Unraveling the Intricacies of Powdery Mildew: Insights into Colonization, Plant Defense Mechanisms, and Future Strategies
Abstract
:1. Introduction
2. Traditional Research on Powdery Mildew
2.1. Symptoms and Effect of Powdery Mildew Infection on Plants
2.1.1. Symptoms of Powdery Mildew Infection
2.1.2. Effects of Powdery Mildew Infection on Plants
2.2. Pathogenesis of Powdery Mildew at Different Stages
2.2.1. Fungal Spore Germination and Appressorium Formation
2.2.2. Mechanisms and Factors Influencing Powdery Mildew Pathogenesis
2.3. Factors Affecting the Colonization of Powdery Mildew Pathogen
2.3.1. Environmental Factors
2.3.2. Host Plant Factors
2.3.3. Physiological Factors
3. Defense Mechanisms in Plants
3.1. Plants’ Defense Mechanisms Against Pathogens
3.2. Mechanisms of Plant Defense Against Pathogenic Fungi
3.2.1. Plants Resist Powdery Mildew Molecular Mechanism of Invasion
3.2.2. Transcriptional Regulation for the Prevention of Powdery Mildew
3.2.3. Powdery Mildew-Infected Plants Involved in AS Regulation
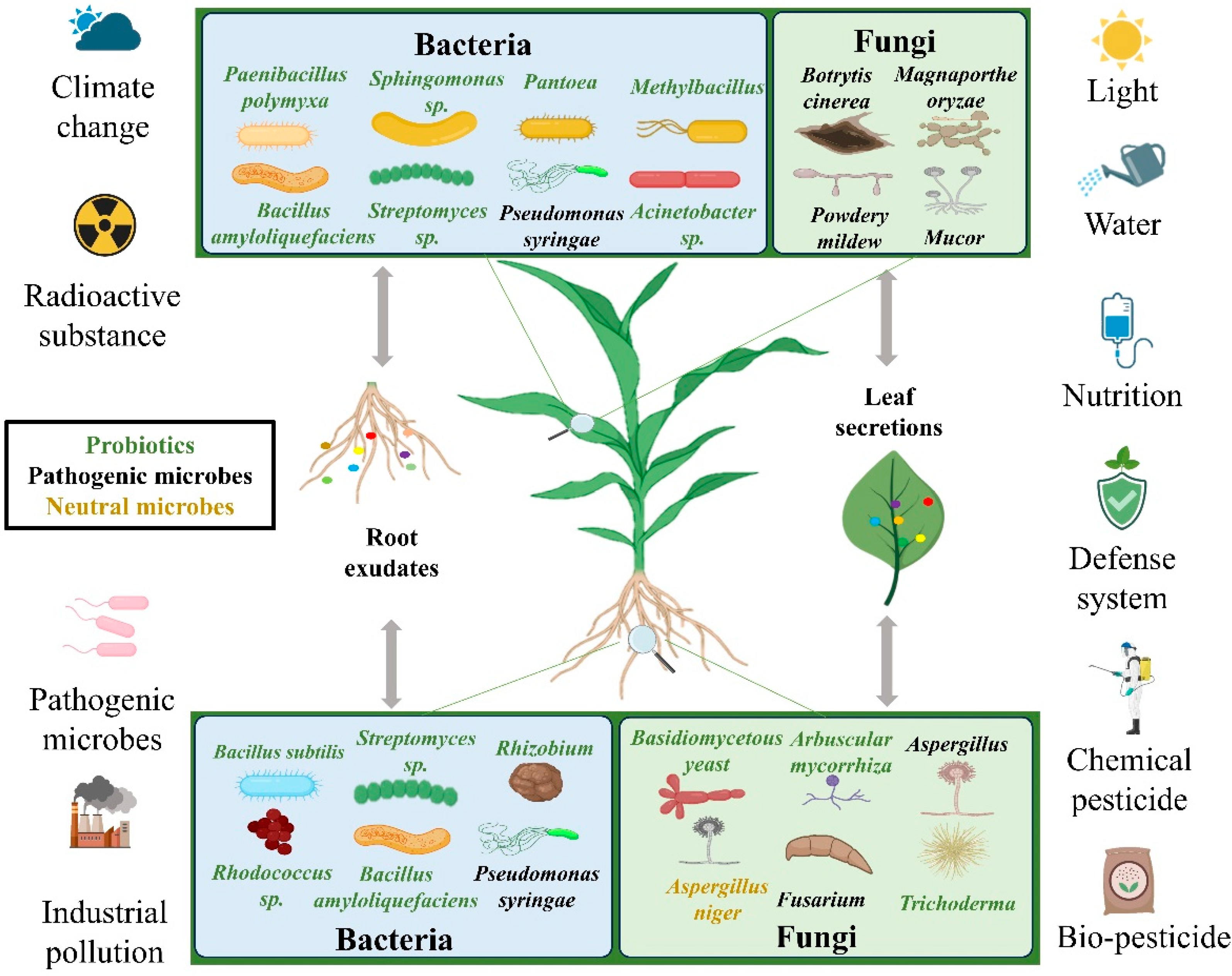
4. Interactions of Powdery Mildew with Symbiotic Microorganism
4.1. The Role of the Microbiome
4.2. Rhizosphere Microorganisms
4.3. Effect on the Structure of Phyllosphere Microorganisms
5. Strategies Used to Control Powdery Mildew
5.1. Methods of Controlling Powdery Mildew and the Need for Biological Control Agents
5.2. Biopesticides
5.3. Environmental Impact of Fungicides vs. Biocontrol
6. Conclusions and Outlook
6.1. Future Directions
6.2. Innovative Microbial Consortia: A Revolutionary Approach to Controlling Powdery Mildew in Agroecosystems
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| MLO | Mildew resistance Locus O |
| AS | Alternative splicing |
| SA | Salicylic acid |
| CWA | Cell Wall Apposition |
| Bgh | Blumeria graminis f. sp. Hordei |
| Bgt | Blumeria graminis f. sp. tritici |
| JA | Jasmonic acid |
| ET | Ethylene |
| NLRs | Nucleotide-binding and leucine-rich repeat receptors |
| PTI | Pattern-triggered immunity |
| ETI | Effector-triggered immunity |
| SP | Spore |
| CW | Cell wall |
| PM | Powdery mildew |
| HR | Hypersensitive response |
| ap | Ascospore |
| PAMP | Pathogen-associated molecular pattern |
| PCD | Programmed cell death |
| MAP | Mitogen-activated protein |
| ATAF1 | As a transcriptional activator, one of the first NAC proteins identified in Arabidopsis |
| TF | Transcription Factor |
| FHB | Fusarium head blight |
| CDA | Chitin deacetylase activity |
| MAMP | Microbe-associated molecular pattern |
| MBCAs | Microbial biological control agents |
| BTH | Benzothiadiazole |
References
- Damicone, J.P.; Sutherland, A.J. First Report of Pepper Powdery Mildew Caused by Leveillula taurica in Oklahoma. Plant Dis. 1999, 83, 1072. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, A.; Bertetti, D.; Frati, S.; Gullino, M.L. First Report of Powdery Mildew Caused by Golovinomyces cichoracearum on Orange Coneflower (Rudbeckia fulgida) in Italy. Plant Dis. 2008, 92, 975. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.; Tobin, P.C. Sequencing Herbarium Specimens of a Common Detrimental Plant Disease (Powdery Mildew). Phytopathology 2020, 110, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Durán, P.; Reinstädler, A.; Rajakrut, A.L.; Hashimoto, M.; Garrido-Oter, R.; Schulze-Lefert, P.; Panstruga, R. A fungal powdery mildew pathogen induces extensive local and marginal systemic changes in the Arabidopsis thaliana microbiota. Environ. Microbiol. 2021, 23, 6292–6308. [Google Scholar] [CrossRef]
- Tan, L.; Song, Q.; Shi, Y.; Wang, J.; Weng, Q.; Liu, Q. First Report of Powdery Mildew on Rosa cymosa Caused by Podosphaera pannosa in Guizhou Province, China. Plant Dis. 2023, 107, 570. [Google Scholar] [CrossRef]
- Sotiropoulos, A.G.; Arango-Isaza, E.; Ban, T.; Barbieri, C.; Bourras, S.; Cowger, C.; Czembor, P.C.; Ben-David, R.; Dinoor, A.; Ellwood, S.R.; et al. Global genomic analyses of wheat powdery mildew reveal association of pathogen spread with historical human migration and trade. Nat. Commun. 2022, 13, 4315. [Google Scholar] [CrossRef]
- Glawe, D.A. The Powdery Mildews: A Review of the World’s Most Familiar (Yet Poorly Known) Plant Pathogens. Annu. Rev. Phytopathol. 2008, 46, 27–51. [Google Scholar] [CrossRef]
- Essling, M.; McKay, S.; Petrie, P.R. Fungicide programs used to manage powdery mildew (Erysiphe necator) in Australian vineyards. Crop. Prot. 2021, 139, 105369. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Li, N.; Gao, J.; Liu, S.; Zhu, S.; Li, Z.; Ren, G.; Kuai, B. Chemical induction of leaf senescence and powdery mildew resistance involves ethylene-mediated chlorophyll degradation and ROS metabolism in cucumber. Hortic. Res. 2022, 9, uhac101. [Google Scholar] [CrossRef]
- Chhuneja, P.; Kumar, K.; Stirnweis, D.; Hurni, S.; Keller, B.; Dhaliwal, H.S.; Singh, K. Identification and mapping of two powdery mildew resistance genes in Triticum boeoticum L. Theor. Appl. Genet. 2012, 124, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Kusch, S.; Qian, J.; Loos, A.; Kümmel, F.; Spanu, P.D.; Panstruga, R. Long-term and rapid evolution in powdery mildew fungi. Mol. Ecol. 2023, 33, e16909. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, Y.; Cai, X.; Zhang, H.; Chen, Y.; Niu, Y.; Wang, Z.; Li, M.; Liu, J.; Wang, H.; et al. Temporal role of the receptor-like cytoplasmic kinase gene Stpk-V in wheat-Blumeria graminis f. sp. tritici interaction by RNA-seq analysis. Plant Growth Regul. 2021, 95, 429–442. [Google Scholar] [CrossRef]
- Mu, B.; Teng, Z.; Tang, R.; Lu, M.; Chen, J.; Xu, X.; Wen, Y.-Q. An effector of Erysiphe necator translocates to chloroplasts and plasma membrane to suppress host immunity in grapevine. Hortic. Res. 2023, 10, uhad163. [Google Scholar] [CrossRef]
- Kusch, S.; Frantzeskakis, L.; Lassen, B.D.; Kümmel, F.; Pesch, L.; Barsoum, M.; Walden, K.D.; Panstruga, R. A fungal plant pathogen overcomes mlo-mediated broad-spectrum disease resistance by rapid gene loss. New Phytol. 2024, 244, 962–979. [Google Scholar] [CrossRef]
- Baillo, E.H.; Kimotho, R.N.; Zhang, Z.; Xu, P. Transcription Factors Associated with Abiotic and Biotic Stress Tolerance and Their Potential for Crops Improvement. Genes 2019, 10, 771. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, R.; Wang, Y.; Zhang, L.; Wang, C.; Lv, S.; Liu, X.; Wang, Y.; Ji, W. Transcriptome-wide alternative splicing modulation during plant-pathogen interactions in wheat. Plant Sci. 2019, 288, 110160. [Google Scholar] [CrossRef]
- Gu, J.; Ma, X.; Ma, Q.; Xia, Z.; Lin, Y.; Yuan, J.; Li, Y.; Li, C.; Chen, Y.; Wang, W.; et al. RNA splicing modulates the postharvest physiological deterioration of cassava storage root. Plant Physiol. 2024, 196, 461–478. [Google Scholar] [CrossRef]
- Amorim, M.d.F.; Willing, E.-M.; Szabo, E.X.; Francisco-Mangilet, A.G.; Droste-Borel, I.; Maček, B.; Schneeberger, K.; Laubinger, S. The U1 snRNP Subunit LUC7 Modulates Plant Development and Stress Responses via Regulation of Alternative Splicing. Plant Cell 2018, 30, 2838–2854. [Google Scholar] [CrossRef]
- Staiger, D.; Brown, J.W. Alternative Splicing at the Intersection of Biological Timing, Development, and Stress Responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef]
- Gassmann, W. Alternative splicing in plant defense. Curr. Top. Microbiol. Immunol. 2008, 326, 219–233. [Google Scholar] [PubMed]
- Liu, Y.; Do, S.; Huynh, H.; Li, J.-X.; Liu, Y.-G.; Du, Z.-Y.; Chen, M.-X. Importance of pre-mRNA splicing and its study tools in plants. Adv. Biotechnol. 2024, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Mei, L.; Wang, F.; Dewayalage, I.K.W.B.; Yang, J.; Dai, L.; Yang, G.; Gao, B.; Cheng, C.; Liu, Y.; et al. PlantSPEAD: A web resource towards comparatively analysing stress-responsive expression of splicing-related proteins in plant. Plant Biotechnol. J. 2020, 19, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Panstruga, R.; Schulze-Lefert, P. Live and let live: Insights into powdery mildew disease and resistance. Mol. Plant Pathol. 2002, 3, 495–502. [Google Scholar] [CrossRef]
- Farinas, C.; Gluck-Thaler, E.; Slot, J.C.; Hand, F.P. Whole-Genome Sequence of the Phlox Powdery Mildew Pathogen Golovinomyces magnicellulatus Strain FPH2017-1. Genome Announc. 2019, 8, e00852-19. [Google Scholar] [CrossRef]
- Ghafoor, A.; McPhee, K. Marker assisted selection (MAS) for developing powdery mildew resistant pea cultivars. Euphytica 2012, 186, 593–607. [Google Scholar] [CrossRef]
- Byrne, D.H. Advances in Rose Breeding and Genetics in North America. In Proceedings of the VI International Symposium on Rose Research and Cultivation, Hannover, Germany, 25–30 August 2015; Volume 1064, pp. 89–98. [Google Scholar]
- Bindschedler, L.V.; Panstruga, R.; Spanu, P.D. Mildew-Omics: How Global Analyses Aid the Understanding of Life and Evolution of Powdery Mildews. Front. Plant Sci. 2016, 7, 123. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Yamada, M.; Kobayashi, I.; Kunoh, H. Actin Microfilaments are Required for the Expression of Nonhost Resistance in Higher Plants. Plant Cell Physiol. 1997, 38, 725–733. [Google Scholar] [CrossRef]
- Schulze-Lefert, P.; Vogel, J. Closing the ranks to attack by powdery mildew. Trends Plant Sci. 2000, 5, 343–348. [Google Scholar] [CrossRef]
- Vogel, J.P.; Raab, T.K.; Somerville, C.R.; Somerville, S.C. Mutations in PMR5 result in powdery mildew resistance and altered cell wall composition. Plant J. 2004, 40, 968–978. [Google Scholar] [CrossRef]
- Zhang, Z.; Henderson, C.; Gurr, S.J. Blumeria graminis secretes an extracellular catalase during infection of barley: Potential role in suppression of host defence. Mol. Plant Pathol. 2004, 5, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Dittgen, J.; Sánchez-Rodríguez, C.; Hou, B.H.; Molina, A.; Schulze-Lefert, P.; Lipka, V.; Somerville, S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 2006, 18, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Krattinger, S.G.; Lagudah, E.S.; Spielmeyer, W.; Singh, R.P.; Huerta-Espino, J.; McFadden, H.; Bossolini, E.; Selter, L.L.; Keller, B. A Putative ABC Transporter Confers Durable Resistance to Multiple Fungal Pathogens in Wheat. Science 2009, 323, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nishimura, M.T.; Zhao, T.; Tang, D. ATG2, an autophagy-related protein, negatively affects powdery mildew resistance and mildew-induced cell death in Arabidopsis. Plant J. 2011, 68, 74–87. [Google Scholar] [CrossRef]
- Mandal, S.N.; Fu, Y.; Zhang, S.; Ji, W. Proteomic Analysis of the Defense Response of Wheat to the Powdery Mildew Fungus, Blumeria graminis f. sp. tritici. Protein J. 2014, 33, 513–524. [Google Scholar] [CrossRef]
- Marchica, A.; Pisuttu, C.; Calzone, A.; Bernardi, R.; Lorenzini, G. First report of powdery mildew caused by Erysiphe platani in Ailanthus altissima, the tree-of-heaven, in the Mediterranean basin, Italy. J. Gen. Plant Pathol. 2020, 86, 428–431. [Google Scholar] [CrossRef]
- Koike, S.T. Powdery Mildew, Caused by Leveillula taurica, on Matilija Poppy in California. Plant Dis. 2007, 91, 329. [Google Scholar] [CrossRef]
- Pramanik, D.; Shelake, R.M.; Park, J.; Kim, M.J.; Hwang, I.; Park, Y.; Kim, J.Y. CRISPR/Cas9-Mediated Generation of Pathogen-Resistant Tomato against Tomato Yellow Leaf Curl Virus and Powdery Mildew. Int. J. Mol. Sci. 2021, 22, 1878. [Google Scholar] [CrossRef]
- Williamson, M.R.; Blake, J.H. First Report of the Teleomorph of an Oidium sp. Causing Powdery Mildew on Flowering Dogwood in South Carolina. Plant Dis. 1999, 83, 200. [Google Scholar] [CrossRef]
- Hoshi, H.; Sato, Y.; Kagiwada, S.; Horie, H. First report of powdery mildew on zinnia, blue torenia, dahurian patrinia and scoparia caused by genus Euoidium in Japan. J. Gen. Plant Pathol. 2013, 79, 89–95. [Google Scholar] [CrossRef]
- Bal, J.S.; Gill, K.S.; Singh, J. The Vegetative and Fruit Characteristics of Hybrid of Indian Jujube. In Proceedings of the II International Jujube Symposium, Xinzheng, China, 3–7 September 2013; Volume 993, pp. 43–46. [Google Scholar]
- Duan, W.; Peng, L.; Jiang, J.; Zhang, H.; Tong, G. Combined transcriptome and metabolome analysis of strawberry fruits in response to powdery mildew infection. Agron. J. 2022, 114, 1027–1039. [Google Scholar] [CrossRef]
- Dracatos, P.M.; Lu, J.; Sánchez-Martín, J.; Wulff, B.B. Resistance that stacks up: Engineering rust and mildew disease control in the cereal crops wheat and barley. Plant Biotechnol. J. 2023, 21, 1938–1951. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Dreyer, E.; Marçais, B. Impact of Erysiphe alphitoides on transpiration and photosynthesis in Quercus robur leaves. Eur. J. Plant Pathol. 2009, 125, 63–72. [Google Scholar] [CrossRef]
- Choi, I.; Abasova, L.; Choi, J.; Park, J.H.; Shin, H.D. Erysiphe lonicerigena sp. nov., a Powdery Mildew Species Found on Lonicera harae. Mycobiology 2023, 51, 67–71. [Google Scholar] [CrossRef]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze-Lefert, P.; et al. Conserved requirement for a plant host cell protein in powdery mildew pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Liang, X.; Wang, B. Wheat Powdery Mildew Spore Images Segmentation Based on U-Net. J. Phys. Conf. Ser. 2020, 1631, 012074. [Google Scholar] [CrossRef]
- Surana, P.; Xu, R.; Fuerst, G.; Chapman, A.V.E.; Nettleton, D.; Wise, R.P. Interchromosomal Transfer of Immune Regulation During Infection of Barley with the Powdery Mildew Pathogen. G3 Genes Genomes Genet. 2017, 7, 3317–3329. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.; Ahmed, H.A.; Hassan, M.A.; El-Fatah, B.E. Influence of foliar application of some salts, phyto-extracts and essential oils for contolling powdery mildew disease of Helianthus annuus. J. Plant Pathol. 2022, 104, 735–747. [Google Scholar] [CrossRef]
- Yi, R.H.; Li, K.Y.; Liang, J.J.; Huang, Y.; Liao, H.Z.; He, P.L.; Li, D. Erysiphe elevata causing powdery mildew on Eucalyptus urophylla × E. camaldulensis in China. Plant Dis. 2023, 107, 3305. [Google Scholar] [CrossRef]
- Wang, L.-F.; Wang, M.; Zhang, Y. Effects of powdery mildew infection on chloroplast and mitochondrial functions in rubber tree. Trop. Plant Pathol. 2014, 39, 242–250. [Google Scholar] [CrossRef]
- Liang, X.; Ma, Z.; Ke, Y.; Wang, J.; Wang, L.; Qin, B.; Tang, C.; Liu, M.; Xian, X.; Yang, Y.; et al. Single-cell transcriptomic analyses reveal cellular and molecular patterns of rubber tree response to early powdery mildew infection. Plant Cell Environ. 2023, 46, 2222–2237. [Google Scholar] [CrossRef]
- Panstruga, R.; Kuhn, H. Mutual interplay between phytopathogenic powdery mildew fungi and other microorganisms. Mol. Plant Pathol. 2019, 20, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Ingvardsen, C.R.; Massange-Sánchez, J.A.; Borum, F.; Füchtbauer, W.S.; Bagge, M.; Knudsen, S.; Gregersen, P.L. Highly effective mlo-based powdery mildew resistance in hexaploid wheat without pleiotropic effects. Plant Sci. 2023, 335, 111785. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Naing, A.H.; Kang, H.; Lee, S.Y.; Li, W.; Chung, M.Y.; Kim, C.K. CRISPR/Cas9-mediated editing of PhMLO1 confers powdery mildew resistance in petunia. Plant Biotechnol. Rep. 2023, 17, 767–775. [Google Scholar] [CrossRef]
- Yaeno, T.; Wahara, M.; Nagano, M.; Wanezaki, H.; Toda, H.; Inoue, H.; Eishima, A.; Nishiguchi, M.; Hisano, H.; Kobayashi, K.; et al. RACE1, a Japanese Blumeria graminis f. sp. hordei isolate, is capable of overcoming partially mlo-mediated penetration resistance in barley in an allele-specific manner. PLoS ONE 2021, 16, e0256574. [Google Scholar] [CrossRef]
- Sharma, G.; Aminedi, R.; Saxena, D.; Gupta, A.; Banerjee, P.; Jain, D.; Chandran, D. Effector mining from the Erysiphe pisi haustorial transcriptome identifies novel candidates involved in pea powdery mildew pathogenesis. Mol. Plant Pathol. 2019, 20, 1506–1522. [Google Scholar] [CrossRef]
- Trupo, M.; Magarelli, R.A.; Martino, M.; Larocca, V.; Giorgianni, A.; Ambrico, A. Crude lipopeptides from culture of Bacillus subtilis strain ET-1 against Podosphaera xanthii on Cucumis melo. J. Nat. Pestic. Res. 2023, 4, 100032. [Google Scholar] [CrossRef]
- Jakuschkin, B.; Fievet, V.; Schwaller, L.; Fort, T.; Robin, C.; Vacher, C. Deciphering the Pathobiome: Intra- and Interkingdom Interactions Involving the Pathogen Erysiphe alphitoides. Microb. Ecol. 2016, 72, 870–880. [Google Scholar] [CrossRef]
- Zou, Y.F.; Qiao, H.B.; Cao, X.R.; Wei, L.; Fan, J.R.; Song, Y.L.; Wang, B.T.; Zhou, Y.L. Regionalization of wheat powdery mildew oversummering in China based on digital elevation. J. Integr. Agric. 2018, 17, 901–910. [Google Scholar] [CrossRef]
- Jacob, D.; David, D.R.; Sztjenberg, A.; Elad, Y. Conditions for development of powdery mildew of tomato caused by Oidium neolycopersici. Phytopathology 2008, 98, 270–281. [Google Scholar] [CrossRef]
- Liyanage, K.K.; Khan, S.; Mortimer, P.E.; Hyde, K.D.; Xu, J.; Brooks, S.; Ming, Z. Powdery mildew disease of rubber tree. For. Pathol. 2016, 46, 90–103. [Google Scholar] [CrossRef]
- Lopes, U.P.; Alonzo, G.; Onofre, R.B.; Mello, P.P.; Gadoury, D.M.; Vallad, G.E.; Peres, N.A. Effective Management of Powdery Mildew in Cantaloupe Plants Using Nighttime Applications of UV Light. Plant Dis. 2023, 107, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Bose, B. Developments in Physiology, Biochemistry and Molecular Biology of Plants; New India Publishing: New Delhi, India, 2008; Volume 2. [Google Scholar]
- Eskandari, S.; Sharifnabi, B. The modifications of cell wall composition and water status of cucumber leaves induced by powdery mildew and manganese nutrition. Plant Physiol. Biochem. 2019, 145, 132–141. [Google Scholar] [CrossRef]
- Huckelhoven, R. Powdery mildew susceptibility and biotrophic infection strategies. FEMS Microbiol. Lett. 2005, 245, 9–17. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Zhang, Z.; Fang, Y.; Dong, L.; Yin, J.; Liu, Y.; Chen, D.; Li, Z.-G.; Liu, W.; et al. The Rubber Tree (Heveae brasiliensis) MLO Protein HbMLO12 Promotes Plant Susceptibility to Sustain Infection by a Powdery Mildew Fungus. Mol. Plant-Microbe Interact. 2023, 36, 273–282. [Google Scholar] [CrossRef]
- Reyad, N.E.; Azoz, S.N.; Ali, A.M.; Sayed, E.G. Mitigation of Powdery Mildew Disease by Integrating Biocontrol Agents and Shikimic Acid with Modulation of Antioxidant Defense System, Anatomical Characterization, and Improvement of Squash Plant Productivity. Horticulturae 2022, 8, 1145. [Google Scholar] [CrossRef]
- Amaro, R.; Diniz, I.; Santos, H.; Pimentel, D.; Rego, C.; Mithöfer, A.; Fortes, A.M. Hormone Changes in Tolerant and Susceptible Grapevine Leaves Under Powdery Mildew Infection. J. Plant Growth Regul. 2023, 42, 3606–3614. [Google Scholar] [CrossRef]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Żyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef]
- Osborne, R.; Rehneke, L.; Lehmann, S.; Roberts, J.; Altmann, M.; Altmann, S.; Zhang, Y.; Köpff, E.; Dominguez-Ferreras, A.; Okechukwu, E.; et al. Symbiont-host interactome mapping reveals effector-targeted modulation of hormone networks and activation of growth promotion. Nat. Commun. 2023, 14, 4065. [Google Scholar] [CrossRef]
- Sharma, A.; Chandran, D. Host nuclear repositioning and actin polarization towards the site of penetration precedes fungal ingress during compatible pea-powdery mildew interactions. Planta 2022, 256, 45. [Google Scholar] [CrossRef]
- Mu, B.; Chen, J.; Wang, H.; Kong, W.; Fan, X.; Wen, Y.-Q. An effector CSEP087 from Erysiphe necator targets arginine decarboxylase VviADC to regulate host immunity in grapevine. Sci. Hortic. 2022, 303, 111205. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H.; Choi, S.; Mazo-Molina, C.; Prokchorchik, M.; Zhang, N.; Kim, B.; Mang, H.; Koehler, N.; Kim, J.; et al. Ptr1 and ZAR1 immune receptors confer overlapping and distinct bacterial pathogen effector specificities. New Phytol. 2023, 239, 1935–1953. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Zhang, X.; Gill, R.A.; Hu, M.; Bai, Z.; Zhao, C.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Functional and evolutionary study of MLO gene family in the regulation of Sclerotinia stem rot resistance in Brassica napus L. Biotechnol. Biofuels Bioprod. 2023, 16, 86. [Google Scholar] [CrossRef]
- von Bongartz, K.; Sabelleck, B.; Forero, A.B.; Kuhn, H.; Leissing, F.; Panstruga, R. Comprehensive comparative assessment of the Arabidopsis thaliana MLO2-calmodulin interaction by various in vitro and in vivo protein-protein interaction assays. bioRxiv 2023, 480, 1615–1638. [Google Scholar]
- Xiang, H.; Stojilkovic, B.; Gheysen, G. Decoding Plant–Pathogen Interactions: A Comprehensive Exploration of Effector–Plant Transcription Factor Dynamics. Mol. Plant Pathol. 2025, 26, e70057. [Google Scholar] [CrossRef]
- Xu, X.; Du, Y.; Li, S.; Tan, M.; Sohail, H.; Liu, X.; Qi, X.; Yang, X.; Chen, X. A genome-wide association study reveals molecular mechanism underlying powdery mildew resistance in cucumber. Genome Biol. 2024, 25, 252. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Ninkuu, V.; Yan, J.; Fu, Z.; Yang, T.; Ziemah, J.; Ullrich, M.S.; Kuhnert, N.; Zeng, H. Lignin and Its Pathway-Associated Phytoalexins Modulate Plant Defense against Fungi. J. Fungi 2022, 9, 52. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Li, B.; Zhang, Z.; Qin, G.; Chen, T.; Tian, S. Molecular mechanisms underlying multi-level defense responses of horticultural crops to fungal pathogens. Hortic. Res. 2022, 9, uhac066. [Google Scholar] [CrossRef]
- Barghahn, S.; Arnal, G.; Jain, N.; Petutschnig, E.; Brumer, H.; Lipka, V. Mixed Linkage β-1,3/1,4-Glucan Oligosaccharides Induce Defense Responses in Hordeum vulgare and Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 682439. [Google Scholar] [CrossRef]
- An, Y.; Zhang, M. Advances in understanding the plant-Ralstonia solanacearum interactions Unraveling the dynamics, mechanisms, and implications for crop disease resistance. New Crops 2024, 1, 100014. [Google Scholar] [CrossRef]
- Collins, N.C.; Thordal-Christensen, H.; Lipka, V.; Bau, S.; Kombrink, E.; Qiu, J.L.; Hückelhoven, R.; Stein, M.; Freialdenhoven, A.; Somerville, S.C.; et al. SNARE-protein-mediated disease resistance at the Plant Cell wall. Nature 2003, 425, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules 2021, 26, 6819. [Google Scholar] [CrossRef]
- Hückelhoven, R.; Panstruga, R. Cell biology of the plant–powdery mildew interaction. Curr. Opin. Plant Biol. 2011, 14, 738–746. [Google Scholar] [CrossRef]
- Ronald, P.C.; Beutler, B. Plant and Animal Sensors of Conserved Microbial Signatures. Science 2010, 330, 1061–1064. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Flor, H.H. Current Status of the Gene-For-Gene Concept. Annu. Rev. Phytopathol. 1971, 9, 275–296. [Google Scholar] [CrossRef]
- Peterhansel, C.; Freialdenhoven, A.; Kurth, J.; Kolsch, R.; Schulze-Lefert, P. Interaction Analyses of Genes Required for Resistance Responses to Powdery Mildew in Barley Reveal Distinct Pathways Leading to Leaf Cell Death. Plant Cell 1997, 9, 1397–1409. [Google Scholar] [CrossRef]
- Kusch, S.; Panstruga, R. mlo-Based Resistance: An Apparently Universal “Weapon” to Defeat Powdery Mildew Disease. Mol. Plant Microbe Interact. 2017, 30, 179–189. [Google Scholar] [CrossRef]
- O’Hara, T.; Steed, A.; Goddard, R.; Gaurav, K.; Arora, S.; Quiroz-Chávez, J.; Ramírez-González, R.; Badgami, R.; Gilbert, D.; Sánchez-Martín, J.; et al. The wheat powdery mildew resistance gene Pm4 also confers resistance to wheat blast. Nat. Plants 2024, 10, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Huang, X.; He, Z.; Zhang, Q.; Meng, H.; Shi, H.; Feng, B.; Zhou, Y.; Zhang, J.; Lu, G.; et al. Phosphorylation of OsTGA5 by casein kinase II compromises its suppression of defense-related gene transcription in rice. Plant Cell 2022, 34, 3425–3442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, T.; E, L.; Xu, M.; Dai, W.; Sun, S.; Ye, J. GT Factor ZmGT-3b Is Associated With Regulation of Photosynthesis and Defense Response to Fusarium graminearum Infection in Maize Seedling. Front. Plant Sci. 2021, 12, 724133. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.N.; Lee, W.H.; Won, S.Y.; Chang, S.; Hong, J.P.; Oh, T.J.; Lee, S.M.; Kang, S.H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Xiang, S.; Chen, Y.; Zhang, H.; Yu, D. The transcription factor WRKY75 positively regulates jasmonate-mediated plant defense to necrotrophic fungal pathogens. J. Exp. Bot. 2021, 72, 1473–1489. [Google Scholar] [CrossRef]
- Chen, H.; He, S.; Zhang, S.; A, R.; Li, W.; Liu, S. The Necrotroph Botrytis cinerea BcSpd1 Plays a Key Role in Modulating Both Fungal Pathogenic Factors and Plant Disease Development. Front. Plant Sci. 2022, 13, 820767. [Google Scholar] [CrossRef]
- Vranic, M.; Perochon, A.; Doohan, F.M. Transcriptional Profiling Reveals the Wheat Defences against Fusarium Head Blight Disease Regulated by a NAC Transcription Factor. Plants 2023, 12, 2708. [Google Scholar] [CrossRef]
- Saxena, S.; Pal, L.; Naik, J.; Singh, Y.; Verma, P.K.; Chattopadhyay, D.; Pandey, A. The R2R3-MYB-SG7 transcription factor CaMYB39 orchestrates surface phenylpropanoid metabolism and pathogen resistance in chickpea. New Phytol. 2023, 238, 798–816. [Google Scholar] [CrossRef]
- Zhao, F.; Yan, Y.; Wang, Y.; Liu, Y.; Yang, R. Splicing complexity as a pivotal feature of alternative exons in mammalian species. BMC Genom. 2023, 24, 198. [Google Scholar] [CrossRef]
- Taylor, K.; Sobczak, K. Intrinsic Regulatory Role of RNA Structural Arrangement in Alternative Splicing Control. Int. J. Mol. Sci. 2020, 21, 5161. [Google Scholar] [CrossRef]
- Uriostegui-Arcos, M.; Mick, S.T.; Shi, Z.; Rahman, R.; Fiszbein, A. Splicing activates transcription from weak promoters upstream of alternative exons. Nat. Commun. 2023, 14, 3435. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Qiu, K.; He, R.; Feng, X.; Liang, X. The occurrence and function of alternative splicing in fungi. Fungal Biol. Rev. 2020, 34, 178–188. [Google Scholar] [CrossRef]
- Abo-Elyousr, K.A.; Seleim, M.A.; Almasoudi, N.M.; Bagy, H.M. Evaluation of Native Bacterial Isolates for Control of Cucumber Powdery Mildew under Greenhouse Conditions. Horticulturae 2022, 8, 1143. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Widrig, V.; Herren, G.; Wicker, T.; Zbinden, H.; Gronnier, J.; Spörri, L.; Praz, C.R.; Heuberger, M.; Kolodziej, M.C.; et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants 2021, 7, 327, Corrected: Nat. Plants 2023, 9, 502–503. [Google Scholar] [CrossRef]
- Fujimura, T.; Sato, S.; Tajima, T.; Arai, M. Powdery mildew resistance in the Japanese domestic tobacco cultivar Kokubu is associated with aberrant splicing of MLO orthologues. Plant Pathol. 2016, 65, 1358–1365. [Google Scholar] [CrossRef]
- Lv, S.; Guo, H.; Zhang, M.; Wang, Q.; Zhang, H.; Ji, W. Large-Scale Cloning and Comparative Analysis of TaNAC Genes in Response to Stripe Rust and Powdery Mildew in Wheat (Triticum aestivum L.). Genes 2020, 11, 1073. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, D. Genome-wide identification of the aspartic protease gene family and their response under powdery mildew stress in wheat. Mol. Biol. Rep. 2020, 47, 8949–8961. [Google Scholar] [CrossRef]
- Gao, C.; Dong, S. New insights into pathogen-mediated modulation of host RNA splicing. Stress Biol. 2022, 2, 34. [Google Scholar] [CrossRef]
- Fu, C.-C.; Huang, B.-X.; Wang, S.-S.; Song, Y.-C.; Metok, D.; Tan, Y.-X.; Fan, T.-P.; Fernie, A.R.; Zargar, M.; Wang, Y.; et al. Deciphering the roles of bacterial and fungal communities in the formation and quality of agarwood. Stress Biol. 2024, 4, 40. [Google Scholar] [CrossRef]
- Maarastawi, S.A.; Frindte, K.; Linnartz, M.; Knief, C. Crop Rotation and Straw Application Impact Microbial Communities in Italian and Philippine Soils and the Rhizosphere of Zea mays. Front. Microbiol. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Steinauer, K.; Thakur, M.P.; Emilia Hannula, S.; Weinhold, A.; Uthe, H.; van Dam, N.M.; Martijn Bezemer, T. Root exudates and rhizosphere microbiomes jointly determine temporal shifts in plant-soil feedbacks. Plant Cell Environ. 2023, 46, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Seo, Y.-S.; Mannaa, M. Recruitment of the rhizo-microbiome army: Assembly determinants and engineering of the rhizosphere microbiome as a key to unlocking plant potential. Front. Microbiol. 2023, 14, 1163832. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, G.H.; Liu, X.B.; Liu, J.D.; Chen, X.L.; Herbert, S.J. Temporal and Spatial Dynamics of Bacterial Community in the Rhizosphere of Soybean Genotypes Grown in a Black Soil. Pedosphere 2009, 19, 808–816. [Google Scholar] [CrossRef]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Kovacs, E.D.; Silaghi-Dumitrescu, L.; Roman, C.; Tian, D. Structural and Metabolic Profiling of Lycopersicon esculentum Rhizosphere Microbiota Artificially Exposed at Commonly Used Non-Steroidal Anti-Inflammatory Drugs. Microorganisms 2022, 10, 254. [Google Scholar] [CrossRef]
- Rudrappa, T.; Czymmek, K.J.; Paré, P.W.; Bais, H.P. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008, 148, 1547–1556. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis thaliana against Leaf-Pathogenic Pseudomonas syringae by Sphingomonas Strains in a Controlled Model System. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef]
- Lorenzi, A.S.; Bonatelli, M.L.; Chia, M.A.; Peressim, L.; Quecine, M.C. Opposite Sides of Pantoea agglomerans and Its Associated Commercial Outlook. Microorganisms 2022, 10, 2072. [Google Scholar] [CrossRef]
- Timmusk, S.; Copolovici, D.; Copolovici, L.; Teder, T.; Nevo, E.; Behers, L. Paenibacillus polymyxa biofilm polysaccharides antagonise Fusarium graminearum. Sci. Rep. 2019, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Li, P.D.; Zhu, Z.R.; Zhang, Y.; Xu, J.; Wang, H.; Wang, Z.; Li, H. The phyllosphere microbiome shifts toward combating melanose pathogen. Microbiome 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Bziuk, N.; Maccario, L.; Sørensen, S.J.; Schikora, A.; Smalla, K. Barley Rhizosphere Microbiome Transplantation—A Strategy to Decrease Susceptibility of Barley Grown in Soils With Low Microbial Diversity to Powdery Mildew. Front. Microbiol. 2022, 13, 830905. [Google Scholar] [CrossRef]
- Adedayo, A.A.; Fadiji, A.E.; Babalola, O.O. Unraveling the functional genes present in rhizosphere microbiomes of Solanum lycopersicum. PeerJ 2023, 11, e15432. [Google Scholar] [CrossRef]
- Samant, S.; Dawson, J.O.; Hahn, D. Growth responses of introduced Frankia strains to edaphic factors. Plant Soil 2016, 400, 123–132. [Google Scholar] [CrossRef]
- Snelders, N.C.; Kettles, G.J.; Rudd, J.J.; Thomma, B.P.H.J. Plant pathogen effector proteins as manipulators of host microbiomes? Mol. Plant Pathol. 2018, 19, 257–259. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, L.; Tan, X.; Kong, X.; Yang, J.; Wang, D.; Zhang, D.; Jin, D.; Liu, Y. Pumpkin powdery mildew disease severity influences the fungal diversity of the phyllosphere. PeerJ 2018, 6, e4559. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, X.; Jin, D.; Yu, H.; Zhu, X.; Su, X.; Wang, P.; Zhang, R.; Jia, M.; Deng, Y. Euonymus japonicus phyllosphere microbiome is significantly changed by powdery mildew. Arch. Microbiol. 2019, 201, 1099–1109. [Google Scholar] [CrossRef]
- Suda, W.; Nagasaki, A.; Shishido, M. Powdery Mildew-Infection Changes Bacterial Community Composition in the Phyllosphere. Microbes Environ. 2009, 24, 217–223. [Google Scholar] [CrossRef]
- Malviya, D.; Thosar, R.; Kokare, N.; Pawar, S.; Singh, U.B.; Saha, S.; Rai, J.P.; Singh, H.V.; Somkuwar, R.G.; Saxena, A.K. A Comparative Analysis of Microbe-Based Technologies Developed at ICAR-NBAIM Against Erysiphe necator Causing Powdery Mildew Disease in Grapes (Vitis vinifera L.). Front. Microbiol. 2022, 13, 871901. [Google Scholar] [CrossRef]
- Rhouma, A.; Mehaoua, M.S.; Mougou, I.; Rhouma, H.; Shah, K.K.; Bedjaoui, H. Combining melon varieties with chemical fungicides for integrated powdery mildew control in Tunisia. Eur. J. Plant Pathol. 2023, 165, 189–201. [Google Scholar] [CrossRef]
- Jia, Z.-C.; Yang, X.; Wu, Y.-K.; Li, M.; Das, D.; Chen, M.-X.; Wu, J. The Art of Finding the Right Drug Target: Emerging Methods and Strategies. Pharmacol. Rev. 2024, 76, 896–914. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, M.; Jia, Z.-C.; Liu, Y.; Wu, S.-F.; Chen, M.-X.; Hao, G.-F.; Yang, Q. Unraveling the secrets: Evolution of resistance mediated by membrane proteins. Drug Resist. Updat. 2024, 77, 101140. [Google Scholar] [CrossRef] [PubMed]
- Barilli, E.; Agudo, F.J.; Masi, M.; Nocera, P.; Evidente, A.; Rubiales, D. Anthraquinones and their analogues as potential biocontrol agents of rust and powdery mildew diseases of field crops. Pest Manag. Sci. 2022, 78, 3489–3497. [Google Scholar] [CrossRef]
- Frem, M.; Nigro, F.; Medawar, S.; El Moujabber, M. Biological Approaches Promise Innovative and Sustainable Management of Powdery Mildew in Lebanese Squash. Sustainability 2022, 14, 2811. [Google Scholar] [CrossRef]
- Yang, H.; Peng, L.; Li, Z.; Huang, C.; Huang, J. Biocontrol of Lysobacter enzymogenes CQ18 against the tobacco powdery mildew fugus, Erysiphe cichoracearum. Chem. Biol. Technol. Agric. 2023, 10, 74. [Google Scholar] [CrossRef]
- Kimura, S.; Komura, T.; Yamaoka, N.; Oka, H. Biological properties of flutianil as a novel fungicide against powdery mildew. J. Pestic. Sci. 2020, 45, 206–215. [Google Scholar] [CrossRef]
- Liang, Q.; Wei, L.; Xu, B.; Liu, J.; Zhang, S.; Liu, L. Induction of resistance of Podosphaera xanthii (hull-less pumpkin powdery mildew) to triazole fungicides and its resistance mechanism. PLoS ONE 2022, 17, e0263068. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Kokko, H.I.; Buchala, A.J.; McDougall, G.J.; Stewart, D.; Kärenlampi, S.O.; Karjalainen, R.O. Benzothiadiazole induces the accumulation of phenolics and improves resistance to powdery mildew in strawberries. J. Agric. Food Chem. 2007, 55, 1862–1870. [Google Scholar] [CrossRef]
- Lam, C.H.; Lim, T.K. Efficacy of hexaconazole for the control of white rust on chrysanthemum and powdery mildew on roses. Int. J. Pest Manag. 1993, 39, 156–160. [Google Scholar] [CrossRef]
- Reuveni, M. Activity of trifloxystrobin against powdery and downy mildew diseases of grapevines. Can. J. Plant Pathol. 2001, 23, 52–59. [Google Scholar] [CrossRef]
- Ghorbani, A.; Ghasemi-Omran, V.O.; Chen, M. The Effect of Glycine Betaine on Nitrogen and Polyamine Metabolisms, Expression of Glycoside-Related Biosynthetic Enzymes, and K/Na Balance of Stevia under Salt Stress. Plants 2023, 12, 1628. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Emamverdian, A.; Pehlivan, N.; Zargar, M.; Razavi, S.M.; Chen, M. Nano-enabled agrochemicals: Mitigating heavy metal toxicity and enhancing crop adaptability for sustainable crop production. J. Nanobiotechnol. 2024, 222, 37. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Emamverdian, A.; Pishkar, L.; Chashmi, K.A.; Salavati, J.; Zargar, M.; Chen, M. Melatonin-mediated nitric oxide signaling enhances adaptation of tomato plants to aluminum stress. S. Afr. J. Bot. 2023, 162, 443–450. [Google Scholar] [CrossRef]
- Ghorbani, A.; Pishkar, L.; Saravi, K.V.; Chen, M. Melatonin-mediated endogenous nitric oxide coordinately boosts stability through proline and nitrogen metabolism, antioxidant capacity, and Na+/K+ transporters in tomato under NaCl stress. Front. Plant Sci. 2023, 14, 1135943. [Google Scholar] [CrossRef]
- Hermosa, R.; Botella, L.; Montero-Barrientos, M.; Alonso-Ramírez, A.; Arbona, V.; Gómez-Cadenas, A.; Monte, E.; Nicolás, C. Biotechnological applications of the gene transfer from the beneficial fungus Trichoderma harzianum spp. to plants. Plant Signal. Behav. 2011, 6, 1235–1236. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- Gupta, G.; Ndiaye, A.; Filteau, M. Leveraging Experimental Strategies to Capture Different Dimensions of Microbial Interactions. Front. Microbiol. 2021, 12, 700752. [Google Scholar] [CrossRef]
- Lam, N.T.; Song, S.; Dung, B.T.; Binh, T.N.; Maleki, A.; Godini, K.; Tang, V.T. Potential Role of Combined Microbial Inoculants and Plant of Limnocharis flava on Eliminating Cadmium from Artificial Contaminated Soil. Sustainability 2022, 14, 12209. [Google Scholar] [CrossRef]
- Ahmed, T.; Wu, Z.; Jiang, H.; Luo, J.; Noman, M.; Shahid, M.; Manzoor, I.; Allemailem, K.S.; Alrumaihi, F.; Li, B. Bioinspired Green Synthesis of Zinc Oxide Nanoparticles from a Native Bacillus cereus Strain RNT6: Characterization and Antibacterial Activity against Rice Panicle Blight Pathogens Burkholderia glumae and B. gladioli. Nanomaterials 2021, 11, 884. [Google Scholar] [CrossRef] [PubMed]
- Hamscher, G.; Sczesny, S.; Höper, H.; Nau, H. Determination of Persistent Tetracycline Residues in Soil Fertilized with Liquid Manure by High-Performance Liquid Chromatography with Electrospray Ionization Tandem Mass Spectrometry. Anal. Chem. 2002, 74, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Yang, Y.J.; Wu, X.; Zhu, C.L.; Lü, H.; Zhao, H.M.; Xiang, L.; Li, H.; Mo, C.H.; Li, Y.W.; et al. Adaptation of bacterial community in maize rhizosphere for enhancing dissipation of phthalic acid esters in agricultural soil. J. Hazard Mater. 2023, 444, 130292. [Google Scholar] [CrossRef] [PubMed]
- El-Nasr, M.K.A.; Nasser, M.A.; Ebrahim, M.; Samaan, M.S.F. Alleviating biotic stress of powdery mildew in mango cv. Keitt by Sulfur nanoparticles and assessing their effect on productivity and disease severity. Sci. Rep. 2025, 15, 5537. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, X.; Kuramae, E.E. Insight into farming native microbiome by bioinoculant in soil-plant system. Microbiol. Res. 2024, 285, 127776. [Google Scholar] [CrossRef]
- Miao, P.; Wang, H.; Wang, W.; Wang, Z.; Ke, H.; Cheng, H.; Ni, J.; Liang, J.; Yao, Y.-F.; Wang, J.; et al. A widespread plant defense compound disarms bacterial type III injectisome assembly. Science 2025, 387, eads0377. [Google Scholar] [CrossRef]
- Fisher, A.; DeGrandi-Hoffman, G.; Smith, B.H.; Ozturk, C.; Kaftanoglu, O.; Fewell, J.H.; Harrison, J.F. Field cross-fostering and in vitro rearing demonstrate negative effects of both larval and adult exposure to a widely used fungicide in honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 2021, 217, 112251. [Google Scholar] [CrossRef]
- Wan, N.-F.; Fu, L.; Dainese, M.; Kiær, L.P.; Hu, Y.-Q.; Xin, F.; Goulson, D.; Woodcock, B.A.; Vanbergen, A.J.; Spurgeon, D.J.; et al. Pesticides have negative effects on non-target organisms. Nat. Commun. 2025, 16, 1360. [Google Scholar] [CrossRef]
- Winding, A.; Binnerup, S.J.; Pritchard, H. Non-target effects of bacterial biological control agents suppressing root pathogenic fungi. FEMS Microbiol. Ecol. 2004, 47, 129–141. [Google Scholar] [CrossRef]
- Panwar, N.; Szczepaniec, A. Endophytic entomopathogenic fungi as biological control agents of insect pests. Pest Manag. Sci. 2024, 80, 6033–6040. [Google Scholar] [CrossRef]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Marrone, P.G. Status of the biopesticide market and prospects for new bioherbicides. Pest Manag. Sci. 2023, 80, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, K.; Kou, Y. Genome editing creates disease-resistant crops without yield penalties. Trends Plant Sci. 2024, 29, 114–116. [Google Scholar] [CrossRef]
- Li, R.; Tang, Y.; Wang, Q.; Zhao, B.; Su, W.; Wang, B.; Li, Q. Inactivation of a Wheat Ribosomal Silencing Factor Gene TaRsfS Confers Resistance to Both Powdery Mildew and Stripe Rust. Plant Cell Environ. 2025, 48, 711–727. [Google Scholar] [CrossRef]
- Pennington, H.G.; Jones, R.; Kwon, S.; Bonciani, G.; Thieron, H.; Chandler, T.; Luong, P.; Morgan, S.N.; Przydacz, M.; Bozkurt, T.; et al. The fungal ribonuclease-like effector protein CSEP0064/BEC1054 represses plant immunity and interferes with degradation of host ribosomal RNA. PLoS Pathog. 2019, 15, e1007620. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, S.; Wang, Y.; Zhang, B.; Zhu, M.; Wang, J.E.; Rajaram, V.; Fang, Y.; Luo, W.; Wang, Y. AIF3 splicing variant elicits mitochondrial malfunction via the concurrent dysregulation of electron transport chain and glutathione-redox homeostasis. Nat. Commun. 2025, 16, 1804. [Google Scholar] [CrossRef]
- Ordon, J.; Logemann, E.; Maier, L.-P.; Lee, T.; Dahms, E.; Oosterwijk, A.; Flores-Uribe, J.; Miyauchi, S.; Paoli, L.; Stolze, S.C.; et al. Conserved immunomodulation and variation in host association by Xanthomonadales commensals in Arabidopsis root microbiota. Nat. Plants 2025, 11, 612–631. [Google Scholar] [CrossRef]
- Du, Y.; Han, X.; Tsuda, K. Microbiome-mediated plant disease resistance: Recent advances and future directions. J. Gen. Plant Pathol. 2025, 91, 1–17. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D. Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): Development of microbial synergistic bio-inoculants in sustainable agriculture. Biol. Control. 2022, 176, 105100. [Google Scholar] [CrossRef]
- Wu, Q.; Ni, M.; Dou, K.; Tang, J.; Ren, J.; Yu, C.; Chen, J. Co-culture of Bacillus amyloliquefaciens ACCC11060 and Trichoderma asperellum GDFS1009 enhanced pathogen-inhibition and amino acid yield. Microb. Cell Factories 2018, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Z.; Liu, R.; Jiang, Y. Effect of powdery mildew on interleaf microbial communities and leaf antioxidant enzyme systems. J. For. Res. 2023, 34, 1535–1547. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
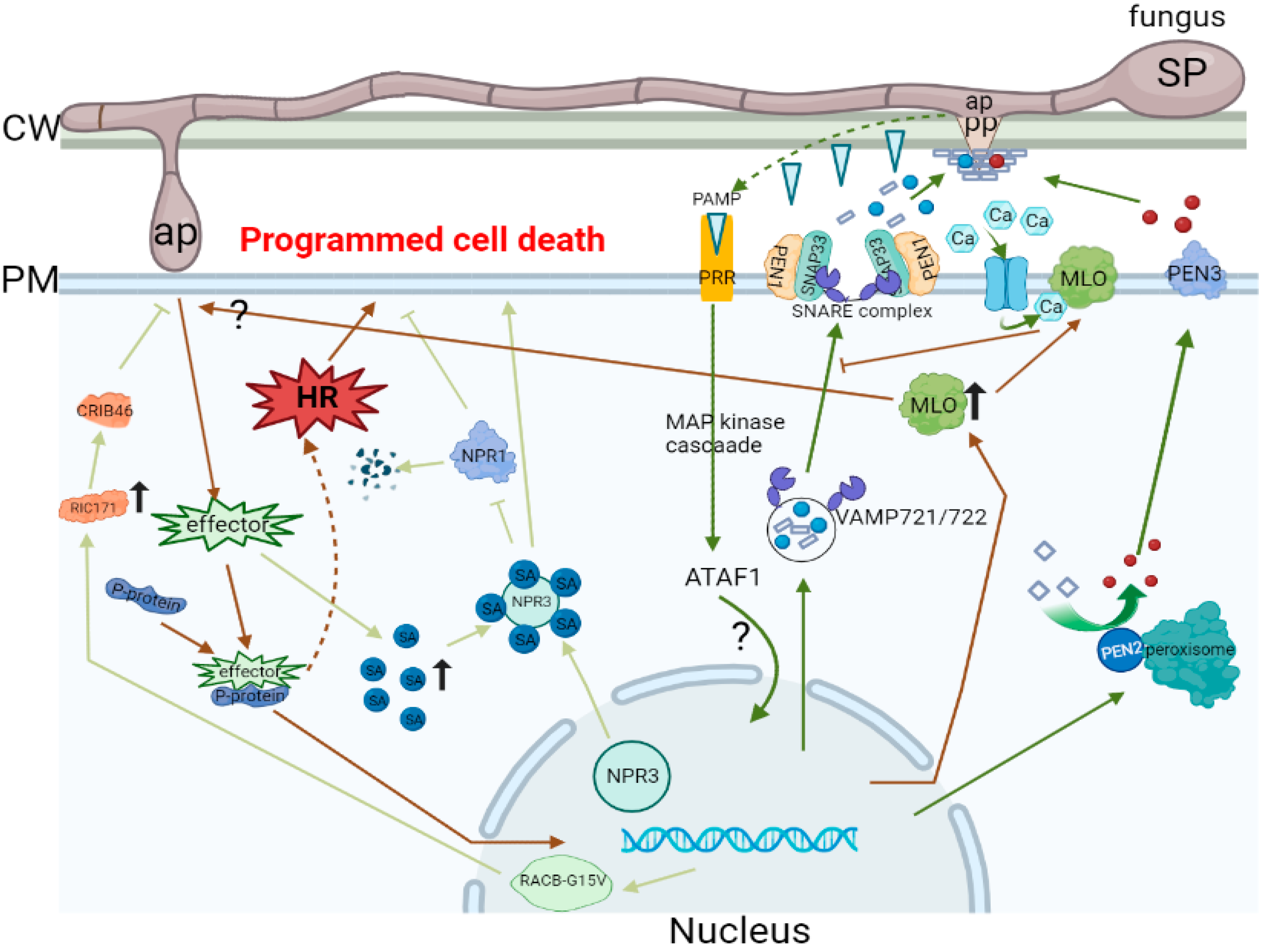
| SL. No. | Study Object | Description | Refs. |
|---|---|---|---|
| 1 | Cytochalasins | It eliminates the polarized radial alignment of host cellular filaments at the place of CWA formation. Furthermore, it induces successful haustorium differentiation. | [29] |
| 2 | Activation of the salicylic acid (SA) pathway | The SA pathway astricts fungal growth even via a mutual effect. | [30] |
| 3 | The pmr5 and pmr6 to activate novel defenses | pmr5 and pmr6 use a similar mechanism to limit the growth of the fungus. | [31] |
| 4 | Blumeria graminis f. sp. Hordei (Bgh) catalase | Bgh catalase potentially plays a role in the removal of H2O2 produced by the host that helps Bgh successfully invade cells. | [32] |
| 5 | A member of the ABC transporter family is PEN3 and PDR8 | PEN3/PDR8 may play a role in the export of toxins to the invasion site, and the activation of the SA pathway may be caused by the accumulation of these toxins in pen3 cells. | [33] |
| 6 | A member of the ABC transporter family is wheat LR34 | A more direct role for LR34 in the resistance process may be through the export of mETEabolites that affect fungal growth. | [34] |
| 7 | The atg2-2 mutant of ATG2 | Few hyphae and conidial peduncles are produced in the leaves of the ATG2-2 mutant, and a large number of foliar cells die. | [35] |
| 8 | Wheat to Blumeria graminis f. sp. tritici (Bgt) infection | Involvement of a defense signaling pathway mediated by the resistance gene Pm3b in triggering race-specific resistance responses to Bgt infection in wheat | [36] |
| Plant Name | Prevention Method | Structure | Molecular Target and Mechanism | Refs. |
|---|---|---|---|---|
| Barley powdery mildew | Flutianil |  | Inhibited haustorium development; affect the host cell’s haustorial formation and nutrient absorption | [140] |
| Hull-less pumpkin powdery mildew | Triazole fungicide | 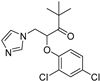 | A mutation of a certain site or multiple sites ofthe CYP51 gene. | [141] |
| Strawberry powdery mildew | Benzothiadiazole | 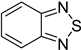 | Benzothiadiazole(BTH) can enhance the accumulation of phenolics in strawberry plants, which may then be involved in the BTH-induced resistance to powdery mildew. | [142] |
| Rose powdery mildew | Hexaconazole | 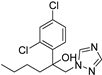 | The excellent foliar efficacy of hexaconazole could be attributed to its properties of protective, eradicative, and translaminar activity with rapid speed of penetration. | [143] |
| Grapevine powdery mildew | Trifloxystrobin | 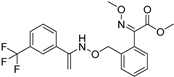 | Trifloxystrobin has a partial effect on zoospore discharge and suppresses zoospore motility and the formation of germ tubes by P. viticola. | [144] |
| Topic | The Focus of the Existing Review | The Innovative Contributions of This Review | Key Cases |
|---|---|---|---|
| Molecular mechanisms | The immunosuppressive function of effector proteins (e.g., CSEP0064 in counteracting host RNA degradation) [169]. | Dynamic AS regulation and effector protein interaction: reveals the interaction network between TF isoforms and effector proteins generated through AS to regulate ROS pathways [74,109,170]. | Wheat Pm4 gene creates two resistant isoforms through AS, each recognizing a different effector protein [94,107]. |
| Microbiome engineering | The antagonistic effect of rhizosphere microorganisms against pathogens [171,172]. | The dynamics of microbial communities and the construction of synthetic microbial communities, analyzing the evolution of the microbiome during powdery mildew infection, and designing synergistic microbial communities [173,174]. | The powdery mildew infection enhances the abundance of bacteria but reduces diversity [175]. |
| Technology integration | CRISPR editing of host R genes (such as MLO knockout) [166,176]. | Synchronized editing of host susceptibility genes (e.g., MLO) and the recruitment of probiotics to achieve dual regulation of “editing-microbiota”. | Gene editing and microbiome customization [177]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, C.-M.; Tang, T.; Zhang, Z.-Y.; Li, M.; Zhao, X.-Q.; Li, S.-Y.; Yan, Y.-W.; Chen, M.-X.; Zhou, X. Unraveling the Intricacies of Powdery Mildew: Insights into Colonization, Plant Defense Mechanisms, and Future Strategies. Int. J. Mol. Sci. 2025, 26, 3513. https://doi.org/10.3390/ijms26083513
Gan C-M, Tang T, Zhang Z-Y, Li M, Zhao X-Q, Li S-Y, Yan Y-W, Chen M-X, Zhou X. Unraveling the Intricacies of Powdery Mildew: Insights into Colonization, Plant Defense Mechanisms, and Future Strategies. International Journal of Molecular Sciences. 2025; 26(8):3513. https://doi.org/10.3390/ijms26083513
Chicago/Turabian StyleGan, Chun-Mei, Ting Tang, Zi-Yu Zhang, Mei Li, Xiao-Qiong Zhao, Shuang-Yu Li, Ya-Wen Yan, Mo-Xian Chen, and Xiang Zhou. 2025. "Unraveling the Intricacies of Powdery Mildew: Insights into Colonization, Plant Defense Mechanisms, and Future Strategies" International Journal of Molecular Sciences 26, no. 8: 3513. https://doi.org/10.3390/ijms26083513
APA StyleGan, C.-M., Tang, T., Zhang, Z.-Y., Li, M., Zhao, X.-Q., Li, S.-Y., Yan, Y.-W., Chen, M.-X., & Zhou, X. (2025). Unraveling the Intricacies of Powdery Mildew: Insights into Colonization, Plant Defense Mechanisms, and Future Strategies. International Journal of Molecular Sciences, 26(8), 3513. https://doi.org/10.3390/ijms26083513









