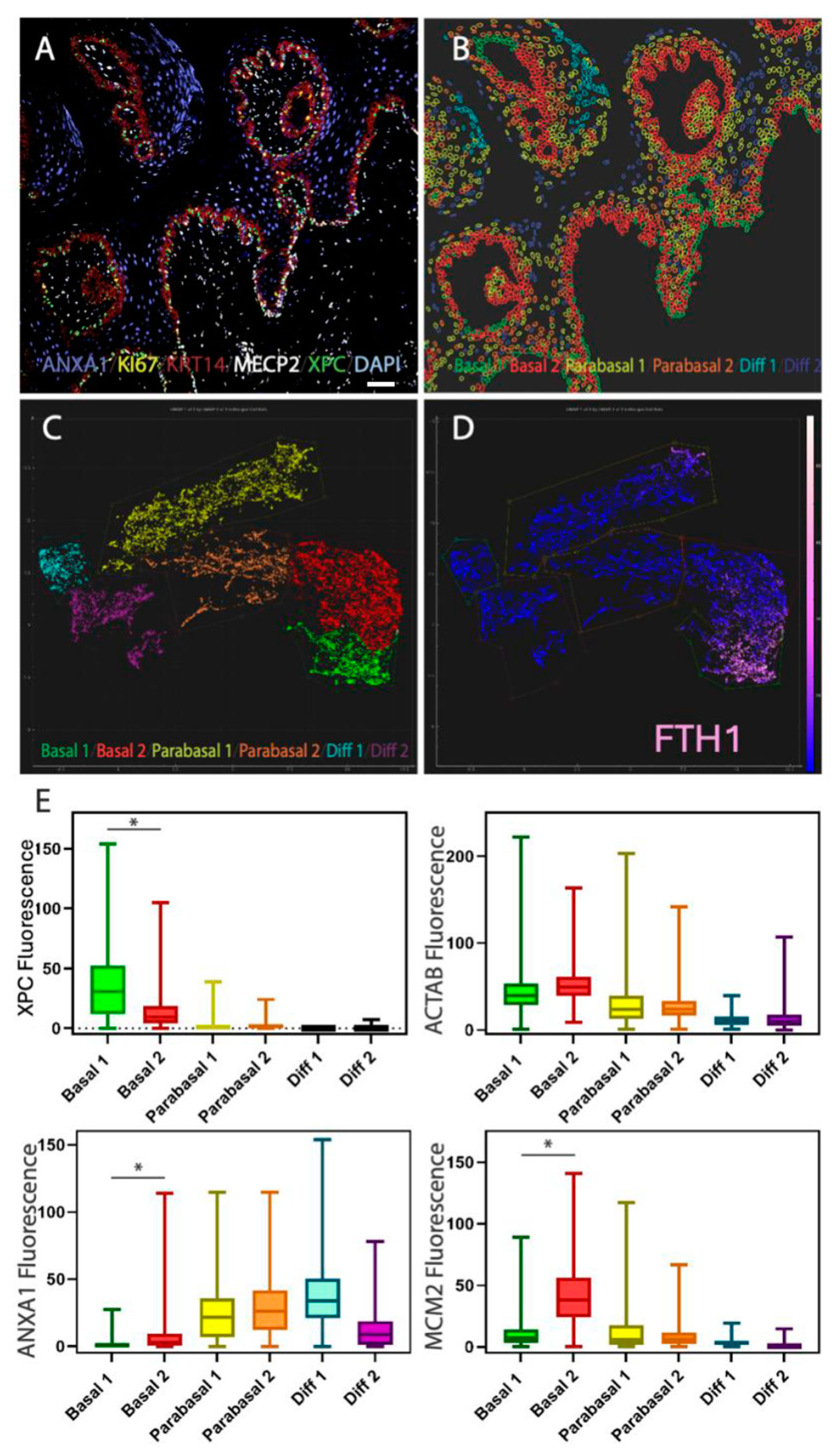
2.3. Single-Cell Analysis of Basal Cells In Situ: Heterogeneity Within the Basal Cell Layer
2.4. Behavioral Heterogeneity in the Basal Cell Layer: Relationship Between Delamination and Proliferation
2.5. Organization of the Basal Cell Layer in Different Species: Canine Oral Basal Cells Exhibit Two Different Types of Basal Cells
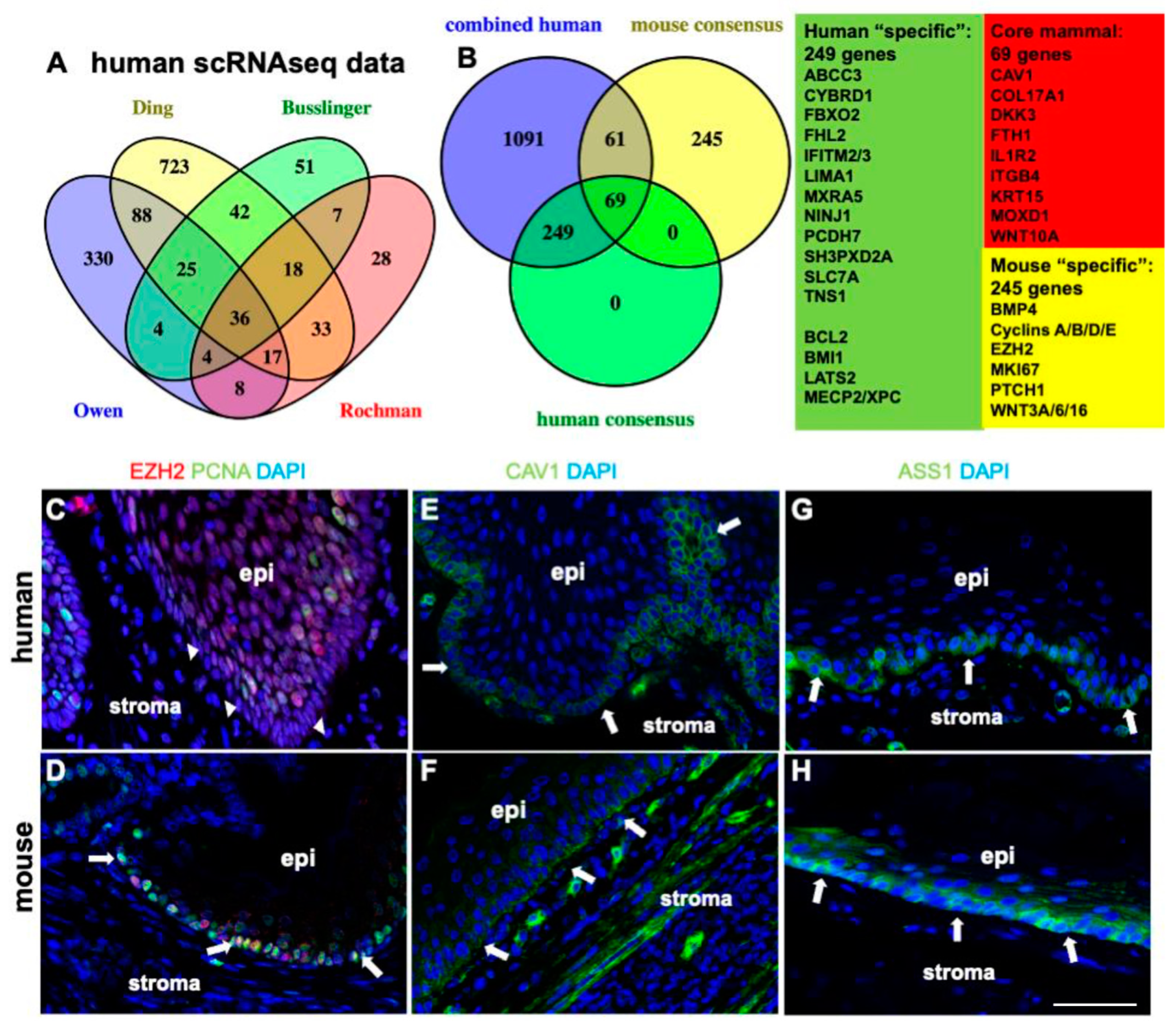
2.6. Comparison of scRNAseq Datasets Confirms Human and Mouse Differences in Basal Cell Proliferation and Proliferation-Associated TGFbeta, WNT, and SHH Pathways
2.7. XPC and MECP2 Interaction Is Restricted to Quiescent Basal Cells
3. Discussion
3.1. The Meaning of Stem Cell Quiescence
3.2. Alternative Explanations
3.3. Proteomic In Situ Analysis Can Detect Putative Features of Longevity in Species Comparisons: XPC and MECP2
3.4. XPC-MECP2 Interactions Are Limited to Quiescent Basal Cells
3.5. Enriched Stem Cell Pathways in Human Squamous Epithelia
3.6. A Combo of Protective Features to Enhance Stem Cell Resilience?
3.7. Limitations of the Study
4. Materials and Methods
4.1. Antibody List
4.2. Antibody Evaluation for Species
4.3. Manual Multiplex Cyclic Immunofluorescence (mcyIF) Stainings
4.4. Automated Multiplex Immunofluorescence Staining Using the Miltenyi MACSima Platform
4.5. Proximity Ligation Assays (PLA)
4.6. Tissues
4.7. Analysis of scRNAseq Data
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Magalhaes, J.P.; Costa, J. A database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 2009, 22, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L. Entropy explains aging, genetic determinism explains longevity, and undefined terminology explains misunderstanding both. PLoS Genet. 2007, 3, e220. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, S.; Rothaus, A.; Zhang, Y.; Luisa Kadekaro, A.; Andl, T.; Andl, C.D. Divide and conquer: Two stem cell populations in squamous epithelia, reserves and the active duty forces. Int. J. Oral. Sci. 2019, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Fushan, A.A.; Turanov, A.A.; Lee, S.G.; Kim, E.B.; Lobanov, A.V.; Yim, S.H.; Buffenstein, R.; Lee, S.R.; Chang, K.T.; Rhee, H.; et al. Gene expression defines natural changes in mammalian lifespan. Aging Cell 2015, 14, 352–365. [Google Scholar] [CrossRef]
- Salmon, A.B.; Leonard, S.; Masamsetti, V.; Pierce, A.; Podlutsky, A.J.; Podlutskaya, N.; Richardson, A.; Austad, S.N.; Chaudhuri, A.R. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009, 23, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Ermolaeva, M.A.; Dakhovnik, A.; Schumacher, B. Quality control mechanisms in cellular and systemic DNA damage responses. Ageing Res. Rev. 2015, 23, 3–11. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? Elife 2021, 10, e62852. [Google Scholar] [CrossRef] [PubMed]
- Ponnappan, S.; Ponnappan, U. Aging and immune function: Molecular mechanisms to interventions. Antioxid. Redox Signal. 2011, 14, 1551–1585. [Google Scholar] [CrossRef]
- Higuchi-Sanabria, R.; Frankino, P.A.; Paul, J.W., 3rd; Tronnes, S.U.; Dillin, A. A Futile Battle? Protein Quality Control and the Stress of Aging. Dev. Cell 2018, 44, 139–163. [Google Scholar] [CrossRef] [PubMed]
- Toren, D.; Kulaga, A.; Jethva, M.; Rubin, E.; Snezhkina, A.V.; Kudryavtseva, A.V.; Nowicki, D.; Tacutu, R.; Moskalev, A.A.; Fraifeld, V.E. Gray whale transcriptome reveals longevity adaptations associated with DNA repair and ubiquitination. Aging Cell 2020, 19, e13158. [Google Scholar] [CrossRef] [PubMed]
- Mertz, T.M.; Harcy, V.; Roberts, S.A. Risks at the DNA Replication Fork: Effects upon Carcinogenesis and Tumor Heterogeneity. Genes 2017, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Seluanov, A.; Hine, C.; Bozzella, M.; Hall, A.; Sasahara, T.H.; Ribeiro, A.A.; Catania, K.C.; Presgraves, D.C.; Gorbunova, V. Distinct tumor suppressor mechanisms evolve in rodent species that differ in size and lifespan. Aging Cell 2008, 7, 813–823. [Google Scholar] [CrossRef]
- Hume, W.J.; Potten, C.S. Advances in epithelial kinetics--an oral view. J. Oral. Pathol. 1979, 8, 3–22. [Google Scholar] [CrossRef]
- Potten, C.S.; Wichmann, H.E.; Loeffler, M.; Dobek, K.; Major, D. Evidence for discrete cell kinetic subpopulations in mouse epidermis based on mathematical analysis. Cell Tissue Kinet. 1982, 15, 305–329. [Google Scholar] [CrossRef]
- Zhang, B.; Hsu, Y.C. Emerging roles of transit-amplifying cells in tissue regeneration and cancer. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e282. [Google Scholar] [CrossRef]
- Carroll, S.B. Evolution at two levels: On genes and form. PLoS Biol. 2005, 3, e245. [Google Scholar] [CrossRef] [PubMed]
- Lubin, A.; Zhang, L.; Chen, H.; White, V.M.; Gong, F. A human XPC protein interactome--a resource. Int. J. Mol. Sci. 2013, 15, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Nemzow, L.; Lubin, A.; Zhang, L.; Gong, F. XPC: Going where no DNA damage sensor has gone before. DNA Repair 2015, 36, 19–27. [Google Scholar] [CrossRef]
- Lucero, R.; Horowitz, D. Xeroderma Pigmentosum. In StatPearls; Treasure Island (FL): Hong Kong, China, 2025. [Google Scholar]
- Jorge-Torres, O.C.; Szczesna, K.; Roa, L.; Casal, C.; Gonzalez-Somermeyer, L.; Soler, M.; Velasco, C.D.; Martinez-San Segundo, P.; Petazzi, P.; Saez, M.A.; et al. Inhibition of Gsk3b Reduces Nfkb1 Signaling and Rescues Synaptic Activity to Improve the Rett Syndrome Phenotype in Mecp2-Knockout Mice. Cell Rep. 2018, 23, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Armstrong, D.; Marsh, E.; Lieberman, D.; Motil, K.; Witt, R.; Standridge, S.; Nues, P.; Lane, J.; Dinkel, T.; et al. Consensus guidelines on managing Rett syndrome across the lifespan. BMJ Paediatr. Open 2020, 4, e000717. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef]
- Andl, C.D.; Le Bras, G.F.; Loomans, H.; Kim, A.S.; Zhou, L.; Zhang, Y.; Andl, T. Association of TGFbeta signaling with the maintenance of a quiescent stem cell niche in human oral mucosa. Histochem. Cell Biol. 2016, 146, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Sa, G.; Xiong, X.; Wu, T.; Yang, J.; He, S.; Zhao, Y. Histological features of oral epithelium in seven animal species: As a reference for selecting animal models. Eur. J. Pharm. Sci. 2016, 81, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kulasinghe, A.; Wood, F.; Belz, G. The seductive allure of spatial biology: Accelerating new discoveries in the life sciences. Immunol. Cell Biol. 2023, 101, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, E.; Borner, G.H.H. Spatial proteomics: A powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 2019, 20, 285–302. [Google Scholar] [CrossRef]
- Berika, M.; Elgayyar, M.E.; El-Hashash, A.H. Asymmetric cell division of stem cells in the lung and other systems. Front. Cell Dev. Biol. 2014, 2, 33. [Google Scholar] [CrossRef]
- Piedrafita, G.; Kostiou, V.; Wabik, A.; Colom, B.; Fernandez-Antoran, D.; Herms, A.; Murai, K.; Hall, B.A.; Jones, P.H. A single-progenitor model as the unifying paradigm of epidermal and esophageal epithelial maintenance in mice. Nat. Commun. 2020, 11, 1429. [Google Scholar] [CrossRef]
- Cockburn, K.; Annusver, K.; Gonzalez, D.G.; Ganesan, S.; May, D.P.; Mesa, K.R.; Kawaguchi, K.; Kasper, M.; Greco, V. Gradual differentiation uncoupled from cell cycle exit generates heterogeneity in the epidermal stem cell layer. Nat. Cell Biol. 2022, 24, 1692–1700. [Google Scholar] [CrossRef]
- Wang, S.; Drummond, M.L.; Guerrero-Juarez, C.F.; Tarapore, E.; MacLean, A.L.; Stabell, A.R.; Wu, S.C.; Gutierrez, G.; That, B.T.; Benavente, C.A.; et al. Single cell transcriptomics of human epidermis identifies basal stem cell transition states. Nat. Commun. 2020, 11, 4239. [Google Scholar] [CrossRef] [PubMed]
- Zebian, A.; El-Dor, M.; Shaito, A.; Mazurier, F.; Rezvani, H.R.; Zibara, K. XPC multifaceted roles beyond DNA damage repair: p53-dependent and p53-independent functions of XPC in cell fate decisions. Mutat. Res. Rev. Mutat. Res. 2022, 789, 108400. [Google Scholar] [CrossRef]
- Enikanolaiye, A.; Ruston, J.; Zeng, R.; Taylor, C.; Schrock, M.; Buchovecky, C.M.; Shendure, J.; Acar, E.; Justice, M.J. Suppressor mutations in Mecp2-null mice implicate the DNA damage response in Rett syndrome pathology. Genome Res. 2020, 30, 540–552. [Google Scholar] [CrossRef]
- Moran-Salvador, E.; Garcia-Macia, M.; Sivaharan, A.; Sabater, L.; Zaki, M.Y.W.; Oakley, F.; Knox, A.; Page, A.; Luli, S.; Mann, J.; et al. Fibrogenic Activity of MECP2 Is Regulated by Phosphorylation in Hepatic Stellate Cells. Gastroenterology 2019, 157, 1398–1412 e1399. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Riccitiello, F.; Squillaro, T.; Capasso, S.; Del Gaudio, S.; Di Bernardo, G.; Cipollaro, M.; Melone, M.A.B.; Peluso, G.; Galderisi, U. Neural stem cells from a mouse model of Rett syndrome are prone to senescence, show reduced capacity to cope with genotoxic stress, and are impaired in the differentiation process. Exp. Mol. Med. 2018, 50, 1. [Google Scholar] [CrossRef] [PubMed]
- Kaddoum, L. La protéine MeCP2: Etude de son Implication dans la Réponse aux Dommages à l’ADN et Développement de Nouveaux Outils pour sa Détection. Ph.D. Thesis, Biochimie et Biologie moléculaire, l’Université Toulouse, Toulouse, France, December 2010. Available online: https://core.ac.uk/download/pdf/12095763.pdf (accessed on 15 January 2024).
- Nasrallah, A.; Rezvani, H.R.; Kobaisi, F.; Hammoud, A.; Rambert, J.; Smits, J.P.H.; Sulpice, E.; Rachidi, W. Generation and characterization of CRISPR-Cas9-mediated XPC gene knockout in human skin cells. Sci. Rep. 2024, 14, 30879. [Google Scholar] [CrossRef] [PubMed]
- DeCasien, A.R.; Barton, R.A.; Higham, J.P. Understanding the human brain: Insights from comparative biology. Trends Cogn. Sci. 2022, 26, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; Denu, J.M. Using comparative biology to understand how aging affects mitochondrial metabolism. Mol. Cell Endocrinol. 2017, 455, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Longworth, K.E. The comparative biology of pulmonary intravascular macrophages. Front. Biosci. 1997, 2, d232–d241. [Google Scholar] [CrossRef]
- Todd, L.; Reh, T.A. Comparative Biology of Vertebrate Retinal Regeneration: Restoration of Vision through Cellular Reprogramming. Cold Spring Harb. Perspect. Biol. 2022, 14, a040816. [Google Scholar] [CrossRef]
- Holderness, J.; Hedges, J.F.; Ramstead, A.; Jutila, M.A. Comparative biology of gammadelta T cell function in humans, mice, and domestic animals. Annu. Rev. Anim. Biosci. 2013, 1, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Cagan, A.; Baez-Ortega, A.; Brzozowska, N.; Abascal, F.; Coorens, T.H.H.; Sanders, M.A.; Lawson, A.R.J.; Harvey, L.M.R.; Bhosle, S.; Jones, D.; et al. Somatic mutation rates scale with lifespan across mammals. Nature 2022, 604, 517–524. [Google Scholar] [CrossRef]
- Kabir, M.F.; Karami, A.L.; Cruz-Acuna, R.; Klochkova, A.; Saxena, R.; Mu, A.; Murray, M.G.; Cruz, J.; Fuller, A.D.; Clevenger, M.H.; et al. Single cell transcriptomic analysis reveals cellular diversity of murine esophageal epithelium. Nat. Commun. 2022, 13, 2167. [Google Scholar] [CrossRef] [PubMed]
- Montazid, S.; Bandyopadhyay, S.; Hart, D.W.; Gao, N.; Johnson, B.; Thrumurthy, S.G.; Penn, D.J.; Wernisch, B.; Bansal, M.; Altrock, P.M.; et al. Adult stem cell activity in naked mole rats for long-term tissue maintenance. Nat. Commun. 2023, 14, 8484. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.N.; Mele, J.; Hornsby, P.J.; Buffenstein, R. Stress resistance in the naked mole-rat: The bare essentials—A mini-review. Gerontology 2012, 58, 453–462. [Google Scholar] [CrossRef]
- Harper, J.M.; Salmon, A.B.; Leiser, S.F.; Galecki, A.T.; Miller, R.A. Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone. Aging Cell 2007, 6, 1–13. [Google Scholar] [CrossRef]
- Bush, S.J.; Nikola, R.; Han, S.; Suzuki, S.; Yoshida, S.; Simons, B.D.; Goriely, A. Adult Human, but Not Rodent, Spermatogonial Stem Cells Retain States with a Foetal-like Signature. Cells 2024, 13, 742. [Google Scholar] [CrossRef]
- Cheung, T.H.; Rando, T.A. Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 2013, 14, 329–340. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Schoennagel, B.; Kacza, J.; Busche, R.; Hornickel, I.N.; Hewicker-Trautwein, M.; Schnapper, A. Keratinization of the esophageal epithelium of domesticated mammals. Acta Histochem. 2014, 116, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Nasjleti, C.J.; Kowalski, C.J.; Castelli, W.A. The effect of mechanical stimulation on the keratinization of sulcular epithelium. J. Periodontol. 1982, 53, 89–92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McIntyre, D.C.; Nance, J. Niche cells regulate primordial germ cell quiescence in response to basement membrane signaling. Development 2023, 150, dev201640. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zheng, Z.; Song, Q. Mesenchymal stem cells: A double-edged sword in radiation-induced lung injury. Thorac. Cancer 2018, 9, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Park, E.S.; Rose, S.A.; Alibo, E.; Sweeney, R.; Dhainaut, M.; Kobayashi, K.S.; Sachidanandam, R.; Baccarini, A.; Merad, M.; et al. Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 2018, 48, 271–285 e275. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Zaikos, T.D.; Collins, K.L. Quiescence Promotes Latent HIV Infection and Resistance to Reactivation from Latency with Histone Deacetylase Inhibitors. J. Virol. 2017, 91, e01080-17. [Google Scholar] [CrossRef]
- Du, Y.; Gupta, P.; Qin, S.; Sieber, M. The role of metabolism in cellular quiescence. J. Cell Sci. 2023, 136, jcs260787. [Google Scholar] [CrossRef]
- Schluter, H.; Paquet-Fifield, S.; Gangatirkar, P.; Li, J.; Kaur, P. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells 2011, 29, 1256–1268. [Google Scholar] [CrossRef]
- Zhang, B.K.; Gines, L. Analysis of Cancer-Resisting Evolutionary Adaptations in Wild Animals and Applications for Human Oncology. J. Mol. Evol. 2024, 92, 685–694. [Google Scholar] [CrossRef]
- Squillaro, T.; Alessio, N.; Cipollaro, M.; Renieri, A.; Giordano, A.; Galderisi, U. Partial silencing of methyl cytosine protein binding 2 (MECP2) in mesenchymal stem cells induces senescence with an increase in damaged DNA. FASEB J. 2010, 24, 1593–1603. [Google Scholar] [CrossRef]
- Baroncelli, L.; Auel, S.; Rinne, L.; Schuster, A.K.; Brand, V.; Kempkes, B.; Dietrich, K.; Muller, M. Oral Feeding of an Antioxidant Cocktail as a Therapeutic Strategy in a Mouse Model of Rett Syndrome: Merits and Limitations of Long-Term Treatment. Antioxidants 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- De Felice, C.; Ciccoli, L.; Leoncini, S.; Signorini, C.; Rossi, M.; Vannuccini, L.; Guazzi, G.; Latini, G.; Comporti, M.; Valacchi, G.; et al. Systemic oxidative stress in classic Rett syndrome. Free Radic. Biol. Med. 2009, 47, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Ciccoli, L.; Signorini, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Hayek, J.; De Felice, C.; Valacchi, G. Increased levels of 4HNE-protein plasma adducts in Rett syndrome. Clin. Biochem. 2011, 44, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, M.; Korsakova, E.; Allen, D.; Lee, P.; Fu, K.; Vargas, B.S.; Cinkornpumin, J.; Salas, C.; Park, J.C.; Germanguz, I.; et al. Loss of MECP2 Leads to Activation of P53 and Neuronal Senescence. Stem Cell Reports 2018, 10, 1453–1463. [Google Scholar] [CrossRef]
- Nan, X.; Tate, P.; Li, E.; Bird, A. DNA methylation specifies chromosomal localization of MeCP2. Mol. Cell Biol. 1996, 16, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.A.; Chew, E.; Flensburg, C.; Zeilemaker, A.; Miller, S.E.; Al Hinai, A.S.; Bajel, A.; Luiken, B.; Rijken, M.; McLennan, T.; et al. MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood 2018, 132, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.; Kernohan, N.M.; Walker, F. Proliferation in the normal cervix and in preinvasive cervical lesions. J. Clin. Pathol. 1996, 49, 667–671. [Google Scholar] [CrossRef][Green Version]
- Dwivedi, N.; Chandra, S.; Kashyap, B.; Raj, V.; Agarwal, A. Suprabasal expression of Ki-67 as a marker for the severity of oral epithelial dysplasia and oral squamous cell carcinoma. Contemp. Clin. Dent. 2013, 4, 7–12. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, H.; Li, X. p53 and Ki-67 combined with periodic acid-Schiff staining for the diagnosis of early stage esophageal squamous cell carcinoma lesions in biopsy specimens. Esophagus 2024, 22, 228–238. [Google Scholar] [CrossRef]
- Kobayashi, T.; Maruyama, S.; Cheng, J.; Ida-Yonemochi, H.; Yagi, M.; Takagi, R.; Saku, T. Histopathological varieties of oral carcinoma in situ: Diagnosis aided by immunohistochemistry dealing with the second basal cell layer as the proliferating center of oral mucosal epithelia. Pathol. Int. 2010, 60, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Shigenaga, M.K.; Park, J.W.; Degan, P.; Ames, B.N. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. USA 1990, 87, 4533–4537. [Google Scholar] [CrossRef]
- Lindahl, T.; Andersson, A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry 1972, 11, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T. Instability and decay of the primary structure of DNA. Nature 1993, 362, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, N.; Kobaisi, F.; Beal, D.; Mahfouf, W.; Ged, C.; Morice-Picard, F.; Fayyad-Kazan, M.; Fayyad-Kazan, H.; Badran, B.; Rezvani, H.R.; et al. Xeroderma Pigmentosum C (XPC) Mutations in Primary Fibroblasts Impair Base Excision Repair Pathway and Increase Oxidative DNA Damage. Front. Genet. 2020, 11, 561687. [Google Scholar] [CrossRef]
- Shell, S.M.; Hawkins, E.K.; Tsai, M.S.; Hlaing, A.S.; Rizzo, C.J.; Chazin, W.J. Xeroderma pigmentosum complementation group C protein (XPC) serves as a general sensor of damaged DNA. DNA Repair 2013, 12, 947–953. [Google Scholar] [CrossRef]
- Abascal, F.; Harvey, L.M.R.; Mitchell, E.; Lawson, A.R.J.; Lensing, S.V.; Ellis, P.; Russell, A.J.C.; Alcantara, R.E.; Baez-Ortega, A.; Wang, Y.; et al. Somatic mutation landscapes at single-molecule resolution. Nature 2021, 593, 405–410. [Google Scholar] [CrossRef]
- Robinson, P.S.; Thomas, L.E.; Abascal, F.; Jung, H.; Harvey, L.M.R.; West, H.D.; Olafsson, S.; Lee, B.C.H.; Coorens, T.H.H.; Lee-Six, H.; et al. Inherited MUTYH mutations cause elevated somatic mutation rates and distinctive mutational signatures in normal human cells. Nat. Commun. 2022, 13, 3949. [Google Scholar] [CrossRef]
- Robinson, P.S.; Coorens, T.H.H.; Palles, C.; Mitchell, E.; Abascal, F.; Olafsson, S.; Lee, B.C.H.; Lawson, A.R.J.; Lee-Six, H.; Moore, L.; et al. Increased somatic mutation burdens in normal human cells due to defective DNA polymerases. Nat. Genet. 2021, 53, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Murat, P.; Perez, C.; Crisp, A.; van Eijk, P.; Reed, S.H.; Guilbaud, G.; Sale, J.E. DNA replication initiation shapes the mutational landscape and expression of the human genome. Sci. Adv. 2022, 8, eadd3686. [Google Scholar] [CrossRef] [PubMed]
- Seplyarskiy, V.B.; Soldatov, R.A.; Koch, E.; McGinty, R.J.; Goldmann, J.M.; Hernandez, R.D.; Barnes, K.; Correa, A.; Burchard, E.G.; Ellinor, P.T.; et al. Population sequencing data reveal a compendium of mutational processes in the human germ line. Science 2021, 373, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Rochman, M.; Wen, T.; Kotliar, M.; Dexheimer, P.J.; Ben-Baruch Morgenstern, N.; Caldwell, J.M.; Lim, H.W.; Rothenberg, M.E. Single-cell RNA-Seq of human esophageal epithelium in homeostasis and allergic inflammation. JCI Insight 2022, 7, e159093. [Google Scholar] [CrossRef]
- Harris, A.; Andl, T. Precancerous Lesions of the Head and Neck Region and Their Stromal Aberrations: Piecemeal Data. Cancers 2023, 15, 2192. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.R.; Key, M.E.; Kalra, K.L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: An enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J. Histochem. Cytochem. 1991, 39, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.Y.; Leong, A.S. How does antigen retrieval work? Adv. Anat. Pathol. 2007, 14, 129–131. [Google Scholar] [CrossRef]
- Owen, R.P.; White, M.J.; Severson, D.T.; Braden, B.; Bailey, A.; Goldin, R.; Wang, L.M.; Ruiz-Puig, C.; Maynard, N.D.; Green, A.; et al. Single cell RNA-seq reveals profound transcriptional similarity between Barrett’s oesophagus and oesophageal submucosal glands. Nat. Commun. 2018, 9, 4261. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Garber, J.J.; Uchida, A.; Lefkovith, A.; Carter, G.T.; Vimalathas, P.; Canha, L.; Dougan, M.; Staller, K.; Yarze, J.; et al. An esophagus cell atlas reveals dynamic rewiring during active eosinophilic esophagitis and remission. Nat. Commun. 2024, 15, 3344. [Google Scholar] [CrossRef]
- Busslinger, G.A.; Weusten, B.L.A.; Bogte, A.; Begthel, H.; Brosens, L.A.A.; Clevers, H. Human gastrointestinal epithelia of the esophagus, stomach, and duodenum resolved at single-cell resolution. Cell Rep. 2021, 34, 108819. [Google Scholar] [CrossRef]
- Pan, X.; Wang, J.; Guo, L.; Na, F.; Du, J.; Chen, X.; Zhong, A.; Zhao, L.; Zhang, L.; Zhang, M.; et al. Identifying a confused cell identity for esophageal squamous cell carcinoma. Signal Transduct. Target. Ther. 2022, 7, 122. [Google Scholar] [CrossRef]
- McGinn, J.; Hallou, A.; Han, S.; Krizic, K.; Ulyanchenko, S.; Iglesias-Bartolome, R.; England, F.J.; Verstreken, C.; Chalut, K.J.; Jensen, K.B.; et al. A biomechanical switch regulates the transition towards homeostasis in oesophageal epithelium. Nat. Cell Biol. 2021, 23, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Hirose, W.; Horiuchi, M.; Li, D.; Motoike, I.N.; Zhang, L.; Nishi, H.; Taniyama, Y.; Kamei, T.; Suzuki, M.; Kinoshita, K.; et al. Selective Elimination of NRF2-Activated Cells by Competition With Neighboring Cells in the Esophageal Epithelium. Cell Mol. Gastroenterol. Hepatol. 2023, 15, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.B.; Furukawa, S.; Marangoni, P.; Ma, H.; Pinkard, H.; D’Urso, R.; Zilionis, R.; Klein, A.M.; Klein, O.D. Quantitative Clonal Analysis and Single-Cell Transcriptomics Reveal Division Kinetics, Hierarchy, and Fate of Oral Epithelial Progenitor Cells. Cell Stem Cell 2019, 24, 183–192.e188. [Google Scholar] [CrossRef] [PubMed]



| Species | MCM2 % | PCNA % | PHH3 % | Ki67 % |
|---|---|---|---|---|
| Human | 40 | 40 | 0 | 20 |
| Dog | 100 | N/A | N/A | 100 |
| Monkey | 50 | N/A | N/A | N/A |
| Mouse | N/A | 100 | 0 | 40 |
| Rat | 100 | 100 | 0 | 50 |
| Basal Cell Marker | Function | Putative Role in Basal Cells | Related Basal Proteins |
|---|---|---|---|
| ABCC3 | cellular waste export of often GSH conjugated molecules; detoxification | enhanced protection against cytotoxic substances. | GSR, GSS |
| ASS1 | urea cycle; arginine synthesis | Without other enzymes of the urea cycle, ASS1 cannot make arginine. However, NOS1 could make arginine into citrulline and ASS1 could use this as substrate to start making arginine again. | SLC3A2 |
| CAV1 | scaffolding protein of caveolae plasma membranes; regulatory function of endocytosis, antiproliferative | enhance adhesion to basal lamina; may sequester growth factor receptors and deepen quiescence; in caveolae: absorbing mechanical stress and preventing cell damage (membrane reservoir); may contribute to deep quiescence due to its antiproliferative effects; differentiation inhibitor; may have link to ferroptosis. | CAV2, CAVIN1, FLOT1, BSG, DST, ITGB1, ITGA6, LIMA1, NGFR, SLC1A3, SLC3A2, SLC7A5, CDH13 |
| CAVIN1 | scaffolding protein of caveolae plasma membranes | enhance adhesion to basal lamina; may sequester growth factor receptors and deepen quiescence; in caveolae: absorbing mechanical stress and preventing cell damage. | CAV1, CAV2, FLOT1 |
| CDH13 | cell membrane and is anchored by a GPI moiety, rather than by a transmembrane domain | may function as an adiponectin receptor? Tumor suppressor? | ITGB1 |
| COL17A1 | attachment of basal keratinocytes to the underlying basement membrane | “household” protein to adhere basal cells to the basal lamina. | DST, LAMC1, ITGB1, ITGB4, |
| CXCL14 | chemoattractant for immune cells; may mediate stromal reprogramming | may mediate antimicrobial activity; problem: how is it kept in an inactive state before needed? Main target may be fibroblasts which express high levels of the putative CXCL14 receptors LRP1 or GPR85. May also be required for the recruitment of dendritic cells and macrophages to sites of infection or inflammation but CXCL4 ko mice have no phenotype; potential anti-tumor activity. Enigma. | |
| CYBRD1 | Iron metabolism, iron uptake | Ferroptosis? Iron metabolism related to FTH1/FTL: reduces iron for storage by ferritin? | FLOT1, LIMA1, FTH1 |
| DKK3 | only receptor for DKK3 is CKAP4. CKAP4 mainly expressed on differentiated keratinocytes, fibroblasts. | ||
| DST | hemidesmosome linker to cytoskeleton | “household” protein to adhere basal cells to the basal lamina | COL17A1, KRT14 |
| FAT1 | cell–cell adhesion, cell polarity, WNT and Hippo signaling | tumor suppressor function may provide a safety feature for stem cells. | LATS1, YAP1 |
| FBXO2 | binds denatured glycoproteins, preferentially those of the high-mannose type | Defense: detection of bacterial surface sugars and viral glycoproteins? Maintenance: Clearance of damaged lysosomes? | |
| FHL2 | scaffolding protein, interaction with dozens of proteins, | maybe advantageous to maintain ER health? Enigma. | |
| IFITM3 | prevents the fusion of viral membranes with host cell membranes | without infection: maintain membrane stability and integrity, Readiness for viral infection and turning of interferon response. Elevated level of readiness. Saves time to reach high enough IFITM3 levels to prevent viral entry. | IFITM1, IFITM2, TLR3, CAV1, |
| IL18 | cytokine | may be “stored” as precursor pro-IL18 to make the cell ready for an emergency and accelerate response times; hypothesis: IL18 exists in its pro-form (supported by PMID: 37068092). | IFITM1, IFITM2, IFITM3 |
| IL1R2 | decoy receptor for IL1 | Dampens inflammatory signaling; maybe protects basal stem cells from activation by “low” level inflammatory signals and maintains a quiescent state even in inflammation (see PMID: 38637492). | |
| ITGB4 | hemidesmosomes | “household” protein to adhere basal cells to the basal lamina. | ITGA6, LAMB2, LAMB3, LAMC1, LAMC2; COL17A1, DST, KRT14 |
| KRT14 | Intermediate filament cytoskeleton | “household” protein to adhere basal cells to the basal lamina. | ITGA6, LAMB2, LAMB3, LAMC1, LAMC2; COL17A1, DST, KRT14 |
| KRT19 | Intermediate filament cytoskeleton | “household” protein to adhere basal cells to the basal lamina. | |
| LAMB3 | basal lamina | “household” protein to adhere basal cells to the basal lamina. | |
| MOXD1 | unknown | Enigma. | |
| MXRA5 | TGF-β1-regulated with anti-inflammatory and anti-fibrotic properties | ||
| NFIB | Transcriptional regulator | Inhibits EZH2, a marker of parabasal cells. May “govern super-enhancer maintenance” specific for quiescent basal stem cells. | SOX2, SOX6 |
| NINJI1 | anti-bacterial host defense by plasma membrane rupture | May supercharge inflammation in case of catastrophic infection of stem cells. | |
| NTRK2 | BDNF receptor | Enigma. | DST, NGFR |
| PCDH7 | cell–cell-adhesion, RHO signaling | MECP2, AHRGEF28 | |
| SH3PXD2A | scaffolding protein prepares a cell for migration, invasion | potentially provides readiness for cell migration. | FSCN1, LIMA1 |
| SLC1A3 | high affinity glutamate transporter | glutamate can be a substrate to make other amino acids or made into glutamine for ammonia detoxification by GLUL. | GLUL |
| SLC1A4 | neutral amino-acid transporter that mediates transport of alanine, serine, cysteine, proline, hydroxyproline and threonine. | Cysteine uptake for GSH production. | GSR, GSS |
| SLC7A5 | uptake of large neutral amino acids | may import amino acids and activate mTOR pathway. | SLC3A2 |
| SOX6 | transcription factor | growth inhibitor; gene targets unknown that may mediate quiescence; may regulate extracellular matrix gene expression; unknown partner in quiescent basal cells. | BCL7A, FLOT1, NFIB, NFIC, SOX2, ZNF503 |
| TNS1 | cell–matrix adhesion | “household” protein to adhere basal cells to the basal lamina. | ITGB1 |
| SUMMARY | 1. Household function in cell–matrix adhesion. 2. Cell protection.3. Many genes that by themselves appear “useless”. | Explanations: 1. readiness for emergencies regarding wounding and infection, nutrient deficiencies; 2. feeder cell function for suprabasal cells (large set of transporter molecules); 3. enhances cellular protection. | IL18, CXCL14, IFITM3, GSR, GSS, SLC3A2, SLC7A5, SLC7A8, SLC1A3, ASS1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, A.; Burnham, K.; Pradhyumnan, R.; Jaishankar, A.; Häkkinen, L.; Góngora-Rosero, R.E.; Piazza, Y.; Andl, C.D.; Andl, T. Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization. Int. J. Mol. Sci. 2025, 26, 3144. https://doi.org/10.3390/ijms26073144
Harris A, Burnham K, Pradhyumnan R, Jaishankar A, Häkkinen L, Góngora-Rosero RE, Piazza Y, Andl CD, Andl T. Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization. International Journal of Molecular Sciences. 2025; 26(7):3144. https://doi.org/10.3390/ijms26073144
Chicago/Turabian StyleHarris, Ashlee, Kaylee Burnham, Ram Pradhyumnan, Arthi Jaishankar, Lari Häkkinen, Rafael E. Góngora-Rosero, Yelena Piazza, Claudia D. Andl, and Thomas Andl. 2025. "Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization" International Journal of Molecular Sciences 26, no. 7: 3144. https://doi.org/10.3390/ijms26073144
APA StyleHarris, A., Burnham, K., Pradhyumnan, R., Jaishankar, A., Häkkinen, L., Góngora-Rosero, R. E., Piazza, Y., Andl, C. D., & Andl, T. (2025). Human-Specific Organization of Proliferation and Stemness in Squamous Epithelia: A Comparative Study to Elucidate Differences in Stem Cell Organization. International Journal of Molecular Sciences, 26(7), 3144. https://doi.org/10.3390/ijms26073144






