Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress
Abstract
1. Introduction
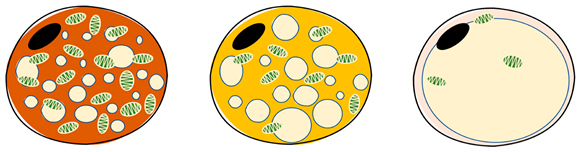
2. Precursor Composition and Function of Brown Adipocytes
| Item | Brown Adipocytes | Beige Adipocytes | White Adipocytes | References |
|---|---|---|---|---|
 | [33,34] | |||
| Location | Interscapular (young animals), supraclavicular, dorsal neck, mediastinum and around kidneys | Supraclavicular, predominantly dispersed in white and brown adipose tissue | Subcutaneous and visceral around | |
| Morphology | Butterfly-shaped, multilocular adipose cell | Spherical, peasized, multilocular adipose cell | Spherical, single vesicular fat cell | |
| Color | Brown | Beige | White | |
| Proportion of mitochondria | High | Medium | Low | |
| Lipid drops | Multilocular, small | Multilocular, small | Unilocular, occupying approximately the entire cell | |
| UCP1 expression | High | Medium | Low/undetectable | |
| Function | Consume energy (triglyceride storage), thermogenesis (non-shivering thermogenesis) | Thermogenic potential | Storing energy | |
| Thermogenetic activity | High | Medium | Low | |
| Role | endocrine organ, energy store | endocrine organ, energy store | endocrine organ, energy store, insulation | |
3. Thermogenesis and Energy Metabolism in Brown Adipose Tissue Under Cold Stress
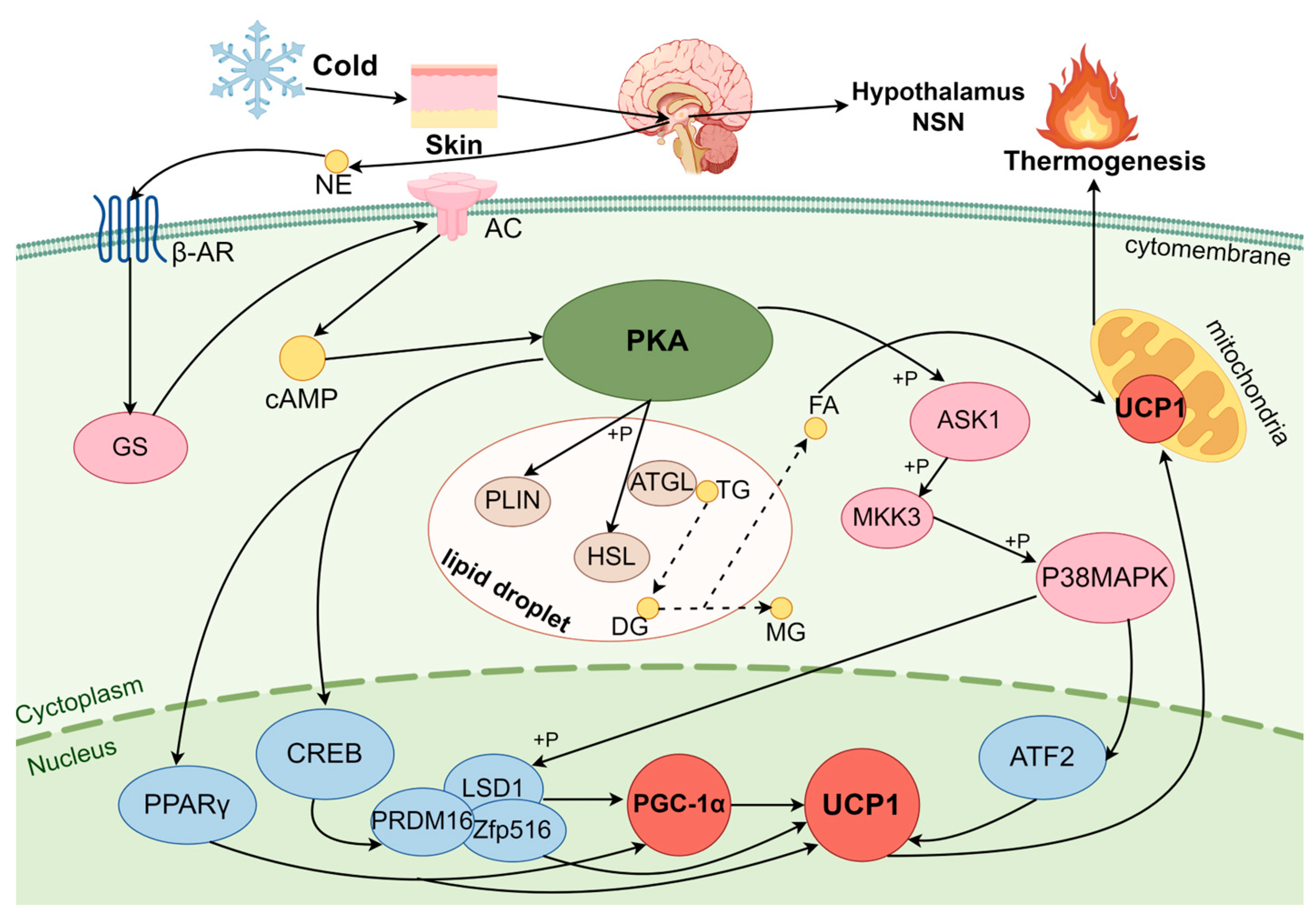
3.1. Regulation of BAT Thermogenesis in Cold Exposure by the cAMP-PKA Pathway
3.1.1. PKA
3.1.2. PKA Pathway Activation Induces BA Thermogenesis
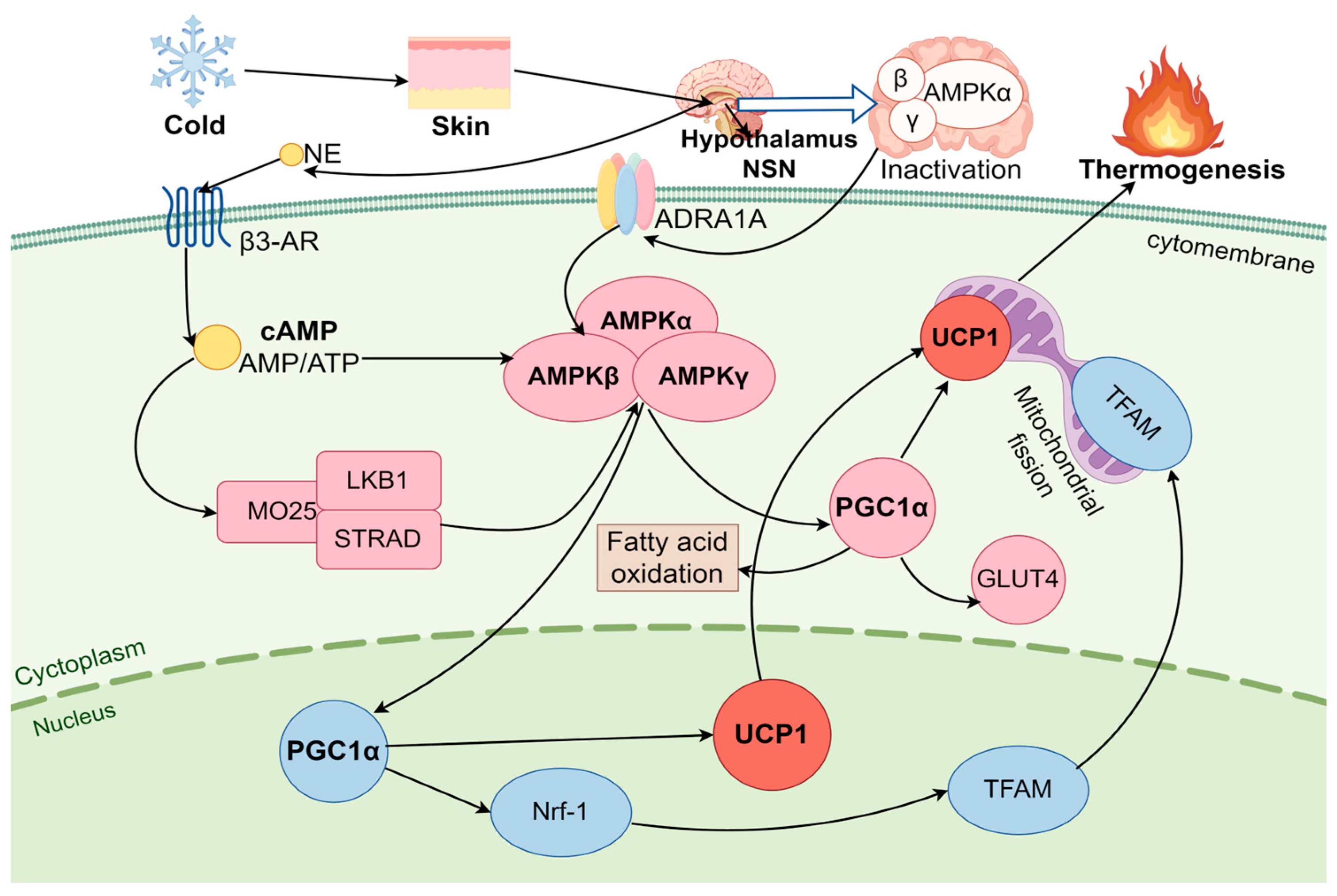
3.2. AMPK Pathway Regulates BAT Thermogenesis Under Cold Stress
3.2.1. AMPK
3.2.2. AMPK Pathway Activation of BA Thermogenesis
4. Thermogenesis and Energy Balance in Brown Adipose Tissue Under Cold Stress
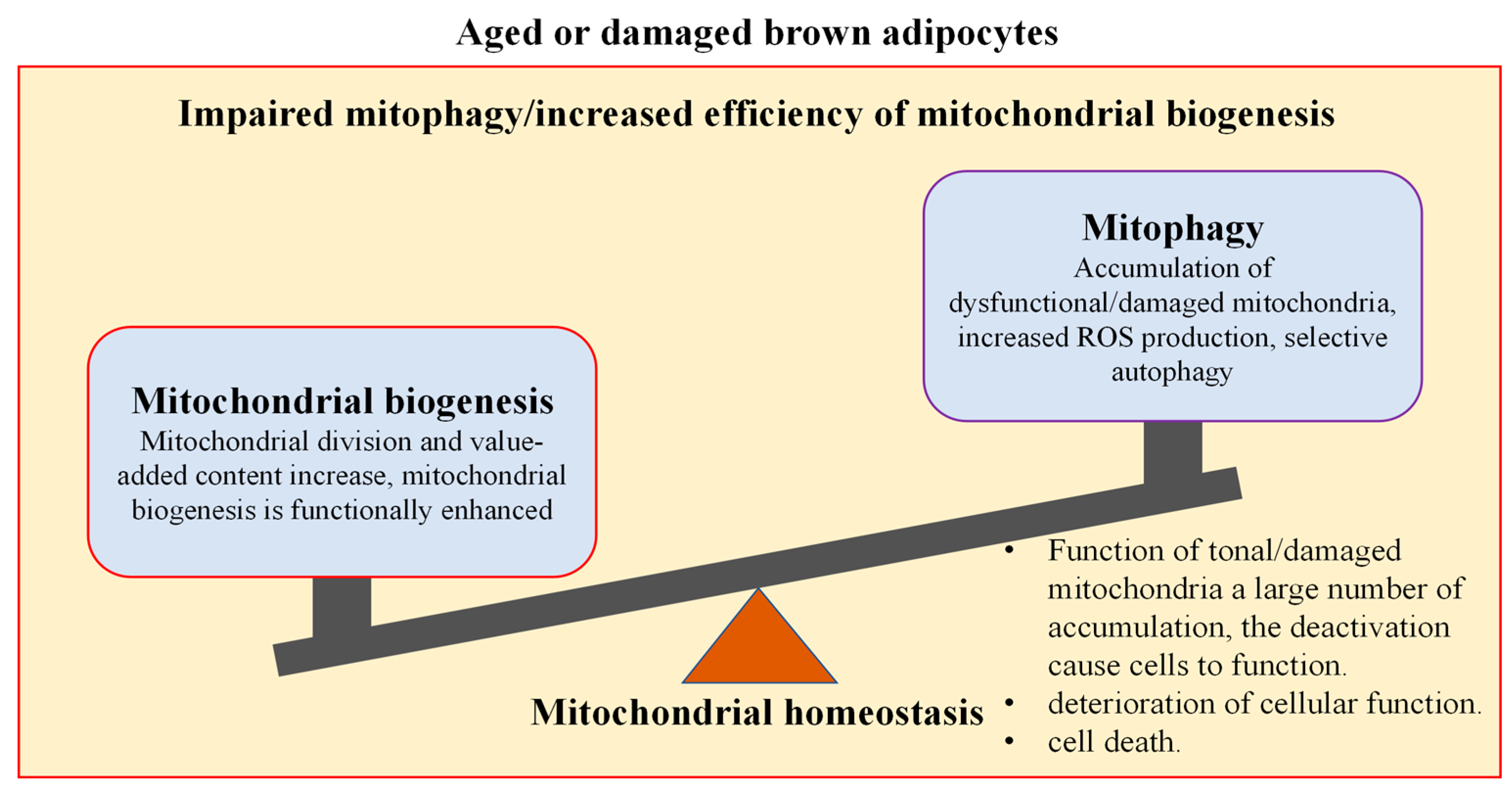
4.1. Thermogenesis and Energy Balance of Mitochondria in Brown Adipocytes
4.1.1. Mitochondrial Biogenesis Regulates Thermogenesis and Energy Metabolism in Brown Adipocytes
4.1.2. Mitophagy Regulates Thermogenesis and Energy Metabolism in Brown Adipocytes
4.1.3. Mitochondria Homeostasis Maintains Thermogenesis and Energy Balance in Brown Adipocytes
4.2. AMPK Signaling Pathway Regulates the Energy Balance in Brown Adipose Tissue
5. Research Methods in the Review
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAT | Brown adipose tissue |
| BA | Brown adipocyte |
| UCP1 | Uncoupling protein 1 |
| NST | Non-shivering thermogenesis |
| PKA | Protein kinase A |
| AMPK | Adenosine monophosphate-activated protein kinase |
| WAT | White adipose tissue |
| NE | Norepinephrine |
| PGC-1α | Peroxisome-proliferator-activated receptor gamma coactivator-1α |
| PGE2 | Prostaglandin E2 |
| Myf-5 | Myogenic factor 5 |
| PRDM16 | PR domain-containing protein 16 |
| Nrf1 | Nuclear respiratory factor 1 |
| C/EBPβ | CCAAT/enhancer binding protein β |
| BMP | Bone morphogenetic protein |
| PPARγ | Peroxisome-proliferator-activated receptor γ |
| MAPK | p38 mitogen-activated protein kinase |
| SNS | Sympathetic nervous system |
| TRP | Transient receptor potential |
| β3-Ars | β3-adrenergic receptors |
| ADRA1A | Adrenergic receptor α1A |
| FFA | Free fatty acid |
| AC | Adenylate cyclase |
| CREB | cAMP response-origin binding protein |
| Zfp516 | Zinc finger protein |
| LSD1 | Demethylase 1 |
| ATF-2 | Transcription factor 2 |
| FAs | Unesterified fatty acids |
| HSL | Hormone-sensitive lipase |
| ATGL | Adipose triglyceride lipase |
| PLIN | Perilipin |
| LKB1 | Liver kinase B1 |
| SKT11 | Serine/threonine kinase 11 |
| PPT1 | Palmitoyl protein thioesterase-1 |
| TFAM | Mitochondria transcription factor A |
| GLUT4 | Glucose transporter 4 |
| BCAAS | Branched-chain amino acids |
| ERR | Estrogen related receptors |
| PINK1 | PTEN-induced putative kinase 1 |
| ATG | Autophagy-related proteins |
| ULK1 | Autophagy-activated kinase |
| MKK3 | MAP kinase 3 |
| MTOR | Mammalian target of rapamycin |
| OMM | Outer mitochondria membrane |
| CPT1 | Carnitine palmitoyl transferase 1 |
| MFF | Mitochondria fission factor |
| E2 | Estradiol |
| T3 | Triiodothyronine |
| Lep | Leptin |
References
- Gao, R.; Shi, L.; Guo, W.; Xu, Y.; Jin, X.; Yan, S.; Shi, B. Effects of Housing and Management Systems on the Growth, Immunity, Antioxidation, and Related Physiological and Biochemical Indicators of Donkeys in Cold Weather. Animals 2022, 12, 2405. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Samet, J.M. Relation between elevated ambient temperature and mortality: A review of the epidemiologic evidence. Epidemiol. Rev. 2002, 24, 190–202. [Google Scholar] [PubMed]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklöv, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [PubMed]
- Liu, X.; Tang, J.; Zhang, R.; Zhan, S.; Zhong, T.; Guo, J.; Wang, Y.; Cao, J.; Li, L.; Zhang, H.; et al. Cold exposure induces lipid dynamics and thermogenesis in brown adipose tissue of goats. BMC Genom. 2022, 23, 528. [Google Scholar]
- Liu, Y.; Xu, B.; Hu, Y.; Liu, P.; Lian, S.; Lv, H.; Yang, Y.; Ji, H.; Yang, H.; Liu, J.; et al. O-GlcNAc/Akt pathway regulates glucose metabolism and reduces apoptosis in liver of piglets with acute cold stress. Cryobiology 2021, 100, 125–132. [Google Scholar]
- Young, B.A. Ruminant cold stress: Effect on production. J. Anim. Sci. 1983, 57, 1601–1607. [Google Scholar]
- Liu, J.; Wu, J.; Qiao, C.; He, Y.; Xia, S.; Zheng, Y.; Lv, H. Impact of chronic cold exposure on lung inflammation, pyroptosis and oxidative stress in mice. Int. Immunopharmacol. 2023, 115, 109590. [Google Scholar]
- Trayhurn, P. Brown Adipose Tissue: A Short Historical Perspective. In Brown Adipose Tissue: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2448, pp. 1–18. [Google Scholar]
- Takeda, Y.; Harada, Y.; Yoshikawa, T.; Dai, P. Mitochondrial Energy Metabolism in the Regulation of Thermogenic Brown Fats and Human Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 1352. [Google Scholar] [CrossRef]
- Tews, D.; Wabitsch, M. Renaissance of brown adipose tissue. Horm. Res. Paediatr. 2011, 75, 231–239. [Google Scholar] [CrossRef]
- Yang, H.; Wu, J.W.; Wang, S.P.; Severi, I.; Sartini, L.; Frizzell, N.; Cinti, S.; Yang, G.; Mitchell, G.A. Adipose-Specific Deficiency of Fumarate Hydratase in Mice Protects Against Obesity, Hepatic Steatosis, and Insulin Resistance. Diabetes 2016, 65, 3396–3409. [Google Scholar]
- Saito, M.; Okamatsu-Ogura, Y. Thermogenic Brown Fat in Humans: Implications in Energy Homeostasis, Obesity and Metabolic Disorders. World J. Mens. Health 2023, 41, 489–507. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [PubMed]
- An, Q.; Wang, X.; Wang, W.L.; Cheng, Z.M.; Zhang, Y.L.; Dai, Y.P. Recent progress in research on the role of lactoferrin in regulating the development and metabolism of adipocytes. Food Sci. 2022, 43, 372–379. [Google Scholar]
- Donnelly, J.R. The productivity of breeding ewes grazing on lucerne or grass and clover pastures on the tablelands of Southern Australia. III. Lamb mortality and weaning percentage. Crop Pasture Sci. 1984, 35, 709–721. [Google Scholar]
- Darby, C.J.; Clarke, L.; Lomax, M.A.; Symonds, M.E. Brown adipose tissue and liver development during early postnatal life in hand-reared and ewe-reared lambs. Reprod. Fertil. Dev. 1996, 8, 137–145. [Google Scholar]
- Xu, Y.; Shi, T.; Cui, X.; Yan, L.; Wang, Q.; Xu, X.; Zhao, Q.; Xu, X.; Tang, Q.Q.; Tang, H.; et al. Asparagine reinforces mTORC1 signaling to boost thermogenesis and glycolysis in adipose tissues. EMBO J. 2021, 40, e108069. [Google Scholar]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar]
- Pfeifer, A.; Hoffmann, L.S. Brown, beige, and white: The new color code of fat and its pharmacological implications. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 207–227. [Google Scholar]
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200. [Google Scholar]
- Shore, A.M.; Karamitri, A.; Kemp, P.; Speakman, J.R.; Graham, N.S.; Lomax, M.A. Cold-induced changes in gene expression in brown adipose tissue, white adipose tissue and liver. PLoS ONE 2013, 8, e68933. [Google Scholar]
- Marlatt, K.L.; Ravussin, E. Brown Adipose Tissue: An Update on Recent Findings. Curr. Obes. Rep. 2017, 6, 389–396. [Google Scholar] [PubMed]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [PubMed]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar]
- Shinde, A.B.; Song, A.; Wang, Q.A. Brown Adipose Tissue Heterogeneity, Energy Metabolism, and Beyond. Front. Endocrinol. 2021, 12, 651763. [Google Scholar]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 2009, 460, 154–158. [Google Scholar]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.M.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar]
- Cohen, P.; Kajimura, S. The cellular and functional complexity of thermogenic fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [PubMed]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef] [PubMed]
- Negroiu, C.E.; Tudorașcu, I.; Bezna, C.M.; Godeanu, S.; Diaconu, M.; Danoiu, R.; Danoiu, S. Beyond the Cold: Activating Brown Adipose Tissue as an Approach to Combat Obesity. J. Clin. Med. 2024, 13, 1973. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.S.; Luo, L.; Guo, Q.; Su, T.; Cheng, P.; Huang, Y. KCTD10 regulates brown adipose tissue thermogenesis and metabolic function via Notch signaling. J. Endocrinol. 2022, 252, 155–166. [Google Scholar]
- Marmol, P.; Krapacher, F.; Ibáñez, C.F. Control of brown adipose tissue adaptation to nutrient stress by the activin receptor ALK7. eLife 2020, 9, e54721. [Google Scholar]
- Cypess, A.M.; Chen, Y.C.; Sze, C.; Wang, K.; English, J.; Chan, O.; Holman, A.R.; Tal, I.; Palmer, M.R.; Kolodny, G.M.; et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 10001–10005. [Google Scholar]
- Huo, C.; Song, Z.; Yin, J.; Zhu, Y.; Miao, X.; Qian, H.; Wang, J.; Ye, L.; Zhou, L. Effect of Acute Cold Exposure on Energy Metabolism and Activity of Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Front. Physiol. 2022, 13, 917084. [Google Scholar]
- Christian, M. Elucidation of the roles of brown and brite fat genes: GPR120 is a modulator of brown adipose tissue function. Exp. Physiol. 2020, 105, 1201–1205. [Google Scholar]
- Shi, M.; Huang, X.Y.; Ren, X.Y.; Wei, X.Y.; Ma, Y.; Lin, Z.Z.; Liu, D.T.; Song, L.; Zhao, T.J.; Li, G.; et al. AIDA directly connects sympatheticc innervation to adaptive thermogenesis by UCP1. Nat. Cell Biol. 2021, 23, 268–277. [Google Scholar]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar]
- Olsen, J.M.; Sato, M.; Dallner, O.S.; Sandström, A.L.; Pisani, D.F.; Chambard, J.C.; Amri, E.Z.; Hutchinson, D.S.; Bengtsson, T. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol. 2014, 207, 365–374. [Google Scholar] [PubMed]
- London, E.; Stratakis, C.A. The regulation of PKA signaling in obesity and in the maintenance of metabolic health. Pharmacol. Ther. 2022, 237, 108113. [Google Scholar] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kazak, L. Promoting metabolic inefficiency for metabolic disease. iScience 2023, 26, 107843. [Google Scholar]
- Sell, H.; Deshaies, Y.; Richard, D. The brown adipocyte: Update on its metabolic role. Int. J. Biochem. Cell Biol. 2004, 36, 2098–2104. [Google Scholar]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; van der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C.; et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623–630. [Google Scholar]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar]
- McNeill, B.T.; Suchacki, K.J.; Stimson, R.H. MECHANISMS IN ENDOCRINOLOGY: Human brown adipose tissue as a therapeutic target: Warming up or cooling down? Eur. J. Endocrinol. 2021, 184, R243–R259. [Google Scholar] [CrossRef]
- Ould Amer, Y.; Hebert-Chatelain, E. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta Bioenerg. 2018, 1859, 868–877. [Google Scholar] [CrossRef]
- McKnight, G.S.; Cummings, D.E.; Amieux, P.S.; Sikorski, M.A.; Brandon, E.P.; Planas, J.V.; Motamed, K.; Idzerda, R.L. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent. Prog. Horm. Res. 1998, 53, 139–159. [Google Scholar]
- Li, Y.; Zhang, K.; Liu, J.; Liu, S.; Nie, C.; Yan, Y.; Guan, Y.; Fan, M.; Qian, H.; Ying, H.; et al. Geniposide suppresses thermogenesis via regulating PKA catalytic subunit in adipocytes. Toxicology 2021, 464, 153014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, D.; Xiang, J.; Zhou, J.; Cao, H.; Che, Q.; Bai, Y.; Guo, J.; Su, Z. Non-shivering Thermogenesis Signalling Regulation and Potential Therapeutic Applications of Brown Adipose Tissue. Int. J. Biol. Sci. 2021, 17, 2853–2870. [Google Scholar] [PubMed]
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130, 2209–2219. [Google Scholar] [PubMed]
- Robidoux, J.; Cao, W.; Quan, H.; Daniel, K.W.; Moukdar, F.; Bai, X.; Floering, L.M.; Collins, S. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38alpha MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol. Cell Biol. 2005, 25, 5466–5479. [Google Scholar]
- Hossain, M.; Imran, K.M.; Rahman, M.S.; Yoon, D.; Marimuthu, V.; Kim, Y.S. Sinapic acid induces the expression of thermogenic signature genes and lipolysis through activation of PKA/CREB signaling in brown adipocytes. BMB Rep. 2020, 53, 142–147. [Google Scholar] [CrossRef]
- Sambeat, A.; Gulyaeva, O.; Dempersmier, J.; Tharp, K.M.; Stahl, A.; Paul, S.M.; Sul, H.S. LSD1 Interacts with Zfp516 to Promote UCP1 Transcription and Brown Fat Program. Cell Rep. 2016, 15, 2536–2549. [Google Scholar]
- Vergnes, L.; Lin, J.Y.; Davies, G.R.; Church, C.D.; Reue, K. Induction of UCP1 and thermogenesis by a small molecule via AKAP1/PKA modulation. J. Biol. Chem. 2020, 295, 15054–15069. [Google Scholar]
- Cao, W.; Daniel, K.W.; Robidoux, J.; Puigserver, P.; Medvedev, A.V.; Bai, X.; Floering, L.M.; Spiegelman, B.M.; Collins, S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell Biol. 2004, 24, 3057–3067. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166126. [Google Scholar]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [PubMed]
- Zhang, G.; Sun, Q.; Liu, C. Influencing Factors of Thermogenic Adipose Tissue Activity. Front. Physiol. 2016, 7, 29. [Google Scholar]
- Dempersmier, J.; Sambeat, A.; Gulyaeva, O.; Paul, S.M.; Hudak, C.S.; Raposo, H.F.; Kwan, H.Y.; Kang, C.; Wong, R.H.; Sul, H.S. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol. Cell 2015, 57, 235–246. [Google Scholar] [PubMed]
- Zimmermann, R.; Lass, A.; Haemmerle, G.; Zechner, R. Fate of fat: The role of adipose triglyceride lipase in lipolysis. Biochim. Biophys. Acta 2009, 1791, 494–500. [Google Scholar]
- Sanders, M.A.; Madoux, F.; Mladenovic, L.; Zhang, H.; Ye, X.; Angrish, M.; Mottillo, E.P.; Caruso, J.A.; Halvorsen, G.; Roush, W.R.; et al. Endogenous and Synthetic ABHD5 Ligands Regulate ABHD5-Perilipin Interactions and Lipolysis in Fat and Muscle. Cell Metab. 2015, 22, 851–860. [Google Scholar]
- Mottillo, E.P.; Bloch, A.E.; Leff, T.; Granneman, J.G. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 2012, 287, 25038–25048. [Google Scholar]
- Gandotra, S.; Lim, K.; Girousse, A.; Saudek, V.; O’Rahilly, S.; Savage, D.B. Human frame shift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator AB-hydrolase-containing 5 (ABHD5). J. Biol. Chem. 2011, 286, 34998–35006. [Google Scholar]
- Bartelt, A.; Heeren, J. The holy grail of metabolic disease: Brown adipose tissue. Curr. Opin. Lipidol. 2012, 23, 190–195. [Google Scholar]
- Yuan, Y.; Li, K.; Ye, X.; Wen, S.; Zhang, Y.; Teng, F.; Zhou, X.; Deng, Y.; Yang, X.; Wang, W.; et al. CLCF1 inhibits energy expenditure via suppressing brown fat thermogenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2310711121. [Google Scholar]
- Hardie, D.G. AMPK as a direct sensor of long-chain fatty acyl-CoA esters. Nat. Metab. 2020, 2, 799–800. [Google Scholar]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar]
- Mulligan, J.D.; Gonzalez, A.A.; Stewart, A.M.; Carey, H.V.; Saupe, K.W. Upregulation of AMPK during cold exposure occurs via distinct mechanisms in brown and white adipose tissue of the mouse. J. Physiol. 2007, 580, 677–684. [Google Scholar] [PubMed]
- van der Vaart, J.I.; Boon, M.R.; Houtkooper, R.H. The Role of AMPK Signaling in Brown Adipose Tissue Activation. Cells 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Liu, M.; Zhou, Y.; Zhang, L.; Li, Y. The New Role of AMP-Activated Protein Kinase in Regulating Fat Metabolism and Energy Expenditure in Adipose Tissue. Biomolecules 2021, 11, 1757. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Desjardins, E.M.; Crane, J.D.; Smith, B.K.; Green, A.E.; Ducommun, S.; Henriksen, T.I.; Rebalka, I.A.; Razi, A.; Sakamoto, K.; et al. Lack of Adipocyte AMPK Exacerbates Insulin Resistance and Hepatic Steatosis through Brown and Beige Adipose Tissue Function. Cell Metab. 2016, 24, 118–129. [Google Scholar]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar]
- López, M. Hypothalamic AMPK and energy balance. Eur. J. Clin. Investig. 2018, 48, e12996. [Google Scholar]
- van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.; Boon, M.R. Regulation of brown fat by AMP-activated protein kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar]
- Marvanova, A.; Kasik, P.; Elsnicova, B.; Tibenska, V.; Galatik, F.; Hornikova, D.; Zvolska, V.; Vebr, P.; Vodicka, P.; Hejnova, L.; et al. Continuous short-term acclimation to moderate cold elicits cardioprotection in rats, and alters β-adrenergic signaling and immune status. Sci. Rep. 2023, 13, 18287. [Google Scholar]
- Min, S.H.; Song, D.K.; Lee, C.H.; Roh, E.; Kim, M.S. Hypothalamic AMP-Activated Protein Kinase as a Whole-Body Energy Sensor and Regulator. Endocrinol. Metab. 2024, 39, 1–11. [Google Scholar]
- Hardie, D.G.; Carling, D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur. J. Biochem. 1997, 246, 259–273. [Google Scholar] [PubMed]
- Liu, X.; Xiao, Z.D.; Han, L.; Zhang, J.; Lee, S.W.; Wang, W.; Lee, H.; Zhuang, L.; Chen, J.; Lin, H.K.; et al. LncRNA NBR2 engages a metabolic checkpoint by regulating AMPK under energy stress. Nat. Cell Biol. 2016, 18, 431–442. [Google Scholar] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z.; Shuai, L.; Li, J.; Li, J. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMP-activated protein kinase: A target for drugs both ancient and modern. Chem. Biol. 2012, 19, 1222–1236. [Google Scholar]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar]
- Okamatsu-Ogura, Y.; Kuroda, M.; Tsutsumi, R.; Tsubota, A.; Saito, M.; Kimura, K.; Sakaue, H. UCP1-dependent and UCP1-independent metabolic changes induced by acute cold exposure in brown adipose tissue of mice. Metabolism 2020, 113, 154396. [Google Scholar]
- Kazak, L.; Chouchani, E.T.; Stavrovskaya, I.G.; Lu, G.Z.; Jedrychowski, M.P.; Egan, D.F.; Kumari, M.; Kong, X.; Erickson, B.K.; Szpyt, J.; et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl. Acad. Sci. USA 2017, 114, 7981–7986. [Google Scholar]
- Kong, X.; Banks, A.; Liu, T.; Kazak, L.; Rao, R.R.; Cohen, P.; Wang, X.; Yu, S.; Lo, J.C.; Tseng, Y.H.; et al. IRF4 is a key thermogenic transcriptional partner of PGC-1α. Cell 2014, 158, 69–83. [Google Scholar]
- Inoue, S.I.; Emmett, M.J.; Lim, H.W.; Midha, M.; Richter, H.J.; Celwyn, I.J.; Mehmood, R.; Chondronikola, M.; Klein, S.; Hauck, A.K.; et al. Short-term cold exposure induces persistent epigenomic memory in brown fat. Cell Metab. 2024, 36, 1764–1778. e9. [Google Scholar]
- Khaibullina, A.; Kenyon, N.; Guptill, V.; Quezado, M.M.; Wang, L.; Koziol, D.; Wesley, R.; Moya, P.R.; Zhang, Z.; Saha, A.; et al. In a model of Batten disease, palmitoyl protein thioesterase-1 deficiency is associated with brown adipose tissue and thermoregulation abnormalities. PLoS ONE 2012, 7, e48733. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Xu, C.Z.; Liu, Y.; Lu, Z.L.; Fu, T.L.; Li, G.R.; Deng, Y.; Luo, G.Q.; Ding, S.; Li, N.; et al. Mitochondrial quality control in human health and disease. Mil. Med. Res. 2024, 11, 32. [Google Scholar] [PubMed]
- Piantadosi, C.A.; Suliman, H.B. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J. Biol. Chem. 2006, 281, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; An, K.; Mao, L. High levels of uric acid inhibit BAT thermogenic capacity through regulation of AMPK. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E376–E389. [Google Scholar] [CrossRef]
- Hill, J.O.; Wyatt, H.R.; Peters, J.C. The Importance of Energy Balance. Eur. Endocrinol. 2013, 9, 111–115. [Google Scholar] [CrossRef]
- Hall, K.D.; Heymsfield, S.B.; Kemnitz, J.W.; Klein, S.; Schoeller, D.A.; Speakman, J.R. Energy balance and its components: Implications for body weight regulation. Am. J. Clin. Nutr. 2012, 95, 989–994. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. Biomed. Res. Int. 2016, 2016, 2365609. [Google Scholar] [CrossRef]
- Wang, W.; Ishibashi, J.; Trefely, S.; Shao, M.; Cowan, A.J.; Sakers, A.; Lim, H.W.; O’Connor, S.; Doan, M.T.; Cohen, P.; et al. A PRDM16-Driven Metabolic Signal from Adipocytes Regulates Precursor Cell Fate. Cell Metab. 2019, 30, 174–189.e5. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.; Wang, D.; Marcet-Rius, M.; Villanueva-García, D.; Gazzano, A.; Domínguez-Oliva, A.; Olmos-Hernández, A.; Hernández-Ávalos, I.; Lezama-García, K.; Verduzco-Mendoza, A.; et al. The Role of Brown Adipose Tissue and Energy Metabolism in Mammalian Thermoregulation during the Perinatal Period. Animals 2023, 13, 2173. [Google Scholar] [CrossRef]
- Simcox, J.; Geoghegan, G.; Maschek, J.A.; Bensard, C.L.; Pasquali, M.; Miao, R.; Lee, S.; Jiang, L.; Huck, I.; Kershaw, E.E.; et al. Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell Metab. 2017, 26, 509–522. e6. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Bulthuis, E.P.; Adjobo-Hermans, M.J.W.; Willems, P.H.G.M.; Koopman, W.J.H. Mitochondrial Morphofunction in Mammalian Cells. Antioxid. Redox Signal. 2019, 30, 2066–2109. [Google Scholar]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [PubMed]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar]
- Kazak, L.; Chouchani, E.T.; Jedrychowski, M.P.; Erickson, B.K.; Shinoda, K.; Cohen, P.; Vetrivelan, R.; Lu, G.Z.; Laznik-Bogoslavski, D.; Hasenfuss, S.C.; et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 2015, 163, 643–655. [Google Scholar]
- Peres Valgas da Silva, C.; Hernández-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome proliferator-activated receptor (PPAR)-alpha: A pharmacological target with a promising future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar]
- Dominy, J.E.; Puigserver, P. Mitochondrial biogenesis through activation of nuclear signaling proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a015008. [Google Scholar]
- Cheng, Y.W.; Liu, J.; Finkel, T. Mitohormesis. Cell Metab. 2023, 35, 1872–1886. [Google Scholar]
- Lowell, B.B.; Spiegelman, B.M. Towards a molecular understanding of adaptive thermogenesis. Nature 2000, 404, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat. Rev. Mol. Cell Biol. 2007, 8, 774–785. [Google Scholar] [PubMed]
- Cairó, M.; Villarroya, J. The role of autophagy in brown and beige adipose tissue plasticity. J. Physiol. Biochem. 2020, 76, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar]
- Wei, H.; Liu, L.; Chen, Q. Selective removal of mitochondria via mitophagy: Distinct pathways for different mitochondrial stresses. Biochim. Biophys. Acta 2015, 1853, 2784–2790. [Google Scholar]
- Lu, Y.; Fujioka, H.; Joshi, D.; Li, Q.; Sangwung, P.; Hsieh, P.; Zhu, J.; Torio, J.; Sweet, D.; Wang, L.; et al. Mitophagy is required for brown adipose tissue mitochondrial homeostasis during cold challenge. Sci. Rep. 2018, 8, 8251. [Google Scholar] [CrossRef]
- Uldry, M.; Yang, W.; St-Pierre, J.; Lin, J.; Seale, P.; Spiegelman, B.M. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006, 3, 333–341. [Google Scholar] [CrossRef]
- Gong, G.; Song, M.; Csordas, G.; Kelly, D.P.; Matkovich, S.J.; Dorn, G.W., 2nd. Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science 2015, 350, aad2459. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, Z.; Zhong, H.; Luo, R.; Liu, W.; Xiong, S.; Liu, Q.; Liu, M. Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment. Hepatol. Commun. 2024, 8, e0484. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Taskintuna, K.; Hum, J.; Gulati, J.; Olaya, S.; Steinman, J.; Golestaneh, N. PGC-1α repression dysregulates lipid metabolism and induces lipid droplet accumulation in retinal pigment epithelium. Cell Death Dis. 2024, 15, 385. [Google Scholar] [PubMed]
- Li, H.; He, Y.; Zhang, C.; Ba, T.; Guo, Z.; Zhuo, Y.; He, L.; Dai, H. NOX1 down-regulation attenuated the autophagy and oxidative damage in pig intestinal epithelial cell following transcriptome analysis of transport stress. Gene 2020, 763, 145071. [Google Scholar] [PubMed]
- Qian, H.; Chen, W.; Yuan, G.; Luo, M.; Zhang, L.; Wu, B.; Huang, H.; Xu, J.; Wang, Q.; Li, M. RTA408 alleviates retinal ganglion cells damage in mouse glaucoma by inhibiting excessive autophagy. PLoS ONE 2024, 19, e0313446. [Google Scholar] [CrossRef]
- Chi, L.; Lee, D.; Leung, S.; Hu, G.; Wen, B.; Delgado-Olguin, P.; Vissa, M.; Li, R.; Brumell, J.H.; Kim, P.K.; et al. Loss of functional peroxisomes leads to increased mitochondrial biogenesis and reduced autophagy that preserve mitochondrial function. Cell Mol. Life Sci. 2023, 80, 183. [Google Scholar]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar]
- Kim, D.; Kim, J.H.; Kang, Y.H.; Kim, J.S.; Yun, S.C.; Kang, S.W.; Song, Y. Suppression of Brown Adipocyte Autophagy Improves Energy Metabolism by Regulating Mitochondrial Turnover. Int. J. Mol. Sci. 2019, 20, 3520. [Google Scholar] [CrossRef]
- Egan, D.F.; Shackelford, D.B.; Mihaylova, M.M.; Gelino, S.; Kohnz, R.A.; Mair, W.; Vasquez, D.S.; Joshi, A.; Gwinn, D.M.; Taylor, R.; et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 2011, 331, 456–461. [Google Scholar]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten Lambert, H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 2021, 593, 435–439. [Google Scholar]
- Kang, J.W.; Hong, J.M.; Lee, S.M. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride-induced liver fibrosis. J. Pineal Res. 2016, 60, 383–393. [Google Scholar]
- Petito, G.; Cioffi, F.; Magnacca, N.; de Lange, P.; Senese, R.; Lanni, A. Adipose Tissue Remodeling in Obesity: An Overview of the Actions of Thyroid Hormones and Their Derivatives. Pharmaceuticals 2023, 16, 572. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, P.; Wang, J.; Zhu, H.; Ren, J.; Chen, Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018, 25, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Wang, J.; Zhang, D.; Wu, H.; Li, W.; Wei, H.; Ta, N.; Fan, Y.; Liu, Y.; et al. Mitophagy receptor FUNDC1 is regulated by PGC-1α/NRF1 to fine tune mitochondrial homeostasis. EMBO Rep. 2021, 22, e50629. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Maraver, J.; Paz, M.V.; Cordero, M.D.; Bautista-Lorite, J.; Oropesa-Ávila, M.; de la Mata, M.; Pavón, A.D.; de Lavera, I.; Alcocer-Gómez, E.; Galán, F.; et al. Critical role of AMP-activated protein kinase in the balance between mitophagy and mitochondrial biogenesis in MELAS disease. Biochim. Biophys. Acta 2015, 1852, 2535–2553. [Google Scholar] [CrossRef]
- Praharaj, P.P.; Panigrahi, D.P.; Bhol, C.S.; Patra, S.; Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Singh, A.; Patil, S.; Bhutia, S.K. Mitochondrial rewiring through mitophagy and mitochondrial biogenesis in cancer stem cells: A potential target for anti-CSC cancer therapy. Cancer Lett. 2021, 498, 217–228. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Kemp, B.E. AMPK in Health and Disease. Physiol. Rev. 2009, 89, 1025–1078. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Zhang, Y.; Zhao, N.; Zhang, W.; Shi, M.; Zhao, Y.; Cai, C.; Lu, C.; Gao, P.; et al. Transcriptome Analysis Revealed Potential Genes of Skeletal Muscle Thermogenesis in Mashen Pigs and Large White Pigs under Cold Stress. Int. J. Mol. Sci. 2023, 24, 15534. [Google Scholar] [CrossRef]
- Ducommun, S.; Deak, M.; Sumpton, D.; Ford, R.J.; Núñez Galindo, A.; Kussmann, M.; Viollet, B.; Steinberg, G.R.; Foretz, M.; Dayon, L.; et al. Motif affinity and mass spectrometry proteomic approach for the discovery of cellular AMPK targets: Identification of mitochondrial fission factor as a new AMPK substrate. Cell Signal. 2015, 27, 978–988. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.P.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef]
- Torres Irizarry, V.C.; Jiang, Y.; He, Y.; Xu, P. Hypothalamic Estrogen Signaling and Adipose Tissue Metabolism in Energy Homeostasis. Front. Endocrinol. 2022, 3, 898139. [Google Scholar]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Tena-Sempere, M. Estradiol effects on hypothalamic AMPK and BAT thermogenesis: A gateway for obesity treatment? Pharmacol. Ther. 2017, 178, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Weiner, L.S.; Roberts-Toler, C.; Franquet Elía, E.; Kessler, S.H.; Kahn, P.A.; English, J.; Chatman, K.; Trauger, S.A.; Doria, A.; et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015, 21, 33–38. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014, 20, 41–53. [Google Scholar]
- Gao, S.; Kinzig, K.P.; Aja, S.; Scott, K.A.; Keung, W.; Kelly, S.; Strynadka, K.; Chohnan, S.; Smith, W.W.; Tamashiro, K.L.; et al. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc. Natl. Acad. Sci. USA 2007, 104, 17358–17363. [Google Scholar]
- López, M.; Nogueiras, R.; Tena-Sempere, M.; Diéguez, C. Hypothalamic AMPK: A canonical regulator of whole-body energy balance. Nat. Rev. Endocrinol. 2016, 12, 421–432. [Google Scholar]
- Wang, Z.; Wang, Q.A.; Liu, Y.; Jiang, L. Energy metabolism in brown adipose tissue. FEBS J. 2021, 288, 3647–3662. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Role of thermogenesis in the regulation of energy balance in relation to obesity. Can. J. Physiol. Pharmacol. 1989, 67, 394–401. [Google Scholar]
- Zhang, Q.; Ye, R.; Zhang, Y.Y.; Fan, C.C.; Wang, J.; Wang, S.; Chen, S.; Liu, X. Brown Adipose Tissue and Novel Management Strategies for Polycystic Ovary Syndrome Therapy. Front. Endocrinol. 2022, 13, 847249. [Google Scholar]
- Zhang, S.; Liu, Y.; Chai, Y.; Xing, L.; Li, J. Effects of intermittent cold stimulation on growth performance, meat quality, antioxidant capacity and liver lipid metabolism in broiler chickens. Poult. Sci. 2024, 103, 103442. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.Y.; Hu, G.S.; Wang, H.Y.; Zhang, G.L.; Cen, X.; Xiang, S.T.; Liu, W.; Li, P.; Ye, H.; et al. DDB1 prepares brown adipocytes for cold-induced thermogenesis. Life Metab. 2022, 1, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Redinger, R.N. Fat storage and the biology of energy expenditure. Transl. Res. 2009, 154, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, J.; Cannon, B. Brown adipose tissue as a heat-producing thermoeffector. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 156, pp. 137–152. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xiao, J.; Jiang, M.; Phillips, C.J.C.; Shi, B. Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress. Int. J. Mol. Sci. 2025, 26, 3233. https://doi.org/10.3390/ijms26073233
Zhang X, Xiao J, Jiang M, Phillips CJC, Shi B. Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress. International Journal of Molecular Sciences. 2025; 26(7):3233. https://doi.org/10.3390/ijms26073233
Chicago/Turabian StyleZhang, Xuekai, Jin Xiao, Min Jiang, Clive J. C. Phillips, and Binlin Shi. 2025. "Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress" International Journal of Molecular Sciences 26, no. 7: 3233. https://doi.org/10.3390/ijms26073233
APA StyleZhang, X., Xiao, J., Jiang, M., Phillips, C. J. C., & Shi, B. (2025). Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress. International Journal of Molecular Sciences, 26(7), 3233. https://doi.org/10.3390/ijms26073233







