Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy
Abstract
1. Introduction
1.1. Basal Ganglia Anatomy and Function
1.1.1. Basal Ganglia Connectivity
1.1.2. Basal Ganglia Microstructure
1.2. Seizures and Status Epilepticus
1.3. Current Clinical and Experimental Data Concerning the Basal Ganglia in EPILEPSY
1.4. Purpose and Objectives
2. Results
2.1. Age-Specific Pattern
2.1.1. In P12
2.1.2. In P15
2.1.3. In P18
2.1.4. In P21 and P25
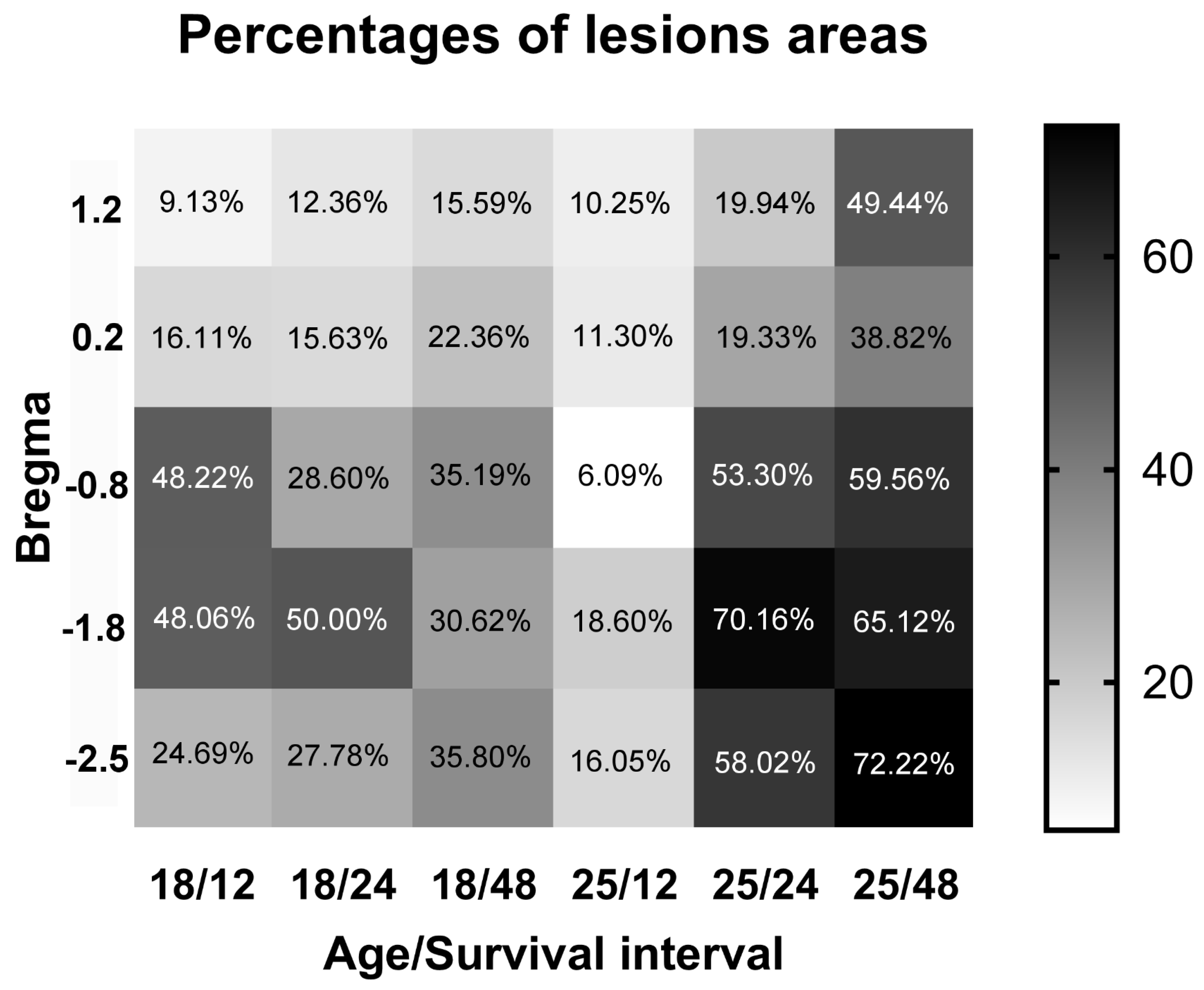
2.2. Neuronal Damage Distribution
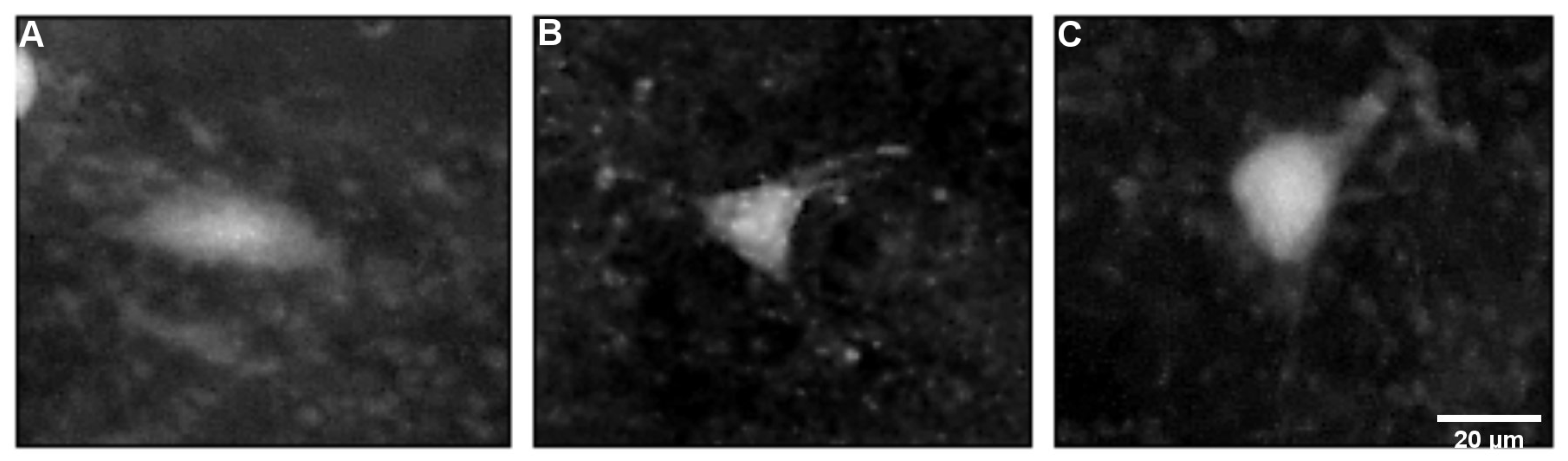
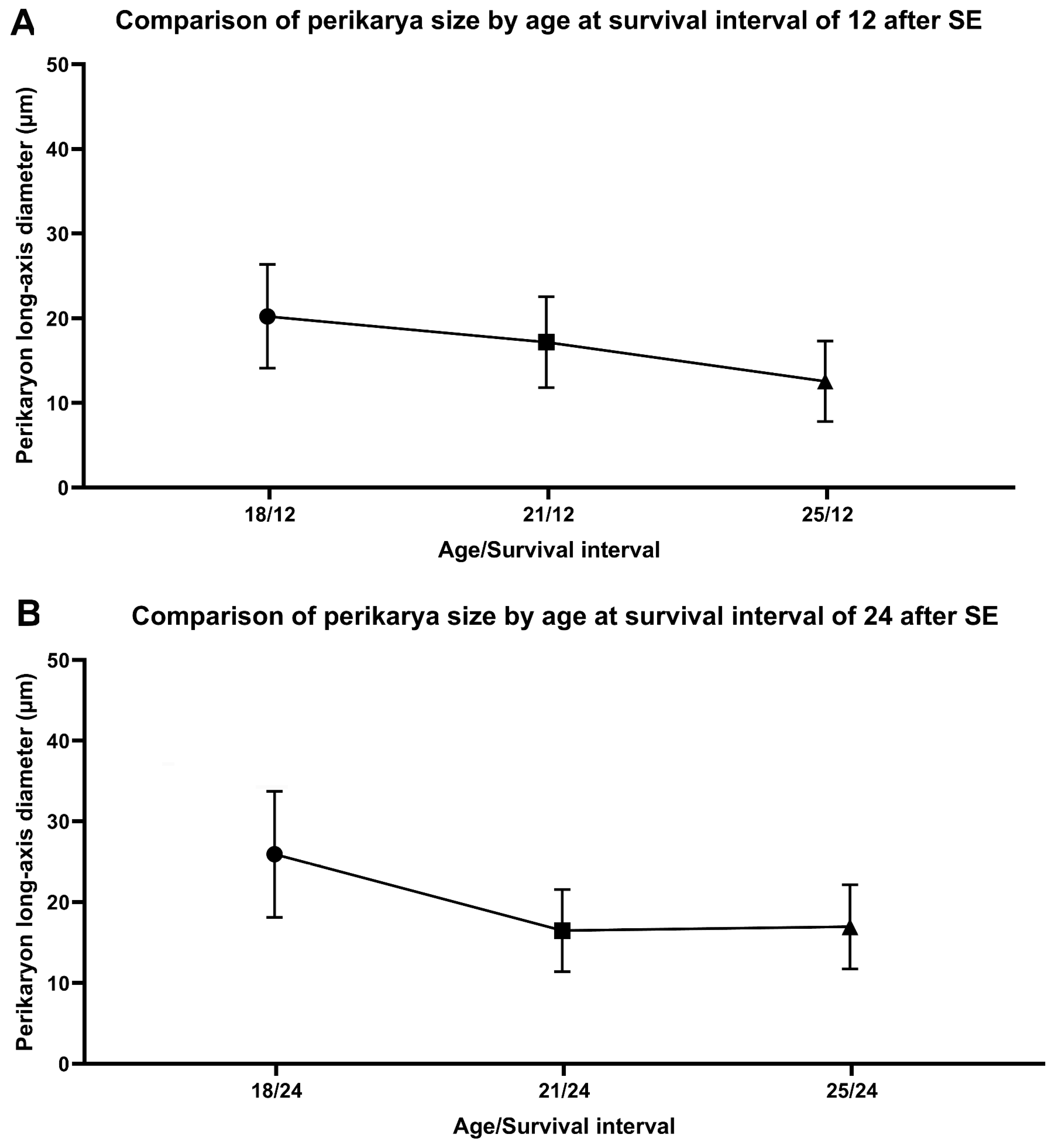
2.3. Characteristics of FJB-Positive Neurons
2.4. Control Animals
3. Discussion
3.1. Age-Specific Pattern of Neuronal Damage in Status Epilepticus
3.2. Neuronal Damage Distribution
3.3. Mechanisms of Neuronal Damage Observed in Status Epilepticus
3.4. BG Neuronal Degeneration in Other Status Epilepticus Experimental Models
3.5. The Limitations of This Study
4. Materials and Methods
4.1. Experimental Animal Model and Design
4.1.1. Brain Handing and Histological Preparation
4.1.2. Statistical Analysis
4.2. Sample Size Statistical Calculation
4.3. Animal Handling and Ethical Policy of Invasive Procedures
4.4. The Procedure and Administered Drugs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SE | status epilepticus |
| BG | basal ganglia |
| DS | dorsal striatum |
| GP | globus pallidus |
| GPe | globus pallidus external segment |
| GPi | globus pallidus internal segments |
| SNc | substantia nigra pars compacta |
| SNr | substantia nigra pars reticulata |
References
- Kubová, H.; Druga, R.; Lukasiuk, K.; Suchomelová, L.; Haugvicová, R.; Jirmanová, I.; Pitkänen, A. Status epilepticus causes necrotic damage in the mediodorsal nucleus of the thalamus in immature rats. J. Neurosci. 2001, 21, 3593–3599. [Google Scholar] [CrossRef]
- Kubová, H.; Druga, R.; Haugvicová, R.; Suchomelová, L.; Pitkanen, A. Dynamic changes of status epilepticus-induced neuronal degeneration in the mediodorsal nucleus of the thalamus during postnatal development of the rat. Epilepsia 2002, 43, 54–60. [Google Scholar] [CrossRef]
- Doczi, J.; Bernásková, K.; Kubová, H.; Détari, L.; Világi, I.; Druga, R.; Mares, P. Long-term changes of activity of cortical neurons after status epilepticus induced at early developmental stages in rats. Neurosci. Lett. 2003, 352, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Blumenfeld, H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002, 3, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Scholl, E.A.; Dudek, F.E.; Ekstrand, J.J. Neuronal degeneration is observed in multiple regions outside the hippocampus after lithium pilocarpine-induced status epilepticus in the immature rat. Neuroscience 2013, 252, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Druga, R.; Mares, P.; Otáhal, J.; Kubová, H. Degenerative neuronal changes in the rat thalamus induced by status epilepticus at different developmental stages. Epilepsy Res. 2005, 63, 43–65. [Google Scholar] [CrossRef]
- Mares, P.; Tsenov, G.; Aleksakhina, K.; Druga, R.; Kubová, H. Changes of cortical interhemispheric responses after status epilepticus in immature rats. Epilepsia 2005, 46, 31–37. [Google Scholar] [CrossRef]
- Druga, R.; Mares, P.; Kubová, H. Time course of neuronal damage in the hippocampus following lithium-pilocarpine status epilepticus in 12-day-old rats. Brain Res. 2010, 1355, 174–179. [Google Scholar] [CrossRef]
- Dematteis, M.; Kahane, P.; Vercueil, L.; Depaulis, A. MRI evidence for the involvement of basal ganglia in epileptic seizures: An hypothesis. Epileptic Disord. 2003, 5, 161–164. [Google Scholar]
- Spencer, S.S. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia 2002, 43, 219–227. [Google Scholar] [CrossRef]
- Briellmann, R.S.; Jackson, G.D.; Pell, G.S.; Mitchell, L.A.; Abbott, D.F. Structural abnormalities remote from the seizure focus: A study using T2 relaxometry at 3 T. Neurology 2004, 63, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Covolan, L.; Mello, L.E. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000, 39, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Holmes, G.L. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006, 5, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bouilleret, V.; Semah, F.; Chassoux, F.; Mantzaridez, M.; Biraben, A.; Trebossen, R.; Ribeiro, M.J. Basal ganglia involvement in temporal lobe epilepsy: A functional and morphologic study. Neurology 2008, 70, 177–184. [Google Scholar] [CrossRef]
- Bekiesinska-Figatowska, M.; Mierzewska, H.; Jurkiewicz, E. Basal ganglia lesions in children and adults. Eur. J. Radiol. 2013, 82, 837–849. [Google Scholar] [CrossRef]
- Graybiel, A.M. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990, 13, 244–254. [Google Scholar] [CrossRef]
- Delgado, J.M.R. Inhibitory functions in the neostriatum. In The Neostriatum; Divac, I., Oberg, R.G.E., Eds.; Pergamon Press: London, UK, 1979; pp. 241–261. [Google Scholar] [CrossRef]
- Alexander, G.E.; DeLong, M.R. Microstimulation of the primate neostriatum. I. Physiological properties of striatal microexcitable zones. J. Neurophysiol. 1985, 53, 1401–1416. [Google Scholar] [CrossRef]
- Alheid, G.F.; Heimer, L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 1988, 27, 1–39. [Google Scholar] [CrossRef]
- Gerfen, C.R. The neostriatal mosaic: Multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 1992, 15, 285–320. [Google Scholar] [CrossRef]
- Haber, S.N. The primate basal ganglia: Parallel and integrative networks. J. Chem. Neuroanat. 2003, 26, 317–330. [Google Scholar] [CrossRef]
- Druga, R. Neocortical inhibitory system. Folia Biol. 2009, 55, 201–217. [Google Scholar]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res. Rev. 1995, 20, 128–154. [Google Scholar] [CrossRef]
- McFarland, N.R.; Haber, S.N. Convergent inputs from thalamic motor nuclei and frontal cortical areas to the dorsal striatum in the primate. J. Neurosci. 2000, 20, 3798–3813. [Google Scholar] [CrossRef] [PubMed]
- Heimer, L.; Switzer, R.D.; Van Hoesen, G.W. Ventral striatum and ventral pallidum: Components of the motor system? TINS 1982, 5, 83–87. [Google Scholar]
- Voorn, P.; Vanderschuren, L.J.; Groenewegen, H.J.; Robbins, T.W.; Pennartz, C.M. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004, 27, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Graybiel, A.M. The basal ganglia: Learning new tricks and loving it. Curr. Opin. Neurobiol. 2005, 15, 638–644. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Grafton, S.T. The striatum: Where skills and habits meet. Cold Spring Harb. Perspect. Biol. 2015, 7, a021691. [Google Scholar] [CrossRef]
- Parent, A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990, 13, 254–258. [Google Scholar] [CrossRef]
- Van der Werf, Y.D.; Witter, M.P.; Groenewegen, H.J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain research. Brain Res. Rev. 2002, 39, 107–140. [Google Scholar] [CrossRef]
- Tepper, J.M.; Wilson, C.J.; Koós, T. Feedforward and feedback inhibition in neostriatal GABAergic spiny neurons. Brain Res. Rev. 2008, 58, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Nauta, W.J.; Mehler, W.R. Projections of the lentiform nucleus in the monkey. Brain Res. 1966, 1, 3–42. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 1991, 44, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McDonald, A.J. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991, 44, 15–33. [Google Scholar] [CrossRef]
- Reep, R.L.; Cheatwood, J.L.; Corwin, J.V. The associative striatum: Organization of cortical projections to the dorsocentral striatum in rats. J. Comp. Neurol. 2003, 467, 271–292. [Google Scholar] [CrossRef]
- Wu, J.H.; Corwin, J.V.; Reep, R.L. Organization of the corticostriatal projection from rat medial agranular cortex to far dorsolateral striatum. Brain Res. 2009, 1280, 69–76. [Google Scholar] [CrossRef]
- Tepper, J.M.; Bolam, J.P. Functional diversity and specificity of neostriatal interneurons. Curr. Opin. Neurobiol. 2004, 14, 685–692. [Google Scholar] [CrossRef]
- Pitkänen, A.; Pikkarainen, M.; Nurminen, N.; Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Ann. N. Y. Acad. Sci. 2000, 911, 369–391. [Google Scholar] [CrossRef]
- Slaght, S.J.; Paz, T.; Mahon, S.; Maurice, N.; Charpier, S.; Deniau, J.M. Functional organization of the circuits connecting the cerebral cortex and the basal ganglia: Implications for the role of the basal ganglia in epilepsy. Epileptic Disord. 2002, 4, S9–S22. [Google Scholar] [CrossRef]
- Löscher, W.; Schirmer, M.; Freichel, C.; Gernert, M. Distribution of GABAergic neurons in the striatum of amygdala-kindled rats: An immunohistochemical and in situ hybridization study. Brain Res. 2006, 1083, 50–60. [Google Scholar] [CrossRef]
- Haber, S.N.; Calzavara, R. The cortico-basal ganglia integrative network: The role of the thalamus. Brain Res. Bull. 2009, 78, 69–74. [Google Scholar] [CrossRef]
- Kincaid, A.E.; Wilson, C.J. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J. Comp. Neurol. 1996, 374, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Graybiel, A.M.; Ragsdale, C.W. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc. Natl. Acad. Sci. USA 1978, 75, 5723–5726. [Google Scholar] [CrossRef] [PubMed]
- Brimblecombe, K.R.; Cragg, S.J. The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem. Neurosci. 2017, 8, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Grofová, I. The identification of striatal and pallidal neurons projecting to substantia nigra. An experimental study by means of retrograde axonal transport of horseradish peroxidase. Brain Res. 1975, 91, 286–291. [Google Scholar] [CrossRef]
- Leontovich, T.A. Neuronal structure of striatopallidum in mammals. J. Nevropatol. Psychiatry 1954, 65, 168–179. [Google Scholar]
- DiFiglia, M.; Aronin, N. Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus. J. Neurosci. 1982, 2, 1267–1274. [Google Scholar] [CrossRef]
- Tepper, J.M.; Sharpe, N.A.; Koós, T.Z.; Trent, F. Postnatal development of the rat neostriatum: Electrophysiological, light- and electron-microscopic studies. Dev. Neurosci. 1998, 20, 125–145. [Google Scholar] [CrossRef]
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef]
- Buckmaster, P.S. Laboratory animal models of temporal lobe epilepsy. Comp. Med. 2004, 54, 473–485. [Google Scholar]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Lévesque, M.; Avoli, M.; Bernard, C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J. Neurosci. Methods 2016, 260, 45–52. [Google Scholar] [CrossRef]
- Garant, D.S.; Gale, K. Lesions of substantia nigra protect against experimentally induced seizures. Brain Res. 1983, 273, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, E.M. Acute and chronic effects of seizures in the developing brain: Lessons from clinical experience. Epilepsia 1999, 40, S42–S66. [Google Scholar] [CrossRef] [PubMed]
- Deransart, C.; Depaulis, A. The control of seizures by the basal ganglia? A review of experimental data. Epileptic Disord. 2002, 4, S61–S72. [Google Scholar]
- Avchalumov, Y.; Kirschstein, T.; Köhling, R. Altered physiology and pharmacology in the corticostriatal system in a model of temporal lobe epilepsy. Epilepsia 2011, 52, 151–157. [Google Scholar] [CrossRef][Green Version]
- Rektor, I.; Kuba, R.; Brázdil, M.; Chrastina, J. Do the basal ganglia inhibit seizure activity in temporal lobe epilepsy? Epilepsy Behav. 2012, 25, 56–59. [Google Scholar] [CrossRef]
- Scott, R.C. What are the effects of prolonged seizures in the brain? Epileptic Disord. 2014, 16, S6–S11. [Google Scholar] [CrossRef]
- Bernasconi, N.; Bernasconi, A.; Caramanos, Z.; Antel, S.B.; Andermann, F.; Arnold, D.L. Mesial temporal damage in temporal lobe epilepsy: A volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 2003, 126 Pt 2, 462–469. [Google Scholar] [CrossRef]
- Goffin, K.; Van Paesschen, W.; Dupont, P.; Van Laere, K. Longitudinal microPET imaging of brain glucose metabolism in rat lithium-pilocarpine model of epilepsy. Exp. Neurol. 2009, 217, 205–209. [Google Scholar] [CrossRef]
- Mraovitch, S.; Calando, Y. Interactions between limbic, thalamo-striatal-cortical, and central autonomic pathways during epileptic seizure progression. J. Comp. Neurol. 1999, 411, 145–161. [Google Scholar] [CrossRef]
- Lee, J.; Park, K.; Lee, S.; Whang, K.; Kang, M.; Park, C.; Huh, Y. Differential changes of calcium binding proteins in the rat striatum after kainic acid-induced seizure. Neurosci. Lett. 2002, 333, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Szyndler, J.; Maciejak, P.; Turzyńska, D.; Sobolewska, A.; Taracha, E.; Skórzewska, A.; Lehner, M.; Bidziński, A.; Hamed, A.; Wisłowska-Stanek, A.; et al. Mapping of c-Fos expression in the rat brain during the evolution of pentylenetetrazol-kindled seizures. Epilepsy Behav. 2009, 16, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Shimizu, S.; Harada, Y.; Morishita, M.; Ishihara, S.; Kumafuji, K.; Sasa, M.; Serikawa, T. Regional expression of Fos-like immunoreactivity following seizures in Noda epileptic rat (NER). Epilepsy Res. 2009, 87, 70–76. [Google Scholar] [CrossRef]
- Yu, W.; Calos, M.; Pilitsis, J.; Shin, D.S. Deconstructing the neural and ionic involvement of seizure-like events in the striatal network. Neurobiol. Dis. 2013, 52, 128–136. [Google Scholar] [CrossRef]
- Nascimento, V.S.; Oliveira, A.A.; Freitas, R.M.; Sousa, F.C.; Vasconcelos, S.M.; Viana, G.S.; Fonteles, M.M. Pilocarpine-induced status epilepticus: Monoamine level, muscarinic and dopaminergic receptors alterations in striatum of young rats. Neurosci. Lett. 2005, 383, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Moeller, F.; Siebner, H.R.; Wolff, S.; Muhle, H.; Boor, R.; Granert, O.; Jansen, O.; Stephani, U.; Siniatchkin, M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. NeuroImage 2008, 39, 1839–1849. [Google Scholar] [CrossRef]
- Velísková, J.; Claudio, O.I.; Galanopoulou, A.S.; Kyrozis, A.; Lado, F.A.; Ravizza, T.; Velísek, L.; Moshé, S.L. Developmental aspects of the basal ganglia and therapeutic perspectives. Epileptic Disord. 2002, 4, S73–S82. [Google Scholar] [CrossRef]
- Cavalheiro, E.A.; Leite, J.P.; Bortolotto, Z.A.; Turski, W.A.; Ikonomidou, C.; Turski, L. Long-term effects of pilocarpine in rats: Structural damage of the brain triggers kindling and spontaneous recurrent seizures. Epilepsia 1991, 32, 778–782. [Google Scholar] [CrossRef]
- Cavalheiro, E.A. The pilocarpine model of epilepsy. Ital. J. Neurol. Sci. 1995, 16, 33–37. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Berendse, H.W.; Galis-de Graaf, Y.; Groenewegen, H.J. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 1992, 316, 314–347. [Google Scholar] [CrossRef] [PubMed]
- Druga, R.; Rokyta, R.; Benes, V., Jr. Thalamocaudate projections in the macaque monkey (a horseradish peroxidase study). J. Hirnforsch. 1991, 32, 765–774. [Google Scholar]
- Suchomelová, L.; Kubová, H.; Haugvicová, R.; Druga, R.; Mares, P. Are acute changes after status epilepticus in immature rats persistent? Physiol. Res. 2002, 51, 185–192. [Google Scholar]
- Hoover, W.B.; Vertes, R.P. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct. Funct. 2007, 212, 149–179. [Google Scholar] [CrossRef]
- Cheatwood, J.L.; Corwin, J.V.; Reep, R.L. Overlap and interdigitation of cortical and thalamic afferents to dorsocentral striatum in the rat. Brain Res. 2005, 1036, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, V.; Maurice, N.; Besson, M.J.; Thierry, A.M.; Deniau, J.M. Effect of a functional impairment of corticostriatal transmission on cortically evoked expression of c-Fos and zif 268 in the rat basal ganglia. Neuroscience 1999, 93, 1313–1321. [Google Scholar] [CrossRef]
- Holopainen, I.E. Seizures in the developing brain: Cellular and molecular mechanisms of neuronal damage, neurogenesis and cellular reorganization. Neurochem. Int. 2008, 52, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, Z.S.; Alloway, K.D. Organization of corticostriatal projections from the vibrissal representations in the primary motor and somatosensory cortical areas of rodents. J. Comp. Neurol. 2001, 439, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Schilman, E.A.; Uylings, H.B.; Galis-de Graaf, Y.; Joel, D.; Groenewegen, H.J. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci. Lett. 2008, 432, 40–45. [Google Scholar] [CrossRef]
- Kamishina, H.; Yurcisin, G.H.; Corwin, J.V.; Reep, R.L. Striatal projections from the rat lateral posterior thalamic nucleus. Brain Res. 2008, 1204, 24–39. [Google Scholar] [CrossRef]
- Brown, L.L.; Smith, D.M.; Goldbloom, L.M. Organizing principles of cortical integration in the rat neostriatum: Corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J. Comp. Neurol. 1998, 392, 468–488. [Google Scholar]
- Turski, W.A.; Cavalheiro, E.A.; Bortolotto, Z.A.; Mello, L.M.; Schwarz, M.; Turski, L. Seizures produced by pilocarpine in mice: A behavioral, electroencephalographic and morphological analysis. Brain Res. 1984, 321, 237–253. [Google Scholar] [CrossRef]
- Naito, A.; Kita, H. The cortico-nigral projection in the rat: An anterograde tracing study with biotinylated dextran amine. Brain Res. 1994, 637, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Côté, P.Y.; Sadikot, A.F.; Parent, A. Complementary Distribution of Calbindin D-28k and Parvalbumin in the Basal Forebrain and Midbrain of the Squirrel Monkey. Eur. J. Neurosci. 1991, 3, 1316–1329. [Google Scholar] [CrossRef]
- Ichinohe, N.; Teng, B.; Kitai, S.T. Morphological study of the tegmental pedunculopontine nucleus, substantia nigra and subthalamic nucleus, and their interconnections in rat organotypic culture. Anat. Embryol. 2000, 201, 435–453. [Google Scholar] [CrossRef]
- Al-Redouan, A.; Salaj, M.; Kubova, H.; Druga, R. Compartmental neuronal degeneration in the ventral striatum induced by status epilepticus in young rats’ brain in comparison with adults. Int. J. Dev. Neurosci. 2024, 84, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade: Novel fluorochromes for detecting toxicant-induced neuronal degeneration. Toxicol. Pathol. 2000, 28, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Festing, M.F. Design and statistical methods in studies using animal models of development. ILAR J. 2006, 47, 5–14. [Google Scholar] [CrossRef]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharm. Pharmacol. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
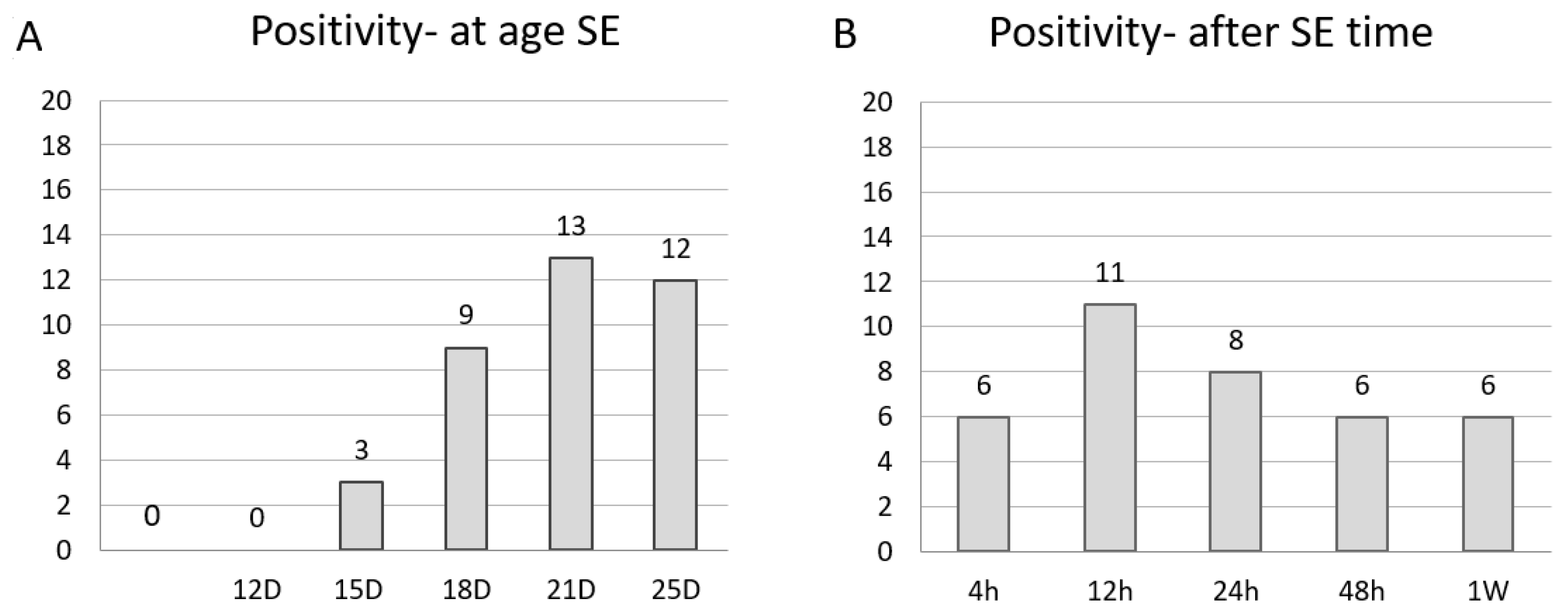
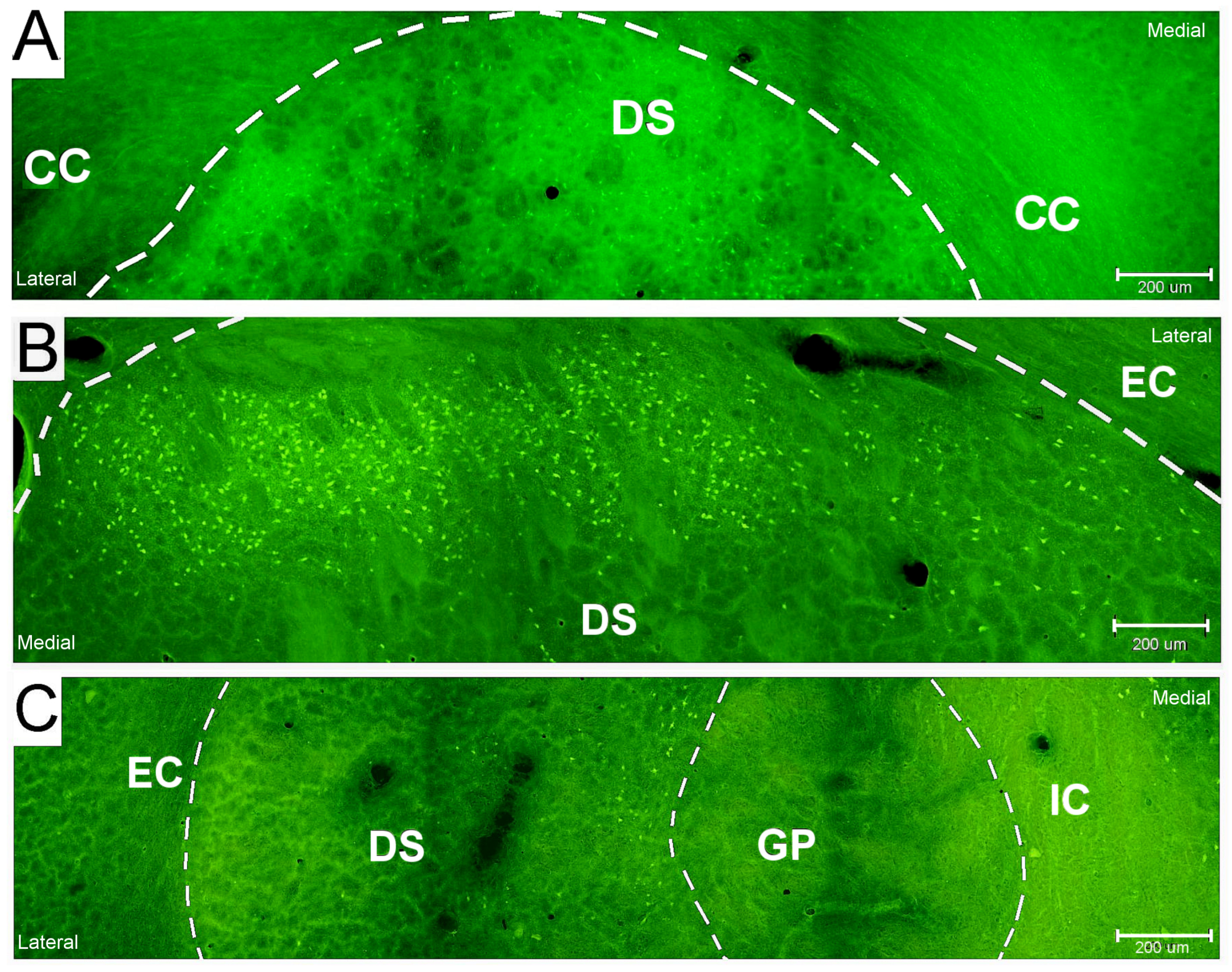
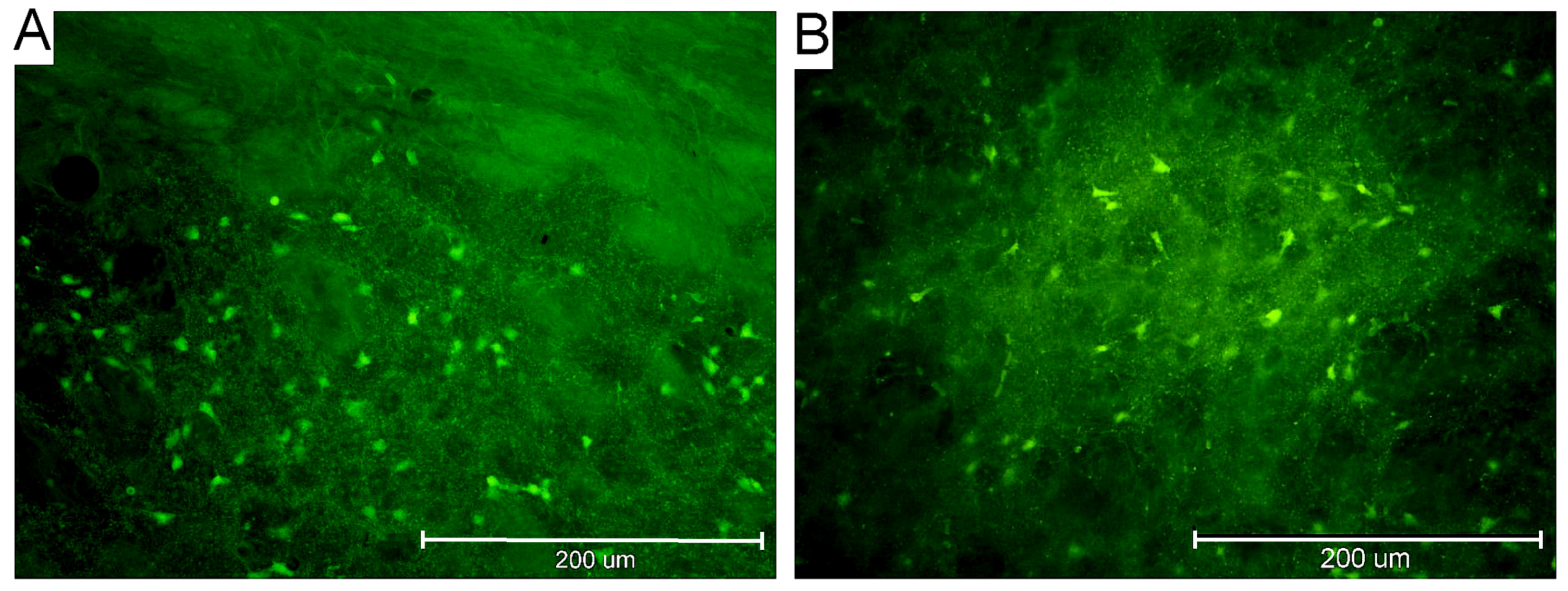
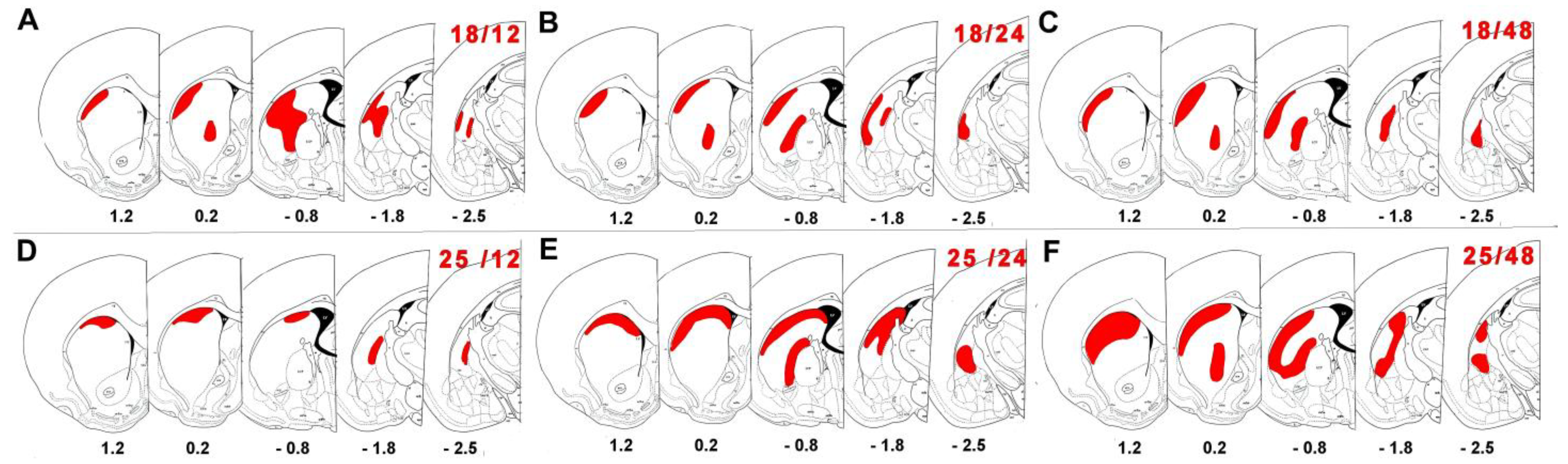
| Age at SE | 12 D | 15 D | 18 D | 21 D | 25 D | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal Ganglia | DS | GP | EP | DS | GP | EP | DS | GP | EP | DS | GP | EP | DS | GP | EP |
| Survival Interval | |||||||||||||||
| 4 h | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| 12 h | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| 24 h | Negative | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| 48 h | Negative | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| 1 W | Negative | Negative | Negative | Negative | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative | Positive | Negative | Negative |
| Age/Survival Interval | 18/12 | 18/24 | 21/12 | 21/24 | 25/12 | 25/24 | p-Value |
|---|---|---|---|---|---|---|---|
| Perimeter (µm) | 20 | 26 | 16 | 17 | 13 | 17 | |
| SD | ±3.28 | ±3.22 | ±3.00 | ±3.00 | ±3.30 | ±2.79 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Redouan, A.; Busch, A.; Salaj, M.; Kubova, H.; Druga, R. Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2025, 26, 3349. https://doi.org/10.3390/ijms26073349
Al-Redouan A, Busch A, Salaj M, Kubova H, Druga R. Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy. International Journal of Molecular Sciences. 2025; 26(7):3349. https://doi.org/10.3390/ijms26073349
Chicago/Turabian StyleAl-Redouan, Azzat, Aaron Busch, Martin Salaj, Hana Kubova, and Rastislav Druga. 2025. "Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy" International Journal of Molecular Sciences 26, no. 7: 3349. https://doi.org/10.3390/ijms26073349
APA StyleAl-Redouan, A., Busch, A., Salaj, M., Kubova, H., & Druga, R. (2025). Dorsal Striatum Is Compromised by Status Epilepticus Induced in Immature Developing Animal Experimental Model of Mesial Temporal Lobe Epilepsy. International Journal of Molecular Sciences, 26(7), 3349. https://doi.org/10.3390/ijms26073349






