Transcriptomic Analysis of Campylobacter jejuni Following Exposure to Gaseous Chlorine Dioxide Reveals an Oxidative Stress Response
Abstract
1. Introduction
2. Results and Discussion
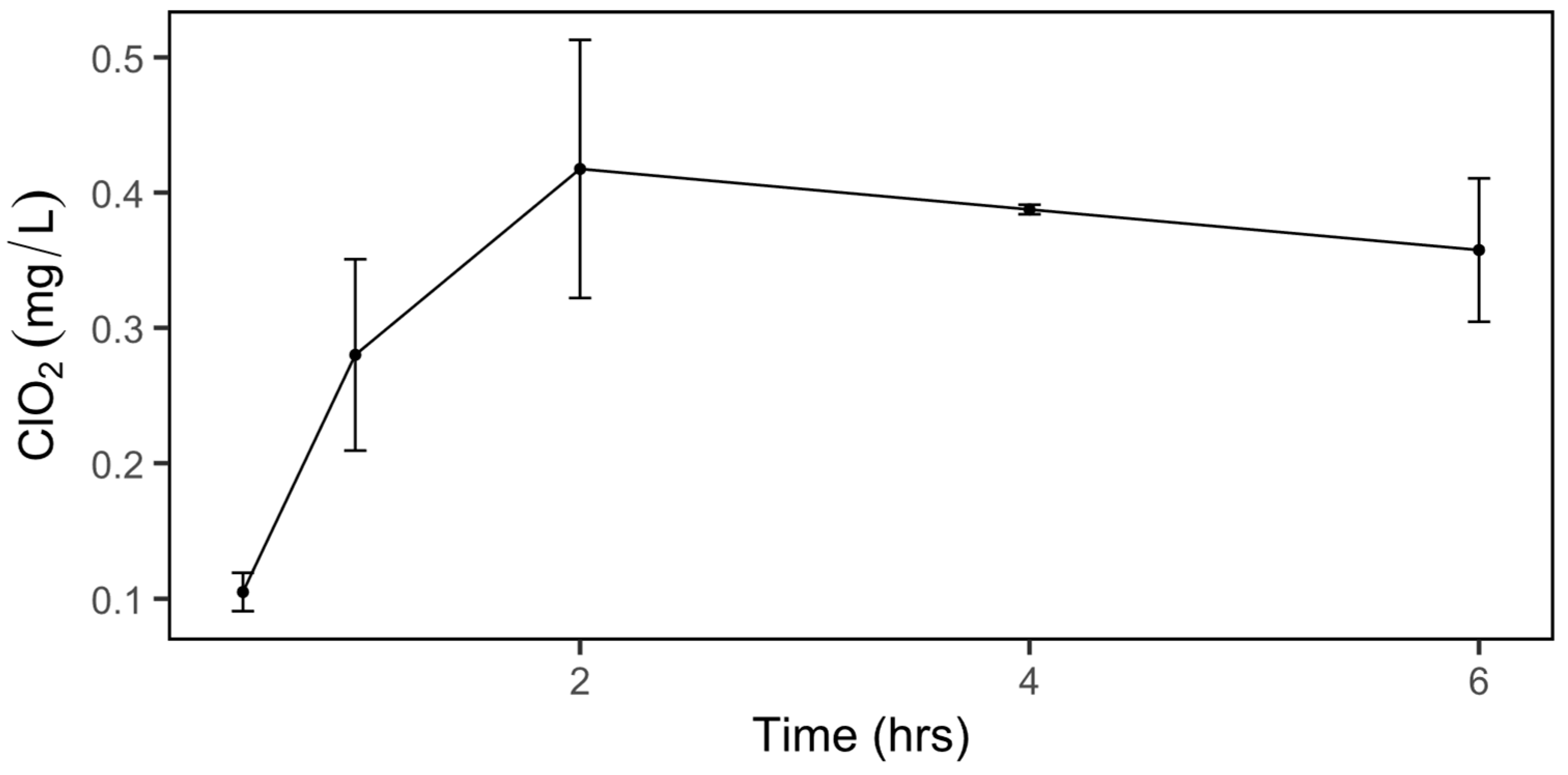
2.1. Dissolved ClO2 in Water
2.2. Exposure of C. jejuni to Gaseous ClO2
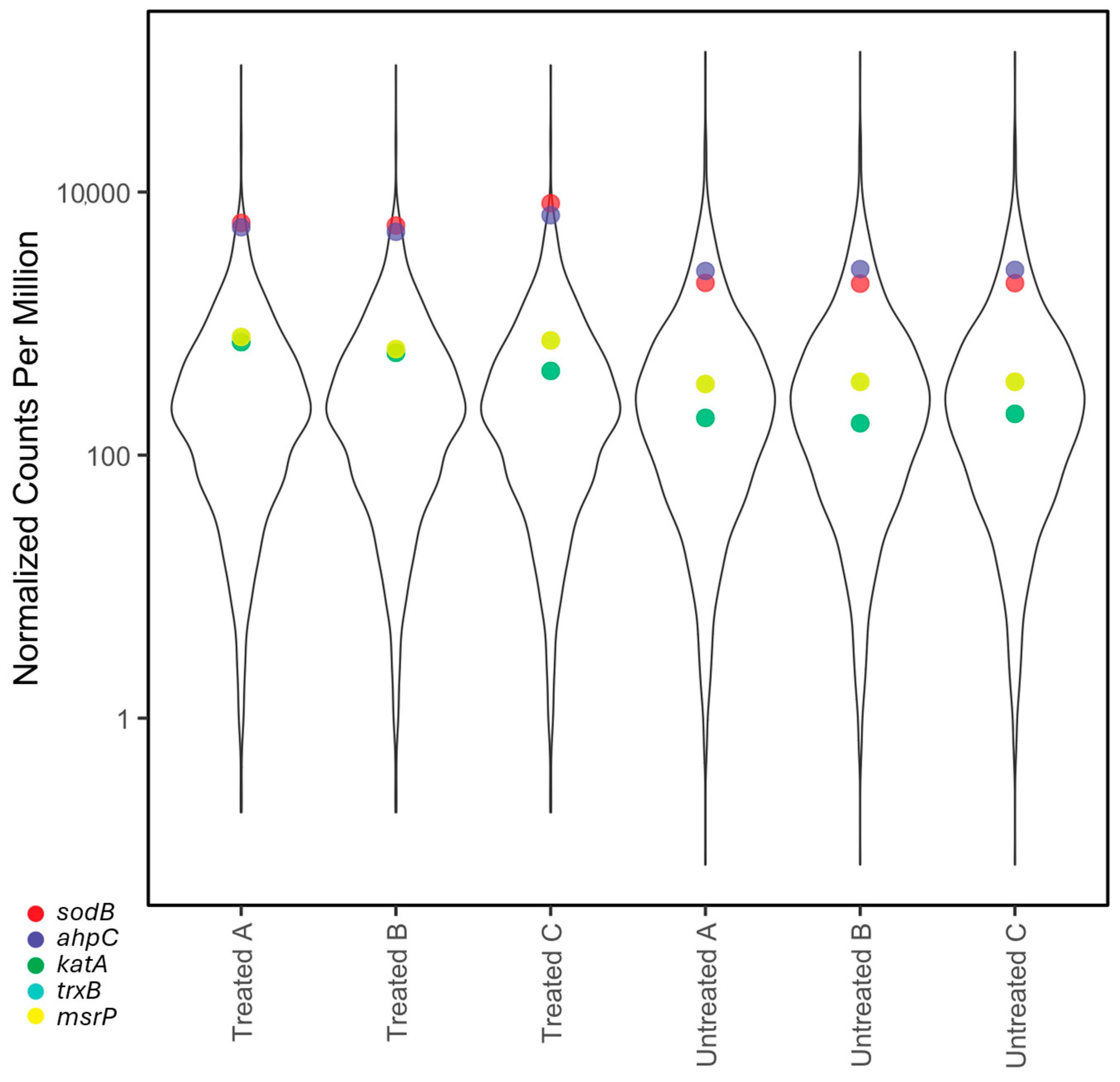
2.3. RNA-Seq
- Oxidative stress response
- b.
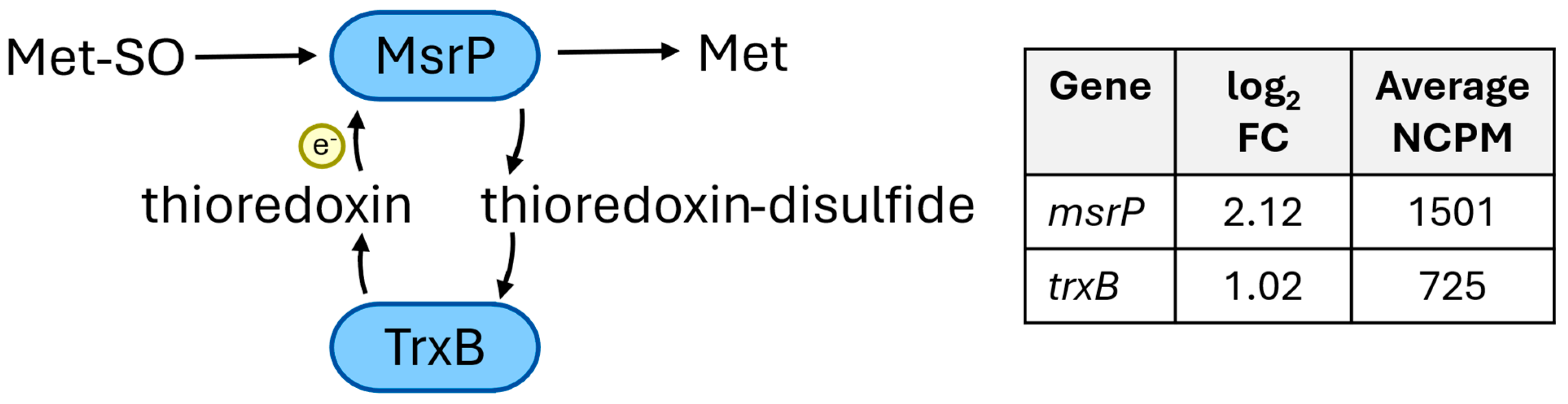
- Methionine sulfoxide reductase
- c.
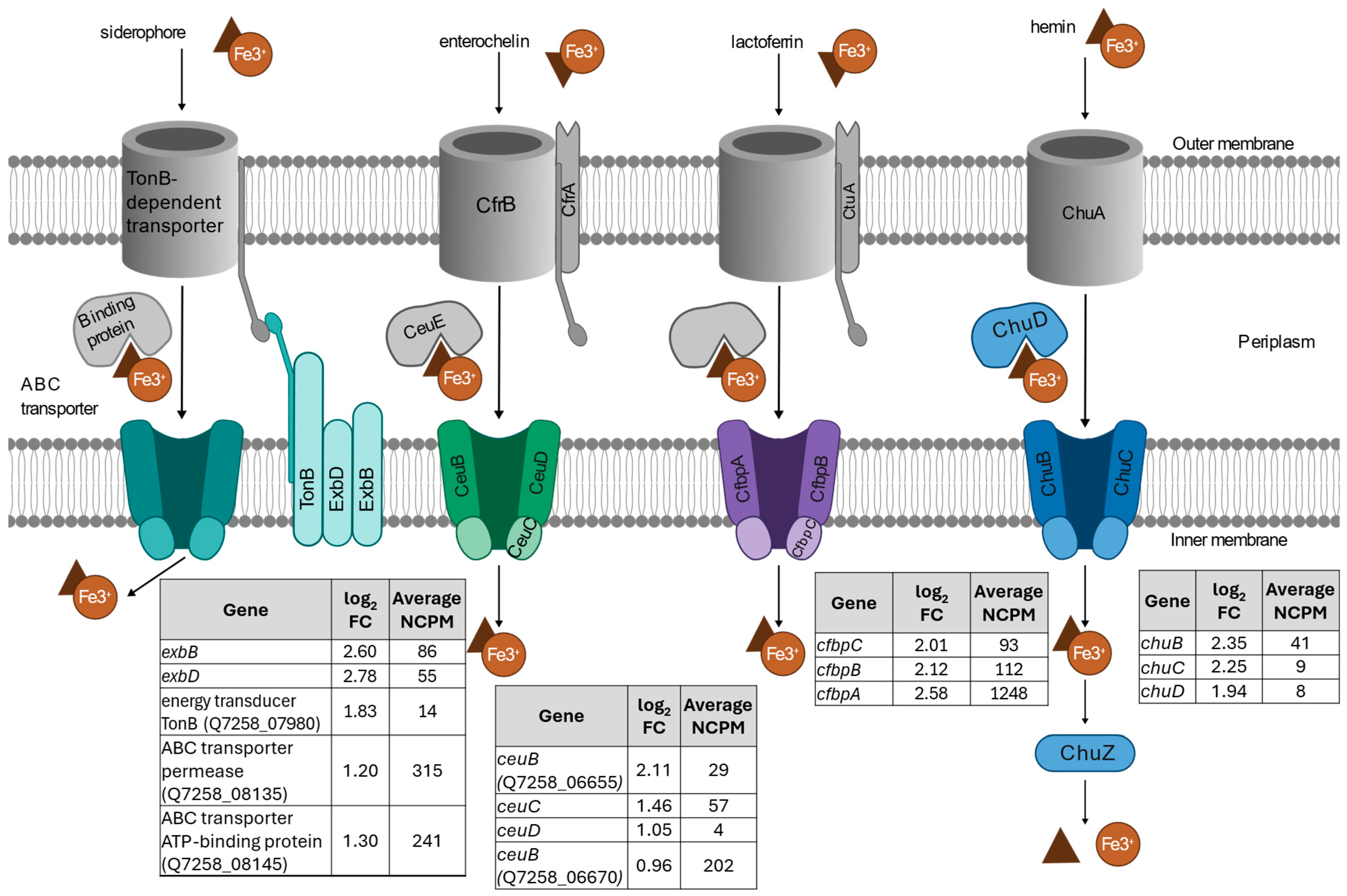
- Iron transport response
- d.
- Phosphate response
- e.
- DNA repair/protection
- f.
- qRT-PCR
3. Materials and Methods
3.1. Culturing Campylobacter jejuni
3.2. Preparation of Chemical Pads
3.3. Measurement of Dissolved Gaseous Chlorine Dioxide
3.4. Inactivation of C. jejuni by Gaseous ClO2
3.5. Sample Treatment with Gaseous ClO2
3.6. Sampling and RNA Preparation
3.7. RT-qPCR
3.8. RNA Sequencing
3.9. Bioinformatics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. About Campylobacter Infection. Available online: https://www.cdc.gov/campylobacter/about/index.html (accessed on 21 March 2025).
- Centers for Disease Control and Prevention. National Center for Emerging and Zoonotic Infectious Diseases (NCEZID). Available online: https://www.cdc.gov/ncezid/dfwed/BEAM-dashboard.html (accessed on 21 March 2025).
- Marasini, D.; Karki, A.B.; Buchheim, M.A.; Fakhr, M.K. Phylogenetic Relatedness Among Plasmids Harbored by Campylobacter jejuni and Campylobacter coli Isolated from Retail Meats. Front. Microbiol. 2018, 9, 2167. [Google Scholar] [CrossRef]
- He, Y.; Dykes, G.E.; Kanrar, S.; Liu, Y.; Gunther, N.W.; Counihan, K.L.; Lee, J.; Capobianco, J.A. Comparative Genomic Analysis of Campylobacter Plasmids Identified in Food Isolates. Microorganisms 2025, 13, 206. [Google Scholar] [CrossRef]
- Ofori, I.; Maddila, S.; Lin, J.; Jonnalagadda, S.B. Chlorine Dioxide Inactivation of Pseudomonas Aeruginosa and Staphylococcus Aureus in Water: The Kinetics and Mechanism. J. Water Process Eng. 2018, 26, 46–54. [Google Scholar] [CrossRef]
- Wang, W.; Smith, D.J.; Ngo, H.; Jin, Z.T.; Mitchell, A.E.; Fan, X. Lipid Oxidation and Volatile Compounds of Almonds as Affected by Gaseous Chlorine Dioxide Treatment to Reduce Salmonella Populations. J. Agric. Food Chem. 2023, 71, 5345–5357. [Google Scholar] [CrossRef]
- Bridges, D.F.; Lacombe, A.; Wu, V.C.H. Integrity of the Escherichia coli O157:H7 Cell Wall and Membranes After Chlorine Dioxide Treatment. Front. Microbiol. 2020, 11, 888. [Google Scholar] [CrossRef]
- Berrang, M.E.; Meinersmann, R.J.; Cox, N.A.; Fedorka-Cray, P.J. Application of Chlorine Dioxide to Lessen Bacterial Contamination during Broiler Defeathering. J. Appl. Poult. Res. 2011, 20, 33–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, J.; Yang, K.; Lu, Y.; Xu, Z.; Yang, H.; Xu, Y.; Wang, L.; Lin, Y.; Tong, X.; et al. Generation, Mechanisms, Kinetics, and Effects of Gaseous Chlorine Dioxide in Food Preservation. Comp. Rev. Food Sci. Food Safe 2023, 22, 3105–3129. [Google Scholar] [CrossRef]
- Bridges, D.F.; Lacombe, A.; Wu, V.C.H. Fundamental Differences in Inactivation Mechanisms of Escherichia coli O157:H7 Between Chlorine Dioxide and Sodium Hypochlorite. Front. Microbiol. 2022, 13, 923964. [Google Scholar] [CrossRef]
- Atack, J.M.; Kelly, D.J. Oxidative Stress in Campylobacter jejuni: Responses, Resistance and Regulation. Future Microbiol. 2009, 4, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, A.H.M.; Ketley, J.M.; Park, S.F.; Penn, C.W. The Role of Iron in Campylobacter Gene Regulation, Metabolism and Oxidative Stress Defense. FEMS Microbiol. Rev. 2002, 26, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Noinaj, N.; Guillier, M.; Barnard, T.J.; Buchanan, S.K. TonB-Dependent Transporters: Regulation, Structure, and Function. Annu. Rev. Microbiol. 2010, 64, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Atack, J.M.; Kelly, D.J. Structure, Mechanism and Physiological Roles of Bacterial Cytochrome c Peroxidases. Adv. Microb. Physiol. 2006, 52, 73–106. [Google Scholar] [CrossRef]
- Wang, H.-W.; Chung, C.-H.; Ma, T.-Y.; Wong, H. Roles of Alkyl Hydroperoxide Reductase Subunit C (AhpC) in Viable but Nonculturable Vibrio parahaemolyticus. Appl. Environ. Microbiol. 2013, 79, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.F.; Mammel, M.K.; McDermott, P.F.; Tartera, C.; White, D.G.; LeClerc, J.E.; Ravel, J.; Cebula, T.A. Comparative Genomics of 28 Salmonella Enterica Isolates: Evidence for CRISPR-Mediated Adaptive Sublineage Evolution. J. Bacteriol. 2011, 193, 3556–3568. [Google Scholar] [CrossRef]
- UniProt Knowledgebase (UniProtKB). Available online: https://www.uniprot.org/uniprotkb/B4TJV1/entry (accessed on 21 March 2025).
- Achard, M.E.S.; Hamilton, A.J.; Dankowski, T.; Heras, B.; Schembri, M.S.; Edwards, J.L.; Jennings, M.P.; McEwan, A.G. A Periplasmic Thioredoxin-Like Protein Plays a Role in Defense against Oxidative Stress in Neisseria gonorrhoeae. Infect. Immun. 2009, 77, 4934–4939. [Google Scholar] [CrossRef]
- Richardson, P.T.; Park, S.F. Enterochelin Acquisition in Campylobacter coli: Characterization of Components of a Binding-Protein-Dependent Transport System. Microbiology 1995, 141, 3181–3191. [Google Scholar] [CrossRef]
- Peralta, D.R.; Adler, C.; Corbalán, N.S.; Paz García, E.C.; Pomares, M.F.; Vincent, P.A. Enterobactin as Part of the Oxidative Stress Response Repertoire. PLoS ONE 2016, 11, e0157799. [Google Scholar] [CrossRef]
- Adler, C.; Corbalan, N.S.; Peralta, D.R.; Pomares, M.F.; De Cristóbal, R.E.; Vincent, P.A. The Alternative Role of Enterobactin as an Oxidative Stress Protector Allows Escherichia coli Colony Development. PLoS ONE 2014, 9, e84734. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Haddad, N.; Chevret, D.; Cappelier, J.-M.; Tresse, O. Comparison of Proteomics Profiles of Campylobacter jejuni Strain Bf under Microaerobic and Aerobic Conditions. Front. Microbiol. 2016, 7, 1596. [Google Scholar] [CrossRef]
- Stahl, M.; Butcher, J.; Stintzi, A. Nutrient Acquisition and Metabolism by Campylobacter jejuni. Front. Cell. Inf. Microbio. 2012, 2, 5. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Molecular Mechanisms of Phosphate Sensing, Transport and Signalling in Streptomyces and Related Actinobacteria. Int. J. Mol. Sci. 2021, 22, 1129. [Google Scholar] [CrossRef] [PubMed]
- Crépin, S.; Lamarche, M.G.; Garneau, P.; Séguin, J.; Proulx, J.; Dozois, C.M.; Harel, J. Genome-Wide Transcriptional Response of an Avian Pathogenic Escherichia coli (APEC) Pst Mutant. BMC Genom. 2008, 9, 568. [Google Scholar] [CrossRef]
- Wang, S.; Deng, K.; Zaremba, S.; Deng, X.; Lin, C.; Wang, Q.; Tortorello, M.L.; Zhang, W. Transcriptomic Response of Escherichia coli O157:H7 to Oxidative Stress. Appl. Environ. Microbiol. 2009, 75, 6110–6123. [Google Scholar] [CrossRef] [PubMed]
- Wösten, M.M.S.M.; Parker, C.T.; Van Mourik, A.; Guilhabert, M.R.; Van Dijk, L.; Van Putten, J.P.M. The Campylobacter jejuni PhosS/PhosR Operon Represents a Non-classical Phosphate-sensitive Two-component System. Mol. Microbiol. 2006, 62, 278–291. [Google Scholar] [CrossRef]
- Chekabab, S.M.; Harel, J.; Dozois, C.M. Interplay between Genetic Regulation of Phosphate Homeostasis and Bacterial Virulence. Virulence 2014, 5, 786–793. [Google Scholar] [CrossRef]
- Gray, M.J.; Jakob, U. Oxidative Stress Protection by Polyphosphate—New Roles for an Old Player. Curr. Opin. Microbiol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Savchenko, A.; Proudfoot, M.; Skarina, T.; Singer, A.; Litvinova, O.; Sanishvili, R.; Brown, G.; Chirgadze, N.; Yakunin, A.F. Molecular Basis of the Antimutagenic Activity of the House-Cleaning Inosine Triphosphate Pyrophosphatase RdgB from Escherichia coli. J. Mol. Biol. 2007, 374, 1091–1103. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Lee, H.-S.; Choi, J.-Y.; Kang, H.-K.; Lee, J.-J.; Hyun, J.-W.; Choi, J.; Ye, S.-K.; Chung, M.-H. MutY Is Down-Regulated by Oxidative Stress in E. coli. Free. Radic. Res. 2003, 37, 873–879. [Google Scholar] [CrossRef]
- He, Y.; Ingudam, S.; Reed, S.; Gehring, A.; Strobaugh, T.P.; Irwin, P. Study on the Mechanism of Antibacterial Action of Magnesium Oxide Nanoparticles against Foodborne Pathogens. J. Nanobiotechnol. 2016, 14, 54. [Google Scholar] [CrossRef]
- He, Y.; Kanrar, S.; Reed, S.; Lee, J.; Capobianco, J. Whole Genome Sequences, De Novo Assembly, and Annotation of Antibiotic Resistant Campylobacter jejuni Strains S27, S33, and S36 Newly Isolated from Chicken Meat. Microorganisms 2024, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Nace, G.W.; Irwin, P.L. A 6×6 Drop Plate Method for Simultaneous Colony Counting and MPN Enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. J. Microbiol. Methods 2003, 55, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential Expression Analysis of Multifactor RNA-Seq Experiments with Respect to Biological Variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]


| Protein | Function | Gene | Primer | Primer Sequence (5′ -> 3′) [11] |
|---|---|---|---|---|
| Gyrase subunit A | Housekeeping | gyrA | Forward | TGCTAAAGTGCGTGAAATCG |
| Reverse | GCATTGGTGCGTTTTCCTAT | |||
| Catalase | Oxidative stress response | katA | Forward | ACCGTTCATGCTAAGGGAAG |
| Reverse | CCTACCAAGTCCCAGTTTCC | |||
| Co-chaperonin | General stress response | groES | Forward | AAACAACAGCCTCAGGCATAA |
| Reverse | TTCTGTTCCACCGTATTTAGCA | |||
| Fe3+ ABC transporter substrate-binding protein | Iron-uptake ABC transport system | cfbpA | Forward | CCACTAATGTTAATATGCGTTCC |
| Reverse | TGTGCTTGATAATCTTGCGACAA | |||
| RelA/spoT family | General stress response | spoT | Forward | GCCCCAATAGCCCATAGAC |
| Reverse | ACCCCAAGCAAATCAAGAAC | |||
| Chaperone | General stress response | dnaK | Forward | CGGTATGCCACAAATCGAAG |
| Reverse | GCTAAGTCCGCTTGAACCTG | |||
| Alkyl hydroperoxide reductase C | Oxidative stress response | ahpC | Forward | AGTTGCCCTTCGTGGTTCGT |
| Reverse | ATCGCCCTTATTCCATCCTG | |||
| Superoxide dismutase | Oxidative stress response | sodB | Forward | TGGCGGTTCATGTCAAAGTA |
| Reverse | ACCAAAACCATCCTGAACCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dykes, G.E.; He, Y.; Jin, T.; Fan, X.; Lee, J.; Reed, S.; Capobianco, J. Transcriptomic Analysis of Campylobacter jejuni Following Exposure to Gaseous Chlorine Dioxide Reveals an Oxidative Stress Response. Int. J. Mol. Sci. 2025, 26, 3254. https://doi.org/10.3390/ijms26073254
Dykes GE, He Y, Jin T, Fan X, Lee J, Reed S, Capobianco J. Transcriptomic Analysis of Campylobacter jejuni Following Exposure to Gaseous Chlorine Dioxide Reveals an Oxidative Stress Response. International Journal of Molecular Sciences. 2025; 26(7):3254. https://doi.org/10.3390/ijms26073254
Chicago/Turabian StyleDykes, Gretchen E., Yiping He, Tony Jin, Xuetong Fan, Joe Lee, Sue Reed, and Joseph Capobianco. 2025. "Transcriptomic Analysis of Campylobacter jejuni Following Exposure to Gaseous Chlorine Dioxide Reveals an Oxidative Stress Response" International Journal of Molecular Sciences 26, no. 7: 3254. https://doi.org/10.3390/ijms26073254
APA StyleDykes, G. E., He, Y., Jin, T., Fan, X., Lee, J., Reed, S., & Capobianco, J. (2025). Transcriptomic Analysis of Campylobacter jejuni Following Exposure to Gaseous Chlorine Dioxide Reveals an Oxidative Stress Response. International Journal of Molecular Sciences, 26(7), 3254. https://doi.org/10.3390/ijms26073254







