Abstract
This study reports the first successful establishment of Perovskia atriplicifolia hairy root cultures using Rhizobium rhizogenes and evaluates their potential for bioactive phenolic acid production, particularly rosmarinic acid (RA). Hairy roots were induced using two R. rhizogenes strains, A4 and ATCC 15834; transformation was confirmed by PCR analysis targeting the rol and aux genes. The A4 strain exhibited higher transformation efficiency (41.3%) than ATCC 15834 (30.2%). Eight transgenic root clones (C1–C8) were established and confirmed as transformed. The clones exhibited significant variation in biomass accumulation and phenolic acid production. RA production was most strongly correlated with PAL, RAS, and CYP98A14 expression. Hierarchical clustering clustered the clones into three groups based on growth, metabolite content, and gene expression. Lines C1 and C2 exhibiting the highest RA, total polyphenol content, and the highest productivity were selected for further experiments. McCown Woody Plant (WP) and Schenk and Hildebrandt (SH) media demonstrated the greatest biomass accumulation, with growth indexes exceeding 13. Conversely, Gamborg (B5) medium enhanced RA content, achieving 38.3 and 40.8 mg/g dry weight (DW) for clones C1 and C2, respectively, representing a fourfold increase compared to the least favorable Murashige and Skoog (MS) medium. These findings establish P. atriplicifolia hairy roots as efficient systems for RA biosynthesis and can provide a basis for metabolic engineering and scale-up production of phenolic acids in medicinal plants.
1. Introduction
Modern society is subject to increasing pressure from civilization diseases, such as cancer, circulatory disorders, diabetes mellitus, obesity, and neurodegenerative diseases; their etiology is closely related to the negative effects of industrialization and Western lifestyles. In many cases, their pathomechanism is significantly associated with chronic oxidative stress, characterized by a disturbed balance between reactive oxygen species (ROS) generation and the body’s antioxidant capacity [1]. Healthy organisms employ several enzymatic mechanisms to minimize ROS-induced damage and protect against their excessive production. Additionally, exogenous antioxidants can be provided as dietary supplementation. Nevertheless, free radicals are continuously produced in aerobic organisms, and this must be counterbalanced by a similar rate of antioxidant consumption [2]. Many plant-derived products act as exogenous antioxidants, neutralizing free radicals and mitigating oxidative stress. Among these, polyphenol-rich products are of particular interest for preventing civilization diseases [2,3]. Rosmarinic acid (RA) is a polyphenol that has received significant attention as an antioxidant supplement and is used in various products, ranging from food preservation to cosmetics and pharmaceuticals [4].
Rosmarinic acid, an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, is one of the most abundant caffeic acid esters in the plant kingdom. First isolated from rosemary (Rosmarinus officinalis L.), RA has since been identified in many species, particularly in the Lamiaceae and Boraginaceae families [5]. RA exhibits numerous biological and pharmacological activities, including anti-inflammatory, antiviral, and antibacterial effects [4,6]. As a potent free radical scavenger, RA demonstrates neuroprotective, cardioprotective, and hepatoprotective properties and shows cognitive-enhancing effects by inhibiting the prolyl oligopeptidase and amyloid-β aggregation pathways. Long-term dietary exposure to RA has shown promise for cancer chemoprevention, with studies indicating therapeutic potential against various cancers. RA has been found to increase the expression of pro-apoptotic genes (BNIP3, TNF, and GADD45A), inhibit the anti-apoptotic protein BIRC5, arrest the cell cycle, and reduce metastatic potential in cancer cells [4,6,7].
Despite its broad occurrence, RA content rarely exceeds 1% of plant dry weight, with the precise value depending on inter alia growth phases, ecological zones, and climatic conditions [7]. The increasing demand for RA has highlighted the need for alternative production methods. Plant biotechnology offers promising solutions, providing a sustainable, environmentally friendly approach to producing valuable plant materials with consistent phytochemical profiles.
Sterilized and standardized plant cultures present an attractive alternative to traditional cultivation methods. In vitro culture allows consistent production irrespective of geographical or seasonal limitations; the product is also free of biological contaminants and enables efficient production within shorter timeframes than field cultivation [8,9]. One of the most promising types of in vitro plant systems is hairy root culture. This approach is characterized by high stability and rapid growth in hormone-free media, and biotechnologically optimized hairy root cultures can produce high amounts of bioactive compounds, often outperforming soil-grown plants [10,11]. Furthermore, in vitro cultures enable enhanced biosynthesis of bioactive compounds through strategies such as genetic modification, selection of high-yielding lines, and optimization of growth conditions [12,13]. Such plant cultures have been found to produce significant levels of RA, often higher than in natural plants [4,7].
The Lamiaceae are rich in RA and other polyphenolic acids and have a wide range of medicinal, culinary, and cosmetic uses [14]. However, in recent years, many natural habitats of medicinal plants have been destroyed due to their over exploration and the environmental pollution. One species whose potential remains underutilized, owing to its low availability and limited scientific research, is Perovskia atriplicifolia Benth.
P. atriplicifolia, reclassified as Salvia yangii B.T. Drew in 2017, is a Central Asian medicinal and aromatic plant. It is traditionally used as a folk medicinal herb in its natural range, including Pakistan, Turkmenistan, and Afghanistan [15]. The plant exhibits antibacterial activity and is used to heal wounds and as a remedy for fever [16]. In traditional Tibetan and Chinese medicine, P. atriplicifolia is reputed to be a powerful analgesic and parasiticide [15,16]. The main active compounds reported in this species include phenolic compounds, terpenoids, steroids, and essential oils [15,17]. Thanks to its traditional uses and its phytochemical diversity, as well as recent advancements in biotechnological approaches used to produce high-quality raw materials, P. atriplicifolia was selected for the present study.
The aim of this study was to establish hairy root cultures of P. atriplicifolia using the soil bacterium Rhizobium rhizogenes, and to examine the RA biosynthetic pathway in the clones by gene expression analysis. Finally, the growth medium was optimized for the selected clones to enhance RA accumulation.
2. Results
2.1. Hairy Root Establishment and Transformation Confirmation
Hairy roots developed at the R. rhizogenes infection sites for both strains as a result of the plasmid DNA fragments integrating with the host plant DNA (Figure 1). However, the A4 strain demonstrated significantly superior transformation efficiency (41.3% vs. 30.2%; p < 0.05) (Table 1). In both cases, adventitious roots began forming at the infected sites during the first week of culture, with the process continuing for up to three weeks. The roots grew slowly and did not exceed 1 cm in length; the roots obtained from shoots infected with ATCC strain were particularly short. Roots were transferred to WP liquid medium supplemented with 500 mg/L cefotaxime and subcultured every 7 days for eight cycles to ensure complete elimination of residual R. rhizogenes. If the roots were not transferred to fresh WP liquid medium containing antibiotics during this period, they died.

Figure 1.
The hairy roots obtained on P. atriplicifolia shoots after infection with R. rhizogenes strain A4 (A) and strain ATCC 15,834 (B). Bar = 1 cm.

Table 1.
Induction of hairy roots of P. atriplicifolia from shoot explants with R. rhizogenes.
Any roots that resumed growth in fresh medium and reached a length of 1.5–2 cm were excised from the explants during the subsequent subculture. After several subcultures, during which the bacteria used for transformation were eliminated, eight root clones (designated as C1–C8) were obtained. Notably, all the clones that were subsequently used for further experiments were initiated using the A4 strain.
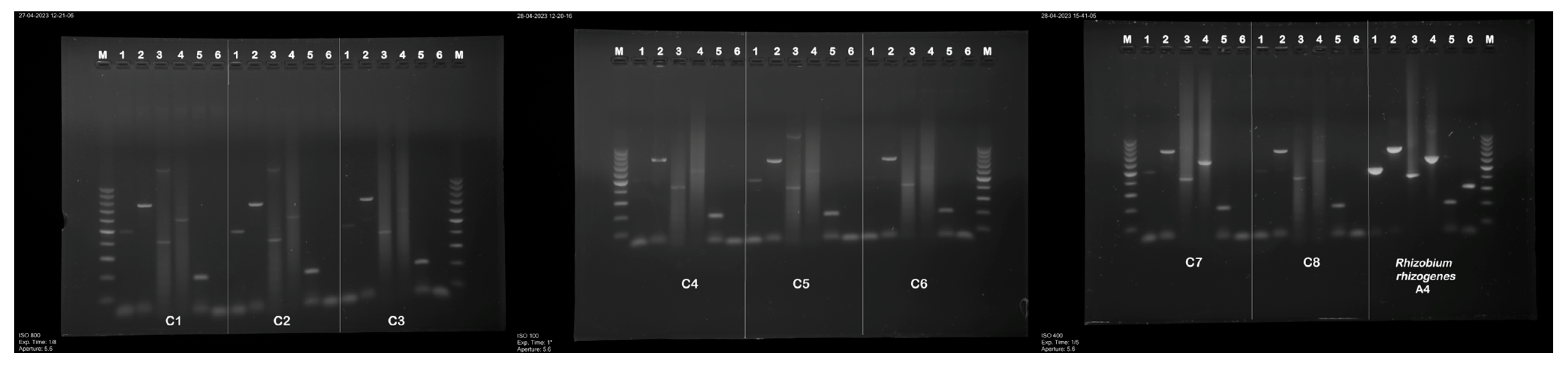
The transgenic status of hairy roots clones (C1–C8) was confirmed by PCR analysis (Figure 2). The PCR analysis also confirmed that hairy roots were not contaminated with R. rhizogenes, and the virG gene, which is involved in the transfer of bacterial T-DNA to the plant cell, was detected only in a positive control.
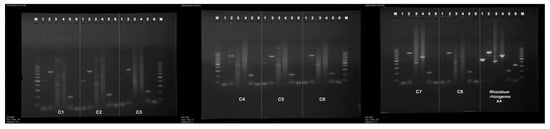
Figure 2.
PCR amplified products in genomic DNA from the hairy root clones of P. atriplicifolia. Lanes: M—molecular weight marker 100–1000 bp DNA ladder, 1—aux1 (500 bp), 2—aux2 (774 bp) 3—rolB gene (386 bp), 4—rolC (582 bp), 5—rolD (204 bp), 6—virG (319 bp).
The 386 bp fragment corresponding to rolB, the 204 bp fragment corresponding to rolD, and 774 bp corresponding to the aux2 gene were present in all hairy root clones C1–C8 (Table 2). The band of expected size 500 bp corresponding to aux1 appeared in lines C1–C3, C5, and C7–C8, while the 582 bp fragment corresponding to rolC was identified in the clones C1–C2 and C4–C8. No amplification was observed in the negative control (genomic DNA of untransformed roots of P. atriplicifolia), confirming the absence of bacterial contamination and primer dimer artifacts.

Table 2.
Confirmation of R. rhizogenes plasmid integration into genomic DNA derived from hairy root clones (C1–C8) of P. atriplicifolia. R. rhizogenes rolB, rolC, rolD, aux1, and aux2 genes were amplified by PCR using the specific primers. Integration status of R. rhizogenes T-DNA genes in hairy root clones (C1–C8): ‘+’ indicates successful amplification; ‘−’ indicates no amplification.
2.2. Growth Measurement of Hairy Root Clones
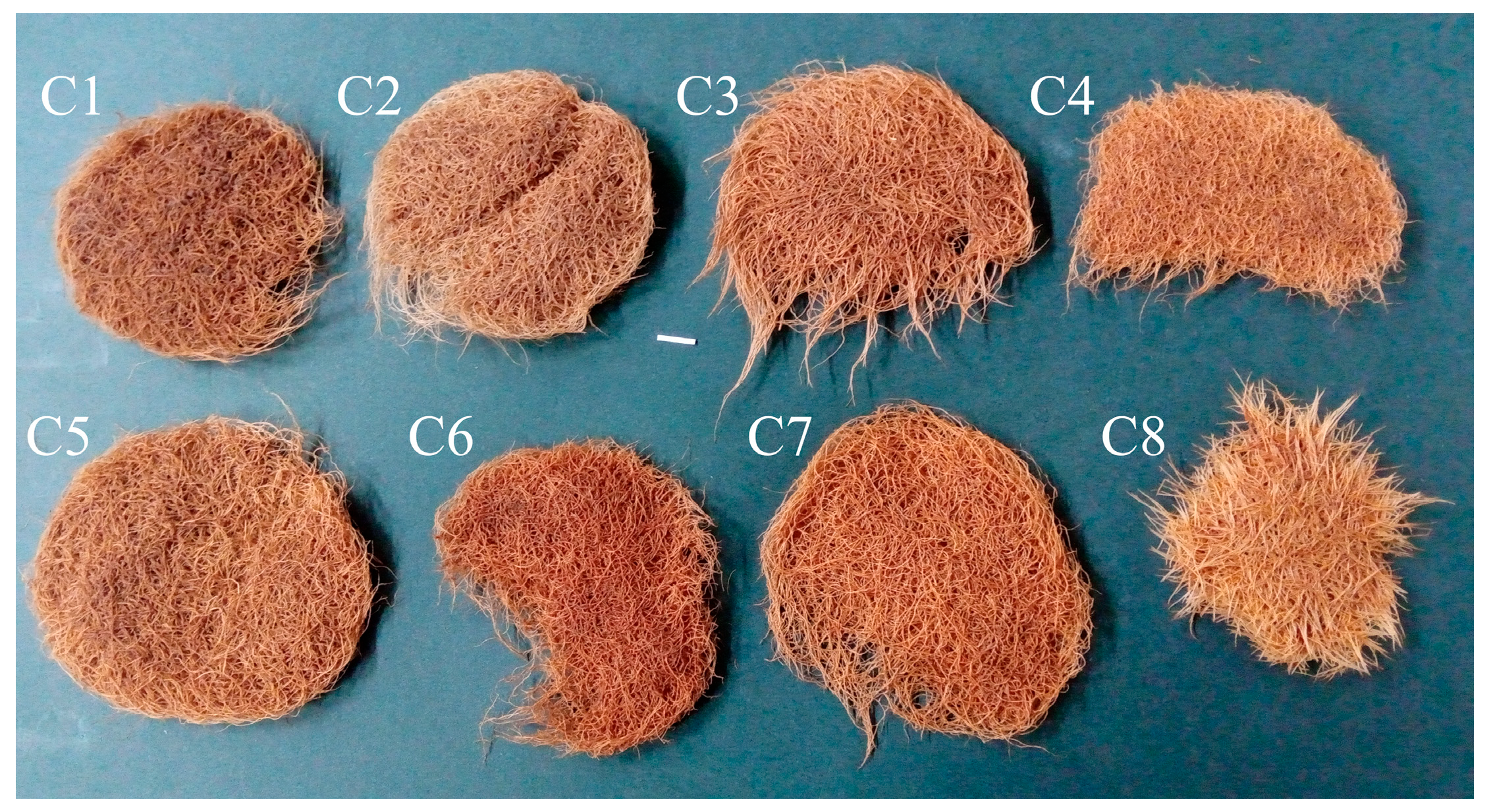
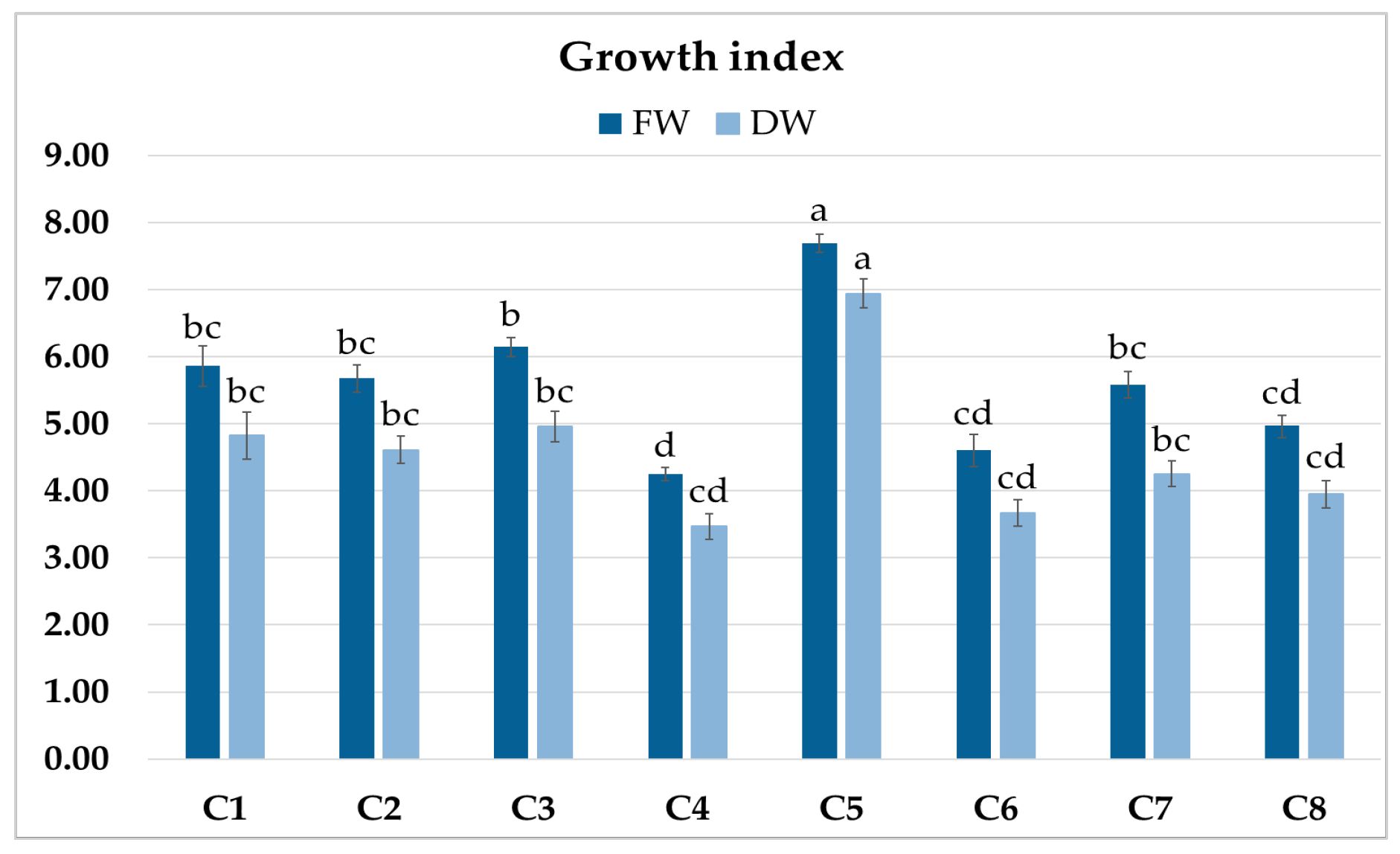
The roots of C1–C8 clones showed morphological features typical of hairy roots. The eight obtained root clones of P. atriplicifolia, confirmed as transformed, were cultured for four weeks in WP medium [18] to evaluate their growth (Figure 3). The growth index of fresh weight (FW) ranged from 4.24 to 7.68, and of dry weight (DW) from 3.42 to 6.88 (Figure 4). The highest values were achieved by clone C5, and the lowest by C4.

Figure 3.
The hairy roots of P. atriplicifolia clone (C1–C8) grown in WP medium after 4 weeks (Bar 1 cm).
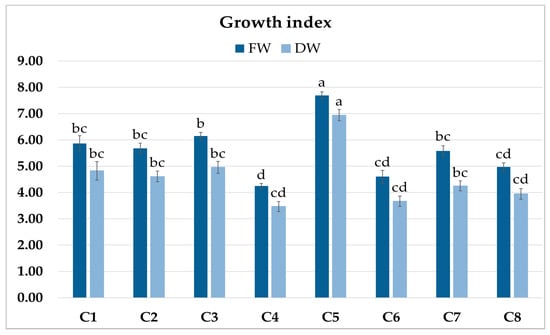
Figure 4.
Growth index (GI) of fresh (FW) and dry weight (DW) of P. atriplicifolia hairy root clones (C1–C8) grown for 4 weeks in WP medium. Data represent a mean ± SEM of three independent experiments (n = 3). The results indicated by the same letter within the same parameter are not significantly different (p ≤ 0.05).
2.3. Phytochemical Studies
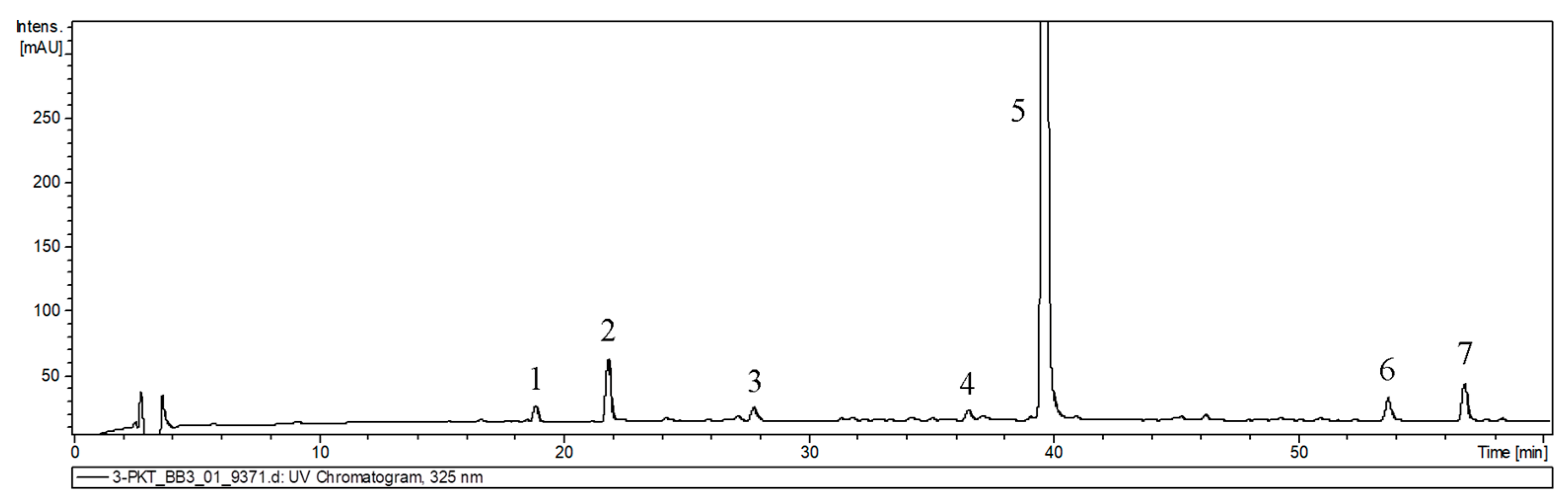
The qualitative analysis of methanol–water extract from hairy root cultures by UPLC-PDA-ESI-MS (ultrahigh performance liquid chromatography–electrospray ionization tandem mass spectrometry) showed that hairy roots could be suitable systems for the production of phenolic acids (Figure 5). Compounds in the extracts were identified based on retention time, as well as UV and mass spectra consistent with the literature [19,20] or by comparing their spectral properties with those observed for commercial standards. The peak characteristics and tentative identities are presented in Table 3.
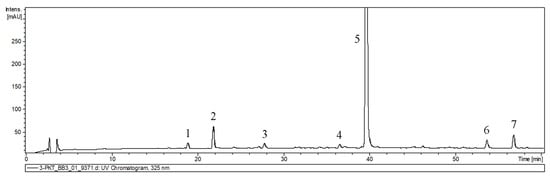
Figure 5.
UPLC-UV chromatograms of extracts derived from hairy roots of P. atriplicifolia used for qualitative analysis. 1—caffeic acid dihexoside; 2—caffeic acid, 3—prolithospermic acid, 4—salvianolic acid isomer, 5—rosmarinic acid, 6—salvianolic acid F isomer I, 7—salvianolic acid F isomer II.

Table 3.
UPLC-PDA-ESI-MS data of polyphenolic compounds detected in P. atriplicifolia hairy roots.
The analyzed extracts were found to contain various phenolic acids. The most prevalent, rosmarinic acid, has been previously identified in the roots of this species [17]. Other compounds, including caffeic acid (CA), caffeic acid dihexoside (CDH), prolithospermic acid (PLS), salvianolic acid isomer (SAI), and salvianolic acid F (SAF) isomers, have been previously identified in other species belonging to the Salvia genus including their hairy root cultures [21,22,23]. The latest phylogenetic studies have placed Perovskia, along with other genera such as Rosmarinus, into the expanded genus Salvia [17].
The most abundant compound in all the obtained clones was RA (Table 4). However, its production varied in individual lines. The highest RA content was found in clone C2 (15.9 mg/g DW), with fivefold lower levels observed in the least productive lines (C4 and C7). The remaining compounds identified in the extracts were biosynthesized in hairy roots at significantly lower levels (Table 4). Although clone C2 demonstrated relatively high biosynthesis of other polyphenols, the most efficient PLS, SAI, and SAF production was observed in C1. Among the identified compounds, caffeic acid dihexoside was present in all analyzed clones, but only in trace amounts.

Table 4.
Polyphenol content of different clones (C1–C8) of P. atriplicifolia hairy roots.
Finally, the highest total studied phenol (TP) content was observed in clone C2 (18.6 mg/g DW) followed by C1 (17.0 mg/g DW). The lowest level was reported for clone C4 (4.4 mg/g DW) (Table 4).
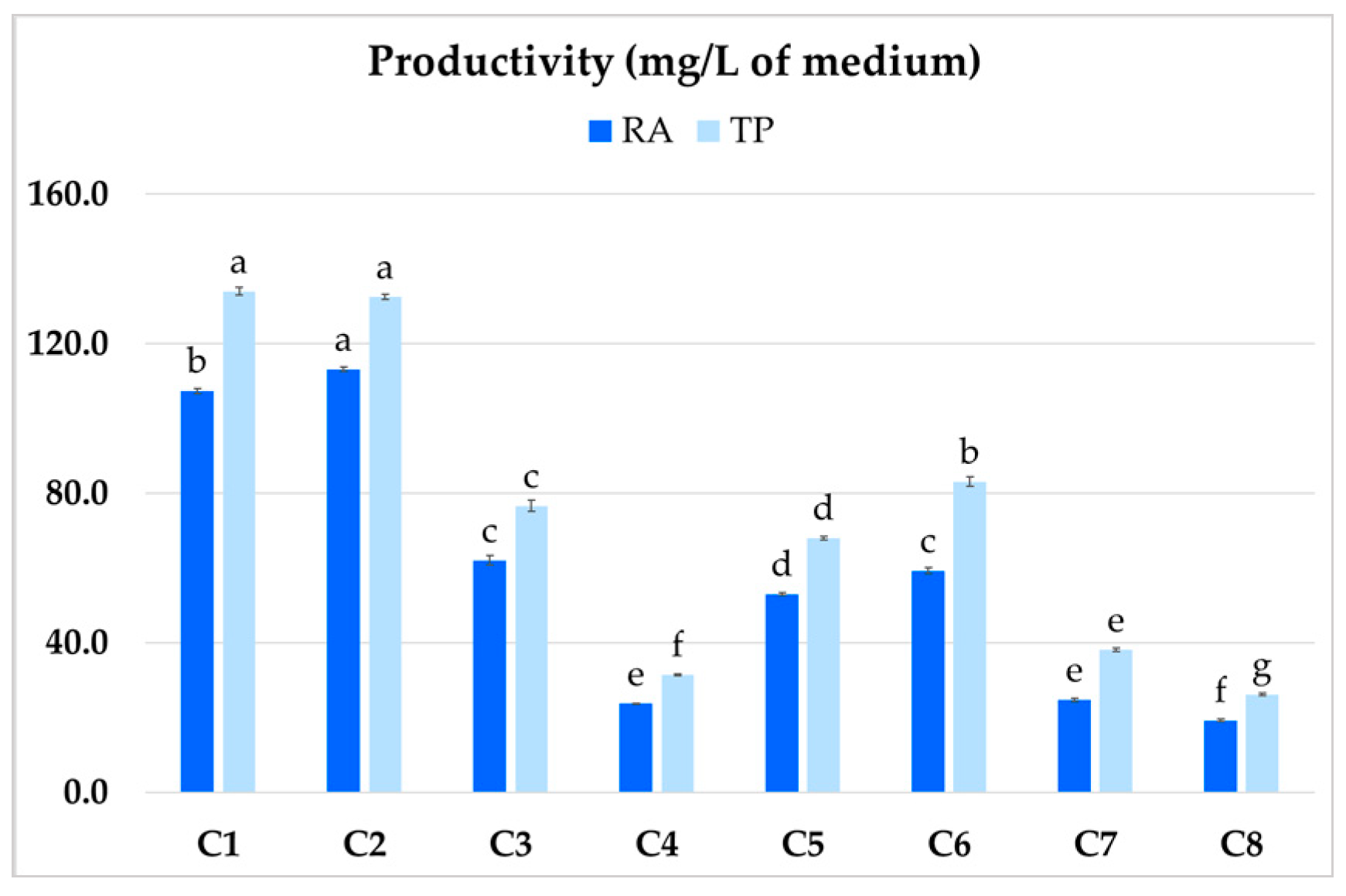
The highest productivity was demonstrated by clones C1 and C2 (Figure 6), which yielded 107.4 and 113.2 mg/L RA, respectively, and 134 and 132.5 mg/L TP content. The least productive clone (C4) achieved 23.7 mg/L for RA and 31.4 mg/L for TP.
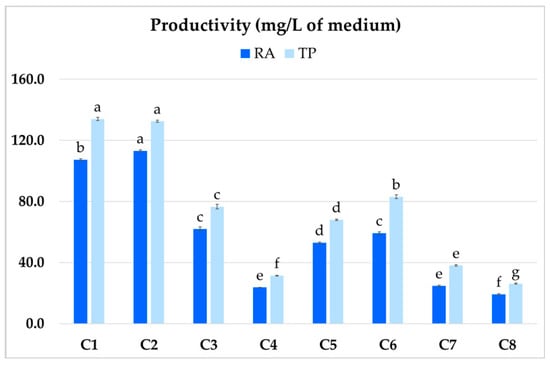
Figure 6.
Total polyphenol (TP) and rosmarinic acid (RA) productivity (mg/L) of different clones (C1–C8) of hairy root of P. atriplicifolia. Data represent a mean ± SEM of three independent experiments (n = 3). The results indicated by the same letter within the same parameter are not significantly different at the level of p ≤ 0.05.
2.4. Expression of Genes Encoding Enzymes of the RA Biosynthetic Pathway
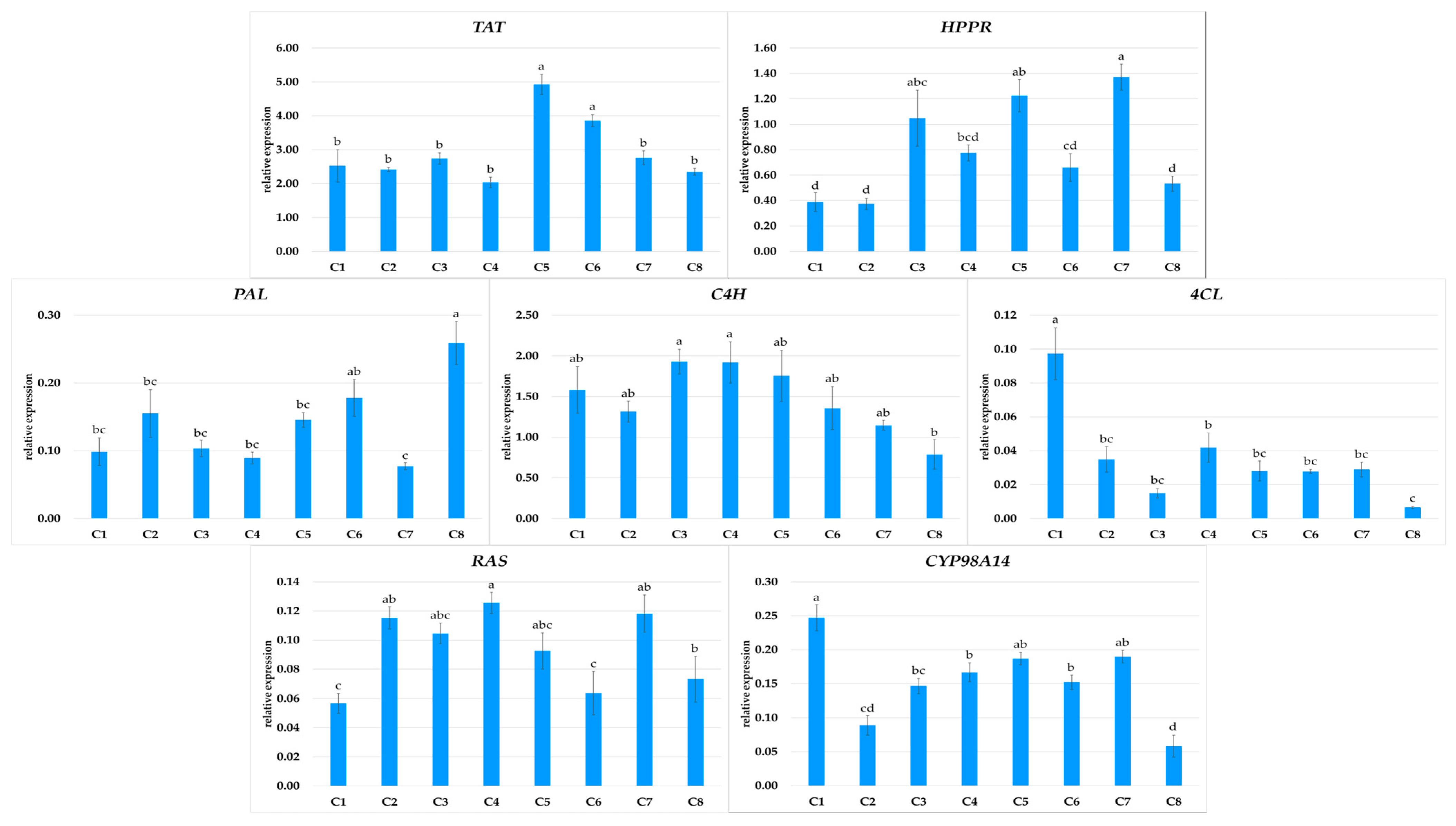
RA, the predominant metabolite in P. atriplicifolia hairy roots, is biosynthesized from L-tyrosine and L-phenylalanine via the phenylpropanoid pathway. In the first branch of the pathway, hydroxyphenyllactic acid is formed by TAT and HPPR; in the second branch, 4-coumaroyl-CoA is produced by reactions catalyzed by PAL, C4H, and then 4CL. These two intermediates condense in a reaction catalyzed by RAS to form 4-coumaroyl-4′-hydroxyphenyllactic acid; this intermediate is subsequently converted to RA by CYP98A14 [24]. The expression of the genes encoding all enzymes in this pathway were analyzed in the eight P. atriplicifolia hairy root clones, and the results were correlated with RA production (Figure 7 and Figure 8).
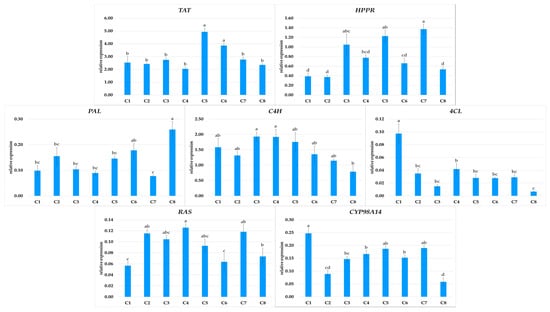
Figure 7.
Expression of TAT, HPPR, PAL, C4H, 4CL, RAS, and CYP98A14 genes in different hairy root clones (C1–C8) of P. atriplicifolia. Data represent a mean ± SEM of three independent experiments (n = 3). The results indicated by the same letter within the same parameter are not significantly different at the level of p ≤ 0.05.
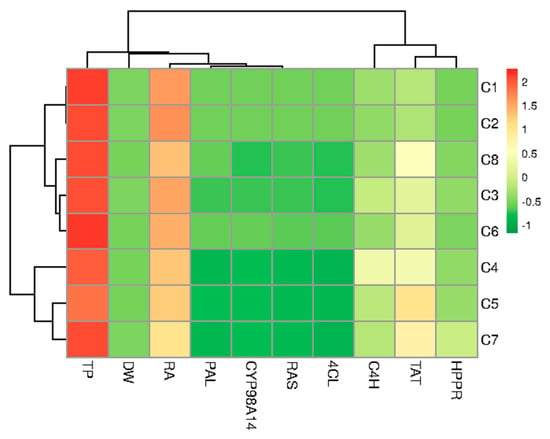
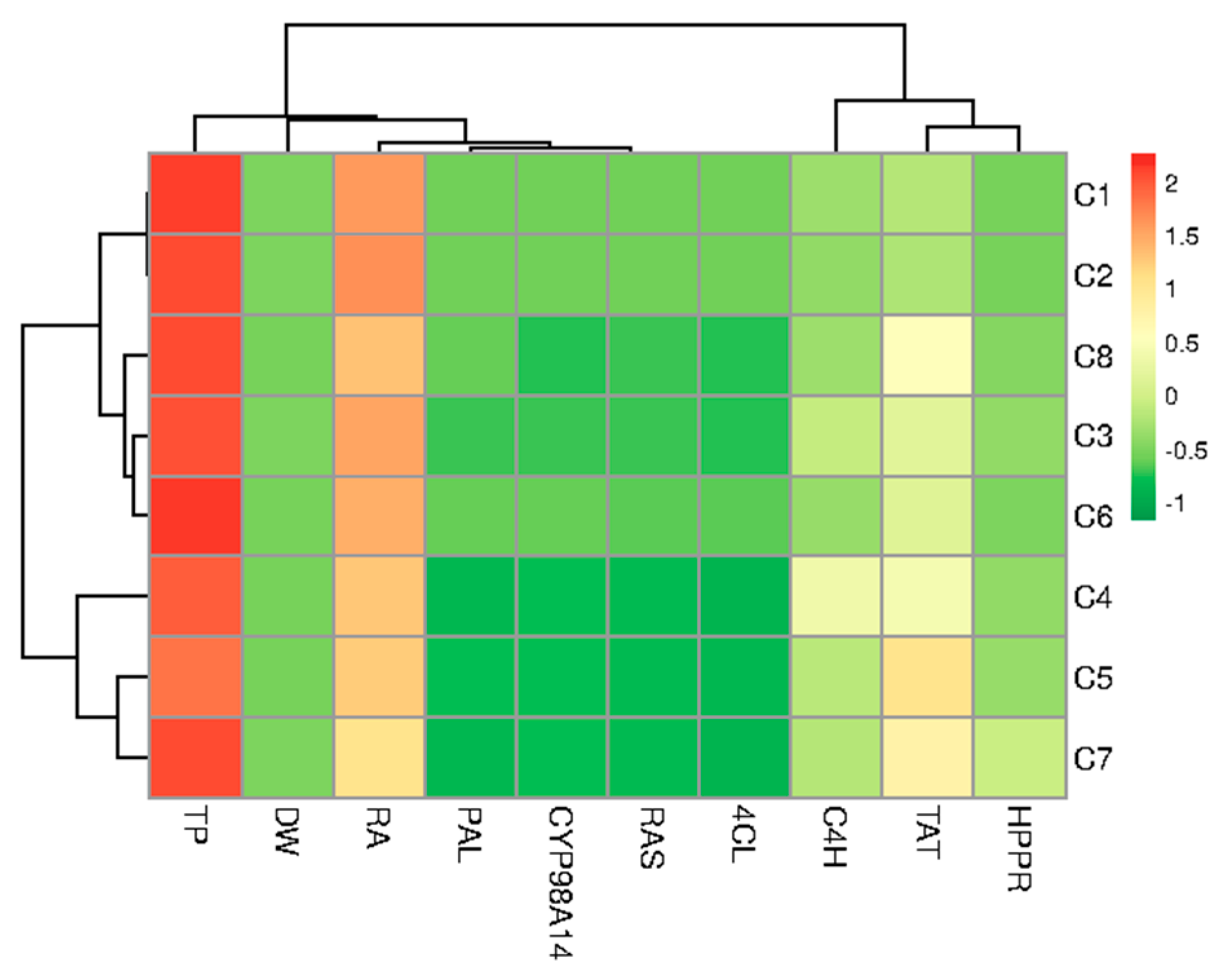
Figure 8.
Heatmap representing the differences between hairy root clones of P. atriplicifolia clustered according to the analyzed parameters (growth, content of RA and TP, gene expression).
The expression patterns of the analyzed genes in the transformed roots were clone-specific (Figure 7). Among the genes encoding the initial steps of the pathway, TAT showed twice the expression in C5 as for the other clones, except for C6. PAL expression was at least twice as high in C8 as in the other clones; however, this clone demonstrated the lowest C4H and CYP98A14 expression. The strongest expression of C4H and CYP98A14, along with 4CL, was noted in C1.
Hierarchical clustering was used to ordinate test samples based on the correlation model into two different stimulus coordinates. From the heat map, it can be seen that the obtained clones formed three different clusters based on growth, metabolite content, and gene expression. Cluster I (C1 and C2) was characterized by high metabolite production and average growth. Cluster II (C3, C6 and C8) had moderate production and growth, while Cluster III included C5 and C7 with C4 (Figure 8).
Moreover, it was found that the production of RA in root clones of P. atriplicifolia is associated with the expression of PAL, as well as with the genes responsible for encoding the final stages of the RA pathway (RAS and CYP98A14) and to a slightly lesser extent with TP content. In addition, a good correlation was observed between the tyrosine-derived genes, TAT and HPPR. The highest RA content observed in clones C1 and C2 correlated with higher PAL, CYP98A14, RAS, and 4CL expression levels but not TAT and HPPR (Figure 8).
2.5. Culture Condition Optimization
The two most productive hairy root clones of P. atriplicifolia (C1 and C2) were selected for production optimization. These were cultured in the following growth media: SH (Schenk and Hildebrandt) [25], MS (Murashige and Skoog) [26], WP (McCown Woody Plant) [18], and B5 (Gamborg) [27].
Morphological differences in the cultures were observed depending on the growth medium. In WP medium, the C1 and C2 roots were light brown, i.e., similar in color to those cultured in MS medium. However, in the MS medium, the roots were brittle and, particularly in the case of clone C2, covered with callus tissue. The presence of callus visibly restricted culture growth and complicated the transfer of roots into fresh medium.
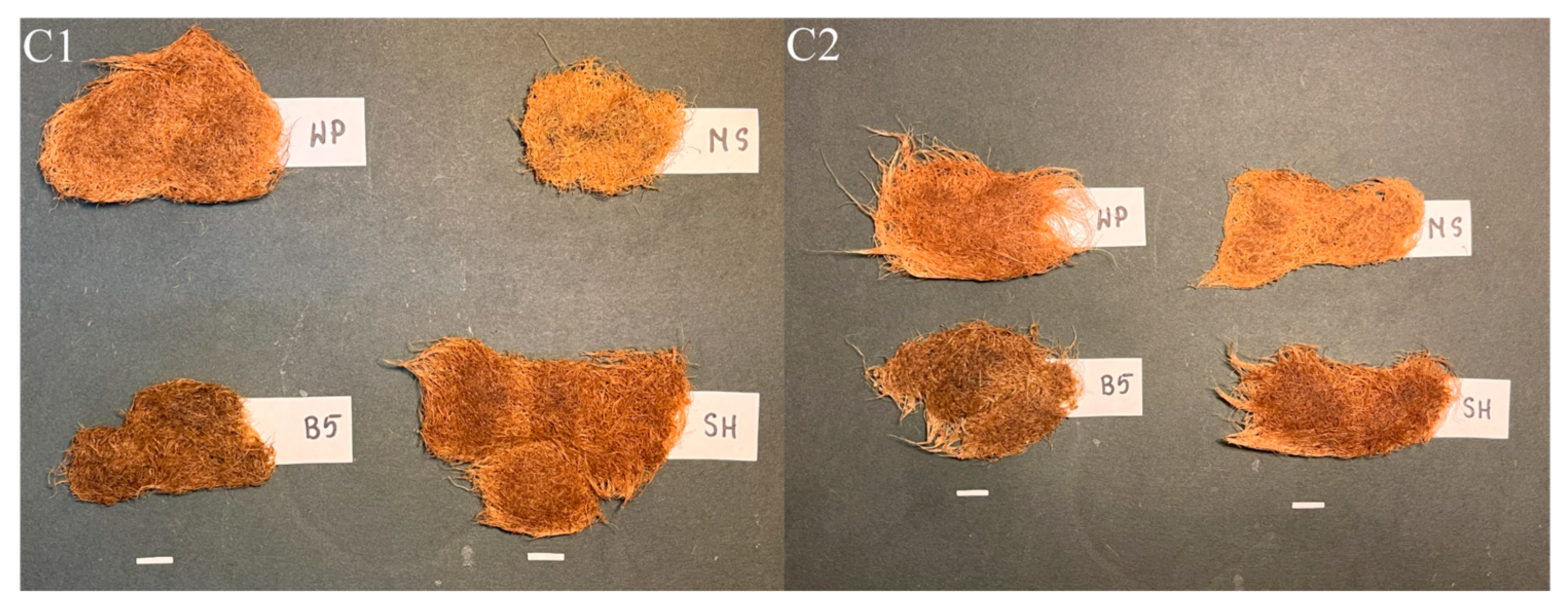
In SH medium, the C1 and C2 hairy roots exhibited a darker, orange-brown coloration (Figure 9). The cultures grown in B5 were the darkest brown in color and significantly shorter than those in WP and SH media, indicating markedly weaker growth, comparable to that achieved in MS medium.
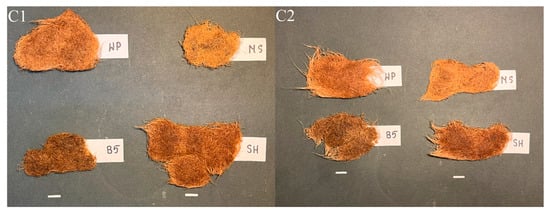
Figure 9.
Hairy root clones of P. atriplicifolia (C1, C2) grown in different basal media after four weeks (Bar 1 cm). WP—Woody Plant, SH—Schenk and Hildebrandt, B5—Gamborg, and MS—Murashige and Skoog medium.
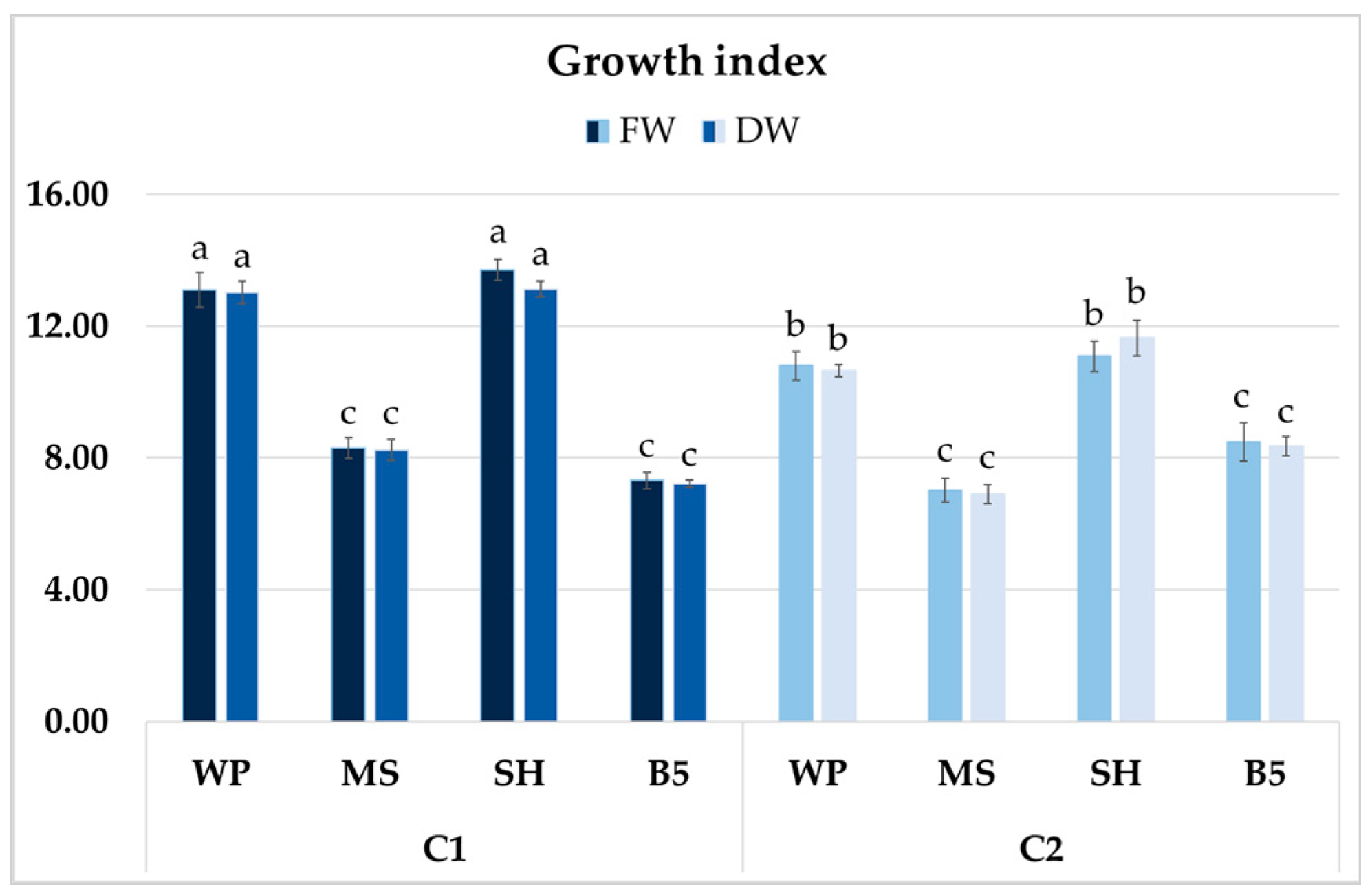
After four weeks of growth, it was determined that in some of the culture media, clone C1 exhibited higher biomass accumulation than clone C2 (Figure 10). However, no significant differences in GIs (FW or DW) were found for the clones cultured in SH and WP media; these values exceeded 13 for C1 and were approximately 11 for C2. The lowest GIs for clone C1 were recorded in B5 medium (approximately 7), a 45% reduction compared to SH medium, and for clone C2 in MS medium, with a 35–40% decrease compared to optimal conditions. The differences between clones cultivated in B5 and MS media were not significant (Figure 10).
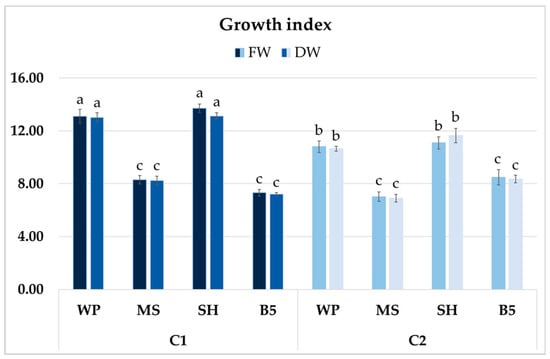
Figure 10.
Growth index (GI) of FW and DW of P. atriplicifolia hairy root clones (C1 and C2) grown for 4 weeks in different basal media: WP, MS, SH, and B5. Data represent a mean ± SEM of three independent experiments (n = 3). The results indicated by the same letter within the same parameter are not significantly different at the level of p ≤ 0.05.
The polyphenolic compound content was analyzed in two P. atriplicifolia hairy root clones collected from various basal media (Table 5).

Table 5.
Polyphenol content of P. atriplicifolia (C1 and C2) hairy roots cultivated in different basal media.
The highest RA content was observed in both root clones cultured in B5 medium (Table 5). In these conditions, the levels of RA were similar for both clones, with no statistically significant differences: 38.31 mg/g DW for clone C1 and 40.84 mg/g DW for clone C2. These values were 3.7- to 4-fold higher than those recorded in the least favorable medium, MS. While the optimal and least favorable media for RA production were consistent for both clones, the WP medium was more favorable for RA accumulation in C1, whereas comparable RA levels were achieved in C2 cultured in SH medium (Table 5). Switching the culture medium from WP to SH for clone C1 or from SH to WP for clone C2 significantly reduced RA accumulation in the roots.
Interestingly, the biosynthesis of other polyphenols often did not correlate with RA biosynthesis. B5 medium, which was particularly beneficial for RA biosynthesis, did not stimulate the production of other metabolites, and sometimes even inhibited it (Table 5), e.g., for both tested clones, SH medium was equally or more beneficial than B5 for the biosynthesis of CA, SAF I, SAF II, and SAI.
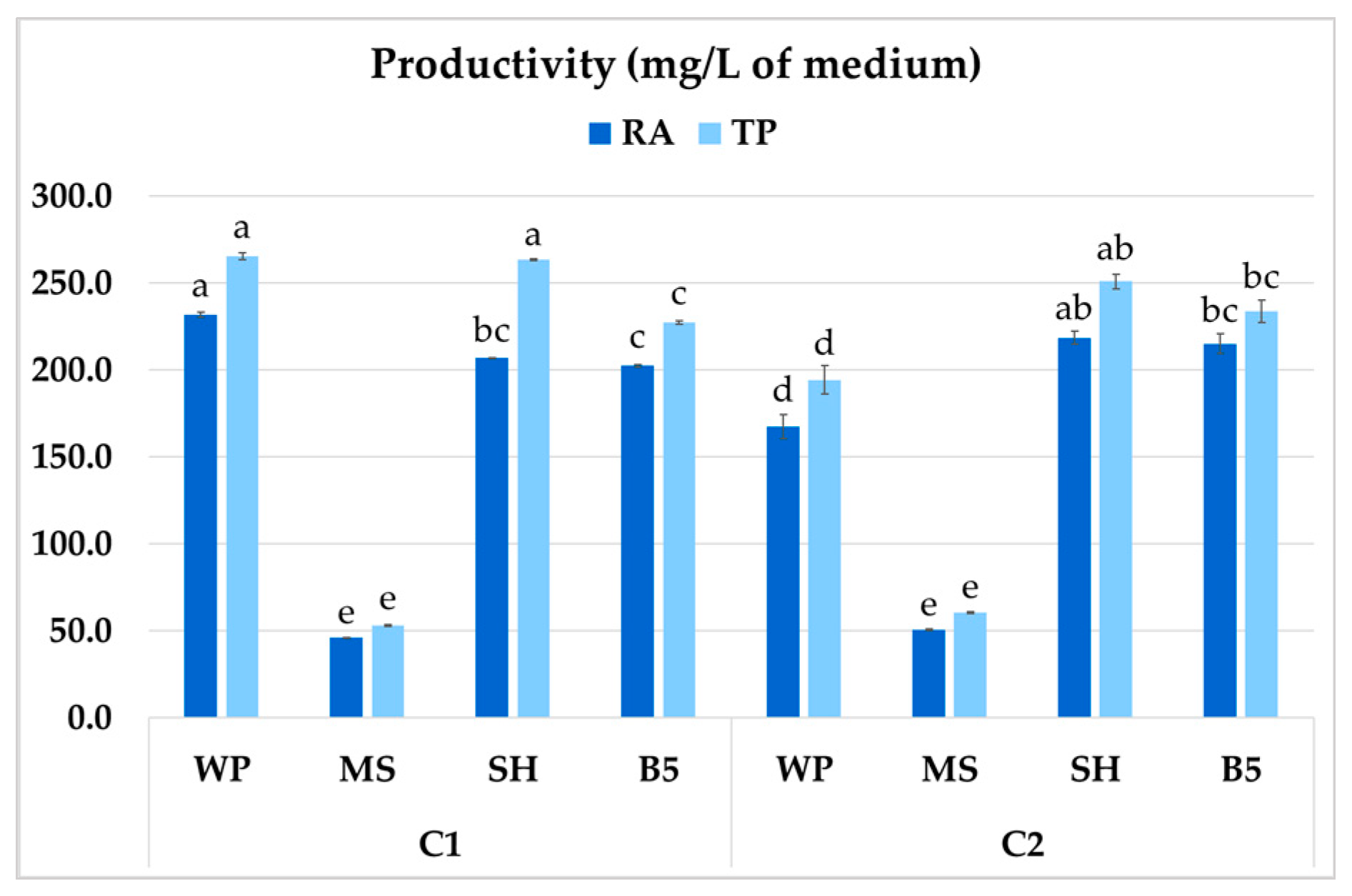
The different medium compositions were tested with regard to their effect on culture productivity, i.e., its growth and secondary compound production (mg per liter of culture). The highest productivity of RA in P. atriplicifolia roots was 231.8 mg/L of medium, observed for clone C1 grown in WP medium (Figure 11). Both WP and SH medium improved total polyphenol productivity in C1 (263.4–265.6 mg/L). In contrast, for C2, both the highest RA and TP productivity were recorded in SH medium (218.7 and 251.0 mg/L, respectively). For both clones, the optimal values were as much as fivefold higher than in MS medium.
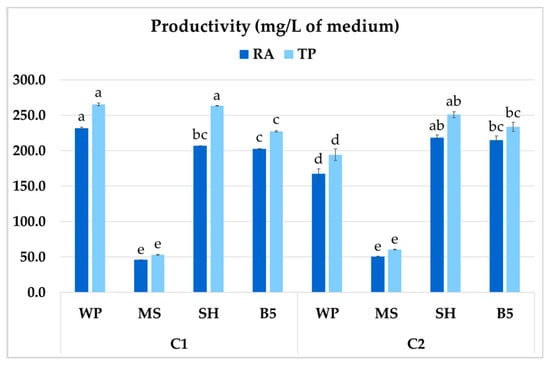
Figure 11.
Total polyphenol (TP) and rosmarinic acid (RA) productivity of P. atriplicifolia hairy roots (C1, C2) cultivated in different basal media. Data represent a mean ± SEM of three independent experiments (n = 3). The results indicated by the same letter within the same parameter are not significantly different at the level of p ≤ 0.05.
3. Discussion
The present study investigated various aspects of hairy root cultures in P. atriplicifolia, focusing on growth conditions optimization and the enhancement of RA production. Our data confirm that Rhizobium-mediated transformation can be performed successfully in Lamiaceae spp., as indicated by the successful establishment of hairy roots in P. atriplicifolia following infection with R. rhizogenes. Both bacterial strains used in the experiment effectively induced root formation, consistent with previous findings in other Lamiaceae spp. [19,28,29,30,31,32].
Strain A4 (41.3%) demonstrated higher transformation efficiency than ATCC 15,834 (30.2%), which is in line with prior reports indicating species-specific differences in transformation potential, as well as strain-specific variations in infection capability and T-DNA transfer efficiency. For instance, R. rhizogenes strains have exhibited varied root induction abilities in different mint species, with the highest transformation efficiency observed in Mentha aquatic L. infected with ATCC 15834, M. piperita L. and M. longifolia L. infected with A4, and M. spicata L. infected with A13 [33]. Similarly, in Ocimum basilicum L., strain A4 demonstrated higher infection efficiency (73%) than ATCC 15,834 (66%) [34], whereas ATCC 15,834 was more effective in Salvia nemorosa L. and Salvia officinalis L. [35,36].
To date, no reports exist on the establishment of hairy root cultures in P. atriplicifolia. However, successful transformation in Perovskia abrotanoides Karel. was noted by strain ATCC 15,834 (47.33%) [37]. These findings underscore the importance of strain selection in optimizing transformation efficiency in vitro culture systems.
R. rhizogenes-mediated transformation is a valuable method for enhancing bioactive compound production. The genetic background is a key determinant of phytochemical synthesis, with environmental factors playing only a secondary role [38]. To establish plants that are highly productive, it is important to screen the metabolite profiles of lines with high biosynthesis of bioactive compounds. In Salvia virgata Jacq. hairy root cultures, biomass accumulation ranged from 0.11 to 2.29 g, and RA content from 2.63 to 5.47 mg/g DW [39]; in contrast, in Salvia viridis L., hairy root clones exhibited biomass levels of 8.6–12.4 g and RA content of 10.9–35.6 mg/g DW [21]. Similar variations have been reported in other species, such as Tylophora indica (Burm. f.) Merrill [40] and Verbascum xanthophoeniceum Griseb. [41]. Line-specific differences are linked to variations in T-DNA genes, including rol and aux genes, which influence cell differentiation, auxin sensitivity, and secondary metabolism [42].
Our present findings indicate that the eight studied hairy root lines of P. atriplicifolia differed in regard to morphological, biomass, and metabolite content, suggesting genetic and epigenetic regulation of metabolic pathways. The obtained root lines clustered into three distinct groups, with C1 and C2 demonstrating the highest secondary metabolite content; these were selected for further research. PCR-based characterization showed the presence of aux1, aux2, rolB, rolC, and rolD in most lines, except C3, C4, and C6, where aux1 or rolC was absent. In the current study, no direct correlation between gene presence and growth or metabolite production was observed, because all analyzed bacterial plasmid genes were found in the genome of the two most productive clones (C1 and C2), as well as in the least productive ones (C7 and C8). On the other hand, differences among root lines with similar rol gene insertions could be attributed to variations in the gene copy number and integration site in the host genome [43]. Studies in Ocimum basilicum L. and Melissa officinalis L. indicate enhanced RA production in clones with multiple rolC copies [44]. Additionally, it is possible that the post-transformation losses of T-DNA fragments of Ri plasmids can also modulate biomass accumulation and metabolite production [45].
However, in our study, we did not analyze quantitatively the copy numbers of aux1, aux2, rolB, rolC, and rolD. The clones were subjected to gene expression analysis to clarify the mechanisms behind the RA biosynthetic pathway. Hierarchical clustering found RA biosynthesis correlated with PAL, RAS, and CYP98A14 transcript levels, but not with TAT level (Figure 8). Our results confirm the previously reported key role of the PAL gene in RA synthesis. For example, PAL RNAi-mediated suppression in S. miltiorrhiza decreased RA production [46]. Other studies found correlations between RA accumulation and the activities of PAL/RAS enzymes in Coleus blumei Benth. cultures [47] or PAL/HPPR enzymes in Lithospermum erythrorhizon [48].
The relationship between RA production and gene expression from the tyrosine-derived pathway (TAT and HPPR) appears even more complex. No prominent relationship between RA accumulation and TAT transcripts were noted in jasmonate-induced Prunella vulgaris L. hairy roots [49]. Also in the present study, the ClustVis algorithm was unable to detect a correlation between RA production and TAT/HPPR levels (Figure 8). However, the algorithm focuses on static data and does not inherently account for complex dynamic changes or regulatory mechanisms, such as negative feedback that might occur in metabolite biosynthesis pathways. Nonetheless, it is worth noting that both clones of P. artiplicifolia with the highest RA production (C1 and C2) demonstrated low expression of the genes encoding two first enzymes of the tyrosine-derived branch (TAT and HPPR) (Figure 7). Interestingly, previous results associated high accumulation of RA with suppressed TAT activity in cultures of Salvia miltiorrhiza Bunge and Lithospermum erythrorhizon Siebold & Zucc. [48,50]. This invites speculation that high RA production may directly or indirectly inhibit the expression of the two initial genes of this pathway: TAT and HPPR.
Another study reported that overexpression of TAT and HPPR in S. miltiorrhiza hairy root cultures significantly increased RA production, suggesting their important roles in promoting RA production [51]. This apparent contradiction may be due to complex regulatory mechanisms, including potential feedback inhibition by RA or intermediates within the pathway or the transcript crosstalk phenomenon [51,52]. Regulation of metabolite levels through negative feedback mechanisms is used by plants to maintain metabolic balance and avoid overproduction of certain compounds. One example of such a regulatory mechanism within the phenylpropanoid pathway involves trans-cinnamic acid, the product of the PAL-catalyzed reaction, known to act as a feedback inhibitor of both PAL enzymatic activity and PAL transcripts [52,53]. The involvement of complex regulatory mechanisms at the transcriptional and post-transcriptional levels remains to be determined.
It is also worth noting that the two clones of P. artiplicifolia with the highest RA production are characterized by high expression of the genes encoding the two last pathway enzymes: CYP98A14 for C1 and RAS for C2; this indicates molecular differences between the lines and that they can promote RA production on different stages. It could also be the reason for the slightly different results regarding the optimization of the culture medium.
Medium components, such as macro- and microelements and vitamins, play an important role in enhancing biomass and the accumulation of target compounds in culture. Therefore, four different media (MS, B5, SH, and WP) were tested to identify the optimal formulations for enhancing the productivity of the two most productive P. atriplicifolia root lines. In both lines, the most effective growth was observed for the WP and SH media. These findings suggest that copper may play an important role in the growth of this culture, as its concentration in WP and SH media is 8–10 times higher than in MS and B5 media. WP and SH media also contain lower amounts of nitrogen compounds, particularly ammonium ions, compared to MS medium, although the same is true for B5 medium. Moreover, WP and SH media have higher levels of pyridoxine and thiamine and lower levels of phosphate ions compared to B5 medium, which may indicate that these factors are important for the growth of P. atriplicifolia roots. Previous research has found that there is a reduction in phosphate levels in the culture-medium-enhanced root growth and daidzein and genistein production in cultures of Psoralea corylifolia L. [54].
In contrast, B5 medium was the most favorable for RA accumulation in the tested P. atriplicifolia hairy roots. Similarly, for S. viridis hairy roots, WP medium was superior for biomass accumulation, while B5 medium was comparable or superior for secondary metabolite production [21]. Also, RA level in Salvia plebeia R. Br. culture was greatest in B5 medium, while the highest biomass accumulation was observed in SH medium [55]. However, the RA content in S. plebeia roots was only 8 mg/g DW, which was approximately 4.5 times lower than that found in our P. atriplicifolia cultures.
On the other hand, WP and SH media provided better conditions for the production of RA and other metabolites than MS medium, which was the least suitable for both growth and metabolite accumulation. Some researchers attribute the poor growth response in MS medium to its high total nitrogen content. Nitrogen is a key factor influencing secondary metabolite production, and reduced nitrate levels have been shown to enhance biosynthesis of polyphenol in Vitis L. species, flavonoid in P. corylifolia, and RA in Halodule pinifolia (Miki) Hartog and S. viridis [54,56,57,58]. However, it has been also demonstrated that hairy roots are often less sensitive to high total nitrogen levels than to elevated ammonium concentration, which can impair cell growth by repressing nitrate assimilation. Both of these effects appear to be important for RA production in P. atriplicifolia cultures, where the highest RA levels were observed in B5 medium, which has the lowest ammonium-to-nitrate ion ratio; in contrast, the lowest RA accumulation occurred in MS medium, which contains the highest total nitrogen level of the tested media and the highest ammonium-to-nitrate ion ratio.
RA, which is the predominant metabolite of hairy root P. atriplicifolia, is also the abundant compound in such cultures of other Lamiaceae species. Highly productive hairy root cultures of Agastache rugosa (Fisch. & C. A. Mey.) Kuntze yielded up to 116 mg RA/g DW [59], while RA levels of 13.6 mg/g DW were reported for Nepeta cataria L. [60], to 35 mg/g DW for S. viridis [21], to 30 mg/g DW for S. miltiorrhiza [61], to 5 mg/g DW for O. basilicum [62], to 20 mg/g DW for Dracocephalum forrestii W. W. Smith [63], and to 16 mg/g DW for S. nemorosa [35].
Hairy roots could demonstrate significantly greater synthesis of secondary metabolites than wild-type roots, and metabolite production in hairy root cultures is generally more stable than in other types of cultures or in conventionally cultivated plants [21,42]. This also appears to be the case for P. atriplicifolia. Optimization of hairy root culture conditions increased RA levels to 40 mg/g DW, which was 20 times higher than in greenhouse-grown P. atriplicifolia roots (up to 2 mg/g DW) [64] and 1.5- to 2-fold higher than in field-grown plants (20–27 mg/g DW) [65]. Such large differences in the RA content in the roots of parent plants reported by two research teams may be attributed to variations in plant variety, growth conditions, or the timing of sample collection. However, they also highlight the strong sensitivity of this species to genetic and environmental factors.
Our study obtained an optimized, stable P. atriplicifolia transformed hairy root culture providing over 230 mg RA per liter of culture within four weeks that may be an effective alternative to field cultivation of this species. As mentioned in the introduction section, thanks to its antioxidant, anti-inflammatory, and antimicrobial properties, RA has numerous industrial applications, e.g., in pharmaceutical formulations (drugs, supplements), cosmetic skincare products, and as a natural preservative in food products [4]. Our results indicate routes of further RA biosynthesis optimization strategies for these applications, such as fine-tuning of the culture medium composition or overexpression of genes known to enhance RA biosynthesis. Additionally, optimal conditions for the metabolite production in hairy roots for industrial needs can be achieved by designing specialized automated systems for monitoring and controlling culture conditions to increase its scale, ensure consistency, and reduce labor costs.
4. Materials and Methods
4.1. Plant Material
The explants were obtained as in vitro shoot cultures of P. atriplicifolia. They were initiated from seeds obtained from the Direction de l’Environnement et du Cadre de Vie, Service Espaces Verts et Nature, Ville de Caen (France). The seeds were sterilized with 15% sodium hypochlorite for 20 min and rinsed three times with sterile distilled water.
For germination, the seeds were placed on MS [26] agar (0.7%) medium supplemented with kinetin (0.02 mg/L) and gibberellic acid (0.1 mg/L) and incubated in a growth chamber at 26 ± 2 °C in darkness. Following germination, the seedlings were maintained under a 16/8 h (light/dark) photoperiod, illuminated by cool-white fluorescent lamps (40 µmol/m2·s).
Shoot tips (0.5 cm in length) were excised from four-week-old seedlings and transferred to agar (0.7%)-solidified SH [25] medium supplemented with sucrose (3%), indole-3-acetic acid (IAA, 0.1 mg/L), and 6-benzylaminopurine (BAP, 0.5 mg/L) to obtain aseptically grown shoot culture. All growth media and growth regulators were purchased from Duchefa Biochemie B.V. (Haarlem, The Netherlands).
The plants were botanically identified by Grąbkowska based on the Flora of Pakistan and Flora of China [66]. A voucher specimen was deposited in the Department of Biology and Pharmaceutical Botany, Medical University of Lodz (Poland).
4.2. Establishment of Hairy Root Cultures
Two agropine strains of R. rhizigenes: ATCC (pRi15834) and A4 (pRiA4) (from LGC Standards Sp. z o.o., Kielpin, Poland), were used for the transformation. The bacteria were grown on YMB [67] solid medium at 26 °C for 48 h.
Aseptic P. atriplicifolia shoots, approximately 1–1.5 cm in length with 2–3 pairs of leaves, were pricked at the nodal regions with a needle immersed in a suspension of the bacterial culture. Control shoots were wounded similarly with a sterile needle without bacteria. The culture was conducted on hormone-free SH agar medium in the dark for three weeks. After this period, the frequency of transformation was evaluated as the percentage of inoculated shoots forming roots relative to the total number of shoots used for transformation. Additionally, the number and length of roots emerging from the responding explants were recorded. No roots developed in the control group.
Roots formed on infected shoots were transferred into 100 mL Erlenmeyer flasks containing 25 mL liquid WP medium [18] supplemented with cefotaxime (500 mg/L) and subcultured every 7 days for eight cycles to eliminate bacteria. The roots were incubated at 26 ± 2 °C in the dark on a rotary shaker at 70 rpm.
Eight root clones (C1–C8) exhibiting vigorous growth were selected for further cultivation.
4.3. Confirmation of the Transformation
The transformation process was confirmed by polymerase chain reaction (PCR). The genomic DNA from eight transformed root clones (C1–C8) and untransformed roots from 12-month-old P. atriplicifolia plants grown in a greenhouse (negative control group) were extracted using a Genomic Mini AX Plant Kit (A&A Biotechnology, Gdynia, Poland). During isolation, 150 mg of fresh plant materials was powdered in liquid nitrogen. The DNA sample was used as a template in PCR analysis to determine the presence of the aux1, aux2, rolB, rolC, and rolD genes. Additionally, PCR analysis for the virG gene was performed to confirm the absence of bacteria in the hairy roots. An Ri plasmid isolated from 24 -hour cultures of R. rhizogenes strain A4 (OD600 = 0.4) using Plasmid Mini AX Kit (A&A Biotechnology, Gdynia, Poland) was used as a positive control. The sequences of primers and PCR conditions have been previously published by Skała et al. [68]. The amplification products were examined by electrophoresis, as described by Wojciechowska et al. [22]. The gels were documented using a FastGene®FAS-Digi PRO imaging system (Nippon Genetics Europe GmbH, Düren, Germany).
4.4. Hairy Root Cultivation
Eight clone hairy roots (C1–C8) were maintained in 300 mL Erlenmeyer flasks with 80 mL of WP medium and subcultured every four weeks by transferring about 0.63–0.84 g fresh weight (FW) of roots (0.08–0.13 g dry weight (DW)) into fresh medium. After 28 days, the growth of hairy roots was measured in terms of FW and DW, and the growth indexes (GIs) were calculated as described by Grzegorczyk-Karolak et al. [69]. The experiment was repeated three times (subcultures 24–26).
4.5. The Phytochemical Analysis
After harvesting, the plant material was lyophilized (Freeze-dryer Alpha 1–2 LD, Martin Christ, Osterode am Harz, Germany) and micronized. The plant material for each treatment (0.1 g) was extracted with 30 mL of 80% methanol in a sonication bath (Techpan, Warsaw, Poland) at 40 °C for 15 min. The extractions were repeated two more times with less solvent (10 mL). The filtered extracts were combined, evaporated to dryness, and stored in a refrigerator (4 °C) before analysis.
The qualitative analysis compounds in the extract was performed with UPLC-PDA-ESI-MS using a UPLC-3000 RS apparatus (Dionex, Germering, Germany) with DAD detection and an AmaZon SL ion trap mass spectrometer with an ESI interface (Bruker Daltonik GmbH, Bremen, Germany) and a Zorbax SB-C18 column (150 × 2.1 mm, 1.9 μm) (Agilent, Santa Clara, CA, USA). The process and details of the analysis have been described earlier [69].
All extracts were quantitatively analyzed using an Agilent Technology 1200 chromatograph with a photodiode array detector (PDA), thermostat, and autosampler (Agilent Technologies, Santa Clara, CA, USA). The analysis was performed using an Eclipse C18 XDB (4.6 × 150 mm) with a particle size of 5 μm or the equivalent column. The mobile phase (A) was 0.1% formic acid in water, and the mobile phase B was acetonitrile. The following gradient system was used for the analysis: 0 min 10% solvent B, 0–5 min 10–18% solvent B, 5–20 min 18–38% solvent B, 20–25 min 38–100% solvent B, 25–30 min 100% solvent B, 30–37 min 100–5% B (equilibration). The flow rate was 1.6 mL/min. The injection volume was arranged as 10 μL. Compounds were detected at λ = 325 nm, and their content was expressed in mg/g DW. The calibration curves for quantitative analysis were constructed based on the peak area. Authentic standards of available compounds (Chem Faces Biochemical Co., Ltd., Wuhan, China) were used for calibration. For the bioactive compounds lacking a commercial standard, quantification was based on the calibration curve of a similar compound from the same group. Total polyphenolic content was obtained as the sum of the contents of all polyphenols quantified in the extracts.
4.6. Gene Expression Analysis (RNA Extraction, Reverse Transcription, and Real-Time PCR (RT-PCR)
RNA was extracted with a Total RNA Midi Kit (A&A BIOTECHNOLOGY, Gdańsk, Poland) according to standard protocol. The concentration and purity of RNA were established photometrically (spectrophotometer NanoReady Touch, Life Real, Hangzhou, China), and cDNA was synthesized following the producer’s instruction using a NG dART RT cDNA synthesis kit (EURx Molecular Biology Products, Gdańsk, Poland) using random hexamer primers (PCR Thermal Cycler T 100, Bio-Rad Laboratories Inc., Hercules, CA, USA). Quantitative real-time PCR analysis was performed with qPCR-HS Mix SYBR® (A&A BIOTECHNOLOGY, Gdańsk, Poland) following the producer’s instructions using a StepOne Plus system (Applied Biosystems, Foster City, CA, USA). Changes in gene expression were determined with the ∆∆Ct method using actin levels for normalization. The primer sequences for P. atriplicifolia have been published previously [24]. The study used primers for the key enzyme-encoding genes involved in phenolic acid biosynthesis, such as phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), hydroxycinnamate coenzyme A ligase (4CL), tyrosine aminotransferase (TAT), 4-hydroxyphenylpyruvate reductase (HPPR), rosmarinic acid synthase (RAS), and a cytochrome P450-dependent monooxygenase (CYP98A14).
4.7. Optimization of Hairy Root Growth Condition
The selected hairy root lines (C1 and C2) were cultivated in different growth media—WP [18], SH [25], MS [26], and B5 [27]—to optimize growth and bioactive compound production (subculture 42–44). After four weeks, culture growth and polyphenol content were analyzed according to the methods described above.
4.8. Data Analysis
The experiments were conducted at least in triplicate for each treatment. The results were calculated as mean ± standard error of the mean (SEM). The outcomes of the different treatments were verified using one-way analysis of variance, followed by post hoc Tukey’s test, assuming min. α = 0.05 (Statistica13.1Pl, StatSoft, Krakow, Poland). Hierarchical clustering was performed using the ClustVis web tool after row centering, with the following settings for both row and column clustering: correlation as the distance metric and average as the clustering method [70]. A heatmap was constructed, where positive values indicate that the corresponding data points are above the mean after row centering for each parameter, while negative values indicate that they are below the mean.
5. Conclusions
This study provides insights into the significance genetic and environmental factors in shaping the growth and metabolic output of hairy root cultures. The superior performance of strain A4 and the differential productivity among clones highlight the potential for transformation optimization, clone selection, and the importance of tailored approaches of genetic engineering in optimizing hairy root systems to maximize metabolite biosynthesis.
Our findings confirm the feasibility of using optimized hairy root cultures of P. atriplicifolia for producing bioactive compounds, particularly RA. Comparative analyses with other Lamiaceae species suggest that P. atriplicifolia hairy roots could serve as a robust platform for the large-scale production of phenolic acids. Further studies integrating transcriptomic and proteomic analyses could elucidate the molecular mechanisms driving these variations and support the development of efficient biotechnological platforms for the production of high-value phytochemicals. Future experiments should focus on further optimization of the Perovskia hairy root culture system and increase its scale to allow higher productivity of RA, given its importance in the field of food, cosmetic, pharmaceutical, and nutraceutical industries.
Author Contributions
Conceptualization, R.G. and I.G.-K.; formal analysis, M.K., K.G.-W. and I.G.-K.; investigation, R.G., M.K., K.G.-W., A.K.K., K.P. and I.G.-K.; methodology, M.K., K.G.-W. and I.G.-K.; project administration, I.G.-K.; resources, I.G.-K.; supervision, I.G.-K.; visualization, R.G., M.K. and I.G.-K.; writing—original draft, I.G.-K.; writing—review and editing, K.G.-W. and I.G.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Medical University of Lodz, grant no. 503/3–012–01/503–31–001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rejkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Oriente, G. Isolamento e costituzione dell’acido rosmarinico (dal rosmarinus off.). Ric. Sci. 1958, 28, 2329–2333. [Google Scholar]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid-from bench to valuable applications in food industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M.P. In vitro plant propagation: A review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar] [CrossRef]
- Song, Z.; Li, X. Expression profiles of rosmarinic acid biosynthesis genes in two Salvia miltiorrhiza lines with differing water-soluble phenolic contents. Ind. Crops Prod. 2015, 71, 24–30. [Google Scholar] [CrossRef]
- Malarz, J.; Yudina, Y.V.; Stojakowska, A. Hairy root cultures as a source of phenolic antioxidants: Simple phenolics, phenolic acids, phenylethanoids, and hydroxycinnamates. Int. J. Mol. Sci. 2023, 24, 6920. [Google Scholar] [CrossRef]
- Krzemińska, M.; Owczarek, A.; Olszewska, M.A.; Grzegorczyk-Karolak, I. In vitro strategy for the enhancement of the production of bioactive polyphenols in transformed roots of Salvia bulleyana. Int. J. Mol. Sci. 2022, 23, 7771. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Zhou, M.L.; Zhu, X.M.; Shao, J.R.; Tang, Y.X.; Wu, Y.M. Production and metabolic engineering of bioactive substances in plant hairy root culture. Appl. Microbiol. Biotechnol. 2011, 90, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.; Haas, C.; Schulz, S.; Pavlov, A. Sage in vitro cultures: A promising tool for the production of bioactive terpenes and phenolic substances. Biotechnol. Lett. 2014, 36, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Mohammadhosseini, M.; Venditti, A.; Akbarzadeh, A. The genus Perovskia Kar.: Ethnobotany, chemotaxonomy and phytochemistry: A review. Toxin Rev. 2019, 40, 484–505. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, J.; Zhu, L.Y.; Zhang, J.R.; Jing, Y.X.; Zhao, J.W.; Huang, X.Z.; Li, G.P.; Jiang, Z.Y.; Xue, D.Y. Four new diterpene glucosides from Perovskia atriplicifolia. Chem. Biodivers. 2017, 14, e1700071. [Google Scholar] [CrossRef]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Ślusarczyk, S. Metabolomics and DNA-based authentication of two traditional Asian medicinal and aromatic species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. International Plant Propagators’ Society 1980, 30, 421–427. [Google Scholar]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical profile and antioxidant activity of aerial and underground parts of Salvia bulleyana Diels. plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef]
- Stanković, J.S.K.; Srećković, N.; Mišić, D.; Gašić, U.; Imbimbo, P.; Monti, D.M.; Mihailović, V. Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae)—A plant rich in rosmarinic acid. Ind. Crops Prod. 2020, 143, 111932. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Skała, E.; Kiss, A.K. Hairy root cultures of Salvia viridis L. for production of polyphenolic compounds. Ind. Crops Prod. 2018, 117, 235–244. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015, 170, 378–385. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Grąbkowska, R.; Gomulski, J.; Żekanowski, C.; Gawęda-Walerych, K. Accumulation of polyphenols and associated gene expression in hairy roots of Salvia viridis exposed to methyl jasmonate. Int. J. Mol. Sci. 2024, 25, 764. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojina, K. Nutrient requirement of suspension cultures of soybean root cell. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Tiwari, R.; Trivedi, M.; Guang, Z.C.; Guo, G.Q.; Zheng, G.C. Agrobacterium rhizogenes mediated transformation of Scutellaria baicalensis and production of flavonoids in hairy roots. Biol. Plant. 2008, 52, 26–35. [Google Scholar] [CrossRef]
- Abadi, M.; Ganjeali, A.; Lahouti, M.; Moshtaghi, N. Influence of different Agrobacterium rhizogenes strains on hairy roots induction and secondary metabolites production in Ocimum basilicum L. J. Hortic. Sci. 2020, 34, 273–284. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag+, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef]
- Fraga, B.M.; Díaz, C.E.; Guadaño, A.; González-Coloma, A. Diterpenes from Salvia broussonetii transformed roots and their insecticidal activity. J. Agric. Food Chem. 2005, 53, 5200–5206. [Google Scholar] [CrossRef]
- Bauer, N.; Kiseljak, D.; Jelaska, S. The effect of yeast extract and methyl jasmonate on rosmarinic acid accumulation in Coleus blumei hairy roots. Biol. Plant. 2009, 53, 650–656. [Google Scholar] [CrossRef]
- Esmaeili, F.; Rahimi, Z.; Yousefian, S.; Farhadpour, M.; Lohrasebi, T. Comparative phenolic profile and antioxidant potential of mentha hairy roots and aerial parts. Biocatal. Agric. Biotechnol. 2025, 63, 103469. [Google Scholar] [CrossRef]
- Sathasivam, R.; Choi, M.; Radhakrishnan, R.; Kwon, H.; Yoon, J.; Yang, S.H.; Kim, J.K.; Chung, Y.S.; Park, S.U. Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front. Plant Sci. 2022, 13, 983776. [Google Scholar] [CrossRef]
- Khoshsokhan, F.; Babalar, M.; Salami, S.A.; Sheikhakbari-Mehr, R.; Mirjalili, M.H. Rosmarinic acid production in hairy root cultures of Salvia nemorosa L. (Lamiaceae). Biocatal. Agric. Biotechnol. 2022, 45, 102494. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia officinalis L. hairy root cultures for the production of rosmarinic acid. Z. Naturforsch. C 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Zaker, A.; Abrishamchi, P.; Bahrami, A.R.; Ganjeali, A.; Sodagar, N. Hairy root induction and secondary metabolite production in Perovskia abrotanoides Karel. J. Plant Process Funct. 2017, 6, 17–26. [Google Scholar]
- Smetanska, I. Sustainable production of polyphenols and antioxidants by plant in vitro cultures. In Bioprocessing of Plant In Vitro Systems; Reference Series in Phytochemistry; Pavlov, A., Bley, T., Eds.; Springer: Cham, Switzerland, 2018; pp. 225–269. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Elicitor-induced phenolic acids accumulation in Salvia virgata Jacq. hairy root cultures. Plant Cell Tissue Organ Cult. 2022, 148, 107–117. [Google Scholar] [CrossRef]
- Chaudhuri, K.N.; Ghosh, B.; Tepfer, D.; Jha, S. Genetic transformation of Tylophora indica with Agrobacterium rhizogenes A4: Growth and tylophorine productivity in different transformed root clones. Plant Cell Rep. 2005, 24, 25–35. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Ludwig-Müller, J.; Alipieva, K.; Lippert, A. Sonication-assisted Agrobacterium rhizogenes-mediated transformation of Verbascum xanthophoeniceum Griseb. for bioactive metabolite accumulation. Plant Cell Rep. 2011, 30, 859–866. [Google Scholar] [CrossRef]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Hairy root research: Recent scenario and exciting prospects. Curr. Opin. Plant Biol. 2006, 9, 341–346. [Google Scholar] [CrossRef]
- Pietrosiuk, A.; Furmanova, M.; Lata, B. Catharanthus roseus: Micropropagation and in vitro techniques. Phytochem. Rev. 2007, 6, 459–473. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Taneja, J.; Jaggi, M.; Wankhede, D.P.; Sinha, A.K. Effect of loss of T-DNA genes on MIA biosynthetic pathway gene regulation and alkaloid accumulation in Catharanthus roseus hairy roots. Plant Cell Rep. 2010, 29, 1119–1129. [Google Scholar] [CrossRef]
- Song, J.; Wang, Z. RNAi-mediated suppression of the phenylalanine ammonia-lyase gene in Salvia miltiorrhiza causes abnormal phenotypes and a reduction in rosmarinic acid biosynthesis. J. Plant Res. 2011, 124, 183–192. [Google Scholar] [CrossRef]
- Szabo, E.; Thelen, A.; Petersen, M. Fungal elicitor preparations and methyl jasmonate enhance rosmarinic acid accumulation in suspension cultures of Coleus blumei. Plant Cell Rep. 1999, 18, 485–489. [Google Scholar] [CrossRef]
- Mizukami, H.; Ogawa, T.; Ellis, B.E. Induction of rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures by yeast extract. Plant Cell Rep. 1992, 11, 480–483. [Google Scholar] [CrossRef]
- Ru, M.; Li, Y.; Guo, M.; Chen, L.; Tan, Y.; Peng, L.; Liang, Z. Increase in rosmarinic acid accumulation and transcriptional responses of synthetic genes in hairy root cultures of Prunella vulgaris induced by methyl jasmonate. Plant Cell Tissue Organ Cult. 2022, 149, 371–379. [Google Scholar] [CrossRef]
- Yan, Q.; Shi, M.; Ng, J.; Wu, J.Y. Elicitor-induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots. Plant Sci. 2006, 170, 853–858. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Gao, S.; Saechao, S.; Di, P.; Chen, J.; Chen, W. The c4h, tat, hppr and hppd genes prompted engineering of rosmarinic acid biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. PLoS ONE 2011, 6, e29713. [Google Scholar] [CrossRef]
- Blount, J.W.; Korth, K.L.; Masoud, S.A.; Rasmussen, S.; Lamb, C.; Dixon, R.A. Altering expression of cinnamic acid 4-hydroxylase in transgenic plants provides evidence for a feedback loop at the entry point into the phenylpropanoid pathway. Plant Physiol. 2000, 122, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Mavandad, M.; Edwards, R.; Liang, X.; Lamb, C.J.; Dixon, R.A. Effects of trans-cinnamic acid on expression of the bean phenylalanine ammonia-lyase gene family. Plant Physiol. 1990, 94, 671–680. [Google Scholar] [CrossRef]
- Shinde, A.N.; Malpathak, N.; Fulzele, D.P. Impact of nutrient components on production of the phytoestrogens daidzein and genistein by hairy roots of Psoralea corylifolia. J. Nat. Med. 2010, 64, 346–353. [Google Scholar] [CrossRef]
- Choi, M.; Yoon, J.; Yang, S.H.; Kim, J.K.; Park, S.U. Production of phenolic compounds and antioxidant activity in hairy root cultures of Salvia plebeia. Plants 2023, 12, 3840. [Google Scholar] [CrossRef]
- Yamakawa, T.; Kato, S.; Ishida, K.; Kodama, T.; Minoda, Y. Production of anthocyanins by Vitis cells in suspension culture. Agric. Biol. Chem. 1983, 47, 2185–2191. [Google Scholar] [CrossRef]
- Danaraj, J.; Mariasingarayan, Y.; Periakaruppan, R.; Krishna, J.; Raja, M.; Ayyappan, S.; Sivaramakrishnan, R.; Incharoensakdi, A. Phenylpropanoid biosynthetic gene expression and nutrient uptake kinetics for enhanced rosmarinic acid production in suspension cultured cells of Halodule pinifolia. Algal Res. 2022, 64, 102675. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I. Optimization of culture conditions and cultivation phase for the growth of Salvia viridis transformed roots and polyphenolic compound production. Plant Cell Tissue Organ Cult. 2020, 142, 571–581. [Google Scholar] [CrossRef]
- Lee, S.Y.; Xu, H.; Kim, Y.K.; Park, S.U. Rosmarinic acid production in hairy root cultures of Agastache rugosa Kuntze. World J. Microbial. Biotechnol. 2008, 24, 969–972. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, C.Y.; Eom, S.H.; Kim, Y.K.; Park, N.; Park, S.U. Rosmarinic acid production from transformed root cultures of Nepeta cataria L. Sci. Res. Essays 2010, 5, 1122–1126. [Google Scholar]
- Xiao, Y.; Gao, S.; Di, P.; Chen, J.; Chen, W.; Zhang, L. Lithospermic acid B is more responsive to silver ions (Ag+) than rosmarinic acid in Salvia miltiorrhiza hairy root cultures. Biosci. Rep. 2010, 30, 33–40. [Google Scholar] [CrossRef]
- Bais, H.P.; Walker, T.S.; Schweizer, H.P.; Vivanco, J.M. Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol. Biochem. 2002, 40, 983–995. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Skała, E.; Olszewska, M.A.; Kiss, A.K.; Balcerczak, E.; Wysokińska, H.; Kicel, A. The identification and quantitative determination of rosmarinic acid and salvianolic acid B in hairy root cultures of Dracocephalum forrestii W.W. Smith. Ind. Crops Prod. 2016, 91, 125–131. [Google Scholar] [CrossRef]
- Khodadadi, F.; Ahmadi, F.S.; Talebi, M.; Matkowski, A.; Szumny, A.; Afshari, M.; Rahimmalek, M. Metabolic and transcriptomic approaches of chitosan and water stress on polyphenolic and terpenoid components and gene expression in Salvia abrotanoides (Karl.) and S. yangii. Int. J. Mol. Sci. 2023, 24, 15426. [Google Scholar] [CrossRef] [PubMed]
- Stafiniak, M.; Ślusarczyk, S.; Pencakowski, B.; Matkowski, A.; Rahimmalek, M.; Bielecka, M. Seasonal variations of rosmarinic acid and its glucoside and expression of genes related to their biosynthesis in two medicinal and aromatic species of Salvia subg. Perovskia. Biology 2021, 10, 458. [Google Scholar] [CrossRef]
- Flora of China/Flora of Pakistan. Available online: http://www.efloras.org/florataxon.aspx?flora_id (accessed on 12 February 2025).
- Hooykaas, P.J.J.; Klapwijk, P.M.; Nuti, M.P.; Schilperoot, R.A.; Rörsch, A. Transfer of the Agrobacterium tumefaciens Ti plasmid to avirulent Agrobacteria and to Rhizobium ex planta. J. Gen. Microbiol. 1977, 98, 477–484. [Google Scholar] [CrossRef]
- Skała, E.; Kicel, A.; Olszewska, M.; Kiss, A.; Wysokińska, H. Establishment of hairy root cultures of Rhaponticum carthamoides (Willd.) Iljin for the production of biomass and caffeic acid derivatives. BioMed Res. Int. 2015, 2015, e181098. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Ejsmont, W.; Kiss, A.K.; Tabaka, P.; Starbała, W.; Krzemińska, M. Improvement of bioactive polyphenol accumulation in callus of Salvia atropatana Bunge. Molecules 2024, 29, 2626. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. Clustvis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).