Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact
Abstract
:1. Introduction
2. Results
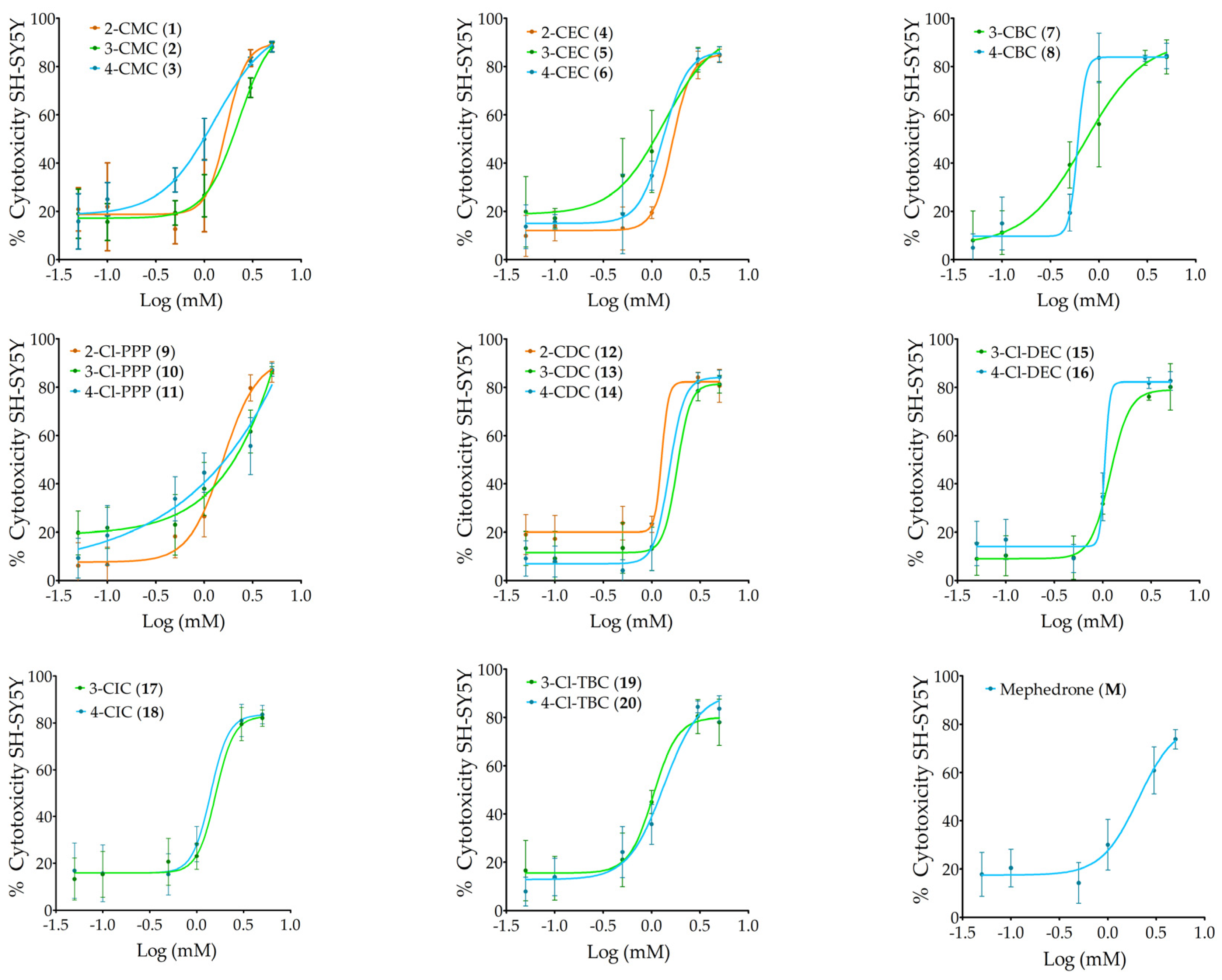
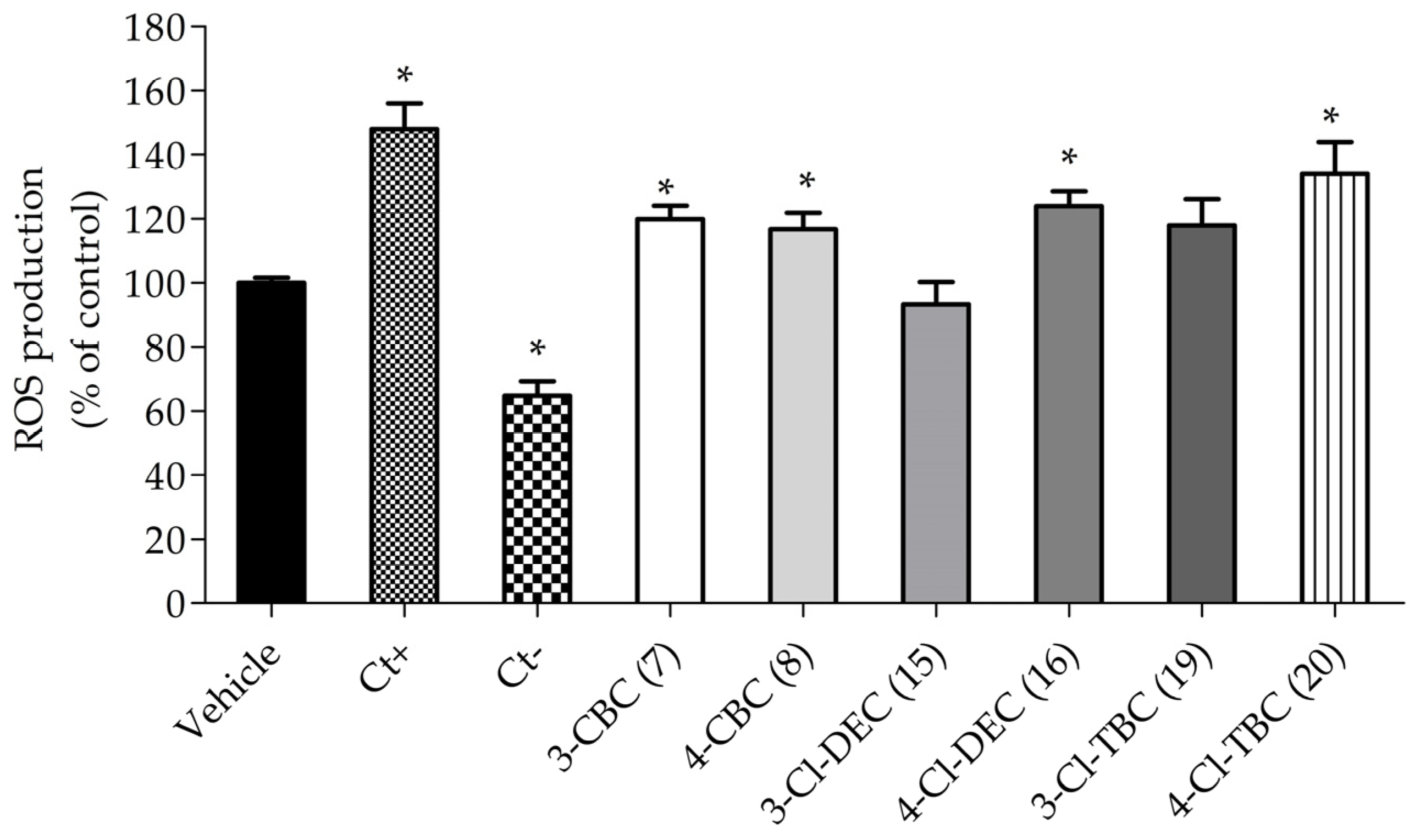
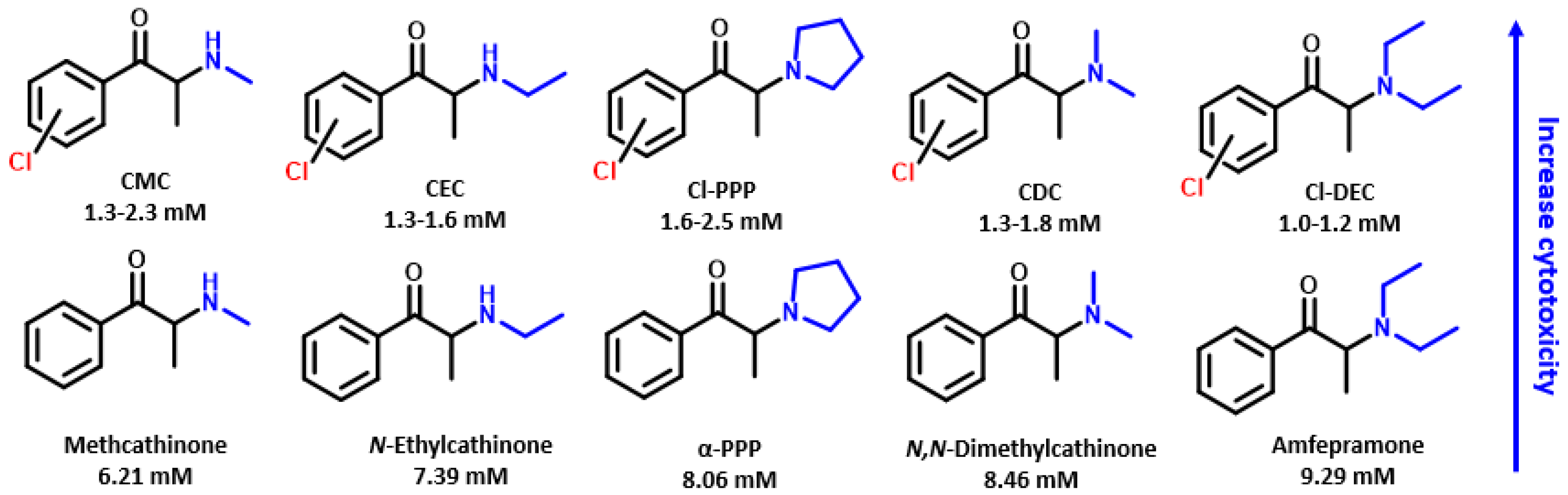
2.1. Neurotoxic Activity on Differentiated SH-SY5Y Cells
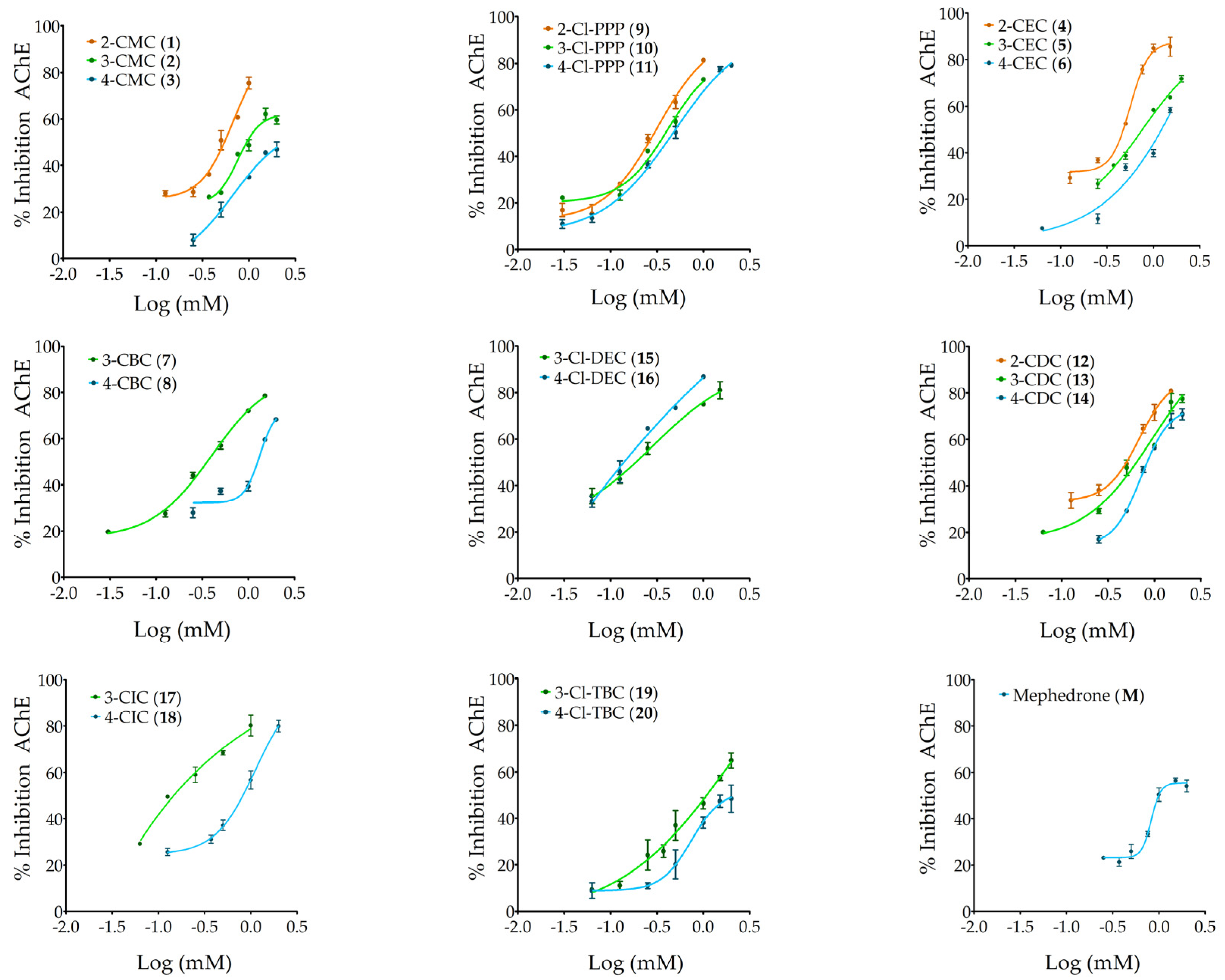
2.2. Acetylcholinesterase (AChE) Inhibitory Activity
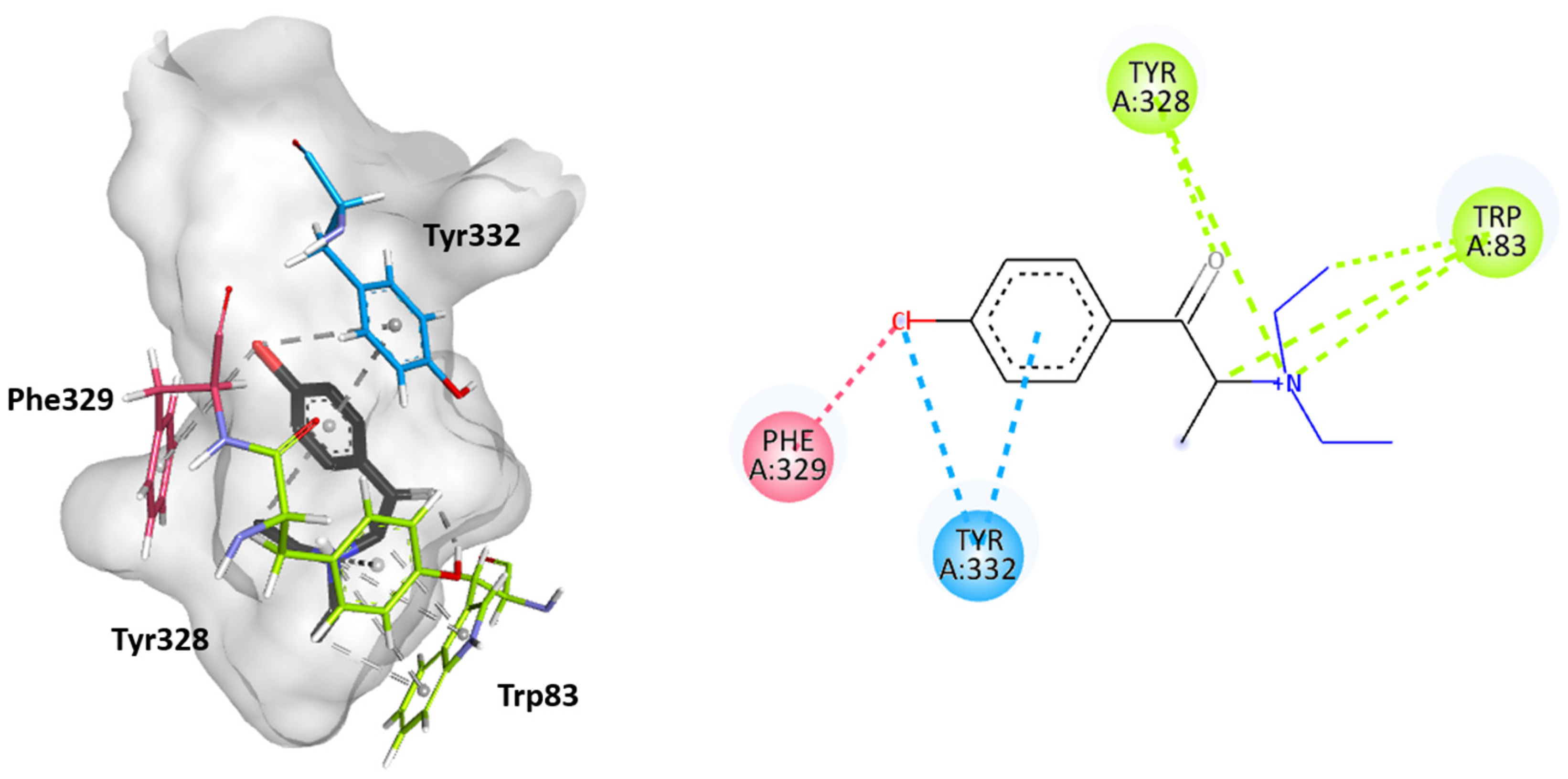
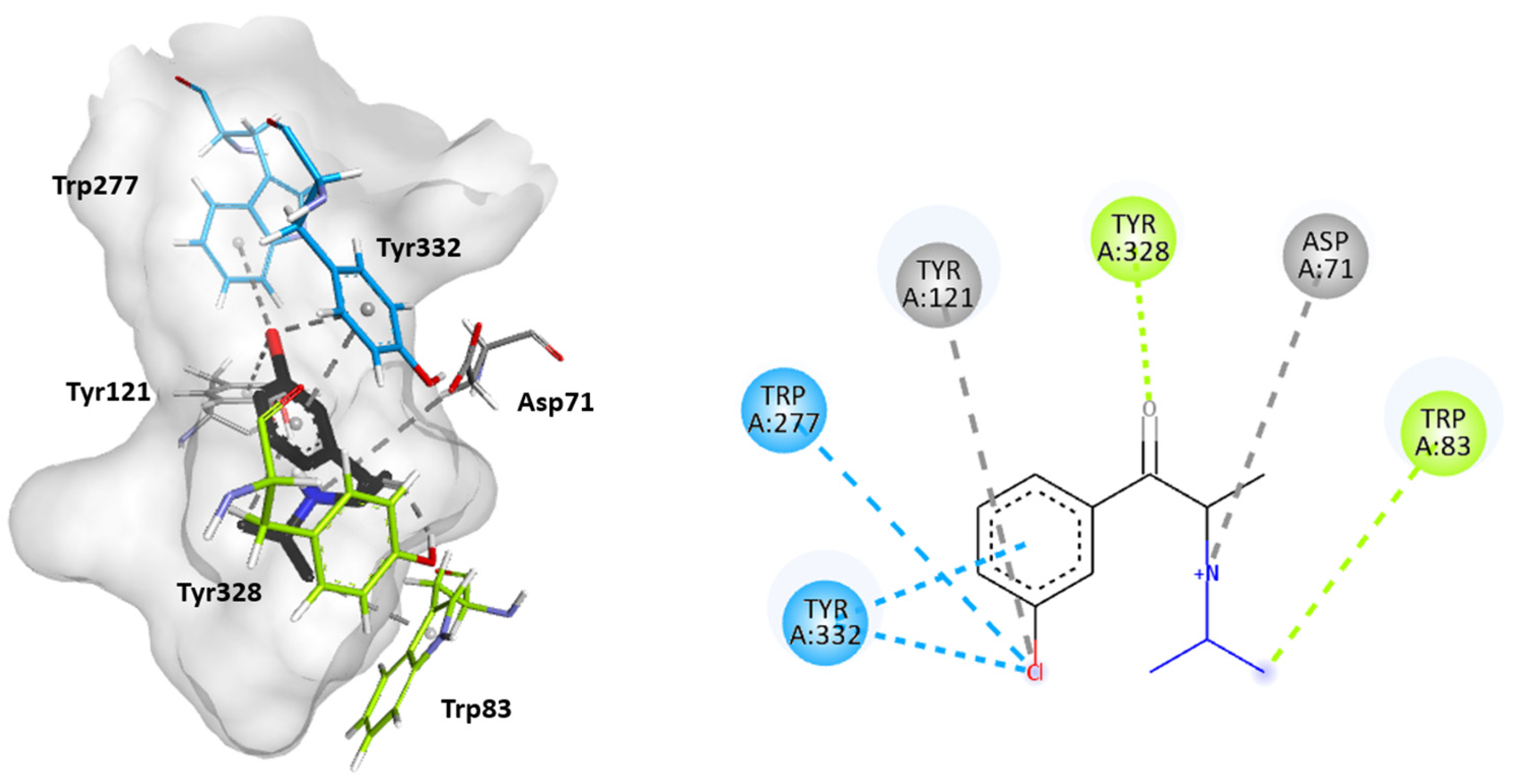
2.3. Molecular Docking Studies
3. Discussion
4. Materials and Methods
4.1. Synthesis and Characterization of Cathinones
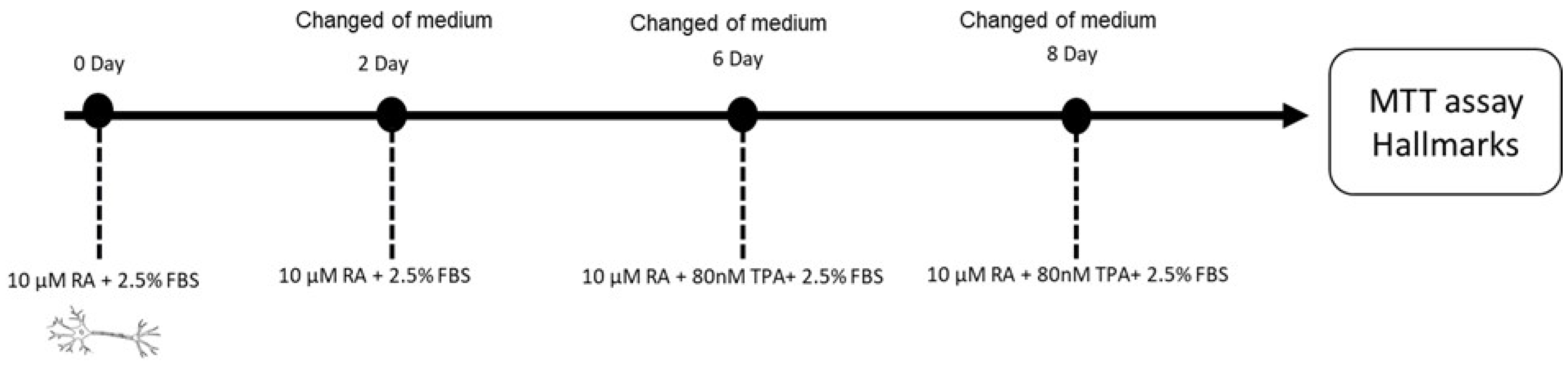
4.2. Neurotoxic Activity in Human Neuroblastoma (SH-SY5Y) Cells
4.2.1. Cell Culture and Maintenance
4.2.2. Differentiation of Human SH-SY5Y Neuronal Cells
4.2.3. Cell Viability on Differentiated SH-SY5Y Cells
4.2.4. 2′,7′-Dichlorodihydrofluorescein Diacetate (H2-DCFDA) Levels
4.2.5. Mitochondrial Membrane Potential (MMP)
4.2.6. Acetylcholinesterase (AChE) Inhibitory Activity
4.3. Statistical Analysis
4.4. Molecular Docking Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valente, M.J.; De Pinho, P.G.; De Lourdes Bastos, M.; Carvalho, F.; Carvalho, M. Khat and synthetic cathinones: A review. Arch. Toxicol. 2014, 88, 15–45. [Google Scholar] [CrossRef]
- Getasetegn, M. Chemical composition of Catha edulis (khat): A review. Phytochem. Rev. 2016, 15, 907–920. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2024: Trends and Developments. Ibid previously European Drugs Report Serie. 2024. Available online: https://www.euda.europa.eu/publications/european-drug-report/2024_en (accessed on 10 September 2024).
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Vari, C.E. Psychoactive drugs—From chemical structure to oxidative stress related to dopaminergic neurotransmission. A review. Antioxidants 2021, 10, 381. [Google Scholar] [CrossRef]
- Cozzi, N.V.; Sievert, M.K.; Shulgin, A.T.; Jacob, P.; Ruoho, A.E. Inhibition of plasma membrane monoamine transporters by β-ketoamphetamines. Eur. J. Pharmacol. 1999, 381, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.H.; Partilla, J.S.; Lehner, K.R.; Thorndike, E.B.; Hoffman, A.F.; Holy, M.; Rothman, R.B.; Goldberg, S.R.; Lupica, C.R.; Sitte, H.H.; et al. Powerful Cocaine-Like Actions of 3,4-Methylenedioxypyrovalerone (MDPV), a Principal Constituent of Psychoactive ‘Bath Salts’ Products. Neuropsychopharmacology 2013, 38, 552–562. [Google Scholar] [CrossRef]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related new psychoactive substances. Neuropharmacology 2018, 134, 4–12. [Google Scholar] [CrossRef]
- Baumann, M.H.; Walters, H.M.; Niello, M.; Sitte, H.H. Neuropharmacology of synthetic cathinones. Handb. Exp. Pharmacol. 2018, 252, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Altun, B.; Çok, İ. Psychoactive bath salts and neurotoxicity risk. Turk. J. Pharm. Sci. 2020, 17, 235–241. [Google Scholar] [CrossRef]
- Prosser, J.M.; Nelson, L.S. The Toxicology of Bath Salts: A Review of Synthetic Cathinones. J. Med. Toxicol. 2012, 8, 33–42. [Google Scholar] [CrossRef]
- Ray, A.; Chitre, N.M.; Daphney, C.M.; Blough, B.E.; Canal, C.E.; Murnane, K.S. Effects of the second-generation “bath salt” cathinone alpha-pyrrolidinopropiophenone (α-PPP) on behavior and monoamine neurochemistry in male mice. Psychopharmacology 2019, 236, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Gannon, B.M.; Baumann, M.H.; Walther, D.; Jimenez-Morigosa, C.; Sulima, A.; Rice, K.C.; Collins, G.T. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology 2018, 43, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Duart-Castells, L.; Nadal-Gratacós, N.; Muralter, M.; Puster, B.; Berzosa, X.; Estrada-Tejedor, R.; Niello, M.; Bhat, S.; Pubill, D.; Camarasa, J.; et al. Role of amino terminal substitutions in the pharmacological, rewarding and psychostimulant profiles of novel synthetic cathinones. Neuropharmacology 2021, 186, 108475. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Gaweł, K.; Jenda, M.; Kotlinska, J.H. The role of acetylcholine in drug addiction. Curr. Issues Pharm. Med. Sci. 2012, 25, 212–217. [Google Scholar] [CrossRef]
- Subramaniyan, M.; Dani, J.A. Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Ann. N. Y. Acad. Sci. 2015, 1349, 46–63. [Google Scholar] [CrossRef]
- Sofuoglu, M.; Mooney, M. Cholinergic Functioning in Stimulant Addiction: Implications for Medications Development. CNS Drugs 2009, 23, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Cholinergic Toxicity, StatPearls Publishing. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539783/ (accessed on 20 December 2023).
- Krotulski, A.J.; Mohr, A.L.A.; Papsun, D.M.; Logan, B.K. Dibutylone (bk-DMBDB): Intoxications, Quantitative Confirmations and Metabolism in Authentic Biological Specimens. J. Anal. Toxicol. 2018, 42, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, N.; Mikus, G.; Czock, D. Effects and Risks Associated with Novel Psychoactive Substances: Mislabeling and sale as bath salts, spice, and research chemicals. Dtsch. Arztebl. Int. 2014, 111, 139–147. [Google Scholar] [CrossRef]
- Coppola, M.; Mondola, R. Synthetic cathinones: Chemistry, pharmacology and toxicology of a new class of designer drugs of abuse marketed as “bath salts” or “plant food”. Toxicol. Lett. 2012, 211, 144–149. [Google Scholar] [CrossRef]
- De La Garza, R.; Shoptaw, S.; Newton, T.F. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Int. J. Neuropsychopharmacol. 2008, 11, 729–741. [Google Scholar] [CrossRef]
- Janowsky, D.S.; Davis, J.M.; El-Yousef, M.K.; Sekerke, H.J. Antagonistic Effects of Physostigmine and Methylphenidate in Man. Am. J. Psychiatry 1973, 130, 1370–1376. [Google Scholar] [CrossRef]
- Diehl, A.; Nakovics, H.; Croissant, B.; Smolka, M.N.; Batra, A.; Mann, K. Galantamine reduces smoking in alcohol-dependent patients: A randomized, placebo-controlled trial. Int. J. Clin. Pharmacol. Ther. 2006, 44, 614–622. [Google Scholar] [CrossRef]
- Majchrzak, M.; Celiński, R.; Kuś, P.; Kowalska, T.; Sajewicz, M. The newest cathinone derivatives as designer drugs: An analytical and toxicological review. Forensic Toxicol. 2018, 36, 33–50. [Google Scholar] [CrossRef]
- Zaami, S.; Giorgetti, R.; Pichini, S.; Pantano, F.; Marinelli, E.; Busardò, F.P. Synthetic cathinones related fatalities: An update. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 268–274. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. EMCDDA–Europol 2017 Annual Report on the Implementation of Council Decision 2005/387/JHA, Implementation Reports; ibid Previously Implementation Reports. 2018. Available online: https://www.euda.europa.eu/publications/implementation-reports/2017_en (accessed on 10 September 2024).
- Howes, S.; Hartmann-Boyce, J.; Livingstone-Banks, J.; Hong, B.; Lindson, N. Antidepressants for smoking cessation. Cochrane Database Syst. Rev. 2020, 2021, CD000031. [Google Scholar] [CrossRef]
- Patel, K.; Allen, S.; Haque, M.N.; Angelescu, I.; Baumeister, D.; Tracy, D.K. Bupropion: A systematic review and meta-analysis of effectiveness as an antidepressant. Ther. Adv. Psychopharmacol. 2016, 6, 99–144. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Moore, S.W. The peripheral anionic site of acetylcholinesterase: Structure, functions and potential role in rational drug design. Curr. Pharm. Des. 2006, 12, 217–225. [Google Scholar] [CrossRef]
- Roca, C.; Requena, C.; Sebastián-Pérez, V.; Malhotra, S.; Radoux, C.; Pérez, C.; Martinez, A.; Páez, J.A.; Blundell, T.L.; Campillo, N.E. Identification of new allosteric sites and modulators of AChE through computational and experimental tools. J. Enzym. Inhib. Med. Chem. 2018, 33, 1034–1047. [Google Scholar] [CrossRef]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Soares, J.; Costa, V.M.; Gaspar, H.; Santos, S.; de Lourdes Bastos, M.; Carvalho, F.; Capela, J.P. Structure-cytotoxicity relationship profile of 13 synthetic cathinones in differentiated human SH-SY5Y neuronal cells. Neurotoxicology 2019, 75, 158–173. [Google Scholar] [CrossRef]
- Chojnacki, M.R.; Thorndike, E.B.; Partilla, J.S.; Rice, K.C.; Schindler, C.W.; Baumann, M.H. Neurochemical and Cardiovascular Effects of 4-Chloro Ring-Substituted Synthetic Cathinones in Rats. J. Pharmacol. Exp. Ther. 2023, 385, 162–170. [Google Scholar] [CrossRef]
- Coccini, T.; Vecchio, S.; Crevani, M.; De Simone, U. Cytotoxic Effects of 3,4-Catechol-PV (One Major MDPV Metabolite) on Human Dopaminergic SH-SY5Y Cells. Neurotox. Res. 2019, 35, 49–62. [Google Scholar] [CrossRef]
- den Hollander, B.; Sundström, M.; Pelander, A.; Siltanen, A.; Ojanperä, I.; Mervaala, E.; Korpi, E.R.; Kankuri, E. Mitochondrial respiratory dysfunction due to the conversion of substituted cathinones to methylbenzamides in SH-SY5Y cells. Sci. Rep. 2015, 5, 14924. [Google Scholar] [CrossRef]
- Leong, H.S.; Philp, M.; Simone, M.; Witting, P.K.; Fu, S. Synthetic cathinones induce cell death in dopaminergic SH-SY5Y cells via stimulating mitochondrial dysfunction. Int. J. Mol. Sci. 2020, 21, 1370. [Google Scholar] [CrossRef]
- de Mello-Sampayo, C.; Vaz, A.R.; Henriques, S.C.; Fernandes, A.; Paradinha, F.; Florindo, P.; Faria, P.; Moreira, R.; Brites, D.A. Lopes, Designer Cathinones N-Ethylhexedrone and Buphedrone Show Different In Vitro Neurotoxicity and Mice Behaviour Impairment. Neurotox. Res. 2021, 39, 392–412. [Google Scholar] [CrossRef]
- Valente, M.J.; de Lourdes Bastos, M.; Fernandes, E.; Carvalho, F.; de Pinho, P.G.; Carvalho, M. Neurotoxicity of β-Keto Amphetamines: Deathly Mechanisms Elicited by Methylone and MDPV in Human Dopaminergic SH-SY5Y Cells. ACS Chem. Neurosci. 2017, 8, 850–859. [Google Scholar] [CrossRef]
- Wojcieszak, J.; Andrzejczak, D.; Kedzierska, M.; Milowska, K.; Zawilska, J.B. Cytotoxicity of α-Pyrrolidinophenones: An Impact of α-Aliphatic Side-chain Length and Changes in the Plasma Membrane Fluidity. Neurotox. Res. 2018, 34, 613–626. [Google Scholar] [CrossRef]
- Li, Q.; He, S.; Chen, Y.; Feng, F.; Qu, W.; Sun, H. Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 158, 463–477. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Abiri, A.; Eskandari, K.; Haghighijoo, Z.; Edraki, N.; Asadipour, A. Phenoxyethyl Piperidine/Morpholine Derivatives as PAS and CAS Inhibitors of Cholinesterases: Insights for Future Drug Design. Sci. Rep. 2019, 9, 19855. [Google Scholar] [CrossRef]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Javed, T.; Habib, R.; Ghafoor, S.; Rumman, B.; Awan, S.; Ntepe, L.J.M.; Batool, S.; Nurulain, S.M. Association of status of acetylcholinesterase and ACHE gene 3′ UTR variants (rs17228602, rs17228616) with drug addiction vulnerability in pakistani population. Chem. Biol. Interact. 2019, 308, 130–136. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Youness, E.R.; Khadrawy, Y.A.; Sleem, A.A. Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both. Asian Pac. J. Trop. Med. 2016, 9, 1089–1094. [Google Scholar] [CrossRef]
- Williams, M.J.; Adinoff, B. The Role of Acetylcholine in Cocaine Addiction. Neuropsychopharmacology 2008, 33, 1779–1797. [Google Scholar] [CrossRef]
- Júlio, S.; Ferro, R.A.; Santos, S.; Alexandre, A.; Caldeira, M.J.; Franco, J.; Barroso, M.; Gaspar, H. Synthesis of emerging cathinones and validation of a SPE GC–MS method for their simultaneous quantification in blood. Anal. Bioanal. Chem. 2023, 415, 571–589. [Google Scholar] [CrossRef]
- Gaspar, H.; Bronze, S.; Oliveira, C.; Victor, B.L.; Machuqueiro, M.; Pacheco, R.; Caldeira, M.J.; Santos, S. Proactive response to tackle the threat of emerging drugs: Synthesis and toxicity evaluation of new cathinones. Forensic Sci. Int. 2018, 290, 146–156. [Google Scholar] [CrossRef]
- Lopes, R.P.; Ferro, R.A.; Milhazes, M.; Figueira, M.; Caldeira, M.J.; Antunes, A.M.M.; Gaspar, H. Metabolic stability and metabolite profiling of emerging synthetic cathinones. Front. Pharmacol. 2023, 14, 1145140. [Google Scholar] [CrossRef]
- Taschwer, M.; Weiß, J.A.; Kunert, O.; Schmid, M.G. Analysis and characterization of the novel psychoactive drug 4-chloromethcathinone (clephedrone). Forensic Sci. Int. 2014, 244, e56–e59. [Google Scholar] [CrossRef]
- Nycz, J.E.; Pazdziorek, T.; Malecki, G.; Szala, M. Identification and derivatization of selected cathinones by spectroscopic studies. Forensic Sci. Int. 2016, 266, 416–426. [Google Scholar] [CrossRef]
- Perrine, D.M.; Ross, J.T.; Nervi, S.J.; Zimmerman, R.H. A Short, One-Pot Synthesis of Bupropion (Zyban, Wellbutrin). J. Chem. Educ. 2000, 77, 1479. [Google Scholar] [CrossRef]
- Khan, S.R.; Berendt, R.T.; Ellison, C.D.; Ciavarella, A.B.; Asafu-Adjaye, E.; Khan, M.A.; Faustino, P.J. Chapter One—Bupropion Hydrochloride. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 1–30. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, B.; Hua, Z.; Xu, P.; Xu, H.; Shen, W.; Di, B.; Wang, Y.; Su, M. Quantification of Cathinone Analogues without Reference Standard Using 1H Quantitative NMR. Anal. Sci. 2021, 37, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Sequeira, M.; de Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC–MS. J. Anal. Toxicol. 2021, 45, 233–242. [Google Scholar] [CrossRef]
- Kuś, P.; Kusz, J.; Książek, M.; Pieprzyca, E.; Rojkiewicz, M. Spectroscopic characterization and crystal structures of two cathinone derivatives: N-ethyl-2-amino-1-phenylpropan-1-one (ethcathinone) hydrochloride and N-ethyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-CEC) hydrochloride. Forensic Toxicol. 2017, 35, 114–124. [Google Scholar] [CrossRef]
- Kuś, P.; Rojkiewicz, M.; Kusz, J.; Książek, M.; Sochanik, A. Spectroscopic characterization and crystal structures of four hydrochloride cathinones: N-ethyl-2-amino-1-phenylhexan-1-one (hexen, NEH), N-methyl-2-amino-1-(4-methylphenyl)-3-methoxypropan-1-one (mexedrone), N-ethyl-2-amino-1-(3,4-methylenedioxyphenyl)pentan-1-one (ephylone) and N-butyl-2-amino-1-(4-chlorophenyl)propan-1-one (4-chlorobutylcathinone). Forensic Toxicol. 2019, 37, 456–464. [Google Scholar] [CrossRef]
- Siczek, M.; Siczek, M.; Szpot, P.; Zawadzki, M.; Wachełko, O. Crystal Structures and Spectroscopic Characterization of Four Synthetic Cathinones: 1-(4-Chlorophenyl)-2-(Dimethylamino)Propan-1-One (N-Methyl-Clephedrone, 4-CDC), 1-(1,3-Benzodioxol-5-yl)-2-(Tert-Butylamino)Propan-1-One (tBuONE, Tertylone, MDPT), 1-(4-Fluorophenyl)-2-(Pyrrolidin-1-yl)Hexan-1-One (4F-PHP) and 2-(Ethylamino)-1-(3-Methylphenyl)Propan-1-One (3-Methyl-Ethylcathinone, 3-MEC). Crystals 2019, 9, 555. [Google Scholar] [CrossRef]
- SWGDRUGS—Monographs. Available online: https://swgdrug.org/monographs.htm (accessed on 20 February 2024).
- Carroll, F.I.; Blough, B.E.; Abraham, P.; Mills, A.C.; Holleman, J.A.; Wolckenhauer, S.A.; Decker, A.M.; Landavazo, A.; McElroy, K.T.; Navarro, H.A.; et al. Synthesis and Biological Evaluation of Bupropion Analogues as Potential Pharmacotherapies for Cocaine Addiction. J. Med. Chem. 2009, 52, 6768–6781. [Google Scholar] [CrossRef]
- Qian, Z.; Jia, W.; Li, T.; Hua, Z.; Liu, C. Identification of five pyrrolidinyl substituted cathinones and the collision-induced dissociation of electrospray-generated pyrrolidinyl substituted cathinones. Drug Test. Anal. 2017, 9, 778–787. [Google Scholar] [CrossRef]
- Błażewicz, A.; Bednarek, E.; Sitkowski, J.; Popławska, M.; Stypułkowska, K.; Bocian, W.; Kozerski, L. Identification and structural characterization of four novel synthetic cathinones: α-methylaminohexanophenone (hexedrone, HEX), 4-bromoethcathinone (4-BEC), 4-chloro-α-pyrrolidinopropiophenone (4-Cl-PPP), and 4-bromo-α-pyrrolidinopentiophenone (4-Br-PVP) after their seizures. Forensic Toxicol. 2017, 35, 317–332. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Nogueira, T.B.; Costa, V.M.; Branco, P.S.; Ferreira, L.M.; Fernandes, E.; Bastos, M.L.; Meisel, A.; Carvalho, F.; Capela, J.P. Neurotoxicity of “ecstasy” and its metabolites in human dopaminergic differentiated SH-SY5Y cells. Toxicol. Lett. 2013, 216, 159–170. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Susano, P.; Simões, M.; Guedes, M.; Martins, A.; Rehfeldt, S.; Gaspar, H.; Goettert, M.; et al. Disclosing the potential of eleganolone for Parkinson’s disease therapeutics: Neuroprotective and anti-inflammatory activities. Pharmacol. Res. 2021, 168, 105589. [Google Scholar] [CrossRef]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L.M. Antioxidant and anti-acetylcholinesterase activity of commercially available medicinal infusions after in vitro gastrointestinal digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef]
- Cabral, R.G.; Viegas, G.; Pacheco, R.; Sousa, A.C.; Robalo, M.P. Sustainable Synthesis, Antiproliferative and Acetylcholinesterase Inhibition of 1,4- and 1,2-Naphthoquinone Derivatives. Molecules 2023, 28, 1232. [Google Scholar] [CrossRef] [PubMed]



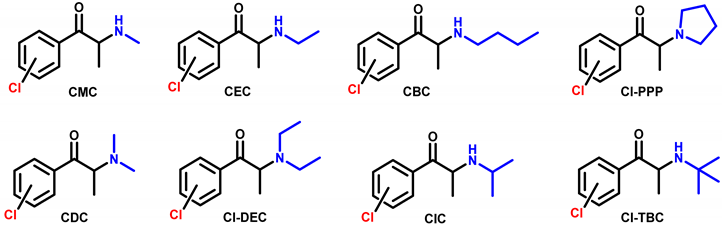 | |||||
|---|---|---|---|---|---|
| Cathinone | (Common Name, IUPAC Name) | Structure | Isomer | EUDA Date | |
| Type | Cl | ||||
| 2-CMC (1) | 2′-chloro-N-methylcathinone (R,S)-1-(2-chlorophenyl)-2-(methylamino)propan-1-one | CMC | 2′ | ortho | 2024 |
| 3-CMC (2) | 3′-chloro-N-methylcathinone (R,S)-1-(3-chlorophenyl)-2-(methylamino)propan-1-one | CMC | 3′ | meta | 2014 |
| 4-CMC (3) | 4′-chloro-N-methylcathinone (R,S)-1-(4-chlorophenyl)-2-(methylamino)propan-1-one | CMC | 4′ | para | 2014 |
| 2-CEC (4) | 2′-chloro-N-ethylcathinone (R,S)-1-(2-chlorophenyl)-2-(ethylamino)propan-1-one | CEC | 2′ | ortho | - |
| 3-CEC (5) | 3′-chloro-N-ethylcathinone (R,S)-1-(3-chlorophenyl)-2-(ethylamino)propan-1-one | CEC | 3′ | meta | 2016 |
| 4-CEC (6) | 4′-chloro-N-ethylcathinone (R,S)-1-(4-chlorophenyl)-2-(ethylamino)propan-1-one | CEC | 4′ | para | 2016 |
| 3-CBC (7) | 3′-chloro-N-butylcathinone (R,S)-2-(butylamino)-1-(3-chlorophenyl)propan-1-one | CBC | 3′ | meta | - |
| 4-CBC (8) | 4′-chloro-N-butylcathinone (R,S)-2-(butylamino)-1-(4-chlorophenyl)propan-1-one | CBC | 4′ | para | 2017 |
| 2-Cl-PPP (9) | 2′-chloro-α-pyrrolidinopropiophenone (R,S)-1-(2-chlorophenyl)-2-(pyrrolidine-1-yl)propan-1-one | Cl-PPP | 2′ | ortho | - |
| 3-Cl-PPP (10) | 3′-chloro-α-pyrrolidinopropiophenone (R,S)-1-(3-chlorophenyl)-2-(pyrrolidine-1-yl)propan-1-one | Cl-PPP | 3′ | meta | - |
| 4-Cl-PPP (11) | 4′-chloro-α-pyrrolidinopropiophenone (R,S)-1-(4-chlorophenyl)-2-(pyrrolidine-1-yl)propan-1-one | Cl-PPP | 4′ | para | 2014 |
| 2-CDC (12) | 2′-chloro-N,N-dimethylcathinone (R,S)-1-(2-chlorophenyl)-2-(dimethylamino)propan-1-one | CDC | 2′ | ortho | - |
| 3-CDC (13) | 3′-chloro-N,N-dimethylcathinone (R,S)-1-(3-chlorophenyl)-2-(dimethylamino)propan-1-one | CDC | 3′ | meta | - |
| 4-CDC (14) | 4′-chloro-N,N-dimethylcathinone (R,S)-1-(4-chlorophenyl)-2-(dimethylamino)propan-1-one | CDC | 4′ | para | 2015 |
| 3-Cl-DEC (15) | 3′-chloro-N,N-diethylcathinone (R,S)-1-(3-chlorophenyl)-2-(diethylamino)propan-1-one | Cl-DEC | 3′ | meta | - |
| 4-Cl-DEC (16) | 4′-chloro-N,N-diethylcathinone (R,S)-1-(4-chlorophenyl)-2-(diethylamino)propan-1-one | Cl-DEC | 4′ | para | - |
| 3-CIC (17) | 3′-chloro-isopropylcathinone (R,S)-1-(3-chlorophenyl)-2-(isopropylamino)propan-1-one | CIC | 3′ | meta | - |
| 4-CIC (18) | 4′-chloro-isopropylcathinone (R,S)-1-(4-chlorophenyl)-2-(isopropylamino)propan-1-one | CIC | 4′ | para | 2016 |
| 3-Cl-TBC (19) | 3′-chloro-tert-butylcathinone (R,S)-2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one | Cl-TBC | 3′ | meta | 2014 |
| 4-Cl-TBC (20) | 4′-chloro-tert-butylcathinone (R,S)-2-(tert-butylamino)-1-(4-chlorophenyl)propan-1-one | Cl-TBC | 4′ | para | - |
| Cathinones | LC50 (m) | [CI] (M) | Hillslope | LOAEL (mM) | B | 2-CMC (1) | 3-CMC (2) | 4-CMC (3) | 2-CEC (4) | 3-CEC (5) | 4-CEC (6) | 3-CBC (7) | 4-CBC (8) | 2-Cl-PPP (9) | 3-Cl-PPP (10) | 4-Cl-PPP (11) | 2-CDC (12) | 3-CDC (13) | 4-CDC (14) | 3-Cl-DEC (15) | 4-Cl-DEC (16) | 3-CIC (17) | 4-CIC (18) | 3-Cl-TBC (19) | 4-Cl-TBC (20) | Mephedrone (M) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | |||||||||||||||||||||||||||
| 2-CMC (1) | 1.7 | [1.2–2.5] | 4.3 | 1.0 | (1) | ||||||||||||||||||||||
| 3-CMC (2) | 2.3 | [1.8–2.9] | 2.5 | 1.0 | (2) | > | |||||||||||||||||||||
| 4-CMC (3) | 1.3 | [0.9–1.9] | 1.6 | 0.1 | (3) | < | < | ||||||||||||||||||||
| 2-CEC (4) | 1.6 | [1.3–2.0] | 4.4 | 0.5 | (4) | NS | < | > | |||||||||||||||||||
| 3-CEC (5) | 1.3 | [0.8–2.3] | 1.7 | 0.5 | (5) | < | < | NS | < | ||||||||||||||||||
| 4-CEC (6) | 1.3 | [1.0–1.7] | 3.4 | 0.1 | (6) | < | < | NS | < | NS | |||||||||||||||||
| 3-CBC (7) | 0.7 | [0.5–1.0] | 1.4 | 0.1 | (7) | < | < | < | < | < | < | ||||||||||||||||
| 4-CBC (8) | 0.6 | [0.4–1.0] | 10.5 | 0.5 | (8) | < | < | < | < | < | < | < | |||||||||||||||
| 2-Cl-PPP (9) | 1.6 | [1.2–1.9] | 2.4 | 0.5 | (9) | NS | < | > | NS | > | > | > | > | ||||||||||||||
| 3-Cl-PPP (10) | 2.5 | [2.0–2.9] | 1.7 | 0.1 | (10) | > | NS | > | > | > | > | > | > | > | |||||||||||||
| 4-Cl-PPP (11) | 1.8 | [1.2–2.5] | 0.9 | 0.5 | (11) | NS | < | > | NS | > | > | > | > | > | < | ||||||||||||
| 2-CDC (12) | 1.3 | [1.1–1.5] | 12.4 | 1.0 | (12) | < | < | NS | < | NS | NS | > | > | < | < | < | |||||||||||
| 3-CDC (13) | 1.8 | [1.2–2.7] | 6.3 | 1.0 | (13) | NS | < | > | > | > | > | > | > | > | < | NS | > | ||||||||||
| 4-CDC (14) | 1.5 | [1.1–2.1] | 5.6 | 1.0 | (14) | NS | < | > | NS | > | > | > | > | NS | < | < | > | < | |||||||||
| 3-Cl-DEC (15) | 1.2 | [1.0–1.5] | 4.3 | 1.0 | (15) | < | < | NS | < | NS | NS | > | > | < | < | < | NS | < | < | ||||||||
| 4-Cl-DEC (16) | 1.0 | [1.0–1.1] | 17.4 | 1.0 | (16) | < | < | < | < | < | < | > | > | < | < | < | < | < | < | < | |||||||
| 3-CIC (17) | 1.6 | [1.2–2.1] | 4.4 | 1.0 | (17) | NS | < | > | NS | > | > | > | > | NS | < | NS | > | < | NS | > | > | ||||||
| 4-CIC (18) | 1.4 | [1.0–2.0] | 4.5 | 1.0 | (18) | < | < | NS | < | NS | NS | > | > | NS | < | < | NS | < | NS | > | > | < | |||||
| 3-Cl-TBC (19) | 1.0 | [0.9–1.2] | 3.5 | 0.5 | (19) | < | < | < | < | < | < | > | > | < | < | < | < | < | < | < | NS | < | < | ||||
| 4-Cl-TBC (20) | 1.3 | [1.1–1.7] | 2.4 | 0.5 | (20) | < | < | NS | < | NS | NS | > | > | < | < | < | NS | < | < | NS | > | < | NS | > | |||
| Mephedrone (M) | 2.1 | [1.4–3.1] | 2.3 | 0.5 | (M) | > | NS | > | > | > | > | > | > | > | < | > | > | > | > | > | > | > | > | > | > | ||
| Cathinones | IC50 (m) | [CI] (M) | Hillslope | LOAEL (mM) | B | 2-CMC (1) | 3-CMC (2) | 4-CMC (3) | 2-CEC (4) | 3-CEC (5) | 4-CEC (6) | 3-CBC (7) | 4-CBC (8) | 2-Cl-PPP (9) | 3-Cl-PPP (10) | 4-Cl-PPP (11) | 2-CDC (12) | 3-CDC (13) | 4-CDC (14) | 3-Cl-DEC (15) | 4-Cl-DEC (16) | 3-CIC (17) | 4-CIC (18) | 3-Cl-TBC (19) | 4-Cl-TBC (20) | Mephedrone (M) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | |||||||||||||||||||||||||||
| 2-CMC (1) | 0.7 | [0.4–1.3] | 2.5 | 0.250 | (1) | ||||||||||||||||||||||
| 3-CMC (2) | 0.8 | [0.6–0.9] | 3.7 | 0.500 | (2) | >NS | |||||||||||||||||||||
| 4-CMC (3) | ~ | 2 * | 1.5 | 0.250 | (3) | > | > | ||||||||||||||||||||
| 2-CEC (4) | 0.6 | [0.5–0.6] | 4.3 | 0.500 | (4) | NS | < | < | |||||||||||||||||||
| 3-CEC (5) | 0.8 | [0.5–1.2] | 1.3 | 0.250 | (5) | NS | NS | < | > | ||||||||||||||||||
| 4-CEC (6) | 1.2 | [1.0–1.4] | 1.2 | 0.250 | (6) | > | > | < | > | > | |||||||||||||||||
| 3-CBC (7) | 0.4 | [0.3–0.5] | 1.4 | 0.125 | (7) | < | < | < | < | < | < | ||||||||||||||||
| 4-CBC (8) | 1.3 | [1.0–1.8] | 5.1 | 0.100 | (8) | > | > | < | > | > | NS | > | |||||||||||||||
| 2-Cl-PPP (9) | 0.3 | [0.2–0.5] | 1.5 | 0.125 | (9) | < | < | < | < | < | < | < | < | ||||||||||||||
| 3-Cl-PPP (10) | 0.4 | [0.2–0.8] | 1.8 | 0.125 | (10) | < | < | < | < | < | < | NS | < | > | |||||||||||||
| 4-Cl-PPP (11) | 0.5 | [0.3–0.7] | 1.1 | 0.250 | (11) | < | < | < | < | < | < | NS | < | > | NS | ||||||||||||
| 2-CDC (12) | 0.7 | [0.6–0.8] | 2.5 | 0.250 | (12) | NS | NS | < | NS | NS | < | > | < | > | > | > | |||||||||||
| 3-CDC (13) | 0.9 | [0.9–1.1] | 1.2 | 0.250 | (13) | > | > | < | > | > | > | > | < | > | > | > | > | ||||||||||
| 4-CDC (14) | 0.7 | [0.7–0.8] | 2.9 | 0.50 | (14) | NS | NS | < | > | NS | < | > | < | > | > | > | >NS | > | |||||||||
| 3-Cl-DEC (15) | 0.3 | [0.1–0.6] | 0.9 | 0.063 | (15) | < | < | < | < | < | < | < | < | NS | < | < | < | < | < | ||||||||
| 4-Cl-DEC (16) | 0.1 | [0.1–0.2] | 0.8 | 0.063 | (16) | < | < | < | < | < | < | < | < | < | < | < | < | < | < | < | |||||||
| 3-CIC (17) | 0.2 | [0.1–0.2] | 0.8 | 0.063 | (17) | < | < | < | < | < | < | < | < | < | < | < | < | < | < | < | NS | ||||||
| 4-CIC (18) | 1.1 | [0.7–1.7] | 2.0 | 0.375 | (18) | > | > | < | > | > | NS | > | NS | > | > | > | > | NS | > | > | > | > | |||||
| 3-Cl-TBC (19) | 1.0 | [0.9–1.2] | 0.9 | 0.250 | (19) | > | > | < | > | > | NS | > | < | > | > | > | > | NS | > | > | > | > | NS | ||||
| 4-Cl-TBC (20) | ~ | 2 ** | 2.7 | 0.500 | (20) | > | > | ND | > | > | > | > | > | > | > | > | > | > | > | > | > | > | > | > | |||
| Mephedrone (M) | 0.8 | [0.8–0.9] | 8.6 | 0.750 | (M) | NS | NS | < | > | NS | < | > | < | > | > | > | NS | NS | NS | > | > | < | < | < | < | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.P.; Ferro, R.; Pinto, D.; Silva, J.; Alves, C.; Pacheco, R.; Gaspar, H. Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact. Int. J. Mol. Sci. 2025, 26, 3540. https://doi.org/10.3390/ijms26083540
Gomes AP, Ferro R, Pinto D, Silva J, Alves C, Pacheco R, Gaspar H. Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact. International Journal of Molecular Sciences. 2025; 26(8):3540. https://doi.org/10.3390/ijms26083540
Chicago/Turabian StyleGomes, Ana Patrícia, Raquel Ferro, Daniela Pinto, Joana Silva, Celso Alves, Rita Pacheco, and Helena Gaspar. 2025. "Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact" International Journal of Molecular Sciences 26, no. 8: 3540. https://doi.org/10.3390/ijms26083540
APA StyleGomes, A. P., Ferro, R., Pinto, D., Silva, J., Alves, C., Pacheco, R., & Gaspar, H. (2025). Synthesis, Characterization, and Biological Effects of Chloro-Cathinones: Toxicity and Potential Neurological Impact. International Journal of Molecular Sciences, 26(8), 3540. https://doi.org/10.3390/ijms26083540











