Advances in Leaf Plant Bioactive Compounds: Modulation of Chronic Inflammation Related to Obesity
Abstract
:1. Introduction
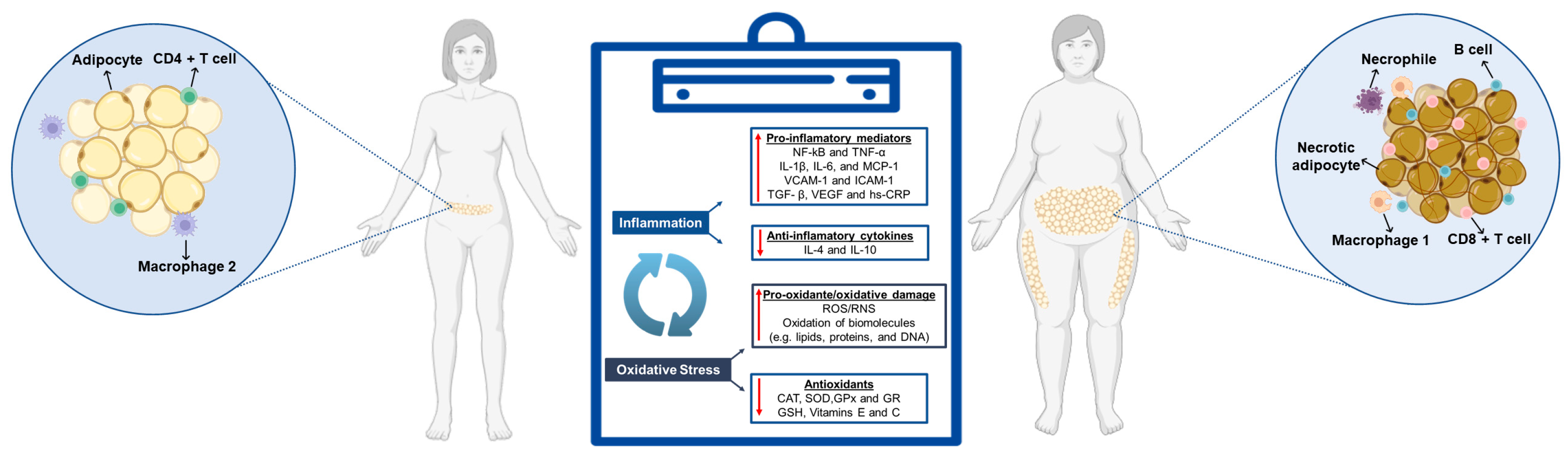
2. Chronic Inflammation and Oxidative Stress Associated with Obesity
2.1. Inflammatory Biomarkers: Mechanisms and Health Impacts
2.1.1. Tumor Necrosis Factor-Alpha (TNF-α)
2.1.2. Interleukin 6 (IL-6)
2.1.3. C-Reactive Protein (CRP)
3. Importance of Dietary Interventions in Managing Chronic Inflammation/Oxidative Stress Related to Excessive Weight and Obesity
| References | Objective/Methods | Target Audience | Study Type | Results |
| [45] | To evaluate the associations between dietary patterns and one or more inflammatory biomarkers (CRP, TNF-α, and IL-6). |
| Meta-Analyses (PRISMA). | Healthy, Mediterranean, and anti-inflammatory diets are most frequently associated with lower levels of inflammation (reduction in CRP, TNF-α, and IL-6). |
| [48] | To characterize the postprandial response of 5 commonly assessed inflammation markers after ingesting a high-fat meal (HFM) in healthy adults. |
| Meta-Analyses and cohort. | Only 1 of these 5 markers, IL-6, consistently increases in the post-HFM period of 4 to 8 h. Specifically, IL-6 will start on average, at a baseline of approximately 1.4 pg/mL and peak at approximately 2.9 pg/mL about 6 h later. In relative terms, IL-6 will increase by approximately 100% in response to an HFM. As for CRP and TNF-α, these rarely show any change. IL-8 and IL-1β also rarely change after HFM consumption in healthy individuals. |
| [52] | To evaluate the association of a diet high in SSB and low in fruits and vegetables with adiposity and a pro-inflammatory adipokine profile. | Mexican-American participants ascendent, with and without a diagnosis of gestational diabetes mellitus (GDM) within 5 years. | Fenland Study. | A diet high in SSB and low in fruits and vegetables may be associated with increased adiposity and a pro-inflammatory adipokine profile characterized by higher leptin, CRP, and MCP-1, and lower anti-inflammatory secreted frizzled-related protein 5 (SFRP-5) in Mexican Americans compared to a diet low in SSB and high in fruits and vegetables. |
| [49] | To evaluate the increase in plasma uric acid levels in overweight and obese individuals consuming sucrose-sweetened sodas for 6 months | 60 eligible participants with overweight and obesity without diabetes. | Meta-Analyses and Randomized Controlled Trials (RCT). | Daily 1 L of sugar-sweetened soft drinks (regular cola/sucrose-sweetened soda) for 6 months increases circulating UA levels. Additionally, it has been demonstrated that changes in plasma UA after the intervention significantly correlate with changes in liver fat levels, triglycerides, and insulin. |
| [50] | To analyze the cross-sectional association between SSB intake and cardiometabolic biomarkers in American women. | 121,700 registered nurses aged between 30 and 55 years, free of diabetes and cardiovascular disease (CVD). | Fenland Study. | The intake of SSB was marginally associated with higher concentrations of CRP and adiponectin (suggesting liver function, lipid metabolism, inflammation, and glucose metabolism as possible pathways). The associations between artificially sweetened beverages (ASB) and fruit juice with cardiometabolic markers were less consistent. |
| [53] | To examine the association of sugars from different sources [beverages (liquids), foods (solids), extrinsic (free) or intrinsic (non-free)] with metabolic and inflammatory markers. | 12,434 individuals born between 1950 and 1975, recruited from general practitioner lists in Cambridgeshire and surrounding areas in the East of England, UK, enrolled between 2005 and 2015. | Fenland Study. | Higher intakes of sugars from non-alcoholic beverages and sugar added to tea, coffee, or cereals were associated with increased blood glucose and CRP. |
| [51] |
| 6856 American adults from the National Health and Nutrition Examination Survey (NHANES) 2007–2010. The average SSB consumption was calculated from 2-day and 24-h dietary recalls. | Fenland Study. | The intake of SSB is positively associated with CRP levels. Obesity may amplify CRP levels in individuals with moderate to high SSB consumption. |
| [39] | To investigate the effect of increased dietary intake of alpha-linolenic acid (ALA) on blood concentration of inflammatory markers, including TNF-α, IL-6, CRP, soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1). | Men and women, adults, both with and without obesity. | Meta-Analyses. | The study found no beneficial effect of ALA supplementation in reducing inflammatory markers, including TNF, IL-6, CRP, sICAM-1, and sVCAM-1. However, in healthy individuals, ALA supplementation may increase CRP concentration. |
| [46] | To evaluate the association of vegan and vegetarian diets with inflammatory biomarkers. | Adult individuals with different dietary habits, ranging from apparently healthy vegans, vegetarians, and omnivores to those with metabolic issues. | Meta-Analyses (PRISMA) included cross-sectional studies, prospective cohort studies, and RCT. | The vegan and vegetarian diets are associated with lower CRP concentrations compared to apparently healthy omnivores and others with metabolic issues. |
| [54] | To examine the associations of dietary macronutrient composition in early childhood with growth and detailed measures of body composition up to 9 years of age. | 3564 Dutch children aged 1 to 9 years. | Cohort Study. | Higher intake of total and animal protein (dairy and non-dairy) is associated with greater height, weight, and BMI up to 9 years of age. The positive association with BMI was fully explained by a higher fat mass index (FMI) and not by the fat-free mass index (FFMI). |
| [47] | To determine changes in body composition and cardiometabolic and inflammatory status of obese participants after 24 weeks of a dietary intervention based on an anti-inflammatory diet with energy reduction and examine the relationship of these changes with changes in the inflammatory potential of the diet. | In the past three months, adult male and female patients from the Clinical Hospital Center Rijeka, Croatia, with BMI ≥ 30 kg/m2, with or without obesity-related complications, and stable body weight. | RCT Study | An anti-inflammatory diet with energy restriction is effective in managing obesity (resulting in high reductions of TNF-α and low levels of hs-CRP and IL-6). Significant reductions in body weight, BMI, total and visceral adipose tissue, and improvements in body composition, cardiometabolic parameters, and inflammatory markers were observed. |
4. Bioactive Compounds in Leafy Vegetables
| Class of Compounds | Annona cherimola Mill. | Ipomoea batata (L.) Poir. | Colocasia esculenta (L.) Schott | Eriobotrya japonica | Cymbopogon citratus | Psidium guajava (L.) | Smallanthus sonchifolius | |
|---|---|---|---|---|---|---|---|---|
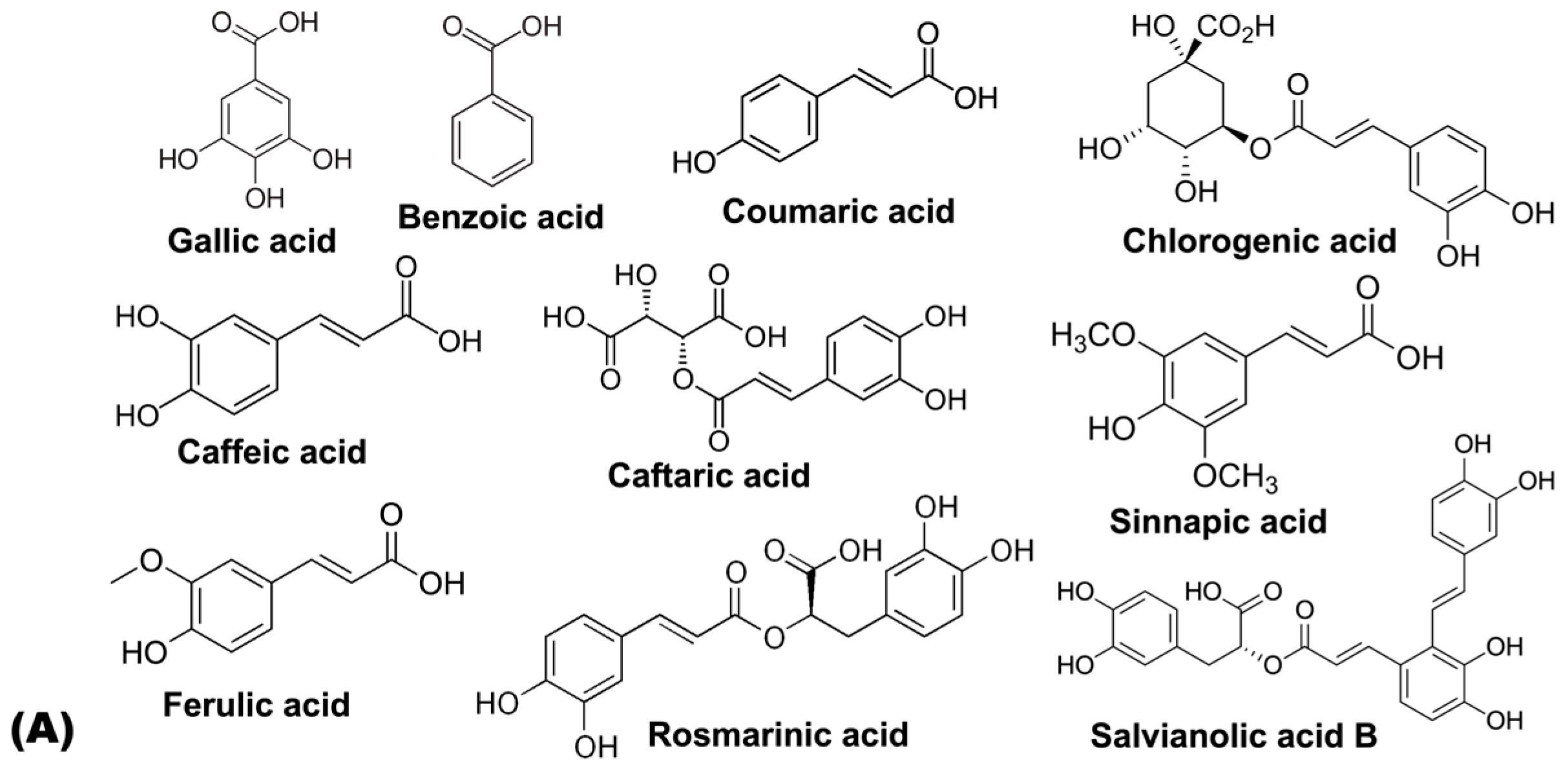
| Hydroxybenzoic acids | Gallic acid | [59] | [60] | [61] | ||||
| Benzoic acid | [59] | |||||||
| Hydroxycinnamic acids | Neochlorogenic acid | [62,63] | [64] | [63] | ||||
| Cryptochlorogenic acid | [62] | |||||||
| p-Coumaric acid | [65] | [63] | [63,64] | [61] | ||||
| 3-O-Coumaroylquinic acid | [62] | |||||||
| Chlorogenic acid | [63] | [63] | [62,63] | [63,64] | [63] | [63,66] | ||
| Caffeic acid hexoside I | [67] | |||||||
| Caffeic acid glucoside | [62] | |||||||
| Caffeic acid | [67] | [65] | [62,63] | [59,63] | [61,63,65] | |||
| Caffeic acid derivative | [62] | |||||||
| 1-caffeoylquinic acid | [67] | [66] | ||||||
| Dicaffeoylquinic acid | [62] | |||||||
| 3-caffeoylquinic acid | [67,68] | |||||||
| 4-caffeoylquinic acid | [67,68] | |||||||
| 3,4-di-O-caffeoylqunic acid | [63,67,68] | [63] | ||||||
| 3,5-di-O-caffeoylqunic acid | [63,67,68] | [66] | ||||||
| 4,5-di-O-caffeoylqunic acid | [67,68] | [66] | ||||||
| 3,4,5-tricaffeoylquinic acid. | [67,68] | |||||||
| 5-O-caffeoylquinic acid | [65] | [66] | ||||||
| Feruloylquinic acid | [62] | [64] | ||||||
| Caftaric acid | [62] | |||||||
| Sinapic acid | [62] | [59] | ||||||
| Ferulic acid | [63] | [62] | [63] | [61,63] | ||||
| Isoferulic acid | [63] | [63] | ||||||
| Rosmarinic acid | [62] | |||||||
| Salvianolic acid B | [62] | |||||||
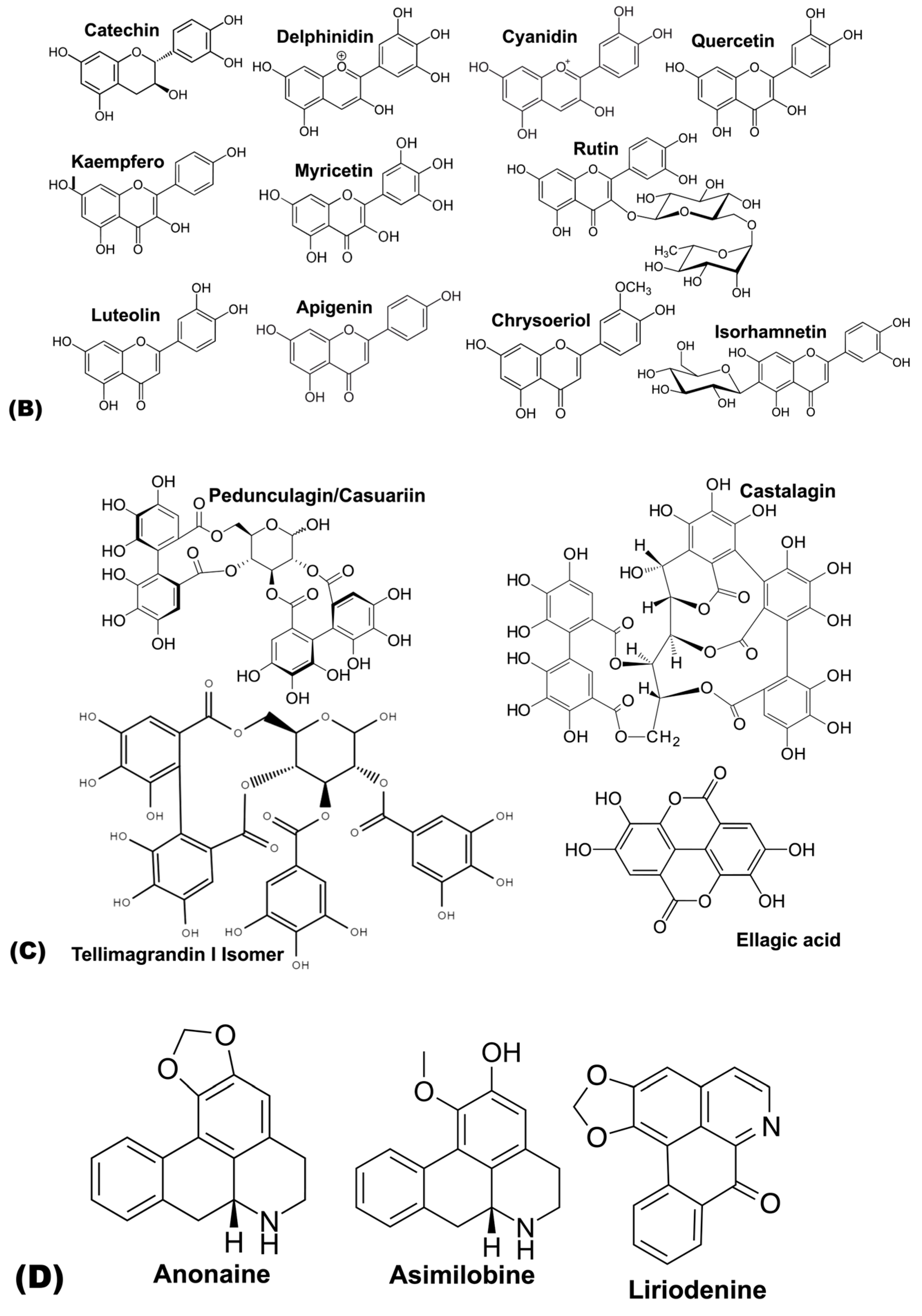
| Flavanol | Catechin | [69] | [63] | [60] | ||||
| Prodelphinidin B Isomer | [60] | |||||||
| Procyanidin B Isomer | [60] | |||||||
| Procyanidin tetramer | [60] | |||||||
| Procyanidin pentamer | [60] | |||||||
| Procyanidin trimer Isomer | [60] | |||||||
| Gallocatechin | [60] | |||||||
| Galloyl-(epi)catechin trimer Isomer | [60] | |||||||
| Procyanidin gallate Isomer | [60] | |||||||
| Galloyl(epi)catechin-(epi)gallocatechin | [60] | |||||||
| Prodelphinidin Dimer Isomer | [60] | |||||||
| Epicatechin | [69] | |||||||
| Flavonols | Quercetin | [59] | [61] | |||||
| Quercetin-3-O-rutinoside | [63] | [63] | [63] | [63] | [63] | [63] | ||
| quercetin 3-O-galactoside | [67,68] | |||||||
| Quercetin 3-O-rutinoside-7-O-glucoside | [69] | |||||||
| Quercetin 3-O-rutinoside-7-O-pentoside | [69] | |||||||
| Quercetin-3-O-glucoside | [63,69] | [63] | [63] | [62,63] | [63] | [63] | [63] | |
| Quercetin-3-O-rhamnoside | [63,69] | [63] | [63] | [63] | ||||
| Quercetin galloylhexoside Isomer | [60] | |||||||
| kaempferol | [59] | [61] | ||||||
| Kaempferol-3-O-glucoside | [69] | [68] | [62] | |||||
| Kaempferol 3-O-rutinoside | [62] | |||||||
| Quercetin-3-O-hexoside | [67,68] | |||||||
| Quercetin-3-O-hexosylhexoside | [67] | |||||||
| Quercetin 3-O-malonylglucoside | [62] | |||||||
| Myricetin | [59] | [61] | ||||||
| Myricetin hexoside Isomer | [60] | |||||||
| Myricetin arabinoside/xylopyranoside Isomer | [60] | |||||||
| Isoschaftoside | [64] | |||||||
| Rutin | [61,66] | |||||||
| Isorhamnetin | [63] | [63] | ||||||
| Flavones | Luteolin-3′,7-di-O-glucoside | [65] | ||||||
| Luteolin-6-C-glucoside | [65] | |||||||
| Luteolin-7-O-glucoside | [63] | [68] | [62] | [63] | [63] | [63] | ||
| Luteolin-8-C-glucoside | [65] | |||||||
| Luteolin-4-O-glucoside | [63] | [63] | [63] | [63] | ||||
| Luteolin 7-O-neohesperidoside | [64] | |||||||
| Luteolin | [69] | [63] | [62,63] | [63] | [63] | [63] | ||
| Luteolin-3-Galactoside-7-Rhamnoside | [69] | |||||||
| Luteolin-3-Glucoside-7-Rhamnoside | [69] | |||||||
| Apigenin | [63] | |||||||
| Apigenin hexoside | [62] | |||||||
| Apigenin derivative isomer 1 | [63] | |||||||
| Apigenin derivative isomer 2 | [63] | |||||||
| Apigenin derivative isomer 3 | [63] | |||||||
| Apigenin derivative isomer 4 | [63] | |||||||
| Isoorientin | [64] | |||||||
| Isoorientin 2-O-rhamnoside | [64] | |||||||
| Chrysoeriol | [65] | [62] | ||||||
| Chrysoeriol rutinoside | [62] | |||||||
| 7-O-malonylglucoside | [62] | |||||||
| Apigenin acetylhexoside | [62] | |||||||
| Apigenin 6-C-glucoside | [65] | |||||||
| Apigenin-6-C-glucoside-7-O-glucoside | [65] | |||||||
| Apigenin 8-C-glucoside | [69] | [65] | ||||||
| Hydrolysable tannins | HHDP glucose Isomer | [60] | ||||||
| Pedunculagin/Casuariin Isomer | [60] | |||||||
| Vescalagin/Castalagin Isomer | [60] | |||||||
| Tellimagrandin I Isomer | [60] | |||||||
| Tellimagrandin I Isomer | [60] | |||||||
| Vescalagin | [60] | |||||||
| Ellagic acid deoxyhexoside | [60] | |||||||
| Casuarinin/Casuarictin Isomer | [60] | |||||||
| Alkaloids | Anonaine | [69] | ||||||
| Asimilobine | [69] | |||||||
| Liriodenine | [69] | |||||||
| Stepharine | [69] | |||||||
| Lanuginosine | [69] | |||||||
| Pronuciferine | [69] |
5. Anti-Inflammatory and Anti-Obesity Properties of Bioactive Compounds Identified in the Leaves of Plants Under Study
5.1. Hydroxybenzoic Acids
5.2. Hydroxycinnamic Acids
5.3. Flavanols
5.4. Flavones
5.5. Flavonols
5.6. Hydrolysable Tannins
6. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell Death and Inflammation during Obesity: “Know My Methods, WAT(Son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Brunilda Nazario WebMD. Available online: https://www.webmd.com/obesity/causes-of-obesity (accessed on 3 December 2024).
- Gonçalves, J.T.T.; Silveira, M.F.; Campos, M.C.C.; Costa, L.H.R. Sobrepeso e Obesidade e Fatores Associados Ao Climatério. Cienc. Saude Coletiva 2016, 21, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Preuss, H.G.; Swaroop, A. Nutraceuticals and Functional Foods in Human Health and Disease Prevention; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pereira, M.d.S. Efeito de Um Programa de Exercício Na Aptidão Física de Indivíduos Obesos Sujeitos a Cirurgia Bariátrica; Universidade de Évora: Évora, Portugal, 2023. [Google Scholar]
- Quintal, C.; Oliveira, J. Socioeconomic Inequalities in Child Obesity and Overweight in Portugal. Int. J. Soc. Econ. 2017, 44, 1377–1389. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística. Inquérito Nacional de Saúde; Instituto Nacional de Estatística: Lisbon, Portugal, 2020; pp. 1–13. [Google Scholar]
- NHS. Available online: https://www.nhs.uk/conditions/obesity/ (accessed on 3 December 2024).
- Peralta, M.; Ramos, M.; Lipert, A.; Martins, J.; Marques, A. Prevalence and Trends of Overweight and Obesity in Older Adults from 10 European Countries from 2005 to 2013. Scand. J. Public Health 2018, 46, 522–529. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 27 July 2024).
- Gentile, D.; Fornai, M.; Pellegrini, C.; Colucci, R.; Blandizzi, C.; Antonioli, L. Dietary Flavonoids as a Potential Intervention to Improve Redox Balance in Obesity and Related Co-Morbidities: A Review. Nutr. Res. Rev. 2018, 31, 239–247. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Ferreira, C.; Vieira, P.; Sá, H.; Malva, J.; Castelo-Branco, M.; Reis, F.; Viana, S. Polyphenols: Immunonutrients Tipping the Balance of Immunometabolism in Chronic Diseases. Front. Immunol. 2024, 15, 1360065. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Beal, T.; Morris, S.S.; Tumilowicz, A. Global Patterns of Adolescent Fruit, Vegetable, Carbonated Soft Drink, and Fast-Food Consumption: A Meta-Analysis of Global School-Based Student Health Surveys. Food Nutr. Bull. 2019, 40, 444–459. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & Inflammation: The Linking Mechanism & the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Samouda, H. Clinical and Biological Risk Factors Associated with Inflammation in Patients with Type 2 Diabetes Mellitus. BMC Endocr. Disord. 2022, 22, 16. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Monteiro, R.; Azevedo, I. Chronic Inflammation in Obesity and the Metabolic Syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar]
- Panic, A.; Stanimirovic, J.; Sudar-Milovanovic, E.; Isenovic, E.R. Oxidative Stress in Obesity and Insulin Resistance. Explor. Med. 2022, 3, 58–70. [Google Scholar]
- Polska, E. PRACE ORYGINALNE/ORIGINAL PAPERS Changes in Inflammatory Biomarkers after Successful Lifestyle Intervention in Obese Children. Endokrynol. Pol. 2011, 62, 499–505. [Google Scholar]
- Tuomisto, K.; Jousilahti, P.; Havulinna, A.S.; Borodulin, K.; Männistö, S.; Salomaa, V. Role of Inflammation Markers in the Prediction of Weight Gain and Development of Obesity in Adults—A Prospective Study. Metabol. Open 2019, 3, 100016. [Google Scholar] [CrossRef]
- Michicotl-Meneses, M.M.; Thompson-Bonilla, M.D.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation Markers in Adipose Tissue and Cardiovascular Risk Reduction by Pomegranate Juice in Obesity Induced by a Hypercaloric Diet in Wistar Rats. Nutrients 2021, 13, 2577. [Google Scholar] [CrossRef]
- Peña, A.; Olson, M.L.; Ayers, S.L.; Sears, D.D.; Vega-López, S.; Colburn, A.T.; Shaibi, G.Q. Inflammatory Mediators and Type 2 Diabetes Risk Factors before and in Response to Lifestyle Intervention among Latino Adolescents with Obesity. Nutrients 2023, 15, 2442. [Google Scholar] [CrossRef]
- Patsalos, O.; Dalton, B.; Leppanen, J.; Ibrahim, M.A.A.; Himmerich, H. Impact of TNF-a Inhibitors on Body Weight and BMI: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 481. [Google Scholar] [CrossRef]
- Alzamil, H. Elevated Serum TNF- α Is Related to Obesity in Type 2 Diabetes Mellitus and Is Associated with Glycemic Control and Insulin Resistance. J. Obes. 2020, 2020, 5076858. [Google Scholar] [CrossRef]
- Kosovski, I.B.; Bacârea, V.; Ghiga, D.; Ciurea, C.N.; Cucoranu, D.C.; Hutanu, A.; Bacârea, A. Exploring the Link between Inflammatory Biomarkers and Adipometrics in Healthy Young Adults Aged 20–35 Years. Nutrients 2024, 16, 257. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, J.Y.; Yu, R. Relationship of Obesity and Visceral Adiposity with Serum Concentrations of CRP, TNF-α and IL-6. Diabetes Res. Clin. Pract. 2005, 69, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kern, L.; Mittenbühler, M.J.; Vesting, A.J.; Ostermann, A.L.; Wunderlich, C.M.; Wunderlich, F.T. Obesity-Induced TNFα and IL-6 Signaling: The Missing Link between Obesity and Inflammation- Driven Liver and Colorectal Cancers. Cancers 2019, 11, 24. [Google Scholar] [CrossRef]
- Tzanavari, T.; Giannogonas, P.; Karalis, K.P. TNF-α and Obesity. Curr. Dir. Autoimmun. 2010, 11, 145–156. [Google Scholar]
- Teixeira, B.C.; Lopes, A.L.; Macedo, R.C.O.; Correa, C.S.; Ramis, T.R.; Ribeiro, J.L.; Reischak-Oliveira, A. Marcadores Inflamatórios, Função Endotelial e Riscos Cardiovasculares. J. Vasc. Bras. 2014, 13, 108–115. [Google Scholar] [CrossRef]
- Sindhu, S.; Thomas, R.; Shihab, P.; Sriraman, D.; Behbehani, K.; Ahmad, R. Obesity Is a Positive Modulator of IL-6R and IL-6 Expression in the Subcutaneous Adipose Tissue: Significance for Metabolic Inflammation. PLoS ONE 2015, 10, e0133494. [Google Scholar] [CrossRef]
- Wueest, S.; Konrad, D. The Controversial Role of IL-6 in Adipose Tissue on Obesity-Induced Dysregulation of Glucose Metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E607–E613. [Google Scholar] [CrossRef]
- El-Mikkawy, D.M.E.; EL-Sadek, M.A.; EL-Badawy, M.A.; Samaha, D. Circulating Level of Interleukin-6 in Relation to Body Mass Indices and Lipid Profile in Egyptian Adults with Overweight and Obesity. Egypt. Rheumatol. Rehabil. 2020, 47, 7. [Google Scholar] [CrossRef]
- Bhadra Reddy, C.; Preeti Nagkumar, K.; Hima Bindu, M.; Mallikarjuna Reddy, C. Association between Obesity and c Reactive Protein among Patients with Risk Factors for Cardiovascular Disease. IP Indian J. Immunol. Respir. Med. 2021, 6, 121–124. [Google Scholar] [CrossRef]
- Verma, S.; Bhatta, M.; Davies, M.; Deanfield, J.E.; Garvey, W.T.; Jensen, C.; Kandler, K.; Kushner, R.F.; Rubino, D.M.; Kosiborod, M.N. Effects of Once-Weekly Semaglutide 2.4 Mg on C-Reactive Protein in Adults with Overweight or Obesity (STEP 1, 2, and 3): Exploratory Analyses of Three Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trials. EClinicalMedicine 2023, 55, 101737. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Jin, Q.; Wang, X. Effect of Dietary Alpha-Linolenic Acid on Blood Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2018, 57, 877–891. [Google Scholar] [CrossRef]
- Rojano-Rodríguez, M.E.; Valenzuela-Salazar, C.; Cárdenas-Lailson, L.E.; Romero Loera, L.S.; Torres-Olalde, M.; Moreno-Portillo, M. C-Reactive Protein Level in Morbidly Obese Patients before and after Bariatric Surgery. Revista de Gastroenterología de México (English Edition) 2014, 79, 90–95. [Google Scholar] [CrossRef]
- Grosso, G.; Laudisio, D.; Frias-Toral, E.; Barrea, L.; Muscogiuri, G.; Savastano, S.; Colao, A. Anti-Inflammatory Nutrients and Obesity-Associated Metabolic-Inflammation: State of the Art and Future Direction. Nutrients 2022, 14, 1137. [Google Scholar] [CrossRef]
- Wagenaar, C.A.; van de Put, M.; Bisschops, M.; Walrabenstein, W.; de Jonge, C.S.; Herrema, H.; van Schaardenburg, D. The Effect of Dietary Interventions on Chronic Inflammatory Diseases in Relation to the Microbiome: A Systematic Review. Nutrients 2021, 13, 3208. [Google Scholar] [CrossRef]
- Steckhan, N.; Hohmann, C.D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, H. Effects of Different Dietary Approaches on Inflammatory Markers in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary Patterns and Associations with Biomarkers of Inflammation in Adults: A Systematic Review of Observational Studies. Nutr. J. 2021, 20, 24. [Google Scholar]
- Menzel, J.; Jabakhanji, A.; Biemann, R.; Mai, K.; Abraham, K.; Weikert, C. Systematic Review and Meta-Analysis of the Associations of Vegan and Vegetarian Diets with Inflammatory Biomarkers. Sci. Rep. 2020, 10, 21736. [Google Scholar] [CrossRef]
- Jovanović, G.K.; Mrakovcic-Sutic, I.; Žeželj, S.P.; Šuša, B.; Rahelić, D.; Majanović, S.K. The Efficacy of an Energy-Restrictedanti-Inflammatory Diet for the Management of Obesity in Younger Adults. Nutrients 2020, 12, 3583. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar]
- Bruun, J.M.; Maersk, M.; Belza, A.; Astrup, A.; Richelsen, B. Consumption of Sucrose-Sweetened Soft Drinks Increases Plasma Levels of Uric Acid in Overweight and Obese Subjects: A 6-Month Randomised Controlled Trial. Eur. J. Clin. Nutr. 2015, 69, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ley, S.H.; Sun, Q.; Hu, F.B.; Malik, V.S. Cross-Sectional Association between Sugar-Sweetened Beverage Intake and Cardiometabolic Biomarkers in US Women. Br. J. Nutr. 2018, 119, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Kao, Y.H.; Sothern, M.S.; Seal, D.W.; Lee, C.H.; Lin, H.Y.; Chen, T.; Tseng, T.S. The Association between Sugar-Sweetened Beverages Intake, Body Mass Index, and Inflammation in US Adults. Int. J. Public. Health 2020, 65, 45–53. [Google Scholar] [CrossRef]
- Koebnick, C.; Black, M.H.; Wu, J.; Shu, Y.H.; Mackay, A.W.; Watanabe, R.M.; Buchanan, T.A.; Xiang, A.H. A Diet High in Sugar-Sweetened Beverage and Low in Fruits and Vegetables Is Associated with Adiposity and a pro-Inflammatory Adipokine Profile. Br. J. Nutr. 2018, 120, 1230–1239. [Google Scholar] [CrossRef]
- O’Connor, L.; Imamura, F.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Intakes and Sources of Dietary Sugars and Their Association with Metabolic and Inflammatory Markers. Clin. Nutr. 2018, 37, 1313–1322. [Google Scholar] [CrossRef]
- Voortman, T.; Braun, K.V.E.; Jaddoe, V.W.V.; Franco, O.H. Macronutrient Composition of Early Childhood Diet Is Associated with Growth and Adiposity. FASEB J. 2017, 31, 29.6. [Google Scholar] [CrossRef]
- Kumar Talapatra, S.; Talapatra, B. Chemistry of Plant Natural Products Stereochemistry, Conformation, Synthesis, Biology, and Medicine; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Adhikary, S.; Dasgupta, N. Role of Secondary Metabolites in Plant Homeostasis during Biotic Stress. Biocatal. Agric. Biotechnol. 2023, 50, 102712. [Google Scholar]
- Rainsford, K.D.; Alamgir, A.N.M. Progress in Drug Research Series Editor: Therapeutic Use of Medicinal Plants and Their Extracts: Volume 2 Phytochemistry and Bioactive Compounds; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Bachheti, A.; Deepti; Bachheti, R.K.; Husen, A. Medicinal Plants and Their Pharmaceutical Properties Under Adverse Environmental Conditions. In Harsh Environment and Plant Resilience; Springer International Publishing: Cham, Switzerland, 2021; pp. 457–502. [Google Scholar]
- Hussain, S.; Javed, W.; Tajammal, A.; Khalid, M.; Rasool, N.; Riaz, M.; Shahid, M.; Ahmad, I.; Muhammad, R.; Shah, S.A.A. Synergistic Antibacterial Screening of Cymbopogon Citratus and Azadirachta Indica: Phytochemical Profiling and Antioxidant and Hemolytic Activities. ACS Omega 2023, 8, 16600–16611. [Google Scholar] [CrossRef]
- Díaz-De-Cerio, E.; Verardo, V.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Exploratory Characterization of Phenolic Compounds with Demonstrated Anti-Diabetic Activity in Guava Leaves at Different Oxidation States. Int. J. Mol. Sci. 2016, 17, 699. [Google Scholar] [CrossRef]
- de Andrade, E.F.; de Souza Leone, R.; Ellendersen, L.N.; Masson, M.L. Phenolic Profile and Antioxidant Activity of Extracts of Leaves and Flowers of Yacon (Smallanthus Sonchifolius). Ind. Crops Prod. 2014, 62, 499–506. [Google Scholar] [CrossRef]
- Pawłowska, A.M.; Żurek, N.; Kapusta, I.; De Leo, M.; Braca, A. Antioxidant and Antiproliferative Activities of Phenolic Extracts of Eriobotrya Japonica (Thunb.) Lindl. Fruits and Leaves. Plants 2023, 12, 3221. [Google Scholar] [CrossRef]
- Barros, J.G.L.; Fernandes, R.; Abraão, A.; Costa, R.D.; Aires, A.; Gouvinhas, I.; Granato, D.; Barros, A.N. Characterization of Azorean Plant Leaves for Sustainable Valorization and Future Advanced Applications in the Food, Cosmetic, and Pharmaceutical Industries. Antioxidants 2024, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Ferreira, J.P.; Vitorino, C.; Pina, M.E.; Sousa, J.J.; Figueiredo, I.V.; Batista, M.T. Polyphenols from Cymbopogon Citratus Leaves as Topical Anti-Inflammatory Agents. J. Ethnopharmacol. 2016, 178, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Gonçalves, R.F.; Gil-Izquierdo, A.; Valentão, P.; Silva, A.M.S.; Silva, J.B.; Santos, D.; Andrade, P.B. Further Knowledge on the Phenolic Profile of Colocasia Esculenta (L.) Shott. J. Agric. Food Chem. 2012, 60, 7005–7015. [Google Scholar] [CrossRef]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milella, L. Evaluation of Antioxidant, Antidiabetic and Anticholinesterase Activities of Smallanthus Sonchifolius Landraces and Correlation with Their Phytochemical Profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef]
- Fu, Z.F.; Tu, Z.C.; Zhang, L.; Wang, H.; Wen, Q.H.; Huang, T. Antioxidant Activities and Polyphenols of Sweet Potato (Ipomoea Batatas L.) Leaves Extracted with Solvents of Various Polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Wu, L.; Zhang, J.; Li, X.; Wu, W. Chemical Characterization and Antioxidant Properties of Ethanolic Extract and Its Fractions from Sweet Potato (Ipomoea Batatas L.) Leaves. Foods 2020, 9, 15. [Google Scholar] [CrossRef]
- Mannino, G.; Gentile, C.; Porcu, A.; Agliassa, C.; Caradonna, F.; Bertea, C.M. Chemical Profile and Biological Activity of Cherimoya (Annona Cherimola Mill.) and Atemoya (Annona Atemoya) Leaves. Molecules 2020, 25, 2612. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2019, 11, 23. [Google Scholar]
- Hsu, C.L.; Yen, G.C. Effect of Gallic Acid on High Fat Diet-Induced Dyslipidaemia, Hepatosteatosis and Oxidative Stress in Rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. P-Hydroxybenzoic Acid Alleviates Inflammatory Responses and Intestinal Mucosal Damage in DSS-Induced Colitis by Activating ERβ Signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic Acid Derivatives: A Potential Class of Natural Compounds for the Management of Lipid Metabolism and Obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar]
- Wei, Z.; Xue, Y.; Xue, Y.; Cheng, J.; Lv, G.; Chu, L.; Ma, Z.; Guan, S. Ferulic Acid Attenuates Non-Alcoholic Steatohepatitis by Reducing Oxidative Stress and Inflammation through Inhibition of the ROCK/NF-ΚB Signaling Pathways. J. Pharmacol. Sci. 2021, 147, 72–80. [Google Scholar] [CrossRef]
- Wang, O.; Zhang, N.; Han, C.; Huang, J. Regular Exercise Combined with Ferulic Acid Exhibits Antiobesity Effect and Regulates Metabolic Profiles in High-Fat Diet-Induced Mice. Front. Nutr. 2022, 9, 957321. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Astiazarán-García, H.; González-Aguilar, G.A.; Loarca-Piña, G.; Ezquerra-Brauer, J.M.; Domínguez Avila, J.A.; Robles-Sánchez, M. Ferulic Acid on Glucose Dysregulation, Dyslipidemia, and Inflammation in Diet-Induced Obese Rats: An Integrated Study. Nutrients 2017, 9, 675. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Ye, L.; Hu, P.; Feng, L.P.; Huang, L.L.; Wang, Y.; Yan, X.; Xiong, J.; Xia, H.L. Protective Effects of Ferulic Acid on Metabolic Syndrome: A Comprehensive Review. Molecules 2023, 28, 281. [Google Scholar] [CrossRef]
- My Tien Truong, T.; Jang, H.J.; Ghosh, M.; Son, Y.O.; Kang, I. P-Coumaric Acid Alleviates Skeletal Muscle Atrophy by Improving Muscular Inflammation and Mitochondrial Dysfunction in High-Fat and High-Sucrose Diet-Fed C57BL/6 Male Mice. J. Funct. Foods 2024, 112, 105979. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sinha, B.; Choudhury, B.P.; Jha, N.K.; Palit, P.; Kundu, S.; Mandal, S.C.; Kolesarova, A.; Yousef, M.I.; Ruokolainen, J.; et al. Scavenging Properties of Plant-Derived Natural Biomolecule Para-Coumaric Acid in the Prevention of Oxidative Stress-Induced Diseases. Antioxidants 2021, 10, 1205. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jo, S.M.; Truong, T.T.M.; Zhang, G.; Kim, D.S.; Lee, M.; Lee, Y.; Kang, I. Peanut Sprout Rich in: P -Coumaric Acid Ameliorates Obesity and Lipopolysaccharide-Induced Inflammation and the Inhibition of Browning in Adipocytes via Mitochondrial Activation. Food Funct. 2021, 12, 5361–5374. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic Acid Improves Glucose Tolerance, Lipid Metabolism, Inflammation and Microbiota Composition in Diabetic Db/Db Mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic Acid Exhibits Anti-Obesity Property and Improves Lipid Metabolism in High-Fat Diet-Induced-Obese Mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Wan, C.W.; Wong, C.N.Y.; Pin, W.K.; Wong, M.H.Y.; Kwok, C.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Chlorogenic Acid Exhibits Cholesterol Lowering and Fatty Liver Attenuating Properties by Up-Regulating the Gene Expression of PPAR-α in Hypercholesterolemic Rats Induced with a High-Cholesterol Diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Wang, Y.; Kaur, G.; Kumar, M.; Kushwah, A.S.; Kabra, A.; Kainth, R. Caffeic Acid Prevents Vascular Oxidative Stress and Atherosclerosis against Atherosclerogenic Diet in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 8913926. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Galleano, M. Linking Biomarkers of Oxidative Stress and Disease with Flavonoid Consumption: From Experimental Models to Humans. Redox Biol. 2021, 42, 101914. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Kang, J.; Lombardo, G.E.; Mostofinejad, Z.; Wang, Z.; Zhu, W.; Oteiza, P.I. (−)-Epicatechin and the Comorbidities of Obesity. Arch. Biochem. Biophys. 2020, 690, 108505. [Google Scholar]
- Kang, J.; Wang, Z.; Oteiza, P.I.; Oteiza, P. (-)-Epicatechin Mitigates High Fat Diet-Induced Neuroinflammation and Altered Behavior in Mice. Food Funct. 2020, 11, 5065–5076. [Google Scholar]
- Connolly, K.; Batacan, R.; Jackson, D.; Fenning, A.S. Effects of Epicatechin on Cardiovascular Function in Middle-Aged Diet-Induced Obese Rat Models of Metabolic Syndrome. Br. J. Nutr. 2024, 131, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Cremonini, E.; Kang, H.; Kang, J.; Haj, F.G.; Oteiza, P.I. Anti-Inflammatory Actions of (−)-Epicatechin in the Adipose Tissue of Obese Mice. Int. J. Biochem. Cell Biol. 2016, 81, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, H.; Yu, J.; Sui, J.; Pan, D.; Wang, S.; Liao, W.; Yang, L.; Sun, G. Effects of Green Tea Catechin on the Blood Pressure and Lipids in Overweight and Obese Population-a Meta-Analysis. Heliyon 2023, 9, e21228. [Google Scholar] [CrossRef]
- Basu, T.; Selman, A.; Reddy, A.P.; Reddy, P.H. Current Status of Obesity: Protective Role of Catechins. Antioxidants 2023, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Anti-Obesity and Anti-Inflammatory Synergistic Effects of Green Tea Catechins and Citrus β-Cryptoxanthin Ingestion in Obese Mice. Int. J. Mol. Sci. 2023, 24, 7054. [Google Scholar] [CrossRef]
- Kim, J.M.; Heo, H.J. The Roles of Catechins in Regulation of Systemic Inflammation. Food Sci. Biotechnol. 2022, 31, 957–970. [Google Scholar] [CrossRef]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; et al. Grape-Seed Procyanidins Prevent Low-Grade Inflammation by Modulating Cytokine Expression in Rats Fed a High-Fat Diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef]
- Pallarès, V.; Cedó, L.; Castell-Auví, A.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M. Effects of Grape Seed Procyanidin Extract over Low-Grade Chronic Inflammation of Obese Zucker Fa/Fa Rats. Food Res. Int. 2013, 53, 319–324. [Google Scholar] [CrossRef]
- Sudhakaran, M.; Doseff, A.I. The Targeted Impact of Flavones on Obesity-Induced Inflammation and the Potential Synergistic Role in Cancer and the Gut Microbiota. Molecules 2020, 25, 2477. [Google Scholar] [CrossRef]
- Kariagina, A.; Doseff, A.I. Anti-Inflammatory Mechanisms of Dietary Flavones: Tapping into Nature to Control Chronic Inflammation in Obesity and Cancer. Int. J. Mol. Sci. 2022, 23, 15753. [Google Scholar] [CrossRef]
- Alam, W.; Rocca, C.; Khan, H.; Hussain, Y.; Aschner, M.; De Bartolo, A.; Amodio, N.; Angelone, T.; Cheang, W.S. Current Status and Future Perspectives on Therapeutic Potential of Apigenin: Focus on Metabolic-Syndrome-Dependent Organ Dysfunction. Antioxidants 2021, 10, 1643. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, Z.; Zhai, Y.; Yan, X.; Zhou, W.; Liu, H.; Guan, L.; Peng, L. Apigenin Alleviates Obesity-Associated Metabolic Syndrome by Regulating the Composition of the Gut Microbiome. Front. Microbiol. 2022, 12, 805827. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Sadeer, N.B.; Hussain, M.; Mahwish; Alsagaby, S.A.; Imran, M.; Mumtaz, T.; Umar, M.; Tauseef, A.; Al Abdulmonem, W.; et al. Therapeutical Properties of Apigenin: A Review on the Experimental Evidence and Basic Mechanisms. Int. J. Food Prop. 2023, 26, 1914–1939. [Google Scholar]
- Xu, Y.; Li, X.; Wang, H. Protective Roles of Apigenin Against Cardiometabolic Diseases: A Systematic Review. Front. Nutr. 2022, 9, 875826. [Google Scholar]
- Kalivarathan, J.; Chandrasekaran, S.P.; Kalaivanan, K.; Ramachandran, V.; Carani Venkatraman, A. Apigenin Attenuates Hippocampal Oxidative Events, Inflammation and Pathological Alterations in Rats Fed High Fat, Fructose Diet. Biomed. Pharmacother. 2017, 89, 323–331. [Google Scholar] [CrossRef]
- Chen, L.Y.; Cheng, H.L.; Liao, C.K.; Kuan, Y.H.; Liang, T.J.; Tseng, T.J.; Lin, H.C. Luteolin Improves Nephropathy in Hyperglycemic Rats through Anti-Oxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms. J. Funct. Foods 2023, 102, 105461. [Google Scholar] [CrossRef]
- Queiroz, M.; Leandro, A.; Azul, L.; Figueirinha, A.; Seiça, R.; Sena, C.M. Luteolin Improves Perivascular Adipose Tissue Profile and Vascular Dysfunction in Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 13671. [Google Scholar] [CrossRef]
- Caporali, S.; De Stefano, A.; Calabrese, C.; Giovannelli, A.; Pieri, M.; Savini, I.; Tesauro, M.; Bernardini, S.; Minieri, M.; Terrinoni, A. Anti-Inflammatory and Active Biological Properties of the Plant-Derived Bioactive Compounds Luteolin and Luteolin 7-Glucoside. Nutrients 2022, 14, 1155. [Google Scholar] [CrossRef]
- García-Barrado, M.J.; Iglesias-Osma, M.C.; Pérez-García, E.; Carrero, S.; Blanco, E.J.; Carretero-Hernández, M.; Carretero, J. Role of Flavonoids in the Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef]
- Sato, S.; Mukai, Y. Modulation of Chronic Inflammation by Quercetin: The Beneficial Effects on Obesity. J. Inflamm. Res. 2020, 13, 421–431. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin Modulates AMPK/SIRT1/NF-κB Signaling to Inhibit Inflammatory/Oxidative Stress Responses in Diabetic High Fat Diet-induced Atherosclerosis in the Rat Carotid Artery. Exp. Ther. Med. 2020, 20, 280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The Positive Correlation of Antioxidant Activity and Prebiotic Effect about Oat Phenolic Compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Geisler, S.; Lisandrelli, R.; Ngoc, H.N.; Ganzera, M.; Schennach, H.; Fuchs, D.; Fuchs, J.E.; Gostner, J.M.; Kurz, K. Pharmacological Targets of Kaempferol within Inflammatory Pathways—A Hint towards the Central Role of Tryptophan Metabolism. Antioxidants 2020, 9, 180. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases (Review). Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Bani Melhim, S.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Xia, S.F.; Le, G.W.; Wang, P.; Qiu, Y.Y.; Jiang, Y.Y.; Tang, X. Regressive Effect of Myricetin on Hepatic Steatosis in Mice Fed a High-Fat Diet. Nutrients 2016, 8, 799. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A Review of the Most Recent Research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The Impact of Ellagitannins and Their Metabolites through Gut Microbiome on the Gut Health and Brain Wellness within the Gut–Brain Axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- Manzoor, F.; Un Nisa, M.; Hussain, H.A.; Anwar, H.; Ahmad, N.; Umbreen, H. Therapeutic Potential of Hydrolysable Tannin on Weight Management Oxidative Stress and Reproductive Health in Polycystic Rats. Food Sci. Technol. 2022, 42, e63720. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.Y.; Fatemi, I.; Kalantari, H.; Mombeini, M.A.; Mehrzadi, S.; Goudarzi, M. Ellagic Acid Prevents Oxidative Stress, Inflammation, and Histopathological Alterations in Acrylamide-Induced Hepatotoxicity in Wistar Rats. J. Diet. Suppl. 2020, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Naraki, K.; Ghasemzadeh Rahbardar, M.; Ajiboye, B.O.; Hosseinzadeh, H. The Effect of Ellagic Acid on the Metabolic Syndrome: A Review Article. Heliyon 2023, 9, e21844. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic Anti-Inflammatory Effects and Mechanisms of Combined Phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Baroutian, S.; Li, J.; Zhang, B.; Ying, T.; Lu, J. Combination of Marine Bioactive Compounds and Extracts for the Prevention and Treatment of Chronic Diseases. Front. Nutr. 2023, 9, 1047026. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, J.; Abraão, A.; Gouvinhas, I.; Granato, D.; Barros, A.N. Advances in Leaf Plant Bioactive Compounds: Modulation of Chronic Inflammation Related to Obesity. Int. J. Mol. Sci. 2025, 26, 3358. https://doi.org/10.3390/ijms26073358
Barros J, Abraão A, Gouvinhas I, Granato D, Barros AN. Advances in Leaf Plant Bioactive Compounds: Modulation of Chronic Inflammation Related to Obesity. International Journal of Molecular Sciences. 2025; 26(7):3358. https://doi.org/10.3390/ijms26073358
Chicago/Turabian StyleBarros, Jorge, Ana Abraão, Irene Gouvinhas, Daniel Granato, and Ana Novo Barros. 2025. "Advances in Leaf Plant Bioactive Compounds: Modulation of Chronic Inflammation Related to Obesity" International Journal of Molecular Sciences 26, no. 7: 3358. https://doi.org/10.3390/ijms26073358
APA StyleBarros, J., Abraão, A., Gouvinhas, I., Granato, D., & Barros, A. N. (2025). Advances in Leaf Plant Bioactive Compounds: Modulation of Chronic Inflammation Related to Obesity. International Journal of Molecular Sciences, 26(7), 3358. https://doi.org/10.3390/ijms26073358









