Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance
Abstract
1. Introduction
2. Case-Study Examples of Emerging GMM Applications
2.1. GM Microalgae for Biofuel Production
Case Study 1: GM Nannochloropsis gaditana and GM Chlamydomonas reinhardtii for the Production of Biofuels
2.2. GM Bacteria for Use as Biofertilizers
Case Study 2: GM Klebsiella variicola and GM Azotobacter vinelandii as Biofertilizing Agents
3. Considerations for the Environmental Risk Assessment (ERA) for GMMs
3.1. Overall Framework for an ERA of GMMs in the European Union
3.2. Protection Goals to Be Considered During ERA in the European Union
3.3. Exposure of the Environment Through the Intended and Unintended Release of GMMs
3.3.1. Exposure of the Environment to GM Microalgae During Production of Biofuels
- Dispersal by air in aerosols, or by wildlife, humans or equipment (e.g., if cultivation ponds are not tightly covered);
- Spillage during handling of algae suspensions during production, harvesting, or transport (e.g., via wastewater, drainage water);
- Spillage of microalgae cultures, e.g., due to extreme weather events or floods;
- Failure of containment (e.g., accidental leakage from bioreactors or cultivation ponds).
3.3.2. Exposure of the Environment to GM Bacteria Used as Biofertilizers
3.4. Relevant Risk Issues for the ERA of GM Microalgae Used for Biofuel Production
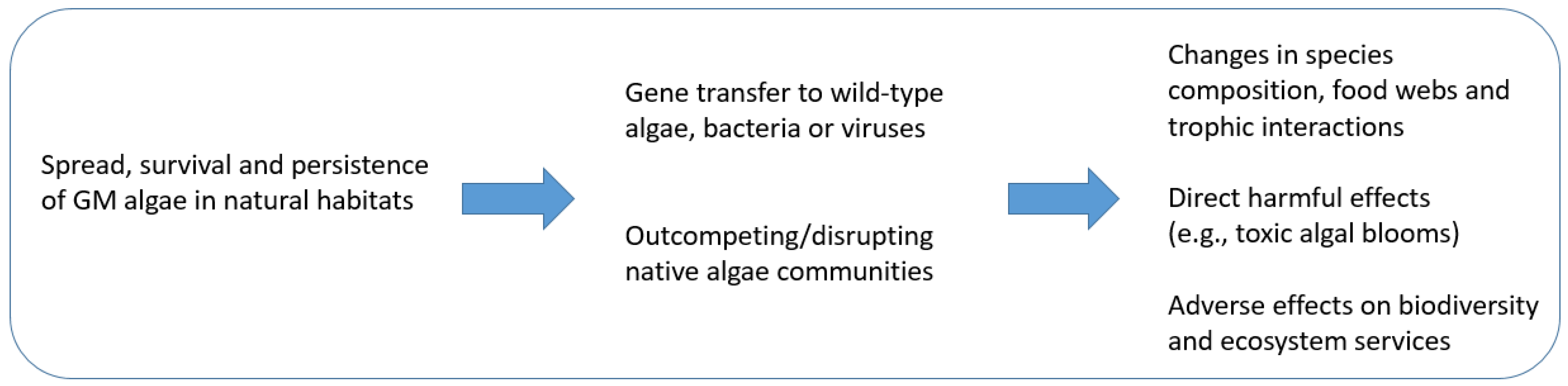
3.4.1. Fitness, Survival, and Persistence of GM Microalgae in Natural Habitats
3.4.2. Gene Transfer to Wild-Type Microalgae, Bacteria, or Viruses
3.4.3. Adverse Effects on Natural Communities, Food Webs, Biodiversity, and Ecosystem Services
3.5. Relevant Risk Issues for the ERA of GM Bacteria Used as Biofertilizers
3.5.1. Fitness, Survival, and Persistence of GM Bacteria from Biofertilizers in Natural Habitats
3.5.2. Genetic Stability and Horizontal Gene Transfer
3.5.3. Adverse Effects on Natural Communities, Biodiversity, and Ecosystem Services
3.5.4. Adverse Effects on Human Health
3.6. Adequacy of the Existing EU Guidance for the ERA of GMMs
3.6.1. A Comparative Assessment May Not Be Applicable for Certain Types of GMMs
3.6.2. The Guidance for Microbial Characterization Needs to Be Updated
3.6.3. The Guidance for the Molecular Characterization of the GMM Needs to Be Updated
3.6.4. The Guidance for the Assessment of Health Effects (Toxicological, Allergenic, and Pathogenic Effects) Needs to Be Further Developed
3.6.5. Guidance for the Assessment of Ecotoxicological Effects Needs to Be Developed
3.6.6. The Exposure Assessment Needs to Address All Potential Receiving Environments
3.6.7. The Guidance for ERA Needs to Be Updated
3.6.8. Specific Guidance for PMEM of GMMs Needs to Be Developed
4. Considerations for a Broader Assessment of GMM Applications Beyond ERA
4.1. Sustainability Analysis of Environmental GMM Applications
4.1.1. Framing of the Sustainability Analysis
Case Study 1: GM Microalgae for Biofuel Production (CS1)
Case Study 2: GM Bacteria for Use as Biofertilizer (CS2)
4.1.2. Issues to Be Addressed by the Sustainability Analysis
Sustainability Issues Relevant for GM Microalgae for Biofuel Production
Sustainability Issues for GM Biofertilizers
4.2. Broader Consideration of Governance Issues Raised by GMM Applications by Technology Assessment (TA)
5. Challenges for the Assessment of GMM Applications, Particularly for ERA, Sustainability Analysis, and Governance of Such Applications
5.1. Open Issues for the ERA of GMMs
5.2. Open Issues Regarding a Broader Assessment of GMM Applications
- The scope and the objectives of a sustainability analysis needs to be defined as a starting point;
- A process to structure such a sustainability analysis has to be devised. This may be best achieved by a multidisciplinary and iterative process as proposed for the sustainability assessment of GM crops by Wohlfender-Bühler et al. [180];
- The nexus to the existing requirements for ERA is not well defined, particularly for issues regarding ecological aspects of a sustainability analysis;
- It also needs to be worked out how the results of a sustainability analysis can be communicated to the regulators and the public.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jackson, D.A.; Symons, R.H.; Berg, P. Biochemical method for inserting new genetic information into DNA of Simian Virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Report of the Multidisciplinary Ad Hoc Technical Expert Group on Synthetic Biology to Support the Process for Broad and Regular Horizon Scanning, Monitoring and Assessment on Its Second Meeting. CBD/SYNBIO/AHTEG/2024/1/3; Montreal, QC, Canada. 2024. Available online: https://www.cbd.int/doc/c/a26e/a6dc/571dd825eb08eef3865f85de/synbio-ahteg-2024-01-03-en.pdf (accessed on 27 November 2024).
- Kumar, K.; Gambhir, G.; Dass, A.; Tripathi, A.K.; Singh, A.; Jha, A.K.; Yadava, P.; Choudhary, M.; Rakshit, S. Genetically modified crops: Current status and future prospects. Planta 2020, 251, 91. [Google Scholar] [CrossRef]
- Berg, P.; Baltimore, D.; Brenner, S.; Roblin, R.O.; Singer, M.F. Summary statement of the Asilomar conference on recombinant DNA molecules. Proc. Natl. Acad. Sci. USA 1975, 72, 1981–1984. [Google Scholar] [CrossRef]
- Ahmad, A.; Munawar, N.; Khan, Z.; Qusmani, A.T.; Khan, S.H.; Jamil, A.; Ashraf, S.; Ghouri, M.Z.; Aslam, S.; Mubarik, M.S.; et al. An Outlook on Global Regulatory Landscape for Genome-Edited Crops. Int. J. Mol. Sci. 2021, 22, 11753. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques-Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.F.; Miklau, M.; Gaugitsch, H. New Plant Breeding Techniques and Risks Associated with Their Application; Environment Agency Austria: Vienna, Austria, 2014. [Google Scholar]
- Eckerstorfer, M.F.; Dolezel, M.; Engelhard, M.; Giovannelli, V.; Grabowski, M.; Heissenberger, A.; Lener, M.; Reichenbecher, W.; Simon, S.; Staiano, G.; et al. Recommendations for the Assessment of Potential Environmental Effects of Genome-Editing Applications in Plants in the EU. Plants 2023, 12, 1764. [Google Scholar] [CrossRef] [PubMed]
- Duensing, N.; Sprink, T.; Parrott, W.A.; Fedorova, M.; Lema, M.A.; Wolt, J.D.; Bartsch, D. Novel Features and Considerations for ERA and Regulation of Crops Produced by Genome Editing. Front. Bioeng. Biotechnol. 2018, 6, 79. [Google Scholar] [CrossRef]
- Sprink, T.; Wilhelm, R. Genome Editing in Biotech Regulations Worldwide. In A Roadmap for Plant Genome Editing; Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., van Laere, K., Woźniak-Gientka, E., Eds.; Springer: Cham, Switzerland, 2024; pp. 425–435. ISBN 978-3-031-46149-1. [Google Scholar]
- OECD. Biosafety and the Environmental Uses of Micro-Organisms: Conference Proceedings, Proceedings of the Conference on Environmental Uses of Micro-Organisms: An Overview of the State-of-the-Art and Implications for Biotechnology Risk/Safety Assessment, Paris, France, 26–27 March 2012; OECD Publishing: Paris, France, 2015; ISBN 9789264204959. [Google Scholar]
- Ballester, A.-R.; Roqué, M.; Ricci-Cabello, I.; Rotger, A.; Malih, N. Horizon scanning on microorganisms and their products obtained by new developments in biotechnology. EFSA Support. Publ. 2023, 20, 8503E. [Google Scholar] [CrossRef]
- SCBD. Synthetic Biology—CBD Technical Series 100. Available online: https://www.cbd.int/doc/publications/cbd-ts-100-en.pdf (accessed on 23 November 2023).
- Miklau, M.; Burn, S.-J.; Eckerstorfer, M.; Dolezel, M.; Greiter, A.; Heissenberger, A.; Hörtenhuber, S.; Zollitsch, W.; Hagen, K. Horizon scanning of potential environmental applications of terrestrial animals, fish, algae and microorganisms produced by genetic modification, including the use of new genomic techniques. Front. Genome Ed. 2024, 6, 1376927. [Google Scholar] [CrossRef]
- Azizoglu, U.; Jouzani, G.S.; Yilmaz, N.; Baz, E.; Ozkok, D. Genetically modified entomopathogenic bacteria, recent developments, benefits and impacts: A review. Sci. Total Environ. 2020, 734, 139169. [Google Scholar] [CrossRef] [PubMed]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.F.; Dolezel, M.; Miklau, M.; Greiter, A.; Heissenberger, A.; Engelhard, M. Scanning the Horizon for Environmental Applications of Genetically Modified Viruses Reveals Challenges for Their Environmental Risk Assessment. Int. J. Mol. Sci. 2024, 25, 1507. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Pan, H.; Zhang, X.; Zhang, Y. Genetically engineered bacterium: Principles, practices, and prospects. Front. Microbiol. 2022, 13, 997587. [Google Scholar] [CrossRef]
- Pant, G.; Garlapati, D.; Agrawal, U.; Prasuna, R.G.; Mathimani, T.; Pugazhendhi, A. Biological approaches practised using genetically engineered microbes for a sustainable environment: A review. J. Hazard. Mater. 2021, 405, 124631. [Google Scholar] [CrossRef]
- Sproles, A.E.; Fields, F.J.; Smalley, T.N.; Le, C.H.; Badary, A.; Mayfield, S.P. Recent advancements in the genetic engineering of microalgae. Algal Res. 2021, 53, 102158. [Google Scholar] [CrossRef]
- Liang, Z.-C.; Liang, M.-H.; Jiang, J.-G. Transgenic microalgae as bioreactors. Crit. Rev. Food Sci. Nutr. 2020, 60, 3195–3213. [Google Scholar] [CrossRef]
- Dolezel, M.; Lang, A.; Greiter, A.; Miklau, M.; Eckerstorfer, M.; Heissenberger, A.; Willée, E.; Züghart, W. Challenges for the Post-Market Environmental Monitoring in the European Union Imposed by Novel Applications of Genetically Modified and Genome-Edited Organisms. BioTech 2024, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Komen, J. The emerging international regulatory framework for biotechnology. GM Crops Food 2012, 3, 78–84. [Google Scholar] [CrossRef]
- Lerner, A.; Benzvi, C.; Vojdani, A. The Potential Harmful Effects of Genetically Engineered Microorganisms (GEMs) on the Intestinal Microbiome and Public Health. Microorganisms 2024, 12, 238. [Google Scholar] [CrossRef]
- Hanlon, P.; Sewalt, V. GEMs: Genetically engineered microorganisms and the regulatory oversight of their uses in modern food production. Crit. Rev. Food Sci. Nutr. 2021, 61, 959–970. [Google Scholar] [CrossRef]
- Shams, A.; Fischer, A.; Bodnar, A.; Kliegman, M. Perspectives on Genetically Engineered Microorganisms and Their Regulation in the United States. ACS Synth. Biol. 2024, 13, 1412–1423. [Google Scholar] [CrossRef]
- Ahmad, J.; Grunden, A.; Kuzma, J. Biotechnology executive order opens door for regulatory reform and social acceptance of genetically engineered microbes in agriculture. GM Crops Food 2024, 15, 248–261. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Jiang, J.-Y.; Shi, T.-Q.; Sun, X.-M.; Zhao, Q.-Y.; Huang, H.; Ren, L.-J. Application of the CRISPR/Cas system for genome editing in microalgae. Appl. Microbiol. Biotechnol. 2019, 103, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.-R.; Jang, J.; Jin, E. Genome engineering via gene editing technologies in microalgae. Bioresour. Technol. 2023, 373, 128701. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, J.; Fa, Y.; Liu, X.; Lindblad, P. Enhancing microalgal lipid accumulation for biofuel production. Front. Microbiol. 2022, 13, 1024441. [Google Scholar] [CrossRef]
- Patel, V.K.; Soni, N.; Prasad, V.; Sapre, A.; Dasgupta, S.; Bhadra, B. CRISPR-Cas9 System for Genome Engineering of Photosynthetic Microalgae. Mol. Biotechnol. 2019, 61, 541–561. [Google Scholar] [CrossRef]
- Vazquez Calderon, F.; Sanchez Lopez, J. An Overview of the Algae Industry in Europe: Producers, Production Systems, Species, Biomass Uses, Other Steps in the Value Chain and Socio-Economic Data; Publications Office of the European Union: Luxembourg, 2022; ISBN 978-92-76-54516-3. [Google Scholar]
- European Commission. Towards a Strong and Sustainable EU Algae Sector: Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions COM(2022) 592 Final. 2022. Available online: https://oceans-and-fisheries.ec.europa.eu/publications/communication-commission-towards-strong-and-sustainable-eu-algae-sector_en (accessed on 9 April 2024).
- Beacham, T.A.; Sweet, J.B.; Allen, M.J. Large scale cultivation of genetically modified microalgae: A new era for environmental risk assessment. Algal Res. 2017, 25, 90–100. [Google Scholar] [CrossRef]
- Naduthodi, M.I.S.; Barbosa, M.J.; van der Oost, J. Progress of CRISPR-Cas Based Genome Editing in Photosynthetic Microbes. Biotechnol. J. 2018, 13, e1700591. [Google Scholar] [CrossRef]
- Fawley, K.P.; Fawley, M.W. Observations on the diversity and ecology of freshwater Nannochloropsis (Eustigmatophyceae), with descriptions of new taxa. Protist 2007, 158, 325–336. [Google Scholar] [CrossRef]
- OGTR. The Biology of Nannochloropsis oceanica Suda & Miyashita (a Microalga). 2019. Available online: https://www.ogtr.gov.au/resources/publications/biology-nannochloropsis-oceanica-suda-miyashita-microalga (accessed on 29 January 2024).
- Radakovits, R.; Jinkerson, R.E.; Fuerstenberg, S.I.; Tae, H.; Settlage, R.E.; Boore, J.L.; Posewitz, M.C. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat. Commun. 2012, 3, 686. [Google Scholar] [CrossRef] [PubMed]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Ajjawi, I.; Verruto, J.; Aqui, M.; Soriaga, L.B.; Coppersmith, J.; Kwok, K.; Peach, L.; Orchard, E.; Kalb, R.; Xu, W.; et al. Lipid production in Nannochloropsis gaditana is doubled by decreasing expression of a single transcriptional regulator. Nat. Biotechnol. 2017, 35, 647–652. [Google Scholar] [CrossRef]
- Verruto, J.; Francis, K.; Wang, Y.; Low, M.C.; Greiner, J.; Tacke, S.; Kuzminov, F.; Lambert, W.; McCarren, J.; Ajjawi, I.; et al. Unrestrained markerless trait stacking in Nannochloropsis gaditana through combined genome editing and marker recycling technologies. Proc. Natl. Acad. Sci. USA 2018, 115, E7015–E7022. [Google Scholar] [CrossRef]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From molecular manipulation of domesticated Chlamydomonas reinhardtii to survival in nature. eLife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Ermilova, E. Cold Stress Response: An Overview in Chlamydomonas. Front. Plant Sci. 2020, 11, 569437. [Google Scholar] [CrossRef]
- Rochaix, J.D. Chlamydomonas reinhardtii as the photosynthetic yeast. Annu. Rev. Genet. 1995, 29, 209–230. [Google Scholar] [CrossRef]
- Flowers, J.M.; Hazzouri, K.M.; Pham, G.M.; Rosas, U.; Bahmani, T.; Khraiwesh, B.; Nelson, D.R.; Jijakli, K.; Abdrabu, R.; Harris, E.H.; et al. Whole-Genome Resequencing Reveals Extensive Natural Variation in the Model Green Alga Chlamydomonas reinhardtii. Plant Cell 2015, 27, 2353–2369. [Google Scholar] [CrossRef]
- Ghribi, M.; Nouemssi, S.B.; Meddeb-Mouelhi, F.; Desgagné-Penix, I. Genome Editing by CRISPR-Cas: A Game Change in the Genetic Manipulation of Chlamydomonas. Life 2020, 10, 295. [Google Scholar] [CrossRef]
- Kao, P.-H.; Ng, I.-S. CRISPRi mediated phosphoenolpyruvate carboxylase regulation to enhance the production of lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef]
- Larson, M.H.; Gilbert, L.A.; Wang, X.; Lim, W.A.; Weissman, J.S.; Qi, L.S. CRISPR interference (CRISPRi) for sequence-specific control of gene expression. Nat. Protoc. 2013, 8, 2180–2196. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Cai, J.; Li, Y.; Fei, X. Expression and knockdown of the PEPC1 gene affect carbon flux in the biosynthesis of triacylglycerols by the green alga Chlamydomonas reinhardtii. Biotechnol. Lett. 2014, 36, 2199–2208. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, H.; Deng, W.; Li, T. Genome-Wide Analysis of the Zn(II)2Cys6 Zinc Cluster-Encoding Gene Family in Tolypocladiumguangdongense and Its Light-Induced Expression. Genes 2019, 10, 179. [Google Scholar] [CrossRef]
- Chollet, R.; Vidal, J.; O’Leary, M.H. PHOSPHOENOLPYRUVATE CARBOXYLASE: A Ubiquitous, Highly Regulated Enzyme in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 273–298. [Google Scholar] [CrossRef]
- Ambrosio, R.; Curatti, L. Deferred control of ammonium cross-feeding in a N2-fixing bacterium-microalga artificial consortium. Appl. Microbiol. Biotechnol. 2021, 105, 2937–2950. [Google Scholar] [CrossRef]
- Das, H.K. Azotobacters as Biofertilizer; Academic Press Inc.: Cambridge, MA, USA, 2019; ISBN 00652164. [Google Scholar]
- OECD. Developments in Delegations on the Safety Assessment of Novel Foods and Feeds, June 2022–April 2023; Series on the Safety of Novel Foods and Feeds No. 36; Environment Directorate; ENV/CBC/MONO(2023)29; OECD: Paris, France, 2023. [Google Scholar]
- Wen, A.; Havens, K.L.; Bloch, S.E.; Shah, N.; Higgins, D.A.; Davis-Richardson, A.G.; Sharon, J.; Rezaei, F.; Mohiti-Asli, M.; Johnson, A.; et al. Enabling Biological Nitrogen Fixation for Cereal Crops in Fertilized Fields. ACS Synth. Biol. 2021, 10, 3264–3277. [Google Scholar] [CrossRef]
- Dasgupta, D.; Kumar, K.; Miglani, R.; Mishra, R.; Panda, A.K.; Bisht, S.S. Chapter 1—Microbial biofertilizers: Recent trends and future outlook. In Recent Advancement in Microbial Biotechnology; de Mandal, S., Passari, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–26. ISBN 978-0-12-822098-6. [Google Scholar]
- Llamas, A.; Leon-Miranda, E.; Tejada-Jimenez, M. Microalgal and Nitrogen-Fixing Bacterial Consortia: From Interaction to Biotechnological Potential. Plants 2023, 12, 2476. [Google Scholar] [CrossRef]
- Barney, B.M.; Plunkett, M.H.; Natarajan, V.; Mus, F.; Knutson, C.M.; Peters, J.W. Transcriptional Analysis of an Ammonium-Excreting Strain of Azotobacter vinelandii Deregulated for Nitrogen Fixation. Appl. Environ. Microbiol. 2017, 83, e01534-17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, S. Transfer of Nitrogen Fixation (nif) Genes to Non-diazotrophic Hosts. Chembiochem 2020, 21, 1717–1722. [Google Scholar] [CrossRef]
- Ryu, M.-H.; Zhang, J.; Toth, T.; Khokhani, D.; Geddes, B.A.; Mus, F.; Garcia-Costas, A.; Peters, J.W.; Poole, P.S.; Ané, J.-M.; et al. Control of nitrogen fixation in bacteria that associate with cereals. Nat. Microbiol. 2020, 5, 314–330. [Google Scholar] [CrossRef]
- Russell, S.J.; Garcia, A.K.; Kaçar, B. A CRISPR interference system for engineering biological nitrogen fixation. mSystems 2024, 9, e0015524. [Google Scholar] [CrossRef] [PubMed]
- Wirth, R.; Pap, B.; Böjti, T.; Shetty, P.; Lakatos, G.; Bagi, Z.; Kovács, K.L.; Maróti, G. Chlorella vulgaris and Its Phycosphere in Wastewater: Microalgae-Bacteria Interactions During Nutrient Removal. Front. Bioeng. Biotechnol. 2020, 8, 557572. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Saranraj, P.; Sivasakthivelan, P. Biological Nitrogen Fixation By Azotobacter sp.—A Review. Indo-Asian J. Multidiscip. Res. 2017, 3, 1274–1284. [Google Scholar] [CrossRef]
- Paul, S.; Paul, B.; Verma, O.P. Effect of Azotobacter Chroococcum on Lepidopteran Insects. New Bot. 2002, 29, 163–168. [Google Scholar]
- Chahal, P.P.K.; Chahal, V.P.S. Biological Control of Root-Knot Nematode of Brinjal (Solanum melongena L.) with Azotobacter chroococcum. In Proceedings of the US-Pakistan International Workshop on Plant Nematology, Karachi, Pakistan, 6–8 April 1986. [Google Scholar]
- Duran-Bedolla, J.; Garza-Ramos, U.; Rodríguez-Medina, N.; Aguilar Vera, A.; Barrios-Camacho, H. Exploring the environmental traits and applications of Klebsiella variicola. Braz. J. Microbiol. 2021, 52, 2233–2245. [Google Scholar] [CrossRef]
- Mukherjee, T.; Banik, A.; Mukhopadhyay, S.K. Plant Growth-Promoting Traits of a Thermophilic Strain of the Klebsiella Group with its Effect on Rice Plant Growth. Curr. Microbiol. 2020, 77, 2613–2622. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, C.; Zhou, J.; Zhen, M.; Wei, X.; Yan, X.; Guo, X.; Zheng, L.; Shao, M.; Li, C.; et al. Pathogen Profile of Klebsiella variicola, the Causative Agent of Banana Sheath Rot. Plant Dis. 2023, 107, 2325–2334. [Google Scholar] [CrossRef] [PubMed]
- Agapito-Tenfen, S.Z.; Okoli, A.S.; Bernstein, M.J.; Wikmark, O.-G.; Myhr, A.I. Revisiting Risk Governance of GM Plants: The Need to Consider New and Emerging Gene-Editing Techniques. Front. Plant Sci. 2018, 9, 1874. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Grabowski, M.; Lener, M.; Engelhard, M.; Simon, S.; Dolezel, M.; Heissenberger, A.; Lüthi, C. Biosafety of Genome Editing Applications in Plant Breeding: Considerations for a Focused Case-Specific Risk Assessment in the EU. BioTech 2021, 10, 10. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms. Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010, 8, 1879. [Google Scholar] [CrossRef]
- Devos, Y.; Craig, W.; Devlin, R.H.; Ippolito, A.; Leggatt, R.A.; Romeis, J.; Shaw, R.; Svendsen, C.; Topping, C.J. Using problem formulation for fit-for-purpose pre-market environmental risk assessments of regulated stressors. EFSA J. 2019, 17, e170708. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Agapito-Tenfen, S.Z. Unintended Genomic Outcomes in Current and Next Generation GM Techniques: A Systematic Review. Plants 2022, 11, 2997. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.; Heissenberger, A. New Genetic Engineering—Possible Unintended Effects; Environment Agency Austria: Vienna, Austria, 2023. [Google Scholar]
- EFSA Panel on Genetically Modified Organisms. Guidance on the risk assessment of genetically modified microorganisms and their products intended for food and feed use. EFSA J. 2011, 9, 2193. [Google Scholar] [CrossRef]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Susanne, H.B.; Koutsoumanis, K.; Machera, K.; Naegeli, H.; et al. Evaluation of existing guidelines for their adequacy for the microbial characterisation and environmental risk assessment of microorganisms obtained through synthetic biology. EFSA J. 2020, 18, e06263. [Google Scholar] [CrossRef]
- Mullins, E.; Bresson, J.-L.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; Naegeli, H.; Nogué, F.; et al. New developments in biotechnology applied to microorganisms. EFSA J. 2024, 22, e8895. [Google Scholar] [CrossRef]
- EFSA. Draft Guidance on the Characterisation and Risk Assessment of Microorganisms Used in the Food Chain. Available online: https://connect.efsa.europa.eu/RM/s/consultations/publicconsultation2/a0lTk0000035F1V/pc1221 (accessed on 3 March 2025).
- OGTR. Limited and Controlled Release of Microalgae Genetically Modified for Increased Production of Fatty Acids. Licence Application No. DIR 169. Office of the Gene Technology Regulator; 2020. Available online: https://www.ogtr.gov.au/gmo-dealings/dealings-involving-intentional-release/dir-169 (accessed on 29 January 2024).
- Devos, Y.; Aguilera, J.; Diveki, Z.; Gomes, A.; Liu, Y.; Paoletti, C.; Du Jardin, P.; Herman, L.; Perry, J.N.; Waigmann, E. EFSA’s scientific activities and achievements on the risk assessment of genetically modified organisms (GMOs) during its first decade of existence: Looking back and ahead. Transgenic Res. 2014, 23, 1–25. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Guidance to develop specific protection goals options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA J. 2016, 14, e04499. [Google Scholar] [CrossRef]
- Richardson, J.S.; Sato, T. Resource subsidy flows across freshwater–terrestrial boundaries and influence on processes linking adjacent ecosystems. Ecohydrology 2015, 8, 406–415. [Google Scholar] [CrossRef]
- Wilkinson, D.M.; Koumoutsaris, S.; Mitchell, E.A.D.; Bey, I. Modelling the effect of size on the aerial dispersal of microorganisms. J. Biogeogr. 2012, 39, 89–97. [Google Scholar] [CrossRef]
- Inoue, H.; Tajima, K.; Mitsumori, C.; Inoue-Kashino, N.; Miura, T.; Ifuku, K.; Hirota, R.; Kashino, Y.; Fujita, K.; Kinoshita, H. Biodiversity risk assessment of genetically modified Chaetoceros gracilis for outdoor cultivation. J. Gen. Appl. Microbiol. 2022, 68, 151–162. [Google Scholar] [CrossRef]
- Matsuwaki, I.; Harayama, S.; Kato, M. Assessment of the biological invasion risks associated with a massive outdoor cultivation of the green alga, Pseudochoricystis ellipsoidea. Algal Res. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Szyjka, S.J.; Mandal, S.; Schoepp, N.G.; Tyler, B.M.; Yohn, C.B.; Poon, Y.S.; Villareal, S.; Burkart, M.D.; Shurin, J.B.; Mayfield, S.P. Evaluation of phenotype stability and ecological risk of a genetically engineered alga in open pond production. Algal Res. 2017, 24, 378–386. [Google Scholar] [CrossRef]
- Finlay, B.J. Global dispersal of free-living microbial eukaryote species. Science 2002, 296, 1061–1063. [Google Scholar] [CrossRef]
- Sebesta, J.; Xiong, W.; Guarnieri, M.T.; Yu, J. Biocontainment of Genetically Engineered Algae. Front. Plant Sci. 2022, 13, 839446. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J.; van der Vlugt, C.J.B.; Bergmans, H.E.N. Cultivated microalgae spills: Hard to predict/easier to mitigate risks. Trends Biotechnol. 2014, 32, 65–69. [Google Scholar] [CrossRef]
- Enzing, C.; Nooijen, A. Algae and Genetic Modification: Research, Production and Risks; CGM 2012-05; Technopolis Group: Amsterdam, The Netherlands, 2012. [Google Scholar]
- González-Morales, S.I.; Pacheco-Gutiérrez, N.B.; Ramírez-Rodríguez, C.A.; Brito-Bello, A.A.; Estrella-Hernández, P.; Herrera-Estrella, L.; López-Arredondo, D.L. Metabolic engineering of phosphite metabolism in Synechococcus elongatus PCC 7942 as an effective measure to control biological contaminants in outdoor raceway ponds. Biotechnol. Biofuels 2020, 13, 119. [Google Scholar] [CrossRef]
- Lee, J.W.; Chan, C.T.Y.; Slomovic, S.; Collins, J.J. Next-generation biocontainment systems for engineered organisms. Nat. Chem. Biol. 2018, 14, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.J.; Mitra, A.; Greenwell, H.C.; Sui, J. Monster potential meets potential monster: Pros and cons of deploying genetically modified microalgae for biofuels production. Interface Focus 2013, 3, 20120037. [Google Scholar] [CrossRef][Green Version]
- Cardador, M.; Krüger, S.; Dunker, S.; Brakel, A.; Hoffmann, R.; Nagel, R.; Jakob, T.; Goss, R.; Sasso, S. Extensive remodeling during Chlamydomonas reinhardtii zygote maturation leads to highly resistant zygospores. Plant J. 2025, 121, e17238. [Google Scholar] [CrossRef]
- Sundqvist, L.; Godhe, A.; Jonsson, P.R.; Sefbom, J. The anchoring effect-long-term dormancy and genetic population structure. ISME J. 2018, 12, 2929–2941. [Google Scholar] [CrossRef]
- Bloch, S.E.; Clark, R.; Gottlieb, S.S.; Wood, L.K.; Shah, N.; Mak, S.-M.; Lorigan, J.G.; Johnson, J.; Davis-Richardson, A.G.; Williams, L.; et al. Biological nitrogen fixation in maize: Optimizing nitrogenase expression in a root-associated diazotroph. J. Exp. Bot. 2020, 71, 4591–4603. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.; Cleenwerck, I.; Venter, S.; Coutinho, T.; De Vos, P. Taxonomic evaluation of the genus Enterobacter based on multilocus sequence analysis (MLSA): Proposal to reclassify E. nimipressuralis and E. amnigenus into Lelliottia gen. nov. as Lelliottia nimipressuralis comb. nov. and Lelliottia amnigena comb. nov., respectively, E. gergoviae and E. pyrinus into Pluralibacter gen. nov. as Pluralibacter gergoviae comb. nov. and Pluralibacter pyrinus comb. nov., respectively, E. cowanii, E. radicincitans, E. oryzae and E. arachidis into Kosakonia gen. nov. as Kosakonia cowanii comb. nov., Kosakonia radicincitans comb. nov., Kosakonia oryzae comb. nov. and Kosakonia arachidis comb. nov., respectively, and E. turicensis, E. helveticus and E. pulveris into Cronobacter as Cronobacter zurichensis nom. nov., Cronobacter helveticus comb. nov. and Cronobacter pulveris comb. nov., respectively, and emended description of the genera Enterobacter and Cronobacter. Syst. Appl. Microbiol. 2013, 36, 309–319. [Google Scholar] [CrossRef]
- Tong, C.Y.; Honda, K.; Derek, C. A review on microalgal-bacterial co-culture: The multifaceted role of beneficial bacteria towards enhancement of microalgal metabolite production. Environ. Res. 2023, 228, 115872. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Eltanahy, E.E.; Liu, H.; Chua, E.T.; Thomas-Hall, S.R.; Wass, T.J.; Pan, K.; Schenk, P.M. Growth-promoting bacteria double eicosapentaenoic acid yield in microalgae. Bioresour. Technol. 2020, 316, 123916. [Google Scholar] [CrossRef]
- Borics, G.; Abonyi, A.; Salmaso, N.; Ptacnik, R. Freshwater phytoplankton diversity: Models, drivers and implications for ecosystem properties. Hydrobiologia 2021, 848, 53–75. [Google Scholar] [CrossRef]
- de Leij, F.; Thomas, C.E.; Bailey, M.J.; Whipps, J.M.; Lynch, J.M. Effect of Insertion Site and Metabolic Load on the Environmental Fitness of a Genetically Modified Pseudomonas fluorescens Isolate. Appl. Environ. Microbiol. 1998, 64, 2634–2638. [Google Scholar] [CrossRef]
- Scholz, M.J.; Weiss, T.L.; Jinkerson, R.E.; Jing, J.; Roth, R.; Goodenough, U.; Posewitz, M.C.; Gerken, H.G. Ultrastructure and composition of the Nannochloropsis gaditana cell wall. Eukaryot. Cell 2014, 13, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cvetkovska, M.; Morgan-Kiss, R.; Hüner, N.P.; Smith, D.R. Draft genome sequence of the Antarctic green alga Chlamydomonas sp. UWO241. iScience 2021, 24, 102084. [Google Scholar] [CrossRef]
- Snow, A.A.; Smith, V.H. Genetically Engineered Algae for Biofuels: A Key Role for Ecologists. BioScience 2012, 62, 765–768. [Google Scholar] [CrossRef]
- Henley, W.J.; Litaker, R.W.; Novoveská, L.; Duke, C.S.; Quemada, H.D.; Sayre, R.T. Initial risk assessment of genetically modified (GM) microalgae for commodity-scale biofuel cultivation. Algal Res. 2013, 2, 66–77. [Google Scholar] [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas reinhardtii, a Reference Organism to Study Algal-Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Wichard, T.; Gerecht, A.; Boersma, M.; Poulet, S.A.; Wiltshire, K.; Pohnert, G. Lipid and fatty acid composition of diatoms revisited: Rapid wound-activated change of food quality parameters influences herbivorous copepod reproductive success. Chembiochem 2007, 8, 1146–1153. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, J.; Curtiss, R. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 6899–6904. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Bloch, S.E.; Ryu, M.-H.; Ozaydin, B.; Broglie, R. Harnessing atmospheric nitrogen for cereal crop production. Curr. Opin. Biotechnol. 2020, 62, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef]
- OECD. Development in Delegations on Biosafety Issues, April 2021–May 2022; Series on the Harmonisation of Regulatory Oversight in Biotechnology No. 71; Environment Directorate; ENV/CBC/MONO(2022)23; OECD: Paris, France, 2022. [Google Scholar]
- OECD. Developments-unclassDevelopments in Delegations on Biosafety Issues, April 2020–March 2021; Series on the Harmonisation of Regulatory Oversight in Biotechnology No. 69; Environment Directorate; ENV/CBC/MONO(2021)19; OECD: Paris, France, 2021. [Google Scholar]
- Soberón-Chávez, G. The Evolution of Bacteria Can Produce Chimeric Creatures: The Case of Azotobacter vinelandii. Front. Young Minds 2019, 7, 135. [Google Scholar] [CrossRef]
- Tariq, M.; Jameel, F.; Ijaz, U.; Abdullah, M.; Rashid, K. Biofertilizer microorganisms accompanying pathogenic attributes: A potential threat. Physiol. Mol. Biol. Plants 2022, 28, 77–90. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, G.; Hao, B.; Newbrough, A.; Clancy, C.J.; Nguyen, M.-H. 1007. Hypermutability in Clinical Strains of Klebsiella pneumoniae: Role of the V76G Mutation in MutH. Open Forum Infect. Dis. 2021, 8, S594. [Google Scholar] [CrossRef]
- González-Casanova, A.; Aguirre-von-Wobeser, E.; Espín, G.; Servín-González, L.; Kurt, N.; Spanò, D.; Blath, J.; Soberón-Chávez, G. Strong seed-bank effects in bacterial evolution. J. Theor. Biol. 2014, 356, 62–70. [Google Scholar] [CrossRef]
- Duran-Bedolla, J.; Rodríguez-Medina, N.; Dunn, M.; Mosqueda-García, D.; Barrios-Camacho, H.; Aguilar-Vera, A.; Aguilar-Vera, E.; Suárez-Rodríguez, R.; Ramírez-Trujillo, J.A.; Garza-Ramos, U. Plasmids of the incompatibility group FIBK occur in Klebsiella variicola from diverse ecological niches. Int. Microbiol. 2023, 26, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Dell’Aversana, C.; Doti, N.; Donadio, G.; Dal Piaz, F.; Izzo, V.; De Filippis, A.; Galdiero, M.; Altucci, L.; Boccia, G.; et al. Outer Membrane Vesicles Derived from Klebsiella pneumoniae Are a Driving Force for Horizontal Gene Transfer. Int. J. Mol. Sci. 2021, 22, 8732. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Luo, W.; Xiang, T.-X.; Jiang, Y.; Liu, P.; Wei, D.-D.; Fan, L.; Huang, S.; Liao, W.; Liu, Y.; et al. Horizontal gene transfer via OMVs co-carrying virulence and antimicrobial-resistant genes is a novel way for the dissemination of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2022, 13, 945972. [Google Scholar] [CrossRef]
- Klümper, U.; Gionchetta, G.; Catão, E.; Bellanger, X.; Dielacher, I.; Elena, A.X.; Fang, P.; Galazka, S.; Goryluk-Salmonowicz, A.; Kneis, D.; et al. Environmental microbiome diversity and stability is a barrier to antimicrobial resistance gene accumulation. Commun. Biol. 2024, 7, 706. [Google Scholar] [CrossRef]
- Podder, M.P.; Rogers, L.; Daley, P.K.; Keefe, G.P.; Whitney, H.G.; Tahlan, K. Klebsiella species associated with bovine mastitis in Newfoundland. PLoS ONE 2014, 9, e106518. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, Y.; McNally, A.; Zong, Z. Occurrence of colistin-resistant hypervirulent Klebsiella variicola. J. Antimicrob. Chemother. 2018, 73, 3001–3004. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms. Guidance for risk assessment of food and feed from genetically modified plants. EFSA J. 2011, 9, 2150. [Google Scholar] [CrossRef]
- USEPA. Algae Supplement to the Guidance Document “Points to Consider in the Preparation of TSCA Biotechnology Submissions for Microorganisms”. Available online: https://www.epa.gov/sites/default/files/2020-10/documents/algae_supplement_091420.pdf (accessed on 27 November 2024).
- Jones, P.R. Genetic instability in cyanobacteria—An elephant in the room? Front. Bioeng. Biotechnol. 2014, 2, 12. [Google Scholar] [CrossRef]
- Steudel, B. Microalgae in Ecology: Ecosystem Functioning Experiments. J. Oceanogr. Mar. Res. 2014, 2, 1000122. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.; He, S. Microalgal diversity enhances water purification efficiency in experimental microcosms. Front. Ecol. Evol. 2023, 11, 1125743. [Google Scholar] [CrossRef]
- Schulze, T.; Ricking, M.; Schröter-Kermani, C.; Körner, A.; Denner, H.-D.; Weinfurtner, K.; Winkler, A.; Pekdeger, A. The German Environmental Specimen Bank. J. Soils Sediments 2007, 7, 361–367. [Google Scholar] [CrossRef]
- Zueghart, W.; Beismann, H.; Schroeder, W. Tools for a scientifically rigorous and efficient monitoring of genetically modified organisms (GMOs)—VDI Guidelines to ensure high quality of GMO-monitoring data. BioRisk 2013, 8, 3–13. [Google Scholar] [CrossRef]
- Eckerstorfer, M.; Gaugitsch, H. Framing Socio-Economic Assessment in GMO & Chemicals Regulation; Environment Agency Austria: Vienna, Austria, 2012. [Google Scholar]
- Editorial in Nature Biotechnology. Can microbes save the planet? Nat. Biotechnol. 2023, 41, 735. [Google Scholar] [CrossRef]
- Beltrán, J.P.; Berbel, J.; Berdaji, I.; Bernabéu, R.; Boix Fayos, C.; Clotet Ballús, R.; Colomer Xena, Y.; Del Castillo Bilbao, M.D.; Flotats Ripoll, X.; Gil, J.C.; et al. The Impact of the European Green Deal from a Sustainable Global Food System Approach. Eur. Food Feed. Law Rev. 2022, 17, 2–38. [Google Scholar]
- Dassler, T.; Myhr, A.I.; Lalyer, C.R.; Frieß, J.L.; Spök, A.; Liebert, W.; Hagen, K.; Engelhard, M.; Giese, B. Structured analysis of broader GMO impacts inspired by technology assessment to inform policy decisions. Agric. Hum. Values 2023, 41, 449–458. [Google Scholar] [CrossRef]
- Gabbert, S.; Scheringer, M.; Ng, C.A.; Stolzenberg, H.-C. Socio-economic analysis for the authorisation of chemicals under REACH: A case of very high concern? Regul. Toxicol. Pharmacol. 2014, 70, 564–571. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Cartagena Protocol on Biosafety to the Convention on Biological Diversity. Text and Annexes, Montreal. 2000. Available online: https://bch.cbd.int/protocol/text (accessed on 27 November 2024).
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Guidance on the Assessment of Socio-Economic Considerations in the Context of Article 26 of the Cartagena Protocol on Biosafety: CBD/CP/MOP/9/10, Annex. 17 August 2018. Available online: https://www.cbd.int/doc/c/0215/0803/cb8d71c24d40c683e6dafb0a/cp-mop-09-10-en.pdf (accessed on 27 November 2024).
- COGEM. Socio-Economic Aspects of GMOs: Building Blocks for an EU Sustainability Assessment of Genetically Modified Crops; COGEM Report CGM/090929-01; Umweltbundesamt GmbH: Vienna, Austria, 2009. [Google Scholar]
- High Council for Biotechnologies, Economic, Ethical and Social Committee (EESC). Main Criteria for Analysis as Established on 13 April 2011; High Council for Biotechnologies: Paris, France, 2011. [Google Scholar]
- Kathage, J.; Gómez-Barbero, M.; Rodríguez-Cerezo, E. Framework for the Socio-Economic Analysis of the Cultivation of Genetically Modified Crops: European GMO Socio-Economics Bureau 1st Reference Document; Publications Office of the European Union: Luxembourg, 2015; ISBN 9789279482465. [Google Scholar]
- Kathage, J.; Gómez-Barbero, M.; Rodríguez-Cerezo, E. Framework for Assessing the Socio-Economic Impacts of Bt Maize Cultivation: European GMO Socio Economics Bureau: 2nd Reference Document; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Gene Technology Act (Act of 2 April 1993 No. 38); Norwegian Ministry of Climate and Environment: Oslo, Norway, 1993; Available online: https://www.regjeringen.no/en/dokumenter/gene-technology-act/id173031/ (accessed on 25 March 2025).
- Myskja, B.K.; Myhr, A.I. Non-safety Assessments of Genome-Edited Organisms: Should They be Included in Regulation? Sci. Eng. Ethics 2020, 26, 2601–2627. [Google Scholar] [CrossRef]
- The Norwegian Biotechnology Advisory Board. Insektresistente Genmodifiserte Planter og Bærekraft (Insect-Resistant Genetically Modified Palnts and Sustainability); The Norwegian Biotechnology Advisory Board: Bergen, Norway, 2011. [Google Scholar]
- The Norwegian Biotechnology Advisory Board. Herbicide-Resistant Genetically Modified Plants and Sustainability; The Norwegian Biotechnology Advisory Board: Bergen, Norway, 2014. [Google Scholar]
- Wang, M.; Ye, X.; Bi, H.; Shen, Z. Microalgae biofuels: Illuminating the path to a sustainable future amidst challenges and opportunities. Biotechnol. Biofuels Bioprod. 2024, 17, 10. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F. Microalgae biofuels production: A systematic review on socioeconomic prospects of microalgae biofuels and policy implications. Environ. Chall. 2021, 5, 100207. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Sustainability Assessment of Food and Agriculture Systems (SAFA): Guidelines. 2014. Available online: https://www.fao.org/3/i3957e/i3957e.pdf (accessed on 27 November 2024).
- Paçarada, R.; Hörtenhuber, S.; Hemme, T.; Wurzinger, M.; Zollitsch, W. Sustainability Assessment Tools for Dairy Supply Chains: A Typology. Sustainability 2024, 16, 4999. [Google Scholar] [CrossRef]
- Culaba, A.B.; Ubando, A.T.; Ching, P.M.L.; Chen, W.-H.; Chang, J.-S. Biofuel from Microalgae: Sustainable Pathways. Sustainability 2020, 12, 8009. [Google Scholar] [CrossRef]
- Bradley, T.; Rajaeifar, M.A.; Kenny, A.; Hainsworth, C.; del Pino, V.; del Valle Inclán, Y.; Povoa, I.; Mendonça, P.; Brown, L.; Smallbone, A.; et al. Life cycle assessment of microalgae-derived biodiesel. Int. J. Life Cycle Assess. 2023, 28, 590–609. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef] [PubMed]
- PIVOT BIO, Inc. Pivot Bio PROVEN® 40: Nitrogen for Corn. Available online: https://www.pivotbio.com/product-proven40-corn (accessed on 18 May 2024).
- FAO. SAFA Guidelines. Sustainability Assessment of Food and Agricultural Systems. Version 3.0. 2014. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/72070c29-47d6-47ef-8059-e78168c2fb69/content (accessed on 25 March 2025).
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathyanarayanan, K.; Khan, M.S.; Park, S.J.; Yoo, H.M.; Cho, S.H.; Ahn, G.; Ahamed, M.A.A.; Padmanabhan, S.; MubarakAli, D.; et al. Recent progress in biotechnological approaches for diverse applications of algae: An overview. Int. J. Environ. Sci. Technol. 2024, 21, 3453–3474. [Google Scholar] [CrossRef]
- Thomas, L.; Singh, I. Microbial Biofertilizers: Types and Applications. In Biofertilizers for Sustainable Agriculture and Environment; Springer: Cham, Switzerland, 2019; Volume 55, pp. 1–19. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, A. Technology Assessment: Concepts and Methods. In Philosophy of Technology and Engineering Sciences: Handbook of the Philosophy of Science; Meijers, A., Ed.; North-Holland: Amsterdam, The Netherlands, 2009; pp. 1103–1146. ISBN 18789846. [Google Scholar]
- Stirling, A. “Opening Up” and “Closing Down”. Sci. Technol. Hum. Values 2007, 33, 262–294. [Google Scholar] [CrossRef]
- International Risk Governance Council. Introduction to the IRGC Risk Governance Framework: Revised Version; EPFL International Risk Governance Center: Lausanne, Switzerland, 2017. [Google Scholar]
- Sarewitz, D. Anticipatory Governance of Emerging Technologies. In The Growing Gap Between Emerging Technologies and Legal-Ethical Oversight: The Pacing Problem; Marchant, G.E., Allenby, B.R., Herkert, J.R., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 95–105. ISBN 978-94-007-1356-7. [Google Scholar]
- Asveld, L.; Stemerding, D. Algae Oil on Trial. Conflicting Views of Technology and Nature; Rathenau Instituut: Den Haag, The Netherlands, 2016. [Google Scholar]
- Schröter-Schlaack, C.; Aicher, C.; Grünwald, R.; Revermann, C.; Schiller, J. Das Potenzial Algenbasierter Kraftstoffe für den Lkw-Verkehr. Sachstandsbericht zum Monitoring »Nachhaltige Potenziale der Bioökonomie—Biokraftstoffe der 3. Generation« (Arbeitsbericht Nr. 181); Büro für Technikfolgen-Abschätzung beim Deutschen Bundestag (TAB): Berlin, Germany, 2019. [Google Scholar]
- Varela Villarreal, J.; Burgués, C.; Rösch, C. Acceptability of genetically engineered algae biofuels in Europe: Opinions of experts and stakeholders. Biotechnol. Biofuels 2020, 13, 92. [Google Scholar] [CrossRef]
- Schiller, J.; Aicher, C.; Feresin, E.; Klauer, B.; Hansjürgens, B.; Sauter, A. Weiße Biotechnologie: Stand und Perspektiven der Industriellen Biotechnologie: Verfahren, Anwendungen, Ökonomische Perspektiven. Innovationsanalyse—Teil II. Stand und Perspektiven der Industriellen Biotechnologie: Umwelt- und Nachhaltigkeitspotenziale (Arbeitsbericht Nr. 169); Büro für Technikfolgen-Abschätzung beim Deutschen Bundestag (TAB): Berlin, Germany, 2016. [Google Scholar]
- Sauter, A. Synthetische Biologie und Genome Editing: Herausforderungen für die Forschungspolitik. TAB-Brief Nr. 46; Büro für Technikfolgen-Abschätzung beim Deutschen Bundestag (TAB): Berlin, Germany, 2015; pp. 27–35. ISBN 21937435. [Google Scholar]
- Sauter, A. Synthetische Biologie: Ein Schlüsselbegriff künftiger Anwendungen der Biotechnologie. TAB-Brief Nr. 46; Büro für Technikfolgen-Abschätzung beim Deutschen Bundestag (TAB): Berlin, Germany, 2015; pp. 35–36. ISBN 21937435. [Google Scholar]
- Wentworth, J. Biological Solutions for Environmental Challenges (Horizon Scanning); UK Parliament: London, UK, 2021. [Google Scholar]
- Bauer, A.; Bogner, A. Let’s (not) talk about synthetic biology: Framing an emerging technology in public and stakeholder dialogues. Public Underst. Sci. 2020, 29, 492–507. [Google Scholar] [CrossRef]
- Ancillotti, M.; Rerimassie, V.; Seitz, S.B.; Steurer, W. An update of public perceptions of synthetic biology: Still undecided? NanoEthics 2016, 10, 309–325. [Google Scholar] [CrossRef]
- Aichinger, H.; Hüsing, B.; Wydra, S. Weiße Biotechnologie: Stand und Perspektiven der Industriellen Biotechnologie: Verfahren, Anwendungen, Ökonomische Perspektiven. Innovationsanalyse—Teil I. Stand und Perspektiven der Industriellen Biotechnologie: Verfahren, Anwendungen, Ökonomische Perspektiven (Arbeitsbericht Nr. 168); Büro für Technikfolgen-Abschätzung beim Deutschen Bundestag (TAB): Berlin, Germany, 2016. [Google Scholar]
- König, H.; Frank, D.; Heil, R.; Coenen, C. Synthetic genomics and synthetic biology applications between hopes and concerns. Curr. Genom. 2013, 14, 11–24. [Google Scholar] [CrossRef][Green Version]
- Rerimassie, V.; Stemerding, D. SynBio Politics. Bringing Synthetic Biology into Debate; Rathenau Instituut: Den Hague, The Netherlands, 2014. [Google Scholar]
- Macnaghten, P.; Davies, S.; Kearnes, M. Narrative and public engagement: Some findings from the DEEPEN project. In Understanding Public Debate on Nanotechnologies: Options for Framing Public Policies; Publications Office of the European Union: Brussels, Belgium, 2010. [Google Scholar]
- Eckerstorfer, M.; Heissenberger, A.; Gaugitsch, H. Considerations for a Precautionary Approach in GMO Policy; Umweltbundesamt Reports REP-0233; Umweltbundesamt: Vienna, Austria, 2010. [Google Scholar]
- Lentzos, F.; Rybicki, E.P.; Engelhard, M.; Paterson, P.; Sandholtz, W.A.; Reeves, R.G. Eroding norms over release of self-spreading viruses. Science 2022, 375, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Wohlfender-Bühler, D.; Feusthuber, E.; Wäger, R.; Mann, S.; Aubry, S.J. Genetically modified crops in Switzerland: Implications for agrosystem sustainability evidenced by multi-criteria model. Agron. Sustain. Dev. 2016, 36, 33. [Google Scholar] [CrossRef][Green Version]
- Kuhlmann, S.; Stegmaier, P.; Konrad, K. The tentative governance of emerging science and technology—A conceptual introduction. Res. Policy 2019, 48, 1091–1097. [Google Scholar] [CrossRef]
- Lyall, C.; Tait, J. Beyond the limits to governance: New rules of engagement for the tentative governance of the life sciences. Res. Policy 2019, 48, 1128–1137. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef]
- Hope, J.A.; Paterson, D.M.; Thrush, S.F. The role of microphytobenthos in soft-sediment ecological networks and their contribution to the delivery of multiple ecosystem services. J. Ecol. 2020, 108, 815–830. [Google Scholar] [CrossRef]


| Protection Goal | Relevant Legislation/ Strategies | Objective | Relevant for RA of CS * |
|---|---|---|---|
| Soil quality and soil health | EU Soil Strategy for 2030 (COM(2021)699) | Protection and restoration of soil quality, health and biodiversity via a sustainable management | CS 1; CS 2 |
| Proposal for a Directive on Soil Monitoring and Resilience (COM(2023)416) | Establish a coherent soil monitoring framework for all soils across the EU to improve soil health | CS 1; CS 2 | |
| Water quality | EU Water Framework Directive (Directive 2000/62/EC) | Improvement of the ecological and chemical status of surface waters | CS 1; CS 2 (run-off water) |
| EU Nitrate Directive 91/676/EEC | Minimizing nitrate input from agricultural sources into water bodies | CS 2 | |
| Ecosystem functions and services | Concept outlined by EFSA Scientific Committee [82] | Protection of relevant supporting, regulating, provisioning, and cultural services | CS 1; CS 2 |
| Nature conservation | EU FFH Directive 92/43/EWG; Regulation (EU) 2024/1991 (Nature restoration law) | Protection and conservation of natural habitats and of wild animals and plants to restore, conserve, and promote biodiversity in natural habitats and to restore degraded terrestrial and aquatic ecosystems | CS 1; CS 2 |
| Risk Issue 1 | Area of Risk | Relevance for GM Microalgae (Risk Hypothesis Is Possible) |
|---|---|---|
| 1/2 | Survival/persistence/proliferation including selective advantage | Microalgae occur in natural habitats; ERA needs to address whether the GM trait provides a selective advantage (e.g., by affecting other microalgae or trophic levels) |
| 3 | Gene transfer (horizontal/vertical) | Relevant for sexually (e.g., Chlamydomonas) and asexually (e.g., Nannochloropsis, Chlamydomonas) reproducing taxa. Horizontal gene transfer to other algae/microorganisms is possible |
| 5 | Impacts on the biotic environment (e.g., non-target organisms) | Toxicological and nutritional effects of the specific changes in lipid composition on other organisms at the same or other trophic levels are possible |
| 6 | Interactions with the abiotic environment | Effects of the specific changes in lipid profile of GM microalgae on nutrient availability are possible |
| 7 | Environmental impacts of the specific techniques used for management of GMM | Relevant in case additional biocontainment traits (genetic or biological) would have adverse environmental impacts |
| 8 | Impacts on human and animal health | Toxicological and nutritional effects of changed levels of certain fatty acids are possible |
| Risk Issue 1 | Area of Risk | Relevance for GM Microalgae (Risk Hypothesis Is Possible) |
|---|---|---|
| 1/2 | Survival/persistence/proliferation including selective advantage | Microalgae occur in natural habitats; ERA needs to address whether the GM trait provides a selective advantage (e.g., by affecting other microalgae or trophic levels) |
| 3 | Gene transfer (horizontal/vertical) | Relevant for sexually (e.g., Chlamydomonas) and asexually (e.g., Nannochloropsis, Chlamydomonas) reproducing taxa. HGT to other algae/microorganisms is possible |
| 4 | Impacts on the biotic environment (target organisms) | Potential plant-pathogenic effects under environmental conditions favoring colonization of certain plant species |
| 5 | Impacts on the biotic environment (non-target organisms) | Effects due to changes in the soil microbiome of the treated crop plots or unintended environments due to transport and spread of the GMMs. Such effects can be due to competition or the increased levels of bioavailable nutrients or nitrogen-compounds |
| 6 | Interactions with the abiotic environment | Effects on nutrient availability due to the nitrogen-fixation ability and other effects related to nutrient cycling in the soil (phosphate/sulfur solubilization, ability to increase the bioavailability of micronutrients) |
| 7 | Environmental impacts of the specific techniques used for management of GMM | Relevant depending on the specific use of the GMMs and the methods used to introduce the GMMs into the intended receiving environments (i.e., the treated soil for crop cultivation) Effects also depend on additional plant management interventions, e.g., further use of synthetic nitrogen fertilizers |
| 8 | Impacts on human and animal health | Increased virulence and pathogenicity may be possible Effects of exposure of the gut microbiome needs consideration |
| PS | CS 1 (Microalgae for Biofuels) | CS 2 (Microbial Biofertilizers in Cereal Production) |
|---|---|---|
| TPS1 | Biofuel production with GM microalgae in open or semi-open pond systems | Supplementary use of GM biofertilizers (standard N fertilization plus GM biofertilizer) |
| TPS2 | Biofuel production with GM microalgae in fully contained, closed-culture systems | Substitution of N fertilizer with GM biofertilizers (N fertilizer partly substituted with GM biofertilizer— minus 7.2 kg N (urea)/ha maize) |
| RPS1 | Biofuel production with non-GM microalgae (in open or closed culture systems) | Biofertilizer use without additional N fertilizer (use of non-GM or GM biofertilizer) |
| RPS2 | Production of standard fossil fuels | Synthetic and organic nitrogen fertilizers (standard use of synthetic/organic N fertilizers) |
| Sustainability Dimension * | Sustainability Themes * | GM Microalgae for Biofuel Production | GM Microbial Biofertilizers in Cereal Production |
|---|---|---|---|
| Ecological integrity | Atmosphere | GHG emissions, air pollutants | GHG emissions, air pollutants |
| Water | Water Withdrawal, water Quality, waste water | Water Quality | |
| Soil | Land Use | Land Degradation | |
| Biodiversity | Ecosystem diversity, species diversity, genetic diversity | Ecosystem diversity, species diversity, genetic diversity | |
| Material and Energy | Energy used in production | Energy used in production | |
| Economic resilience and efficiency | Production efficiency | Volume of production | Volume of production |
| Economic resilience | Costs, energy security, affordability, and independence | Costs, affordability, and independence | |
| Social sustainability | Decent Livelihood | Job creation, income | Farm income |
| Equality, non-discrimination, gender equality, vulnerable groups | Human mobility | Opportunities for low-income and subsistence farmers | |
| Good governance | Compliance with energy and climate policies | Compliance with climate policies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckerstorfer, M.F.; Dolezel, M.; Miklau, M.; Greiter, A.; Heissenberger, A.; Kastenhofer, K.; Schulz, F.; Hagen, K.; Otto, M.; Engelhard, M. Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance. Int. J. Mol. Sci. 2025, 26, 3174. https://doi.org/10.3390/ijms26073174
Eckerstorfer MF, Dolezel M, Miklau M, Greiter A, Heissenberger A, Kastenhofer K, Schulz F, Hagen K, Otto M, Engelhard M. Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance. International Journal of Molecular Sciences. 2025; 26(7):3174. https://doi.org/10.3390/ijms26073174
Chicago/Turabian StyleEckerstorfer, Michael F., Marion Dolezel, Marianne Miklau, Anita Greiter, Andreas Heissenberger, Karen Kastenhofer, Freya Schulz, Kristin Hagen, Mathias Otto, and Margret Engelhard. 2025. "Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance" International Journal of Molecular Sciences 26, no. 7: 3174. https://doi.org/10.3390/ijms26073174
APA StyleEckerstorfer, M. F., Dolezel, M., Miklau, M., Greiter, A., Heissenberger, A., Kastenhofer, K., Schulz, F., Hagen, K., Otto, M., & Engelhard, M. (2025). Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance. International Journal of Molecular Sciences, 26(7), 3174. https://doi.org/10.3390/ijms26073174








