Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands
Abstract
:1. Introduction
2. Results
2.1. Syntheses and Characterization of Complexes 2a–2e
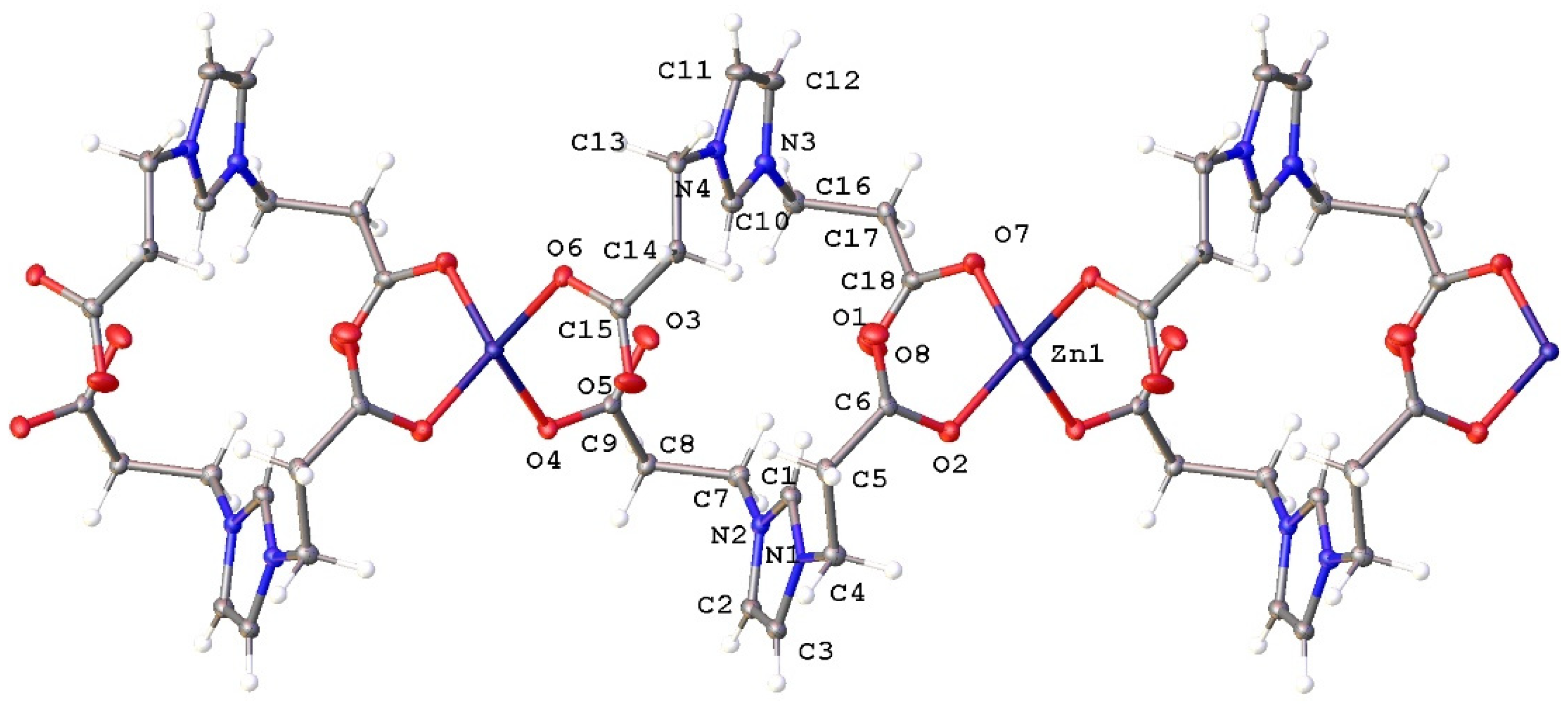
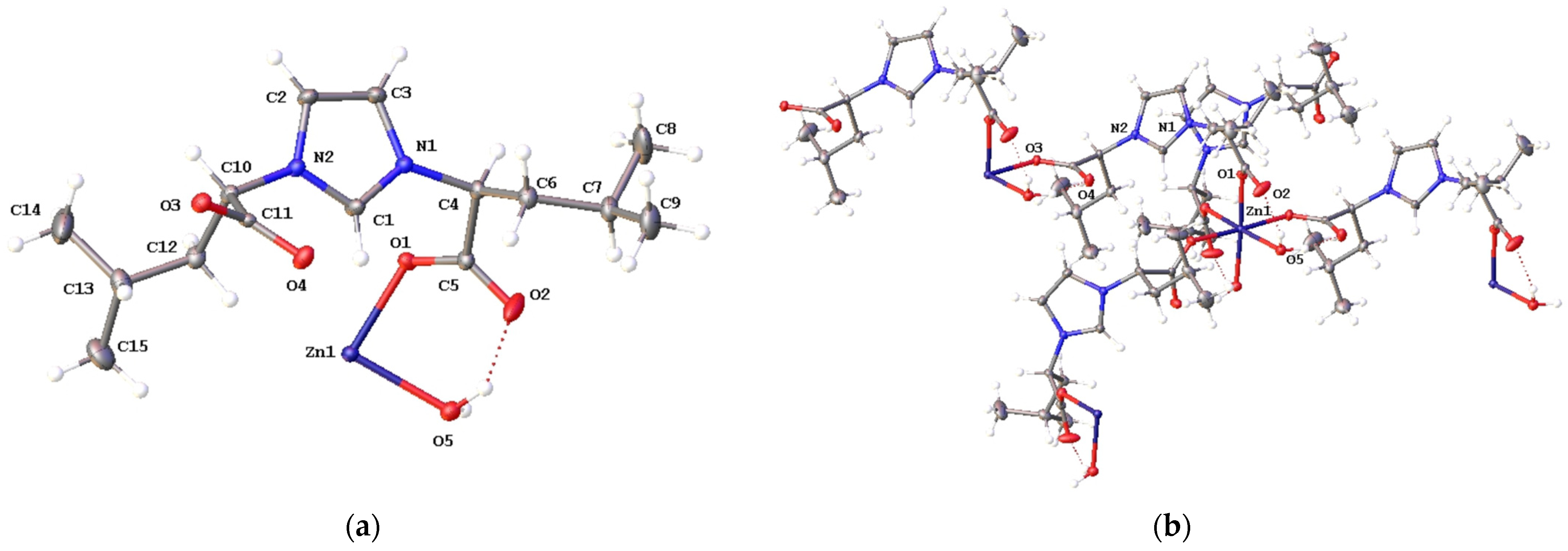
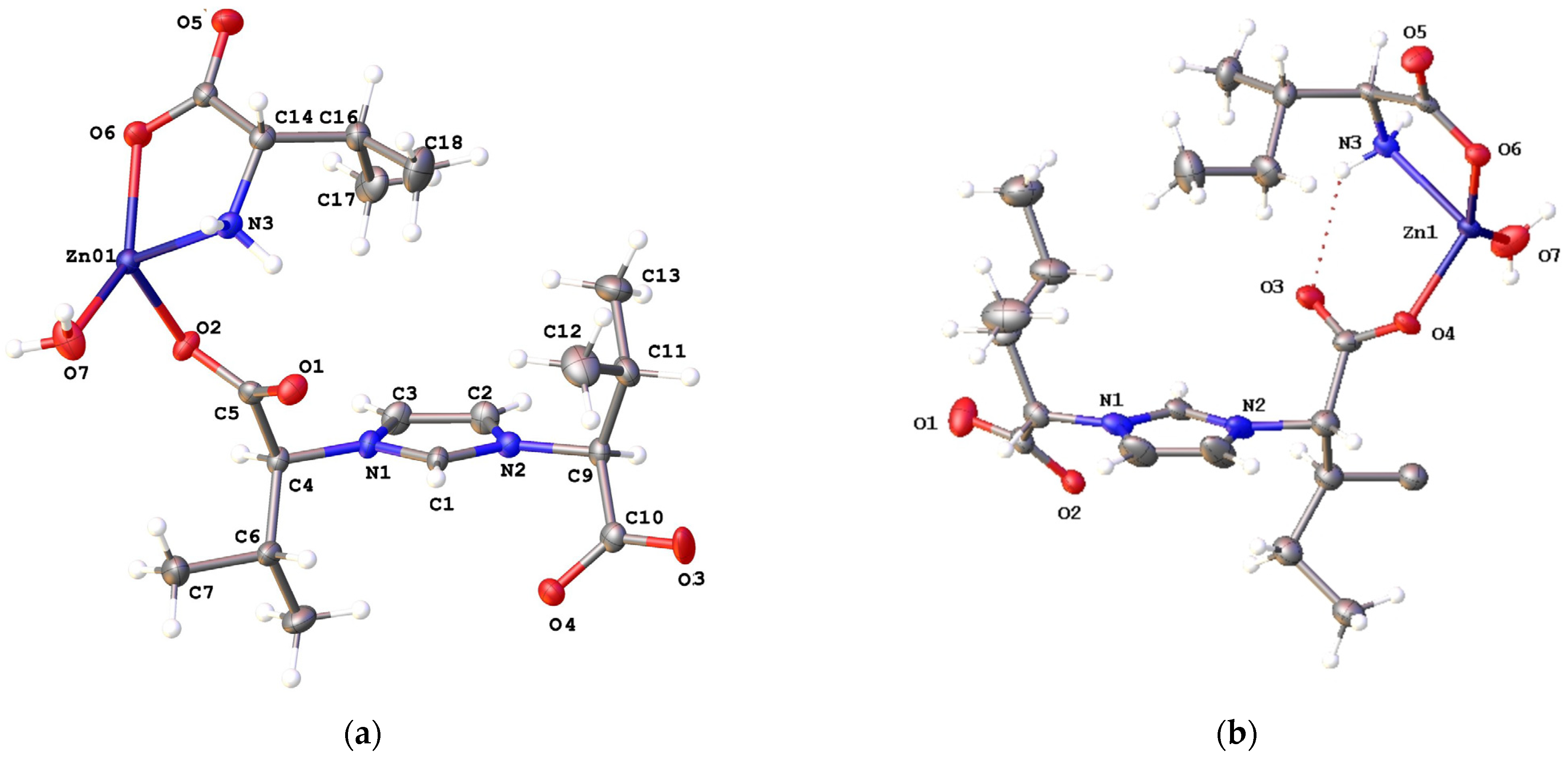
2.2. Structural Characterization of Complexes 2a–2e
2.3. Evaluation of Anticancer Activity
3. Materials and Methods
3.1. General
3.2. Synthesis
3.2.1. Complexes [Zn(LR)2]n, 2a and 2b
3.2.2. Complex [Zn(LLeu)2(H2O)2]n, 2c
3.2.3. Complexes [Zn(LR)(aa)(H2O)]n, 2d and 2e
- [Zn(LVal)(Val)(H2O)]n, 2d.
- [Zn(LIle)(Ile)(H2O)]n, 2e.
3.3. Cell Lines and Cell Viability Assays
3.4. Single-Crystal X-Ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruijnincx, P.C.; Sadler, P.J. New Trends for Metal Complexes with Anticancer Activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. On the Medicinal Chemistry of Gold Complexes as Anticancer Drugs. Coord. Chem. Rev. 2009, 253, 1670–1681. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene-Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Del Bello, F.; Santini, C. Zinc Complexes with Nitrogen Donor Ligands as Anticancer Agents. Molecules 2020, 25, 5814. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc Coordination Complexes as Anticancer Agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I. A Bioinformatics View of Zinc Enzymes. J. Inorg. Biochem. 2012, 111, 150–156. [Google Scholar] [CrossRef]
- Lipscomb, W.N.; Sträter, N. Recent Advances in Zinc Enzymology. Chem. Rev. 1996, 96, 2375–2433. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The Biochemical Basis of Zinc Physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Prince, R.H. Zinc: Inorganic & coordination chemistry. In Encyclopedia of Inorganic Chemistry; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Sadow, A.D. 3.06-Zinc. In Comprehensive Coordination Chemistry III, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 139–185. [Google Scholar] [CrossRef]
- Abendrot, M.; Chȩcińska, L.; Kusz, J.; Lisowska, K.; Zawadzka, K.; Felczak, A.; Kalinowska-Lis, U. Zinc(II) Complexes with Amino Acids for Potential Use in Dermatology: Synthesis, Crystal Structures, and Antibacterial Activity. Molecules 2020, 25, 951. [Google Scholar] [CrossRef] [PubMed]
- Caballero, P.; Colodrero, R.M.P.; Conejo, M.d.M.; Pastor, A.; Álvarez, E.; Montilla, F.; Carrasco, C.J.; Nicasio, A.I.; Galindo, A. Homochiral Imidazolium-Based Dicarboxylate Compounds: Structure and Solution Behaviour. Inorganica Chim. Acta 2020, 513, 119923. [Google Scholar] [CrossRef]
- Nicasio, A.I.; Montilla, F.; Álvarez, E.; Colodrero, R.P.; Galindo, A. Synthesis and Structural Characterization of Homochiral 2D Coordination Polymers of Zinc and Copper with Conformationally Flexible Ditopic Imidazolium-Based Dicarboxylate Ligands. Dalton Trans. 2017, 46, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Borrego, E.; Nicasio, A.I.; Álvarez, E.; Montilla, F.; Córdoba, J.M.; Galindo, A. Synthesis and Structural Characterization of Homochiral Coordination Polymers with Imidazole-Based Monocarboxylate Ligands. Dalton Trans. 2019, 48, 8731–8739. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Conejo, M.d.M.; Pastor, A.; Galindo, A. Recent Developments in Amino Acid-Derived Imidazole-, Imidazolium- and N-Heterocyclic Carbene-Carboxylate Complexes. Inorganica Chim. Acta 2023, 557, 121717. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Galindo, A.; Pérez-Aranda, M.; Pajuelo, E.; Alcudia, A. Homochiral Imidazolium-Based Dicarboxylate Silver(I) Compounds: Synthesis, Characterisation and Antimicrobial Properties. Dalton Trans. 2022, 51, 5061–5071. [Google Scholar] [CrossRef]
- Sánchez, A.; Carrasco, C.J.; Montilla, F.; Álvarez, E.; Galindo, A.; Pérez-Aranda, M.; Pajuelo, E.; Alcudia, A. Antimicrobial Properties of Amino-Acid-Derived N-Heterocyclic Carbene Silver Complexes. Pharmaceutics 2022, 14, 748. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Álvarez, E.; Calderón-Montaño, J.M.; López-Lázaro, M.; Galindo, A. Chirality Influence on the Cytotoxic Properties of Anionic Chiral Bis(N-Heterocyclic Carbene)Silver Complexes. J. Inorg. Biochem. 2022, 235, 111924. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer Statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Sutton, C.C.R.; Da Silva, G.; Franks, G.V. Modeling the IR Spectra of Aqueous Metal Carboxylate Complexes: Correlation between Bonding Geometry and Stretching Mode Wavenumber Shifts. Chem.—Eur. J. 2015, 21, 6801–6805. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Ang, W.H.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J. Ionic Solid-State Dimers and Polymers Derived from Imidazolium Dicarboxylic Acids. Chem.—Eur. J. 2006, 12, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- Babu, C.N.; Sathyanarayana, A.; Mobin, S.M.; Prabusankar, G. Structurally Characterized Zwitterionic Chiral Zinc Imidazolium [4,4] Grid. Inorg. Chem. Commun. 2013, 37, 222–224. [Google Scholar] [CrossRef]
- Kühl, O.; Palm, G. Imidazolium Salts from Amino Acids-a New Route to Chiral Zwitterionic Carbene Precursors? Tetrahedron Asymmetry 2010, 21, 393–397. [Google Scholar] [CrossRef]
- Kühl, O.; Millinghaus, S.; Wehage, P. Functionalised, Chiral Imidazolium Compounds from Proteinogenic Amino Acids. Open Chem. 2010, 8, 1223–1226. [Google Scholar] [CrossRef]
- Farger, P.; Guillot, R.; Leroux, F.; Parizel, N.; Gallart, M.; Gilliot, P.; Rogez, G.; Delahaye, E.; Rabu, P. Imidazolium Dicarboxylate Based Metal-Organic Frameworks Obtained by Solvo-Ionothermal Reaction. Eur. J. Inorg. Chem. 2015, 5342–5350. [Google Scholar] [CrossRef]
- Fei, Z.; Zhao, D.; Geldbach, T.J.; Scopelliti, R.; Dyson, P.J.; Antonijevic, S.; Bodenhausen, G. A Synthetic Zwitterionic Water Channel: Characterization in the Solid State by X-Ray Crystallography and NMR Spectroscopy. Angew. Chem. Int. Ed. 2005, 44, 5720–5725. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. A Simple and Reliable Approach for Assessing Anticancer Activity In Vitro. Curr. Med. Chem. 2015, 22, 1324–1334. [Google Scholar] [CrossRef]
- Martínez, V.R.; Aguirre, M.V.; Todaro, J.S.; Ferrer, E.G.; Williams, P.A.M. Improvement of the Anticancer Activities of Telmisartan by Zn(II) Complexation and Mechanisms of Action. Biol. Trace Elem. Res. 2020, 197, 454–463. [Google Scholar] [CrossRef]
- Andres, S.A.; Bajaj, K.; Vishnosky, N.S.; Peterson, M.A.; Mashuta, M.S.; Buchanan, R.M.; Bates, P.J.; Grapperhaus, C.A. Synthesis, Characterization, and Biological Activity of Hybrid Thiosemicarbazone-Alkylthiocarbamate Metal Complexes. Inorg. Chem. 2020, 59, 4924–4935. [Google Scholar] [CrossRef] [PubMed]
- Naskar, B.; Modak, R.; Maiti, D.K.; Drew, M.G.B.; Bauzá, A.; Frontera, A.; Das Mukhopadhyay, C.; Mishra, S.; Das Saha, K.; Goswami, S. A Schiff Base Platform: Structures, Sensing of Zn(II) and PPi in Aqueous Medium and Anticancer Activity. Dalton Trans. 2017, 46, 9498–9510. [Google Scholar] [CrossRef]
- Tan, Y.S.; Ooi, K.K.; Ang, K.P.; Akim, A.M.; Cheah, Y.K.; Halim, S.N.A.; Seng, H.L.; Tiekink, E.R.T. Molecular Mechanisms of Apoptosis and Cell Selectivity of Zinc Dithiocarbamates Functionalized with Hydroxyethyl Substituents. J. Inorg. Biochem. 2015, 150, 48–62. [Google Scholar] [CrossRef]
- Yu, P.; Deng, J.; Cai, J.; Zhang, Z.; Zhang, J.; Hamid Khan, M.; Liang, H.; Yang, F. Anticancer and Biological Properties of a Zn-2,6-Diacetylpyridine Bis(Thiosemicarbazone) Complex. Metallomics 2019, 11, 1372–1386. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.L.; Shen, W.Y.; Chen, Z.F.; Zhao, L.F.; Qin, Q.P.; Yu, Y.C.; Liang, H. Oxoaporphine Metal Complexes (CoII, NiII, ZnII) with High Antitumor Activity by Inducing Mitochondria-Mediated Apoptosis and S-Phase Arrest in HepG2. Sci. Rep. 2017, 7, 46056. [Google Scholar] [CrossRef]
- Xhafa, S.; Di Nicola, C.; Tombesi, A.; Pettinari, R.; Pettinari, C.; Scarpelli, F.; Crispini, A.; La Deda, M.; Candreva, A.; Garufi, A.; et al. Pyrazolone-Based Zn(II) Complexes Display Antitumor Effects in Mutant P53-Carrying Cancer Cells. J. Med. Chem. 2024, 67, 15676–15690. [Google Scholar] [CrossRef]
- Carrasco, C.J.; Montilla, F.; Villalobo, E.; Angulo, M.; Álvarez, E.; Galindo, A. Antimicrobial Activity of Anionic Bis(N-Heterocyclic Carbene) Silver Complexes. Molecules 2024, 29, 4608. [Google Scholar] [CrossRef]
- Bruker SAINT+. SAINT+; Bruker AXS Inc.: Madison, Wisconsin, USA, 2007.
- Sheldrick, G.M. SADABS, Programs for Scaling and Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Burla, M.C.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2002: The Program. J. Appl. Crystallogr. 2003, 36, 1103. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar]
- Van Der Sluis, P.; Spek, A.L. BYPASS: An Effective Method for the Refinement of Crystal Structures Containing Disordered Solvent Regions. Acta Crystallogr. Sect. A 1990, 46, 194–201. [Google Scholar] [CrossRef]


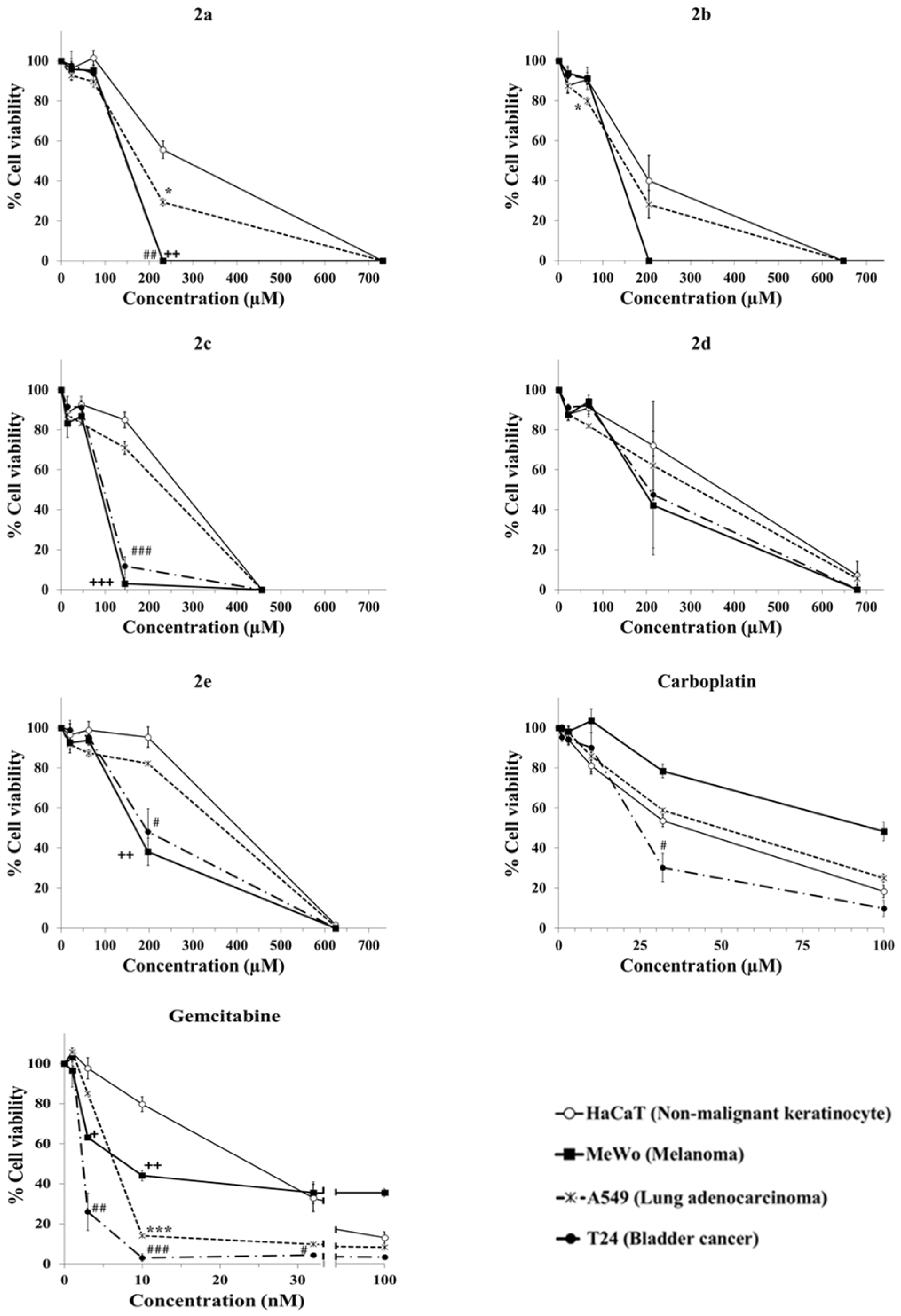
| Complex | IC50 (Mean ± SEM; p Value vs. HaCaT) (Selectivity Index, Mean ± SEM) | |||
| HaCaT (Non-Malignant Keratinocyte) | A549 (Lung Adenocarcinoma) | MeWo (Melanoma) | T24 (Bladder Cancer) | |
| 2a | 260.2 ± 20.0 | 156.3 ± 3.7; 0.0214 (1.7 ± 0.2) | 126.5 ± 1.9; 0.0071 (2.1 ± 0.2) | 124.4 ± 5.5; 0.0101 (2.1 ± 0.2) |
| 2b | 185.0 ± 33.7 | 131.8 ± 11.2; 0.1048 (1.4 ± 0.1) | 108.7 ± 2.2; 0.1139 (1.7 ± 0.3) | 108.5 ± 3.9; 0.1183 (1.7 ± 0.3) |
| 2c | 231.4 ± 7.4 | 202.4 ± 7.6; 0.0965 (1.1 ± 0.1) | 75.7 ± 1.9; 0.0001 (3.1 ± 0.0) | 83.2 ± 3.8; 0.0001 (2.8 ± 0.1) |
| 2d | 328.9 ± 77.8 | 283.3 ± 59.7; 0.1027 (1.1 ± 0.1) | 227.7 ± 66.4; 0.1403 (1.6 ± 0.5) | 238.9 ± 74.5; 0.2019 (1.6 ± 0.5) |
| 2e | 343.9 ± 12.5 | 311.7 ± 3.2; 0.1299 (1.1 ± 0.1) | 162.3 ± 19.3; 0.0032 (2.2 ± 0.3) | 194.6 ± 37.7; 0.0157 (1.9 ± 0.3) |
| Carboplatin | 35.9 ± 3.8 | 42.9 ± 1.4; 0.2188 (0.8 ± 0.1) | 100.4 ± 17.4; 0.0182 (0.4 ± 0.0) | 22.1 ± 2.6; 0.0130 (1.7 ± 0.2) |
| Gemcitabine (nM) | 22.1 ± 3.1 | 5.6 ± 0.1; 0.0130 (4.0 ± 0.5) | 7.3 ± 0.7; 0.0270 (3.3 ± 0.8) | 2.3 ± 0.3; 0.0062 (9.3 ± 0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, C.J.; Pastor, A.; Conejo, M.d.M.; Álvarez, E.; Calderón-Montaño, J.M.; López-Lázaro, M.; Galindo, A. Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands. Int. J. Mol. Sci. 2025, 26, 3202. https://doi.org/10.3390/ijms26073202
Carrasco CJ, Pastor A, Conejo MdM, Álvarez E, Calderón-Montaño JM, López-Lázaro M, Galindo A. Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands. International Journal of Molecular Sciences. 2025; 26(7):3202. https://doi.org/10.3390/ijms26073202
Chicago/Turabian StyleCarrasco, Carlos J., Antonio Pastor, María del Mar Conejo, Eleuterio Álvarez, José Manuel Calderón-Montaño, Miguel López-Lázaro, and Agustín Galindo. 2025. "Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands" International Journal of Molecular Sciences 26, no. 7: 3202. https://doi.org/10.3390/ijms26073202
APA StyleCarrasco, C. J., Pastor, A., Conejo, M. d. M., Álvarez, E., Calderón-Montaño, J. M., López-Lázaro, M., & Galindo, A. (2025). Synthesis, Characterization, and Preliminary In Vitro Anticancer Activity of Zinc Complexes Containing Amino Acid-Derived Imidazolium-Based Dicarboxylate Ligands. International Journal of Molecular Sciences, 26(7), 3202. https://doi.org/10.3390/ijms26073202



_Kim.png)




