Role of Hypoxia-Inducible Factors in Respiratory Syncytial Virus Infection-Associated Lung Disease
Abstract
1. Introduction
2. Results
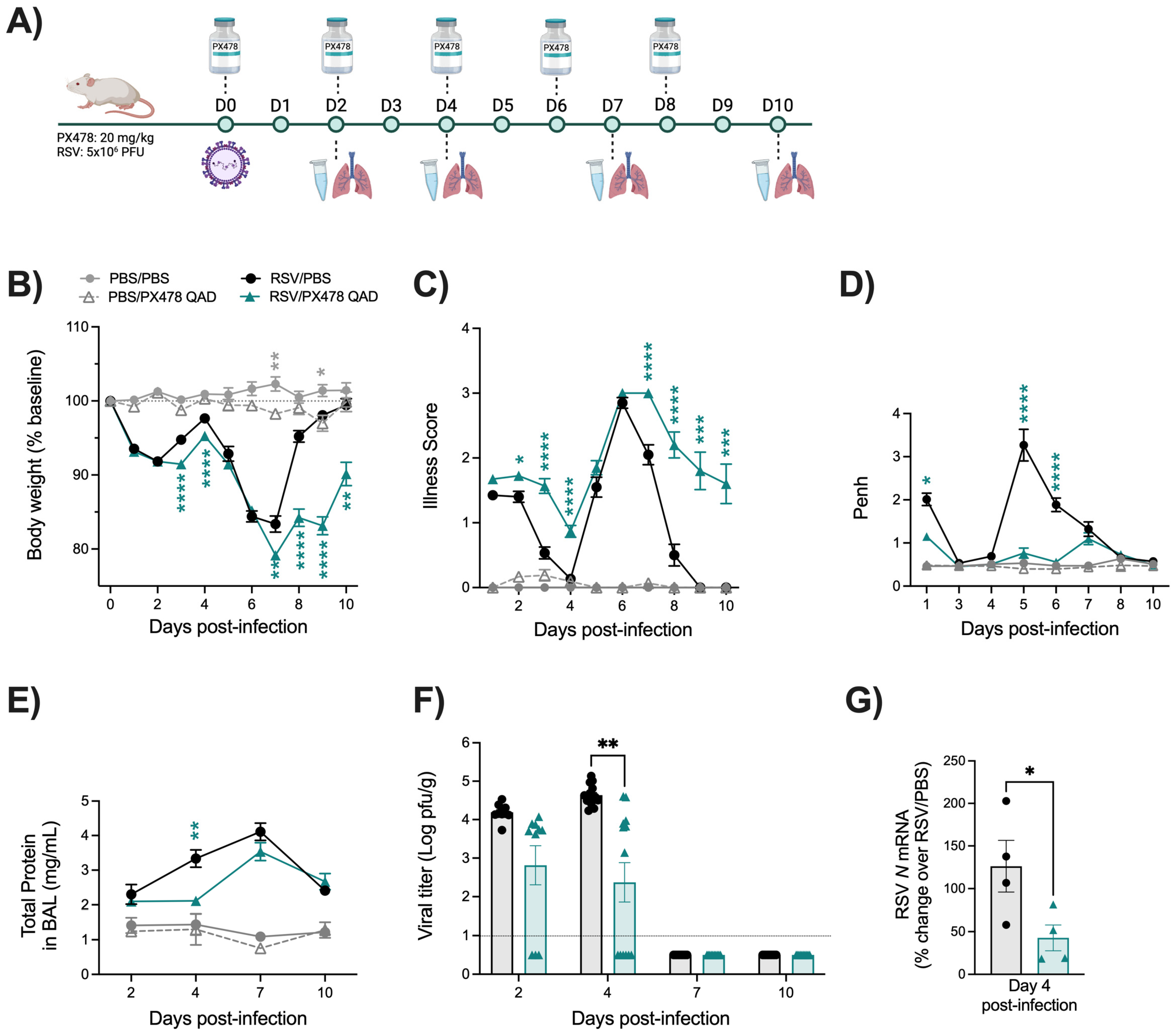
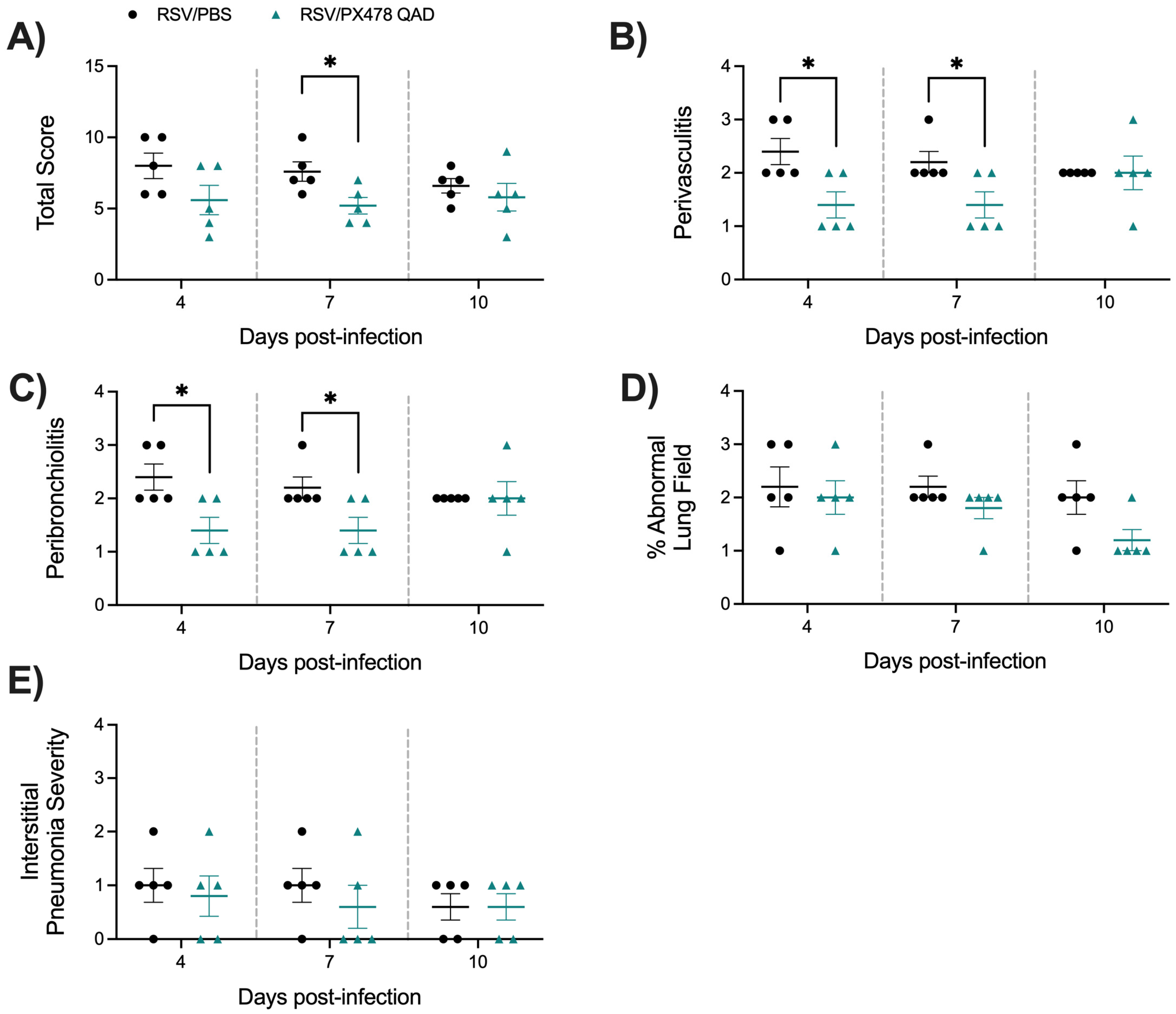
2.1. Effect of PX478 Administration on RSV Infection-Associated Disease Parameters and Viral Replication
2.2. Effect of PX478 Administration on RSV-Induced HIF-1a Target Genes
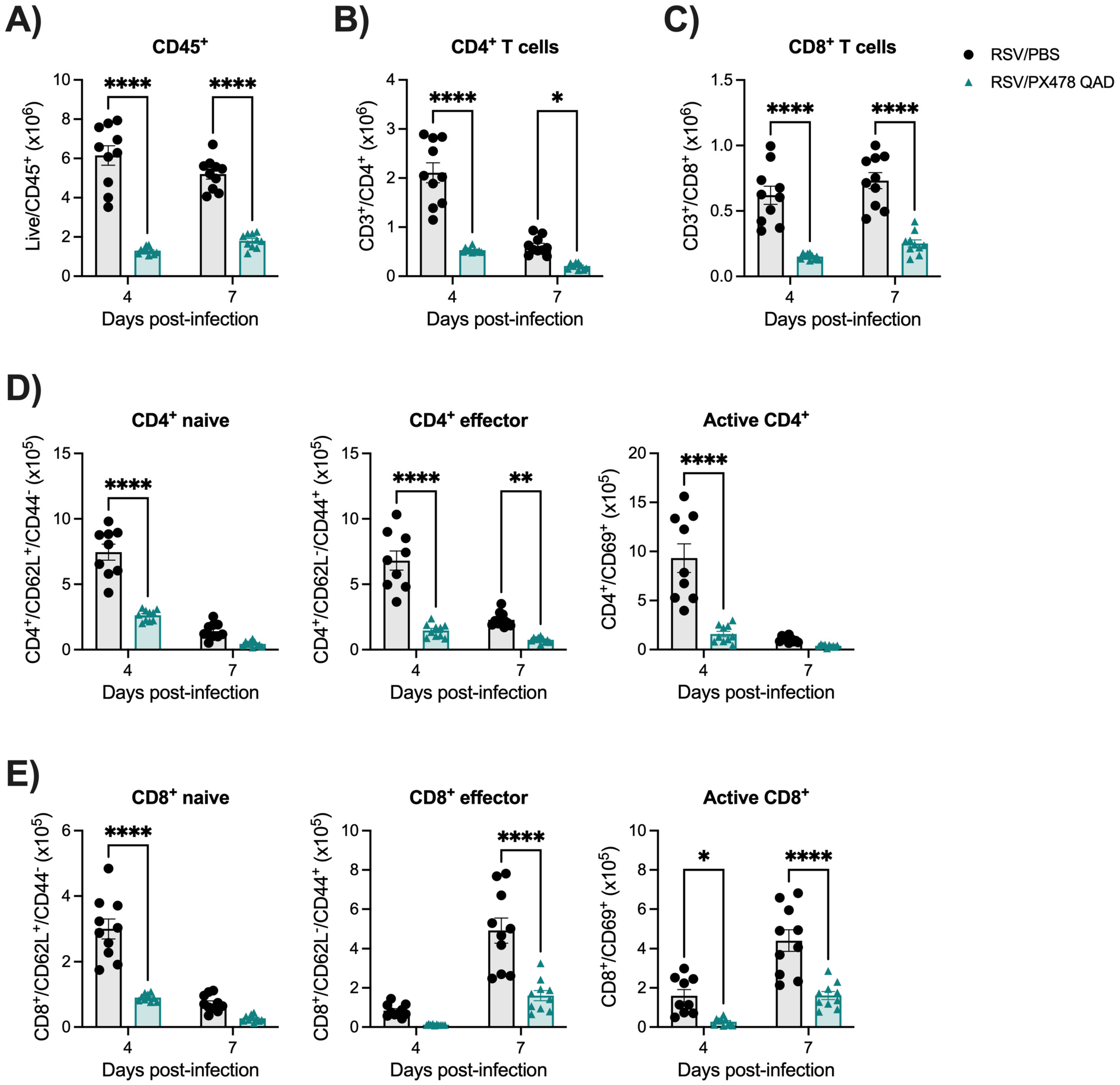
2.3. Inhibition of HIF-1a Leads to Reduced Immune Responses Following RSV Infection
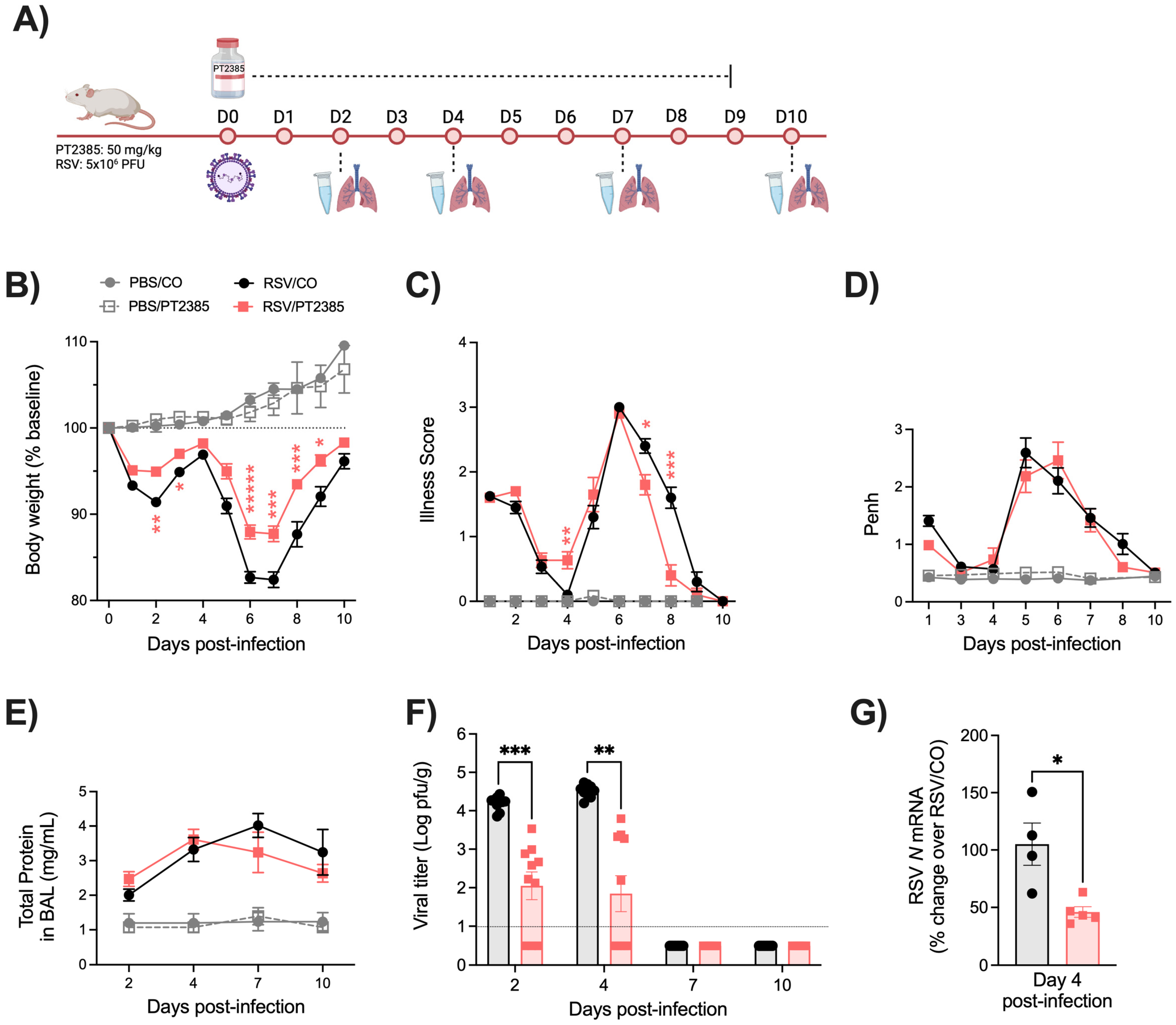
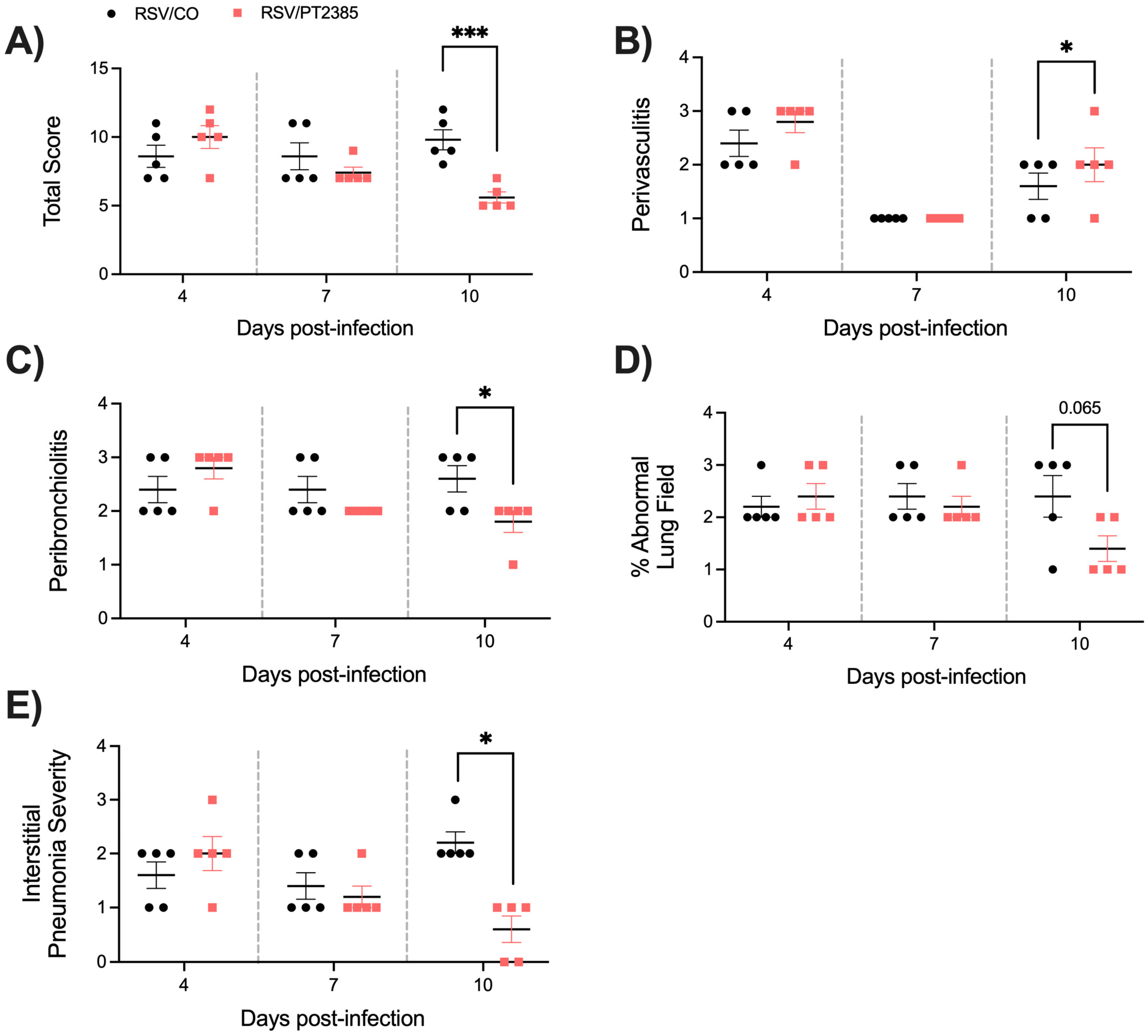
2.4. Effect of PT2385 Administration on RSV Infection-Associated Disease Parameters and Viral Replication
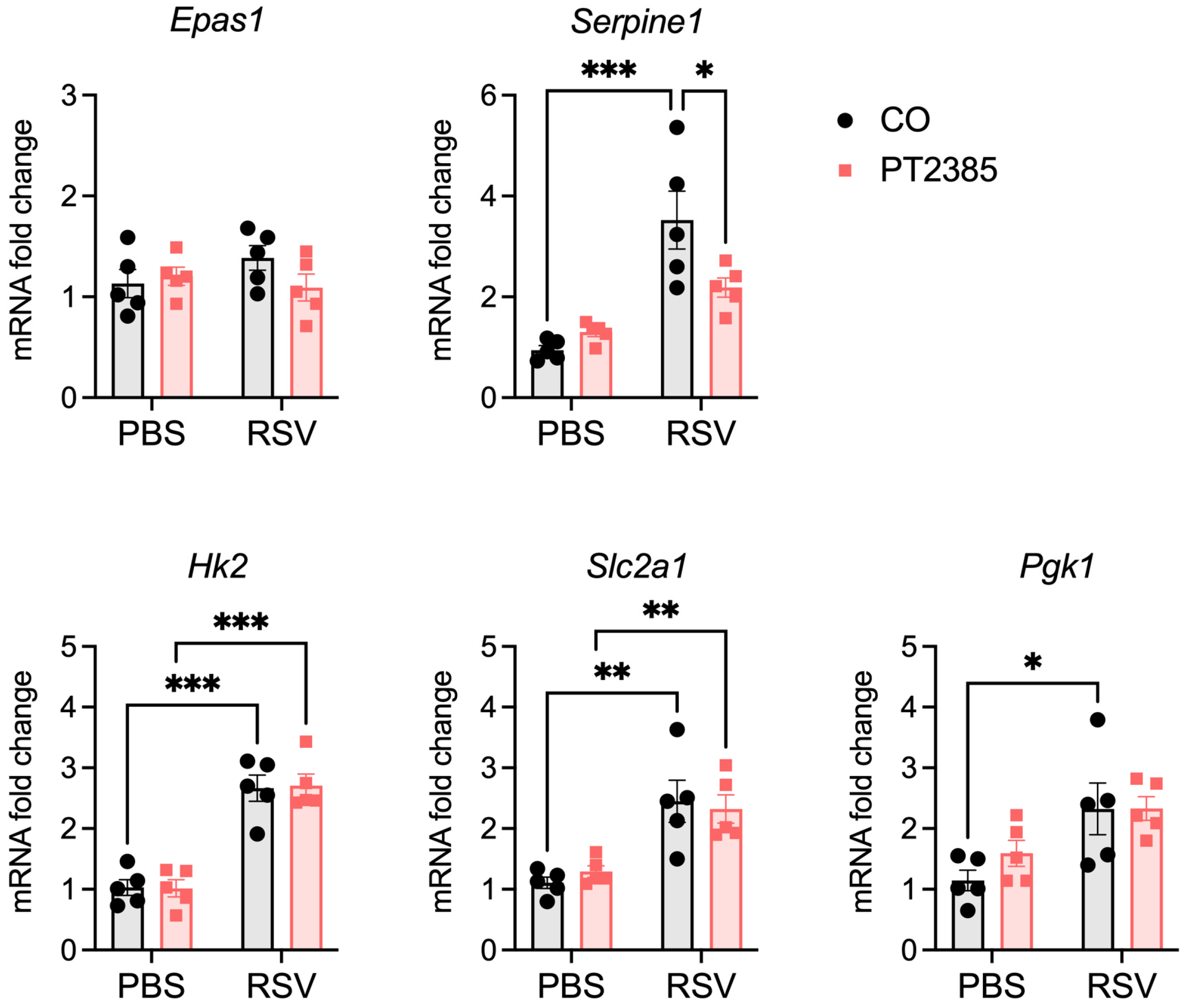
2.5. Effect of PT2385 Administration on RSV-Induced HIF-2a Target Genes
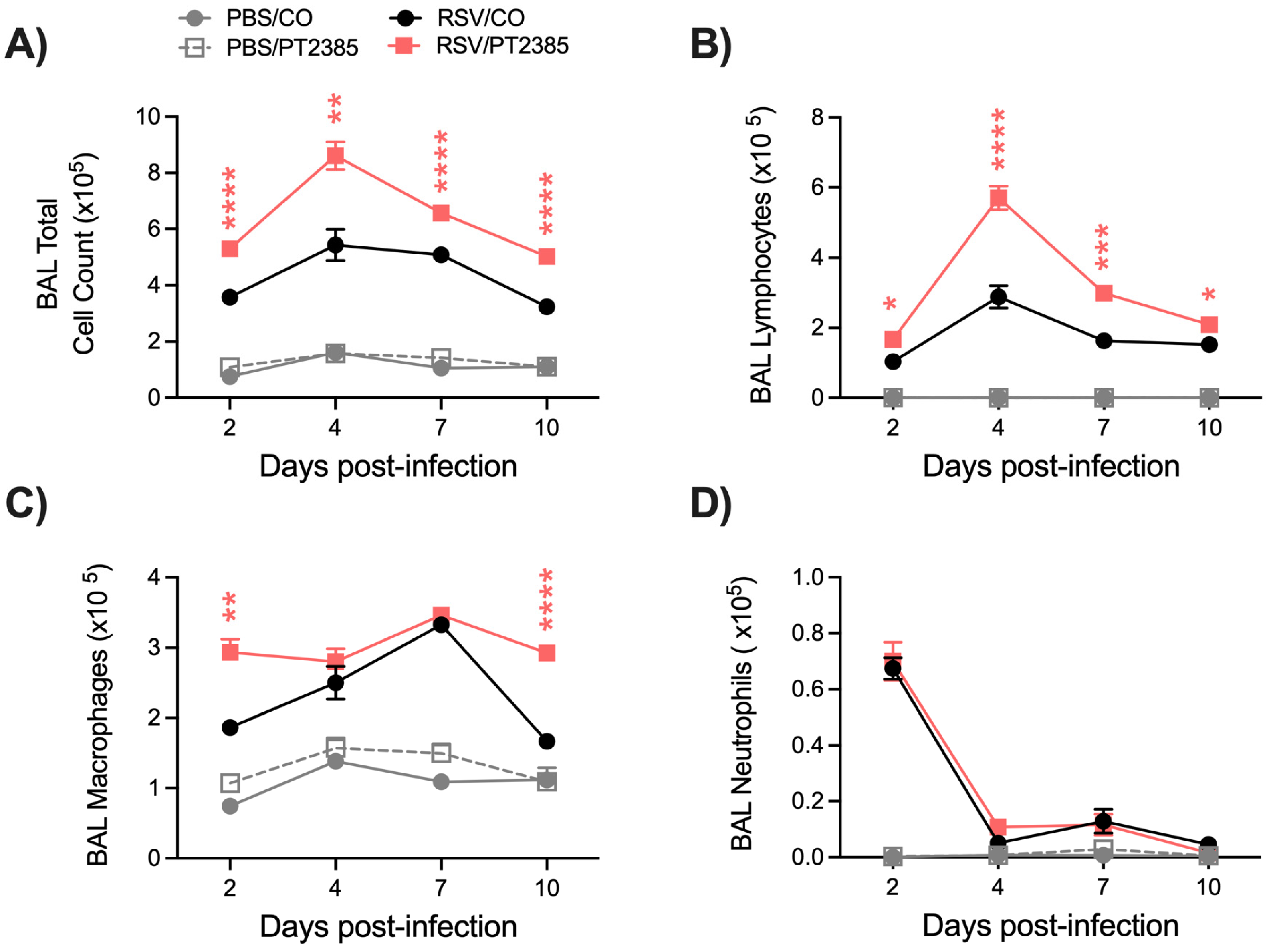
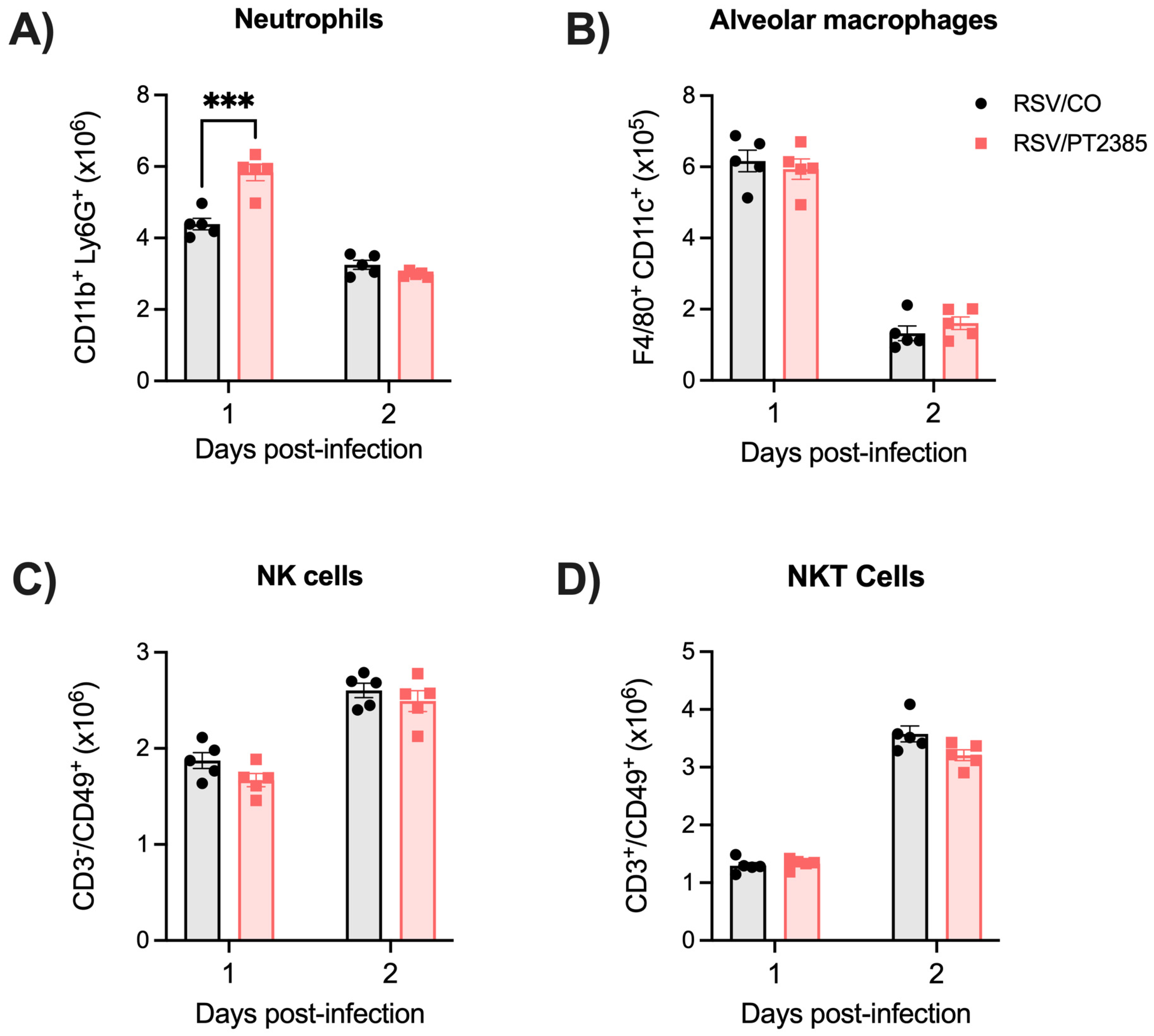
2.6. Inhibition of HIF-2a Leads to Increased Immune Activity in the BAL and Lung Tissue of RSV-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. RNA Extraction and Reverse Transcription–Quantitative PCR (RT-qPCR)
4.3. Bronchoalveolar Lavage and Viral Replication
4.4. Flow Cytometry
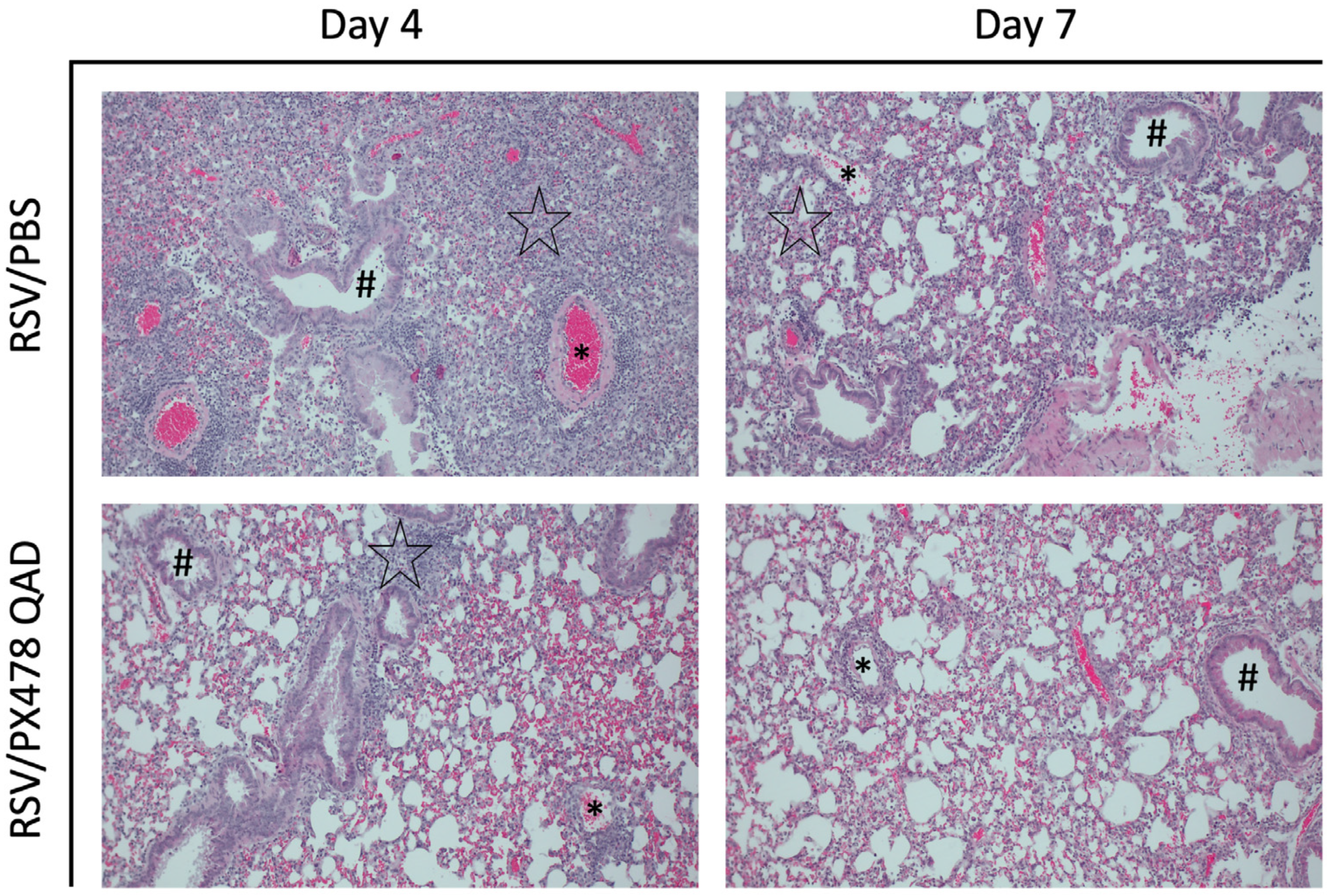
4.5. Assessment of Airway Function and Lung Histopathology
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Muller, W.J.; Madhi, S.A.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Dagan, R.; Hammitt, L.L.; Llapur, C.J.; Novoa, J.M.; Saez Llorens, X.; et al. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N. Engl. J. Med. 2023, 388, 1533–1534. [Google Scholar] [CrossRef]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Reyes, A.; Corrales, N.; Gálvez, N.M.S.; Bueno, S.M.; Kalergis, A.M.; González, P.A. Contribution of hypoxia inducible factor-1 during viral infections. Virulence 2020, 11, 1482–1500. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Huestis, M.; Gan, E.S.; Ooi, E.E.; Ohh, M. Hypoxia and viral infectious diseases. JCI Insight 2021, 6, e147190. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Pharmacologic Targeting of Hypoxia-Inducible Factors. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 379–403. [Google Scholar] [CrossRef]
- Reyes, A.; Duarte, L.F.; Farías, M.A.; Tognarelli, E.; Kalergis, A.M.; Bueno, S.M.; González, P.A. Impact of Hypoxia over Human Viral Infections and Key Cellular Processes. Int. J. Mol. Sci. 2021, 22, 7954. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Pouysségur, J. HIF at a glance. J. Cell Sci. 2009, 122, 1055–1057. [Google Scholar] [CrossRef]
- Infantino, V.; Santarsiero, A.; Convertini, P.; Todisco, S.; Iacobazzi, V. Cancer Cell Metabolism in Hypoxia: Role of HIF-1 as Key Regulator and Therapeutic Target. Int. J. Mol. Sci. 2021, 22, 5703. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Powis, G. Passing the baton: The HIF switch. Trends Biochem. Sci. 2012, 37, 364–372. [Google Scholar] [CrossRef]
- Zhang, P.; Yao, Q.; Lu, L.; Li, Y.; Chen, P.-J.; Duan, C. Hypoxia-Inducible Factor 3 Is an Oxygen-Dependent Transcription Activator and Regulates a Distinct Transcriptional Response to Hypoxia. Cell Rep. 2014, 6, 1110–1121. [Google Scholar] [CrossRef]
- Downes, N.L.; Laham-Karam, N.; Kaikkonen, M.U.; Yla-Herttuala, S. Differential but Complementary HIF1alpha and HIF2alpha Transcriptional Regulation. Mol. Ther. 2018, 26, 1735–1745. [Google Scholar] [CrossRef]
- Morris, D.R.; Qu, Y.; Agrawal, A.; Garofalo, R.P.; Casola, A. HIF-1alpha Modulates Core Metabolism and Virus Replication in Primary Airway Epithelial Cells Infected with Respiratory Syncytial Virus. Viruses 2020, 12, 1088. [Google Scholar] [CrossRef]
- Morris, D.R.; Ansar, M.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Selective Blockade of TNFR1 Improves Clinical Disease and Bronchoconstriction in Experimental RSV Infection. Viruses 2020, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Unger, S.A.; Walton, M.; Schwarze, J. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev. 2017, 30, 481–502. [Google Scholar] [CrossRef]
- De, C.; Pickles, R.J.; Yao, W.; Liao, B.; Boone, A.; Choi, M.; Battaglia, D.M.; Askin, F.B.; Whitmire, J.K.; Silvestri, G.; et al. Human T cells efficiently control RSV infection. JCI Insight 2023, 8, e168110. [Google Scholar] [CrossRef]
- McGinley, J.; Thwaites, R.; Brebner, W.; Greenan-Barrett, L.; Aerssens, J.; Öner, D.; Bont, L.; Wildenbeest, J.; Martinón-Torres, F.; Nair, H.; et al. A Systematic Review and Meta-analysis of Animal Studies Investigating the Relationship Between Serum Antibody, T Lymphocytes, and Respiratory Syncytial Virus Disease. J. Infect. Dis. 2021, 226, S117–S129. [Google Scholar] [CrossRef]
- Welsh, S.; Williams, R.; Kirkpatrick, L.; Paine-Murrieta, G.; Powis, G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol. Cancer Ther. 2004, 3, 233–244. [Google Scholar] [CrossRef]
- Palayoor, S.T.; Mitchell, J.B.; Cerna, D.; DeGraff, W.; John-Aryankalayil, M.; Coleman, C.N. PX-478, an inhibitor of hypoxia-inducible factor-1α, enhances radiosensitivity of prostate carcinoma cells. Int. J. Cancer 2008, 123, 2430–2437. [Google Scholar] [CrossRef] [PubMed]
- Villa-Roel, N.; Ryu, K.; Gu, L.; Fernandez Esmerats, J.; Kang, D.-W.; Kumar, S.; Jo, H. Hypoxia inducible factor 1α inhibitor PX-478 reduces atherosclerosis in mice. Atherosclerosis 2022, 344, 20–30. [Google Scholar] [CrossRef]
- Sun, K.; Halberg, N.; Khan, M.; Magalang, U.J.; Scherer, P.E. Selective Inhibition of Hypoxia-Inducible Factor 1α Ameliorates Adipose Tissue Dysfunction. Mol. Cell. Biol. 2013, 33, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Ilegems, E.; Bryzgalova, G.; Correia, J.; Yesildag, B.; Berra, E.; Ruas, J.L.; Pereira, T.S.; Berggren, P.-O. HIF-1a inhibitor PX-478 preserves pancreatic beta cell function in diabetes. Sci. Transl. Med. 2022, 14, eaba9112. [Google Scholar] [CrossRef]
- McGettrick, A.F.; O’Neill, L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020, 32, 524–536. [Google Scholar] [CrossRef]
- Bhat, R.; Farrag, M.A.; Almajhdi, F.N. Double-edged role of natural killer cells during RSV infection. Int. Rev. Immunol. 2020, 39, 233–244. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Varga, S.M. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection. Cytokine 2020, 133, 154481. [Google Scholar] [CrossRef]
- Currie, S.M.; Findlay, E.G.; McHugh, B.J.; Mackellar, A.; Man, T.; Macmillan, D.; Wang, H.; Fitch, P.M.; Schwarze, J.; Davidson, D.J. The human cathelicidin LL-37 has antiviral activity against respiratory syncytial virus. PLoS ONE 2013, 8, e73659. [Google Scholar] [CrossRef]
- Dabo, A.J.; Cummins, N.; Eden, E.; Geraghty, P. Matrix Metalloproteinase 9 Exerts Antiviral Activity against Respiratory Syncytial Virus. PLoS ONE 2015, 10, e0135970. [Google Scholar] [CrossRef] [PubMed]
- Hartshorn, K.L.; White, M.R.; Tecle, T.; Holmskov, U.; Crouch, E.C. Innate defense against influenza A virus: Activity of human neutrophil defensins and interactions of defensins with surfactant protein D. J. Immunol. 2006, 176, 6962–6972. [Google Scholar] [CrossRef] [PubMed]
- Dos Ramos Almeida, C.J.L.; Veras, F.P.; Paiva, I.M.; Schneider, A.H.; da Costa Silva, J.; Gomes, G.F.; Costa, V.F.; Silva, B.M.S.; Caetite, D.B.; Silva, C.M.S.; et al. Neutrophil Virucidal Activity Against SARS-CoV-2 Is Mediated by Neutrophil Extracellular Traps. J. Infect. Dis. 2024, 229, 1352–1365. [Google Scholar] [CrossRef]
- Hsu, T.-S.; Lin, Y.-L.; Wang, Y.-A.; Mo, S.-T.; Chi, P.-Y.; Lai, A.C.-Y.; Pan, H.-Y.; Chang, Y.-J.; Lai, M.-Z. HIF-2α is indispensable for regulatory T cell function. Nat. Commun. 2020, 11, 5005. [Google Scholar] [CrossRef] [PubMed]
- Ajouaou, Y.; Azouz, A.; Taquin, A.; Denanglaire, S.; Hussein, H.; Krayem, M.; Andris, F.; Moser, M.; Goriely, S.; Leo, O. The oxygen sensor prolyl hydroxylase domain 2 regulates the in vivo suppressive capacity of regulatory T cells. eLife 2022, 11, e70555. [Google Scholar] [CrossRef]
- Bowlin, A.; Roys, H.; Wanjala, H.; Bettadapura, M.; Venugopal, G.; Surma, J.; Simon, M.C.; Weinkopff, T. Hypoxia-Inducible Factor Signaling in Macrophages Promotes Lymphangiogenesis in Leishmania major Infection. Infect. Immun. 2021, 89, e0012421. [Google Scholar] [CrossRef]
- Czopik, A.K.; McNamee, E.N.; Vaughn, V.; Huang, X.; Bang, I.H.; Clark, T.; Wang, Y.; Ruan, W.; Nguyen, T.; Masterson, J.C.; et al. HIF-2α-dependent induction of miR-29a restrains T(H)1 activity during T cell dependent colitis. Nat. Commun. 2024, 15, 8042. [Google Scholar] [CrossRef]
- Ahmad, T.; Kumar, M.; Mabalirajan, U.; Pattnaik, B.; Aggarwal, S.; Singh, R.; Singh, S.; Mukerji, M.; Ghosh, B.; Agrawal, A. Hypoxia response in asthma: Differential modulation on inflammation and epithelial injury. Am. J. Respir. Cell Mol. Biol. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Dewitz, C.; McEachern, E.; Shin, S.; Akong, K.; Nagle, D.G.; Broide, D.H.; Akuthota, P.; Crotty Alexander, L.E. Hypoxia-inducible factor-1α inhibition modulates airway hyperresponsiveness and nitric oxide levels in a BALB/c mouse model of asthma. Clin. Immunol. 2017, 176, 94–99. [Google Scholar] [CrossRef]
- Pan, S.; Conaway, S., Jr.; Deshpande, D.A. Mitochondrial regulation of airway smooth muscle functions in health and pulmonary diseases. Arch. Biochem. Biophys. 2019, 663, 109–119. [Google Scholar] [CrossRef]
- Bartman, C.M.; Matveyenko, A.; Pabelick, C.; Prakash, Y.S. Cellular clocks in hyperoxia effects on [Ca2+]i regulation in developing human airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L451–L466. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E. Hypoxia-Inducible Factor Signaling in Inflammatory Lung Injury and Repair. Cells 2022, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Watts, D.; Gaete, D.; Sormendi, S.; Wielockx, B. Hypoxia Pathway Proteins and Their Impact on the Blood Vasculature. Int. J. Mol. Sci. 2021, 22, 9191. [Google Scholar] [CrossRef]
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target. Ther. 2021, 6, 308. [Google Scholar] [CrossRef]
- Wing, P.A.C.; Keeley, T.P.; Zhuang, X.; Lee, J.Y.; Prange-Barczynska, M.; Tsukuda, S.; Morgan, S.B.; Harding, A.C.; Argles, I.L.A.; Kurlekar, S.; et al. Hypoxic and pharmacological activation of HIF inhibits SARS-CoV-2 infection of lung epithelial cells. Cell Rep. 2021, 35, 109020. [Google Scholar] [CrossRef]
- Wing, P.A.C.; Prange-Barczynska, M.; Cross, A.; Crotta, S.; Orbegozo Rubio, C.; Cheng, X.; Harris, J.M.; Zhuang, X.; Johnson, R.L.; Ryan, K.A.; et al. Hypoxia inducible factors regulate infectious SARS-CoV-2, epithelial damage and respiratory symptoms in a hamster COVID-19 model. PLoS Pathog. 2022, 18, e1010807. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, W.; Han, P.; Zhang, J.; Zhu, Y.; Meng, X.; Zhang, J.; Hu, Y.; Yi, Z.; Wang, R. Influenza A virus (H1N1) triggers a hypoxic response by stabilizing hypoxia-inducible factor-1α via inhibition of proteasome. Virology 2019, 530, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, W.; Zhang, J.; Zhang, J.; Zhang, H.; Zhu, Y.; Meng, X.; Yi, Z.; Wang, R. Influenza A Virus (H1N1) Infection Induces Glycolysis to Facilitate Viral Replication. Virol. Sin. 2021, 36, 1532–1542. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.; Cheng, L.; Xu, K.; Yang, Y.; Su, X. Deficiency of HIF-1α enhances influenza A virus replication by promoting autophagy in alveolar type II epithelial cells. Emerg. Microbes Infect. 2020, 9, 691–706. [Google Scholar] [CrossRef]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef]
- Ueba, O. Respiratory syncytial virus. I. Concentration and purification of the infectious virus. Acta Med. Okayama 1978, 32, 265–272. [Google Scholar]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef]
- Qu, Y.; Haas de Mello, A.; Morris, D.R.; Jones-Hall, Y.L.; Ivanciuc, T.; Sattler, R.A.; Paessler, S.; Menachery, V.D.; Garofalo, R.P.; Casola, A. SARS-CoV-2 Inhibits NRF2-Mediated Antioxidant Responses in Airway Epithelial Cells and in the Lung of a Murine Model of Infection. Microbiol. Spectr. 2023, 11, e0037823. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, D.R.; Qu, Y.; Haas de Mello, A.; Jones-Hall, Y.L.; Liu, T.; Weglarz, M.; Ivanciuc, T.; Garofalo, R.P.; Casola, A. Role of Hypoxia-Inducible Factors in Respiratory Syncytial Virus Infection-Associated Lung Disease. Int. J. Mol. Sci. 2025, 26, 3182. https://doi.org/10.3390/ijms26073182
Morris DR, Qu Y, Haas de Mello A, Jones-Hall YL, Liu T, Weglarz M, Ivanciuc T, Garofalo RP, Casola A. Role of Hypoxia-Inducible Factors in Respiratory Syncytial Virus Infection-Associated Lung Disease. International Journal of Molecular Sciences. 2025; 26(7):3182. https://doi.org/10.3390/ijms26073182
Chicago/Turabian StyleMorris, Dorothea R., Yue Qu, Aline Haas de Mello, Yava L. Jones-Hall, Tianshuang Liu, Meredith Weglarz, Teodora Ivanciuc, Roberto P. Garofalo, and Antonella Casola. 2025. "Role of Hypoxia-Inducible Factors in Respiratory Syncytial Virus Infection-Associated Lung Disease" International Journal of Molecular Sciences 26, no. 7: 3182. https://doi.org/10.3390/ijms26073182
APA StyleMorris, D. R., Qu, Y., Haas de Mello, A., Jones-Hall, Y. L., Liu, T., Weglarz, M., Ivanciuc, T., Garofalo, R. P., & Casola, A. (2025). Role of Hypoxia-Inducible Factors in Respiratory Syncytial Virus Infection-Associated Lung Disease. International Journal of Molecular Sciences, 26(7), 3182. https://doi.org/10.3390/ijms26073182








