Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials
Abstract
1. Introduction
2. Materials and Methods
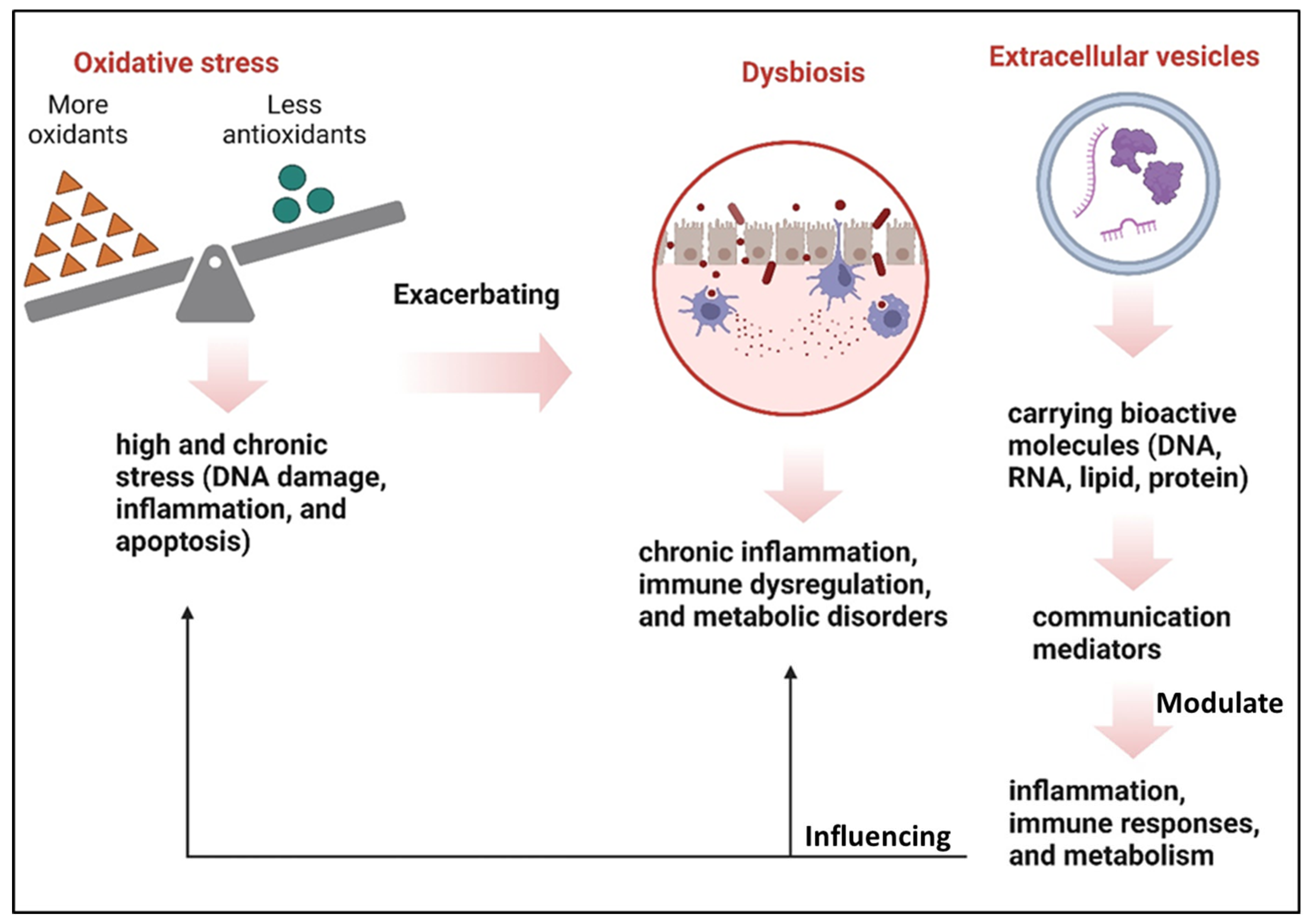
3. Oxidative Stress (OS)
4. Gut Microbiota: Structure and Function
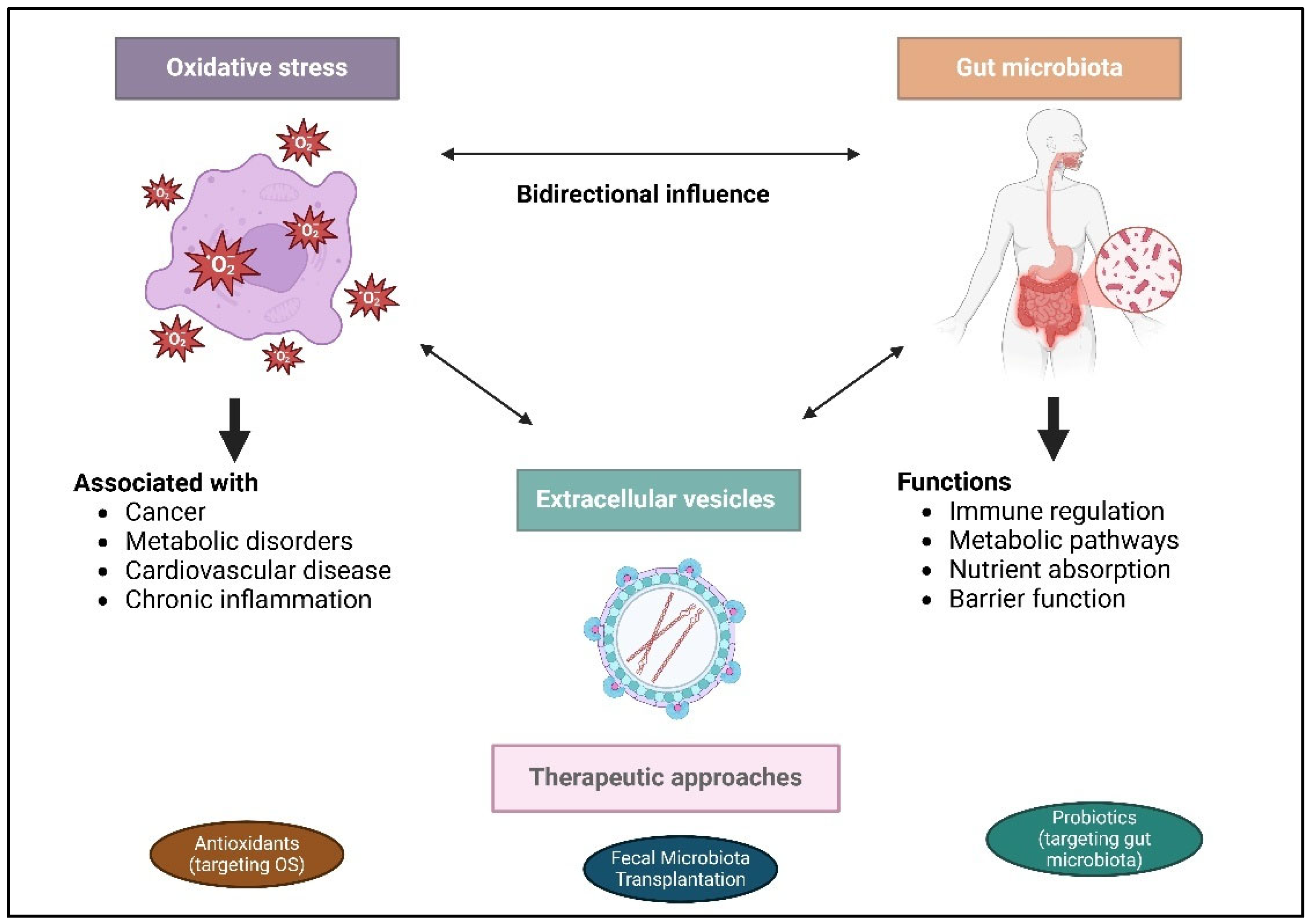
5. Interactions Between OS and Gut Microbiota
6. Extracellular Vesicles as Mediators in Signaling Communication
Interaction of EVs in Gut Microbiota and OS
7. Personalized Therapeutic
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef] [PubMed]
- Kunst, C.; Schmid, S.; Michalski, M.; Tümen, D.; Buttenschön, J.; Müller, M.; Gülow, K. The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines 2023, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short chain fatty acids: Key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef]
- Sultan, S.; Mottawea, W.; Yeo, J.; Hammami, R. Gut Microbiota Extracellular Vesicles as Signaling Molecules Mediating Host-Microbiota Communications. Int. J. Mol. Sci. 2021, 22, 13166. [Google Scholar] [CrossRef]
- Sun, B.; Sawant, H.; Borthakur, A.; Bihl, J.C. Emerging therapeutic role of gut microbial extracellular vesicles in neurological disorders. Front. Neurosci. 2023, 17, 1241418. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Liu, J.; Han, X.; Zhang, T.; Tian, K.; Li, Z.; Luo, F. Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: From mechanism to therapy. J. Hematol. Oncol. 2023, 16, 116. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Ausserlechner, M.J.; Hagenbuchner, J. The Complex Interplay between Mitochondria, ROS and Entire Cellular Metabolism. Antioxidants 2022, 11, 1995. [Google Scholar] [CrossRef] [PubMed]
- Gusti AM, T.; Qusti, S.Y.; Alshammari, E.M.; Toraih, E.A.; Fawzy, M.S. Antioxidants-Related Superoxide Dismutase (SOD), Catalase (CAT), Glutathione Peroxidase (GPX), Glutathione-S-Transferase (GST), and Nitric Oxide Synthase (NOS) Gene Variants Analysis in an Obese Population: A Preliminary Case-Control Study. Antioxidants 2021, 10, 595. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano GA, D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.C.; Hoffmann, C.; Mota, J.F. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1–24. [Google Scholar] [CrossRef]
- Volmer, J.G.; McRae, H.; Morrison, M. The evolving role of methanogenic archaea in mammalian microbiomes. Front. Microbiol. 2023, 14, 1268451. [Google Scholar] [CrossRef]
- Zhang, L.; Zhan, H.; Xu, W.; Yan, S.; Ng, S.C. The role of gut mycobiome in health and diseases. Ther. Adv. Gastroenterol. 2021, 14, 17562848211047130. [Google Scholar] [CrossRef]
- Cao, Z.; Sugimura, N.; Burgermeister, E.; Ebert, M.P.; Zuo, T.; Lan, P. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022, 81, 104113. [Google Scholar] [CrossRef]
- Pargin, E.; Roach, M.J.; Skye, A.; Papudeshi, B.; Inglis, L.K.; Mallawaarachchi, V.; Grigson, S.R.; Harker, C.; Edwards, R.A.; Giles, S.K. The human gut virome: Composition, colonization, interactions, and impacts on human health. Front. Microbiol. 2023, 14, 963173. [Google Scholar] [CrossRef]
- do Socorro Fôro Ramos, E.; de Oliveira Ribeiro, G.; Villanova, F.; de Padua Milagres, F.A.; Brustulin, R.; Araújo EL, L.; Pandey, R.P.; Raj, V.S.; Deng, X.; Delwart, E.; et al. Composition of Eukaryotic Viruses and Bacteriophages in Individuals with Acute Gastroenteritis. Viruses 2021, 13, 2365. [Google Scholar] [CrossRef]
- Metzger, R.N.; Krug, A.B.; Eisenächer, K. Enteric Virome Sensing-Its Role in Intestinal Homeostasis and Immunity. Viruses 2018, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Mihindukulasuriya, K.A.; Mars RA, T.; Johnson, A.J.; Ward, T.; Priya, S.; Lekatz, H.R.; Kalari, K.R.; Droit, L.; Zheng, T.; Blekhman, R.; et al. Multi-Omics Analyses Show Disease, Diet, and Transcriptome Interactions With the Virome. Gastroenterology 2021, 161, 1194–1207.e8. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nature reviews. Microbiology 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Aykur, M.; Malatyalı, E.; Demirel, F.; Cömert-Koçak, B.; Gentekaki, E.; Tsaousis, A.D.; Dogruman-Al, F. Blastocystis: A Mysterious Member of the Gut Microbiome. Microorganisms 2024, 12, 461. [Google Scholar] [CrossRef]
- Watanabe, S.; Kameoka, S.; Shinozaki, N.O.; Kubo, R.; Nishida, A.; Kuriyama, M.; Takeda, A.K. A cross-sectional analysis from the Mykinso Cohort Study: Establishing reference ranges for Japanese gut microbial indices. Biosci. Microbiota Food Health 2021, 40, 123–134. [Google Scholar] [CrossRef]
- Grant, E.T.; Boudaud, M.; Muller, A.; Macpherson, A.J.; Desai, M.S. Maternal diet and gut microbiome composition modulate early-life immune development. EMBO Mol. Med. 2023, 15, e17241. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim MA, K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Bradley, E.; Haran, J. The human gut microbiome and aging. Gut Microbes 2024, 16, 2359677. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. iScience 2022, 26, 105905. [Google Scholar] [CrossRef]
- Fan, P.; Bian, B.; Teng, L.; Nelson, C.D.; Driver, J.; Elzo, M.A.; Jeong, K.C. Host genetic effects upon the early gut microbiota in a bovine model with graduated spectrum of genetic variation. ISME J. 2020, 14, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. MicrobiologyOpen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 572912. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef]
- Quaglio AE, V.; Grillo, T.G.; De Oliveira EC, S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, A.; Wu, X.; Deng, X.; Yang, J.; Xue, J. Heterogeneous changes in gut and tumor microbiota in patients with pancreatic cancer: Insights from clinical evidence. BMC Cancer 2024, 24, 478. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Olteanu, G.; Ciucă-Pană, M.A.; Busnatu, Ș.S.; Lupuliasa, D.; Neacșu, S.M.; Mititelu, M.; Musuc, A.M.; Ioniță-Mîndrican, C.B.; Boroghină, S.C. Unraveling the Microbiome-Human Body Axis: A Comprehensive Examination of Therapeutic Strategies, Interactions and Implications. Int. J. Mol. Sci. 2024, 25, 5561. [Google Scholar] [CrossRef]
- Xiong, R.G.; Li, J.; Cheng, J.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Gan, R.Y.; Li, H.B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kong, L.; Huang, H.; Pan, Y.; Zhang, D.; Jiang, J.; Shen, Y.; Xi, C.; Lai, J.; Ng, C.H.; et al. Gut Microbiota—A Potential Contributor in the Pathogenesis of Bipolar Disorder. Front. Neurosci. 2022, 16, 830748. [Google Scholar] [CrossRef]
- Kandeel, W.A.; Meguid, N.A.; Bjørklund, G.; Eid, E.M.; Farid, M.; Mohamed, S.K.; Wakeel, K.E.; Chirumbolo, S.; Elsaeid, A.; Hammad, D.Y. Impact of Clostridium Bacteria in Children with Autism Spectrum Disorder and Their Anthropometric Measurements. J. Mol. Neurosci. MN 2020, 70, 897–907. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Wang, J.; Chen, S.; Xie, L.; Wu, X. Schizophrenia and obesity: May the gut microbiota serve as a link for the pathogenesis? iMeta 2023, 2, e99. [Google Scholar] [CrossRef] [PubMed]
- Katsimichas, T.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. Gut Microbiota and Coronary Artery Disease: Current Therapeutic Perspectives. Metabolites 2023, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Branchereau, M.; Burcelin, R.; Heymes, C. The gut microbiome and heart failure: A better gut for a better heart. Rev. Endocr. Metab. Disord. 2019, 20, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Kamo, T.; Akazawa, H.; Suda, W.; Saga-Kamo, A.; Shimizu, Y.; Yagi, H.; Liu, Q.; Nomura, S.; Naito, A.T.; Takeda, N.; et al. Dysbiosis and compositional alterations with aging in the gut microbiota of patients with heart failure. PLoS ONE 2017, 12, e0174099. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Toor, D.; Wsson, M.K.; Kumar, P.; Karthikeyan, G.; Kaushik, N.K.; Goel, C.; Singh, S.; Kumar, A.; Prakash, H. Dysbiosis Disrupts Gut Immune Homeostasis and Promotes Gastric Diseases. Int. J. Mol. Sci. 2019, 20, 2432. [Google Scholar] [CrossRef] [PubMed]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Li, L.; Zhong, C.; Zhang, Y.; Yang, X.; Li, M.; Yang, C. The role of gut microbiota in intestinal disease: From an oxidative stress perspective. Front. Microbiol. 2024, 15, 1328324. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, M.; Rout, A.; Kingsley, T.; Kirchoff, R.; Singh, A.; Verma, V.; Kant, R.; Chaudhary, R. Role of gut microbiota in cardiovascular diseases. World J. Cardiol. 2020, 12, 110–122. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, H.; Gao, Y.; An, N.; Li, X.; Pan, X.; Yang, X.; Tian, L.; Sun, J.; Xiong, X.; et al. Gut microbiota-derived short-chain fatty acids and hypertension: Mechanism and treatment. Biomed. Pharmacother.=Biomed. Pharmacother. 2020, 130, 110503. [Google Scholar] [CrossRef]
- Paeslack, N.; Mimmler, M.; Becker, S.; Gao, Z.; Khuu, M.P.; Mann, A.; Malinarich, F.; Regen, T.; Reinhardt, C. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, M. Trimethylamine N-Oxide Generated by the Gut Microbiota Is Associated with Vascular Inflammation: New Insights into Atherosclerosis. Mediat. Inflamm. 2020, 2020, 4634172. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Chiang JY, L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F. The role of the gut microbiota in tumor, immunity, and immunotherapy. Front. Immunol. 2024, 15, 1410928. [Google Scholar] [CrossRef] [PubMed]
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The role of bile acids in carcinogenesis. Cell. Mol. Life Sci. CMLS 2022, 79, 243. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Berni Canani, R.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Acevedo-León, D.; Monzó-Beltrán, L.; Pérez-Sánchez, L.; Naranjo-Morillo, E.; Gómez-Abril, S.Á.; Estañ-Capell, N.; Bañuls, C.; Sáez, G. Oxidative Stress and DNA Damage Markers in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 11664. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, K.; Sudhakar, U.; Balakrishnan, T.; Almasri, M.A.; Al-Ahmari, M.M.; Al Dira, H.S.; Suhluli, M.; Dubey, A.; Mujoo, S.; Khurshid, Z.; et al. Malondialdehyde, an Oxidative Stress Marker in Oral Squamous Cell Carcinoma-A Systematic Review and Meta-Analysis. Curr. Issues Mol. Biol. 2021, 43, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.; Bhalla, P.; Suraishkumar, G.K. Oxidative stress decreases the redox ratio and folate content in the gut microbe, Enterococcus durans (MTCC 3031). Sci. Rep. 2018, 8, 12138. [Google Scholar] [CrossRef]
- Li, X.; Hong, J.; Wang, Y.; Pei, M.; Wang, L.; Gong, Z. Trimethylamine-N-Oxide Pathway: A Potential Target for the Treatment of MAFLD. Front. Mol. Biosci. 2021, 8, 733507. [Google Scholar] [CrossRef]
- Das, T.K.; Ganesh, B.P. Interlink between the gut microbiota and inflammation in the context of oxidative stress in Alzheimer’s disease progression. Gut Microbes 2023, 15, 2206504. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing I A 2023, 20, 67. [Google Scholar] [CrossRef]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, J.; Liu, X.; Zhong, Y.; Cai, X.; Long, L. Review: The Role of Intestinal Dysbiosis in Parkinson’s Disease. Front. Cell. Infect. Microbiol. 2021, 11, 615075. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas SL, B.; Ribeiro, B.E.; Marinho, Y.; de Souza HS, P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The Impact of Gut Microbiota Disorders on the Blood-Brain Barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing intestinal barrier efficiency: A novel metabolic diseases therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospíšil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Rigottier-Gois, L. Dysbiosis in inflammatory bowel diseases: The oxygen hypothesis. ISME J. 2013, 7, 1256–1261. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Kempiński, R.; Bromke, M.A.; Neubauer, K. Oxidative Stress Markers in Inflammatory Bowel Diseases: Systematic Review. Diagnostics 2020, 10, 601. [Google Scholar] [CrossRef]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The emerging role of oxidative stress in inflammatory bowel disease. Front. Endocrinol. 2024, 15, 1390351. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos JC, F.; Araújo OR, P.; Goulart MO, F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Ding, Z.; Ishaq, M.; Bacha, A.S.; Khan, I.; Hanif, A.; Li, W.; Guo, X. Understanding the Effects of Gut Microbiota Dysbiosis on Nonalcoholic Fatty Liver Disease and the Possible Probiotics Role: Recent Updates. Int. J. Biol. Sci. 2021, 17, 818–833. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Solis-Urra, P.; Aragón-Vela, J.; Rodríguez-Rodríguez, F.; Olivares-Arancibia, J.; Álvarez-Mercado, A.I. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines 2021, 9, 145. [Google Scholar] [CrossRef]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2022, 26, 671–683. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef]
- Schwenger, K.J.; Clermont-Dejean, N.; Allard, J.P. The role of the gut microbiome in chronic liver disease: The clinical evidence revised. JHEP Rep. Innov. Hepatol. 2019, 1, 214–226. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Yang, J.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Mariappan, T.; Jeyaraman, N.; Muthu, S.; Ramasubramanian, S.; Santos, G.S.; da Fonseca, L.F.; Lana, J.F. Gut microbiome: A revolution in type II diabetes mellitus. World J. Diabetes 2024, 15, 1874–1888. [Google Scholar] [CrossRef] [PubMed]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Wu, J.; Yang, K.; Fan, H.; Wei, M.; Xiong, Q. Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1114424. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Yan, M.; Man, S.; Sun, B.; Ma, L.; Guo, L.; Huang, L.; Gao, W. Gut liver brain axis in diseases: The implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 443. [Google Scholar] [CrossRef]
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhou, P.; Wang, J.; Lu, X.; Chen, Y. The Characteristics, Mechanisms and Therapeutics: Exploring the Role of Gut Microbiota in Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2023, 16, 3691–3705. [Google Scholar] [CrossRef]
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of Next-Generation Probiotics. Microorganisms 2022, 10, 452. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Cryan, J.F.; Schellekens, H. Gut peptides and the microbiome: Focus on ghrelin. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sharma, P.; Sarma, D.K.; Kumawat, M.; Tiwari, R.; Verma, V.; Nagpal, R.; Kumar, M. Implication of Obesity and Gut Microbiome Dysbiosis in the Etiology of Colorectal Cancer. Cancers 2023, 15, 1913. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; García-Cárdenas, J.M.; Paz-Y-Miño, C. Understanding Celiac Disease From Genetics to the Future Diagnostic Strategies. Clin. Med. Insights. Gastroenterol. 2017, 10, 1179552217712249. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef]
- Anand, N.; Gorantla, V.R.; Chidambaram, S.B. The Role of Gut Dysbiosis in the Pathophysiology of Neuropsychiatric Disorders. Cells 2022, 12, 54. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhou, R.; Wang, X.; Song, L.; Huang, S.; Wang, G.; Xia, B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J. Clin. Microbiol. 2014, 52, 398–406. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Belei, O.; Jugănaru, I.; Basaca, D.G.; Munteanu, A.I.; Mărginean, O. The Role of Intestinal Microbiota in Celiac Disease and Further Therapeutic Perspectives. Life 2023, 13, 2039. [Google Scholar] [CrossRef]
- Moretti, S.; Mrakic-Sposta, S.; Roncoroni, L.; Vezzoli, A.; Dellanoce, C.; Monguzzi, E.; Branchi, F.; Ferretti, F.; Lombardo, V.; Doneda, L.; et al. Oxidative stress as a biomarker for monitoring treated celiac disease. Clin. Transl. Gastroenterol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Zhao, T.; Wei, Y.; Zhu, Y.; Xie, Z.; Hai, Q.; Li, Z.; Qin, D. Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Front. Immunol. 2022, 13, 1007165. [Google Scholar] [CrossRef]
- Wiertsema, S.P.; van Bergenhenegouwen, J.; Garssen, J.; Knippels LM, J. The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies. Nutrients 2021, 13, 886. [Google Scholar] [CrossRef]
- Larsen, J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology 2017, 151, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Mahroum, N.; Seida, R.; Shoenfeld, Y. Triggers and regulation: The gut microbiome in rheumatoid arthritis. Expert Rev. Clin. Immunol. 2023, 19, 1449–1456. [Google Scholar] [CrossRef]
- Merino de Paz, N.; Quevedo-Abeledo, J.C.; Gómez-Bernal, F.; de Vera-González, A.; Abreu-González, P.; Martín-González, C.; González-Gay, M.Á.; Ferraz-Amaro, I. Malondialdehyde Serum Levels in a Full Characterized Series of 430 Rheumatoid Arthritis Patients. J. Clin. Med. 2024, 13, 901. [Google Scholar] [CrossRef]
- Yisireyili, M.; Takeshita, K.; Saito, S.; Murohara, T.; Niwa, T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J. Med. Sci. 2017, 79, 477–486. [Google Scholar] [CrossRef]
- Cao, C.; Zhu, H.; Yao, Y.; Zeng, R. Gut Dysbiosis and Kidney Diseases. Front. Med. 2022, 9, 829349. [Google Scholar] [CrossRef]
- Du, L.; Qi, R.; Wang, J.; Liu, Z.; Wu, Z. Indole-3-Propionic Acid, a Functional Metabolite of Clostridium sporogenes, Promotes Muscle Tissue Development and Reduces Muscle Cell Inflammation. Int. J. Mol. Sci. 2021, 22, 12435. [Google Scholar] [CrossRef]
- Upadhya, D.; Shetty, A.K. MISEV2023 provides an updated and key reference for researchers studying the basic biology and applications of extracellular vesicles. Stem Cells Transl. Med. 2024, 13, 848–850. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan DC, I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks TA, P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Chen, Z. Emerging role of exosomes in cancer therapy: Progress and challenges. Mol. Cancer 2025, 24, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Extracellular Vesicles in Breast Cancer: From Intercellular Communication to Therapeutic Opportunities. Pharmaceutics 2024, 16, 654. [Google Scholar] [CrossRef]
- Babuta, M.; Szabo, G. Extracellular vesicles in inflammation: Focus on the microRNA cargo of EVs in modulation of liver diseases. J. Leukoc. Biol. 2022, 111, 75–92. [Google Scholar] [CrossRef]
- Fyfe, J.; Casari, I.; Manfredi, M.; Falasca, M. Role of lipid signalling in extracellular vesicles-mediated cell-to-cell communication. Cytokine Growth Factor Rev. 2023, 73, 20–26. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, T.; Zhao, C.; Li, G. The Regulation of Exosome Generation and Function in Physiological and Pathological Processes. Int. J. Mol. Sci. 2023, 25, 255. [Google Scholar] [CrossRef]
- Hsu, M.T.; Wang, Y.K.; Tseng, Y.J. Exosomal Proteins and Lipids as Potential Biomarkers for Lung Cancer Diagnosis, Prognosis, and Treatment. Cancers 2022, 14, 732. [Google Scholar] [CrossRef] [PubMed]
- Muttiah, B.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Beyond Blood Clotting: The Many Roles of Platelet-Derived Extracellular Vesicles. Biomedicines 2024, 12, 1850. [Google Scholar] [CrossRef]
- Montanari, M.; Guescini, M.; Gundogdu, O.; Luchetti, F.; Lanuti, P.; Ciacci, C.; Burattini, S.; Campana, R.; Ortolani, C.; Papa, S.; et al. Extracellular Vesicles from Campylobacter jejuni CDT-Treated Caco-2 Cells Inhibit Proliferation of Tumour Intestinal Caco-2 Cells and Myeloid U937 Cells: Detailing the Global Cell Response for Potential Application in Anti-Tumour Strategies. Int. J. Mol. Sci. 2022, 24, 487. [Google Scholar] [CrossRef] [PubMed]
- Melo-Marques, I.; Cardoso, S.M.; Empadinhas, N. Bacterial extracellular vesicles at the interface of gut microbiota and immunity. Gut Microbes 2024, 16, 2396494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, J.; Jiang, M.; Peng, D.; Dou, X.; Song, Y.; Shi, J. The Potential Role of Gut Microbial-Derived Exosomes in Metabolic-Associated Fatty Liver Disease: Implications for Treatment. Front. Immunol. 2022, 13, 893617. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra SV, O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Chiaradia, E.; Tancini, B.; Emiliani, C.; Delo, F.; Pellegrino, R.M.; Tognoloni, A.; Urbanelli, L.; Buratta, S. Extracellular Vesicles under Oxidative Stress Conditions: Biological Properties and Physiological Roles. Cells 2021, 10, 1763. [Google Scholar] [CrossRef]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium butyricum-Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol. Nutr. Food Res. 2023, 67, e2200884. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Akhavan Sepahi, A.; Mousavi, S.F.; Vaziri, F.; Siadat, S.D. The effect of Faecalibacterium prausnitzii and its extracellular vesicles on the permeability of intestinal epithelial cells and expression of PPARs and ANGPTL4 in the Caco-2 cell culture model. J. Diabetes Metab. Disord. 2020, 19, 1061–1069. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Jin, H.; Liu, J.; Wang, D. Antioxidant Potential of Exosomes in Animal Nutrition. Antioxidants 2024, 13, 964. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Gul, L.; Modos, D.; Fonseca, S.; Madgwick, M.; Thomas, J.P.; Sudhakar, P.; Booth, C.; Stentz, R.; Carding, S.R.; Korcsmaros, T. Extracellular vesicles produced by the human commensal gut bacterium Bacteroides thetaiotaomicron affect host immune pathways in a cell-type specific manner that are altered in inflammatory bowel disease. J. Extracell. Vesicles 2022, 11, e12189. [Google Scholar] [CrossRef]
- Krishaa, L.; Ng TK, S.; Wee, H.N.; Ching, J. Gut-brain axis through the lens of gut microbiota and their relationships with Alzheimer’s disease pathology: Review and recommendations. Mech. Ageing Dev. 2023, 211, 111787. [Google Scholar] [CrossRef]
- Tran, S.M.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood-brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef] [PubMed]
- Kasarello, K.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Communication of gut microbiota and brain via immune and neuroendocrine signaling. Front. Microbiol. 2023, 14, 1118529. [Google Scholar] [CrossRef]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. (Landmark Ed.) 2010, 15, 25–34. [Google Scholar] [CrossRef]
- Fonseca, S.; Carvalho, A.L.; Miquel-Clopés, A.; Jones, E.J.; Juodeikis, R.; Stentz, R.; Carding, S.R. Extracellular vesicles produced by the human gut commensal bacterium Bacteroides thetaiotaomicron elicit anti-inflammatory responses from innate immune cells. Front. Microbiol. 2022, 13, 1050271. [Google Scholar] [CrossRef]
- Zheng, T.; Hao, H.; Liu, Q.; Li, J.; Yao, Y.; Liu, Y.; Zhang, T.; Zhang, Z.; Yi, H. Effect of Extracelluar Vesicles Derived from Akkermansia muciniphila on Intestinal Barrier in Colitis Mice. Nutrients 2023, 15, 4722. [Google Scholar] [CrossRef]
- Quadri, Z.; Elsherbini, A.; Crivelli, S.M.; El-Amouri, S.S.; Tripathi, P.; Zhu, Z.; Ren, X.; Zhang, L.; Spassieva, S.D.; Nikolova-Karakashian, M.; et al. Ceramide-mediated orchestration of oxidative stress response through filopodia-derived small extracellular vesicles. J. Extracell. Vesicles 2024, 13, e12477. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Bucci, C.; Marzetti, E. Mitochondrial-Derived Vesicles: The Good, the Bad, and the Ugly. Int. J. Mol. Sci. 2023, 24, 13835. [Google Scholar] [CrossRef]
- Jimenez-Trinidad, F.R.; Calvo-Gomez, S.; Sabaté, M.; Brugaletta, S.; Campuzano, V.; Egea, G.; Dantas, A.P. Extracellular Vesicles as Mediators of Endothelial Dysfunction in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 1008. [Google Scholar] [CrossRef]
- Eustes, A.S.; Dayal, S. The Role of Platelet-Derived Extracellular Vesicles in Immune-Mediated Thrombosis. Int. J. Mol. Sci. 2022, 23, 7837. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Bhat, A.A. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Ponneri Babuharisankar, A.; Lin, Y.C.; Lien, H.W.; Lo, Y.K.; Chou, H.Y.; Tangeda, V.; Cheng, L.C.; Cheng, A.N.; Lee, A.Y. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Wu, Q.; Kan, J.; Fu, C.; Liu, X.; Cui, Z.; Wang, S.; Le, Y.; Li, Z.; Liu, Q.; Zhang, Y.; et al. Insights into the unique roles of extracellular vesicles for gut health modulation: Mechanisms, challenges, and perspectives. Curr. Res. Microb. Sci. 2024, 7, 100301. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, C.; Patil, S.; Dong, S. Unveiling the therapeutic symphony of probiotics, prebiotics, and postbiotics in gut-immune harmony. Front. Nutr. 2024, 11, 1355542. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef]
- Lian, W.S.; Wang, F.S.; Chen, Y.S.; Tsai, M.H.; Chao, H.R.; Jahr, H.; Wu, R.W.; Ko, J.Y. Gut Microbiota Ecosystem Governance of Host Inflammation, Mitochondrial Respiration and Skeletal Homeostasis. Biomedicines 2022, 10, 860. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S.; Sajjad, S.; Sharma, S. Probiotics in Irritable Bowel Syndrome: A Review Article. Cureus 2023, 15, e36565. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef]
- Ballway, J.W.; Song, B.J. Translational Approaches with Antioxidant Phytochemicals against Alcohol-Mediated Oxidative Stress, Gut Dysbiosis, Intestinal Barrier Dysfunction, and Fatty Liver Disease. Antioxidants 2021, 10, 384. [Google Scholar] [CrossRef]
- de Andrade, K.Q.; Moura, F.A.; dos Santos, J.M.; de Araújo, O.R.; de Farias Santos, J.C.; Goulart, M.O. Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef] [PubMed]
- Sahle, Z.; Engidaye, G.; Shenkute Gebreyes, D.; Adenew, B.; Abebe, T.A. Fecal microbiota transplantation and next-generation therapies: A review on targeting dysbiosis in metabolic disorders and beyond. SAGE Open Med. 2024, 12, 20503121241257486. [Google Scholar] [CrossRef]
- Zu, M.; Liu, G.; Xu, H.; Zhu, Z.; Zhen, J.; Li, B.; Shi, X.; Shahbazi, M.A.; Reis, R.L.; Kundu, S.C.; et al. Extracellular Vesicles from Nanomedicine-Trained Intestinal Microbiota Substitute for Fecal Microbiota Transplant in Treating Ulcerative Colitis. Adv. Mater. 2024, 36, e2409138. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
| Health Condition | Gut Microbiota Changes | Potential Effects | References |
|---|---|---|---|
| Irritable Bowel Syndrome (IBS) | ↑ Enterobacteriaceae, Lactobacillaceae, Bacteroides ↓ Faecalibacterium, Bifidobacterium | Abdominal pain, bloating, altered bowel habits | [36,37] |
| Pancreatic Diseases | ↑ Escherichia-Shigella, Firmicutes and Actinobacteria ↓ Faecalibacterium, Bifidobacterium | Chronic inflammation, pancreatic fibrosis, increased cancer risk | [38] |
| Obesity | ↑ Bacillota/Bacteroidetes ratio (>1) ↓ Faecalibacterium prausnitzii | Increased obesity risk, insulin resistance | [39] |
| Anxiety Disorders | ↓ Microbial richness and diversity ↓ Bacillota ↑ Bacteroidetes, Fusobacteria | Associated with higher anxiety levels | [40] |
| Depression | ↓ Dialister, Coprococcus ↑ Klebsiella | Linked to depressive symptoms | [41] |
| Bipolar Disorder | ↓ Faecalibacterium, Ruminococcaceae, Christensenellaceae ↑ Clostridiaceae, Collinsella, Flavonifractor, Pseudomonadaceae | Associated with bipolar disorder symptoms | [42] |
| Autism Spectrum Disorder (ASD) | ↑ Clostridium species | May influence ASD symptoms | [43] |
| Schizophrenia | ↑ Proteobacteria ↓ Faecalibacterium | Associated with schizophrenia symptoms | [44] |
| Atherosclerosis & Coronary Artery Disease | ↑ TMAO-producing bacteria, Enterobacteriaceae | Promotes systemic inflammation, endothelial dysfunction, lipid imbalance | [45] |
| Heart Failure (HF) | ↓ Faecalibacterium prausnitzii, SCFA producers ↑ Gram-negative bacteria (Escherichia, Shigella) | Increased intestinal permeability, systemic inflammation, worsening cardiac function | [46] |
| ↑ Blautia, Dialister (in milder cases) ↓ Bacteroidota, ↑ Pseudomonadota (in older patients) | [47] | ||
| Changes in Ruminococcus, Collinsella, Eubacterium, Lachnospiraceae | [48] |
| Disease | Microbiota Changes | Oxidative Stress Markers | Key Interactions | References |
|---|---|---|---|---|
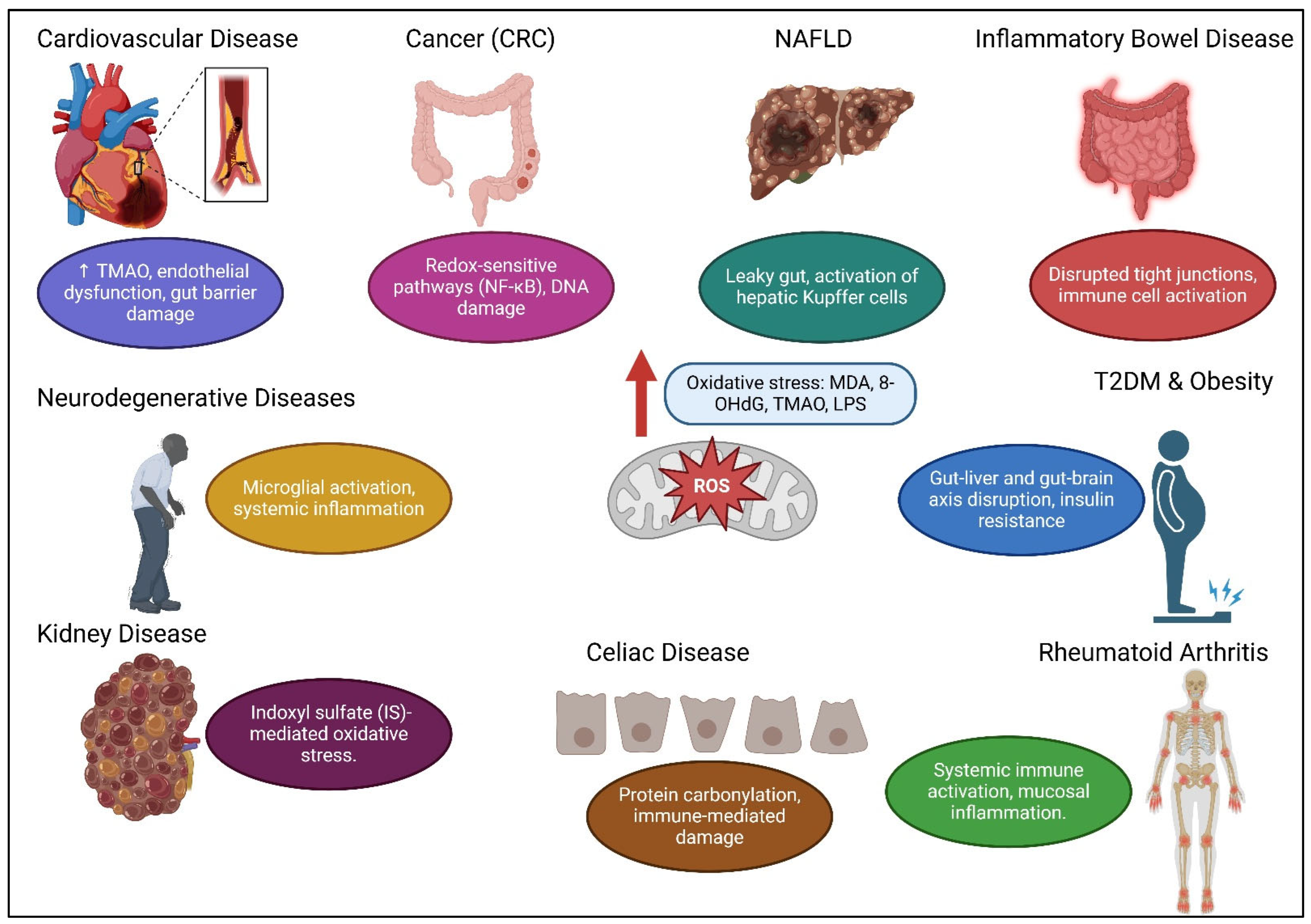
| Cardiovascular Disease | • Loss of microbial diversity • Increase in pro-inflammatory species • Decreased SCFA production | • Increased ROS • Elevated TMAO • Increased LPS | • Compromised gut barrier allows bacterial toxins to enter circulation • Reduced SCFAs | [54,55,56,57,58,59,60] |
| Cancer (particularly CRC) | • Increased pro-inflammatory metabolites • Elevated secondary bile acids and LPS | • Decreased TAC, GSH, and CAT • Increased MDA and ROS | • Activation of redox-sensitive pathways (NF-κB) • Chronic inflammation and DNA damage | [61,62,63,64,65,66,67] |
| Neurodegenerative Diseases (AD, PD) | • Enhanced gut permeability • Disturbed gut microbiota composition | • Increased ROS generation • OS-mediated mitochondrial dysfunction | • Bacterial endotoxins trigger systemic inflammation • Microglial activation via TLR4 and NF-κB | [68,69,70,71,72] |
| Inflammatory Bowel Disease (IBD) | • Depletion of beneficial microbes (Firmicutes, Bacteroidetes) • Overrepresentation of pro-inflammatory taxa (Proteobacteria, Enterobacteriaceae) | • Elevated TOS and OSI • Increased MDA and 8-OHdG • Reduced GSH and SOD | • Compromised tight junctions and intestinal barrier • Microbial translocation stimulates immune cells | [73,74,75,76,77,78,79,80,81,82,83,84] |
| Non-alcoholic Fatty Liver Disease (NAFLD) | • Increased Firmicutes • Decreased Bacteroidetes • Increased intestinal permeability | • Elevated TLR4 • Increased PAMPs (e.g., LPS) | • Microbial metabolites enter bloodstream through “leaky gut” • Activation of hepatic Kupffer cells and hepatocytes | [85,86,87,88,89,90,91] |
| Type 2 Diabetes Mellitus (T2DM) | • Reduced beneficial Firmicutes • Overgrowth of pathogenic Proteobacteria | • Increased ROS | • Systemic inflammation and insulin resistance • Disruption of gut-liver and gut-brain axes | [92,93,94,95,96,97] |
| Obesity | • Increased Firmicutes-to-Bacteroidetes ratio • Altered intestinal barrier function | • Systemic inflammation | • Greater energy extraction from food • Changes in gut peptides involved in satiety (ghrelin, peptide YY) | [98,99,100,101,102] |
| Celiac Disease (CD) | • Lower levels of beneficial bacteria (Bifidobacterium, Lactobacillus) • Increased pro-inflammatory bacteria (Bacteroides, E. coli, Staphylococcus) | • Elevated ROS levels • Increased lipid peroxidation products (MDA) | • Oxidative modification of proteins (protein carbonylation) • Immune-mediated damage | [103,104,105,106,107,108,109,110,111,112,113] |
| Rheumatoid Arthritis (RA) | • Increased Prevotella copri (pro-inflammatory) • Decreased Bacteroides fragilis (anti-inflammatory) | • Increased MDA | • Disrupted immune homeostasis • Systemic immune activation • Mucosal inflammation | [114,115,116,117,118] |
| Kidney Disease | • Increased proteolytic bacteria • Altered tryptophan metabolism | • Increased indoxyl sulfate (IS) | • IS activates AHR and Stat3 pathways • IPA (antioxidant) prevents Stat3 phosphorylation | [119,120,121] |
| Feature | Small EVs (sEVs) | Large EVs (lEVs) |
|---|---|---|
| Size | <200 nm | >200 nm (typically 200–1000 nm, including apoptotic bodies >1000 nm) |
| Biogenesis | Endosomal origin (formed via multivesicular bodies—MVBs and released by exocytosis) | Plasma membrane shedding or direct budding; apoptotic bodies result from cell fragmentation |
| Isolation Methods | Ultracentrifugation, size-exclusion chromatography (SEC), density gradient centrifugation | Differential centrifugation, filtration, size-exclusion chromatography |
| Membrane Markers | CD9, CD63, CD81 (tetraspanins) | Integrins (ITGB1, ITGA2B), Annexins (ANXA5), Flotillin-1 (FLOT1) |
| Cytoskeletal Proteins | Actin, TSG101, ALIX | Actinin-4 (ACTN4), Tubulin, Myosin |
| Lipid Raft-Associated Proteins | Flotillin-1 (FLOT1), Caveolin-1 (CAV1) | Flotillin-1 (FLOT1), Caveolin-1 (CAV1) (also found in lEVs) |
| Membrane Trafficking Proteins | Rab GTPases (Rab27a, Rab5), ESCRT components (TSG101, ALIX) | ARF6, VAMP3, Rab22A |
| Apoptotic Markers | Absent (unless from dying cells) | Histones (H3, H2B), Caspase-3, Annexin V (specific to apoptotic bodies) |
| Mitochondrial Markers | Usually absent or very low | TOMM20, ATP5A (may be enriched in lEVs, but should be validated to avoid contamination) |
| RNA Content | Enriched in miRNAs, lncRNAs, mRNAs | Contains mRNAs, rRNAs, and some miRNAs but varies based on origin |
| Protein Content | Enriched in cytosolic and membrane proteins (e.g., ALIX, TSG101, CD63) | Contains cytoskeletal and apoptotic proteins (e.g., actin, caspases, histones) |
| Functional Roles | Intercellular communication, cargo delivery, immune modulation, tumor progression | Cell signaling, immune modulation, removal of cellular debris, apoptosis |
| Type of EV | Source | Effects on OS | Mechanisms | Associated Diseases |
|---|---|---|---|---|
| MDEVs | Lactobacillus, Bifidobacterium | Reduce OS | Antioxidant enzyme transfer (SOD, CAT, GPx), SCFAs, miRNA-mediated OS regulation | IBD, CRC, metabolic disorders |
| sEVs | Cancer cells, immune cells | Promote OS | Ceramide signaling, oxidative DNA damage, immune evasion | Cancer progression |
| MDVs | Stressed mitochondria | Promote OS | Transfer of oxidized mitochondrial constituents, mitochondrial dysfunction | Diabetic foot ulcers, metabolic diseases |
| Erythrocyte-derived EVs | Senescent RBCs | Promote OS | Free iron, heme, oxidative enzymes disrupt NO signaling | CVD, Type 2 diabetes |
| Platelet-derived EVs | Activated platelets | Promote OS | Thromboxane, NADPH oxidase activation, vascular oxidative stress | Thrombotic disorders, inflammation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Barathan, M.; Ng, M.H.; Law, J.X. Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. Int. J. Mol. Sci. 2025, 26, 3148. https://doi.org/10.3390/ijms26073148
Ma B, Barathan M, Ng MH, Law JX. Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. International Journal of Molecular Sciences. 2025; 26(7):3148. https://doi.org/10.3390/ijms26073148
Chicago/Turabian StyleMa, Bo, Muttiah Barathan, Min Hwei Ng, and Jia Xian Law. 2025. "Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials" International Journal of Molecular Sciences 26, no. 7: 3148. https://doi.org/10.3390/ijms26073148
APA StyleMa, B., Barathan, M., Ng, M. H., & Law, J. X. (2025). Oxidative Stress, Gut Microbiota, and Extracellular Vesicles: Interconnected Pathways and Therapeutic Potentials. International Journal of Molecular Sciences, 26(7), 3148. https://doi.org/10.3390/ijms26073148







