The Effect of Valine on the Synthesis of α-Casein in MAC-T Cells and the Expression and Phosphorylation of Genes Related to the mTOR Signaling Pathway
Abstract
:1. Introduction
2. Results
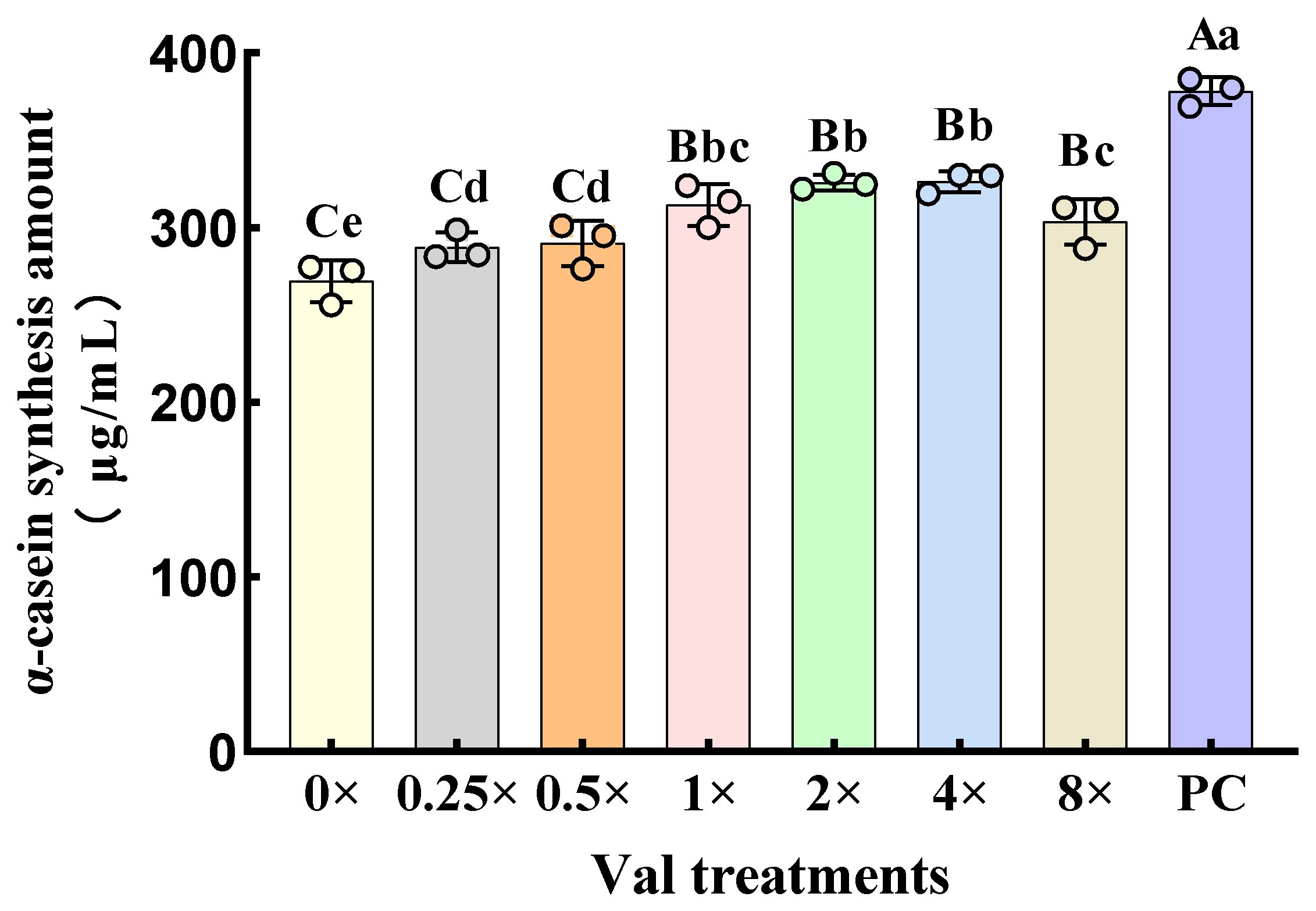
2.1. Effects of Varying Valine Concentrations on α-Casein Synthesis in MAC-T Cells
2.2. Effects of Varying Valine Concentrations on the Relative Expression Level of CSN1S1 and CSN1S2
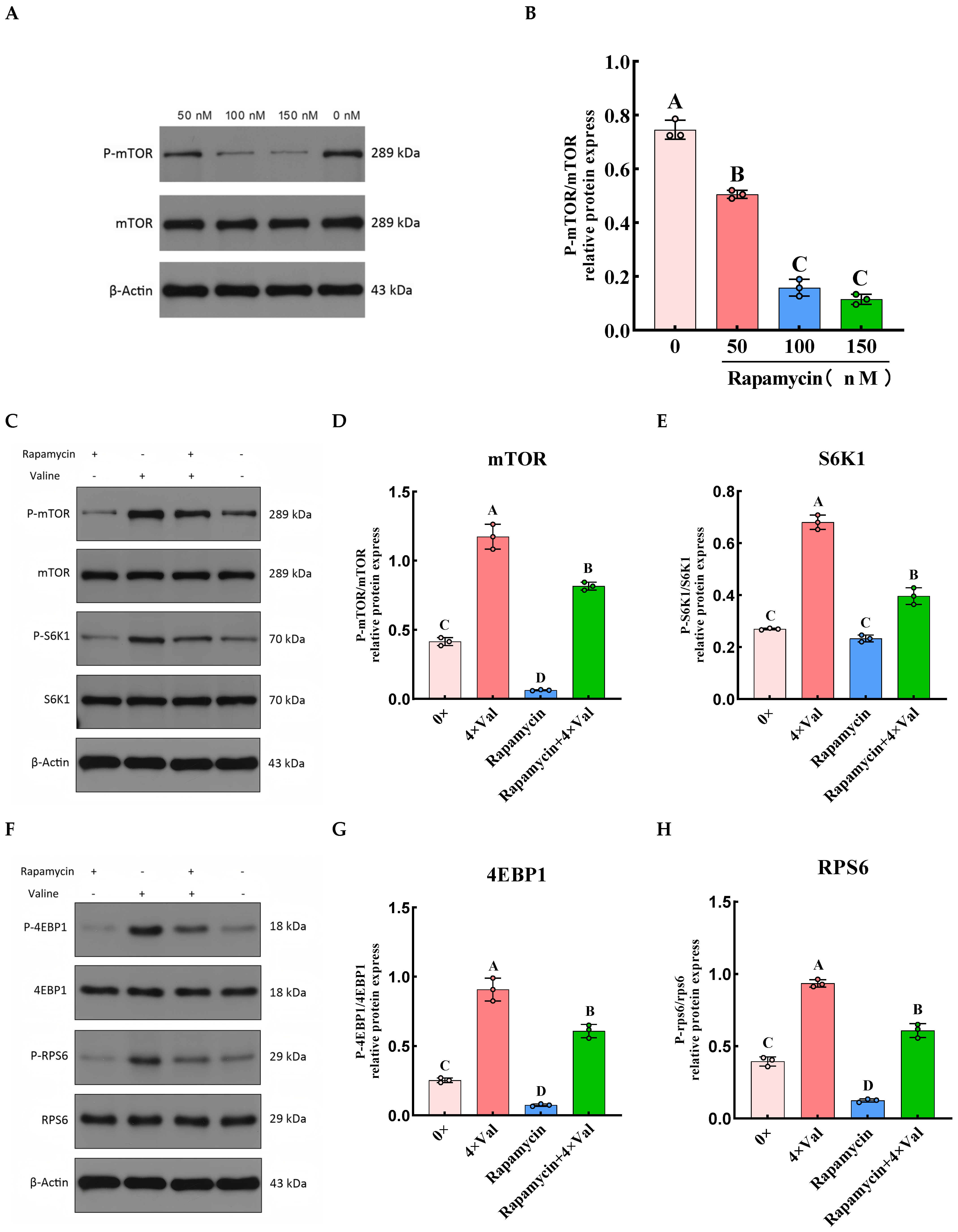
2.3. Effects of Varying Valine Concentrations on the Expression Levels of mTOR Signaling Pathway-Related Genes and Protein Phosphorylation
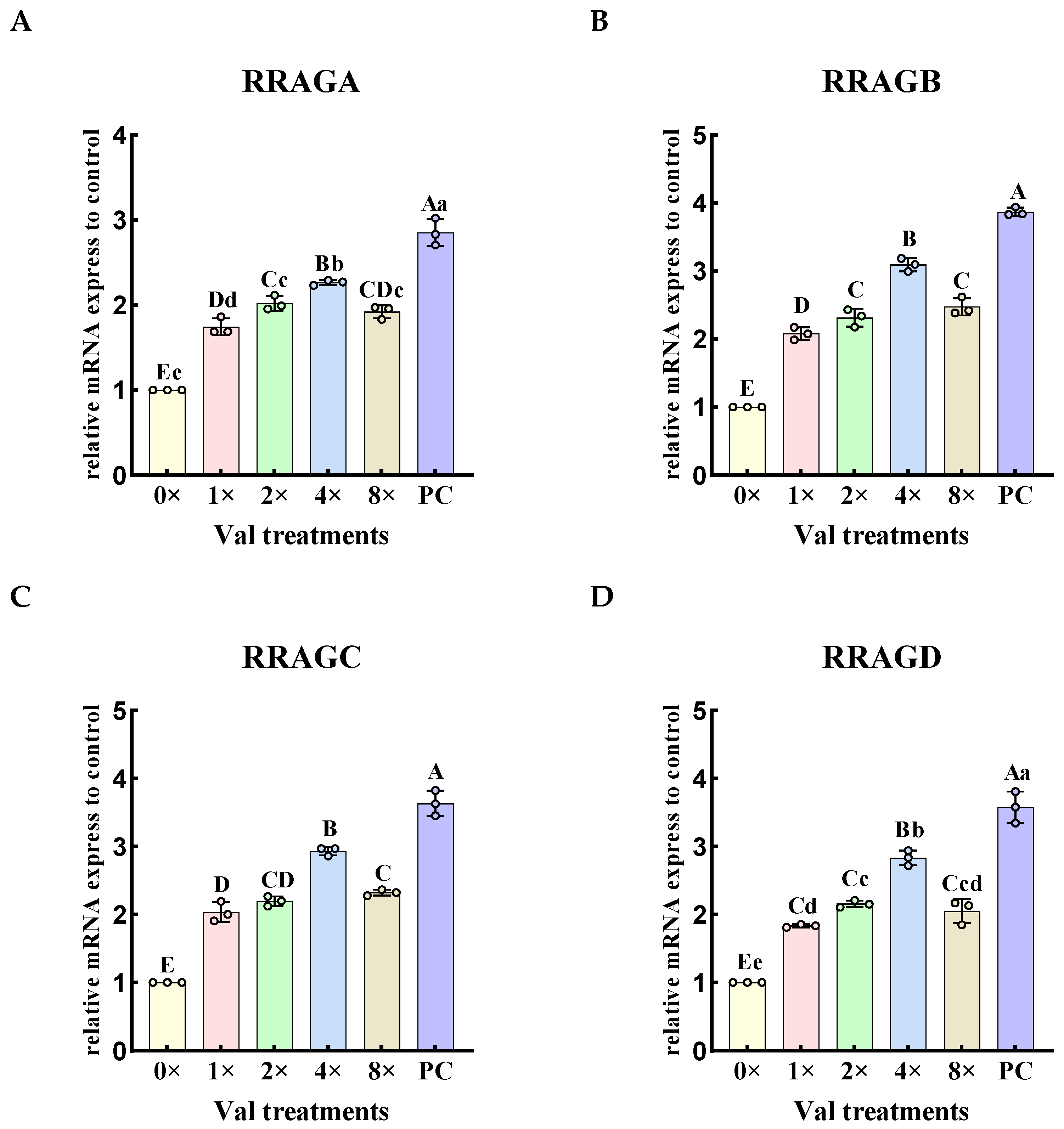
2.3.1. Effects of Varying Valine Concentrations on the Expression Levels of Rag Small G Protein Genes Upstream in the mTOR Signaling Pathway
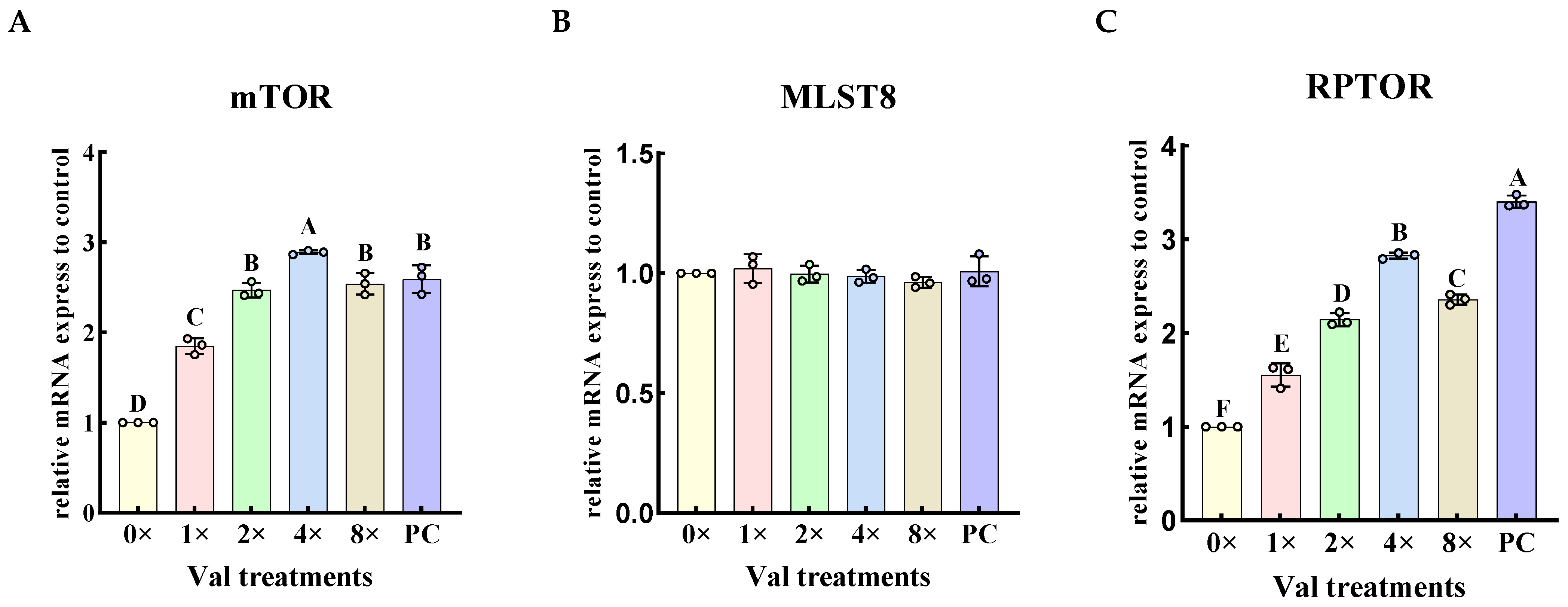
2.3.2. Effects of Varying Valine Concentrations on the Relative Expression Levels of mTORC1-Related Genes
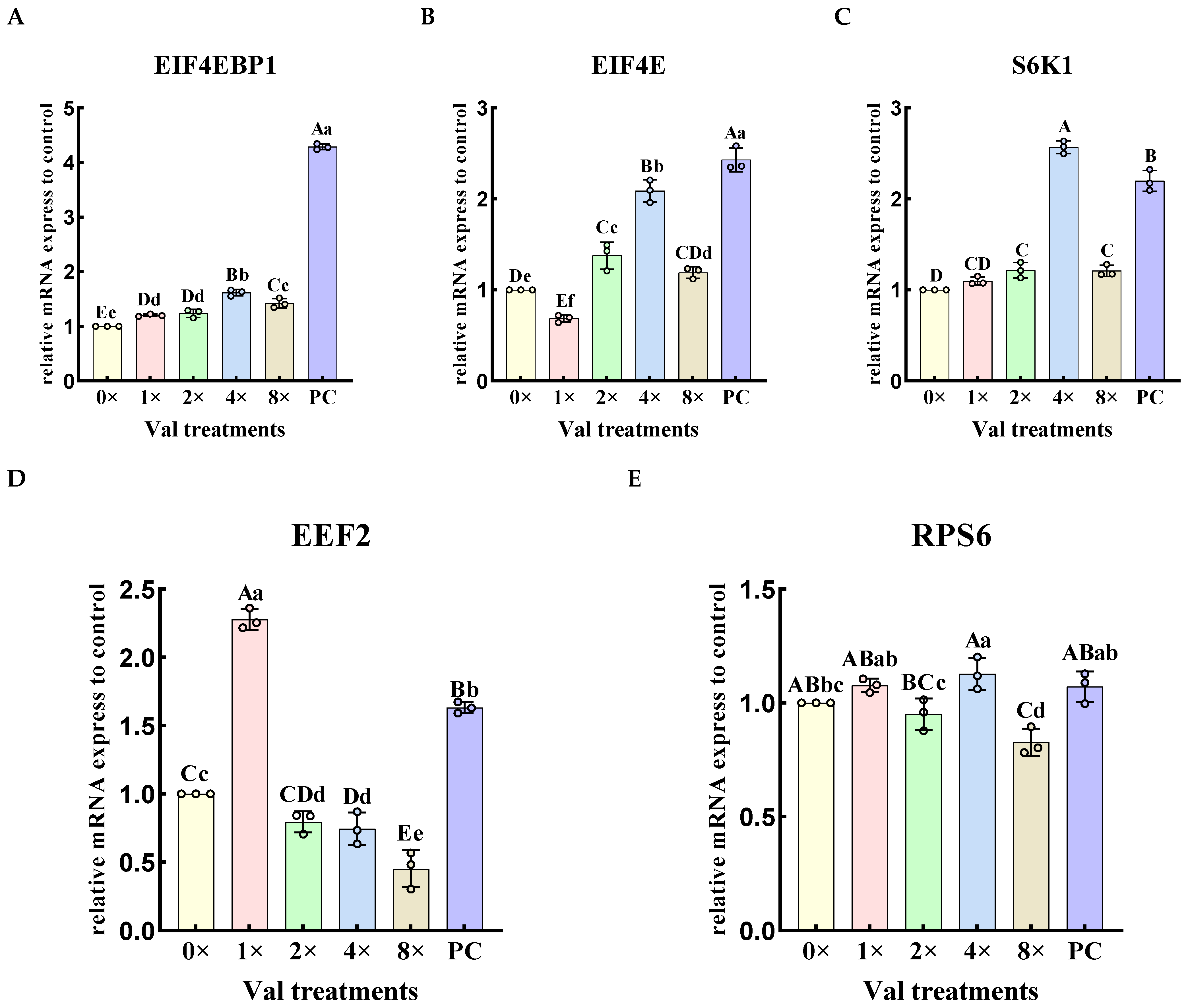
2.3.3. Effects of Varying Valine Concentrations on the Relative Expression Levels of Downstream Genes in the mTOR Signaling Pathway
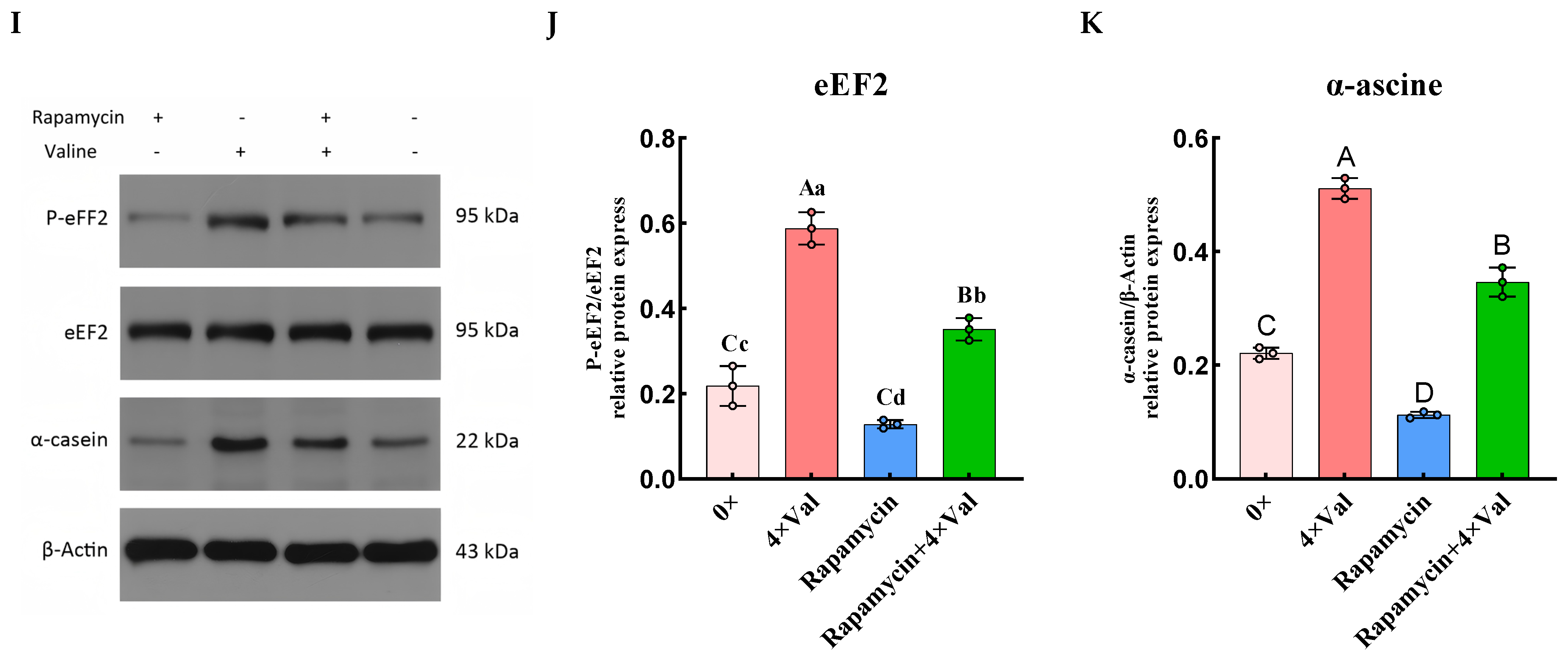
2.3.4. Effects of Varying Valine Concentrations on the Phosphorylation of Downstream Proteins in the mTOR Signaling Pathway
2.4. To Investigate the Role of the mTOR Signaling Pathway in Valine-Induced α-Casein Synthesis in MAC-T Cells by Inhibiting mTOR
3. Discussion
3.1. Effects of Different Concentrations of Valine on the Synthesis of α-Casein
3.2. Effects of Varying Valine Concentrations on the Relative Expression Level of the α-Casein Gene
3.3. Effects of Varying Valine Concentrations on the Expression and Phosphorylation Levels of Genes Related to α-Casein Synthesis Mediated by the mTOR Signaling Pathway
3.4. To Investigate the Role of the mTOR Signaling Pathway in Valine-Induced α-Casein Synthesis in MAC-T Cells, We Conducted Experiments to Inhibit the mTOR Signaling Pathway
4. Materials and Methods
4.1. Materials
4.2. MAC-T Cells
4.3. Experimental Design
4.4. Detection Indicators and Methods
4.4.1. Determination of the Synthesis Quantity of α-Casein
4.4.2. Determination of the Expression Levels of Related Genes
4.4.3. Determination of Phosphorylation of Related Proteins
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAC-T cells | Bovine mammary epithelial cells line |
| BCAA | Branched-chain amino acids |
| RT-qPCR | Real-time quantitative PCR |
| Val | Valine |
| FBS | Fetal bovine serum |
| 10%FBS | 10% fetal bovine serum |
| ELISA | Enzyme-linked immunosorbent assay |
| mTOR | Mammalian target of rapamycin |
| S6K1 | Ribosomal protein S6 kinase 1 |
| 4EBP1 | Eukaryotic initiation factor 4E-binding protein 1 |
| RPS6 | Ribosomal protein S6 |
| eEF2 | Eukaryotic translation elongation factor 2 |
| EIF4E | Eukaryotic initiation factor 4E |
| EEF2 | Eukaryotic elongation factor 2 |
| BMECs | Bovine mammary epithelial cells |
| RPS6 | Ribosomal protein S6 |
References
- Cemin, H.S.; Tokach, M.D.; Woodworth, J.C.; Dritz, S.S.; DeRouchey, J.M.; Goodband, R.D. Branched-chain amino acid interactions in growing pig diets. Transl. Anim. Sci. 2019, 3, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, S.; Huang, J.; Peng, P.; Liu, Y.; Han, B.; Sun, D. Identification of the Potential Role of the Rumen Microbiome in Milk Protein and Fat Synthesis in Dairy Cows Using Metagenomic Sequencing. Animals 2021, 11, 1247. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Rulquin, H.; Lemosquet, S. Milk protein responses in dairy cows to changes in postruminal supplies of arginine, isoleucine, and valine. J. Dairy Sci. 2013, 96, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, J.; Zeng, H.; Han, Z. Enhancement of BCAT2-Mediated Valine Catabolism Stimulates β-Casein Synthesis via the AMPK-mTOR Signaling Axis in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2022, 70, 9898–9907. [Google Scholar] [CrossRef]
- Toerien, C.A.; Trout, D.R.; Cant, J.P. Nutritional stimulation of milk protein yield of cows is associated with changes in phosphorylation of mammary eukaryotic initiation factor 2 and ribosomal s6 kinase 1. J. Nutr. 2010, 140, 285–292. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nature reviews. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Adel, P. The role of gut microbiota on regulation of growth and metabolism by branched-chain amino acids. J. Anim. Sci. 2024, 102, 199. [Google Scholar] [CrossRef]
- Petrova, S.Y.; Khlgatian, S.V.; Emelyanova, O.Y.; Pishchulina, L.A.; Berzhets, V.M. Structure and biological functions of milk caseins. Russ. Open Med. J. 2022, 11, 209. [Google Scholar]
- Backwell, F.R.; Bequette, B.J.; Wilson, D.; Metcalf, J.A.; Franklin, M.F.; Beever, D.E.; Lobley, G.E.; MacRae, J.C. Evidence for the utilization of peptides for milk protein synthesis in the lactating dairy goat in vivo. Am. J. Physiol. 1996, 271, R955–R960. [Google Scholar] [CrossRef]
- Pszczolkowski, V.L.; Arriola Apelo, S.I. The market for amino acids: Understanding supply and demand of substrate for more efficient milk protein synthesis. J. Anim. Sci. Biotechnol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, F.; Liu, J.; Liu, H. Dipeptide (Methionyl-Methionine) Transport and Its Effect on β-Casein Synthesis in Bovine Mammary Epithelial Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 49, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Argüello, B.; Ruiz-Zárate, F.; Guerrero-Rodríguez, A.; López-Trujillo, R.; García-Elizondo, R.; Rodríguez, J.M.F. Degradación y absorción de fuentes proteicas en la cinética ruminal de los ovinos. Agraria 2014, 11, 103–109. [Google Scholar] [CrossRef]
- Wang, M.; Xu, B.; Wang, H.; Bu, D.; Wang, J.; Loor, J.J. Effects of Arginine concentration on the in vitro expression of Casein and mTOR pathway related genes in mammary epithelial cells from dairy cattle. PLoS ONE 2014, 9, e95985. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M. Role of amino acid transporters in amino acid sensing. Am. J. Clin. Nutr. 2014, 99, 223S–230S. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, L.; Zhao, F.; Liu, H. Regulation of Milk Protein Synthesis by Free and Peptide-Bound Amino Acids in Dairy Cows. Biology 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.H. Effects of Branched-Chain Amino Acids on Casein Synthesis in Bovine Mammary Epithelial Cells. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2023. (In Chinese) [Google Scholar] [CrossRef]
- Cao, Y.; Hu, G.; Long, X.; Li, F.; Wang, J.; Sun, M.; Xie, Y.; Ge, Y.; Guo, W.; Liu, J.; et al. Valine promotes milk synthesis by regulating PKM2 nuclear accumulation and histone H3 acetylation through the TAS1R1-mTOR-DDX39B signaling pathway. Int. J. Biol. Macromol. 2024, 254, 127786. [Google Scholar] [CrossRef]
- Jiangfeng, F.; Yuzhu, L.; Sijiu, Y.; Yan, C.; Gengquan, X.; Libin, W.; Yangyang, P.; Honghong, H. Transcriptional profiling of two different physiological states of the yak mammary gland using RNA sequencing. PLoS ONE 2018, 13, e0201628. [Google Scholar] [CrossRef]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nature reviews. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Bell, A.L.; Nayananjalie, W.A.; Escobar, J.; Hanigan, M.D. Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J. Nutr. 2011, 141, 1209–1215. [Google Scholar] [CrossRef]
- Taylor, P.M. Amino acid transporters: Éminences grises of nutrient signalling mechanisms? Biochem. Soc. Trans. 2009, 37, 237–241. [Google Scholar] [CrossRef]
- Zheng, X.; Liang, Y.; He, Q.; Yao, R.; Bao, W.; Bao, L.; Wang, Y.; Wang, Z. Current models of mammalian target of rapamycin complex 1 (mTORC1) activation by growth factors and amino acids. Int. J. Mol. Sci. 2014, 15, 20753–20769. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, X.; Liu, H.; Jia, Q.; Chen, J.; Ding, Y.; Sun, M.; Zhu, H. SNAT2-mediated regulation of estrogen and progesterone in the proliferation of goat mammary epithelial cells. Amino Acids 2024, 56, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Vandewalle, N.; De Veirman, K.; Vanderkerken, K.; Menu, E.; De Bruyne, E. Targeting mTOR signaling pathways in multiple myeloma: Biology and implication for therapy. Cell Commun. Signal. CCS 2024, 22, 320. [Google Scholar] [CrossRef]
- Xie, J.; Proud, C.G. Signaling crosstalk between the mTOR complexes. Translation 2014, 2, e28174. [Google Scholar] [CrossRef]
- Machen, K. The Role of EIF4E in Nociception of Drosophila Melanogaster. Master’s Thesis, Appalachian State University, Boone, NC, USA, 2021. [Google Scholar]
- Lee, C.M.; Lee, H.Y.; Jarrell, Z.R.; Smith, M.R.; Jones, D.P.; Go, Y.M. Mechanistic role for mTORC1 signaling in profibrotic toxicity of low-dose cadmium. Toxicol. Appl. Pharmacol. 2025, 494, 117159. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Z.C.; Cheng, S.; Liu, A.; Zuo, J.; Xia, S.; Liu, X.; Liu, W.; Jia, Z.; Xie, W.; et al. PQBP1 promotes translational elongation and regulates hippocampal mGluR-LTD by suppressing eEF2 phosphorylation. Mol. Cell 2021, 81, 1425–1438.e10. [Google Scholar] [CrossRef] [PubMed]
- Pigott, C.R.; Mikolajek, H.; Moore, C.E.; Finn, S.J.; Phippen, C.W.; Werner, J.M.; Proud, C.G. Insights into the regulation of eukaryotic elongation factor 2 kinase and the interplay between its domains. Biochem. J. 2012, 442, 105–118. [Google Scholar] [CrossRef]
- Appuhamy, J.A.; Nayananjalie, W.A.; England, E.M.; Gerrard, D.E.; Akers, R.M.; Hanigan, M.D. Effects of AMP-activated protein kinase (AMPK) signaling and essential amino acids on mammalian target of rapamycin (mTOR) signaling and protein synthesis rates in mammary cells. J. Dairy Sci. 2014, 97, 419–429. [Google Scholar] [CrossRef]
- van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past—Present—Future. ALTEX 2018, 35, 99–118. [Google Scholar] [CrossRef]
- Yang, C.C.; Masai, H. Claspin is Required for Growth Recovery from Serum Starvation through Regulating the PI3K-PDK1-mTOR Pathway in Mammalian Cells. Mol. Cell. Biol. 2023, 43, 1–21. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Che, L.; Xu, M.; Gao, K.; Zhu, C.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z.; Wu, D. Valine increases milk fat synthesis in mammary gland of gilts through stimulating AKT/MTOR/SREBP1 pathway. Biol. Reprod. 2019, 101, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Xu, M.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Xiao, H.; Jiang, Z.; Wu, D. Valine supplementation during late pregnancy in gilts increases colostral protein synthesis through stimulating mTOR signaling pathway in mammary cells. Amino Acids 2019, 51, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xie, C.; Zhang, X.; Liu, J.; Liu, J.; Xia, Z. Advances in the mTOR signaling pathway and its inhibitor rapamycin in epilepsy. Brain Behav. 2023, 13, e2995. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, S.; Jin, Q.; Lv, X.; Sun, W. PAPPA2 Promote the Proliferation of Dermal Papilla Cells in Hu Sheep (Ovis aries) by Regulating IGFBP5. Genes 2021, 12, 1490. [Google Scholar] [CrossRef]
- Yang, X.; Lu, X.; Wang, L.; Bai, L.; Yao, R.; Jia, Z.; Ma, Y.; Chen, Y.; Hao, H.; Wu, X.; et al. Stearic acid promotes lipid synthesis through CD36/Fyn/FAK/mTORC1 axis in bovine mammary epithelial cells. Int. J. Biol. Macromol. 2023, 253, 127324. [Google Scholar] [CrossRef]
- Pu, P.; Zheng, X.; Jiao, L.; Chen, L.; Yang, H.; Zhang, Y.; Liang, G. Six flavonoids inhibit the antigenicity of β-lactoglobulin by noncovalent interactions: A spectroscopic and molecular docking study. Food Chem. 2021, 339, 128106. [Google Scholar] [CrossRef]
- Gao, H.; Han, R.; Zheng, N.; Hu, H.; Li, F.; Wang, J. Effects of Lysine on the Expression of Casein and mTOR Signaling Pathway-related Genes in Primary Bovine Mammary Epithelial Cells. J. Gansu Agric. Univ. 2015, 50, 7–15. (In Chinese) [Google Scholar] [CrossRef]


| Items | Groups | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0× Val | 0.25× Val | 0.5× Val | 1× Val | 2× Val | 4× Val | 8× Val | PC | Rapamycin | Rapamycin + Val | Val | |
| Valine (mM) | 0.000 | 1.596 | 3.192 | 6.384 | 12.768 | 25.536 | 51.072 | 0.000 | 0.000 | 25.536 | 25.536 |
| Rapamycin (mM) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 100.000 | 100.000 | 0.000 |
| Fetal Bovine Serum (%) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 10.000 | 0.000 | 0.000 | 0.000 |
| Genes | Gene Accession Number | Primer Sequence |
|---|---|---|
| mTOR | XM_001788228.1 | F: ATGCTGTCCCTGGTCCTTATG |
| R: GGGTCAGAGAGTGGCCTTCAA | ||
| MLST8 | NM_001035411 | F: ATCTGTACGCGAACTGTGCAG |
| R: CATACATGCGAATGTGCTGG | ||
| RPTOR | NM_001192130 | F: GTTTGGAATGCTGGATTGATC |
| R: CTGAGTGTAGTTCTTGTTGAAGACC | ||
| RRAGA | NM_001035499 | F: GAGGTTCTGATTTATGTGTTCGAC |
| R: ACAATACTGGACCAGGCTTTGTAG | ||
| RRAGB | NM_001075279 | F: GGGTAAGACCAGCATGAGGTCT |
| R: CACAGTCCCACAGGTTCAATACC | ||
| RRAGC | NM_001076456 | F: CATCCAGAAGGTGGTGTTTCAT |
| R: GCATCAATGACATATATCAATGCTC | ||
| RRAGD | NM_001192828 | F: CAGAGGTAAAGCCGAGGATCC |
| R: TCCAAGAACAGAGTTTCGTTGG | ||
| S6K1 | NM_205816.1 | F: CTGGGGAAGAGGTGCTTCAG |
| R: GTGCTCTGGTCGTTTGGAGA | ||
| EIF4EBP1 | BC120290.1 | F: GAACTCACCTGTGACCAAGA |
| R: CTCAAACTGTGACTCTTCACC | ||
| EIF4E | NM_174310.1 | F: ACGAAGTGACCTCGATCGTT |
| R: AGTAGCTGTGTCTGCATGGG | ||
| EEF2 | NW_618521.1 | F: CTCTACCAAACCTTCCAGCG |
| R: GCTGTTGGCTGACTTGCTGA | ||
| RPS6 | NM_001010.2 | F: AAGAGCTAGCAGAATCCGCA |
| R: CGTGGAGTAACAAGACGCTG | ||
| CSN1S1 | NM_181029.2 | F: TCAACCCAGCTTGCTGCTTCTTCC |
| R: GCCTAGCAAGAGCAACAGCCACAA | ||
| CSN1S2 | NM_174528.2 | F: AGCAGCTCTCCACCAGTGAGGAAA |
| R: TGGGGCAAGGCGAATTTCTGGT | ||
| β-actin | NM_173979.3 | F: GTCATCACCATCGGCAATGAG |
| R: AATGCCGCAGGATTCCATG |
| Primary Antibody | Antibody | Source | Catalog Number | Dilution Method | Dilution Ratio |
|---|---|---|---|---|---|
| β-Actin | Tian De Yue | TDY051 | 5% fat-free milk | 1:10,000 | |
| P-mTOR | bioss | bs-3494R | 5% egg white | 1:500 | |
| P-S6K1 | affbiotech | AF3228 | 5% egg white | 1:1000 | |
| S6K1 | affbiotech | AF6226 | 5% egg white | 1:2000 | |
| P-4EBP1 | affbiotech | AF3432 | 5% egg white | 1:1000 | |
| 4EBP1 | affbiotech | AF6432 | 5% egg white | 1:2000 | |
| P-RPS6 | affbiotech | AF3354 | 5% egg white | 1:500 | |
| RPS6 | biorbyt | orb585017 | 5% egg white | 1:1000 | |
| P-eFF2 | affbiotech | AF7220 | 5% egg white | 1:500 | |
| eEF2 | biorbyt | orb584002 | 5% egg white | 1:1000 | |
| Secondary antibody | HRP-Goat anti Rabbit | ASPEN | AS1107 | 5% fat-free milk | 1:10,000 |
| HRP-Goat anti Mouse | ASPEN | AS1106 | 5% fat-free milk | 1:10,000 | |
| HRP-Rabbit anti Goat | ASPEN | AS1108 | 5% fat-free milk | 1:10,000 | |
| HRP-Goat anti Rat | ASPEN | AS1093 | 5% fat-free milk | 1:10,000 | |
| HRP-Rabbit anti Sheep | ASPEN | AS1245 | 5% fat-free milk | 1:10,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhang, X.; Ding, Y.; Yang, L.; Ren, W.; Gao, Y.; Yao, K.; Zhou, Y.; Shao, W. The Effect of Valine on the Synthesis of α-Casein in MAC-T Cells and the Expression and Phosphorylation of Genes Related to the mTOR Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 3179. https://doi.org/10.3390/ijms26073179
Yang M, Zhang X, Ding Y, Yang L, Ren W, Gao Y, Yao K, Zhou Y, Shao W. The Effect of Valine on the Synthesis of α-Casein in MAC-T Cells and the Expression and Phosphorylation of Genes Related to the mTOR Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(7):3179. https://doi.org/10.3390/ijms26073179
Chicago/Turabian StyleYang, Min, Xinyu Zhang, Yu Ding, Liang Yang, Wanping Ren, Yu Gao, Kangyu Yao, Yuxin Zhou, and Wei Shao. 2025. "The Effect of Valine on the Synthesis of α-Casein in MAC-T Cells and the Expression and Phosphorylation of Genes Related to the mTOR Signaling Pathway" International Journal of Molecular Sciences 26, no. 7: 3179. https://doi.org/10.3390/ijms26073179
APA StyleYang, M., Zhang, X., Ding, Y., Yang, L., Ren, W., Gao, Y., Yao, K., Zhou, Y., & Shao, W. (2025). The Effect of Valine on the Synthesis of α-Casein in MAC-T Cells and the Expression and Phosphorylation of Genes Related to the mTOR Signaling Pathway. International Journal of Molecular Sciences, 26(7), 3179. https://doi.org/10.3390/ijms26073179





