Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa?
Abstract
1. Introduction
2. Results
2.1. Biological Parameters of White and Yellow Turkey Sperm
2.2. Correlations Between Selected Parameters of Spermatozoa Derived from White and Yellow Semen
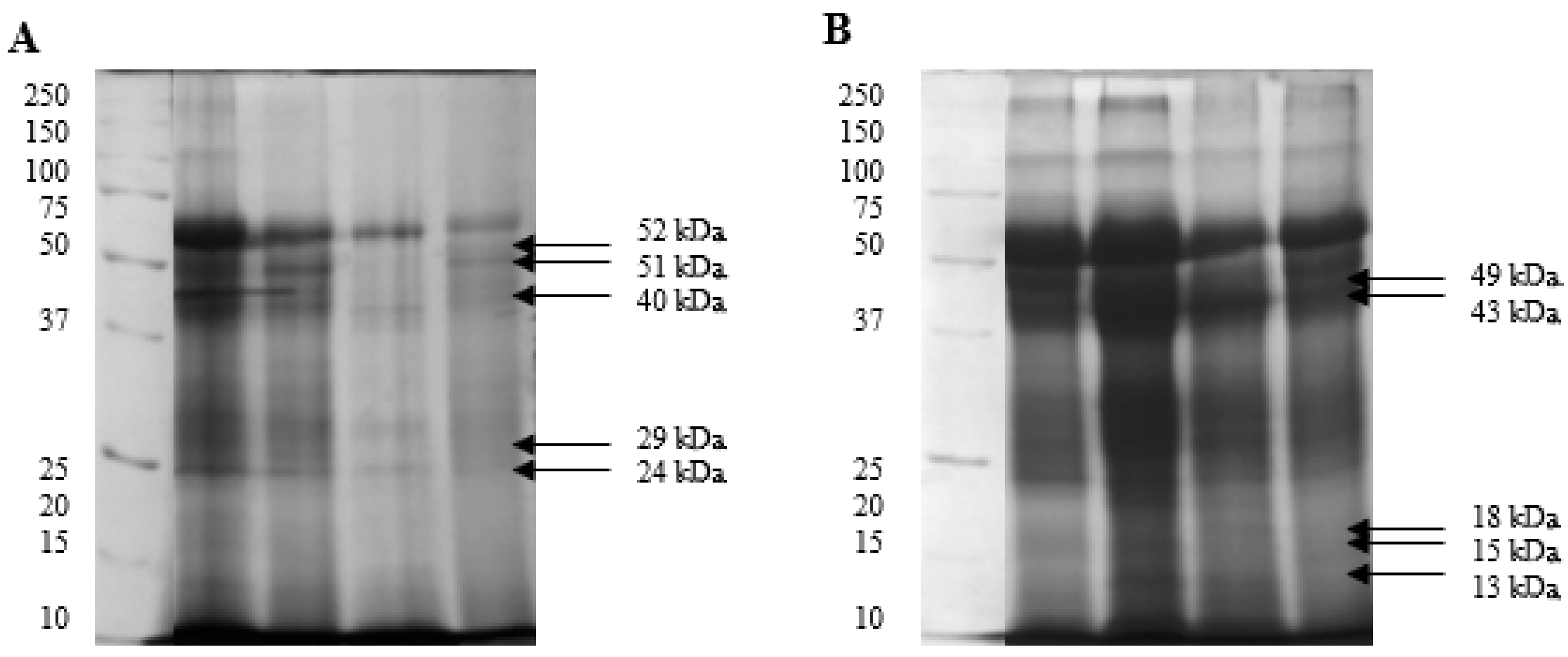
2.3. Isolation and Identification of Sperm Phosphoproteins from White and Yellow Semen
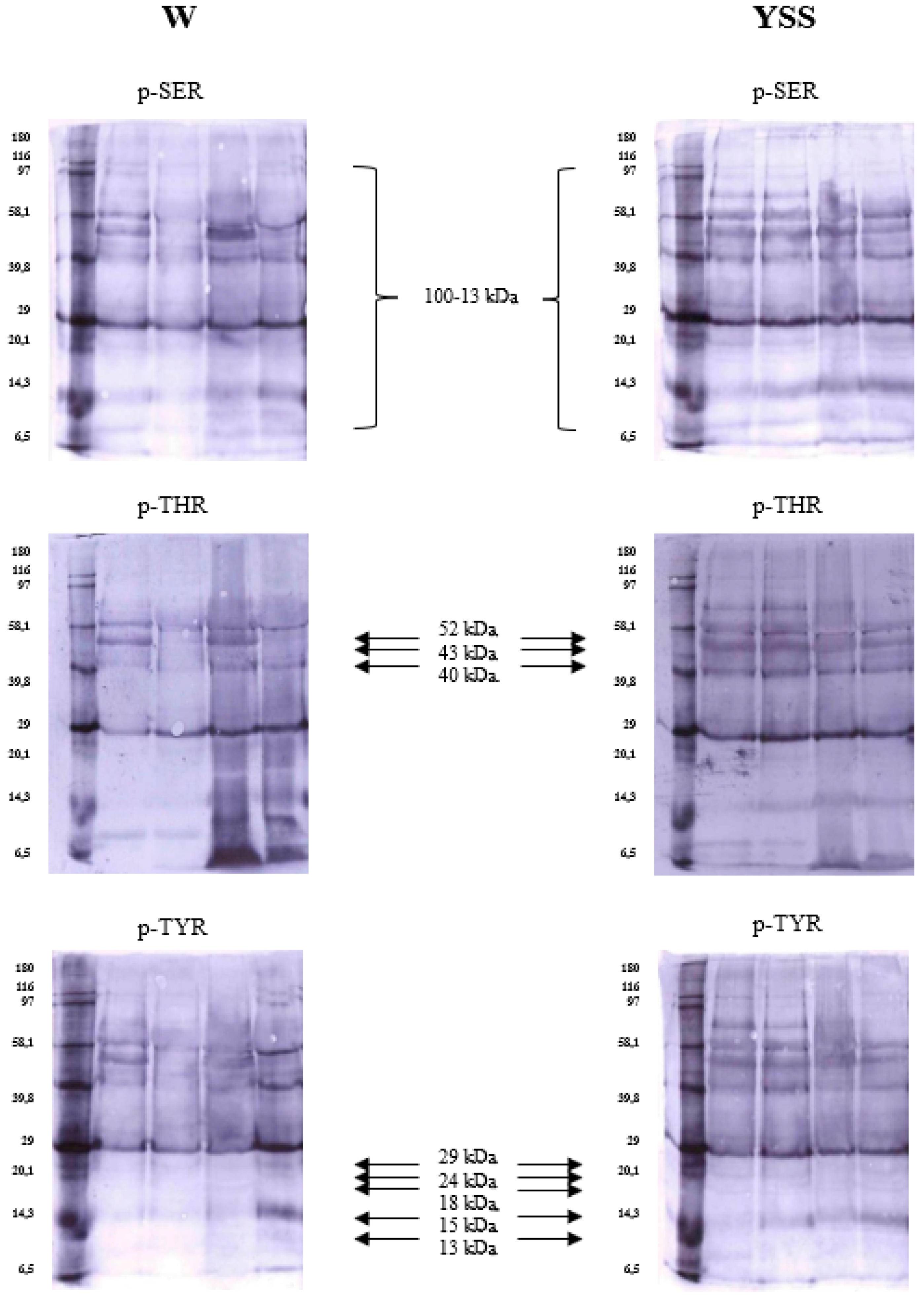
2.4. Immunodetection of Sperm Phosphoproteins in White and Yellow Semen
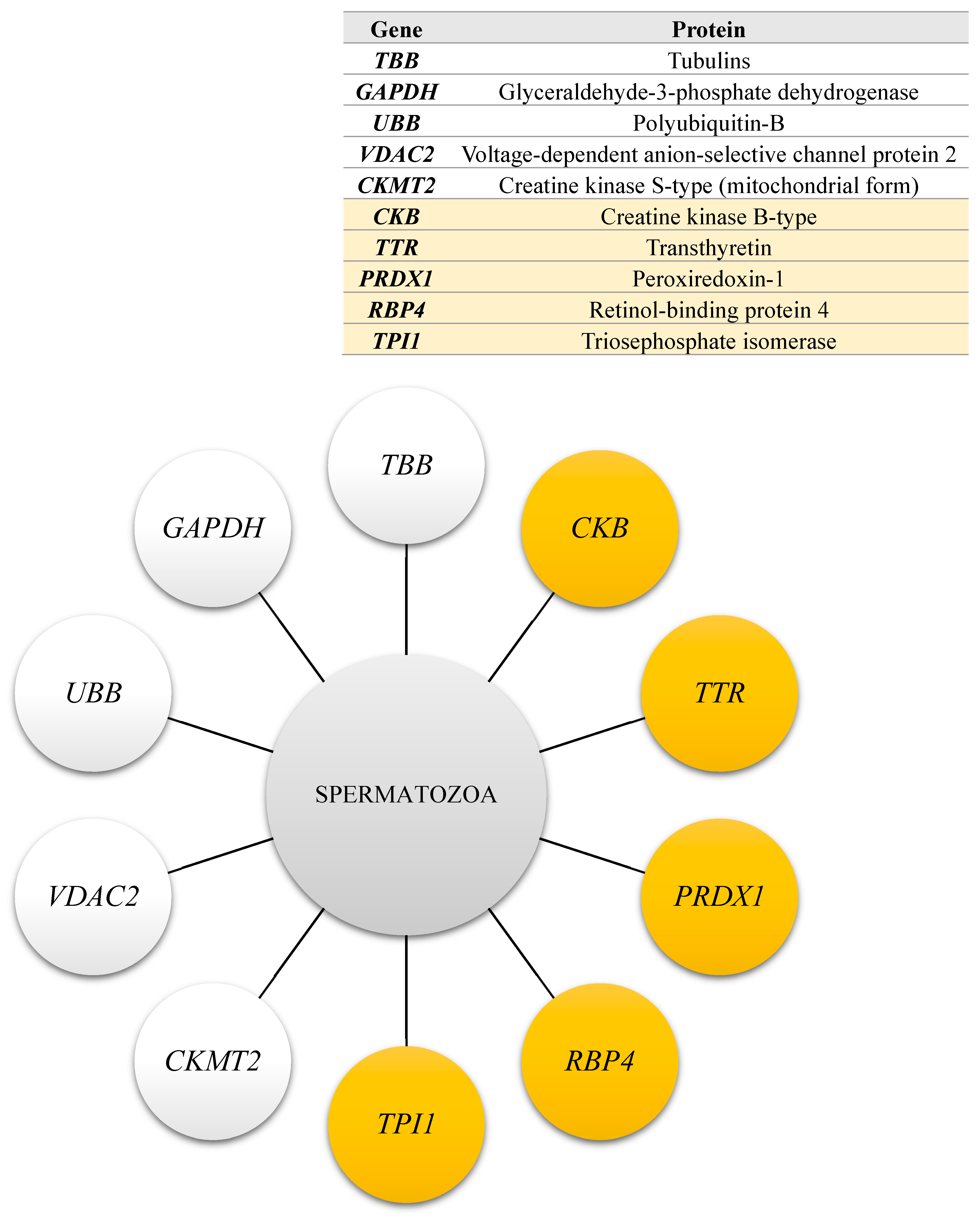
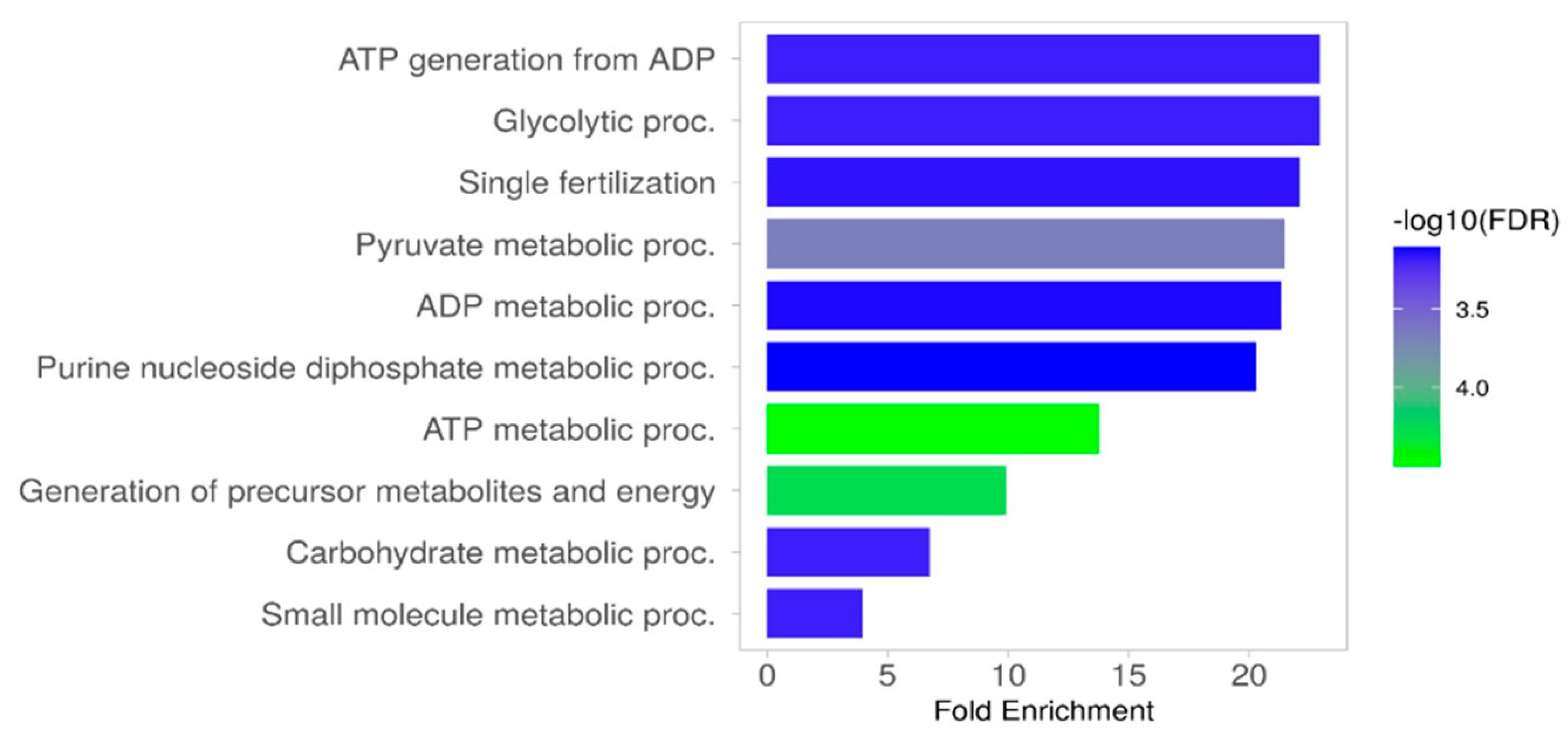
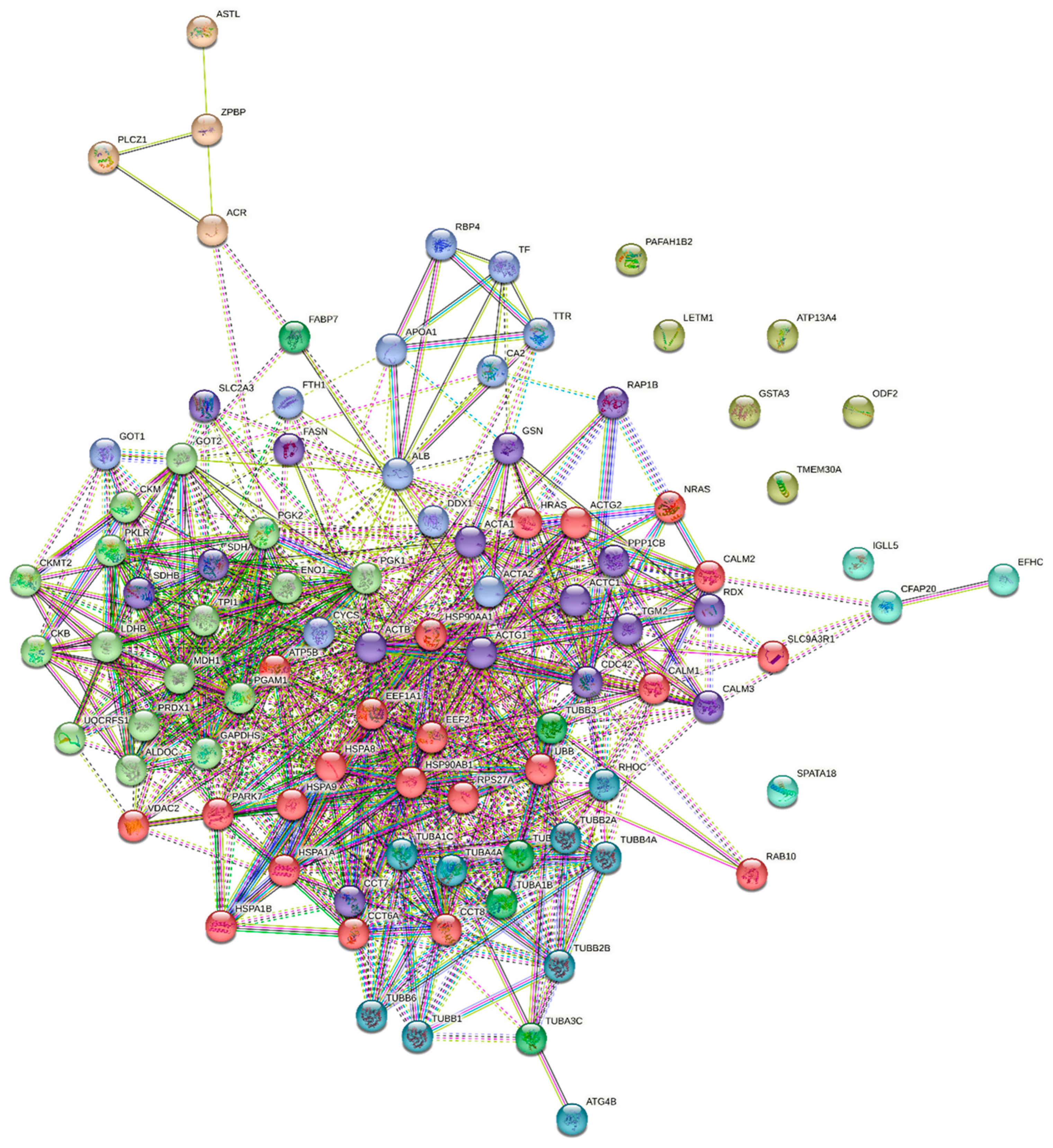
2.5. The Results of Functional Analysis of the Identified Sperm Phosphoproteins
3. Discussion
3.1. The Differences in Biological Parameters of White and Yellow Turkey Sperm
3.2. The Role of Protein Phosphorylation/Dephosphorylation Processes in Turkey Spermatozoa
3.3. The Role of Selected Sperm Phosphoproteins in Turkey Spermatozoa
3.4. Effect of Ejaculate Type on the Phosphorylation Profiles of Sperm Proteins
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Determination of Sperm Concentration
5.3. Determination of Sperm Motility Parameters (CASA)
5.4. Assessment of Plasma Membrane Integrity
5.5. Assessment of Mitochondrial Function in Spermatozoa
5.6. Assessment of Pro-Apoptotic Changes in Spermatozoa
5.7. Estimation of the Percentage of Sperm Producing Nitric Oxide
5.8. Antioxidant Capacity of Sperm
5.8.1. Determination of Superoxide Dismutase (SOD) Activity
5.8.2. Determination of Glutathione Peroxidase (GPx) Activity
5.8.3. Determination of Catalase Activity (CAT)
5.8.4. Determination of Reduced Glutathione (GSH) Content
5.8.5. Determination of Malondialdehyde (MDA) Levels
5.9. Evaluation of Total Protein Content in Sperm Extracts
5.10. Isolation of Phosphoproteins by Immobilised Fe3+ Affinity Chromatography on PHOS-Select Iron Affinity Gel Beads
5.11. Determination of the Molecular Weight of Sperm Phosphoproteins by One-Dimensional SDS Gel Electrophoresis (SDS-PAGE)
5.12. Electrotransfer and Immunodetection of Sperm Phosphoproteins
5.13. Identification of Sperm Phosphoproteins by Nano LC-MS/MS
5.14. Functional Analysis of the Identified Sperm Phosphoproteins
5.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thurston, R.J.; Korn, N. Semen quality in the domestic turkey: The yellow semen syndrome. Avian Poult. Biol. Rev. 1997, 8, 109–121. [Google Scholar]
- Thurston, R.J.; Hess, R.A.; Froman, D.P.; Biellier, H.V. Elevated seminal plasma protein: A characteristic of yellow turkey semen. Poult. Sci. 1982, 61, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Thurston, R.J. Protein, cholesterol, acid phosphatase and aspartate aminotransaminase in the seminal plasma of turkeys (Meleagris gallopavo) producing normal white or abnormal yellow semen. Biol. Reprod. 1984, 31, 239–243. [Google Scholar]
- Hess, R.A.; Thurston, R.J. Detection and incidence of yellow turkey semen on commercial breeder farms. Poult. Sci. 1984, 63, 2084–2086. [Google Scholar] [CrossRef] [PubMed]
- Słowińska, M.; Kozłowski, K.; Jankowski, J.; Ciereszko, A. Proteomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo). J. Anim. Sci. 2015, 93, 2785–2795. [Google Scholar] [CrossRef]
- Słowińska, M.; Nynca, J.; Arnold, G.J.; Fröhlich, T.; Jankowski, J.; Kozłowski, K.; Mostek, A.; Ciereszko, A. Proteomic identification of turkey (Meleagris gallopavo) seminal plasma proteins. Poult. Sci. 2017, 96, 3422–3435. [Google Scholar] [CrossRef]
- Słowińska, M.; Hejmej, A.; Bukowska, J.; Liszewska, E.; Bilińska, B.; Hliwa, P.; Kozłowski, L.; Jankowski, J.; Ciereszko, A. Expression and secretion of albumin in male turkey (Meleagris gallopavo) reproductive tract in relation to yellow semen syndrome. Poult. Sci. 2019, 98, 1872–1882. [Google Scholar] [CrossRef]
- Słowińska, M.; Sallem, H.; Clench, M.R.; Ciereszko, A. Metabolomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo). Poult. Sci. 2018, 97, 1059–1065. [Google Scholar] [CrossRef]
- Słowińska, M.; Jankowski, J.; Dietrich, G.J.; Karol, H.; Liszewska, E.; Glogowski, J.; Kozłowski, K.; Sartowska, K.; Ciereszko, A. Effect of organic and inorganic forms of selenium in diets on turkey semen quality. Poult. Sci. 2011, 90, 181–190. [Google Scholar] [CrossRef]
- Serrano, R.; Garcia-Marin, L.J.; Bragado, M.J. Sperm Phosphoproteome: Unraveling Male Infertility. Biology 2022, 11, 659. [Google Scholar] [CrossRef]
- Porambo, J.R.; Salicioni, A.M.; Visconti, P.E.; Platt, M.D. Sperm phosphoproteomics: Historical perspectives and current methodologies. Expert. Rev. Proteom. 2012, 9, 533–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, J.; Ling, X.; Liu, M.; Zhou, T. The human sperm proteome 2.0: An integrated resource for studying sperm functions at the level of posttranslational modification. Proteomics 2016, 16, 2597–2601. [Google Scholar] [CrossRef]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm-zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum. Reprod. 2006, 21, 1002–1008. [Google Scholar] [CrossRef]
- Buffone, M.G.; Verstraeten, S.V.; Calamera, J.C.; Doncel, G.F. High cholesterol content and decreased membrane fluidity in human spermatozoa are associated with protein tyrosine phosphorylation and functional deficiencies. J. Androl. 2009, 30, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Albero, M.C.; González-Brusi, L.; Cots, P.; Luongo, C.; Abril-Sánchez, S.; Ros-Santaella, J.L.; Pintus, E.; Ruiz-Díaz, S.; Barros-García, C.; Sánchez-Calabuig, M.J.; et al. Protein Identification of Spermatozoa and Seminal Plasma in Bottlenose Dolphin (Tursiops truncatus). Front. Cell Dev. Biol. 2021, 9, 673961. [Google Scholar] [CrossRef]
- Labas, V.; Grasseau, I.; Cahier, K.; Gargaros, A.; Harichaux, G.; Teixeira-Gomes, A.P.; Alves, S.; Bourin, M.; Gérard, N.; Blesbois, E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteomics 2015, 112, 313–335. [Google Scholar] [CrossRef]
- Fréour, T.; Jean, M.; Mirallié, S.; Dubourdieu, S.; Barrière, P. Computer-Assisted Sperm Analysis (CASA) parameters and their evolution during preparation as predictors of pregnancy in intrauterine insemination with frozen-thawed donor semen cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 149, 186–189. [Google Scholar] [CrossRef]
- Domosławska, A.; Zduńczyk, S.; Niżański, W.; Janowski, T. Assessment of semen quality in infertile dogs using computer-assisted sperm analysis by the Hamilton-Thorne Semen Analyser. J. Vet. Res. 2013, 57, 429–432. [Google Scholar] [CrossRef]
- Verstegen, J.; Iguer–Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef]
- Fetterlof, P.M.; Rogers, B.J. Prediction of human sperm penetrating ability using computerized motion parameters. Mol. Reprod. Dev. 1990, 2, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Pilane, C.M.; Mapeka, M.H. The response of cockerel semen kinematic parameters LIN, STR, WOB, ALH and BCF to induced oxidative stress. Open J. Anim. Sci. 2021, 11, 292–303. [Google Scholar] [CrossRef]
- Ayad, B.M.; Oyeyipo, I.P.; Van der Horst, G.; Du Plessis, S.S. Cementing the relationship between conventional and advanced semen parameters. Middle East. Fertil. Soc. J. 2021, 26, 39. [Google Scholar] [CrossRef]
- Hess, R.A.; Thurston, R.J.; Biellier, H.V. Morphology of the epididymal region and ductus deferens of the turkey (Meleagris gallopavo). J. Anat. 1976, 122, 241–252. [Google Scholar] [PubMed]
- Surai, P.F.; Blesbois, E.; Grasseau, I.; Chalah, T.; Brillard, J.P.; Wishart, G.J.; Cerolini, S.; Sparks, N.H. Fatty acid composition, glutathione peroxidase and superoxide dismutase activity and total antioxidant activity of avian semen. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 527–533. [Google Scholar] [CrossRef]
- Khan, R.U.; Laudadio, V.; Tufarelli, V. Semen traits and seminal plasma biochemical parameters in white leghorn layer breeders. Reprod. Domest. Anim. 2012, 47, 190–195. [Google Scholar] [CrossRef]
- Rafalska, K.T.; Orzołek, A.; Ner-Kluza, J.; Wysocki, P. A comparison of white and yellow seminal plasma phosphoproteomes obtained from turkey (Meleagris gallopavo) Semen. Int. J. Mol. Sci. 2024, 25, 9941. [Google Scholar] [CrossRef]
- Koziorowska-Gilun, M.; Orzołek, A.; Rafalska, K.; Miksa, M.; Dziekońska, A.; Kordan, W. Comparison of selected biological properties and antioxidative status of turkey (Meleagris gallopavo) spermatozoa derived from white and yellow semen. Reprod. Domest. Anim. 2022, 57, 74. [Google Scholar]
- Kobayashi, T.; Miyazaki, T.; Natori, M.; Nozawa, S. Protective role of superoxide dismutase in human sperm motility. Superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum. Reprod. 1991, 6, 987–991. [Google Scholar] [CrossRef]
- Zhang, H.; Limphong, P.; Pieper, J.; Liu, Q.; Rodesch, C.K.; Christians, E.; Benjamin, I.J. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J. 2012, 26, 1442–1451. [Google Scholar] [CrossRef]
- Evenson, D.P.; Darzynkiewicz, Z.; Melamed, M.R. Simultaneous measurement by flow cytometry of sperm cell viability and mitochondrial membrane potential related to cell motility. J. Histochem. Cytochem. 1982, 30, 279–280. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Jiang, H.; Zini, A.; Kodama, H.; Gagnon, C. Reactive oxygen species and sperm physiology. Rev. Reprod. 1997, 2, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Irvine, D.S.; Aitken, R.J. Evaluation of a spectrophotometric assay for the measurement of malondialdehyde and 4-hydroxyalkenals in human spermatozoa: Relationships with semen quality and sperm function. Int. J. Androl. 1998, 21, 81–94. [Google Scholar] [CrossRef]
- Alvarez, J.G.; Touchstone, J.C.; Blasco, L.; Storey, B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987, 8, 338–348. [Google Scholar] [CrossRef]
- Buffone, M.G.; Doncel, G.F.; Marin Briggiler, C.I.; Vazquez-Levin, M.H.; Calamera, J.C. Human sperm subpopulations: Relationship between functional quality and protein tyrosine phosphorylation. Hum. Reprod. 2004, 19, 139–146. [Google Scholar] [CrossRef]
- Jha, K.N.; Salicioni, A.M.; Arcelay, E.; Chertihin, O.; Kumari, S.; Herr, J.C.; Visconti, P.E. Evidence for the involvement of proline-directed serine/threonine phosphorylation in sperm capacitation. Mol. Hum. Reprod. 2006, 12, 781–789. [Google Scholar] [CrossRef]
- Hughes, K.; Nikolakaki, E.; Plyte, S.; Totty, N.F.; Woodgett, J.R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993, 12, 803–808. [Google Scholar] [CrossRef]
- Woodgett, J.R. Judging a protein by more than its name: GSK-3. Sci. STKE 2001, 18, re12. [Google Scholar] [CrossRef]
- Somanath, P.R.; Jack, S.L.; Vijayaraghavan, S. Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. J. Androl. 2013, 25, 605–617. [Google Scholar] [CrossRef]
- Visconti, P.E.; Galantino-Homer, H.; Moore, G.D.; Bailey, J.L.; Ning, X.; Fornes, M.; Kopf, G.S. The molecular basis of sperm capacitation. J. Androl. 1998, 19, 242–248. [Google Scholar] [CrossRef]
- Chan, C.C.; Shui, H.; Wu, C.H.; Wang, C.Y.; Sun, G.H.; Chen, H.M.; Wu, G.J. Motility and protein phosphorylation in healthy and asthenozoospermic sperm. J. Proteome Res. 2009, 8, 5382–5386. [Google Scholar] [CrossRef]
- Naz, R.K. Involvement of protein serine and threonine phosphorylation in human sperm capacitation. Biol. Reprod. 1999, 60, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Serrano, R.; Zaragoza, C.; Garcia-Marin, L.J.; Bragado, M.J. Human sperm phosphoproteome reveals differential phosphoprotein signatures that regulate human sperm motility. J. Proteom. 2020, 215, 103654. [Google Scholar] [CrossRef]
- Abdulsamad, H.M.R.; Murtaza, Z.F.; AlMuhairi, H.M.; Bafleh, W.S.; AlMansoori, S.A.; AlQubaisi, S.A.; Hamdan, H.; Kashir, J. The therapeutic and diagnostic potential of phospholipase C zeta, oocyte activation, and calcium in treating human infertility. Pharmaceuticals 2023, 16, 441. [Google Scholar] [CrossRef] [PubMed]
- Vasisth, R.; Mukesh, M.; Sodhi, M.; Kataria, R.S. Semen quality biomarkers for improvement of production in livestock. Adv. Anim. Exp. Model. 2022, 7, 77–84. [Google Scholar] [CrossRef]
- Wang, T.; Cao, B.; Cai, Y.; Chen, S.; Wang, B.; Yuan, Y.; Zhang, Q. Plcz1 Deficiency Decreased Fertility in Male Mice Which Is Associated with Sperm Quality Decline and Abnormal Cytoskeleton in Epididymis. Int. J. Mol. Sci. 2023, 24, 314. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Kastelic, J.P.; Thundathil, J.C. Ouabain-induced activation of phospholipase C zeta and its contributions to bovine sperm capacitation. Cell Tissue Res. 2021, 385, 785–801. [Google Scholar] [CrossRef]
- Wang, Z.; Grange, M.; Wagner, T.; Kho, A.L.; Gautel, M.; Rausner, S. The molecular basis for sarcomere organization in vertebrate skeletal muscle. Cell 2021, 184, 2135–2150. [Google Scholar] [CrossRef]
- Breitbert, H.; Finkelstein, M. Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [Google Scholar] [CrossRef]
- Reyes-Miguel, T.; Roa-Espitia, A.L.; Baltiérrez-Hoyos, R.; Hernández-González, E.O. CDC42 drives RHOA activity and actin polymerization during capacitation. Reproduction 2020, 160, 393–404. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Q.; Ma, C.; Wang, Y.; Zhang, P.; Li, C.; Cheng, X.; Xu, H. Comparative proteomics and phosphoproteomics analysis reveal the possible breed difference in Yorkshire and Duroc boar spermatozoa. Front. Cell Dev. Biol. 2021, 9, 652809. [Google Scholar] [CrossRef] [PubMed]
- Hinsch, K.D.; De Pinto, V.; Aires, V.A.; Schneider, X.; Messina, A.; Hinsch, E. Voltage-dependent anion-selective channels VDAC2 and VDAC3 are abundant proteins in bovine outer dense fibers, a cytoskeletal component of the sperm flagellum. J. Biol. Chem. 2004, 279, 15281–15288. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Sasanami, T. Sperm motility regulation in male and female bird genital tracts. J. Poult. Sci. 2022, 59, 1–7. [Google Scholar] [CrossRef]
- Liu, B.; Wang, P.; Wang, Z.; Jia, Y.; Niu, X.; Wang, W.; Zhang, W. Analysis and difference of voltage-dependent anion channel mRNA in ejaculated spermatozoa from normozoospermic fertile donors and infertile patients with idiopathic asthenozoospermia. J. Assist. Reprod. Genet. 2010, 27, 719–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, B.; Jung, B.K.; Park, S.H.; Song, K.J.; Anwar, M.A.; Ryu, K.Y.; Kim, K.P. Polyubiquitin gene Ubb is required for upregulation of Piwi protein level during mouse testis development. Cell Death Discov. 2021, 7, 194. [Google Scholar] [CrossRef] [PubMed]
- Naletova, I.; Schmalhausen, E.; Tomasello, B.; Pozdyshev, D.; Attanasio, F.; Muronetz, V. The role of sperm-specific glyceraldehyde-3-phosphate dehydrogenase in the development of pathologies—From asthenozoospermia to carcinogenesis. Front. Mol. Biosci. 2023, 30, 1256963. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Melnikova, A.K.; Barinova, K.V.; Schmalhausen, E.V. Inhibitors of Glyceraldehyde 3-Phosphate Dehydrogenase and Unexpected Effects of Its Reduced Activity. Biochemistry 2019, 84, 1268–1279. [Google Scholar] [CrossRef]
- Tisdale, E.J. Glyceraldehyde-3-phosphate dehydrogenase is phosphorylated by protein kinase Ciota /lambda and plays a role in microtubule dynamics in the early secretory pathway. J. Biol. Chem. 2002, 277, 3334–3341. [Google Scholar] [CrossRef]
- Inaba, K. Molecular architecture of the sperm flagella: Molecules for motility and signaling. Zool. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef]
- Audebert, S.; White, D.; Cosson, J.; Huitorel, P.; Eddé, B.; Gagnon, C. The carboxy-terminal sequence Asp427-Glu432 of beta-tubulin plays an important function in axonemal motility. Eur. J. Biochem. 1999, 261, 48–56. [Google Scholar] [CrossRef]
- Słowińska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Bukowska, J.; Kozłowski, K.; Jankowski, J.; Ciereszko, A. Transcriptome analysis of turkey (Meleagris gallopavo) reproductive tract revealed key pathways regulating spermatogenesis and post-testicular sperm maturation. Poult. Sci. 2020, 99, 6094–6118. [Google Scholar] [CrossRef]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta 2006, 1762, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Kekelidze, T.; Khait, I.; Togliatti, A.; Benzecry, J.M.; Wieringa, B.; Holtzman, D. Altered brain phosphocreatine and ATP regulation when mitochondrial creatine kinase is absent. J. Neurosci. Res. 2001, 66, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Fraga, D.; Edmiston, P.L. Regulation of creatine kinase activity by phosphorylation of serine-199 by AMP-activated kinase. FASEB J. 2008, 22, S1. [Google Scholar] [CrossRef]
- Chida, K.; Tsunenaga, M.; Kasahara, K.; Kohno, Y.; Kuroki, T. Regulation of creatine phosphokinase B activity by protein kinase C. Biochem. Biophys. Res. Commun. 1990, 173, 346–350. [Google Scholar] [CrossRef]
- Ríos, S.R.; Lamarche, F.; Cottet-Rousselle, C.; Klaus, A.; Tuerk, R.; Thali, R.; Auchli, Y.; Brunisholz, R.; Neumann, D.; Barret, L.; et al. Regulation of brain-type creatine kinase by AMP-activated protein kinase: Interaction, phosphorylation and ER localization. Biochim. Biophys. Acta 2014, 1837, 1271–1283. [Google Scholar] [CrossRef]
- Nasrallah, F.; Hammami, M.B.; Omar, S.; Aribia, H.B.; Sanhaji, H.; Feki, M. Semen creatine and creatine kinase activity as an indicator of sperm quality. Clin. Lab. 2020, 66, 1751–1757. [Google Scholar] [CrossRef]
- Rao, B.; Soufir, J.C.; Martin, M.; David, G. Lipid peroxidation in human spermatozoa as related to mid piece abnormalities and motility. Gamete Res. 1989, 24, 127–134. [Google Scholar] [CrossRef]
- Dandekar, S.P.; Parkar, G.M. Correlation between creatine kinase activity, lipid-peroxidation and water test in male infertility. J. Postgrad. Med. 1999, 45, 42–48. [Google Scholar]
- Saponaro, F.; Kim, J.H.; Chiellini, G. Transthyretin Stabilization: An Emerging Strategy for the Treatment of Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 8672. [Google Scholar] [CrossRef]
- Zhao, L.; Buxbaum, J.N.; Reixach, N. Age-related oxidative modifications of transthyretin modulate its amyloidogenicity. Biochemistry 2013, 52, 1913–1926. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, Z.; Nakashima, A.; Sharma, S. Gestational Age-Dependent Regulation of Transthyretin in Mice during Pregnancy. Biology 2023, 12, 1048. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Kang, D.; Yu, D.-Y.; Rhee, S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signalling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Albrecht, E.; Dannenberger, D.; Hammon, H.M.; Kuehn, C.; Sauerwein, H.; Yang, R.; Zhao, Z.; Maak, S. Retinol binding protein 4 abundance in plasma and tissues is related to body fat deposition in cattle. Sci. Rep. 2019, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- Cope, F.O.; Staller, J.M.; Mahsem, R.A.; Boutwell, R.K. Retinoid-binding proteins are phosphorylated in vitro by soluble Ca2+- and phosphatidylserine-dependent protein kinase from mouse brain. Biochem. Biophys. Res. Commun. 1984, 120, 593–601. [Google Scholar] [CrossRef]
- Ninomiya, M.; Fujiki, H.; Paik, N.S.; Horiuchi, T.; Boutwell, R.K. Absence of phosphorylation of retinoid-binding proteins by protein kinase C in vitro. Biochem. Biophys. Res. Commun. 1986, 138, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Vilagran, I.; Castillo-Martín, M.; Prieto-Martínez, N.; Bonet, S.; Yeste, M. Triosephosphate isomerase (TPI) and epididymal secretory glutathione peroxidase (GPX5) are markers for boar sperm quality. Anim. Reprod. Sci. 2016, 165, 22–30. [Google Scholar] [CrossRef]
- Schachner, L.F.; Des Soye, B.; Ro, S.; Kenney, G.E.; Ives, A.N.; Su, T.; Goo, Y.A.; Jewett, M.C.; Rosenzweig, A.C.; Kelleher, N.L. Revving an Engine of Human Metabolism: Activity Enhancement of Triosephosphate Isomerase via Hemi-Phosphorylation. ACS Chem. Biol. 2022, 17, 2769–2780. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol. Cell Proteom. 2015, 14, 1230–1240. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. The Collection of Spermatozoa from the Domestic Fowl and Turkey. Poult. Sci. 1937, 16, 19–24. [Google Scholar] [CrossRef]
- Garner, D.L.; Johnson, L.A. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 1995, 53, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.; Lecewicz, M.; Strzeżek, J. Fluorometric assessments of viability and mitochondrial status of boar spermatozoa following liquid storage. Pol. J. Vet. Sci. 2002, 5, 85–92. [Google Scholar]
- Thomas, C.A.; Garner, D.L.; DeJarnette, J.M.; Marshall, C.E. Effect of cryopreservation of bovine sperm organelle function and viability as determined by flow cytometry. Biol. Reprod. 1998, 58, 786–793. [Google Scholar] [CrossRef]
- Trzcińska, M.; Bryła, M. Apoptotic-like changes of boar spermatozoa in freezing media supplemented with different antioxidants. Pol. J. Vet. Sci. 2015, 18, 473–480. [Google Scholar] [CrossRef]
- Wasilewska, K.; Fraser, L. Boar variability in sperm cryo-tolerance after cooling of semen in different long-term extenders at various temperatures. Anim. Reprod. Sci. 2017, 185, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Lampiao, F.; Strijdom, H.; du Plessis, S.S. Direct nitric oxide measurement in human spermatozoa: Flow cytometric analysis using the fluorescent probe, diaminofluorescein. Int. J. Androl. 2006, 29, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
| White | Yellow | |
|---|---|---|
| TMOT (%) | 68.43 ± 3.58 a | 57.43 ± 3.91 b |
| PMOT (%) | 35.22 ± 2.34 | 32.37 ± 3.37 |
| VAP (µm/s) | 77.68 ± 1.16 | 80.19 ± 1.95 |
| VSL (µm/s) | 66.55 ± 1.45 b | 72.44 ± 2.14 a |
| VCL (µm/s) | 113.07 ± 1.59 A | 105.23 ± 2.17 B |
| ALH (µm) | 3.63 ± 0.07 | 3.59 ± 0.11 |
| BCF (Hz) | 17.64 ± 0.67 A | 14.25 ± 0.83 B |
| STR (%) | 84.60 ± 0.87 B | 89.37 ± 0.90 A |
| LIN (%) | 62.29 ± 1.43 B | 70.69 ± 1.69 A |
| Sperm concentration (×109) | 3.51 ± 0.12 A | 2.18 ± 0.13 B |
| PMI (%) | 85.78 ± 0.82 a | 81.58 ± 1.56 b |
| MMP (%) | 83.73 ± 1.02 A | 79.01 ± 1.44 B |
| NO-producing sperm (%) | 42.65 ± 3.36 | 44.09 ± 3.75 |
| Viable sperm (%) | 85.91 ± 1.01 A | 75.66 ± 0.71 B |
| Pro-apoptotic spermatozoa (%) | 12.16 ± 0.95 B | 21.03 ±0.63 A |
| Necrotic spermatozoa (%) | 1.93 ± 0.33 B | 3.32 ± 0.27 A |
| SOD activity (U/109 sperm.) | 0.42 ± 0.04 B | 0.86 ± 0.14 A |
| GPx activity (U/109 sperm.) | 99.36 ± 13.39 b | 168.68 ± 30.11 a |
| CAT activity (µM/min/109 sperm.) | 1.84 ± 0.78 | 5.95 ± 2.49 |
| GSH content (µM/109 sperm.) | 85.39 ± 6.39 B | 136.13 ± 21.69 A |
| MDA levels (µM/109 sperm.) | 15.31 ± 1.87 | 12.71 ± 1.72 |
| TMOT (%) | PMOT (%) | VAP (µm/s) | VSL (µm/s) | VCL (µm/s) | ALH (µm) | BCF (Hz) | STR (%) | LIN (%) | |
|---|---|---|---|---|---|---|---|---|---|
| SOD activity (U/109 sperm.) | −0.11 | −0.02 | 0.10 | 0.12 | −0.06 | −0.05 | 0.04 | 0.11 | 0.11 |
| GPx activity (U/109 sperm.) | 0.02 | 0.10 | −0.26 | −0.25 | 0.05 | 0.06 | 0.32 | −0.22 | −0.24 |
| CAT activity (µM/min/109 sperm.) | 0.13 | 0.29 | 0.15 | 0.13 | 0.14 | −0.05 | 0.22 | 0.07 | 0.05 |
| GSH content (µM/109 sperm.) | −0.16 | −0.15 | −0.31 | −0.21 | −0.21 | −0.19 | 0.27 | −0.03 | −0.08 |
| MDA levels (µM/109 sperm.) | −0.10 | −0.04 | −0.02 | −0.03 | −0.01 | 0.11 | 0.10 | −0.04 | −0.06 |
| TMOT (%) | PMOT (%) | VAP (µm/s) | VSL (µm/s) | VCL (µm/s) | ALH (µm) | BCF (Hz) | STR (%) | LIN (%) | |
|---|---|---|---|---|---|---|---|---|---|
| SOD activity (U/109 sperm.) | 0.15 | 0.03 | −0.07 | −0.02 | −0.28 | −0.05 | −0.21 | 0.18 | 0.14 |
| GPx activity (U/109 sperm.) | 0.12 | 0.13 | −0.33 | −0.24 | −0.35 | 0.19 | −0.02 | 0.03 | −0.04 |
| CAT activity (µM/min/109 sperm.) | 0.52 * | 0.56 * | −0.15 | −0.13 | 0.05 | −0.17 | 0.63 | −0.03 | −0.18 |
| GSH content (µM/109 sperm.) | 0.21 | 0.24 | 0.62 * | 0.63 * | 0.14 | 0.48 | 0.06 | 0.51 * | 0.52 * |
| MDA levels (µM/109 sperm.) | 0.20 | 0.32 | 0.24 | 0.38 | 0.51 * | −0.19 | −0.28 | 0.51 * | 0.60 * |
| Sperm Concentration (×106 sperm./mL) | PMI (%) | MMP (%) | NO (%) | Viable (%) | Pro-Apoptotic (%) | Necrotic (%) | |
|---|---|---|---|---|---|---|---|
| SOD activity (U/109 sperm.) | −0.44 * | 0.15 | 0.14 | 0.44 * | 0.28 | −0.33 | 0.20 |
| GPx activity (U/109 sperm.) | −0.37 * | 0.10 | 0.08 | 0.06 | 0.15 | −0.15 | 0.00 |
| CAT activity (µM/min/109 sperm.) | 0.08 | 0.08 | 0.28 | 0.00 | −0.43 * | 0.45 * | −0.08 |
| GSH content (µM/109 sperm.) | −0.14 | 0.09 | 0.02 | −0.18 | 0.09 | −0.04 | −0.17 |
| MDA levels (µM/109 sperm.) | 0.36 * | −0.02 | 0.14 | 0.05 | 0.00 | 0.01 | −0.06 |
| Sperm Concentration (×106 sperm./mL) | PMI (%) | MMP (%) | NO (%) | Viable (%) | Pro-Apoptotic (%) | Necrotic (%) | |
|---|---|---|---|---|---|---|---|
| SOD activity (U/109 sperm.) | −0.25 | 0.32 | 0.44 | 0.33 | 0.04 | −0.20 | 0.33 |
| GPx activity (U/109 sperm.) | −0.35 | 0.14 | 0.10 | 0.18 | −0.17 | 0.22 | −0.12 |
| CAT activity (µM/min/109 sperm.) | −0.25 | 0.16 | 0.28 | −0.20 | 0.09 | 0.04 | −0.26 |
| GSH content (µM/109 sperm.) | −0.07 | −0.14 | −0.10 | 0.34 | −0.03 | −0.10 | 0.26 |
| MDA levels (µM/109 sperm.) | 0.38 * | −0.37 | −0.34 | 0.57 * | −0.04 | −0.09 | 0.19 |
| Protein Band [kDa] | White | Yellow |
|---|---|---|
| 52 | 0.48 | 0.32 |
| 51 | 0.43 | 0.23 |
| 49 | 0.24 | 0.45 |
| 43 | 0.30 | 0.44 |
| 40 | 0.49 | 0.27 |
| 29 | 0.38 | 0.25 |
| 24 | 0.33 | 0.16 |
| 18 | 0.22 | 0.29 |
| 15 | 0.24 | 0.28 |
| 13 | 0.23 | 0.35 |
| Protein Band | Molecular Weight (Multi-Analyst) | Residue | Type of Semen | Arithmetic Mean | ±SEM |
|---|---|---|---|---|---|
| 15 | 52 kDa | SER | W | 0.17 | 0.03 |
| Y | 0.16 | 0.04 | |||
| THR | W | 0.16 * | 0.02 | ||
| Y | 0.27 * | 0.03 | |||
| TYR | W | 0.14 | 0.04 | ||
| Y | 0.20 | 0.03 | |||
| 16 | 51 kDa | SER | W | 0.18 | 0.03 |
| Y | 0.17 | 0.04 | |||
| THR | W | 0.17 | 0.03 | ||
| Y | 0.27 | 0.03 | |||
| TYR | W | 0.16 | 0.05 | ||
| Y | 0.21 | 0.03 | |||
| 17 | 49 kDa | SER | W | 0.18 | 0.03 |
| Y | 0.15 | 0.03 | |||
| THR | W | 0.18 | 0.03 | ||
| Y | 0.27 | 0.03 | |||
| TYR | W | 0.15 | 0.04 | ||
| Y | 0.20 | 0.03 | |||
| 18 | 43 kDa | SER | W | 0.17 | 0.03 |
| Y | 0.14 | 0.02 | |||
| THR | W | 0.16 * | 0.02 | ||
| Y | 0.25 * | 0.02 | |||
| TYR | W | 0.13 | 0.03 | ||
| Y | 0.18 | 0.02 | |||
| 19 | 40 kDa | SER | W | 0.20 | 0.04 |
| Y | 0.18 | 0.03 | |||
| THR | W | 0.17 * | 0.03 | ||
| Y | 0.27 * | 0.03 | |||
| TYR | W | 0.16 | 0.04 | ||
| Y | 0.20 | 0.03 | |||
| 20 | 29 kDa | SER | W | 0.31 | 0.08 |
| Y | 0.40 | 0.09 | |||
| THR | W | 0.30 | 0.06 | ||
| Y | 0.42 | 0.07 | |||
| TYR | W | 0.29 | 0.07 | ||
| Y | 0.33 | 0.06 | |||
| 21 | 24 kDa | SER | W | 0.11 | 0.03 |
| Y | 0.09 | 0.02 | |||
| THR | W | 0.16 | 0.03 | ||
| Y | 0.19 | 0.01 | |||
| TYR | W | 0.11 | 0.02 | ||
| Y | 0.10 | 0.02 | |||
| 22 | 18 kDa | SER | W | 0.07 | 0.01 |
| Y | 0.05 | 0.01 | |||
| THR | W | 0.13 | 0.03 | ||
| Y | 0.18 | 0.01 | |||
| TYR | W | 0.07 | 0.02 | ||
| Y | 0.08 | 0.01 | |||
| 23 | 15 kDa | SER | W | 0.10 | 0.03 |
| Y | 0.12 | 0.04 | |||
| THR | W | 0.15 | 0.04 | ||
| Y | 0.18 | 0.01 | |||
| TYR | W | 0.08 | 0.03 | ||
| Y | 0.10 | 0.02 | |||
| 24 | 13 kDa | SER | W | 0.04 | 0.01 |
| Y | 0.05 | 0.02 | |||
| THR | W | 0.14 | 0.03 | ||
| Y | 0.16 | 0.01 | |||
| TYR | W | 0.07 | 0.02 | ||
| Y | 0.06 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafalska, K.T.; Orzołek, A.; Ner-Kluza, J.; Wysocki, P. Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa? Int. J. Mol. Sci. 2025, 26, 3467. https://doi.org/10.3390/ijms26083467
Rafalska KT, Orzołek A, Ner-Kluza J, Wysocki P. Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa? International Journal of Molecular Sciences. 2025; 26(8):3467. https://doi.org/10.3390/ijms26083467
Chicago/Turabian StyleRafalska, Katarzyna T., Aleksandra Orzołek, Joanna Ner-Kluza, and Paweł Wysocki. 2025. "Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa?" International Journal of Molecular Sciences 26, no. 8: 3467. https://doi.org/10.3390/ijms26083467
APA StyleRafalska, K. T., Orzołek, A., Ner-Kluza, J., & Wysocki, P. (2025). Does the Type of Semen Affect the Phosphoproteome of Turkey (Meleagris gallopavo) Spermatozoa? International Journal of Molecular Sciences, 26(8), 3467. https://doi.org/10.3390/ijms26083467






