The Ancestral KEAP1-NRF Pathway in Amphioxus Branchiostoma japonicum: Implications for the Evolution of Antioxidant Defense System
Abstract
:1. Introduction
2. Results
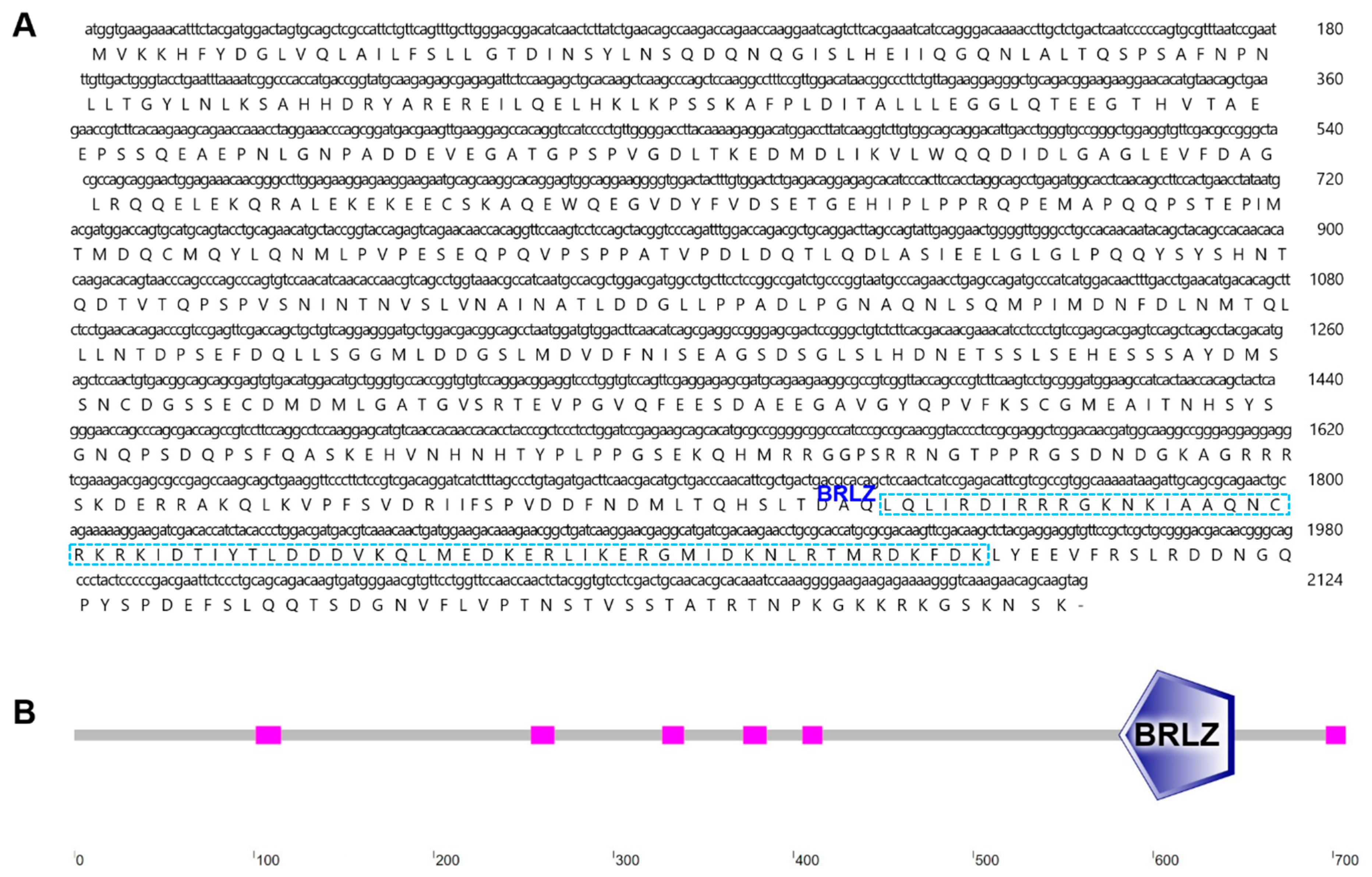
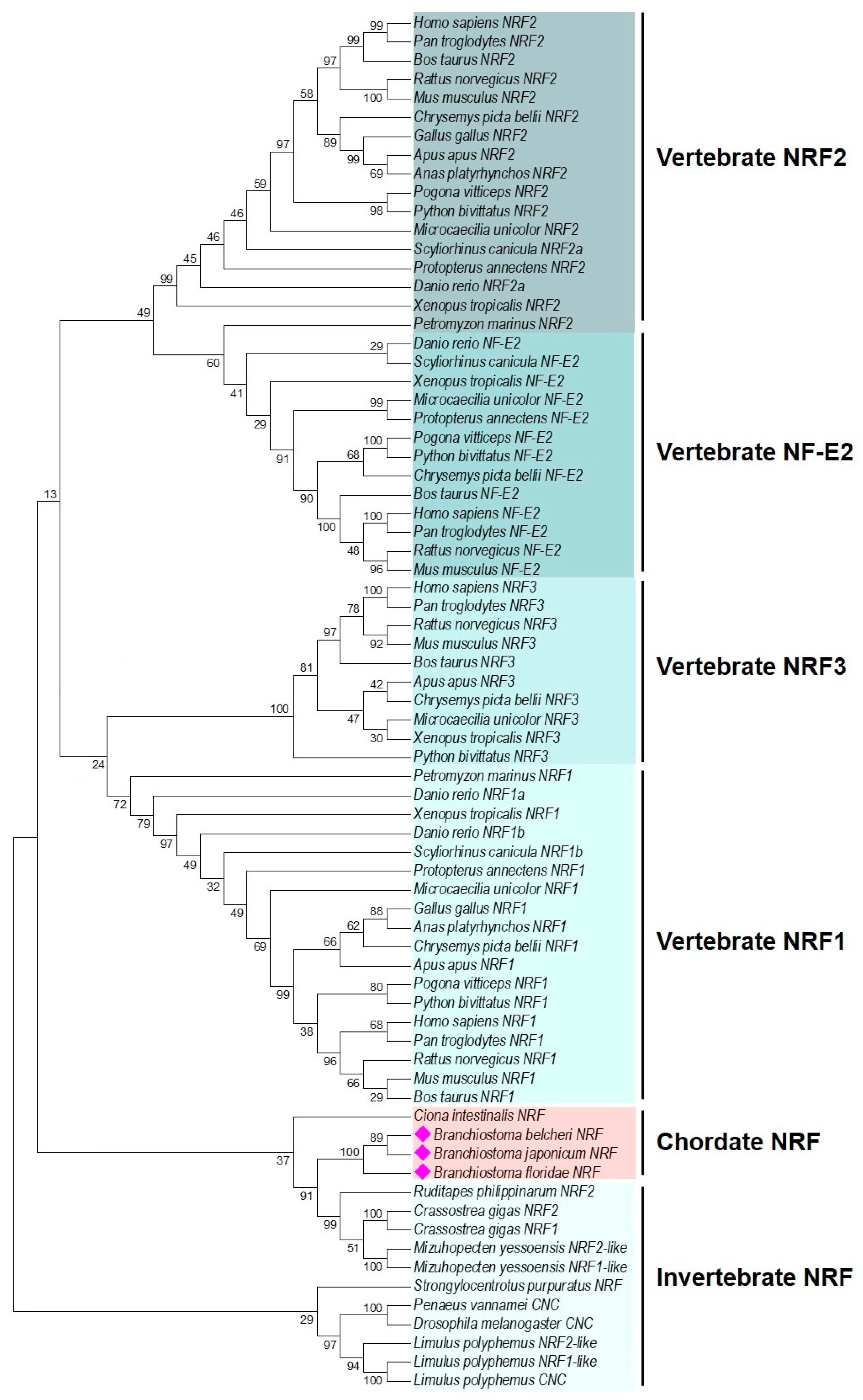
2.1. Characterization and Phylogenetic Analysis of B. japonicum NRF
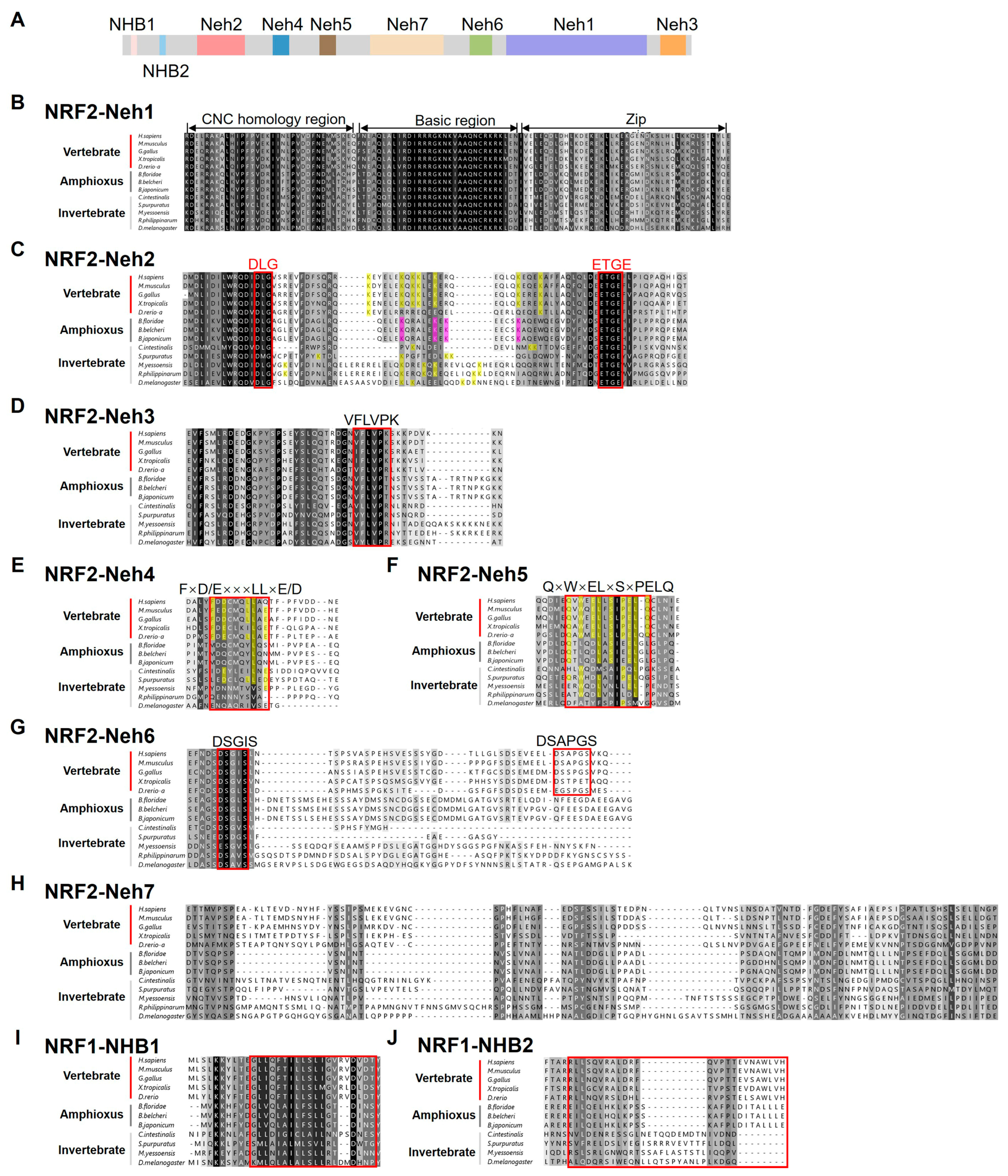
2.2. Domain Structure Analysis of BjNRF
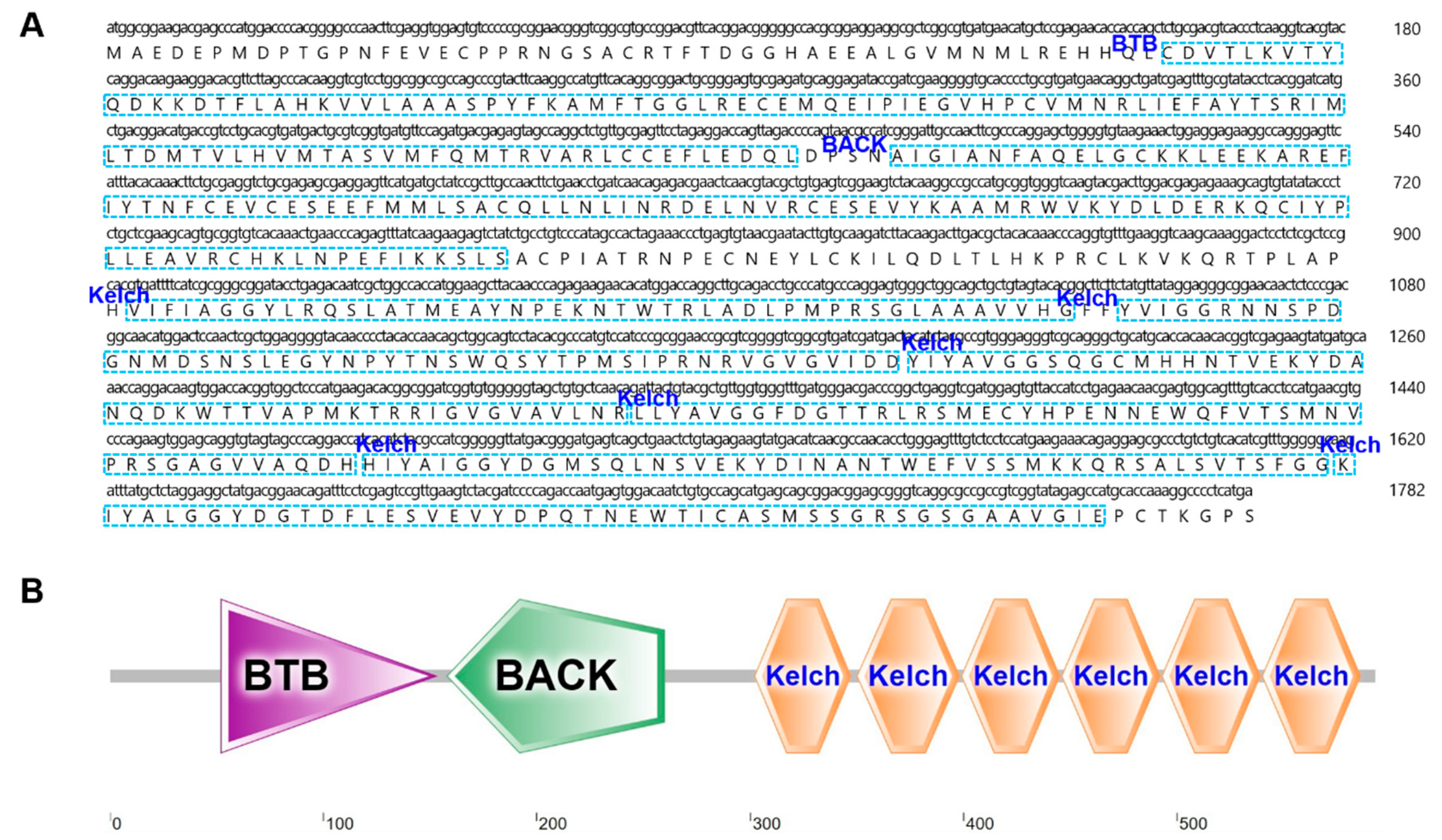
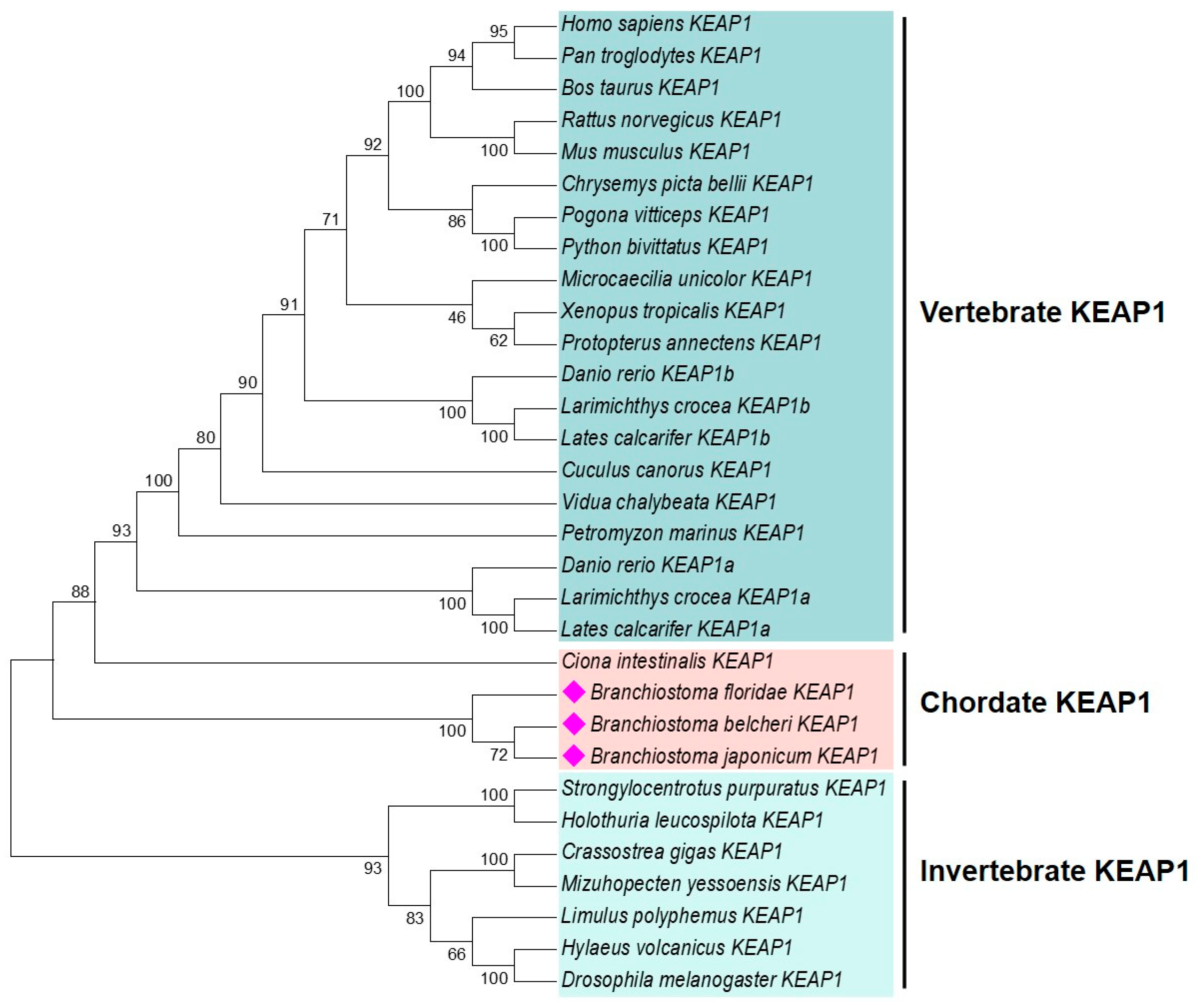
2.3. Characterization and Phylogenetic Analysis of B. japonicum KEAP1
2.4. Domain Structure Analysis of BjKEAP1
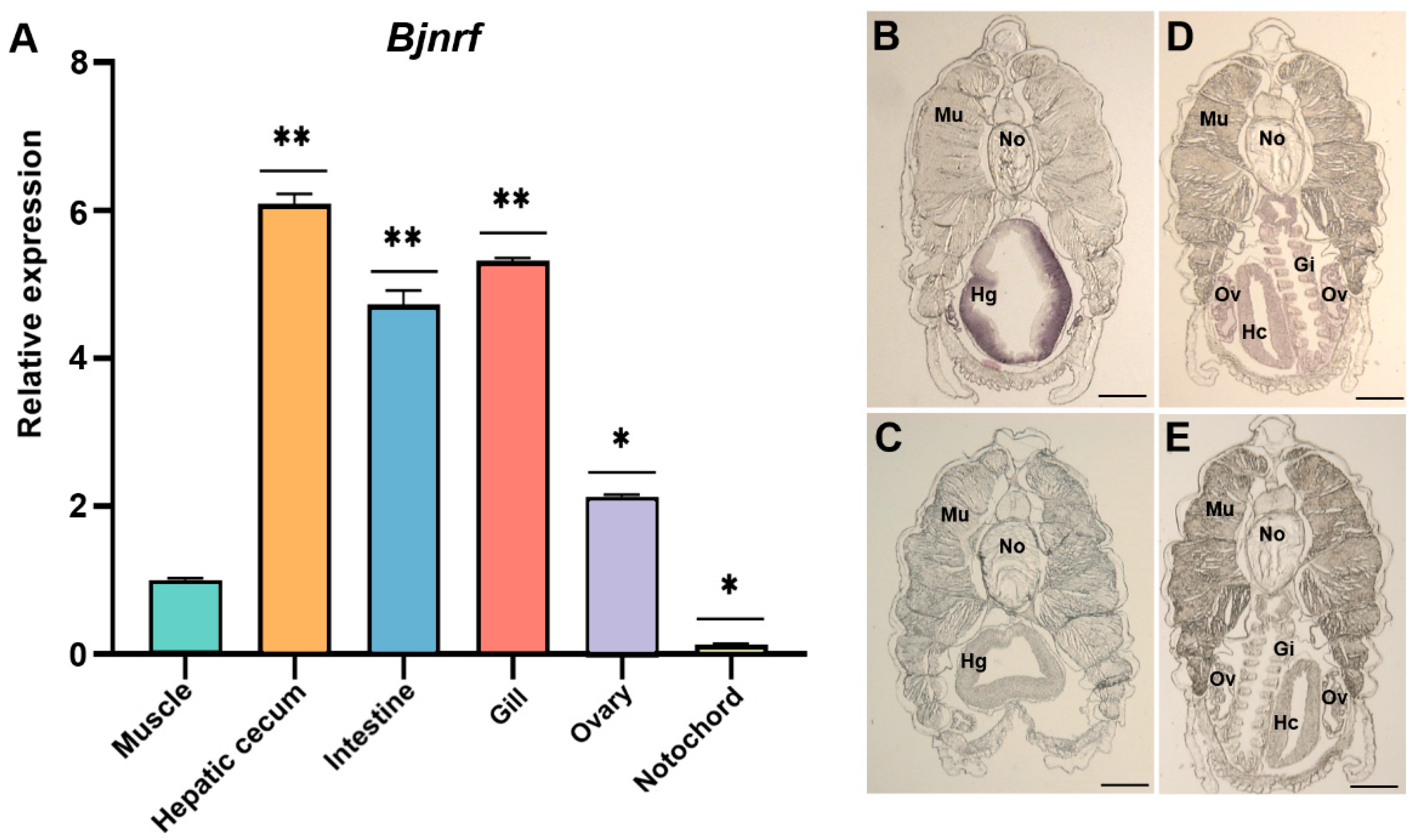
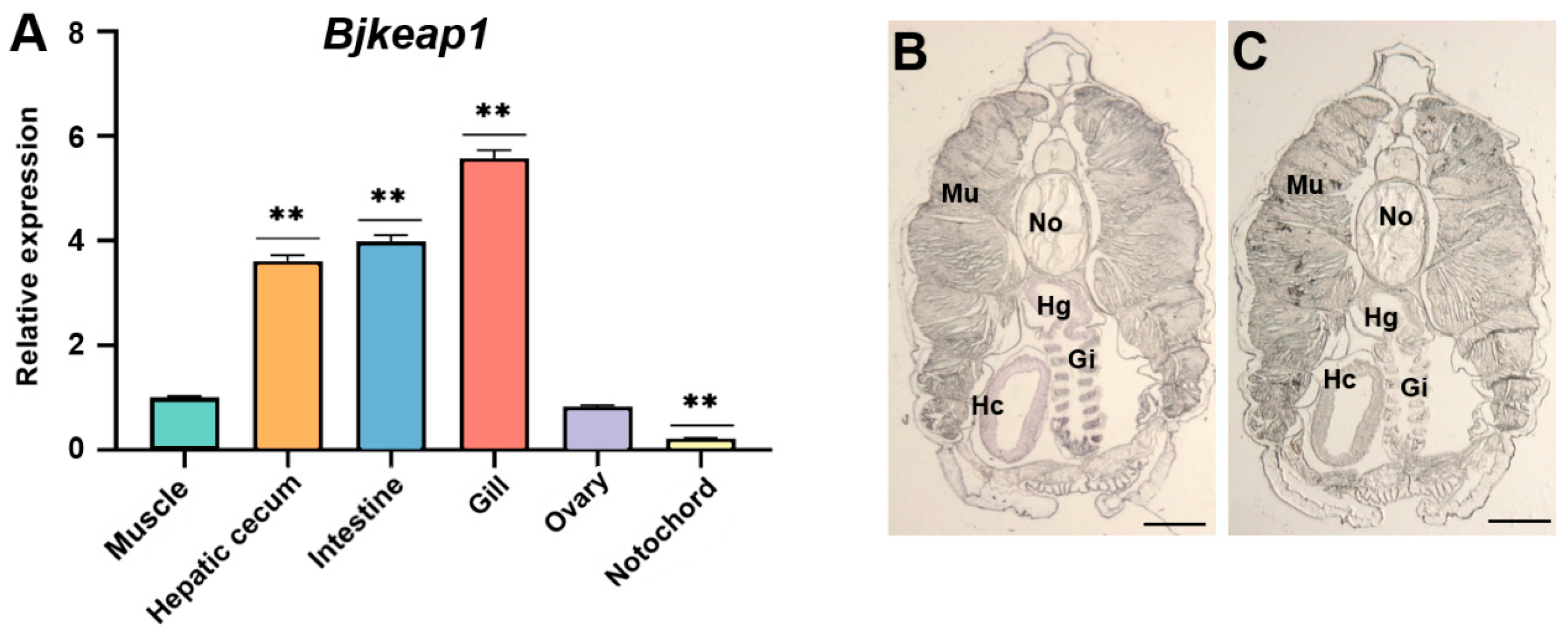
2.5. Tissue-Specific Expression of Bjnrf and Bjkeap1
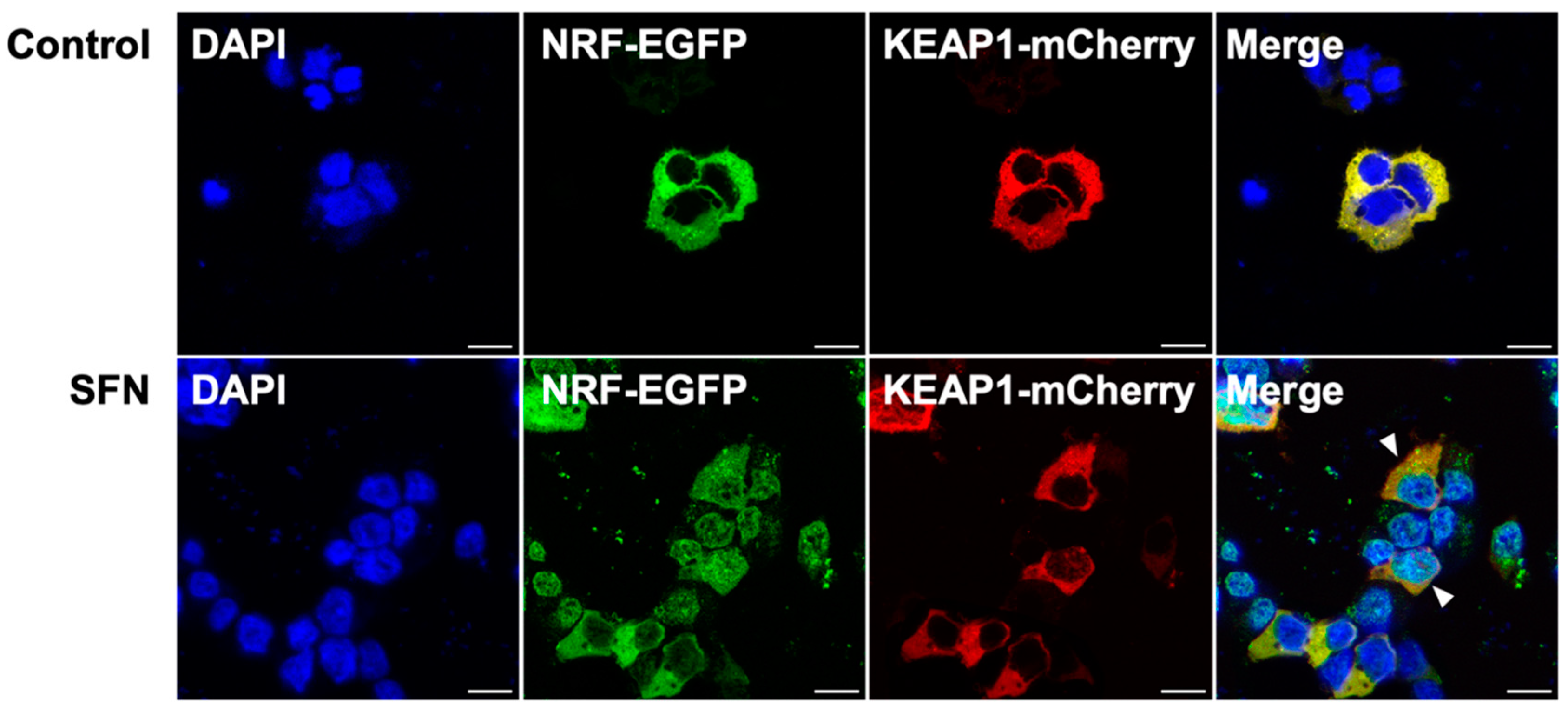
2.6. Subcellular Localization of BjNRF and BjKEAP1
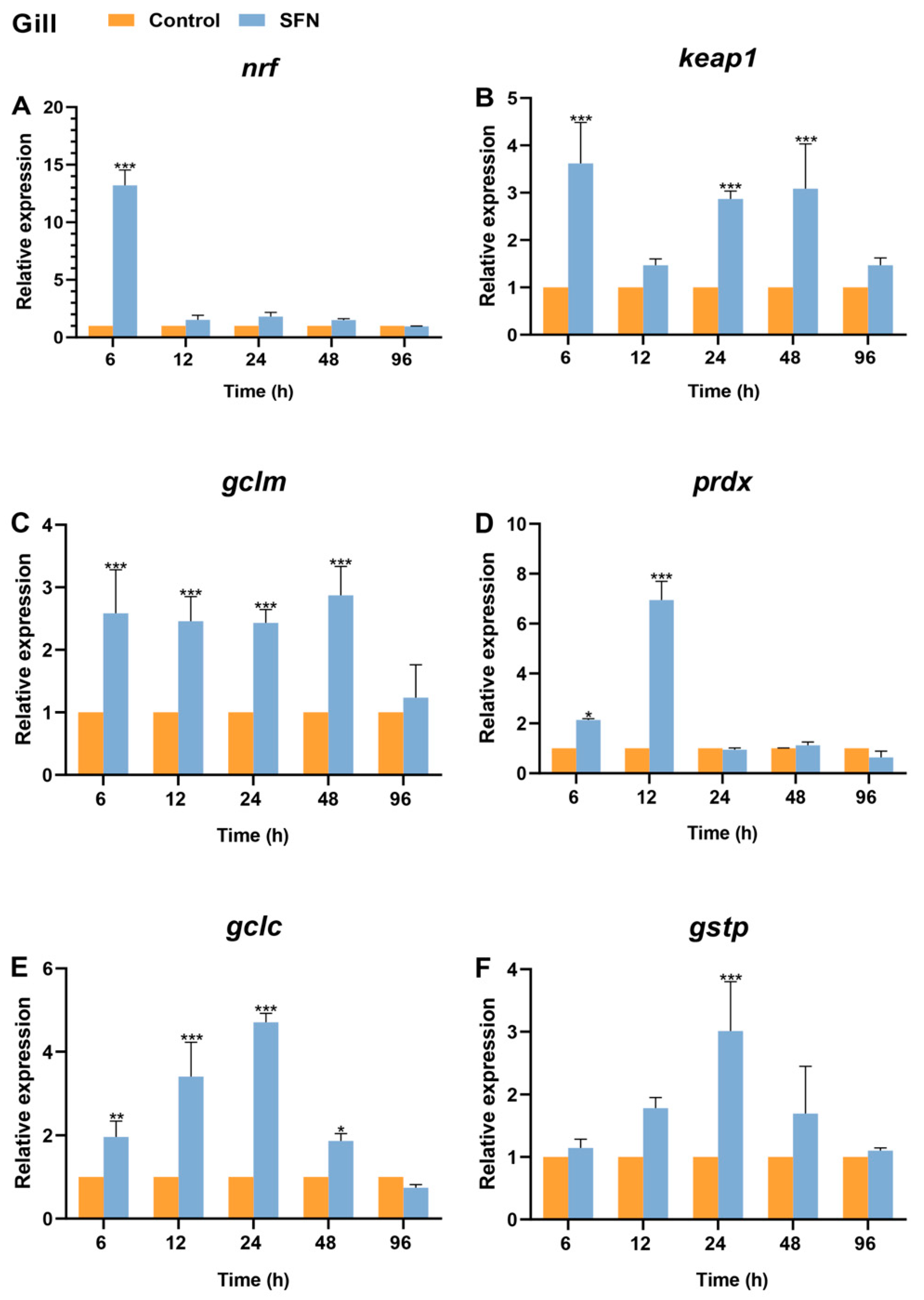
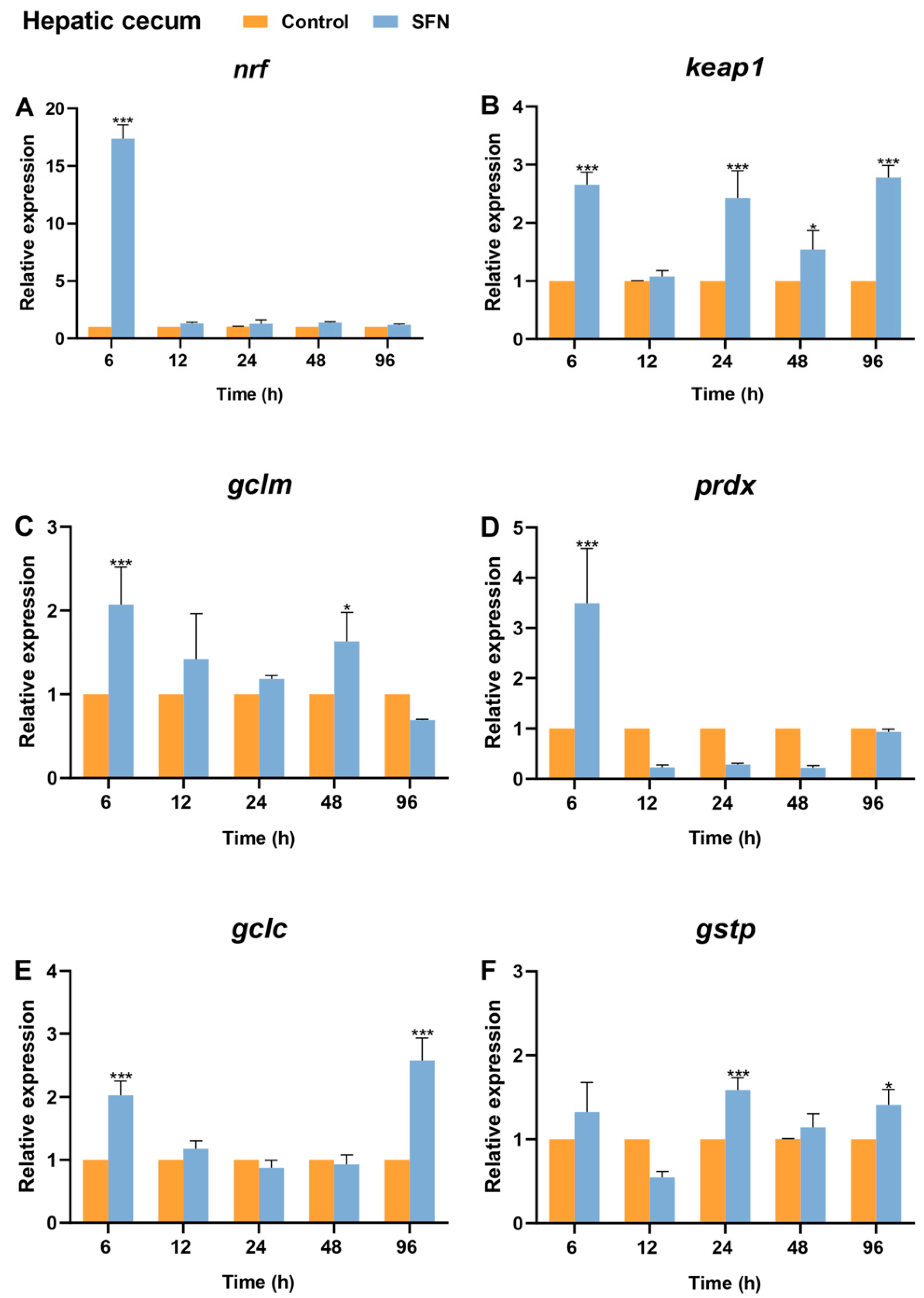
2.7. Expression of Bjnrf, Bjkeap1, and Phase II Detoxification Genes in Response to SFN Treatment
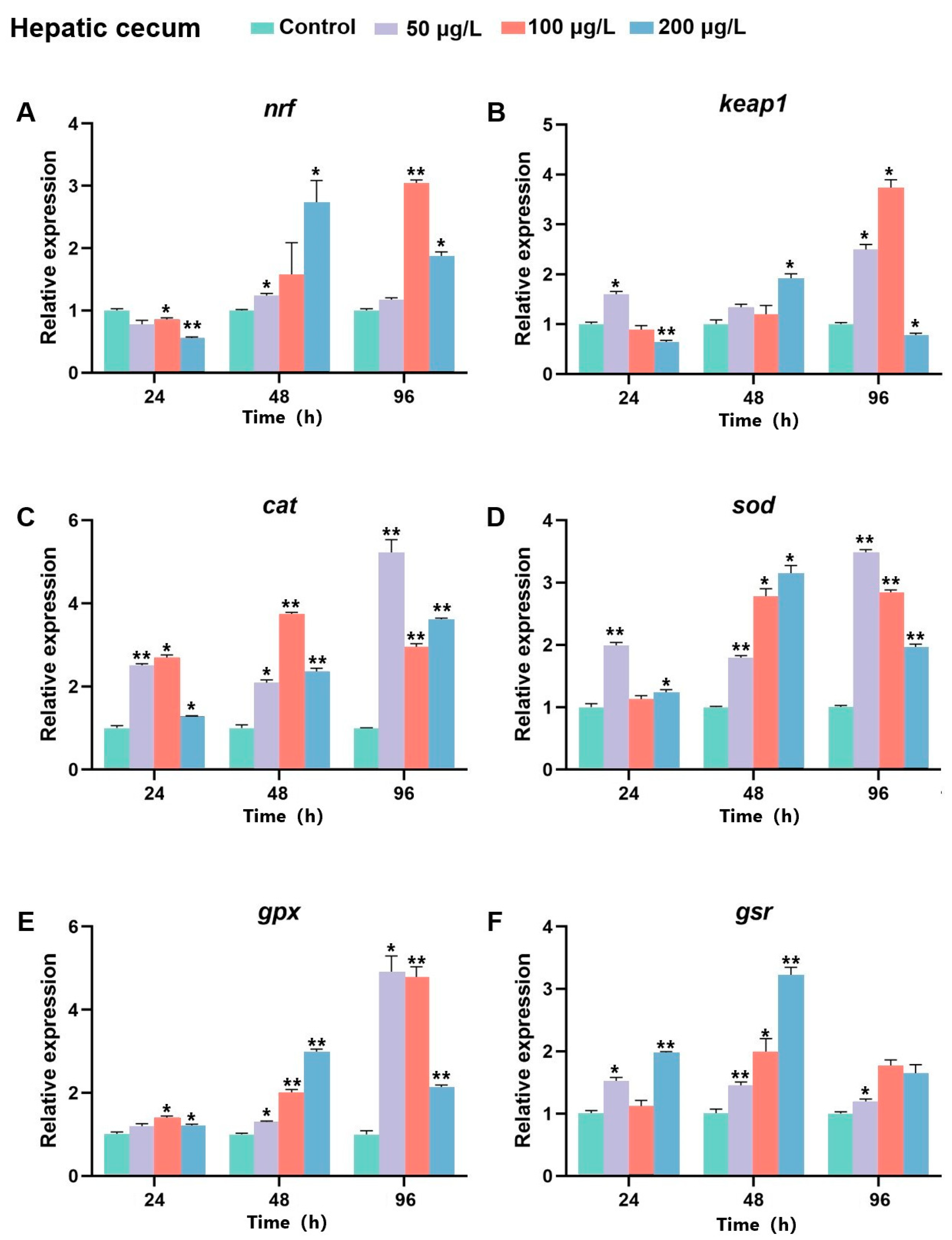
2.8. The Expression of Bjnrf, Bjkeap1, and Antioxidant Genes After BaP Exposure
3. Discussion
4. Materials and Methods
4.1. Animal and Cell Culture
4.2. Cloning and Sequencing of B. japonicum nrf and keap1 cDNAs
4.3. Sequence Analysis
4.4. In Situ Hybridization
4.5. Plasmid Construction
4.6. Subcellular Localization
4.7. SFN Treatment and Sample Collection
4.8. BaP Exposure and Sample Collection
4.9. qRT-PCR
4.10. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aoki, Y.; Sato, H.; Nishimura, N.; Takahashi, S.; Itoh, K.; Yamamoto, M. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharm. 2001, 173, 154–160. [Google Scholar] [CrossRef]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Kelleher, M.O.; Eggleston, I.M.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol. Appl. Pharm. 2009, 237, 267–280. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994, 91, 9926–9930. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Cederbaum, A.I. Transcription factor Nrf2 protects HepG2 cells against CYP2E1 plus arachidonic acid-dependent toxicity. J. Biol. Chem. 2006, 281, 14573–14579. [Google Scholar] [CrossRef]
- Kwak, M.K.; Kensler, T.W. Targeting NRF2 signaling for cancer chemoprevention. Toxicol. Appl. Pharmacol. 2010, 244, 66–76. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Itoh, K.; Mimura, J.; Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 2010, 13, 1665–1678. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Katsuoka, F.; Funayama, R.; Nagashima, T.; Nishida, Y.; Nakayama, K.; Engel, J.D.; Yamamoto, M. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012, 40, 10228–10239. [Google Scholar] [CrossRef]
- Kim, J.H.; Yu, S.; Chen, J.D.; Kong, A.N. The nuclear cofactor RAC3/AIB1/SRC-3 enhances Nrf2 signaling by interacting with transactivation domains. Oncogene 2013, 32, 514–527. [Google Scholar] [CrossRef]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Choe, K.P.; Przybysz, A.J.; Strange, K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol. 2009, 29, 2704–2715. [Google Scholar] [CrossRef] [PubMed]
- Danielli, N.M.; Trevisan, R.; Mello, D.F.; Fischer, K.; Deconto, V.S.; da Silva Acosta, D.; Bianchini, A.; Bainy, A.C.; Dafre, A.L. Upregulating Nrf2-dependent antioxidant defenses in Pacific oysters Crassostrea gigas: Investigating the Nrf2/Keap1 pathway in bivalves. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 195, 16–26. [Google Scholar] [CrossRef]
- Mohler, J.; Mahaffey, J.W.; Deutsch, E.; Vani, K. Control of Drosophila head segment identity by the bZIP homeotic gene cnc. Development 1995, 121, 237–247. [Google Scholar] [CrossRef]
- Oliveira, R.P.; Porter Abate, J.; Dilks, K.; Landis, J.; Ashraf, J.; Murphy, C.T.; Blackwell, T.K. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell 2009, 8, 524–541. [Google Scholar] [CrossRef]
- Pitoniak, A.; Bohmann, D. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic. Biol. Med. 2015, 88, 302–313. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef]
- Qi, P.; Tang, Z. The Nrf2 molecule trigger antioxidant defense against acute benzo(a)pyrene exposure in the thick shell mussel Mytilus coruscus. Aquat. Toxicol. 2020, 226, 105554. [Google Scholar] [CrossRef] [PubMed]
- Sykiotis, G.P.; Bohmann, D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 2008, 14, 76–85. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Wang, H.; Mao, T.; Li, J.; Cheng, X.; Hu, J.; Xue, B.; Li, B. Cloning and Functional Analysis of CncC and Keap1 Genes in Silkworm. J. Agric. Food Chem. 2018, 66, 2630–2636. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef]
- Inoue, H.; Hisamoto, N.; An, J.H.; Oliveira, R.P.; Nishida, E.; Blackwell, T.K.; Matsumoto, K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005, 19, 2278–2283. [Google Scholar] [CrossRef]
- Grimberg, K.B.; Beskow, A.; Lundin, D.; Davis, M.M.; Young, P. Basic leucine zipper protein Cnc-C is a substrate and transcriptional regulator of the Drosophila 26S proteasome. Mol. Cell. Biol. 2011, 31, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Tsakiri, E.N.; Sykiotis, G.P.; Papassideri, I.S.; Gorgoulis, V.G.; Bohmann, D.; Trougakos, I.P. Differential regulation of proteasome functionality in reproductive vs. somatic tissues of Drosophila during aging or oxidative stress. FASEB J. 2013, 27, 2407–2420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Evolutionary Biology of Amphioxus: Tracing Origin of Vertebrate; Science Press: Beijing, China, 2020; pp. 1–49. ISBN 978-7-03-066112-8. [Google Scholar]
- D’Aniello, S.; Bertrand, S.; Escriva, H. Amphioxus as a model to study the evolution of development in chordates. eLife 2023, 12, e87028. [Google Scholar] [CrossRef]
- Holland, L.Z.; Laudet, V.; Schubert, M. The chordate amphioxus: An emerging model organism for developmental biology. Cell. Mol. Life Sci. 2004, 61, 2290–2308. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S. Hepatic caecum of amphioxus and origin of vertebrate liver. Hereditas 2010, 32, 437–442. [Google Scholar] [CrossRef]
- Sekine, H.; Motohashi, H. Unique and overlapping roles of NRF2 and NRF1 in transcriptional regulation. J. Clin. Biochem. Nutr. 2024, 74, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Katoh, Y.; Kusunoki, H.; Itoh, K.; Tanaka, T.; Yamamoto, M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: Characterization of the two-site molecular recognition model. Mol. Cell. Biol. 2006, 26, 2887–2900. [Google Scholar] [CrossRef]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two domains of Nrf2 cooperatively bind CBP, a CREB binding protein, and synergistically activate transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hosoya, T.; Maruyama, A.; Nishikawa, K.; Maher, J.M.; Ohta, T.; Motohashi, H.; Fukamizu, A.; Shibahara, S.; Itoh, K.; et al. Nrf2 Neh5 domain is differentially utilized in the transactivation of cytoprotective genes. Biochem. J. 2007, 404, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Chowdhry, S.; Zhang, Y.; McMahon, M.; Sutherland, C.; Cuadrado, A.; Hayes, J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 2013, 32, 3765–3781. [Google Scholar] [CrossRef]
- Zhang, Y.; Lucocq, J.M.; Yamamoto, M.; Hayes, J.D. The NHB1 (N-terminal homology box 1) sequence in transcription factor Nrf1 is required to anchor it to the endoplasmic reticulum and also to enable its asparagine-glycosylation. Biochem. J. 2007, 408, 161–172. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. The Keap1 BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J. Biol. Chem. 2002, 277, 36544–36552. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Suzuki, T.; Hiramoto, K.; Asami, S.; Naganuma, E.; Suda, H.; Iso, T.; Yamamoto, H.; Morita, M.; Baird, L.; et al. Characterizations of Three Major Cysteine Sensors of Keap1 in Stress Response. Mol. Cell. Biol. 2015, 36, 271–284. [Google Scholar] [CrossRef]
- Takaya, K.; Suzuki, T.; Motohashi, H.; Onodera, K.; Satomi, S.; Kensler, T.W.; Yamamoto, M. Validation of the multiple sensor mechanism of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2012, 53, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Suzuki, T.; Kobayashi, A.; Wakabayashi, J.; Maher, J.; Motohashi, H.; Yamamoto, M. Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol. Cell. Biol. 2008, 28, 2758–2770. [Google Scholar] [CrossRef]
- Madden, S.K.; Itzhaki, L.S. Structural and mechanistic insights into the Keap1-Nrf2 system as a route to drug discovery. Biochim. Biophys. Acta Proteins Proteom. 2020, 1868, 140405. [Google Scholar] [CrossRef]
- Velichkova, M.; Hasson, T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005, 25, 4501–4513. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Moriyama, Y.; Koshiba-Takeuchi, K. Significance of whole-genome duplications on the emergence of evolutionary novelties. Brief Funct. Genom. 2018, 17, 329–338. [Google Scholar] [CrossRef]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem. 2019, 294, 18131–18149. [Google Scholar] [CrossRef]
- Tian, W.; Rojo de la Vega, M.; Schmidlin, C.J.; Ooi, A.; Zhang, D.D. Kelch-like ECH-associated protein 1 (KEAP1) differentially regulates nuclear factor erythroid-2-related factors 1 and 2 (NRF1 and NRF2). J. Biol. Chem. 2018, 293, 2029–2040. [Google Scholar] [CrossRef]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different electrostatic potentials define ETGE and DLG motifs as hinge and latch in oxidative stress response. Mol. Cell. Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.I.; Kobayashi, A.; Katsuoka, F.; Yamamoto, M. Two-site substrate recognition model for the Keap1-Nrf2 system: A hinge and latch mechanism. J. Biol. Chem. 2006, 387, 1311–1320. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The carboxy terminal Neh3 domain of Nrf2 is required for transcriptional activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chan, J.Y. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J. Biol. Chem. 2006, 281, 19676–19687. [Google Scholar] [CrossRef]
- Zhang, Y.; Crouch, D.H.; Yamamoto, M.; Hayes, J.D. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: Nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem. J. 2006, 399, 373–385. [Google Scholar] [CrossRef]
- Kang, M.I.; Kobayashi, A.; Wakabayashi, N.; Kim, S.G.; Yamamoto, M. Scaffolding of Keap1 to the actin cytoskeleton controls the function of Nrf2 as key regulator of cytoprotective phase 2 genes. Proc. Natl. Acad. Sci. USA 2004, 101, 2046–2051. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology 2008, 246, 24–33. [Google Scholar] [CrossRef]
- Biswas, M.; Chan, J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010, 244, 16–20. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Otoo, R.A.; Allen, A.R. Sulforaphane’s Multifaceted Potential: From Neuroprotection to Anticancer Action. Molecules 2023, 28, 6902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Thirty years of NRF2: Advances and therapeutic challenges. Nature reviews. Nat. Rev. Drug Discov. 2025. [Google Scholar] [CrossRef]
- Hu, C.; Eggler, A.L.; Mesecar, A.D.; van Breemen, R.B. Modification of keap1 cysteine residues by sulforaphane. Chem. Res. Toxicol. 2011, 24, 515–521. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Z.Y.; Khor, T.O.; Shu, L.; Kong, A.N. Sulforaphane enhances Nrf2 expression in prostate cancer TRAMP C1 cells through epigenetic regulation. Biochem. Pharmacol. 2013, 85, 1398–1404. [Google Scholar] [CrossRef]
- Su, Z.Y.; Zhang, C.; Lee, J.H.; Shu, L.; Wu, T.Y.; Khor, T.O.; Conney, A.H.; Lu, Y.P.; Kong, A.N. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev. Res. 2014, 7, 319–329. [Google Scholar] [CrossRef]
- Ribeiro, M.; Alvarenga, L.; Coutinho-Wolino, K.S.; Nakao, L.S.; Cardozo, L.F.; Mafra, D. Sulforaphane upregulates the mRNA expression of NRF2 and NQO1 in non-dialysis patients with chronic kidney disease. Free Radic. Biol. Med. 2024, 221, 181–187. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, T.; Sun, Y.; Chen, S.; Hao, H.; Du, W.; Zou, H.; Yu, D.; Zhu, H.; Pang, Y. Sulforaphane suppresses paraquat-induced oxidative damage in bovine in vitro-matured oocytes through Nrf2 transduction pathway. Ecotoxicol. Environ. Saf. 2023, 254, 114747. [Google Scholar] [CrossRef]
- Shao, J.; Huang, J.; Guo, Y.; Li, L.; Liu, X.; Chen, X.; Yuan, J. Up-regulation of nuclear factor E2-related factor 2 (Nrf2) represses the replication of SVCV. Fish Shellfish Immunol. 2016, 58, 474–482. [Google Scholar] [CrossRef]
- Lee, O.H.; Jain, A.K.; Papusha, V.; Jaiswal, A.K. An auto-regulatory loop between stress sensors INrf2 and Nrf2 controls their cellular abundance. J. Biol. Chem. 2007, 282, 36412–36420. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol. Cell. Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef]
- McMahon, M.; Itoh, K.; Yamamoto, M.; Chanas, S.A.; Henderson, C.J.; McLellan, L.I.; Wolf, C.R.; Cavin, C.; Hayes, J.D. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res. 2001, 61, 3299–3307. [Google Scholar]
- Morgenstern, C.; Lastres-Becker, I.; Demirdöğen, B.C.; Costa, V.M.; Daiber, A.; Foresti, R.; Motterlini, R.; Kalyoncu, S.; Arioz, B.I.; Genc, S.; et al. Biomarkers of NRF2 signalling: Current status and future challenges. Redox Biol. 2024, 72, 103134. [Google Scholar] [CrossRef]
- Aloke, C.; Onisuru, O.O.; Achilonu, I. Glutathione S-transferase: A versatile and dynamic enzyme. Biochem. Biophys. Res. Commun. 2024, 734, 150774. [Google Scholar] [CrossRef]
- Ikeda, H.; Serria, M.S.; Kakizaki, I.; Hatayama, I.; Satoh, K.; Tsuchida, S.; Muramatsu, M.; Nishi, S.; Sakai, M. Activation of mouse Pi-class glutathione S-transferase gene by Nrf2 (NF-E2-related factor 2) and androgen. Biochem. J. 2002, 364, 563–570. [Google Scholar] [CrossRef]
- Rhee, S.G.; Woo, H.A. Multiple functions of peroxiredoxins: Peroxidases, sensors and regulators of the intracellular messenger HO, and protein chaperones. Antioxid. Redox Signal. 2011, 15, 781–794. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sivasantosh, S.; Sathiyaseelan, A.; Sankaranarayanan, A.; Naveen, K.V.; Zhang, X.; Jamla, M.; Vijayasarathy, S.; Vishnu Priya, V.; MubarakAli, D.; et al. Impact of benzo[a]pyrene with other pollutants induce the molecular alternation in the biological system: Existence, detection, and remediation methods. Environ. Pollut. 2022, 304, 119207. [Google Scholar] [CrossRef]
- Lim, J.; Ortiz, L.; Nakamura, B.N.; Hoang, Y.D.; Banuelos, J.; Flores, V.N.; Chan, J.Y.; Luderer, U. Effects of deletion of the transcription factor Nrf2 and benzo [a] pyrene treatment on ovarian follicles and ovarian surface epithelial cells in mice. Reprod. Toxicol. 2015, 58, 24–32. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Si, L.; Miao, J. The role of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by PAHs in the calm Ruditapes philippinarum. Fish Shellfish Immunol. 2018, 80, 325–334. [Google Scholar] [CrossRef]
- Wuputra, K.; Hsu, W.H.; Ku, C.C.; Yang, Y.H.; Kuo, K.K.; Yu, F.J.; Yu, H.S.; Nagata, K.; Wu, D.C.; Kuo, C.H.; et al. The AHR-NRF2-JDP2 gene battery: Ligand-induced AHR transcriptional activation. Biochem. Pharmacol. 2025, 233, 116761. [Google Scholar] [CrossRef]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef]
- Wang, H.; Pan, L.; Xu, R.; Si, L.; Zhang, X. The molecular mechanism of Nrf2-Keap1 signaling pathway in the antioxidant defense response induced by BaP in the scallop Chlamys farreri. Fish Shellfish Immunol. 2019, 92, 489–499. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Regoli, F. Identification of the Nrf2-Keap1 pathway in the European eel Anguilla anguilla: Role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat. Toxicol. 2014, 150, 117–123. [Google Scholar] [CrossRef]
- Giuliani, M.E.; Benedetti, M.; Nigro, M.; Regoli, F. Nrf2 and regulation of the antioxidant system in the Antarctic silverfish, Pleuragramma antarctica: Adaptation to environmental changes of pro-oxidant pressure. Mar. Environ. Res. 2017, 129, 1–13. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. 2010, 115, 391–400. [Google Scholar] [CrossRef]
- Pi, J.; Qu, W.; Reece, J.M.; Kumagai, Y.; Waalkes, M.P. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: Involvement of hydrogen peroxide. Exp. Cell Res. 2003, 290, 234–245. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Regoli, F.; Giuliani, M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014, 93, 106–117. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, W.D.; Liu, Y.; Chen, G.F.; Jiang, J.; Li, S.H.; Feng, L.; Zhou, X.Q. Effect of choline on antioxidant defenses and gene expressions of Nrf2 signaling molecule in the spleen and head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2014, 38, 374–382. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S.; Yang, Q.; Liu, Z.; Zhang, S. Functional characterization of thyrostimulin in amphioxus suggests an ancestral origin of the TH signaling pathway. Endocrinology 2018, 159, 3536–3548. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Liang, X.; Xiang, K.; Li, H.; Zhang, Y. The Ancestral KEAP1-NRF Pathway in Amphioxus Branchiostoma japonicum: Implications for the Evolution of Antioxidant Defense System. Int. J. Mol. Sci. 2025, 26, 3427. https://doi.org/10.3390/ijms26073427
Li W, Liang X, Xiang K, Li H, Zhang Y. The Ancestral KEAP1-NRF Pathway in Amphioxus Branchiostoma japonicum: Implications for the Evolution of Antioxidant Defense System. International Journal of Molecular Sciences. 2025; 26(7):3427. https://doi.org/10.3390/ijms26073427
Chicago/Turabian StyleLi, Weichen, Xiaoqian Liang, Keyu Xiang, Hongyan Li, and Yu Zhang. 2025. "The Ancestral KEAP1-NRF Pathway in Amphioxus Branchiostoma japonicum: Implications for the Evolution of Antioxidant Defense System" International Journal of Molecular Sciences 26, no. 7: 3427. https://doi.org/10.3390/ijms26073427
APA StyleLi, W., Liang, X., Xiang, K., Li, H., & Zhang, Y. (2025). The Ancestral KEAP1-NRF Pathway in Amphioxus Branchiostoma japonicum: Implications for the Evolution of Antioxidant Defense System. International Journal of Molecular Sciences, 26(7), 3427. https://doi.org/10.3390/ijms26073427





