Mechanical Force Triggers Macrophage Pyroptosis and Sterile Inflammation by Disrupting Cellular Energy Metabolism
Abstract
:1. Introduction
2. Results
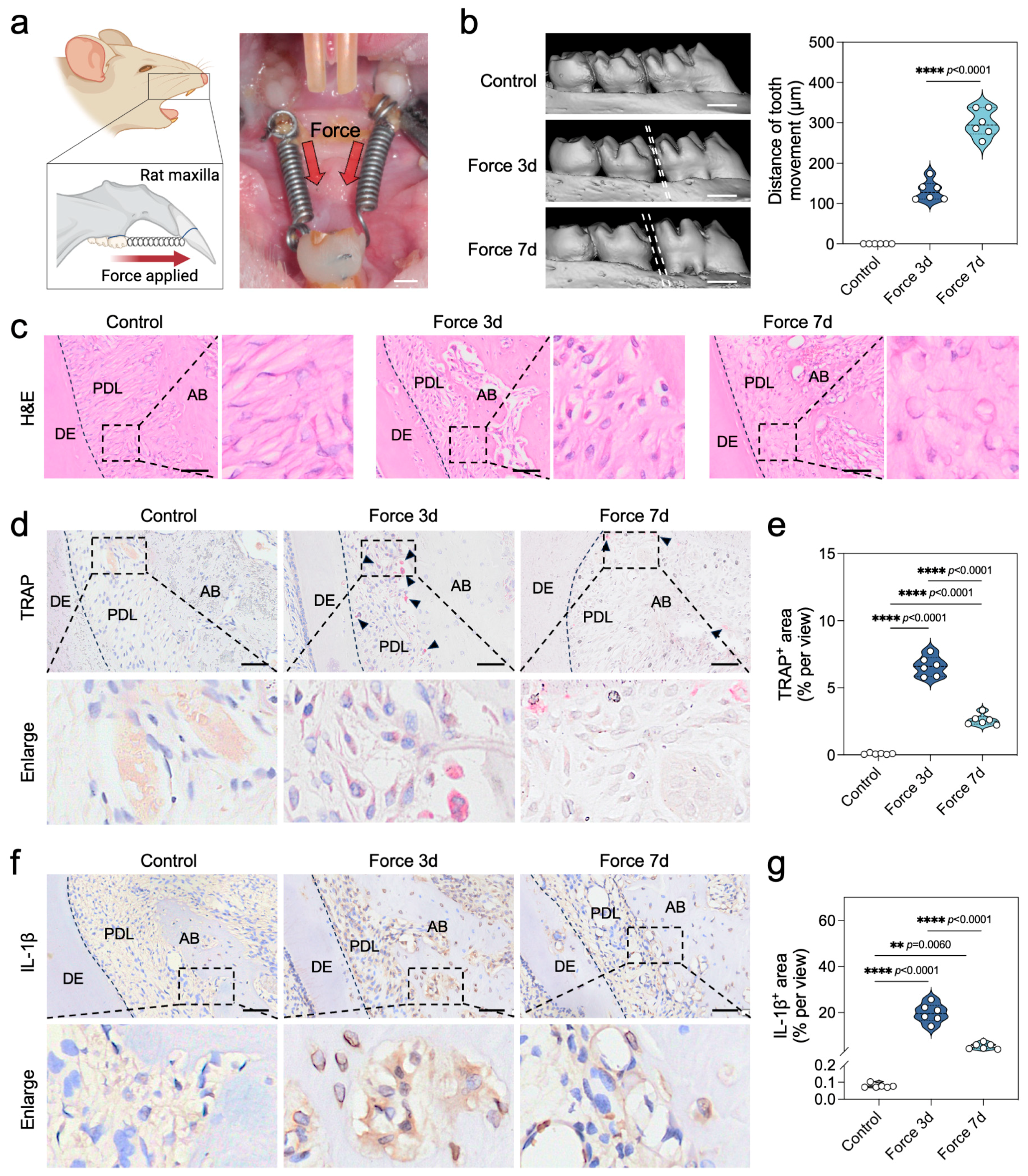
2.1. Force Triggers Sterile Inflammation During the Bone Remodeling Process of OTM
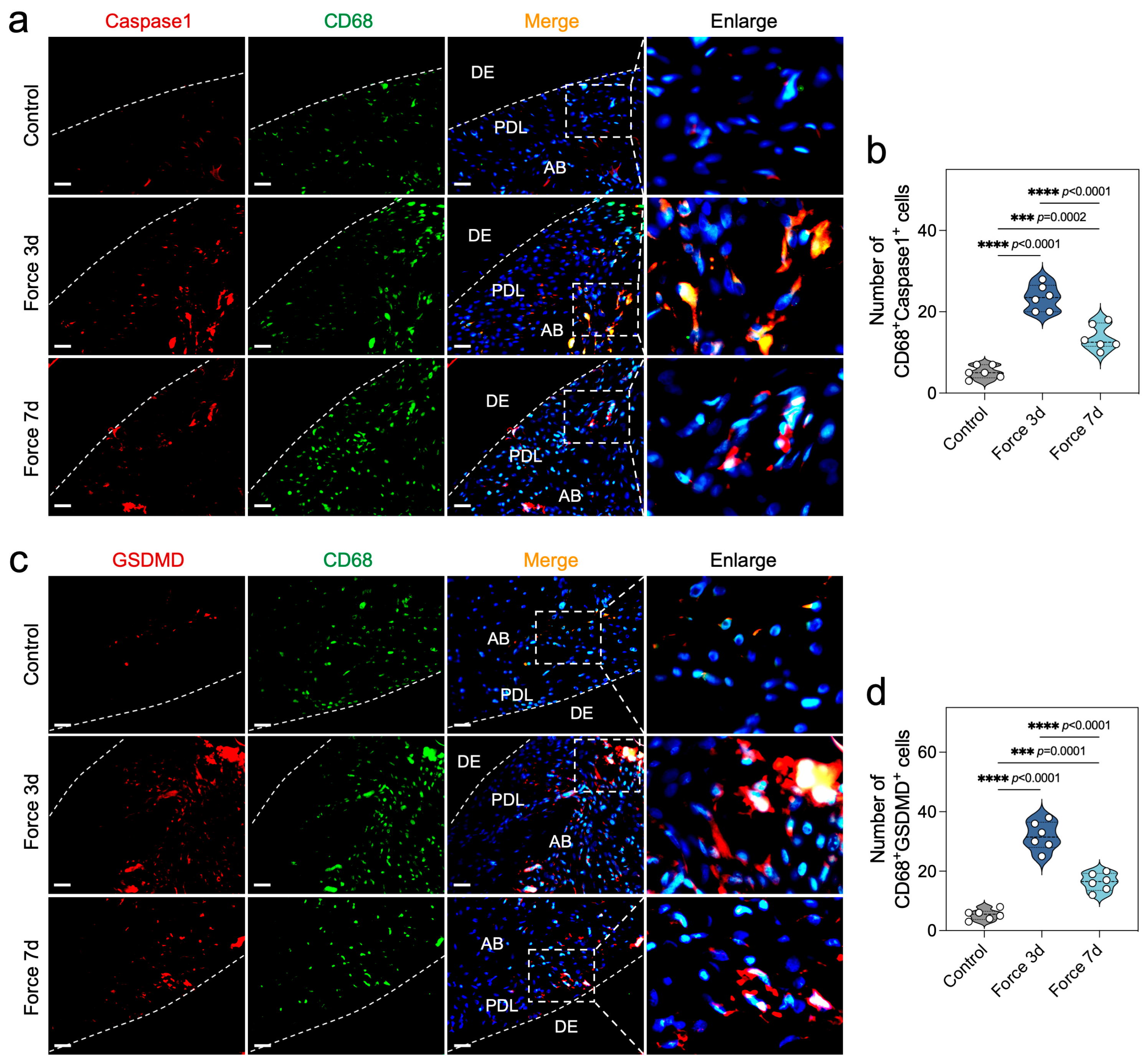
2.2. Force Induces Macrophage Pyroptosis During OTM
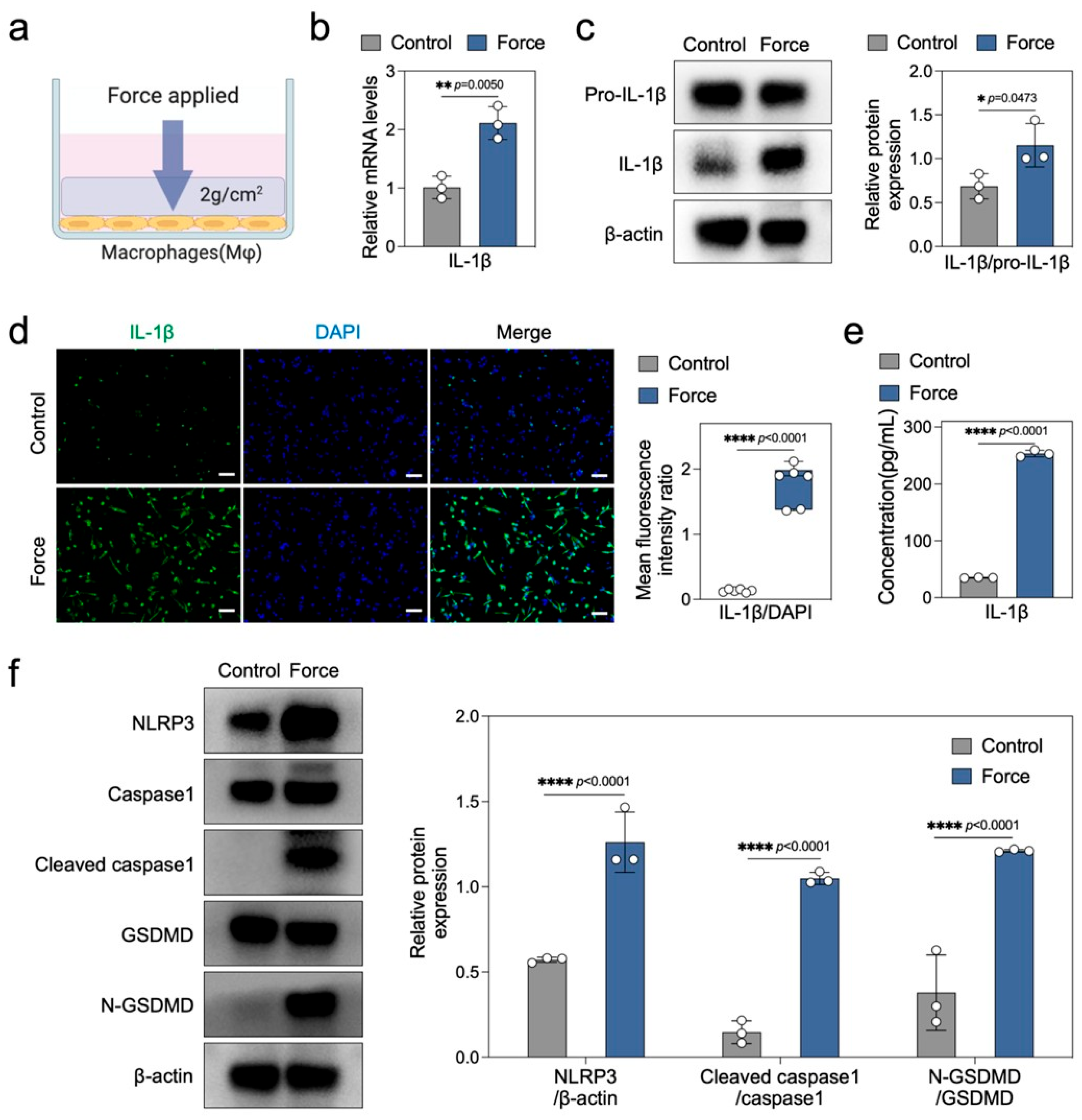
2.3. Force Induces Macrophage Pyroptosis and Sterile Inflammation In Vitro
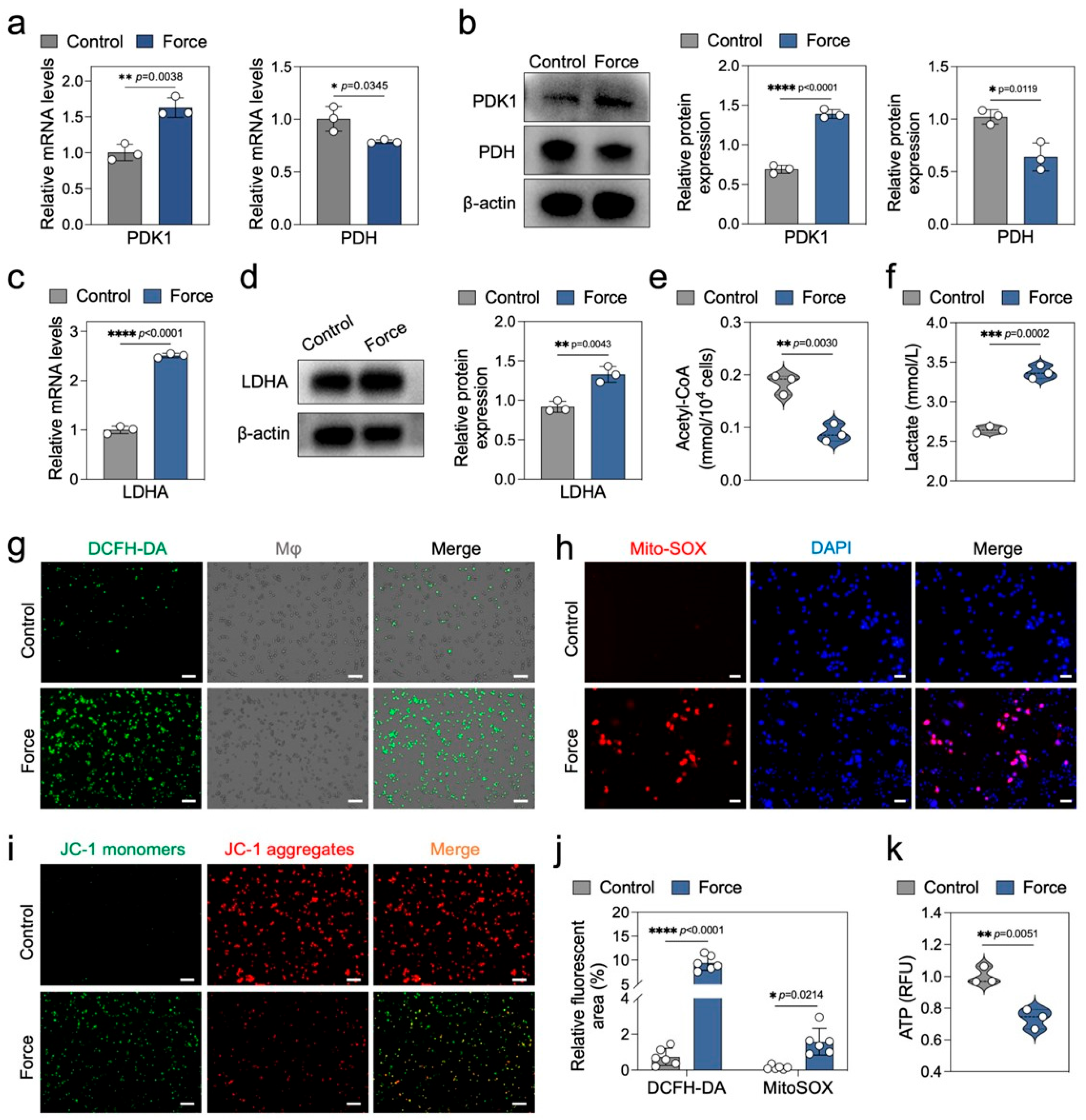
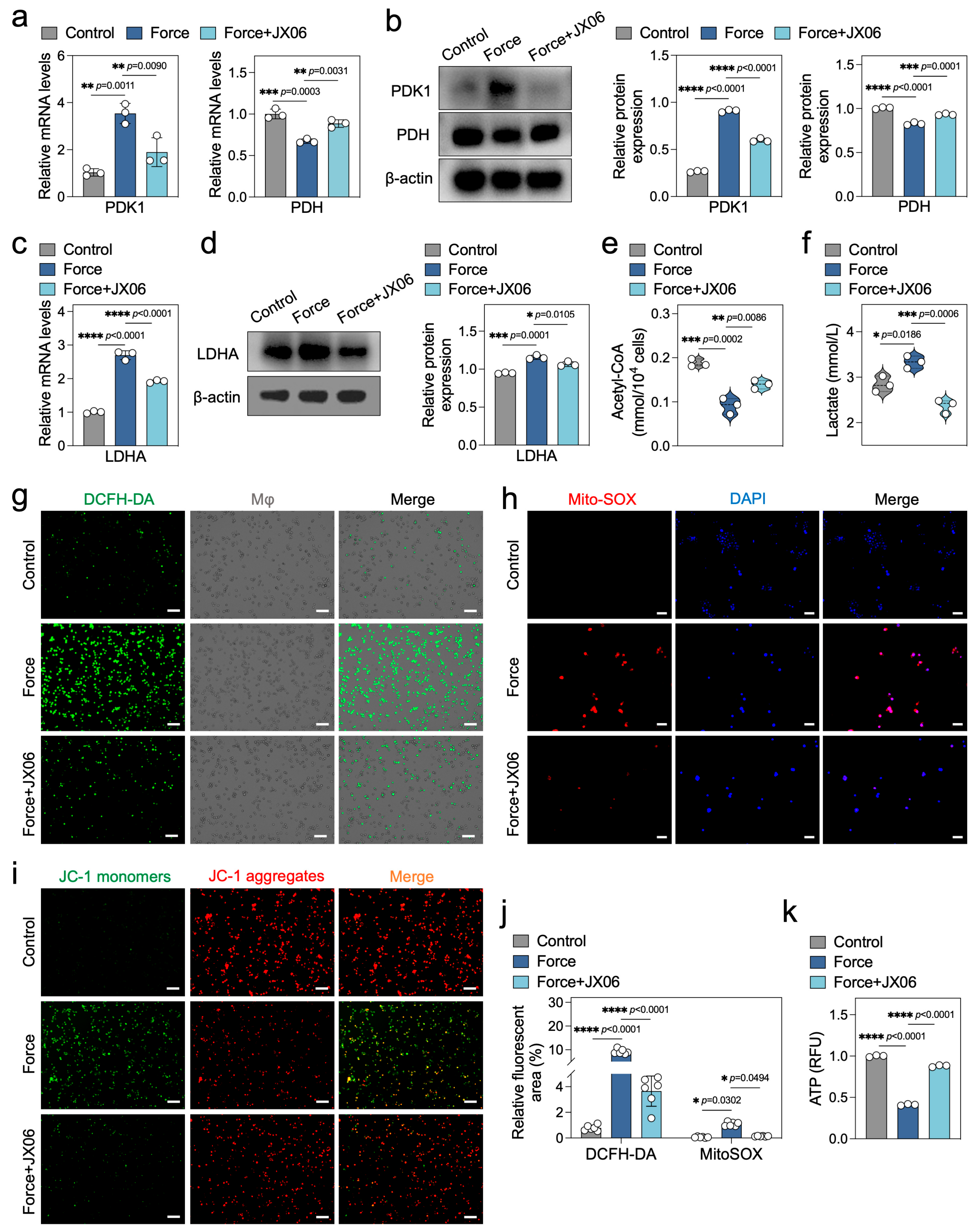
2.4. Force Disrupts Macrophage Energy Metabolism
2.5. Inhibition of PDK1 Rectifies the Energy Metabolism Disorders in Force-Loaded Macrophages
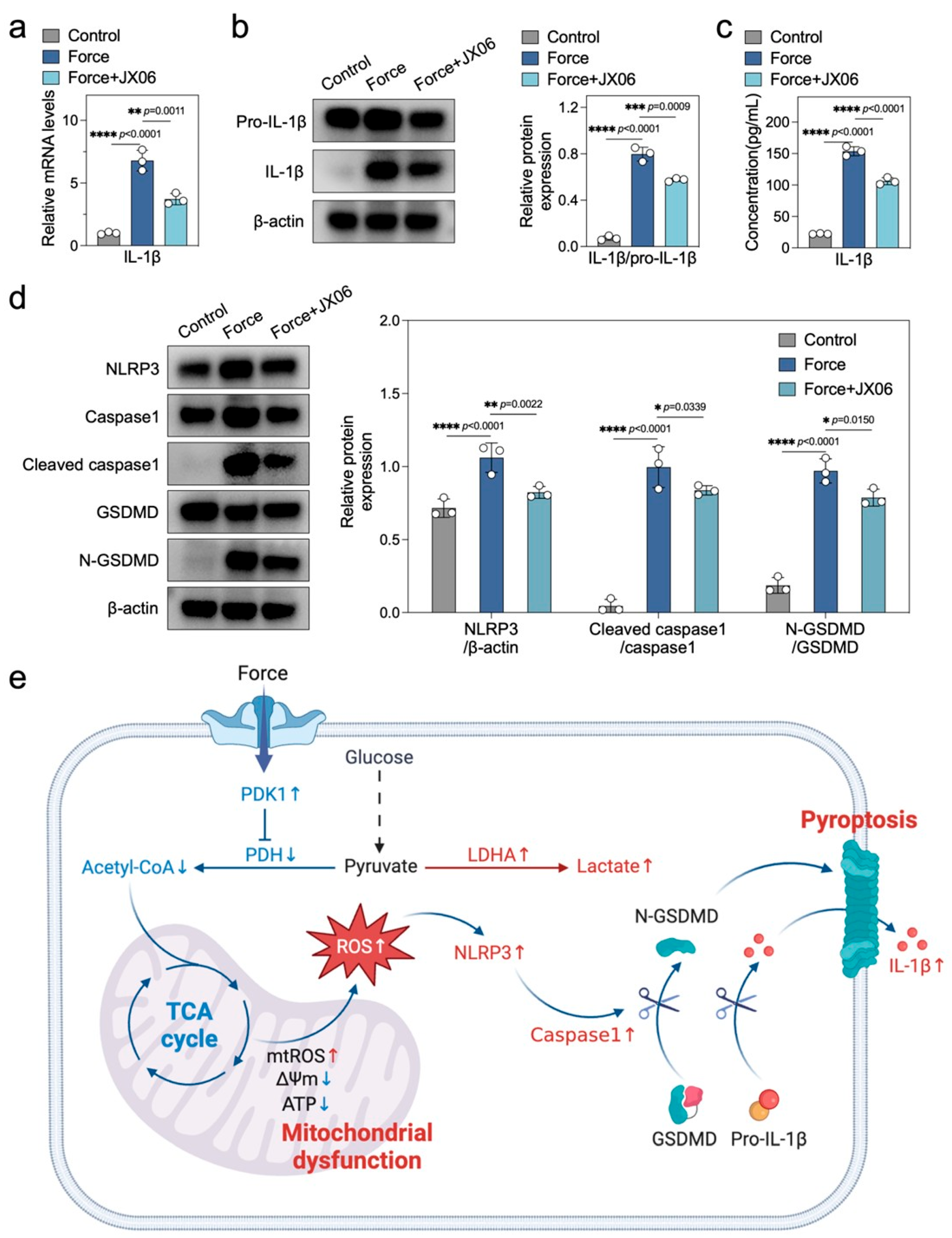
2.6. Inhibiting PDK1 Alleviates Force-Induced Macrophage Pyroptosis and Sterile Inflammation
3. Discussion
4. Materials and Methods
4.1. Animals and Orthodontic Force Treatment
4.2. Micro-CT Scanning and Measurement of Tooth Movement Distance
4.3. Hematoxylin and Eosin (H&E) Staining
4.4. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
4.5. Immunohistochemical Staining
4.6. Immunofluorescence Staining of Tissues
4.7. Force Loading and Treatments on Macrophages In Vitro
4.8. Quantitative Reverse Transcription-PCR (RT-qPCR)
4.9. Western Blotting Analysis
4.10. Immunofluorescence Staining of Cells
4.11. Enzyme Linked Immunosorbent Assay (ELISA)
4.12. Acetyl-CoA and Lactate Content Detection
4.13. ROS Detection
4.14. Mitochondrial Membrane Potential Measurement
4.15. ATP Level Determination
4.16. NAD+ and NADH Detection
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhan, Q.; Bao, M.; Yi, J.; Li, Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.H.; Huang, X.; Rojas Cortez, L.; Sripinun, P.; Lee, J.M.; Hong, J.J.; Graves, D.T. Inflammation and mechanical force-induced bone remodeling. Periodontol. 2000 2024, online ahead of print. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, W.; Zhou, R. DAMP sensing and sterile inflammation: Intracellular, intercellular and inter-organ pathways. Nat. Rev. Immunol. 2024, 24, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J. Dent. Res. 2022, 101, 11–20. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Kou, X.; Yang, R.; Liu, D.; Wang, X.; Luo, Q.; Song, Y.; Liu, F.; Yan, Y.; Gan, Y.; et al. M1-like Macrophage Polarization Promotes Orthodontic Tooth Movement. J. Dent. Res. 2015, 94, 1286–1294. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Mázló, A.; Jenei, V.; Burai, S.; Molnár, T.; Bácsi, A.; Koncz, G. Types of necroinflammation, the effect of cell death modalities on sterile inflammation. Cell Death Dis. 2022, 13, 423. [Google Scholar] [CrossRef]
- Toldo, S.; Abbate, A. The role of the NLRP3 inflammasome and pyroptosis in cardiovascular diseases. Nat. Rev. Cardiol. 2024, 21, 219–237. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Chen, L.; Yu, H.; Li, Z.; Wang, Y.; Jin, S.; Yu, M.; Zhu, L.; Ding, C.; Wu, X.; Wu, T.; et al. Force-induced Caspase-1-dependent pyroptosis regulates orthodontic tooth movement. Int. J. Oral Sci. 2024, 16, 3. [Google Scholar] [CrossRef]
- Zhao, D.; Wu, Y.; Zhuang, J.; Xu, C.; Zhang, F. Activation of NLRP1 and NLRP3 inflammasomes contributed to cyclic stretch-induced pyroptosis and release of IL-1β in human periodontal ligament cells. Oncotarget 2016, 7, 68292–68302. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Gao, J.; Li, P.; Wang, Y.; Qi, Q.; Liu, X.; Li, J.; Wang, C.; Du, L. Pyroptosis, metabolism, and tumor immune microenvironment. Clin. Transl. Med. 2021, 11, e492. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, Y.; Xiong, L.; Jiang, P.; Wang, J.; Li, C. Metabolism, metabolites, and macrophages in cancer. J. Hematol. Oncol. 2023, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.T.; Funai, K. Mitochondrial membrane lipids in the regulation of bioenergetic flux. Cell Metab. 2024, 36, 1963–1978. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A.M.K. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar] [CrossRef]
- Sha, J.F.; Xie, Q.M.; Chen, N.; Song, S.M.; Ruan, Y.; Zhao, C.C.; Liu, Q.; Shi, R.H.; Jiang, X.Q.; Fei, G.H.; et al. TLR2-hif1α-mediated glycolysis contributes to pyroptosis and oxidative stress in allergic airway inflammation. Free Radic. Biol. Med. 2023, 200, 102–116. [Google Scholar] [CrossRef]
- Meyers, A.K.; Wang, Z.; Han, W.; Zhao, Q.; Zabalawi, M.; Duan, L.; Liu, J.; Zhang, Q.; Manne, R.K.; Lorenzo, F.; et al. Pyruvate dehydrogenase kinase supports macrophage NLRP3 inflammasome activation during acute inflammation. Cell Rep. 2023, 42, 111941. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, B.; Ye, B.; Sun, Z.; Qian, Z.; Yu, L.; Bi, Y.; Ma, L.; Ding, Y.; Du, Y.; et al. Mitochondrial-Targeted Delivery of Polyphenol-Mediated Antioxidases Complexes against Pyroptosis and Inflammatory Diseases. Adv. Mater. 2023, 35, e2208571. [Google Scholar] [CrossRef]
- Weindel, C.G.; Martinez, E.L.; Zhao, X.; Mabry, C.J.; Bell, S.L.; Vail, K.J.; Coleman, A.K.; Van Portfliet, J.J.; Zhao, B.; Wagner, A.R.; et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 2022, 185, 3214–3231. [Google Scholar] [CrossRef]
- Elias, E.E.; Lyons, B.; Muruve, D.A. Gasdermins and pyroptosis in the kidney. Nat. Rev. Nephrol. 2023, 19, 337–350. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, C.; Tai, Y.; Li, B.; Lan, T.; Lai, E.; Dai, W.; Guo, Y.; Gan, C.; Kostallari, E.; et al. STING mediates hepatocyte pyroptosis in liver fibrosis by Epigenetically activating the NLRP3 inflammasome. Redox Biol. 2023, 62, 102691. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Bakiri, L.; Bilban, M.; Toegel, S.; Haschemi, A.; Yuan, H.; Kasper, M.; Windhager, R.; Wagner, E.F. Metabolic rewiring controlled by c-Fos governs cartilage integrity in osteoarthritis. Ann. Rheum. Dis. 2023, 82, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Xu, J.; Yin, Y.; Shu, Y.; Li, Y.; Li, T.; Zou, Z.; Wang, Z.; Li, F.; Zhang, M.; et al. Targeting the PDK/PDH axis to reverse metabolic abnormalities by structure-based virtual screening with in vitro and in vivo experiments. Int. J. Biol. Macromol. 2024, 262 Pt 1, 129970. [Google Scholar] [CrossRef]
- Bernard, N.J. Mitochondria control pyroptosis. Nat. Immunol. 2021, 22, 1071. [Google Scholar] [CrossRef]
- Morse, P.T.; Arroum, T.; Wan, J.; Pham, L.; Vaishnav, A.; Bell, J.; Pavelich, L.; Malek, M.H.; Sanderson, T.H.; Edwards, B.F.P.; et al. Phosphorylations and Acetylations of Cytochrome c Control Mitochondrial Respiration, Mitochondrial Membrane Potential, Energy, ROS, and Apoptosis. Cells 2024, 13, 493. [Google Scholar] [CrossRef]
- Garcia-Canaveras, J.C.; Heo, D.; Trefely, S.; Leferovich, J.; Xu, C.; Philipson, B.I.; Ghassemi, S.; Milone, M.C.; Moon, E.K.; Snyder, N.W.; et al. CAR T-Cells Depend on the Coupling of NADH Oxidation with ATP Production. Cells 2021, 10, 2334. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Zhou, J.; Zhang, L.; Li, X.; Wang, Z.; Yin, S.; Zhai, L.; Huang, T.; Wu, X.; et al. Targeting Cancer Metabolism Plasticity with JX06 Nanoparticles via Inhibiting PDK1 Combined with Metformin for Endometrial Cancer Patients with Diabetes. Adv. Sci. 2022, 9, e2104472. [Google Scholar] [CrossRef]
- Semba, H.; Takeda, N.; Isagawa, T.; Sugiura, Y.; Honda, K.; Wake, M.; Miyazawa, H.; Yamaguchi, Y.; Miura, M.; Jenkins, D.M.; et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016, 7, 11635. [Google Scholar] [CrossRef]
- Tan, M.S.; Tan, L.; Jiang, T.; Zhu, X.C.; Wang, H.F.; Jia, C.D.; Yu, J.T. Amyloid-β induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014, 5, e1382. [Google Scholar] [CrossRef]
- Zhai, Z.; Yang, F.; Xu, W.; Han, J.; Luo, G.; Li, Y.; Zhuang, J.; Jie, H.; Li, X.; Shi, X.; et al. Attenuation of Rheumatoid Arthritis Through the Inhibition of Tumor Necrosis Factor-Induced Caspase 3/Gasdermin E-Mediated Pyroptosis. Arthritis Rheumatol. 2022, 74, 427–440. [Google Scholar] [CrossRef]
- Sun, S.J.; Jiao, X.D.; Chen, Z.G.; Cao, Q.; Zhu, J.H.; Shen, Q.R.; Liu, Y.; Zhang, Z.; Xu, F.F.; Shi, Y.; et al. Gasdermin-E-mediated pyroptosis drives immune checkpoint inhibitor-associated myocarditis via cGAS-STING activation. Nat. Commun. 2024, 15, 6640. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Men, X.; Ji, L.; Chen, X.; He, S.; Zhang, P.; Chen, S. NLRP3-mediated periodontal ligament cell pyroptosis promotes root resorption. J. Clin. Periodontol. 2024, 51, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, F.Y.; Davis, C.T.; Liu, W.F. Physical and mechanical regulation of macrophage phenotype and function. Cell. Mol. Life Sci. 2015, 72, 1303–1316. [Google Scholar] [CrossRef]
- Schröder, A.; Käppler, P.; Nazet, U.; Jantsch, J.; Proff, P.; Cieplik, F.; Deschner, J.; Kirschneck, C. Effects of Compressive and Tensile Strain on Macrophages during Simulated Orthodontic Tooth Movement. Mediators Inflamm. 2020, 2020, 2814015. [Google Scholar] [CrossRef]
- Zhu, G.; Yu, H.; Peng, T.; Yang, K.; Xu, X.; Gu, W. Glycolytic enzyme PGK1 promotes M1 macrophage polarization and induces pyroptosis of acute lung injury via regulation of NLRP3. Respir. Res. 2024, 25, 291. [Google Scholar] [CrossRef]
- Haque, P.S.; Kapur, N.; Barrett, T.A.; Theiss, A.L. Mitochondrial function and gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 537–555. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, W.; Chen, G.; Wang, P.; Chen, Z.; Zhou, Y.; Ogasawara, M.; Trachootham, D.; Feng, L.; Pelicano, H.; et al. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 2012, 22, 399–412. [Google Scholar] [CrossRef]
- Hsu, S.N.; Stephen, L.A.; Phadwal, K.; Dillon, S.; Carter, R.; Morton, N.M.; Luijten, I.; Emelianova, K.; Amin, A.K.; Macrae, V.E.; et al. Mitochondrial dysfunction and mitophagy blockade contribute to renal osteodystrophy in chronic kidney disease-mineral bone disorder. Kidney Int. 2025, online ahead of print. [Google Scholar] [CrossRef]
- Li, J.; Shi, X.; Xu, J.; Wang, K.; Hou, F.; Luan, X.; Chen, L. Aldehyde Dehydrogenase 2 Lactylation Aggravates Mitochondrial Dysfunction by Disrupting PHB2 Mediated Mitophagy in Acute Kidney Injury. Adv. Sci. 2025, 12, e2411943. [Google Scholar] [CrossRef]
- Lin, Z.J.; Dong, X.; He, H.; Jiang, J.L.; Guan, Z.J.; Li, X.; Lu, L.; Li, H.; Huang, Y.S.; Xian, S.X.; et al. A simplified herbal decoction attenuates myocardial infarction by regulating macrophage metabolic reprogramming and phenotypic differentiation via modulation of the HIF-1α/PDK1 axis. Chin. Med. 2024, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, F.; Tabariès, S.; Andrzejewski, S.; Dong, Z.; Blagih, J.; Annis, M.G.; Omeroglu, A.; Gao, D.; Leung, S.; Amir, E.; et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab. 2015, 22, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Jiang, C.; Chang, W.Y.; Zhang, H.; An, J.; Ho, F.; Chen, P.; Zhang, H.; Junqueira, C.; Amgalan, D.; et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity 2023, 56, 2523–2541. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Liu, F.; Cui, S.; Jiang, N.; Yu, H.; Zhou, Y.; Liu, Y.; Kou, X. Mechanical load-induced H2S production by periodontal ligament stem cells activates M1 macrophages to promote bone remodeling and tooth movement via STAT1. Stem Cell Res. Ther. 2020, 11, 112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, H.; Yang, G.; Zhu, Y.; He, X.; Yang, L.; Hu, Y.; Zheng, L. Mechanical Force Triggers Macrophage Pyroptosis and Sterile Inflammation by Disrupting Cellular Energy Metabolism. Int. J. Mol. Sci. 2025, 26, 3321. https://doi.org/10.3390/ijms26073321
Tan H, Yang G, Zhu Y, He X, Yang L, Hu Y, Zheng L. Mechanical Force Triggers Macrophage Pyroptosis and Sterile Inflammation by Disrupting Cellular Energy Metabolism. International Journal of Molecular Sciences. 2025; 26(7):3321. https://doi.org/10.3390/ijms26073321
Chicago/Turabian StyleTan, Hao, Guoyin Yang, Ye Zhu, Xinyi He, Lan Yang, Yun Hu, and Leilei Zheng. 2025. "Mechanical Force Triggers Macrophage Pyroptosis and Sterile Inflammation by Disrupting Cellular Energy Metabolism" International Journal of Molecular Sciences 26, no. 7: 3321. https://doi.org/10.3390/ijms26073321
APA StyleTan, H., Yang, G., Zhu, Y., He, X., Yang, L., Hu, Y., & Zheng, L. (2025). Mechanical Force Triggers Macrophage Pyroptosis and Sterile Inflammation by Disrupting Cellular Energy Metabolism. International Journal of Molecular Sciences, 26(7), 3321. https://doi.org/10.3390/ijms26073321





